EPDR1 Links Fibroblast Dysfunction to Disease Severity in Idiopathic Pulmonary Fibrosis
Abstract
Highlights
- EPDR1 is significantly upregulated in IPF lung fibroblasts, tissues, BALF, and serum, and it is associated with poor prognosis.
- EPDR1 knockdown restores lysosomal acidification, improves autophagic flux, and reduces cellular senescence in IPF fibroblasts.
- EPDR1 contributes to lysosomal dysfunction and fibroblast senescence, which are key pathogenic features of IPF.
- EPDR1 may serve as a novel biomarker and potential therapeutic target in idiopathic pulmonary fibrosis.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Culture of Primary Human Fibroblasts Obtained from Biopsy Specimens
2.3. Silencing of EPDR1 via Electroporation of siRNA
2.4. Quantitative Polymerase Chain Reaction (qPCR)
2.5. Western Blot Analyses
2.6. Measurement of EPDR1 Protein in BALF
2.7. Immunofluorescence (IF) Double Stain of EPDR1 with α-SMA, COL1A1, and FN1
2.8. Analysis of Lysosomal Acidity and EPDR1 Colocalization Using LysoTracker
2.9. Senescence-Associated β-Galactosidase (SA-β-Gal) Activity
2.10. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Groups
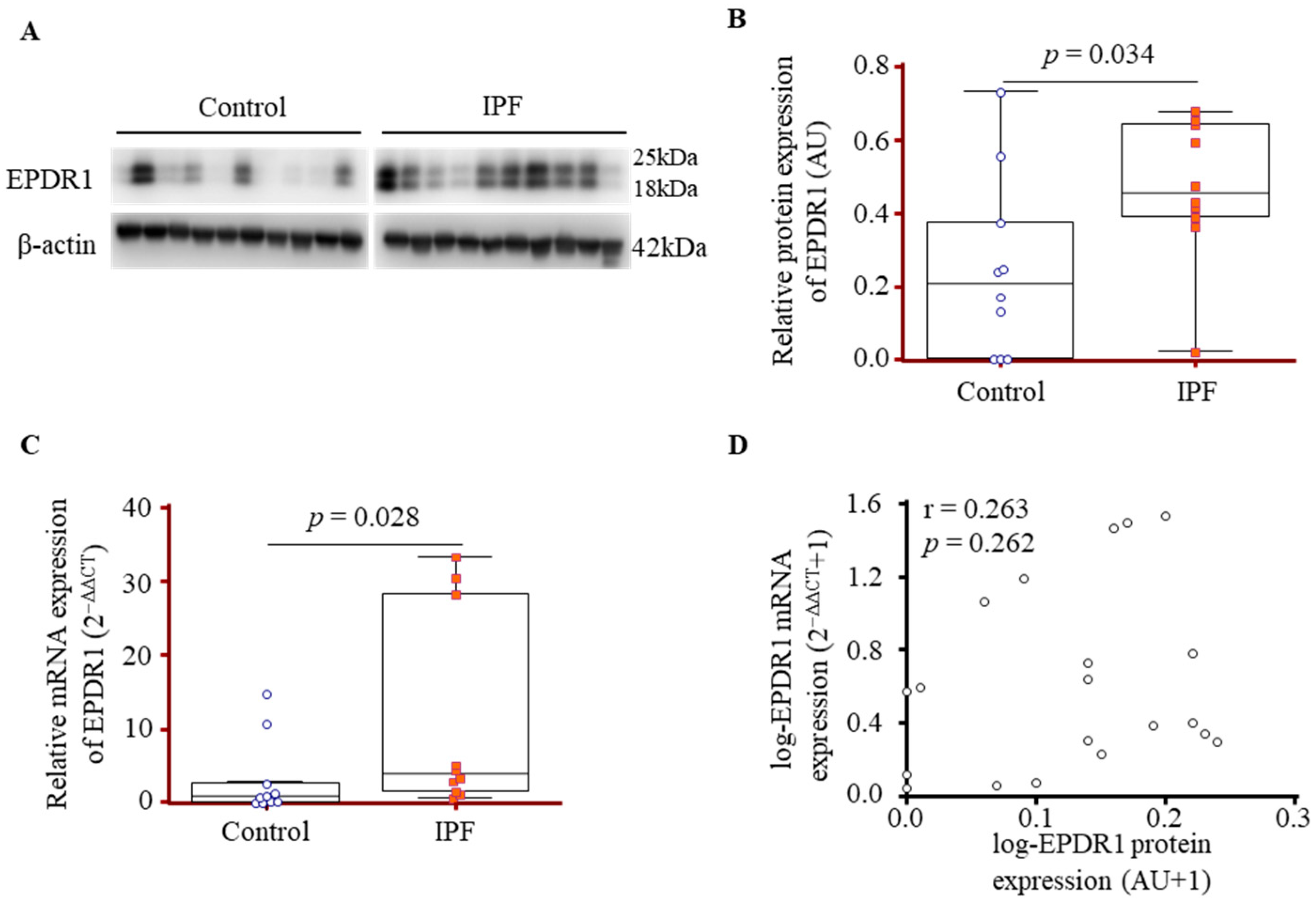
3.2. Comparison of EPDR1 Expression in Lung-Tissue-Derived Fibroblasts from IPF Patients and Controls
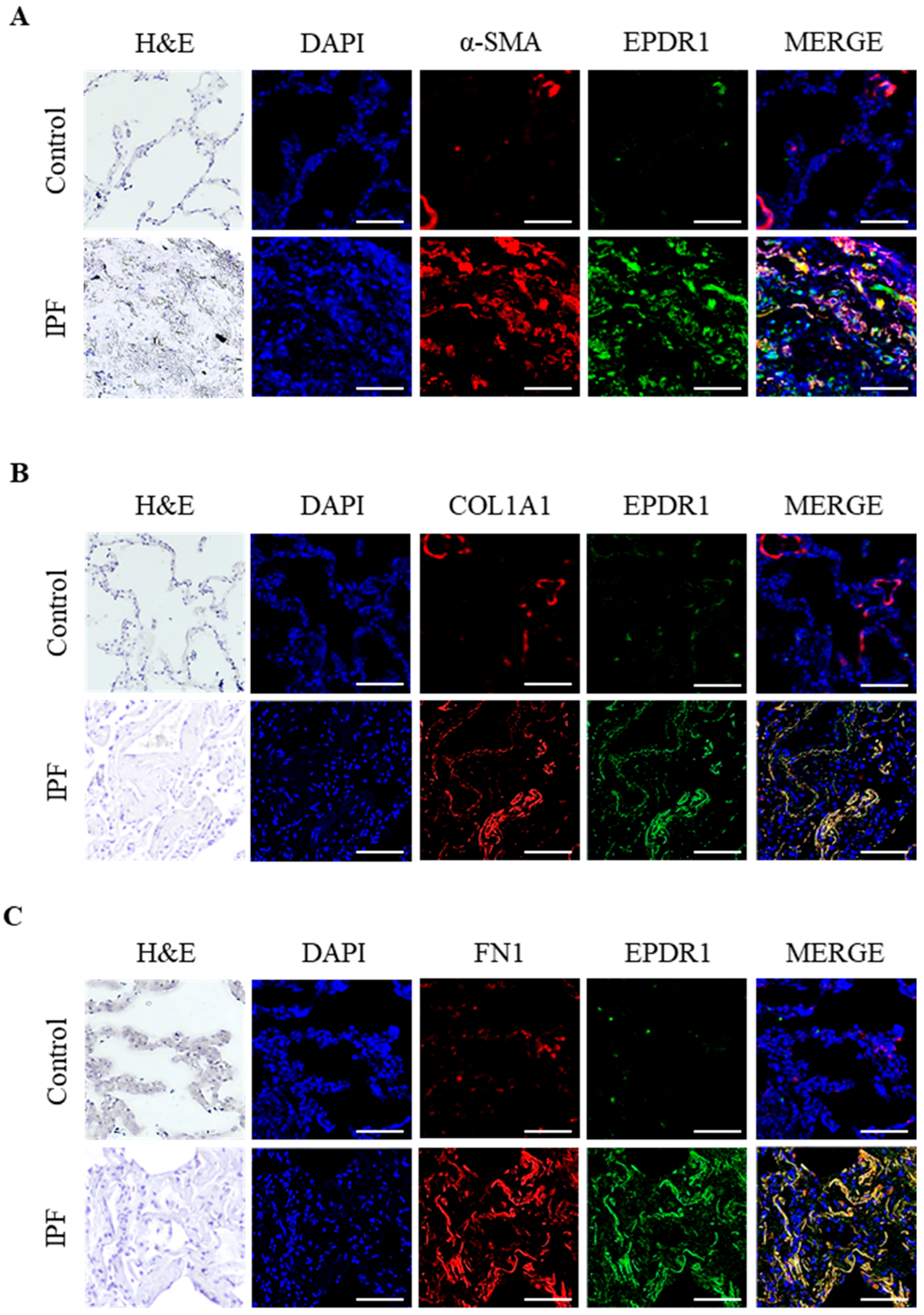
3.3. IF Localization of EPDR1 Protein in Lung Tissues
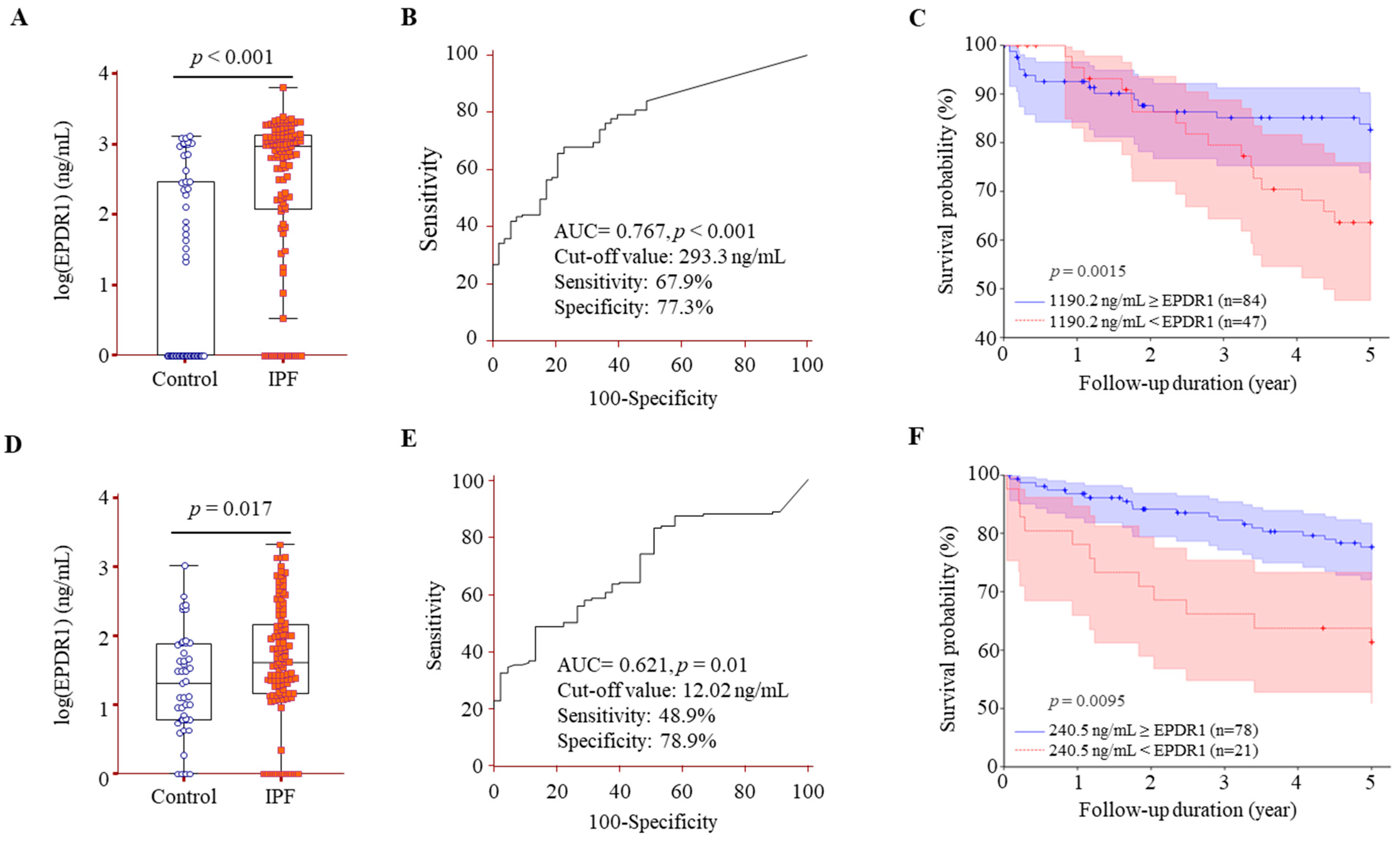
3.4. Comparison of EPDR1 Protein Levels in BALF and Serum Between IPF Patients and Controls, and Their Association with Survival in IPF
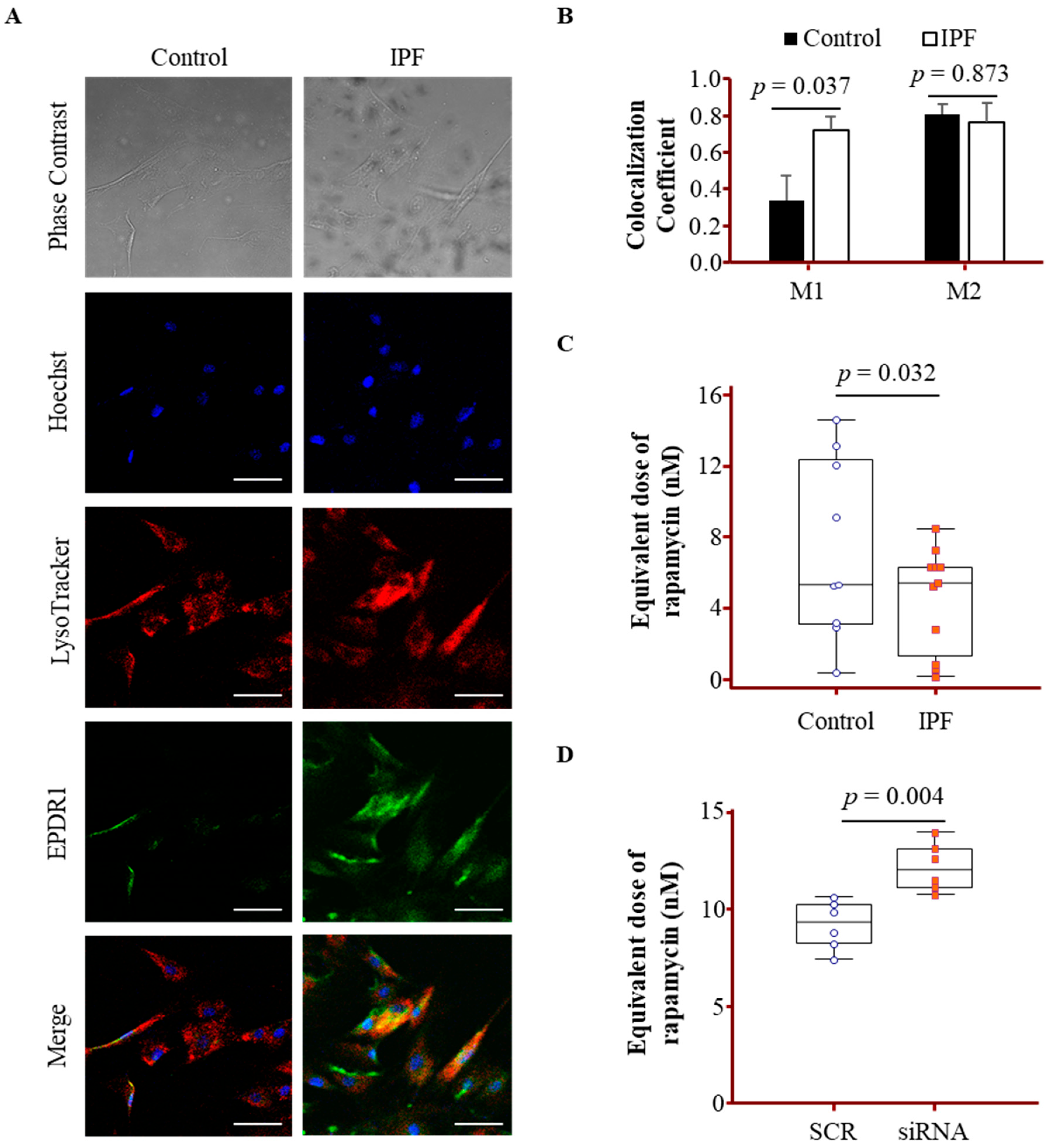
3.5. Lysosomal Localization and Activity Changes Associated with EPDR1 in IPF Fibroblasts
