Beyond Molecular Markers: The Therapeutic Significance of Mesenchymal Stem Cell Deformability in Regenerative Medicine
Abstract
Highlights
- Cellular deformability is an integrative, functional biomarker of MSC quality, correlated with stemness, homing efficiency, early differentiation, and aging status.
- Real-time deformability cytometry (RT-DC) and emerging AI-based imaging predictors represent the most translatable tools for mechanotype assessment. A practical GMP-oriented framework is proposed.
- Incorporating deformability into ATMP quality control and sorting can enrich preparations with therapeutically potent MSC subpopulations, reduce heterogeneity, and improve clinical outcomes.
- Standardized protocols and validation, combined with multi-omics integration, can enable personalized, mechanotype-guided manufacturing of MSC therapies.
Abstract
1. Introduction
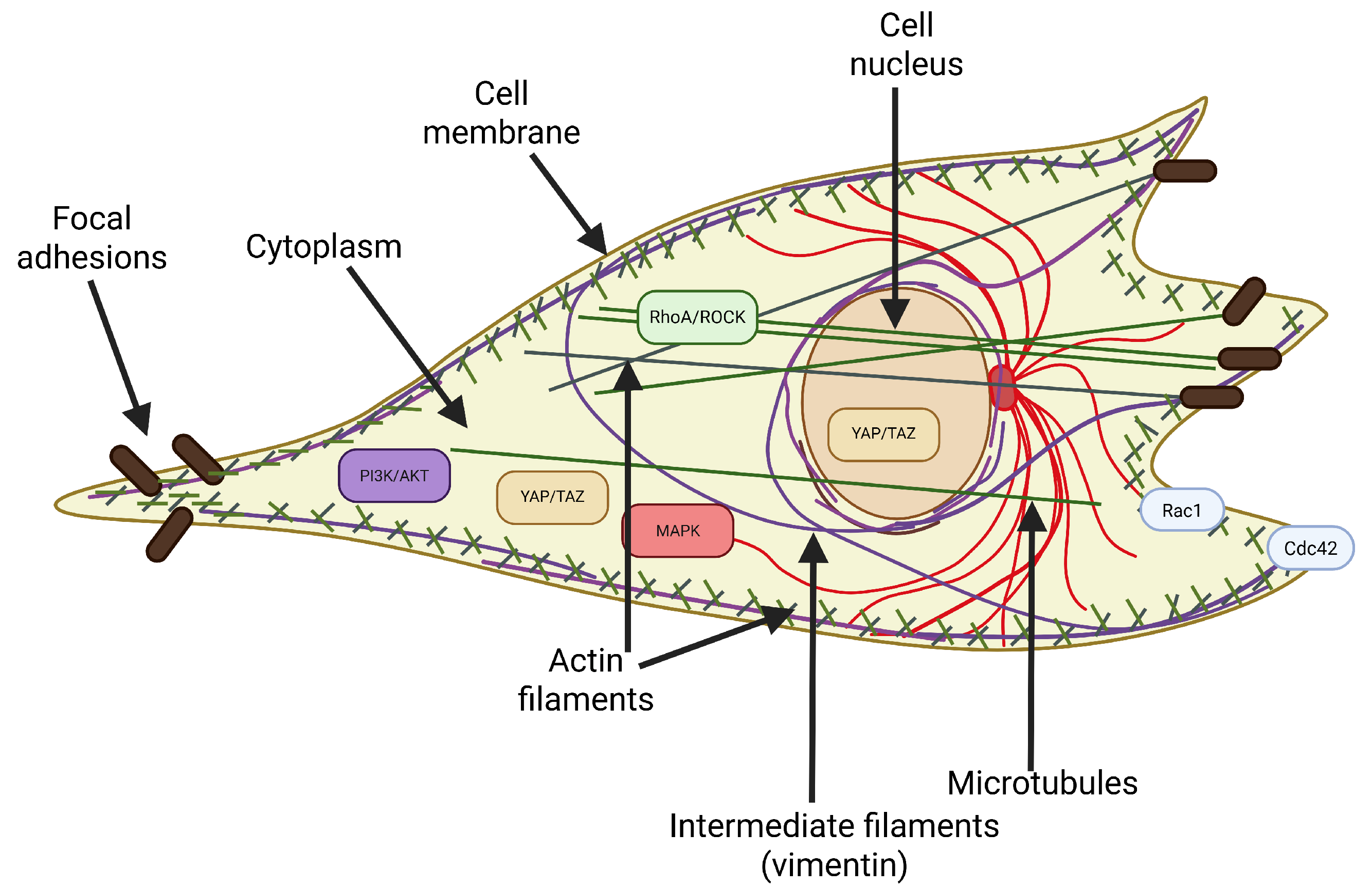
2. Determinants of Cellular Deformability
2.1. The Cytoskeleton as the Primary Determinant of Deformability
- Actin filaments (F-actin) form a dense network just below the cell membrane, called the actin cortex, responsible for the cell’s resistance to deformation under low forces. The remodeling of this network, including the formation of stress fibers, directly affects the cell’s deformability.
- Microtubules ensure the spatial stability of the cell and its resistance to compressive forces. They also play an important role in the organization of organelles and intracellular transport, and their depolymerization can indirectly affect the tension of the actin cortex.
- Intermediate filaments (mainly vimentin) are responsible for the cell’s resistance to tensile and compressive deformation. Vimentin transfers mechanical forces from the cytoplasm to the nucleus and stabilizes the cell nucleus’s position, especially during migration through narrow spaces.
2.2. Other Structural Components Affecting Deformability
- The cell membrane. Its lipid composition, the presence of cholesterol, and interactions with the cytoskeleton affect its deformability and susceptibility to deformation. The lipid bilayer is intrinsically heterogeneous; cholesterol-rich “lipid-rigid” domains coexist with more compliant regions. This mosaic organization generates local variations in bending rigidity and modulates cytoskeletal anchoring. As a result, membrane composition can bias downstream cytoskeletal responses, for example, by stabilizing stress fibers in rigid lipid domains or promoting lamellipodia in more fluid regions [44,45,46]. Greater membrane fluidity may promote local deformation, but it plays a key role in the overall mechanics of the cell only in combination with the dynamic actin cortex.
- The cell nucleus. It is the largest and hardest organelle, often limiting the cell’s ability to pass through narrow spaces. The stiffness of the nucleus, which depends on the level of lamin A/C and chromatin condensation, can determine the cell’s overall deformability [47]. Mutations in LMNA (lamin A/C), reported in laminopathies, alter nuclear stiffness and compromise nuclear adaptability [48]. Such alterations may block efficient passage through confined tissue spaces, diminishing their homing efficiency and regenerative potential [49]. Pathological stiffening of the nuclear envelope also impairs chromatin organization, thereby influencing lineage commitment and accelerating features of premature senescence [50].
- Cytoplasmic viscosity determines the ease with which organelles and macromolecules move within the cytosolic space and, consequently, how the whole cell responds to deformation [51]. The cytoplasm is not a simple fluid but a crowded, viscoelastic medium whose properties are shaped by protein concentration, cytoskeletal crosslinking, and metabolic activity [52]. Increases in macromolecular crowding, aggregation of structural proteins, and oxidative stress can elevate viscosity, thereby restricting intracellular flow and reducing compliance. Conversely, active ATP-dependent processes, such as actin turnover and vesicle trafficking, can transiently fluidize the cytoplasm, enhancing its ability to deform [53].
