Using 0.1 THz Radiation Regulates Collagen Synthesis Through TGF-β/Smad Signaling Pathway in Human Fetal Scleral Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
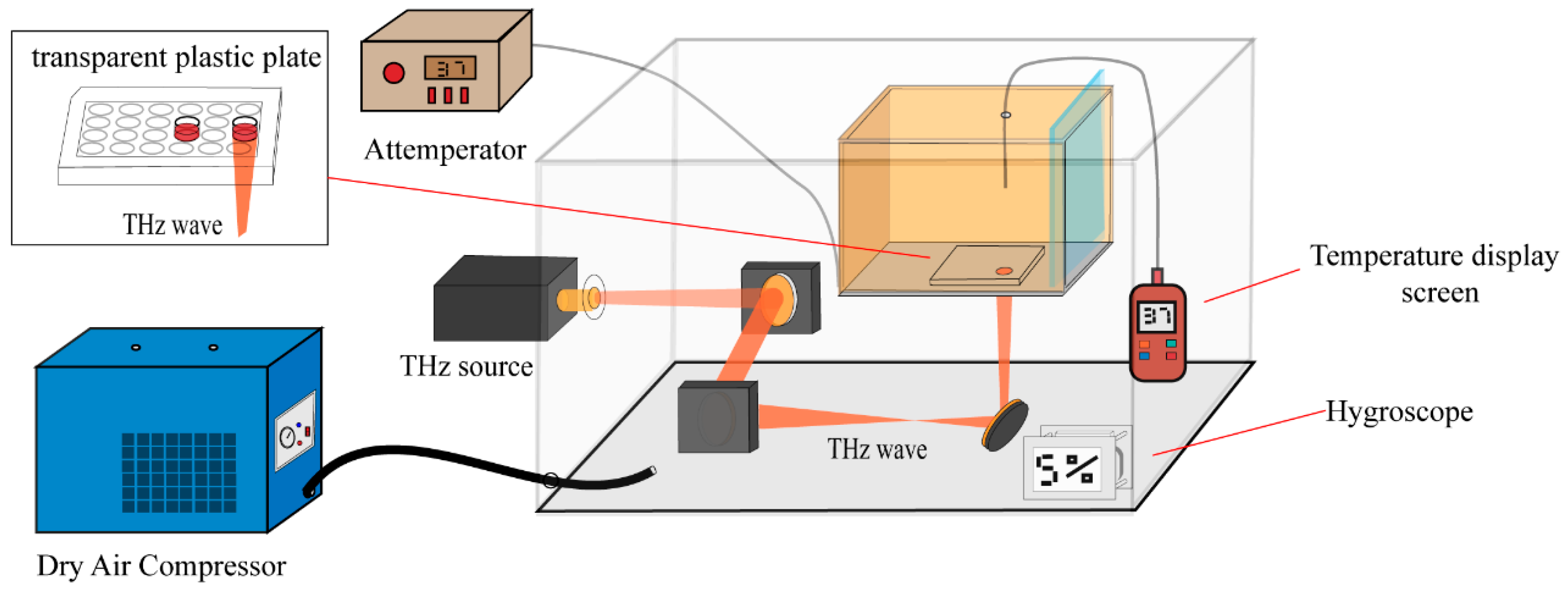
2.2. THz Sources and Irradiation
2.3. Cell Viability Assay
2.4. Proteomics Analysis
2.5. RNA Extraction and qPCR
2.6. Statistical Data Analysis
3. Results
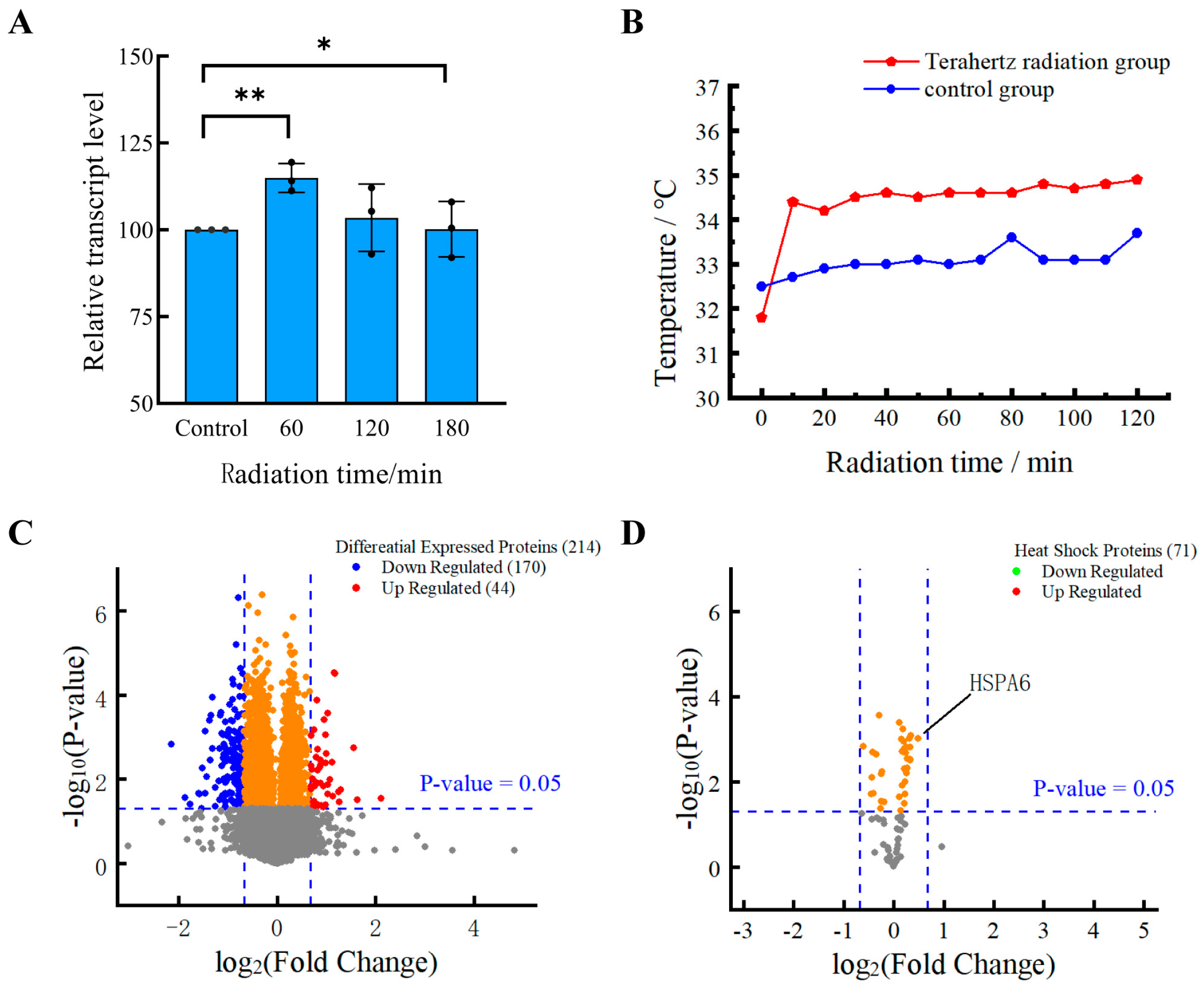
3.1. The Vitality of HFSFs Is Improved by Terahertz Radiation
3.2. The Different Expression of Proteins Induced by THz Irradiation
3.3. Heat Shock Proteins Do Not Respond to Terahertz Radiation
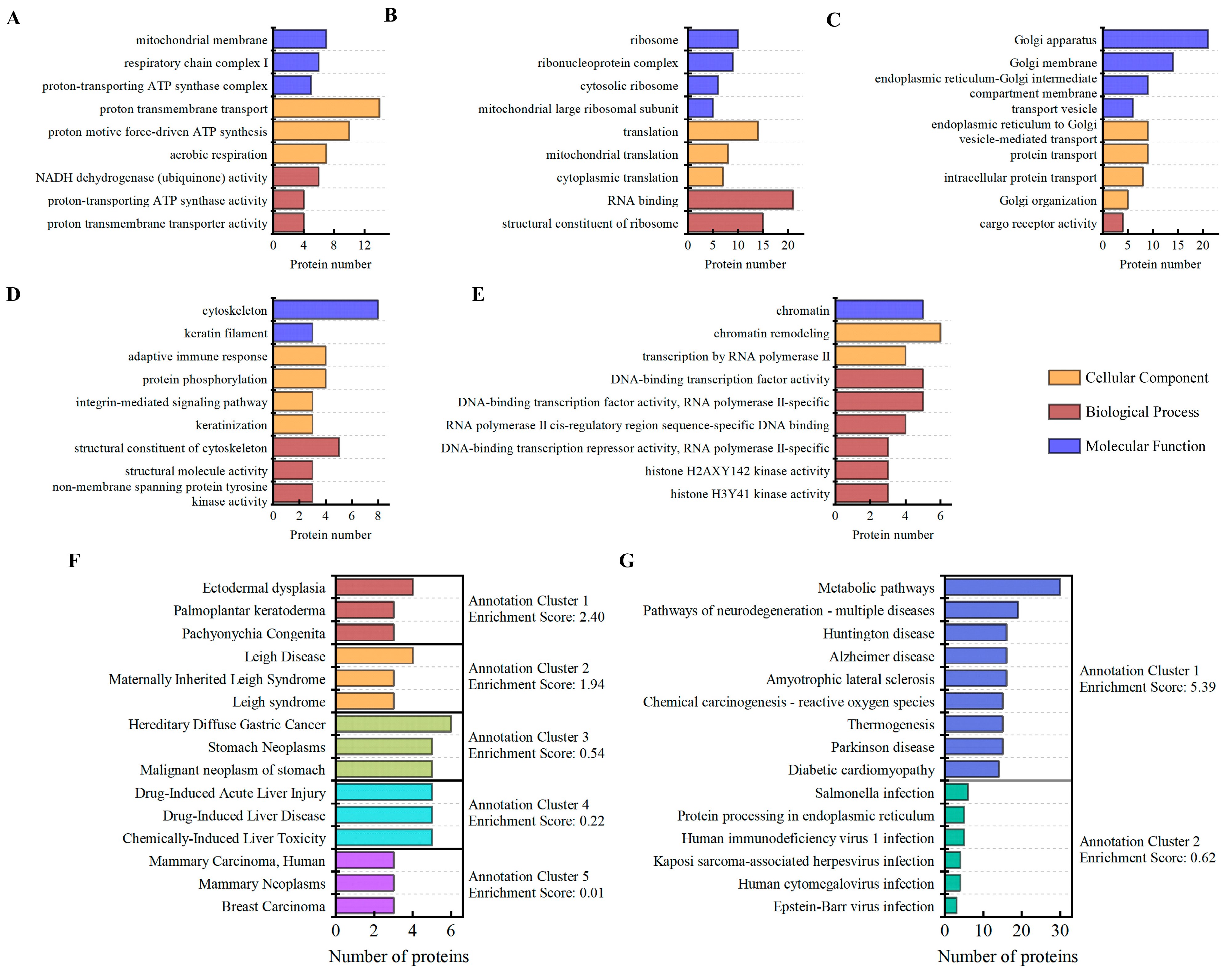
3.4. Gene Ontology (GO) Analysis
3.5. KEGG and Disease Annotation Analysis
3.6. Protein–Protein Interaction (PPI) Analysis
3.7. Terahertz Radiation Activates the Transforming Growth Factor-β/Smad Signaling Pathway
3.8. Terahertz Radiation Inhibits Collagen Synthesis and Degradation in HFSFs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HFSFs | human fetal scleral fibroblasts |
| DEPs | differentially expressed proteins |
| ECM | extracellular matrix |
| HSPs | heat shock protein |
| TGF-β | Transforming growth factor-β |
| COL1A1 | type I collagen |
| MMP-2 | matrix metalloproteinase 2 |
| TIMP-2 | tissue inhibitor of matrix metalloproteinase-2 |
References
- Globus, T.R.; Woolard, D.L.; Khromova, T.; Crowe, T.W.; Bykhovskaia, M.; Gelmont, B.L.; Hesler, J.; Samuels, A.C. THz-Spectroscopy of Biological Molecules. J. Biol. Phys. 2003, 29, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, X.; Yang, K.; Liu, Y.; Liu, Y.; Fu, W.; Luo, Y. Biomedical Applications of Terahertz Spectroscopy and Imaging. Trends Biotechnol. 2016, 34, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Nikitkina, A.I.; Bikmulina, P.Y.; Gafarova, E.R.; Kosheleva, N.V.; Efremov, Y.M.; Bezrukov, E.A.; Butnaru, D.V.; Dolganova, I.N.; Chernomyrdin, N.V.; Cherkasova, O.P.; et al. Terahertz radiation and the skin: A review. J. Biomed. Opt. 2021, 26, 043005. [Google Scholar] [CrossRef]