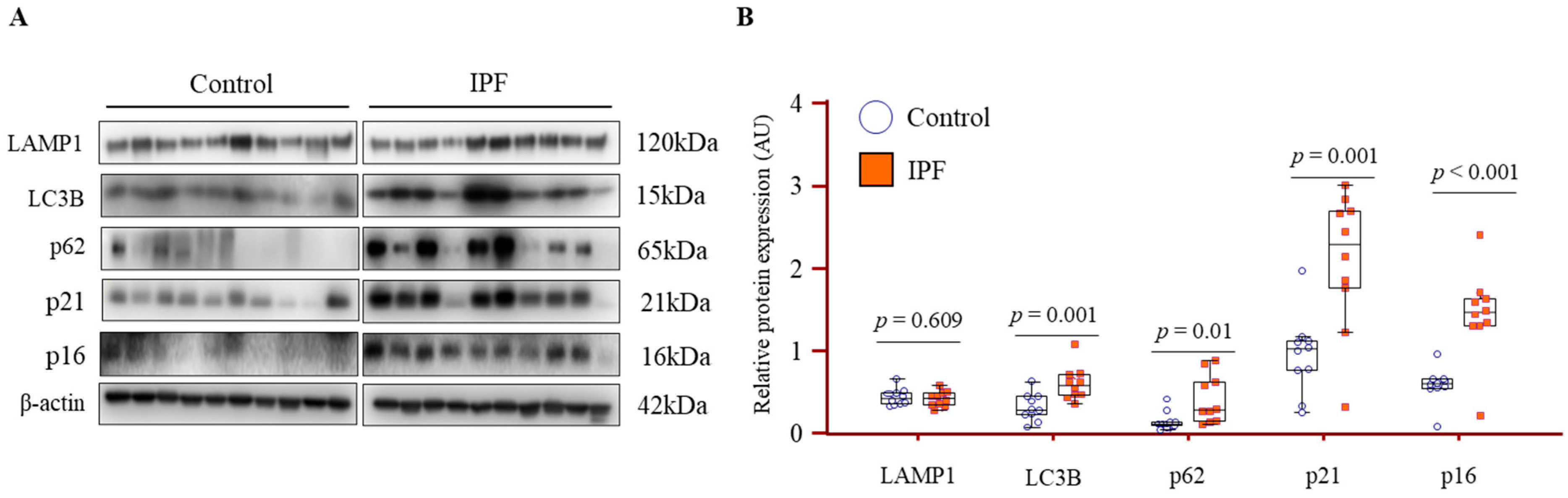
3.6. Increased Expression of Autophagy and Senescence Markers in IPF Lung Fibroblasts
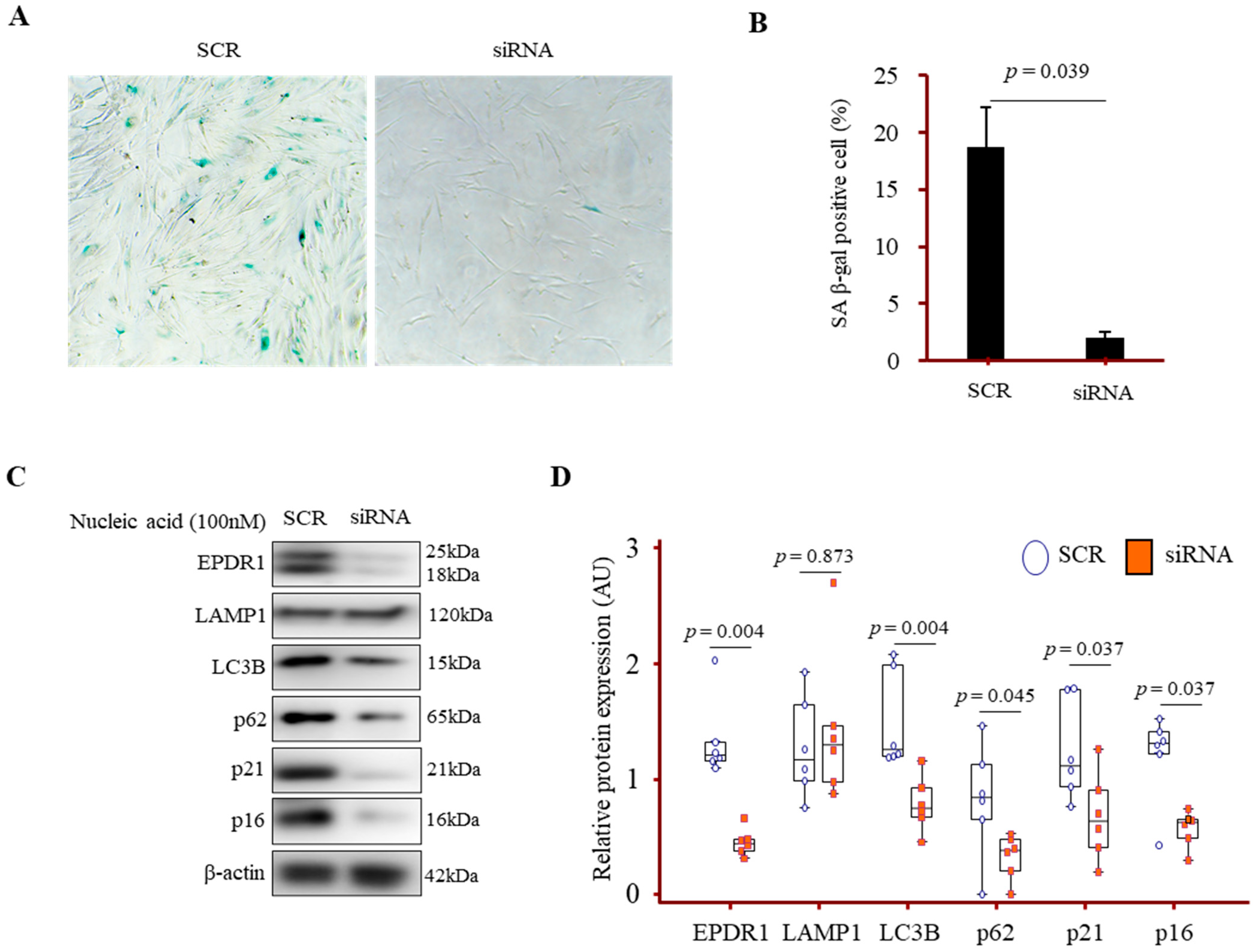
3.7. EPDR1 Knockdown Attenuated Autophagy and Senescence Marker Expression in IPF Fibroblasts
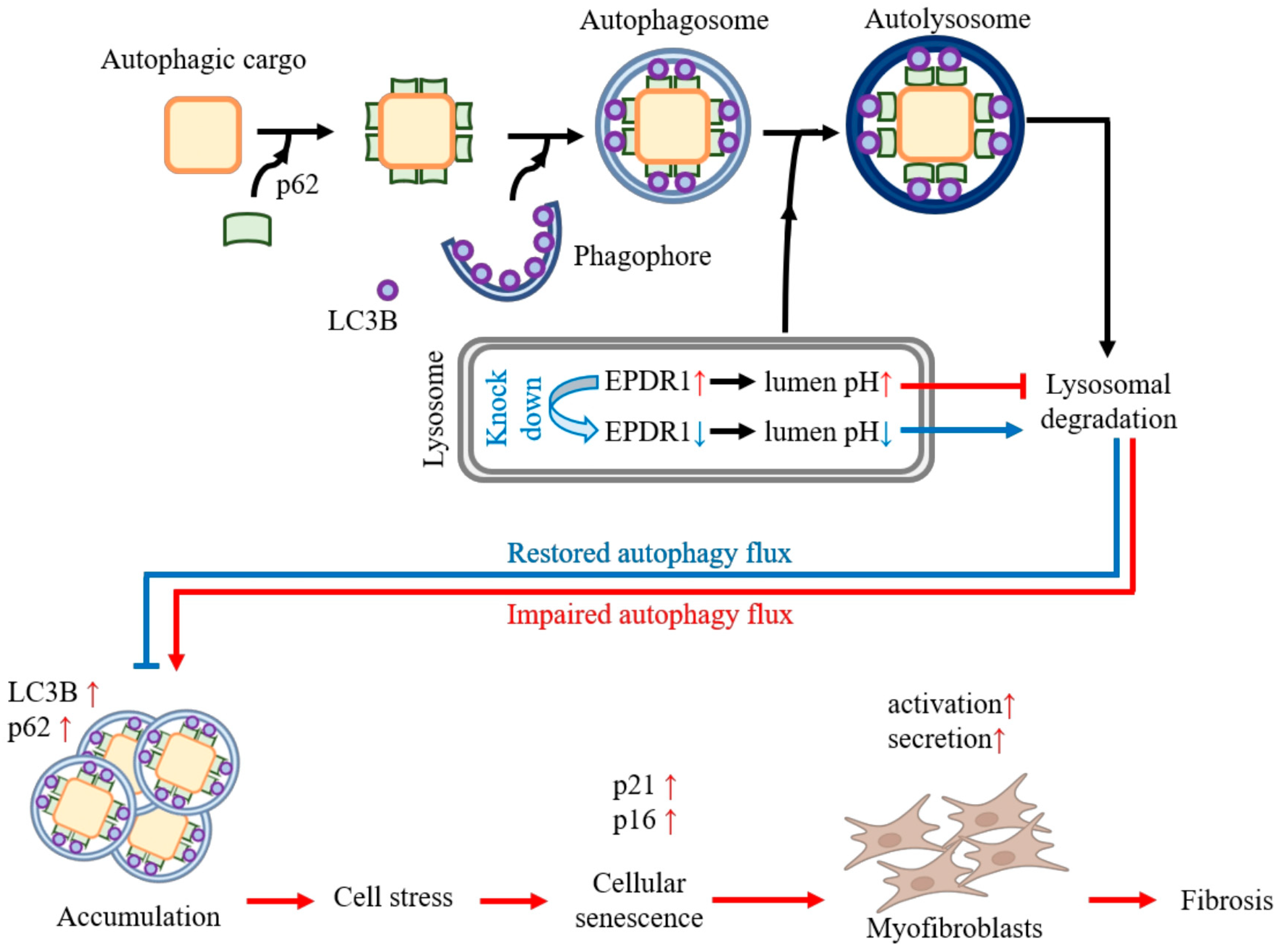
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moss, B.J.; Ryter, S.W.; Rosas, I.O. Pathogenic Mechanisms Underlying Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. 2022, 17, 515–546. [Google Scholar] [CrossRef]
- Herrera, J.; Henke, C.A.; Bitterman, P.B. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Investig. 2018, 128, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gonzalez De Los Santos, F.; Hirsch, M.; Wu, Z.; Phan, S.H. Noncanonical Wnt Signaling Promotes Myofibroblast Differentiation in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2021, 65, 489–499. [Google Scholar] [CrossRef]
- Jin, C.; Chen, Y.; Wang, Y.; Li, J.; Liang, J.; Zheng, S.; Zhang, L.; Li, Q.; Wang, Y.; Ling, F.; et al. Single-cell RNA sequencing reveals special basal cells and fibroblasts in idiopathic pulmonary fibrosis. Sci. Rep. 2024, 14, 15778. [Google Scholar] [CrossRef]
- Hanmandlu, A.; Zhu, L.; Mertens, T.C.J.; Collum, S.; Bi, W.; Xiong, F.; Wang, R.; Amirthalingam, R.T.; Ren, D.; Han, L.; et al. Transcriptomic and Epigenetic Profiling of Fibroblasts in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2022, 66, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, M.; Zhang, M.; Ye, X.; Gu, H.; Jiang, C.; Zhu, H.; Ye, X.; Li, Q.; Huang, X.; et al. The gene expression of CALD1, CDH2, and POSTN in fibroblast are related to idiopathic pulmonary fibrosis. Front. Immunol. 2024, 15, 1275064. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Cheong, H.S.; Shim, E.Y.; Bae, D.J.; Chang, H.S.; Uh, S.T.; Kim, Y.H.; Park, J.S.; Lee, B.; Shin, H.D.; et al. Gene profile of fibroblasts identify relation of CCL8 with idiopathic pulmonary fibrosis. Respir. Res. 2017, 18, 3. [Google Scholar] [CrossRef]
- Tsukui, T.; Sun, K.H.; Wetter, J.B.; Wilson-Kanamori, J.R.; Hazelwood, L.A.; Henderson, N.C.; Adams, T.S.; Schupp, J.C.; Poli, S.D.; Rosas, I.O.; et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat. Commun. 2020, 11, 1920. [Google Scholar] [CrossRef]
- Staats, K.A.; Wu, T.; Gan, B.S.; O’Gorman, D.B.; Ophoff, R.A. Dupuytren’s disease susceptibility gene, EPDR1, is involved in myofibroblast contractility. J. Dermatol. Sci. 2016, 83, 131–137. [Google Scholar] [CrossRef]
- Della Valle, M.C.; Sleat, D.E.; Sohar, I.; Wen, T.; Pintar, J.E.; Jadot, M.; Lobel, P. Demonstration of lysosomal localization for the mammalian ependymin-related protein using classical approaches combined with a novel density shift method. J. Biol. Chem. 2006, 281, 35436–35445. [Google Scholar] [CrossRef]
- Wei, Y.; Xiong, Z.J.; Li, J.; Zou, C.; Cairo, C.W.; Klassen, J.S.; Privé, G.G. Crystal structures of human lysosomal EPDR1 reveal homology with the superfamily of bacterial lipoprotein transporters. Commun. Biol. 2019, 2, 52. [Google Scholar] [CrossRef]
- Takaya, K.; Asou, T.; Kishi, K. Aging Fibroblasts Adversely Affect Extracellular Matrix Formation via the Senescent Humoral Factor Ependymin-Related Protein 1. Cells 2022, 11, 3749. [Google Scholar] [CrossRef]
- Kolter, T.; Sandhoff, K. Lysosomal degradation of membrane lipids. FEBS Lett. 2010, 584, 1700–1712. [Google Scholar] [CrossRef]
