Unique and Conserved Endoplasmic Reticulum Stress Responses in Neuroendocrine Cells
Abstract
Highlights
- ER stress induces core unfolded protein response genes across neuroendocrine cell types, as well as unique transcriptional programs.
- Comparative transcriptomics reveals concordant and discordant responses to ER stress, highlighting potential roles of novel gene candidates.
- Discordant gene expression may explain differences in susceptibility to ER stress-induced dysfunction and death.
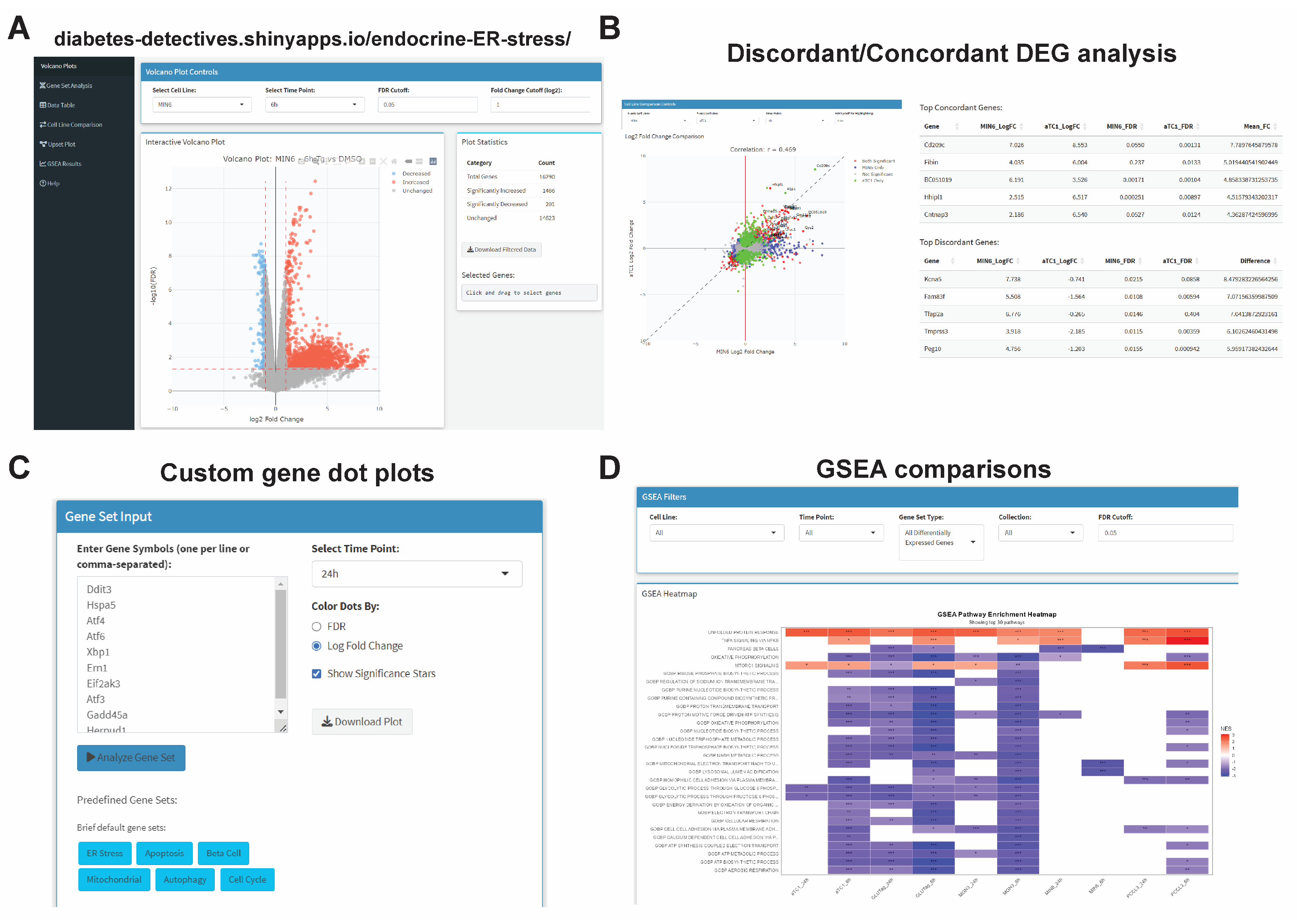
- An interactive web tool enables custom end-user analysis of neuroendocrine cell ER stress responses.