- De Amicis, A.; De Sanctis, S.; Di Cristofaro, S.; Franchini, V.; Lista, F.; Regalbuto, E.; Giovenale, E.; Gallerano, G.P.; Nenzi, P.; Bei, R.; et al. Biological effects of in vitro THz radiation exposure in human foetal fibroblasts. Mutat. Res. Toxicol. Environ. Mutagen. 2015, 793, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Franchini, V.; De Sanctis, S.; Marinaccio, J.; De Amicis, A.; Coluzzi, E.; Di Cristofaro, S.; Lista, F.; Regalbuto, E.; Doria, A.; Giovenale, E.; et al. Study of the effects of 0.15 terahertz radiation on genome integrity of adult fibroblasts. Environ. Mol. Mutagen. 2018, 59, 476–487. [Google Scholar] [CrossRef]
- Wilmink, G.J.; Rivest, B.D.; Roth, C.C.; Ibey, B.L.; Payne, J.A.; Cundin, L.X.; Grundt, J.E.; Peralta, X.; Mixon, D.G.; Roach, W.P. In vitro investigation of the biological effects associated with human dermal fibroblasts exposed to 2.52 THz radiation. Lasers Surg. Med. 2010, 43, 152–163. [Google Scholar] [CrossRef]
- Wilmink, G.J.; Grundt, J.E. Invited Review Article: Current State of Research on Biological Effects of Terahertz Radiation. J. Infrared Millim. Terahertz Waves 2011, 32, 1074–1122. [Google Scholar] [CrossRef]
- Kim, K.T.; Park, J.; Jo, S.J.; Jung, S.; Kwon, O.S.; Gallerano, G.P.; Park, W.Y.; Park, G.S. High-power femtosecond-terahertz pulse induces a wound response in mouse skin. Sci. Rep. 2013, 3, 2296. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, M.; Chen, C.; Ma, Q.; Zhang, Y. Investigation of the effect of terahertz radiation on acute wounds in mice. Chin. J. Aesthetic Plast. Surg. 2025, 36, 96–102. [Google Scholar] [CrossRef]