- Schulze, H.; Sandhoff, K. Sphingolipids and lysosomal pathologies. Biochim. Biophys. Acta 2014, 1841, 799–810. [Google Scholar] [CrossRef]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Park, C.S.; Chung, S.W.; Ki, S.Y.; Lim, G.I.; Uh, S.T.; Kim, Y.H.; Choi, D.I.; Park, J.S.; Lee, D.W.; Kitaichi, M. Increased levels of interleukin-6 are associated with lymphocytosis in bronchoalveolar lavage fluids of idiopathic nonspecific interstitial pneumonia. Am. J. Respir. Crit. Care Med. 2000, 162, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Adar, Y.; Stark, M.; Bram, E.E.; Nowak-Sliwinska, P.; van den Bergh, H.; Szewczyk, G.; Sarna, T.; Skladanowski, A.; Griffioen, A.W.; Assaraf, Y.G. Imidazoacridinone-dependent lysosomal photodestruction: A pharmacological Trojan horse approach to eradicate multidrug-resistant cancers. Cell Death Dis. 2012, 3, e293. [Google Scholar] [CrossRef]
- Fang, C.; Weng, T.; Hu, S.; Yuan, Z.; Xiong, H.; Huang, B.; Cai, Y.; Li, L.; Fu, X. IFN-γ-induced ER stress impairs autophagy and triggers apoptosis in lung cancer cells. Oncoimmunology 2021, 10, 1962591. [Google Scholar] [CrossRef] [PubMed]
- Shakhov, A.S.; Kovaleva, P.A.; Churkina, A.S.; Kireev, I.I.; Alieva, I.B. Colocalization Analysis of Cytoplasmic Actin Isoforms Distribution in Endothelial Cells. Biomedicines 2022, 10, 3194. [Google Scholar] [CrossRef]
- Álvarez, D.; Cárdenes, N.; Sellarés, J.; Bueno, M.; Corey, C.; Hanumanthu, V.S.; Peng, Y.; D’Cunha, H.; Sembrat, J.; Nouraie, M.; et al. IPF lung fibroblasts have a senescent phenotype. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L1164–L1173. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Adams, T.S.; Schupp, J.C.; Poli, S.; Ayaub, E.A.; Neumark, N.; Ahangari, F.; Chu, S.G.; Raby, B.A.; DeIuliis, G.; Januszyk, M.; et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci. Adv. 2020, 6, eaba1983. [Google Scholar] [CrossRef]
