Immunological Mechanisms Underlying Allergy Predisposition After SARS-CoV-2 Infection in Children
Abstract
1. Introduction
2. Incidence of Post–COVID New-Onset Allergies or Asthma
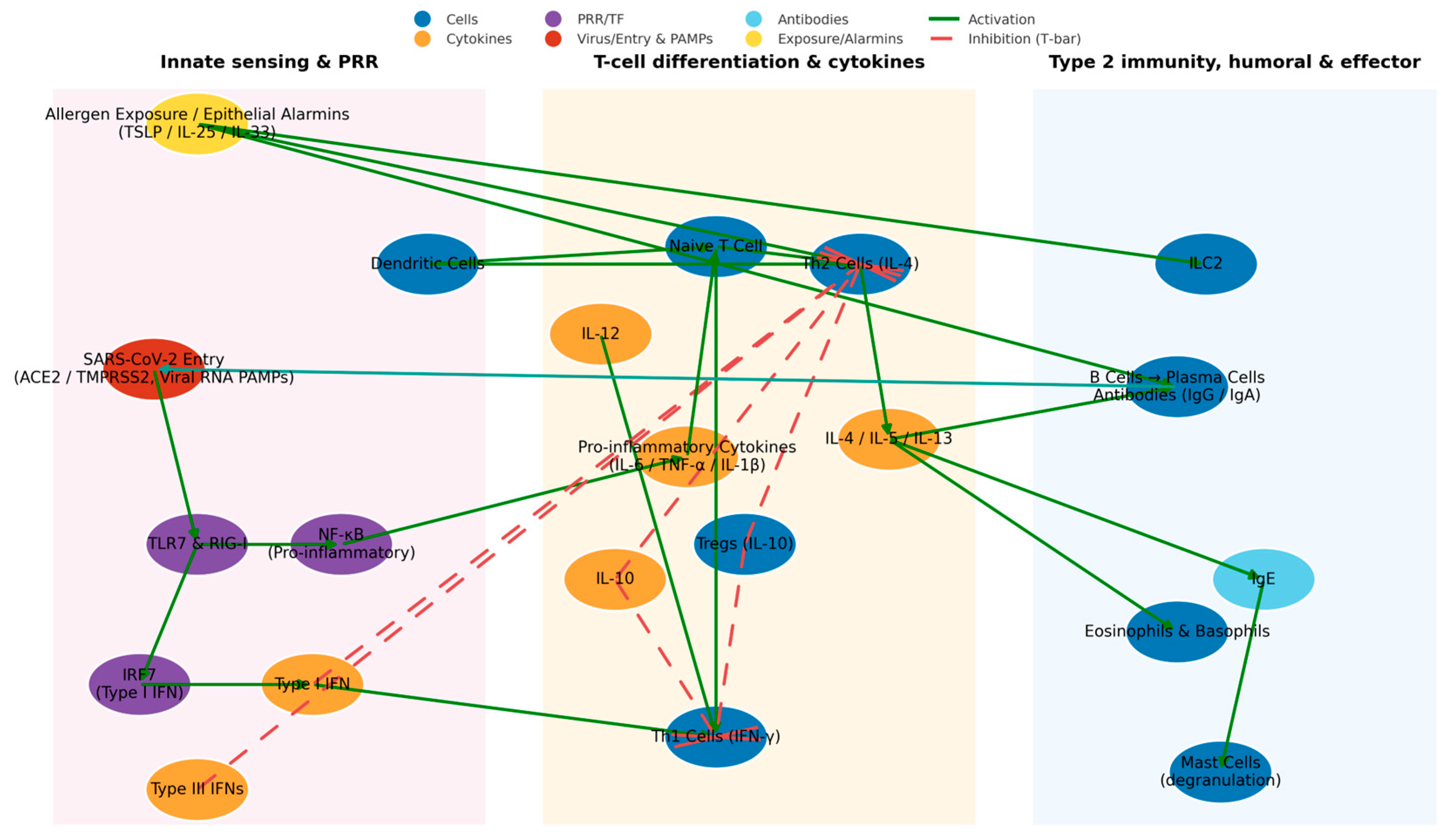
3. Immunological Mechanisms
4. Immunopathogenesis: Cytokines, Receptors, and Immune Cells
5. Immunological and Biological Mechanisms
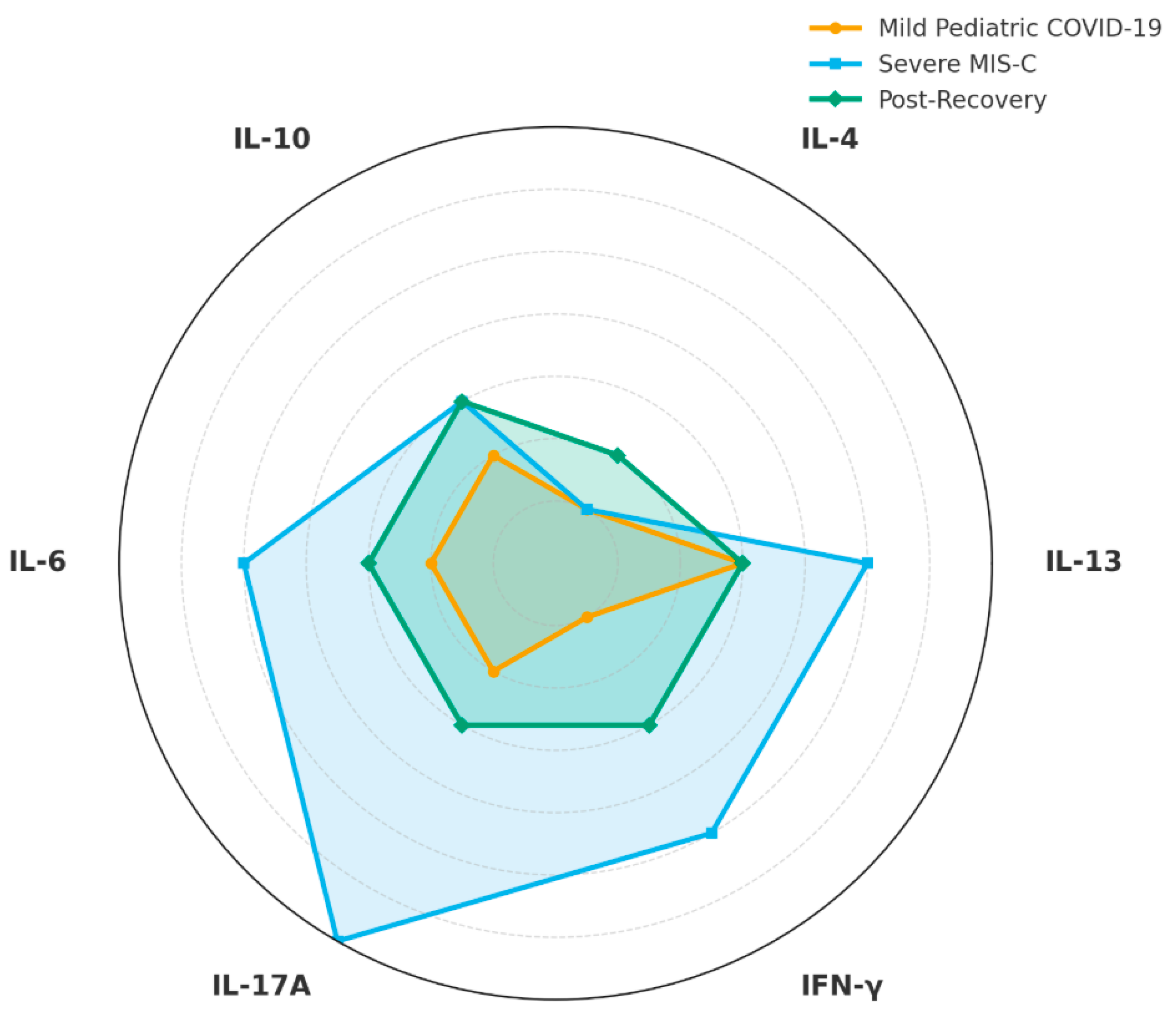
6. Cytokine Profiles and Persistent Inflammation
7. Regulatory T Cells and the Disruption of Tolerance
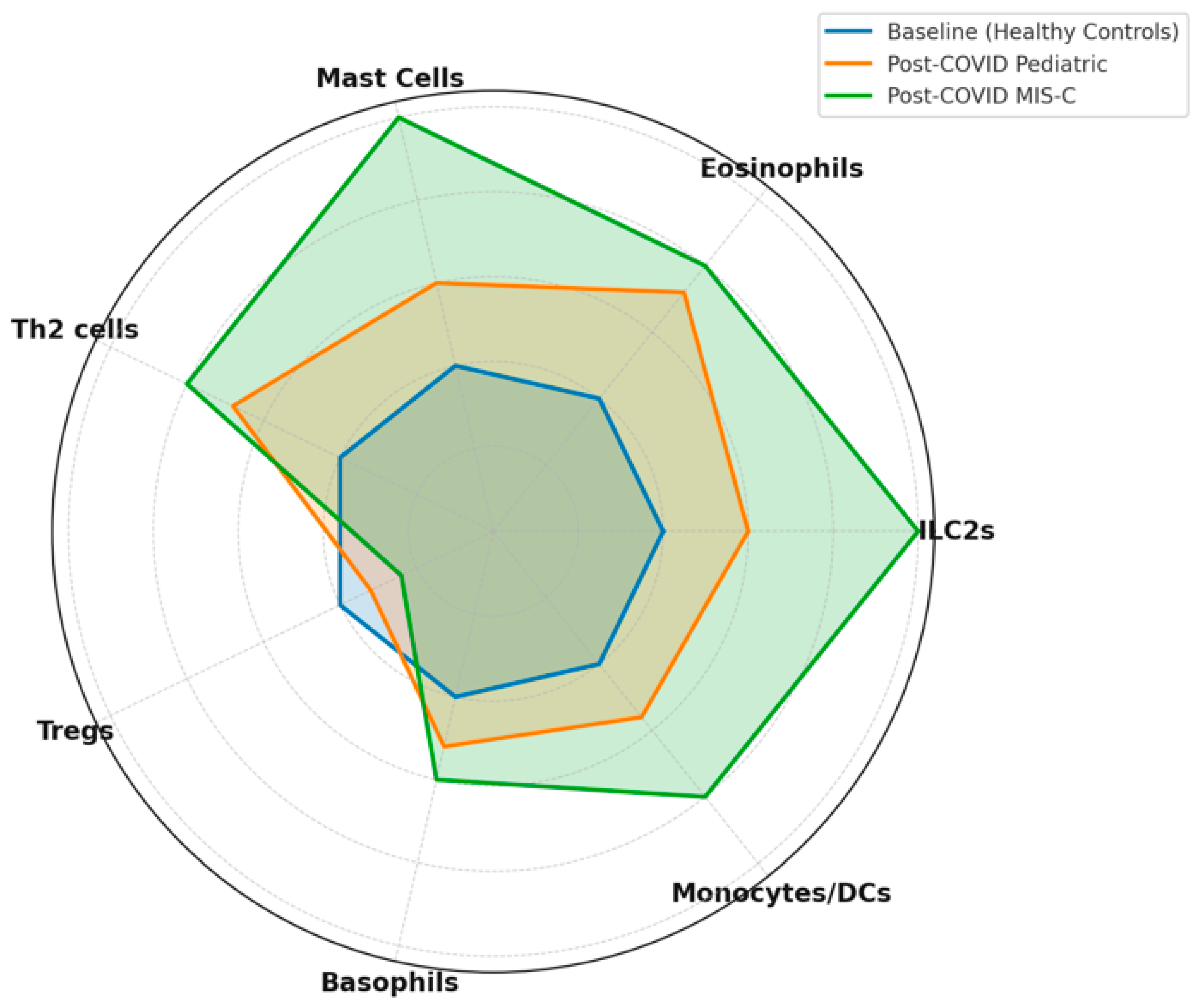
8. Imbalances of Th1, Th2, and Th17 Cells and Atopic Predisposition
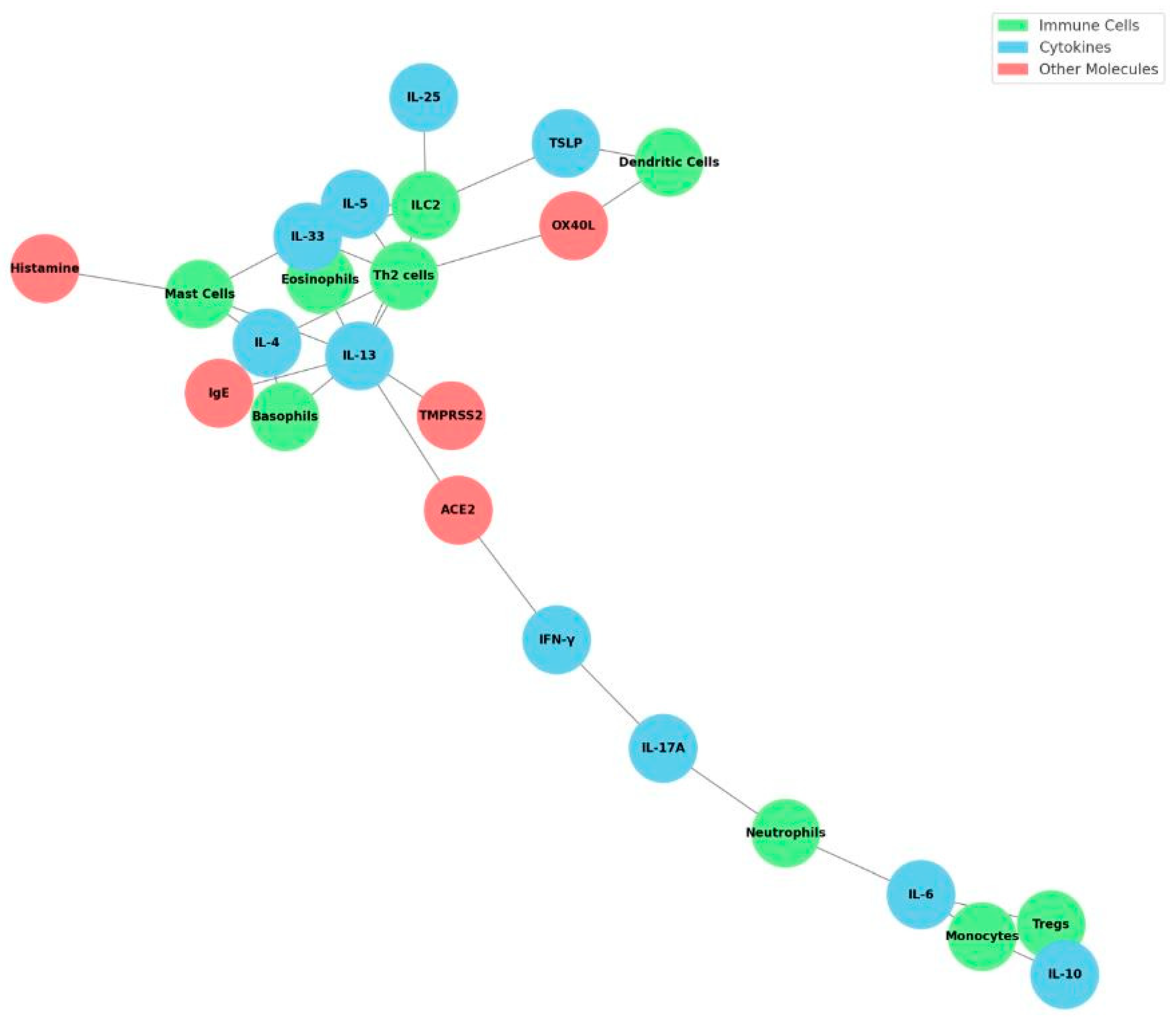
9. Cytokine Pathways and Immune Networks
10. Implications for Allergy Development and Persistence
11. Key Immune Cell Players
12. Cytokine Cross-Regulation and Immune Checkpoints
13. Molecular Mimicry and Autoantibodies
14. Trained Immunity, Epigenetic and Transcriptomic Reprogramming: Distinct Epigenetic Profiles Connecting COVID-19 to Atopic Susceptibility
15. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zimmermann, P.; Curtis, N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Dis. Child. 2021, 106, 429–439. [Google Scholar] [CrossRef]
- Kompaniyets, L.; Agathis, N.T.; Nelson, J.M.; Preston, L.E.; Ko, J.Y.; Belay, B.; Pennington, A.F.; Danielson, M.L.; DeSisto, C.L.; Chevinsky, J.R.; et al. Underlying Medical Conditions Associated With Severe COVID-19 Illness Among Children. JAMA Netw. Open 2021, 4, e2111182. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- Brodin, P. Immune determinants of COVID-19 disease presentation in children. Nat. Med. 2022, 28, 819–828. [Google Scholar] [CrossRef]
- Gern, J.E. Viral respiratory infection and the link to asthma. Pediatr. Infect. Dis. J. 2008, 27 (Suppl. 10), S97–S103. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; Gangnon, R.E.; Evans, M.D.; Roberg, K.A.; Anderson, E.L.; Pappas, T.E.; Printz, M.C.; Lee, W.M.; Shult, P.A.; Reisdorf, E.; et al. Wheezing rhinovirus illnesses in early life predict asthma development. Am. J. Respir. Crit. Care Med. 2008, 178, 667–672. [Google Scholar] [CrossRef]