- Sun, Y.; Sha, Y.; Yang, J.; Fu, H.; Hou, X.; Li, Z.; Xie, Y.; Wang, G. Collagen is crucial target protein for scleral remodeling and biomechanical change in myopia progression and control. Heliyon 2024, 10, e35313. [Google Scholar] [CrossRef]
- Ku, H.; Chen, J.J.-Y.; Chen, W.; Tien, P.-T.; Lin, H.-J.; Wan, L.; Xu, G. The role of transforming growth factor beta in myopia development. Mol. Immunol. 2024, 167, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Mors, M.T.; Wenckebach, T.; Planken, P.C.M. Terahertz dielectric properties of polystyrene foam. J. Opt. Soc. Am. B 2002, 19, 1476–1479. [Google Scholar] [CrossRef]
- Zhang, X.; Chang, T.; Cui, H.-L.; Sun, Z.; Yang, C.; Yang, X.; Liu, L.; Fan, W. A Free-Space Measurement Technique of Terahertz Dielectric Properties. J. Infrared Millim. Terahertz Waves 2016, 38, 356–365. [Google Scholar] [CrossRef]
- Nguyen, N.N.Y.; Kim, S.S.; Jo, Y.H. Deregulated Mitochondrial DNA in Diseases. DNA Cell Biol. 2020, 39, 1385–1400. [Google Scholar] [CrossRef]
- Shang, S.; Gao, F.; Zhang, Q.; Song, T.; Wang, W.; Liu, D.; Gong, Y.; Lu, X. 0.263 terahertz irradiation induced genes expression changes in Caenorhabditis elegans. iScience 2024, 27, 109391. [Google Scholar] [CrossRef]
- Hough, C.M.; Purschke, D.N.; Huang, C.; Titova, L.V.; Kovalchuk, O.; Warkentin, B.J.; Hegmann, F.A. Topology-Based Prediction of Pathway Dysregulation Induced by Intense Terahertz Pulses in Human Skin Tissue Models. J. Infrared Millim. Terahertz Waves 2018, 39, 887–898. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, L.; Zhou, J.; Liu, X.; Xiao, M.; Chen, N.; Huang, X.; Chen, H.; Pei, X.; Zhang, H. Targeting the KRT16-vimentin axis for metastasis in lung cancer. Pharmacol. Res. 2023, 193, 106818. [Google Scholar] [CrossRef]
- Tajerian, M.; Hung, V.; Khan, H.; Lahey, L.J.; Sun, Y.; Birklein, F.; Krämer, H.H.; Robinson, W.H.; Kingery, W.S.; Clark, J.D. Identification of KRT16 as a target of an autoantibody response in complex regional pain syndrome. Exp. Neurol. 2017, 287, 14–20. [Google Scholar] [CrossRef]