- Habermann, A.C.; Gutierrez, A.J.; Bui, L.T.; Yahn, S.L.; Winters, N.I.; Calvi, C.L.; Peter, L.; Chung, M.I.; Taylor, C.J.; Jetter, C.; et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci. Adv. 2020, 6, eaba1972. [Google Scholar] [CrossRef]
- Reyfman, P.A.; Walter, J.M.; Joshi, N.; Anekalla, K.R.; McQuattie-Pimentel, A.C.; Chiu, S.; Fernandez, R.; Akbarpour, M.; Chen, C.I.; Ren, Z.; et al. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1517–1536. [Google Scholar] [CrossRef]
- Morse, C.; Tabib, T.; Sembrat, J.; Buschur, K.L.; Bittar, H.T.; Valenzi, E.; Jiang, Y.; Kass, D.J.; Gibson, K.; Chen, W.; et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur. Respir. J. 2019, 54, 1802441. [Google Scholar] [CrossRef]
- Maher, T.M.; Strek, M.E. Antifibrotic therapy for idiopathic pulmonary fibrosis: Time to treat. Respir. Res. 2019, 20, 205. [Google Scholar] [CrossRef]
- D’Agnano, V.; Mariniello, D.F.; Ruotolo, M.; Quarcio, G.; Moriello, A.; Conte, S.; Sorrentino, A.; Sanduzzi Zamparelli, S.; Bianco, A.; Perrotta, F. Targeting Progression in Pulmonary Fibrosis: An Overview of Underlying Mechanisms, Molecular Biomarkers, and Therapeutic Intervention. Life 2024, 14, 229. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, H.; Zhu, H.; Yuan, D.; Zhang, H.; Liu, Z.; Zhao, F.; Liang, G. EPDR1 levels and tumor budding predict and affect the prognosis of bladder carcinoma. Front. Oncol. 2022, 12, 986006. [Google Scholar] [CrossRef]