- Osmotic pressure provides another layer of regulation of cellular deformability by directly modulating intracellular volume and hydrostatic balance. Mechano-osmotic coupling ensures that ion and water fluxes, mediated by channels and transporters such as aquaporins, Na+/K+-ATPase, and mechanosensitive ion channels (i.e., Piezo1, TRPV4), continuously adjust intracellular pressure in response to mechanical stress [54,55]. Swelling under hypo-osmotic conditions can reduce cortical tension and increase deformability, while shrinkage under hyper-osmotic stress can stiffen the cell and hinder passage through confined environments [56].
- Focal adhesions are the mechanical interface between the cytoskeleton and the substrate. Their size, number, and maturity affect the cell’s tension and ability to change shape. Strongly anchored cells have limited deformability [57].
2.3. Pathways Regulating Cell Deformability
2.3.1. Canonical Regulators (RhoA/ROCK, Rac1/Cdc42, MAPK, and PI3K/AKT)
2.3.2. Hippo, YAP/TAZ, and Integrators of Mechanotransduction
2.3.3. Developmental and Differentiation Pathways (Wnt/β-Catenin, TGF-β/Smad, and Notch)
2.3.4. Mechanosensors (Integrin–FAK Signaling, Piezo/TRP Channels, and GPCRs)
2.3.5. Stress and Metabolic Regulators (NF-κB, mTOR, AMPK, ROS, p53/p21, HIF-1α, and JAK/STAT)
2.3.6. Epigenetic Regulation and Nuclear Mechanics
2.4. Matrix Elasticity as a Determinant of Deformability
3. Deformability of MSCs and Their Regenerative Competence
3.1. Deformability Reflects Stemness and Functional Immaturity
3.2. Deformability Is a Determinant of Migration and Homing
3.3. Deformability and Differentiation Status of MSCs
4. Measurement Techniques: From Biophysical Tools to Translational Applications
4.1. High-Resolution, Low-Throughput Techniques
4.2. Medium-Throughput Techniques
4.3. High-Throughput Techniques
4.4. Next-Generation Techniques
5. Translational Applications
5.1. Deformability as a Quality Control Criterion
5.2. Selection of Subpopulations with Increased Therapeutic Efficacy
5.3. Integration of Deformability with Multiomics Approaches
5.4. Clinical and Regulatory Perspectives
5.5. Practical Framework for Implementing MSC Deformability Assessment in ATMP Manufacturing
- Initial cell harvesting and isolation: Assess the initial deformability of freshly isolated MSCs to establish donor- and tissue-specific reference values.
- Expansion phase monitoring: Perform periodic assessments of MSCs’ deformability during culture using RT-DC to detect early mechanical changes associated with aging or unintended differentiation.
- Quality control before clinical introduction: Determine the final deformability profile at the batch level before product launch and verify and exclude subpopulations with increased stiffness, indicating reduced self-guidance or regeneration capacity.
- Post-thaw verification (if cryopreserved): Assess deformability after thawing to confirm recovery of the mechanical phenotype prior to administration.
6. Challenges and Future Directions
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| AI | Artificial Intelligence |
| ATMP | Advanced Therapy Medicinal Product |
| EMA | European Medicines Agency |
| FAK | Focal adhesion kinase |
| FDA | Food and Drug Administration |
| FRET | Forster Resonance Energy Transfer |
| GMP | Good Manufacturing Practice |
| GPCR | G-protein coupled receptors |
| IL-1β | Interleukin 1 β |
| IL-6 | Interleukin 6 |
| ITSC | International Society for Cellular Therapy |
| MAPK | Mitogen-Activated Protein Kinase |
| MLC | Myosin light chain |
| MSC | Mesenchymal Stem Cell |
| RT-DC | Real-Time Deformability Cytometry |
| TAZ | Transcriptional Coactivator with PDZ-binding motif |
| YAP | Yes-Associated Protein |
References
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Pînzariu, A.C.; Moscalu, R.; Soroceanu, R.P.; Maranduca, M.A.; Drochioi, I.C.; Vlasceanu, V.I.; Timofeiov, S.; Timofte, D.V.; Huzum, B.; Moscalu, M.; et al. The Therapeutic Use and Potential of MSCs: Advances in Regenerative Medicine. Int. J. Mol. Sci. 2025, 26, 3084. [Google Scholar] [CrossRef]
- Maldonado, V.V.; Patel, N.H.; Smith, E.E.; Barnes, C.L.; Gustafson, M.P.; Rao, R.R.; Samsonraj, R.M. Clinical Utility of Mesenchymal Stem/Stromal Cells in Regenerative Medicine and Cellular Therapy. J. Biol. Eng. 2023, 17, 44. [Google Scholar] [CrossRef]
- Szydlak, R. Mesenchymal Stem Cells as Modern Off-the-Shelf Products: From Research Perspectives to Clinical Practice. In Handbook of Stem Cell Applications; Springer Nature: Singapore, 2023; pp. 1–30. [Google Scholar]
- Szydlak, R. Mesenchymal Stem Cells’ Homing and Cardiac Tissue Repair. Acta Biochim. Pol. 2019, 66, 483–489. [Google Scholar] [CrossRef]
- Mabotuwana, N.S.; Rech, L.; Lim, J.; Hardy, S.A.; Murtha, L.A.; Rainer, P.P.; Boyle, A.J. Paracrine Factors Released by Stem Cells of Mesenchymal Origin and Their Effects in Cardiovascular Disease: A Systematic Review of Pre-Clinical Studies. Stem Cell Rev. Rep. 2022, 18, 2606–2628. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Krampera, M.; Galipeau, J.; Shi, Y.; Tarte, K.; Sensebe, L. Immunological Characterization of Multipotent Mesenchymal Stromal Cells—The International Society for Cellular Therapy (ISCT) Working Proposal. Cytotherapy 2013, 15, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Renesme, L.; Pierro, M.; Cobey, K.D.; Mital, R.; Nangle, K.; Shorr, R.; Lalu, M.M.; Thébaud, B. Definition and Characteristics of Mesenchymal Stromal Cells in Preclinical and Clinical Studies: A Scoping Review. Stem Cells Transl. Med. 2022, 11, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the Nomenclature for MSC: The International Society for Cellular Therapy Position Statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Cao, Y.; Boss, A.L.; Bolam, S.M.; Munro, J.T.; Crawford, H.; Dalbeth, N.; Poulsen, R.C.; Matthews, B.G. In Vitro Cell Surface Marker Expression on Mesenchymal Stem Cell Cultures Does Not Reflect Their Ex Vivo Phenotype. Stem Cell Rev. Rep. 2024, 20, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Rand, E.; Webster, A.J.; Genever, P.G. Characterisation of Mesenchymal Stromal Cells in Clinical Trial Reports: Analysis of Published Descriptors. Stem Cell Res. Ther. 2021, 12, 360. [Google Scholar] [CrossRef]