- Celebi Sozener, Z.; Ozdel Ozturk, B.; Cerci, P.; Turk, M.; Gorgulu Akin, B.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial barrier hypothesis: Effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy 2022, 77, 1418–1449. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R. Viral Infection and Airway Epithelial Immunity in Asthma. Int. J. Mol. Sci. 2022, 23, 9914. [Google Scholar] [CrossRef]
- Bordon, Y. A dead calm. Nat. Rev. Immunol. 2012, 13, 4–5. [Google Scholar] [CrossRef]
- Fischer, A.; Rausell, A. What do primary immunodeficiencies tell us about the essentiality/redundancy of immune responses? Semin. Immunol. 2018, 36, 13–16. [Google Scholar] [CrossRef]
- Welham, A.; Chorvinsky, E.; Bhattacharya, S.; Bera, B.S.; Salka, K.; Weinstock, J.; Chen, X.X.; Perez, G.F.; Pillai, D.K.; Gutierrez, M.J.; et al. High TSLP responses in the human infant airways are associated with pre-activated airway epithelial IFN antiviral immunity. Immunology 2025, 176, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zou, G.L.; Zhang, W.; Lai, X.N.; Chen, H.W.; Xiong, L.X. Interleukin-33: Its Emerging Role in Allergic Diseases. Molecules 2018, 23, 1665. [Google Scholar] [CrossRef]
- Porsbjerg, C.M.; Sverrild, A.; Lloyd, C.M.; Menzies-Gow, A.N.; Bel, E.H. Anti-alarmins in asthma: Targeting the airway epithelium with next-generation biologics. Eur. Respir. J. 2020, 56, 2000260. [Google Scholar] [CrossRef]
- Filippatos, F.; Tatsi, E.-B.; Dourdouna, M.-M.; Zoumakis, E.; Margeli, A.; Syriopoulou, V.; Michos, A. SARS-CoV-2 Seroepidemiology and Antibody Levels in Children during BA.5 Predominance Period. Diagnostics 2024, 14, 1039. [Google Scholar] [CrossRef]