- Gimeno-Valiente, F.; Riffo-Campos, Á.L.; Ayala, G.; Tarazona, N.; Gambardella, V.; Rodríguez, F.M.; Huerta, M.; Martínez-Ciarpaglini, C.; Montón-Bueno, J.; Roselló, S.; et al. EPDR1 up-regulation in human colorectal cancer is related to staging and favours cell proliferation and invasiveness. Sci. Rep. 2020, 10, 3723. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Y. EPDR1 correlates with immune cell infiltration in hepatocellular carcinoma and can be used as a prognostic biomarker. J. Cell Mol. Med. 2020, 24, 12107–12118. [Google Scholar] [CrossRef]
- Min, H.Y.; Sim, J.Y.; Ahn, J.H.; Kang, N.W.; Boo, H.J.; Kim, J.; Yu, N.Y.; Bottacin, M.; Huh, J.; Park, C.S.; et al. Gaylussacin, a stilbene glycoside, inhibits chronic obstructive pulmonary disease in mice. Redox Biol. 2025, 85, 103744. [Google Scholar] [CrossRef]
- Patel, A.S.; Lin, L.; Geyer, A.; Haspel, J.A.; An, C.H.; Cao, J.; Rosas, I.O.; Morse, D. Autophagy in idiopathic pulmonary fibrosis. PLoS ONE 2012, 7, e41394. [Google Scholar] [CrossRef]
- Lehmann, M.; Korfei, M.; Mutze, K.; Klee, S.; Skronska-Wasek, W.; Alsafadi, H.N.; Ota, C.; Costa, R.; Schiller, H.B.; Lindner, M.; et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur. Respir. J. 2017, 50, 1602367. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, Z. Fibroblast Senescence in Idiopathic Pulmonary Fibrosis. Front. Cell Dev. Biol. 2020, 8, 593283. [Google Scholar] [CrossRef]
- Ahmed, T.; Flores, P.C.; Pan, C.C.; Ortiz, H.R.; Lee, Y.S.; Langlais, P.R.; Mythreye, K.; Lee, N.Y. EPDR1 is a noncanonical effector of insulin-mediated angiogenesis regulated by an endothelial-specific TGF-β receptor complex. J. Biol. Chem. 2022, 298, 102297. [Google Scholar] [CrossRef]
- Kimura, S.; Noda, T.; Yoshimori, T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 2007, 3, 452–460. [Google Scholar] [CrossRef]