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Cell Culture, Treatments, and RNA Isolation
2.3. Human Islet Culture and Treatment
2.4. RNA Isolation and RT-qPCR Validation
2.5. Transcriptomics
2.6. Data Processing and Analysis
2.7. Interactive Web Tool Design
2.8. Statistical Analysis
3. Results
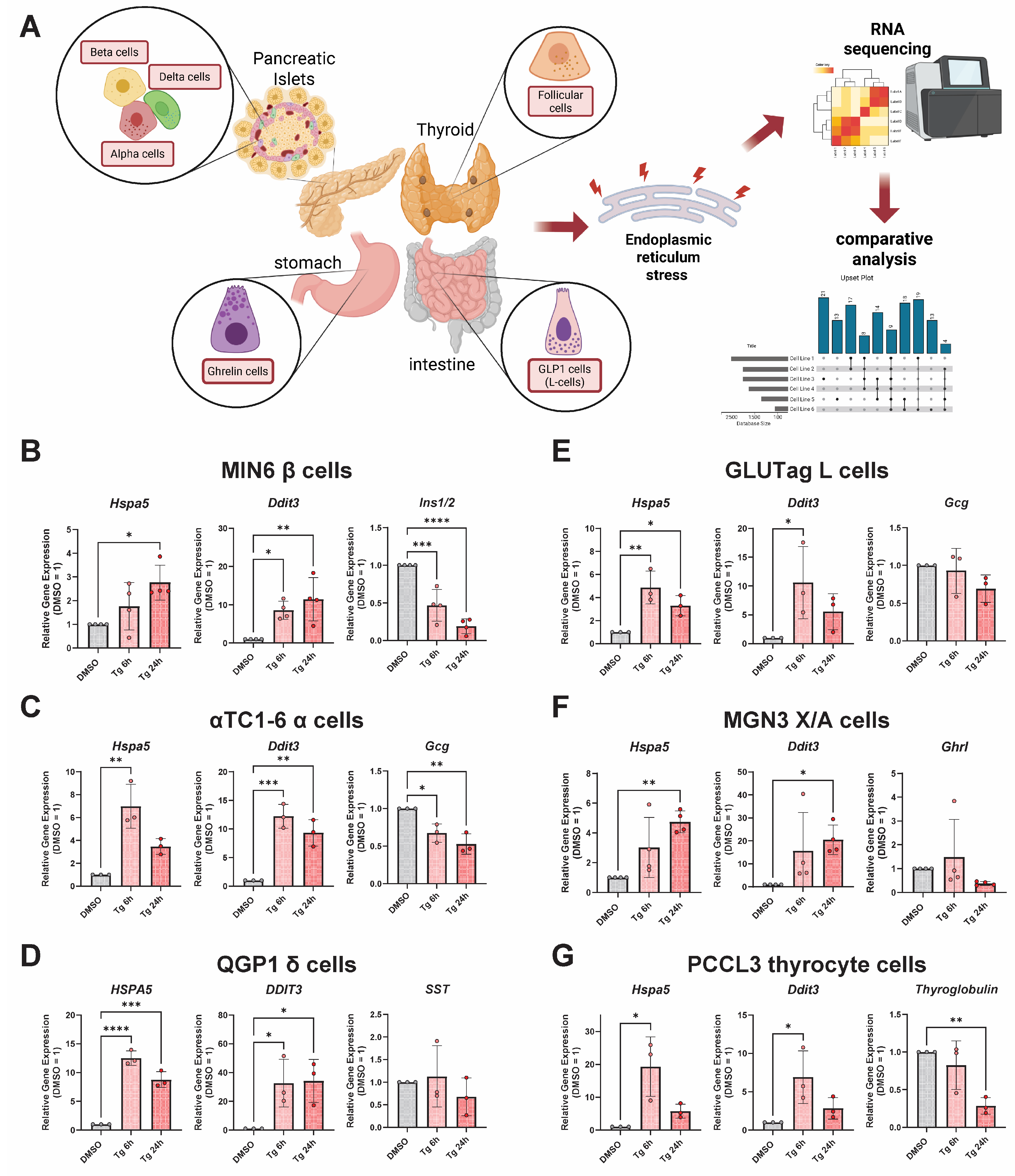
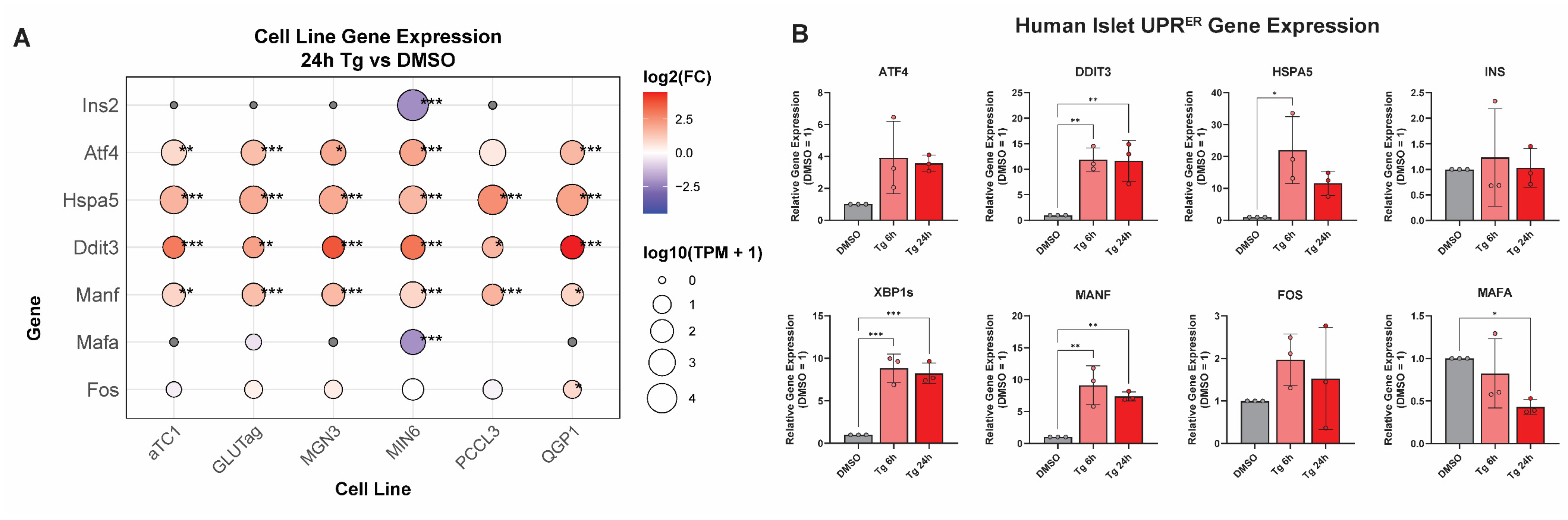
3.1. Thapsigargin Induces ER Stress in Multiple Endocrine Cell Lines from Distinct Tissue Types
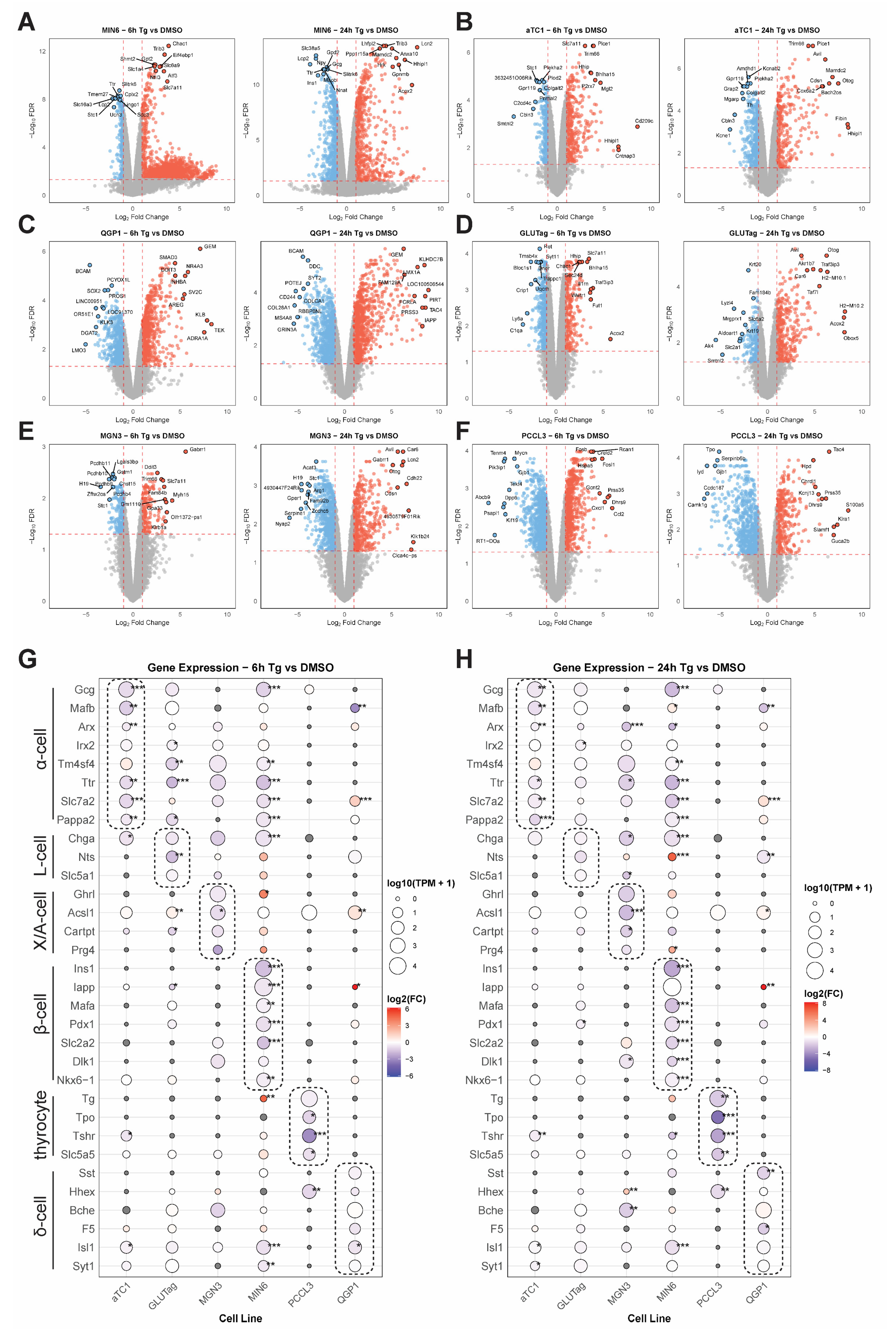
3.2. Integrating Cell-Type-Specific UPRER Transcriptomic Responses Identifies Common and Unique Gene Signatures
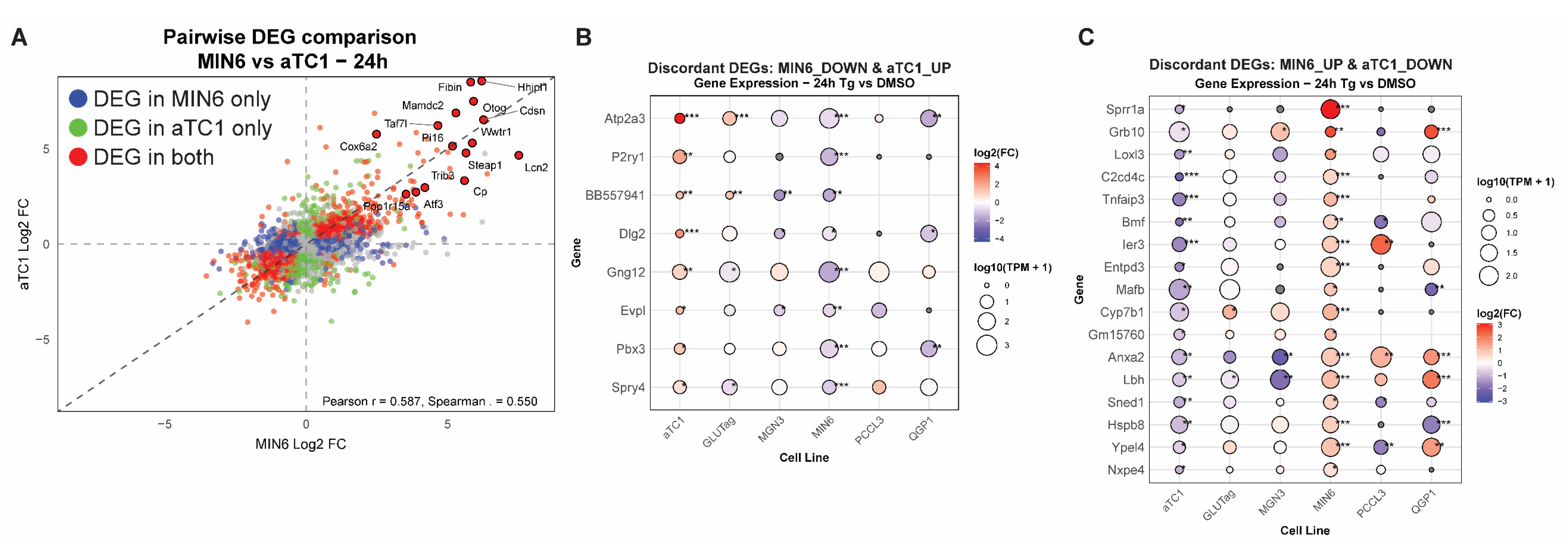
3.3. Cell-Type Comparisons Identify Concordant and Discordant Responses to UPRER in β-Cells Versus α-Cells
3.4. Generation of an Interactive Transcriptomics Browser for Endocrine UPRER
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UPRER | Unfolded protein response of the endoplasmic reticulum |
| T1D | Type 1 diabetes |
| T2D | Type 2 diabetes |
| GSEA | Gene set enrichment analysis |
| GLP1 | Glucagon-like peptide 1 |
| Tg | Thapsigargin |
References
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Hollien, J. Evolution of the unfolded protein response. Biochim. Biophys. Acta 2013, 1833, 2458–2463. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Axten, J.M.; Patterson, J.B. Pharmacological targeting of the unfolded protein response for disease intervention. Nat. Chem. Biol. 2019, 15, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Kabil, O.; Young, K.Z.; Kellogg, A.P.; Chang, A.; Arvan, P. Thyrocyte cell survival and adaptation to chronic endoplasmic reticulum stress due to misfolded thyroglobulin. J. Biol. Chem. 2020, 295, 6876–6887. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, D.T.; Kaufman, R.J. That which does not kill me makes me stronger: Adapting to chronic ER stress. Trends Biochem. Sci. 2007, 32, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic beta-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Kalwat, M.A.; Scheuner, D.; Rodrigues-Dos-Santos, K.; Eizirik, D.L.; Cobb, M.H. The