- Vergouwen, D.P.C.; Ten Berge, J.C.; Guzel, C.; van den Bosch, T.P.P.; Verdijk, R.M.; Rothova, A.; Luider, T.M.; Schreurs, M.W.J. Scleral Proteome in Noninfectious Scleritis Unravels Upregulation of Filaggrin-2 and Signs of Neovascularization. Investig. Opthalmology Vis. Sci. 2023, 64, 27. [Google Scholar] [CrossRef] [PubMed]
- Metlapally, R.; Park, H.N.; Chakraborty, R.; Wang, K.K.; Tan, C.C.; Light, J.G.; Pardue, M.T.; Wildsoet, C.F. Genome-Wide Scleral Micro- and Messenger-RNA Regulation During Myopia Development in the Mouse. Investig. Opthalmology Vis. Sci. 2016, 57, 6089–6097. [Google Scholar] [CrossRef]
- Romashin, D.D.; Tolstova, T.V.; Varshaver, A.M.; Kozhin, P.M.; Rusanov, A.L.; Luzgina, N.G. Keratins 6, 16, and 17 in Health and Disease: A Summary of Recent Findings. Curr. Issues Mol. Biol. 2024, 46, 8627–8641. [Google Scholar] [CrossRef]
- Ye, W.; Qin, M.; Qiu, R.; Li, J. Keratin-based wound dressings: From waste to wealth. Int. J. Biol. Macromol. 2022, 211, 183–197. [Google Scholar] [CrossRef]
- Pecoraro, A.; Pagano, M.; Russo, G.; Russo, A. Ribosome Biogenesis and Cancer: Overview on Ribosomal Proteins. Int. J. Mol. Sci. 2021, 22, 5496. [Google Scholar] [CrossRef] [PubMed]
- Bogomazova, A.N.; Vassina, E.M.; Goryachkovskaya, T.N.; Popik, V.M.; Sokolov, A.S.; Kolchanov, N.A.; Lagarkova, M.A.; Kiselev, S.L.; Peltek, S.E. No DNA damage response and negligible genome-wide transcriptional changes in human embryonic stem cells exposed to terahertz radiation. Sci. Rep. 2015, 5, 7749. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Wu, X.; Zhang, Q.; Zhao, J.; Hu, E.; Wang, L.; Lu, X. 0.1 THz exposure affects primary hippocampus neuron gene expression via alternating transcription factor binding. Biomed. Opt. Express 2021, 12, 3729–3742. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Zhang, T.; Lu, X.; Zhao, X.; Wang, H.; Long, J.; Lu, Z. Membrane-mediated modulation of mitochondrial physiology by terahertz waves. Biomed. Opt. Express 2024, 15, 4065–4080. [Google Scholar] [CrossRef]