- Kim, B.-G.; Lee, H.; Yeom, S.W.; Jeong, C.Y.; Park, D.W.; Park, T.S.; Moon, J.-Y.; Kim, T.-H.; Sohn, J.W.; Yoon, H.J.; et al. Increased Risk of New-Onset Asthma After COVID-19: A Nationwide Population-Based Cohort Study. J. Allergy Clin. Immunol. Pract. 2023, 12, 120–132.e5. [Google Scholar] [CrossRef]
- Oh, J.; Lee, M.; Kim, M.; Kim, H.J.; Lee, S.W.; Rhee, S.Y.; Koyanagi, A.; Smith, L.; Kim, M.S.; Lee, H.; et al. Incident allergic diseases in post-COVID-19 condition: Multinational cohort studies from South Korea, Japan and the UK. Nat. Commun. 2024, 15, 2830. [Google Scholar] [CrossRef]
- Kompaniyets, L.; Bull-Otterson, L.; Boehmer, T.K.; Baca, S.; Alvarez, P.; Hong, K.; Hsu, J.; Harris, A.M.; Gundlapalli, A.V.; Saydah, S. Post–COVID-19 Symptoms and Conditions Among Children and Adolescents—United States, March 1, 2020–January 31, 2022. MMWR-Morb. Mortal. Wkly. Rep. 2022, 71, 993–999. [Google Scholar] [CrossRef]
- Senter, J.P.; Aisenberg, L.K.; Dudley, J.W.; Luan, X.; Huang, J.; Kenyon, C.C.; Hill, D.A. COVID-19 and Asthma Onset in Children. Pediatrics 2024, 153, e2023064615. [Google Scholar] [CrossRef]
- Horton, D.B.; Neikirk, A.L.; Yang, Y.; Huang, C.; Panettieri, R.A.; Crystal, S.; Strom, B.L.; Parlett, L.E. Childhood asthma diagnoses declined during the COVID-19 pandemic in the United States. Respir. Res. 2023, 24, 72. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.J.; Williams, D.M.; Walker, A.J.; Mitchell, R.E.; Niedzwiedz, C.L.; Yang, T.C.; Huggins, C.F.; Kwong, A.S.F.; Silverwood, R.J.; Di Gessa, G.; et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat. Commun. 2022, 13, 3528. [Google Scholar] [CrossRef]
- Slob, E.M.A.; Longo, C.; Vijverberg, S.J.H.; van Beijsterveldt, T.C.E.M.; Bartels, M.; Hottenga, J.J.; Pijnenburg, M.W.; Koppelman, G.H.; der Zee, A.M.; Dolan, C.V.; et al. Persistence of parental-reported asthma at early ages: A longitudinal twin study. Pediatr. Allergy Immunol. 2022, 33, e13762. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.K.; Palm, P.; Nygaard, U.; Bundgaard, H.; Petersen, M.N.S.; Rosenkilde, S.; Thorsted, A.B.; Ersbøll, A.K.; Thygesen, L.C.; Nielsen, S.D.; et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0–14 years and matched controls in Denmark (LongCOVIDKidsDK): A national, cross-sectional study. Lancet Child Adolesc. Health 2022, 6, 614–623. [Google Scholar] [CrossRef]