Pancreatic ss-cell Response to Secretory Demands and Adaption to Stress. Endocrinology 2021, 162, bqab173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eizirik, D.L.; Szymczak, F.; Mallone, R. Why does the immune system destroy pancreatic beta-cells but not alpha-cells in type 1 diabetes? Nat. Rev. Endocrinol. 2023, 19, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, H.; Hayashi, I.; Kono, A. A Somatostatin-secreting Cell Line Established from a Human Pancreatic Islet Cell Carcinoma (Somatostatinoma): Release Experiment and Immunohistochemical Study. Cancer Res. 1990, 50, 3691–3693. [Google Scholar]
- Iwakura, H.; Li, Y.; Ariyasu, H.; Hosoda, H.; Kanamoto, N.; Bando, M.; Yamada, G.; Hosoda, K.; Nakao, K.; Kangawa, K.; et al. Establishment of a novel ghrelin-producing cell line. Endocrinology 2010, 151, 2940–2945. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, K.; Leiter, E.H. Comparison of cytokine effects on mouse pancreatic alpha-cell and beta-cell lines. Viability, secretory function, and MHC antigen expression. Diabetes 1990, 39, 415–425. [Google Scholar] [CrossRef]
- Morishita, Y.; Kellogg, A.P.; Larkin, D.; Chen, W.; Vadrevu, S.; Satin, L.; Liu, M.; Arvan, P. Cell death-associated lipid droplet protein CIDE-A is a noncanonical marker of endoplasmic reticulum stress. JCI Insight 2021, 6, e143980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lei, X.; Zhang, S.; Bohrer, A.; Barbour, S.E.; Ramanadham, S. Role of calcium-independent phospholipase A(2)beta in human pancreatic islet beta-cell apoptosis. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1386–E1395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abadpour, S.; Gopel, S.O.; Schive, S.W.; Korsgren, O.; Foss, A.; Scholz, H. Glial cell-line derived neurotrophic factor protects human islets from nutrient deprivation and endoplasmic reticulum stress induced apoptosis. Sci. Rep. 2017, 7, 1575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weldemariam, M.M.; Sudhir, P.R.; Woo, J.; Zhang, Q. Effects of multiple stressors on pancreatic human islets