- Srinivasan, A.; Sathiyanathan, P.; Yin, L.; Liu, T.M.; Lam, A.; Ravikumar, M.; Smith, R.A.A.; Loh, H.P.; Zhang, Y.; Ling, L.; et al. Strategies to Enhance Immunomodulatory Properties and Reduce Heterogeneity in Mesenchymal Stromal Cells during Ex Vivo Expansion. Cytotherapy 2022, 24, 456–472. [Google Scholar] [CrossRef]
- Nolta, J.A.; Galipeau, J.; Phinney, D.G. Improving Mesenchymal Stem/Stromal Cell Potency and Survival. Cytotherapy 2020, 22, 123–126. [Google Scholar] [CrossRef]
- Urbanska, M.; Guck, J. Single-Cell Mechanics: Structural Determinants and Functional Relevance. Annu. Rev. Biophys. 2025, 33, 54. [Google Scholar] [CrossRef]
- Vilar, A.; Hodgson-Garms, M.; Kusuma, G.D.; Donderwinkel, I.; Carthew, J.; Tan, J.L.; Lim, R.; Frith, J.E. Substrate Mechanical Properties Bias MSC Paracrine Activity and Therapeutic Potential. Acta Biomater. 2023, 168, 144–158. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.-F.; Lian, Q. Paracrine Mechanisms of Mesenchymal Stem Cell-Based Therapy: Current Status and Perspectives. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Titushkin, I.; Cho, M. Modulation of Cellular Mechanics during Osteogenic Differentiation of Human Mesenchymal Stem Cells. Biophys. J. 2007, 93, 3693–3702. [Google Scholar] [CrossRef]
- Qi, X.; Ma, S.; Jiang, X.; Wu, H.; Zheng, J.; Wang, S.; Han, K.; Zhang, T.; Gao, J.; Li, X. Single-Cell Characterization of Deformation and Dynamics of Mesenchymal Stem Cells in Microfluidic Systems: A Computational Study. Phys. Rev. E 2023, 108, 054402. [Google Scholar] [CrossRef]
- Szydlak, R.; Majka, M.; Lekka, M.; Kot, M.; Laidler, P. AFM-Based Analysis of Wharton’s Jelly Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 4351. [Google Scholar] [CrossRef] [PubMed]
- Szydlak, R. Biological, Chemical and Mechanical Factors Regulating Migration and Homing of Mesenchymal Stem Cells. World J. Stem Cells 2021, 13, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Maloney, J.M.; Nikova, D.; Lautenschläger, F.; Clarke, E.; Langer, R.; Guck, J.; Van Vliet, K.J. Mesenchymal Stem Cell Mechanics from the Attached to the Suspended State. Biophys. J. 2010, 99, 2479–2487. [Google Scholar] [CrossRef] [PubMed]
- LeBlon, C.E.; Casey, M.E.; Fodor, C.R.; Zhang, T.; Zhang, X.; Jedlicka, S.S. Correlation between In Vitro Expansion-Related Cell Stiffening and Differentiation Potential of Human Mesenchymal Stem Cells. Differentiation 2015, 90, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Riehl, B.D.; Bouzid, T.; Yang, R.; Duan, B.; Donahue, H.J.; Lim, J.Y. YAP Mechanotransduction under Cyclic Mechanical Stretch Loading for Mesenchymal Stem Cell Osteogenesis Is Regulated by ROCK. Front. Bioeng. Biotechnol. 2024, 11, 1306002. [Google Scholar] [CrossRef]
- Yang, Y.-H.K.; Ogando, C.R.; Wang See, C.; Chang, T.-Y.; Barabino, G.A. Changes in Phenotype and Differentiation Potential of Human Mesenchymal Stem Cells Aging in Vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef]
- Wu, P.-H.; Aroush, D.R.-B.; Asnacios, A.; Chen, W.-C.; Dokukin, M.E.; Doss, B.L.; Durand-Smet, P.; Ekpenyong, A.; Guck, J.; Guz, N.V.; et al. A Comparison of Methods to Assess Cell Mechanical Properties. Nat. Methods 2018, 15, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Oevreeide, I.H.; Szydlak, R.; Luty, M.; Ahmed, H.; Prot, V.; Skallerud, B.H.; Zemła, J.; Lekka, M.; Stokke, B.T. On the Determination of Mechanical Properties of Aqueous Microgels—Towards High-Throughput Characterization. Gels 2021, 7, 64. [Google Scholar] [CrossRef]
- Pérez-Domínguez, S.; Kulkarni, S.G.; Pabijan, J.; Gnanachandran, K.; Holuigue, H.; Eroles, M.; Lorenc, E.; Berardi, M.; Antonovaite, N.; Marini, M.L.; et al. Reliable, Standardized Measurements for Cell Mechanical Properties. Nanoscale 2023, 15, 16371–16380. [Google Scholar] [CrossRef]
- Sarem, M.; Otto, O.; Tanaka, S.; Shastri, V.P. Cell Number in Mesenchymal Stem Cell Aggregates Dictates Cell Stiffness and Chondrogenesis. Stem Cell Res. Ther. 2019, 10, 10. [Google Scholar] [CrossRef]
- Øvreeide, I.H.; Sturdy, J.; Szydlak, R.; Hines, T.G.; Ahmed, H.; Totlani, K.; Zemla, J.; Luty, M.; Prot, V.E.; Lekka, M.; et al. Microfluidic Constriction-Based Mechanoprofiling of Alginate Microgels and Bladder Cancer Cells. Polymer 2025, 337, 128958. [Google Scholar] [CrossRef]
- Elsayad, K.; Polakova, S.; Gregan, J. Probing Mechanical Properties in Biology Using Brillouin Microscopy. Trends Cell Biol. 2019, 29, 608–611. [Google Scholar] [CrossRef]
- Meng, F.; Sachs, F. Orientation-Based FRET Sensor for Real-Time Imaging of Cellular Forces. J. Cell Sci. 2012, 125, 743–750. [Google Scholar] [CrossRef]
- Wu, Z.; Feng, Y.; Bi, R.; Liu, Z.; Niu, Y.; Jin, Y.; Li, W.; Chen, H.; Shi, Y.; Du, Y. Image-Based Evaluation of Single-Cell Mechanics Using Deep Learning. Cell Regen. 2025, 14, 21. [Google Scholar] [CrossRef]