- Marchesi, N.; Fahmideh, F.; Boschi, F.; Pascale, A.; Barbieri, A. Ocular Neurodegenerative Diseases: Interconnection between Retina and Cortical Areas. Cells 2021, 10, 2394. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.; LÜ, J.; Xu, R.; Le, W. Terahertz technology: A new frontier in Alzheimer’s disease therapy. Innov. Life 2024, 2, 100084-1–100084-8. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Zhao, Y.; Wang, P.; Ding, H.; Liu, C.; Lyu, J.; Le, W. Terahertz Irradiation Improves Cognitive Impairments and Attenuates Alzheimer’s Neuropathology in the APPSWE/PS1DE9 Mouse: A Novel Therapeutic Intervention for Alzheimer’s Disease. Neurosci. Bull. 2023, 40, 857–871. [Google Scholar] [CrossRef]
- Liang, R.; Li, T.; Shi, W.; Gao, H.; Ai, B.; Li, B.; Zhou, X. Proteomic insights into myopia development: Differential protein expression and the role of calcium signaling in form deprivation myopia in Guinea pigs. Biomed. Technol. 2024, 7, 15–24. [Google Scholar] [CrossRef]
- Ji, S.; Ye, L.; Yuan, J.; Feng, Q.; Dai, J. Integrative Transcriptome and Proteome Analyses Elucidate the Mechanism of Lens-Induced Myopia in Mice. Investig. Opthalmology Vis. Sci. 2023, 64, 15. [Google Scholar] [CrossRef]
- Wang, M.-K.; Sun, H.-Q.; Xiang, Y.-C.; Jiang, F.; Su, Y.-P.; Zou, Z.-M. Different roles of TGF-β in the multi-lineage differentiation of stem cells. World J. Stem Cells 2012, 4, 28–34. [Google Scholar] [CrossRef]