- Filippatos, F.; Tatsi, E.-B.; Michos, A. Immunology of Multisystem Inflammatory Syndrome after COVID-19 in Children: A Review of the Current Evidence. Int. J. Mol. Sci. 2023, 24, 5711. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, V.G.; Lukassen, S.; Drechsler, M.; Loske, J.; Burkart, S.S.; Wüst, S.; Jacobsen, E.M.; Röhmel, J.; Mall, M.A.; Debatin, K.M.; et al. Immune-epithelial cell cross-talk enhances antiviral responsiveness to SARS-CoV-2 in children. EMBO Rep. 2023, 24, e57912. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Connors, T.J.; Zhu, Y.; Baldwin, M.R.; Lin, W.H.; Wontakal, S.; Szabo, P.A.; Wells, S.B.; Dogra, P.; Gray, J.; et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2021, 22, 25–31. [Google Scholar] [CrossRef]
- Sajuthi, S.P.; DeFord, P.; Li, Y.; Jackson, N.D.; Montgomery, M.T.; Everman, J.L.; Rios, C.L.; Pruesse, E.; Nolin, J.D.; Plender, E.G.; et al. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat. Commun. 2020, 11, 5139. [Google Scholar] [CrossRef]
- Bradding, P.; Richardson, M.; Hinks, T.S.C.; Howarth, P.H.; Choy, D.F.; Arron, J.R.; Wenzel, S.E.; Siddiqui, S. ACE2, TMPRSS2, and furin gene expression in the airways of people with asthma-implications for COVID-19. J. Allergy Clin. Immunol. 2020, 146, 208–211. [Google Scholar] [CrossRef]
- Kimura, H.; Francisco, D.; Conway, M.; Martinez, F.D.; Vercelli, D.; Polverino, F.; Billheimer, D.; Kraft, M. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J. Allergy Clin. Immunol. 2020, 146, 80–88.e8. [Google Scholar] [CrossRef]