- Guazzelli, N.; Cacopardo, L.; Ahluwalia, A. Precision Design of Dextran-Permeated Agarose Hydrogels Matching Adipose Stem Cell Adhesion Timescales. Mater. Today Bio 2025, 32, 101832. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Galie, P.A.; Georges, P.C.; Janmey, P.A. How Do Cells Stiffen? Biochem. J. 2022, 479, 1825–1842. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; McCulloch, C.A. Cell Mechanics: Integrating Cell Responses to Mechanical Stimuli. Annu. Rev. Biomed. Eng. 2007, 9, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, A.F.; Janmey, P.; Weitz, D.A. Mechanical Properties of the Cytoskeleton and Cells. Cold Spring Harb. Perspect. Biol. 2017, 9, a022038. [Google Scholar] [CrossRef] [PubMed]
- Milan, J.L.; Wendling-Mansuy, S.; Jean, M.; Chabrand, P. Divided Medium-Based Model for Analyzing the Dynamic Reorganization of the Cytoskeleton during Cell Deformation. Biomech. Model. Mechanobiol. 2007, 6, 373–390. [Google Scholar] [CrossRef]
- Grady, M.E.; Composto, R.J.; Eckmann, D.M. Cell Elasticity with Altered Cytoskeletal Architectures across Multiple Cell Types. J. Mech. Behav. Biomed. Mater. 2016, 61, 197–207. [Google Scholar] [CrossRef]
- Polemidiotou, K.; Kulkarni, S.G.; Szydlak, R.; Lekka, M.; Radmacher, M.; Gkretsi, V.; Stylianopoulos, T.; Stylianou, A. Assessing Sarcoma Cell Cytoskeleton Remodeling in Response to Varying Collagen Concentration. Int. J. Biol. Macromol. 2024, 282, 136770. [Google Scholar] [CrossRef]
- Lasota, M.; Bentke-Imiolek, A.; Skrzypek, K.; Bobrowska, J.; Jagusiak, A.; Bryniarska-Kubiak, N.; Zagajewski, J.; Kot, M.; Szydlak, R.; Lekka, M.; et al. Small-Molecule Inhibitor—Tyrphostin AG1296 Regulates Proliferation, Survival and Migration of Rhabdomyosarcoma Cells. J. Physiol. Pharmacol. 2021, 72, 881–893. [Google Scholar] [CrossRef]
- Luty, M.; Szydlak, R.; Pabijan, J.; Zemła, J.; Oevreeide, I.H.; Prot, V.E.; Stokke, B.T.; Lekka, M.; Zapotoczny, B. Tubulin-Targeted Therapy in Melanoma Increases the Cell Migration Potential by Activation of the Actomyosin Cytoskeleton—An In Vitro Study. ACS Biomater. Sci. Eng. 2024, 10, 7155–7166. [Google Scholar] [CrossRef]
- Doole, F.T.; Kumarage, T.; Ashkar, R.; Brown, M.F. Cholesterol Stiffening of Lipid Membranes. J. Membr. Biol. 2022, 255, 385–405. [Google Scholar] [CrossRef]
- Head, B.P.; Patel, H.H.; Insel, P.A. Interaction of Membrane/Lipid Rafts with the Cytoskeleton: Impact on Signaling and Function. Biochim. Biophys. Acta (BBA)—Biomembr. 2014, 1838, 532–545. [Google Scholar] [CrossRef]
- Bi, J.; Wang, R.; Zeng, X. Lipid Rafts Regulate the Lamellipodia Formation of Melanoma A375 Cells via Actin Cytoskeleton-mediated Recruitment of Β1 and Β3 Integrin. Oncol. Lett. 2018, 16, 6540–6546. [Google Scholar] [CrossRef]
- Dingal, P.C.D.P.; Bradshaw, A.M.; Cho, S.; Raab, M.; Buxboim, A.; Swift, J.; Discher, D.E. Fractal Heterogeneity in Minimal Matrix Models of Scars Modulates Stiff-Niche Stem-Cell Responses via Nuclear Exit of a Mechanorepressor. Nat. Mater. 2015, 14, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Zwerger, M.; Jaalouk, D.E.; Lombardi, M.L.; Isermann, P.; Mauermann, M.; Dialynas, G.; Herrmann, H.; Wallrath, L.L.; Lammerding, J. Myopathic Lamin Mutations Impair Nuclear Stability in Cells and Tissue and Disrupt Nucleo-Cytoskeletal Coupling. Hum. Mol. Genet. 2013, 22, 2335–2349. [Google Scholar] [CrossRef] [PubMed]
- Alcorta-Sevillano, N.; Macías, I.; Rodríguez, C.I.; Infante, A. Crucial Role of Lamin A/C in the Migration and Differentiation of MSCs in Bone. Cells 2020, 9, 1330. [Google Scholar] [CrossRef] [PubMed]
- Malashicheva, A.; Bogdanova, M.; Zabirnyk, A.; Smolina, N.; Ignatieva, E.; Freilikhman, O.; Fedorov, A.; Dmitrieva, R.; Sjöberg, G.; Sejersen, T.; et al. Various Lamin A/C Mutations Alter Expression Profile of Mesenchymal Stem Cells in Mutation Specific Manner. Mol. Genet. Metab. 2015, 115, 118–127. [Google Scholar] [CrossRef]
- Betterton, M.D. A New View of How Cytoplasmic Viscosity Affects Microtubule Dynamics. Dev. Cell 2022, 57, 419–420. [Google Scholar] [CrossRef]
- Gerum, R.; Mirzahossein, E.; Eroles, M.; Elsterer, J.; Mainka, A.; Bauer, A.; Sonntag, S.; Winterl, A.; Bartl, J.; Fischer, L.; et al. Viscoelastic Properties of Suspended Cells Measured with Shear Flow Deformation Cytometry. Elife 2022, 11, e78823. [Google Scholar] [CrossRef] [PubMed]
- Ebata, H.; Umeda, K.; Nishizawa, K.; Nagao, W.; Inokuchi, S.; Sugino, Y.; Miyamoto, T.; Mizuno, D. Activity-Dependent Glassy Cell Mechanics I: Mechanical Properties Measured with Active Microrheology. Biophys. J. 2023, 122, 1781–1793. [Google Scholar] [CrossRef]
- Venkova, L.; Vishen, A.S.; Lembo, S.; Srivastava, N.; Duchamp, B.; Ruppel, A.; Williart, A.; Vassilopoulos, S.; Deslys, A.; Garcia Arcos, J.M.; et al. A Mechano-Osmotic Feedback Couples Cell Volume to the Rate of Cell Deformation. Elife 2022, 11, e72381. [Google Scholar] [CrossRef]
- Liao, H.-S.; Wen, P.J.; Wu, L.-G.; Jin, A.J. Effect of Osmotic Pressure on Cellular Stiffness as Evaluated Through Force Mapping Measurements. J. Biomech. Eng. 2018, 140, 054502. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, W.; Feng, S.; Chen, Y.; Wu, X.; Zhang, Q.; Wu, S. Dynamic Response of the Cell Traction Force to Osmotic Shock. Microsyst. Nanoeng. 2023, 9, 131. [Google Scholar] [CrossRef]
- Lekka, M.; Navajas, D.; Radmacher, M.; Podestà, A. Mechanics of Cells and Tissues in Diseases. Biomedical Applications; Lekka, M., Navajas, D., Radmacher, M., Podestà, A., Eds.; De Gruyter: Berlin, Germany, 2023; ISBN 9783110989380. [Google Scholar]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth Factors, Matrices, and Forces Combine and Control Stem Cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef]