proteome reveal new insights into the pathways involved. Proteomics 2023, 23, e2300022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oleson, B.J.; McGraw, J.A.; Broniowska, K.A.; Annamalai, M.; Chen, J.; Bushkofsky, J.R.; Davis, D.B.; Corbett, J.A.; Mathews, C.E. Distinct differences in the responses of the human pancreatic beta-cell line EndoC-betaH1 and human islets to proinflammatory cytokines. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R525–R534. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alvelos, M.I.; Szymczak, F.; Castela, A.; Marin-Canas, S.; de Souza, B.M.; Gkantounas, I.; Colli, M.; Fantuzzi, F.; Cosentino, C.; Igoillo-Esteve, M.; et al. A functional genomic approach to identify reference genes for human pancreatic beta cell real-time quantitative RT-PCR analysis. Islets 2021, 13, 51–65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roy, G.; Ordonez, A.; Binns, D.D.; Rodrigues-Dos-Santos, K.; Kwakye, M.B.; King, G.C.; Kuntz, R.L.; Mukherjee, N.; Templin, A.T.; Tan, Z.; et al. VDAC1 is a target for pharmacologically induced insulin hypersecretion in beta cells. Cell Rep. 2025, 44, 115834. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Breese, M.R.; Liu, Y. NGSUtils: A software suite for analyzing and manipulating next-generation sequencing datasets. Bioinformatics 2013, 29, 494–496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.; Huber, W.; Pages, H.; Aboyoun, P.; Carlson, M.; Gentleman, R.; Morgan, M.T.; Carey, V.J. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 2013, 9, e1003118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szklarczyk, D.; Nastou, K.; Koutrouli, M.; Kirsch, R.; Mehryary, F.; Hachilif, R.; Hu, D.; Peluso, M.E.; Huang, Q.; Fang, T.; et al. The STRING database in 2025: Protein networks with directionality of regulation. Nucleic Acids Res. 2025, 53, D730–D737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodrigues-Dos-Santos, K.; Roy, G.; Binns, D.D.; Grzemska, M.G.; Barella, L.F.; Armoo, F.; McCoy, M.K.; Huynh, A.V.; Yang, J.Z.; Posner, B.A.; et al. Small Molecule-mediated Insulin