- Peters, M.C.; Sajuthi, S.; Deford, P.; Christenson, S.; Rios, C.L.; Montgomery, M.T.; Woodruff, P.G.; Mauger, D.T.; Erzurum, S.C.; Johansson, M.W.; et al. COVID-19-related Genes in Sputum Cells in Asthma. Relationship to Demographic Features and Corticosteroids. Am. J. Respir. Crit. Care Med. 2020, 202, 83–90. [Google Scholar] [CrossRef]
- Hamey, F.K.; Lau, W.W.; Kucinski, I.; Wang, X.; Diamanti, E.; Wilson, N.K.; Göttgens, B.; Dahlin, J.S. Single-cell molecular profiling provides a high-resolution map of basophil and mast cell development. Allergy 2020, 76, 1731–1742. [Google Scholar] [CrossRef]
- Sasaki, H.; Miyata, J.; Kawana, A.; Fukunaga, K. Antiviral roles of eosinophils in asthma and respiratory viral infection. Front. Allergy 2025, 6, 1548338. [Google Scholar] [CrossRef] [PubMed]
- Asmara, I.G.Y.; Agustriadi, I.G.N.O.; Sujaya, I.M.; Thalib, S.S.; Lestari, R.; Fatrullah, S.P.; Widiasari, K.S.R.; Ajmala, I.E. Eosinopenia as a prognostic factor of mortality for COVID-19 in end-stage kidney disease patients. Caspian J. Intern. Med. 2024, 15, 273–279. [Google Scholar] [CrossRef]
- Moya, B.; Riggioni, C.; Kalayci, Ö.; Eigenmann, P. Editorial comment on: Diet-associated vertically transferred metabolites and risk of asthma, allergy, eczema, and infections in early childhood. Pediatr. Allergy Immunol. 2023, 34, e13947. [Google Scholar] [CrossRef]
- Macchia, I.; La Sorsa, V.; Urbani, F.; Moretti, S.; Antonucci, C.; Afferni, C.; Schiavoni, G. Eosinophils as potential biomarkers in respiratory viral infections. Front. Immunol. 2023, 14, 1170035. [Google Scholar] [CrossRef]
- Park, A.; Iwasaki, A. Type I and Type III Interferons—Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe. 2020, 27, 870–878. [Google Scholar] [CrossRef]
- Maiti, A.K. MDA5 Is a Major Determinant of Developing Symptoms in Critically Ill COVID-19 Patients. Clin. Rev. Allergy Immunol. 2024, 67, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Gadotti, A.C.; de Castro Deus, M.; Telles, J.P.; Wind, R.; Goes, M.; Ossoski, R.G.C.; de Padua, A.M.; de Noronha, L.; Moreno-Amaral, A.; Baena, C.P.; et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020, 289, 198171. [Google Scholar] [CrossRef] [PubMed]