- Saidova, A.A.; Vorobjev, I.A. Lineage Commitment, Signaling Pathways, and the Cytoskeleton Systems in Mesenchymal Stem Cells. Tissue Eng. Part B Rev. 2020, 26, 13–25. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, K.; Wang, M.; Wang, N.; Song, Y.; Xiong, W.; Guo, S.; Yi, Z.; Wang, Q.; Yang, S. Integrating Physicomechanical and Biological Strategies for BTE: Biomaterials-Induced Osteogenic Differentiation of MSCs. Theranostics 2023, 13, 3245–3275. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Cannon, R.D.; Coates, D.E.; Mei, L. Effect of the Rho-Kinase/ROCK Signaling Pathway on Cytoskeleton Components. Genes 2023, 14, 272. [Google Scholar] [CrossRef]
- Noguchi, M.; Hosoda, K.; Fujikura, J.; Fujimoto, M.; Iwakura, H.; Tomita, T.; Ishii, T.; Arai, N.; Hirata, M.; Ebihara, K.; et al. Genetic and Pharmacological Inhibition of Rho-Associated Kinase II Enhances Adipogenesis. J. Biol. Chem. 2007, 282, 29574–29583. [Google Scholar] [CrossRef] [PubMed]
- Nobes, C.D.; Hall, A. Rho, Rac and Cdc42 GTPases: Regulators of Actin Structures, Cell Adhesion and Motility. Biochem. Soc. Trans. 1995, 23, 456–459. [Google Scholar] [CrossRef]
- Lim Lam, V.K.; Hin Wong, J.Y.; Chew, S.Y.; Chan, B.P. Rac1-GTPase Regulates Compression-Induced Actin Protrusions (CAPs) of Mesenchymal Stem Cells in 3D Collagen Micro-Tissues. Biomaterials 2021, 274, 120829. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Crawford, R.; Chen, C.; Xiao, Y. The Key Regulatory Roles of the PI3K/Akt Signaling Pathway in the Functionalities of Mesenchymal Stem Cells and Applications in Tissue Regeneration. Tissue Eng. Part B Rev. 2013, 19, 516–528. [Google Scholar] [CrossRef]
- Wang, L.; Ruan, M.; Bu, Q.; Zhao, C. Signaling Pathways Driving MSC Osteogenesis: Mechanisms, Regulation, and Translational Applications. Int. J. Mol. Sci. 2025, 26, 1311. [Google Scholar] [CrossRef]
- Samakova, A.; Gazova, A.; Sabova, N.; Valaskova, S.; Jurikova, M.; Kyselovic, J. The PI3k/Akt Pathway Is Associated with Angiogenesis, Oxidative Stress and Survival of Mesenchymal Stem Cells in Pathophysiologic Condition in Ischemia. Physiol. Res. 2019, 68, S131–S138. [Google Scholar] [CrossRef]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Tumaneng, K.; Schlegelmilch, K.; Russell, R.C.; Yimlamai, D.; Basnet, H.; Mahadevan, N.; Fitamant, J.; Bardeesy, N.; Camargo, F.D.; Guan, K.-L. YAP Mediates Crosstalk between the Hippo and PI(3)K–TOR Pathways by Suppressing PTEN via MiR-29. Nat. Cell Biol. 2012, 14, 1322–1329. [Google Scholar] [CrossRef]
- Azzolin, L.; Panciera, T.; Soligo, S.; Enzo, E.; Bicciato, S.; Dupont, S.; Bresolin, S.; Frasson, C.; Basso, G.; Guzzardo, V.; et al. YAP/TAZ Incorporation in the β-Catenin Destruction Complex Orchestrates the Wnt Response. Cell 2014, 158, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Xu, M.; Lu, F.; Chang, Q. Adipogenesis or Osteogenesis: Destiny Decision Made by Mechanical Properties of Biomaterials. RSC Adv. 2022, 12, 24501–24510. [Google Scholar] [CrossRef] [PubMed]
- Bienz, M. β-Catenin: A Pivot between Cell Adhesion and Wnt Signalling. Current Biology 2005, 15, R64–R67. [Google Scholar] [CrossRef] [PubMed]
- Day, T.F.; Guo, X.; Garrett-Beal, L.; Yang, Y. Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis. Dev. Cell 2005, 8, 739–750. [Google Scholar] [CrossRef]
- de Winter, T.J.J.; Nusse, R. Running Against the Wnt: How Wnt/β-Catenin Suppresses Adipogenesis. Front. Cell Dev. Biol. 2021, 9, 627429. [Google Scholar] [CrossRef]
- Du, J.; Zu, Y.; Li, J.; Du, S.; Xu, Y.; Zhang, L.; Jiang, L.; Wang, Z.; Chien, S.; Yang, C. Extracellular Matrix Stiffness Dictates Wnt Expression through Integrin Pathway. Sci. Rep. 2016, 6, 20395. [Google Scholar] [CrossRef]
- Xu, R.; Wu, M.; Wang, Y.; Li, C.; Zeng, L.; Wang, Y.; Xiao, M.; Chen, X.; Geng, S.; Lai, P.; et al. Mesenchymal Stem Cells Reversibly De-Differentiate Myofibroblasts to Fibroblast-like Cells by Inhibiting the TGF-β-SMAD2/3 Pathway. Mol. Med. 2023, 29, 59. [Google Scholar] [CrossRef]
- Wei, E.; Hu, M.; Wu, L.; Pan, X.; Zhu, Q.; Liu, H.; Liu, Y. TGF-β Signaling Regulates Differentiation of MSCs in Bone Metabolism: Disputes among Viewpoints. Stem Cell Res. Ther. 2024, 15, 156. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Zhao, S.; Zhao, X.-Y.; Min, P.-X.; Ma, Y.-D.; Wang, Y.-Y.; Chen, Y.; Tang, S.-J.; Zhang, Y.-J.; et al. Non-Canonical Notch Signaling Regulates Actin Remodeling in Cell Migration by Activating PI3K/AKT/Cdc42 Pathway. Front. Pharmacol. 2019, 10, 370. [Google Scholar] [CrossRef]
- Xie, J.; Wang, W.; Si, J.-W.; Miao, X.-Y.; Li, J.-C.; Wang, Y.-C.; Wang, Z.-R.; Ma, J.; Zhao, X.-C.; Li, Z.; et al. Notch Signaling Regulates CXCR4 Expression and the Migration of Mesenchymal Stem Cells. Cell Immunol. 2013, 281, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Chi, Y.; Li, X.; Du, W.; Han, Z.-B.; Tian, J.; Li, J.; Chen, F.; Wu, H.; Han, L.; et al. Inhibition of Notch Signaling Promotes the Adipogenic Differentiation of Mesenchymal Stem Cells Through Autophagy Activation and PTEN-PI3K/AKT/MTOR Pathway. Cell. Physiol. Biochem. 2015, 36, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Perdigoto, C.N.; Bardin, A.J. Sending the Right Signal: Notch and Stem Cells. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2013, 1830, 2307–2322. [Google Scholar] [CrossRef]
- Shih, Y.-R.V.; Tseng, K.-F.; Lai, H.-Y.; Lin, C.-H.; Lee, O.K. Matrix Stiffness Regulation of Integrin-Mediated Mechanotransduction during Osteogenic Differentiation of Human Mesenchymal Stem Cells. J. Bone Miner. Res. 2011, 26, 730–738. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, F.; Song, R.; Zhuang, L.; Yang, M.; Suo, J.; Li, L. Integrins in the Regulation of Mesenchymal Stem Cell Differentiation by Mechanical Signals. Stem Cell Rev. Rep. 2022, 18, 126–141. [Google Scholar] [CrossRef]