Hypersecretion Induces Transient ER Stress Response and Loss of Beta Cell Function. Endocrinology 2022, 163, bqac081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romitti, M.; Eski, S.E.; Fonseca, B.F.; Gillotay, P.; Singh, S.P.; Costagliola, S. Single-Cell Trajectory Inference Guided Enhancement of Thyroid Maturation In Vitro Using TGF-Beta Inhibition. Front. Endocrinol. 2021, 12, 657195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beumer, J.; Puschhof, J.; Bauza-Martinez, J.; Martinez-Silgado, A.; Elmentaite, R.; James, K.R.; Ross, A.; Hendriks, D.; Artegiani, B.; Busslinger, G.A.; et al. High-Resolution mRNA and Secretome Atlas of Human Enteroendocrine Cells. Cell 2020, 181, 1291–1306.e19. [Google Scholar] [CrossRef] [PubMed]
- Elgamal, R.M.; Kudtarkar, P.; Melton, R.L.; Mummey, H.M.; Benaglio, P.; Okino, M.L.; Gaulton, K.J. An Integrated Map of Cell Type-Specific Gene Expression in Pancreatic Islets. Diabetes 2023, 72, 1719–1728. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tritschler, S.; Thomas, M.; Böttcher, A.; Ludwig, B.; Schmid, J.; Schubert, U.; Kemter, E.; Wolf, E.; Lickert, H.; Theis, F.J. A transcriptional cross species map of pancreatic islet cells. Mol. Metab. 2022, 66, 101595. [Google Scholar] [CrossRef] [PubMed]
- Coomans de Brachene, A.; Scoubeau, C.; Musuaya, A.E.; Costa-Junior, J.M.; Castela, A.; Carpentier, J.; Faoro, V.; Klass, M.; Cnop, M.; Eizirik, D.L. Exercise as a non-pharmacological intervention to protect pancreatic beta cells in individuals with type 1 and type 2 diabetes. Diabetologia 2023, 66, 450–460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panzhinskiy, E.; Skovsø, S.; Cen, H.H.; Chu, K.Y.; MacDonald, K.; Soukhatcheva, G.; Dionne, D.A.; Hallmaier-Wacker, L.K.; Wildi, J.S.; Marcil, S.; et al. Eukaryotic translation initiation factor 2A protects pancreatic beta cells during endoplasmic reticulum stress while rescuing translation inhibition. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lytton, J.; Westlin, M.; Hanley, M.R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J. Biol. Chem. 1991, 266, 17067–17071. [Google Scholar] [CrossRef]