- Araya, J.; Kojima, J.; Takasaka, N.; Ito, S.; Fujii, S.; Hara, H.; Yanagisawa, H.; Kobayashi, K.; Tsurushige, C.; Kawaishi, M.; et al. Insufficient autophagy in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L56–L69. [Google Scholar] [CrossRef]
- Tai, H.; Wang, Z.; Gong, H.; Han, X.; Zhou, J.; Wang, X.; Wei, X.; Ding, Y.; Huang, N.; Qin, J.; et al. Autophagy impairment with lysosomal and mitochondrial dysfunction is an important characteristic of oxidative stress-induced senescence. Autophagy 2017, 13, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Neppl, C.; Keller, M.D.; Schmid, R.A.; Tschan, M.P.; Berezowska, S. Expression Analysis of Autophagy Related Markers LC3B, p62 and HMGB1 Indicate an Autophagy-Independent Negative Prognostic Impact of High p62 Expression in Pulmonary Squamous Cell Carcinomas. Cancers 2018, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.L.; Zhang, M.Y.; Liu, J.Y.; Fang, L.J.; Qu, Y.Q. The role of autophagy in idiopathic pulmonary fibrosis: From mechanisms to therapies. Ther. Adv. Respir. Dis. 2022, 16, 17534666221140972. [Google Scholar] [CrossRef]
| Clinical Parameters | Control | IPF |
|---|---|---|
| No. | 52 | 131 |
| Age (year) | 52 (44–62) | 65 (59–72) |
| Sex (male/female) | 34/18 | 86/45 |
| Smoke (CS/ES/NS) | 28/11/13 | 58/45/28 |
| Survival/death | ND | 79/52 |
| Follow up duration (years) | ND | 4.77 (1.64–7.46) |
| FVC (% pred.) | 87.26 ± 10.26 | 73.6 ± 16.77 *# |
| DLCO (% pred.) | 88.4 ± 19.75 | 70.11 ± 20.98 *# |
| BAL total cell count (×105) | 0.81 ± 0.8 | 5.63 ± 5.92 * |
| Macrophages (%) | 94.49 ± 3.53 | 63.77 ± 25.17 * |
| Neutrophils (%) | 2.55 ± 2.58 | 28.05 ± 25.19 * |
| Eosinophils (%) | 0.42 ± 0.82 | 3.2 ± 6.94 * |
| Lymphocytes (%) | 2.54 ± 2 | 4.98 ± 5.18 * |