- Corrigan, M.A.; Johnson, G.P.; Stavenschi, E.; Riffault, M.; Labour, M.-N.; Hoey, D.A. TRPV4-Mediates Oscillatory Fluid Shear Mechanotransduction in Mesenchymal Stem Cells in Part via the Primary Cilium. Sci. Rep. 2018, 8, 3824. [Google Scholar] [CrossRef]
- Sugimoto, A.; Miyazaki, A.; Kawarabayashi, K.; Shono, M.; Akazawa, Y.; Hasegawa, T.; Ueda-Yamaguchi, K.; Kitamura, T.; Yoshizaki, K.; Fukumoto, S.; et al. Piezo Type Mechanosensitive Ion Channel Component 1 Functions as a Regulator of the Cell Fate Determination of Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 17696. [Google Scholar] [CrossRef] [PubMed]
- Kamm, K.E.; Stull, J.T. Dedicated Myosin Light Chain Kinases with Diverse Cellular Functions. J. Biol. Chem. 2001, 276, 4527–4530. [Google Scholar] [CrossRef]
- Badri, L.; Lama, V.N. Lysophosphatidic Acid Induces Migration of Human Lung-Resident Mesenchymal Stem Cells Through the β-Catenin Pathway. Stem Cells 2012, 30, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Hardman, K.; Goldman, A.; Pliotas, C. Membrane Force Reception: Mechanosensation in G Protein-Coupled Receptors and Tools to Address It. Curr. Opin. Physiol. 2023, 35, 100689. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Wu, X.; Tian, L.; Zhou, J.; Li, X.; Wang, B. Lysophosphatidic Acid Receptor 4 Regulates Osteogenic and Adipogenic Differentiation of Progenitor Cells via Inactivation of RhoA/ROCK1/β-Catenin Signaling. Stem Cells 2020, 38, 451–463. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Shi, J.; Xu, X.; Xu, J. The Role of TNF-α in the Fate Regulation and Functional Reprogramming of Mesenchymal Stem Cells in an Inflammatory Microenvironment. Front. Immunol. 2023, 14, 1074863. [Google Scholar] [CrossRef]
- Uchibori, R.; Tsukahara, T.; Mizuguchi, H.; Saga, Y.; Urabe, M.; Mizukami, H.; Kume, A.; Ozawa, K. NF-ΚB Activity Regulates Mesenchymal Stem Cell Accumulation at Tumor Sites. Cancer Res. 2013, 73, 364–372. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, P.J.; Zhang, S.; Zhou, H.; Stoica, L.; Galiano, M.; Krnjević, K.; Roman, G.; Costa-Mattioli, M. MTORC2 Controls Actin Polymerization Required for Consolidation of Long-Term Memory. Nat. Neurosci. 2013, 16, 441–448. [Google Scholar] [CrossRef]
- Gharibi, B.; Farzadi, S.; Ghuman, M.; Hughes, F.J. Inhibition of Akt/MTOR Attenuates Age-Related Changes in Mesenchymal Stem Cells. Stem Cells 2014, 32, 2256–2266. [Google Scholar] [CrossRef]
- Chen, X.; Yan, J.; He, F.; Zhong, D.; Yang, H.; Pei, M.; Luo, Z.-P. Mechanical Stretch Induces Antioxidant Responses and Osteogenic Differentiation in Human Mesenchymal Stem Cells through Activation of the AMPK-SIRT1 Signaling Pathway. Free Radic. Biol. Med. 2018, 126, 187–201. [Google Scholar] [CrossRef]
- Borodkina, A.V.; Shatrova, A.N.; Nikolsky, N.N.; Burova, E.B. Role of P38 Map-Kinase in the Stress-Induced Senescence Progression of Human Endometrium-Derived Mesenchymal Stem Cells. Tsitologiia 2016, 58, 429–435. [Google Scholar] [PubMed]
- Denu, R.A.; Hematti, P. Optimization of Oxidative Stress for Mesenchymal Stromal/Stem Cell Engraftment, Function and Longevity. Free Radic. Biol. Med. 2021, 167, 193–200. [Google Scholar] [CrossRef]
- Gu, Z.; Jiang, J.; Tan, W.; Xia, Y.; Cao, H.; Meng, Y.; Da, Z.; Liu, H.; Cheng, C. P53/P21 Pathway Involved in Mediating Cellular Senescence of Bone Marrow-Derived Mesenchymal Stem Cells from Systemic Lupus Erythematosus Patients. Clin. Dev. Immunol. 2013, 2013, 134243. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yoon, Y.; Lee, S. Hypoxic Preconditioning Promotes the Bioactivities of Mesenchymal Stem Cells via the HIF-1α-GRP78-Akt Axis. Int. J. Mol. Sci. 2017, 18, 1320. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Yuan, H.; Peng, L.; Dai, Z.; Sun, Y.; Liu, R.; Li, W.; Li, J.; Zhu, C. Hypoxia Preconditioning of Human Amniotic Mesenchymal Stem Cells Enhances Proliferation and Migration and Promotes Their Homing via the HGF/C-MET Signaling Axis to Augment the Repair of Acute Liver Failure. Tissue Cell 2024, 87, 102326. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Jiang, W.; Wang, H.; Li, H.; Tang, B.; Liu, B.; Jiang, H.; Sun, X. The IL-6/STAT3 Pathway Regulates Adhesion Molecules and Cytoskeleton of Endothelial Cells in Thromboangiitis Obliterans. Cell Signal 2018, 44, 118–126. [Google Scholar] [CrossRef]
- Na, H.; Im, K.-I.; Kim, N.; Lee, J.; Gil, S.; Min, G.-J.; Cho, S.-G. The IL-6 Signaling Pathway Contributes Critically to the Immunomodulatory Mechanism of Human Decidua-Derived Mesenchymal Stromal Cells. iScience 2024, 27, 109783. [Google Scholar] [CrossRef]
- Tagay, Y. BPS2025—Cracking the Code of Nuclear Rigidity: New Frontiers in Cell Migration and Therapy. Biophys. J. 2025, 124, 302a–303a. [Google Scholar] [CrossRef]
- Xia, J.; Zhao, J.Z.; Strom, A.R.; Brangwynne, C.P. Chromatin Heterogeneity Modulates Nuclear Condensate Dynamics and Phase Behavior. Nat. Commun. 2025, 16, 6406. [Google Scholar] [CrossRef]
- Swift, J.; Ivanovska, I.L.; Buxboim, A.; Harada, T.; Dingal, P.C.D.P.; Pinter, J.; Pajerowski, J.D.; Spinler, K.R.; Shin, J.-W.; Tewari, M.; et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science 2013, 341, 1240104. [Google Scholar] [CrossRef]
- Pajerowski, J.D.; Dahl, K.N.; Zhong, F.L.; Sammak, P.J.; Discher, D.E. Physical Plasticity of the Nucleus in Stem Cell Differentiation. Proc. Natl. Acad. Sci. USA 2007, 104, 15619–15624. [Google Scholar] [CrossRef]
- Spagnol, S.T.; Lin, W.-C.; Booth, E.A.; Ladoux, B.; Lazarus, H.M.; Dahl, K.N. Early Passage Dependence of Mesenchymal Stem Cell Mechanics Influences Cellular Invasion and Migration. Ann. Biomed. Eng. 2016, 44, 2123–2131. [Google Scholar] [CrossRef]
- Rando, T.A.; Brunet, A.; Goodell, M.A. Hallmarks of Stem Cell Aging. Cell Stem Cell 2025, 32, 1038–1054. [Google Scholar] [CrossRef]