- Maestas, M.M.; Ishahak, M.; Augsornworawat, P.; Veronese-Paniagua, D.A.; Maxwell, K.G.; Velazco-Cruz, L.; Marquez, E.; Sun, J.; Shunkarova, M.; Gale, S.E.; et al. Identification of unique cell type responses in pancreatic islets to stress. Nat. Commun. 2024, 15, 5567. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, W.; Cheng, J.; Allaire, J.; Sievert, C.; Schloerke, B.; Aden-Buie, G.; Xie, Y.; Allen, J.; McPherson, J.; Dipert, A.; et al. Shiny: Web Application Framework for R, R package version 1.11.1.9000; R Project: Vienna, Austria, 2025. [Google Scholar]
- Marhfour, I.; Lopez, X.M.; Lefkaditis, D.; Salmon, I.; Allagnat, F.; Richardson, S.J.; Morgan, N.G.; Eizirik, D.L. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia 2012, 55, 2417–2420. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, R.J.; Back, S.H.; Song, B.; Han, J.; Hassler, J. The unfolded protein response is required to maintain the integrity of the endoplasmic reticulum, prevent oxidative stress and preserve differentiation in beta-cells. Diabetes Obes. Metab. 2010, 12 (Suppl. S2), 99–107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, K.; Chan, J.Y.; Liang, C.; Ip, C.K.; Shi, Y.C.; Herzog, H.; Hughes, W.E.; Bensellam, M.; Delghingaro-Augusto, V.; Koina, M.E.; et al. XBP1 maintains beta cell identity, represses beta-to-alpha cell transdifferentiation and protects against diabetic beta cell failure during metabolic stress in mice. Diabetologia 2022, 65, 984–996. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.; Lee, Y.S.; Harenda, Q.; Pietrzak, S.; Oktay, H.Z.; Schreiber, S.; Liao, Y.; Sonthalia, S.; Ciecko, A.E.; Chen, Y.G.; et al. Beta Cell Dedifferentiation Induced by IRE1alpha Deletion Prevents Type 1 Diabetes. Cell Metab. 2020, 31, 822–836 e825. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haataja, L.; Arunagiri, A.; Hassan, A.; Regan, K.; Tsai, B.; Dhayalan, B.; Weiss, M.A.; Liu, M.; Arvan, P. Distinct states of proinsulin misfolding in MIDY. Cell. Mol. Life Sci. 2021, 78, 6017–6031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, M.; Weiss, M.A.; Arunagiri, A.; Yong, J.; Rege, N.; Sun, J.; Haataja, L.; Kaufman, R.J.; Arvan, P. Biosynthesis, structure, and folding of the insulin precursor protein. Diabetes Obes. Metab. 