- Sriram, U.; Xu, J.; Chain, R.W.; Varghese, L.; Chakhtoura, M.; Bennett, H.L.; Zoltick, P.W.; Gallucci, S. IL-4 suppresses the responses to TLR7 and TLR9 stimulation and increases the permissiveness to retroviral infection of murine conventional dendritic cells. PLoS ONE 2014, 9, e87668. [Google Scholar] [CrossRef]
- Suprun, M.; Kearney, P.; Hayward, C.; Butler, H.; Getts, R.; Sicherer, S.H.; Turner, P.J.; Campbell, D.E.; Sampson, H.A. Predicting probability of tolerating discrete amounts of peanut protein in allergic children using epitope-specific IgE antibody profiling. Allergy 2022, 77, 3061–3069. [Google Scholar] [CrossRef]
- Kombe Kombe, A.J.; Fotoohabadi, L.; Gerasimova, Y.; Nanduri, R.; Lama Tamang, P.; Kandala, M.; Kelesidis, T. The Role of Inflammation in the Pathogenesis of Viral Respiratory Infections. Microorganisms 2024, 12, 2526. [Google Scholar] [CrossRef]
- Budnevsky, A.V.; Avdeev, S.N.; Kosanovic, D.; Ovsyannikov, E.S.; Savushkina, I.A.; Alekseeva, N.G.; Feigelman, S.N.; Shishkina, V.V.; Filin, A.A.; Esaulenko, D.I.; et al. Involvement of Mast Cells in the Pathology of COVID-19: Clinical and Laboratory Parallels. Cells 2024, 13, 711. [Google Scholar] [CrossRef] [PubMed]
- Kakavas, S.; Karayiannis, D.; Mastora, Z. The Complex Interplay between Immunonutrition, Mast Cells, and Histamine Signaling in COVID-19. Nutrients 2021, 13, 3458. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Rubiey, H.F.; Al-Buhadily, A.K.; Al-Gareeb, A.I. Anti-histamines and COVID-19: Hype or Hope. J. Pak. Med. Assoc. 2021, 71 (Suppl. 8), S144–S148. [Google Scholar]
- Morán Blanco, J.I.; Alvarenga Bonilla, J.A.; Fremont-Smith, P.; Villar Gómez de Las Heras, K. Antihistamines as an early treatment for COVID-19. Heliyon 2023, 9, e15772. [Google Scholar] [CrossRef]