- Dione, M.N.; Zhang, Q.; Shang, S.; Lu, X. Transcriptomic Analysis of Blood Collagen-Induced Arthritis Mice Exposed to 0.1 THz Reveals Inhibition of Genes and Pathways Involved in Rheumatoid Arthritis. Int. J. Mol. Sci. 2024, 25, 12812. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Wotton, D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000, 19, 1745–1754. [Google Scholar] [CrossRef]
- Shi, Y.; Massagué, J. Mechanisms of TGF-β Signaling from Cell Membrane to the Nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, B.; Dai, L.; Wang, Z.; Feng, L.; Zhao, J. Association between TGF-β gene polymorphism and myopia: A systematic review and meta-analysis. Medicine 2022, 101, e29961. [Google Scholar] [CrossRef]
- Wang, J.; Cui, J.; Zhu, H. Suppression of type I collagen in human scleral fibroblasts treated with extremely low-frequency electromagnetic fields. Mol. Vis. 2013, 19, 885–893. [Google Scholar]
- Shelton, L.; Rada, J.A. Inhibition of Human Scleral Fibroblast Cell Attachment to Collagen Type I by TGFBIp. Investig. Opthalmology Vis. Sci. 2009, 50, 3542–3552. [Google Scholar] [CrossRef]
- Hsiao, Y.T.; Lee, J.J.; Yang, I.H.; Wu, P.C.; Ke, M.C.; Lo, J. Ultraviolet A at levels experienced outdoors suppresses transforming growth factor-beta signaling and collagen production in human scleral fibroblasts. Biochem. Biophys. Res. Commun. 2022, 641, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Jobling, A.I.; Nguyen, M.; Gentle, A.; McBrien, N.A. Isoform-specific Changes in Scleral Transforming Growth Factor-β Expression and the Regulation of Collagen Synthesis during Myopia Progression. J. Biol. Chem. 2004, 279, 18121–18126. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yuan, Y.; Chen, Q.; Me, R.; Gu, Q.; Yu, Y.; Sheng, M.; Ke, B. Expression of Wnt/β-Catenin Signaling Pathway and Its Regulatory Role in Type I Collagen with TGF-β1 in Scleral Fibroblasts from an Experimentally Induced Myopia Guinea Pig Model. J. Ophthalmol. 2016, 2016, 5126560. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zheng, Q.; He, J.; Li, L.; Xie, X.; Liang, H. Hsa-miR-142-3p reduces collagen I in human scleral fibroblasts by targeting TGF-β1 in high myopia. Exp. Eye Res. 2022, 219, 109023. [Google Scholar] [CrossRef] [PubMed]