2018, 20 (Suppl. S2), 28–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michels, A.W.; Brusko, T.M.; Evans-Molina, C.; Homann, D.; Richardson, S.J.; Powers, A.C. Challenges and Opportunities for Understanding the Pathogenesis of Type 1 Diabetes: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2025, 110, 2496–2508. [Google Scholar] [CrossRef] [PubMed]
- Arredouani, A.; Guiot, Y.; Jonas, J.C.; Liu, L.H.; Nenquin, M.; Pertusa, J.A.; Rahier, J.; Rolland, J.F.; Shull, G.E.; Stevens, M.; et al. SERCA3 ablation does not impair insulin secretion but suggests distinct roles of different sarcoendoplasmic reticulum Ca(2+) pumps for Ca(2+) homeostasis in pancreatic beta-cells. Diabetes 2002, 51, 3245–3253. [Google Scholar] [CrossRef] [PubMed]
- Mawla, A.M.; Huising, M.O. Navigating the Depths and Avoiding the Shallows of Pancreatic Islet Cell Transcriptomes. Diabetes 2019, 68, 1380–1393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, H.T.; Noriega Polo, C.; Wiederkehr, A.; Wollheim, C.B.; Park, K.S. CDN1163, an activator of sarco/endoplasmic reticulum Ca(2+) ATPase, up-regulates mitochondrial functions and protects against lipotoxicity in pancreatic beta-cells. Br. J. Pharmacol. 2023, 180, 2762–2776. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.N.; Reissaus, C.A.; Kono, T.; Evans-Molina, C. 2112-P: Sarco/Endoplasmic Reticulum ATPase (SERCA2) Deficiency in the Nonobese Diabetic (NOD) Mouse Accelerates Type 1 Diabetes Development. Diabetes 2020, 69, 2112. [Google Scholar] [CrossRef]
- Huang, H.; Weng, H.; Sun, W.; Qin, X.; Shi, H.; Wu, H.; Zhao, B.S.; Mesquita, A.; Liu, C.; Yuan, C.L.; et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018, 20, 285–295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Jesus, D.F.; Zhang, Z.; Kahraman, S.; Brown, N.K.; Chen, M.; Hu, J.; Gupta, M.K.; He, C.; Kulkarni, R.N. m(6)A mRNA Methylation Regulates Human beta-Cell Biology in Physiological States and in Type 2 Diabetes. Nat. Metab. 