- Drouin, O.; Smyrnova, A.; Bétinjané, N.; Ducharme, F.M. Adherence to inhaled corticosteroids prescribed once vs twice daily in children with asthma. Ann. Allergy Asthma Immunol. 2022, 128, 423–431.e3. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Zheng, Y.; Fu, Z.; Wang, J.; Zhang, Y.; Wang, C.; Qi, X.; Gong, T.; Ma, L.; Lin, X.; et al. Ventral hippocampal CA1 modulates pain behaviors in mice with peripheral inflammation. Cell Rep. 2023, 42, 112017. [Google Scholar] [CrossRef]
- Pavord, I.D.; Bourdin, A.; Papi, A.; Domingo, C.; Corren, J.; Altincatal, A.; Radwan, A.; Pandit-Abid, N.; Jacob-Nara, J.A.; Deniz, Y.; et al. Dupilumab sustains efficacy in patients with moderate-to-severe type 2 asthma regardless of inhaled corticosteroids dose. Allergy 2023, 78, 2921–2932. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Yoshida, M.; Worlock, K.B.; Huang, N.; Lindeboom, R.G.H.; Butler, C.R.; Kumasaka, N.; Dominguez Conde, C.; Mamanova, L.; Bolt, L.; Richardson, L.; et al. Local and systemic responses to SARS-CoV-2 infection in children and adults. Nature 2022, 602, 321–327. [Google Scholar] [CrossRef]
- Benamar, M.; Chen, Q.; Chou, J.; Julé, A.M.; Boudra, R.; Contini, P.; Crestani, E.; Lai, P.S.; Wang, M.; Fong, J.; et al. The Notch1/CD22 signaling axis disrupts Treg function in SARS-CoV-2-associated multisystem inflammatory syndrome in children. J. Clin. Investig. 2023, 133, e163235. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Shanahan, F. Fecal microbiota-based treatment for recurrent Clostridioides difficile infection. Cell 2023, 186, 1087. [Google Scholar] [CrossRef]
- Liew, F.; Efstathiou, C.; Fontanella, S.; Richardson, M.; Saunders, R.; Swieboda, D.; Sidhu, J.K.; Ascough, S.; Moore, S.C.; Mohamed, N.; et al. Large-scale phenotyping of patients with long COVID post-hospitalization reveals mechanistic subtypes of disease. Nat. Immunol. 2024, 25, 607–621. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Ham, J.; Sohn, K.; Lee, S.; Chung, D.H.; Cho, S.; Kang, H.R.; Kim, H.Y. The effect of air pollutants on airway innate immune cells in patients with asthma. Allergy 2020, 75, 2372–2376. [Google Scholar] [CrossRef]