| Parameter | HR | 95% CI | p-Value |
|---|---|---|---|
| Univariate analysis | |||
| Age (year) | 1.01 | 0.97–1.04 | 0.605 |
| Sex (male vs. female) | 0.71 | 0.35–1.43 | 0.343 |
| Smoke (yes vs. none) | 1.69 | 0.83–3.41 | 0.143 |
| BMI, kg/m2 | 1.01 | 0.89–1.15 | 0.849 |
| FVC (% pred) | 0.96 | 0.94–0.98 | 0.001 |
| DLCO (% pred) | 0.97 | 0.95–0.99 | 0.006 |
| BALF EPDR1 ≥ 1190.2 ng/mL | 2.19 | 1.08–4.43 | 0.029 |
| Serum EPDR1 ≥ 240.5 ng/mL | 2.65 | 1.29–5.45 | 0.008 |
| Multivariate analysis | |||
| BALF EPDR1 ≥ 1190.2 ng/mL | 2.21 | 1.04–4.67 | 0.038 |
| Serum EPDR1 ≥ 240.5 ng/mL | 2.61 | 1.13–5.95 | 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-U.; Park, S.-L.; Kim, M.K.; Seo, E.; Hwang, H.-G.; Kim, J.H.; Chang, H.S.; Park, C.-S. EPDR1 Links Fibroblast Dysfunction to Disease Severity in Idiopathic Pulmonary Fibrosis. Cells 2025, 14, 1515. https://doi.org/10.3390/cells14191515
Lee J-U, Park S-L, Kim MK, Seo E, Hwang H-G, Kim JH, Chang HS, Park C-S. EPDR1 Links Fibroblast Dysfunction to Disease Severity in Idiopathic Pulmonary Fibrosis. Cells. 2025; 14(19):1515. https://doi.org/10.3390/cells14191515
Chicago/Turabian StyleLee, Jong-Uk, Seung-Lee Park, Min Kyung Kim, Eunjeong Seo, Hun-Gyu Hwang, Jung Hyun Kim, Hun Soo Chang, and Choon-Sik Park. 2025. "EPDR1 Links Fibroblast Dysfunction to Disease Severity in Idiopathic Pulmonary Fibrosis" Cells 14, no. 19: 1515. https://doi.org/10.3390/cells14191515
APA StyleLee, J.-U., Park, S.-L., Kim, M. K., Seo, E., Hwang, H.-G., Kim, J. H., Chang, H. S., & Park, C.-S. (2025). EPDR1 Links Fibroblast Dysfunction to Disease Severity in Idiopathic Pulmonary Fibrosis. Cells, 14(19), 1515. https://doi.org/10.3390/cells14191515