2019, 1, 765–774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Jesus, D.F.; Zhang, Z.; Brown, N.K.; Li, X.; Gaffrey, M.J.; Kahraman, S.; Wei, J.; Hu, J.; Basile, G.; Xiao, L.; et al. Redox Regulation of m (6) A Methyltransferase METTL3 in Human beta-cells Controls the Innate Immune Response in Type 1 Diabetes. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gibbs, S.; Fijneman, R.; Wiegant, J.; van Kessel, A.G.; van De Putte, P.; Backendorf, C. Molecular characterization and evolution of the SPRR family of keratinocyte differentiation markers encoding small proline-rich proteins. Genomics 1993, 16, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, L.; Miyatsuka, T.; Himuro, M.; Wakabayashi, Y.; Osonoi, S.; Miura, M.; Katahira, T.; Fujitani, Y.; Iida, H.; Mizukami, H.; et al. Cumulative autophagy insufficiency in mice leads to progression of beta-cell failure. Biochem. Biophys. Res. Commun. 2022, 611, 38–45. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues-dos-Santos, K.; Roy, G.; Geisinger, A.; Somalraju, S.; Johnson, T.S.; Kalwat, M.A. Unique and Conserved Endoplasmic Reticulum Stress Responses in Neuroendocrine Cells. Cells 2025, 14, 1529. https://doi.org/10.3390/cells14191529
Rodrigues-dos-Santos K, Roy G, Geisinger A, Somalraju S, Johnson TS, Kalwat MA. Unique and Conserved Endoplasmic Reticulum Stress Responses in Neuroendocrine Cells. Cells. 2025; 14(19):1529. https://doi.org/10.3390/cells14191529
Chicago/Turabian StyleRodrigues-dos-Santos, Karina, Gitanjali Roy, Anna Geisinger, Sahiti Somalraju, Travis S. Johnson, and Michael A. Kalwat. 2025. "Unique and Conserved Endoplasmic Reticulum Stress Responses in Neuroendocrine Cells" Cells 14, no. 19: 1529. https://doi.org/10.3390/cells14191529
APA StyleRodrigues-dos-Santos, K., Roy, G., Geisinger, A., Somalraju, S., Johnson, T. S., & Kalwat, M. A. (2025). Unique and Conserved Endoplasmic Reticulum Stress Responses in Neuroendocrine Cells. Cells, 14(19), 1529. https://doi.org/10.3390/cells14191529