- Siegwart, J.T.; Norton, T.T. Steady state mRNA levels in tree shrew sclera with form-deprivation myopia and during recovery. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1153–1159. [Google Scholar]


| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| GAPDH | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA |
| COL1A1 | CCCGGGTTTCAGAGACAACTTC | TCCACATGCTTTATTCCAGCAATC |
| MMP2 | CTCATCGCAGATGCCTGGAA | TTCAGGTAATAGGCACCCTTGAAGA |
| TGFB2 | TTACACTGTCCCTGCTGCACTT | GGTATATGTGGAGGTGCCATCAA |
| TIMP2 | GGAGCACTGTGTTTATGCTGGAA | GAGACATGCGCAGTCTGCTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Sun, L.; Wang, L.; Zhao, J.; She, S.; Hou, P.; He, M. Using 0.1 THz Radiation Regulates Collagen Synthesis Through TGF-β/Smad Signaling Pathway in Human Fetal Scleral Fibroblasts. Cells 2025, 14, 1512. https://doi.org/10.3390/cells14191512
Wang W, Sun L, Wang L, Zhao J, She S, Hou P, He M. Using 0.1 THz Radiation Regulates Collagen Synthesis Through TGF-β/Smad Signaling Pathway in Human Fetal Scleral Fibroblasts. Cells. 2025; 14(19):1512. https://doi.org/10.3390/cells14191512
Chicago/Turabian StyleWang, Wenxia, Liu Sun, Lei Wang, Jinwu Zhao, Shuocheng She, Pandeng Hou, and Mingxia He. 2025. "Using 0.1 THz Radiation Regulates Collagen Synthesis Through TGF-β/Smad Signaling Pathway in Human Fetal Scleral Fibroblasts" Cells 14, no. 19: 1512. https://doi.org/10.3390/cells14191512
APA StyleWang, W., Sun, L., Wang, L., Zhao, J., She, S., Hou, P., & He, M. (2025). Using 0.1 THz Radiation Regulates Collagen Synthesis Through TGF-β/Smad Signaling Pathway in Human Fetal Scleral Fibroblasts. Cells, 14(19), 1512. https://doi.org/10.3390/cells14191512