- Elmi, F.; Soltanmohammadi, F.; Fayeghi, T.; Farajnia, S.; Alizadeh, E. Preventing MSC Aging and Enhancing Immunomodulation: Novel Strategies for Cell-Based Therapies. Regen. Ther. 2025, 29, 517–539. [Google Scholar] [CrossRef]
- Doolin, M.T.; Moriarty, R.A.; Stroka, K.M. Mechanosensing of Mechanical Confinement by Mesenchymal-Like Cells. Front. Physiol. 2020, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Lipowsky, H.H.; Bowers, D.T.; Banik, B.L.; Brown, J.L. Mesenchymal Stem Cell Deformability and Implications for Microvascular Sequestration. Ann. Biomed. Eng. 2018, 46, 640–654. [Google Scholar] [CrossRef]
- Krueger, T.E.G.; Thorek, D.L.J.; Denmeade, S.R.; Isaacs, J.T.; Brennen, W.N. Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise. Stem Cells Transl. Med. 2018, 7, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S. Pulmonary Passage Is a Major Obstacle for Intravenous Stem Cell Delivery: The Pulmonary First-Pass Effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef]
- Gao, J.; Dennis, J.E.; Muzic, R.F.; Lundberg, M.; Caplan, A.I. The Dynamic in Vivo Distribution of Bone Marrow-Derived Mesenchymal Stem Cells after Infusion. Cells Tissues Organs 2001, 169, 12–20. [Google Scholar] [CrossRef]
- Tietze, S.; Kräter, M.; Jacobi, A.; Taubenberger, A.; Herbig, M.; Wehner, R.; Schmitz, M.; Otto, O.; List, C.; Kaya, B.; et al. Spheroid Culture of Mesenchymal Stromal Cells Results in Morphorheological Properties Appropriate for Improved Microcirculation. Adv. Sci. 2019, 6, 1802104. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Z.; Shi, Y.; Yao, S.; He, F.; Cong, X.; Teng, F. Modulating Cellular Deformability via 3D Dextran Hydrogel Cultivation to Regulate the Microcirculation of Mesenchymal Stem Cells in Murine Spleen and Liver. Exp. Mol. Pathol. 2025, 143, 104987. [Google Scholar] [CrossRef]
- Toma, C.; Wagner, W.R.; Bowry, S.; Schwartz, A.; Villanueva, F. Fate Of Culture-Expanded Mesenchymal Stem Cells in the Microvasculature. Circ. Res. 2009, 104, 398–402. [Google Scholar] [CrossRef]
- Nitzsche, F.; Müller, C.; Lukomska, B.; Jolkkonen, J.; Deten, A.; Boltze, J. Concise Review: MSC Adhesion Cascade—Insights into Homing and Transendothelial Migration. Stem Cells 2017, 35, 1446–1460. [Google Scholar] [CrossRef]
- Ryu, C.H.; Park, S.A.; Kim, S.M.; Lim, J.Y.; Jeong, C.H.; Jun, J.A.; Oh, J.H.; Park, S.H.; Oh, W.; Jeun, S.-S. Migration of Human Umbilical Cord Blood Mesenchymal Stem Cells Mediated by Stromal Cell-Derived Factor-1/CXCR4 Axis via Akt, ERK, and P38 Signal Transduction Pathways. Biochem. Biophys. Res. Commun. 2010, 398, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, H.; Guo, L.; Wang, S.; Cheng, W.; Wan, L.; Zhang, Z.; Xing, L.; Zhou, Q.; Yang, X.; et al. SDF-1 Secreted by Mesenchymal Stem Cells Promotes the Migration of Endothelial Progenitor Cells via CXCR4/PI3K/AKT Pathway. J. Mol. Histol. 2021, 52, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Hou, J.; Liu, D.; Tang, D.; Zhang, Y.; Zeng, Q.; Pan, H.; Fan, L. Important Role of the SDF-1/CXCR4 Axis in the Homing of Systemically Transplanted Human Amnion-Derived Mesenchymal Stem Cells (HAD-MSCs) to Ovaries in Rats with Chemotherapy-Induced Premature Ovarian Insufficiency (POI). Stem Cell Res. Ther. 2022, 13, 79. [Google Scholar] [CrossRef]
- Neuss, S.; Becher, E.; Wöltje, M.; Tietze, L.; Jahnen-Dechent, W. Functional Expression of HGF and HGF Receptor/C-met in Adult Human Mesenchymal Stem Cells Suggests a Role in Cell Mobilization, Tissue Repair, and Wound Healing. Stem Cells 2004, 22, 405–414. [Google Scholar] [CrossRef]
- Ambriz, X.; de Lanerolle, P.; Ambrosio, J.R. The Mechanobiology of the Actin Cytoskeleton in Stem Cells during Differentiation and Interaction with Biomaterials. Stem Cells Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Yen, M.-H.; Chen, Y.-H.; Liu, Y.-S.; Lee, O.K.-S. Alteration of Young’s Modulus in Mesenchymal Stromal Cells during Osteogenesis Measured by Atomic Force Microscopy. Biochem. Biophys. Res. Commun. 2020, 526, 827–832. [Google Scholar] [CrossRef]
- He, F.; Yang, C.; Liu, H.; Wang, J. Changes in the Mechanical Properties of Human Mesenchymal Stem Cells during Differentiation. R. Soc. Open Sci. 2023, 10, 220607. [Google Scholar] [CrossRef] [PubMed]
- Putra, V.D.L.; Kilian, K.A.; Knothe Tate, M.L. Biomechanical, Biophysical and Biochemical Modulators of Cytoskeletal Remodelling and Emergent Stem Cell Lineage Commitment. Commun. Biol. 2023, 6, 75. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Khan, A.U.; Qu, R.; Fan, T.; Ouyang, J.; Dai, J. A Glance on the Role of Actin in Osteogenic and Adipogenic Differentiation of Mesenchymal Stem Cells. Stem Cell Res. Ther. 2020, 11, 283. [Google Scholar] [CrossRef]
- Potolitsyna, E.; Pickering, S.H.; Bellanger, A.; Germier, T.; Collas, P.; Briand, N. Cytoskeletal Rearrangement Precedes Nucleolar Remodeling during Adipogenesis. Commun. Biol. 2024, 7, 458. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, J.; Wan, L.; Li, J. The Effect of Matrix Stiffness on the Chondrogenic Differentiation of Mesenchymal Stem Cells. J. Mol. Histol. 2022, 53, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Li, L.; Sun, M.; Zhang, Y.; Chen, L.; Rong, Y.; Li, Y. Mechanism of Regulation of Stem Cell Differentiation by Matrix Stiffness. Stem Cell Res. Ther. 2015, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Lorthongpanich, C.; Thumanu, K.; Tangkiettrakul, K.; Jiamvoraphong, N.; Laowtammathron, C.; Damkham, N.; U-pratya, Y.; Issaragrisil, S. YAP as a Key Regulator of Adipo-Osteogenic Differentiation in Human MSCs. Stem Cell Res. Ther. 2019, 10, 402. [Google Scholar] [CrossRef]
- McGrail, D.J.; McAndrews, K.M.; Dawson, M.R. Biomechanical Analysis Predicts Decreased Human Mesenchymal Stem Cell Function before Molecular Differences. Exp. Cell Res. 2013, 319, 684–696. [Google Scholar] [CrossRef]
- Khani, M.-M.; Tafazzoli-Shadpour, M.; Goli-Malekabadi, Z.; Haghighipour, N. Mechanical Characterization of Human Mesenchymal Stem Cells Subjected to Cyclic Uniaxial Strain and TGF-Β1. J. Mech. Behav. Biomed. Mater. 2015, 43, 18–25. [Google Scholar] [CrossRef]
- Mierke, C.T. The Role of the Optical Stretcher Is Crucial in the Investigation of Cell Mechanics Regulating Cell Adhesion and Motility. Front. Cell Dev. Biol. 2019, 7, 184. [Google Scholar] [CrossRef]
- Sraj, I.; Szatmary, A.C.; Desai, S.A.; Marr, D.W.M.; Eggleton, C.D. Erythrocyte Deformation in High-Throughput Optical Stretchers. Phys. Rev. E 2012, 85, 041923. [Google Scholar] [CrossRef]
- Otto, O.; Rosendahl, P.; Mietke, A.; Golfier, S.; Herold, C.; Klaue, D.; Girardo, S.; Pagliara, S.; Ekpenyong, A.; Jacobi, A.; et al. Real-Time Deformability Cytometry: On-the-Fly Cell Mechanical Phenotyping. Nat. Methods 2015, 12, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, P.; Plak, K.; Jacobi, A.; Kraeter, M.; Toepfner, N.; Otto, O.; Herold, C.; Winzi, M.; Herbig, M.; Ge, Y.; et al. Real-Time Fluorescence and Deformability Cytometry. Nat. Methods 2018, 15, 355–358. [Google Scholar] [CrossRef]
- Mokbel, M.; Mokbel, D.; Mietke, A.; Träber, N.; Girardo, S.; Otto, O.; Guck, J.; Aland, S. Numerical Simulation of Real-Time Deformability Cytometry To Extract Cell Mechanical Properties. ACS Biomater. Sci. Eng. 2017, 3, 2962–2973. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wang, L.; Zhong, Y.; Xu, Q.; Yan, J.; Pan, D.; Xu, Y.; Chen, C.; Wang, J.; Wang, G.; et al. Impact of Mesenchymal Stem Cell Size and Adhesion Modulation on in Vivo Distribution: Insights from Quantitative PET Imaging. Stem Cell Res. Ther. 2024, 15, 456. [Google Scholar] [CrossRef] [PubMed]
- De Becker, A.; Riet, I. Van Homing and Migration of Mesenchymal Stromal Cells: How to Improve the Efficacy of Cell Therapy? World J. Stem Cells 2016, 8, 73. [Google Scholar] [CrossRef]
- Shiomi, A.; Kaneko, T.; Nishikawa, K.; Tsuchida, A.; Isoshima, T.; Sato, M.; Toyooka, K.; Doi, K.; Nishikii, H.; Shintaku, H. High-Throughput Mechanical Phenotyping and Transcriptomics of Single Cells. Nat. Commun. 2024, 15, 3812. [Google Scholar] [CrossRef]
- Young, K.M.; Xu, C.; Ahkee, K.; Mezencev, R.; Swingle, S.P.; Yu, T.; Paikeday, A.; Kim, C.; McDonald, J.F.; Qiu, P.; et al. Correlating Mechanical and Gene Expression Data on the Single Cell Level to Investigate Metastatic Phenotypes. iScience 2023, 26, 106393. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chi, G.; Xu, J.; Tan, Y.; Xu, J.; Lv, S.; Xu, Z.; Xia, Y.; Li, L.; Li, Y. Extracellular Matrix Stiffness Controls Osteogenic Differentiation of Mesenchymal Stem Cells Mediated by Integrin A5. Stem Cell Res. Ther. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Tai, C.; Wang, Z.; Yang, Z.; Chen, X.; Zhang, J.; Zheng, L.; Fan, Y. Stiff Extracellular Matrix Drives the Differentiation of Mesenchymal Stem Cells toward Osteogenesis by the Multiscale 3D Genome Reorganization. Biomaterials 2025, 312, 122715. [Google Scholar] [CrossRef]
- Heyman, E.; Olenic, M.; De Vlieghere, E.; De Smet, S.; Devriendt, B.; Thorrez, L.; De Schauwer, C. Donor Age and Breed Determine Mesenchymal Stromal Cell Characteristics. Stem Cell Res. Ther. 2025, 16, 99. [Google Scholar] [CrossRef] [PubMed]

| Technique | Scale | Throughput | Invasiveness | GMP Applicability | Advantages | Limitations |
|---|---|---|---|---|---|---|
| Atomic force microscopy | Local and global * | Very low (<30 cells/h) | Contact-based, label-free | Low | High precision, subcellular mapping, detects subtle changes | Time-consuming, requires a skilled operator, low throughput, requires adherent cells |
| Micropipette aspiration | Local and global | Very low (<10 cells/h) | Contact-based, label-free | Low | Simple principle, direct viscoelastic measurement | Manual operation, limited scalability, low throughput, requires adherent cells |
| Optical stretching | Global | Moderate (<1000 cells/h) | Contact-free, label-free | Medium | Non-contact, suitable for suspended cells | Requires specialized optics, moderate throughput |
| Real-Time Deformability Cytometry | Global | Very high (<10,000 cells/s) | Hydrodynamic contact, label-free | High | High speed, label-free, suitable for suspended cells, enables sorting | Requires microfluidic setup, lacks universal standards |
| AI-based imaging prediction | Local and global | Very high (imaging-limited) | Non-invasive, label-free | Potentially high | Non-invasive, scalable, low cost after training, no physical manipulation | Requires a large annotated dataset, indirect measurement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szydlak, R. Beyond Molecular Markers: The Therapeutic Significance of Mesenchymal Stem Cell Deformability in Regenerative Medicine. Cells 2025, 14, 1516. https://doi.org/10.3390/cells14191516
Szydlak R. Beyond Molecular Markers: The Therapeutic Significance of Mesenchymal Stem Cell Deformability in Regenerative Medicine. Cells. 2025; 14(19):1516. https://doi.org/10.3390/cells14191516
Chicago/Turabian StyleSzydlak, Renata. 2025. "Beyond Molecular Markers: The Therapeutic Significance of Mesenchymal Stem Cell Deformability in Regenerative Medicine" Cells 14, no. 19: 1516. https://doi.org/10.3390/cells14191516
APA StyleSzydlak, R. (2025). Beyond Molecular Markers: The Therapeutic Significance of Mesenchymal Stem Cell Deformability in Regenerative Medicine. Cells, 14(19), 1516. https://doi.org/10.3390/cells14191516