- Bartemes, K.R.; Kita, H. Roles of innate lymphoid cells (ILCs) in allergic diseases: The 10-year anniversary for ILC2s. J. Allergy Clin. Immunol. 2021, 147, 1531–1547. [Google Scholar] [CrossRef]
- Vanderheiden, A.; Ralfs, P.; Chirkova, T.; Upadhyay, A.A.; Zimmerman, M.G.; Bedoya, S.; Aoued, H.; Tharp, G.M.; Pellegrini, K.L.; Manfredi, C.; et al. Type I and Type III Interferons Restrict SARS-CoV-2 Infection of Human Airway Epithelial Cultures. J. Virol. 2020, 94, e00985-20. [Google Scholar] [CrossRef]
- Wang, M.; Bu, X.; Fang, G.; Luan, G.; Huang, Y.; Akdis, C.A.; Wang, C.; Zhang, L. Distinct expression of SARS-CoV-2 receptor ACE2 correlates with endotypes of chronic rhinosinusitis with nasal polyps. Allergy 2021, 76, 789–803. [Google Scholar] [CrossRef]
- Richard, D.; Muthuirulan, P.; Aguiar, J.; Doxey, A.C.; Banerjee, A.; Mossman, K.; Hirota, J.; Capellini, T.D. Intronic regulation of SARS-CoV-2 receptor (ACE2) expression mediated by immune signaling and oxidative stress pathways. iScience 2022, 25, 104614. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, N.J.; Wang, Y.; Manickas-Hill, Z.; Carbone, C.; Dauphin, A.; Boribong, B.P.; Loiselle, M.; Davis, J.; Leonard, M.M.; Kuri-Cervantes, L.; et al. Innate lymphoid cells and COVID-19 severity in SARS-CoV-2 infection. eLife 2022, 11, e74681. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.K.; Wang, C.; Lin, P.Q.; Hu, L.; Ye, J.; Gao, Z.G.; Lin, R.; Li, H.M.; Shu, Q.; Huang, L.S.; et al. Severe pediatric COVID-19: A review from the clinical and immunopathophysiological perspectives. World J. Pediatr. 2024, 20, 307–324. [Google Scholar] [CrossRef]
- Eijmael, M.; Janssens, N.; le Cessie, S.; van Dooren, Y.; Koster, T.; Karim, F. Coronavirus disease 2019 and peripheral blood eosinophil counts: A retrospective study. Infection 2021, 49, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Krysko, O.; Bourne, J.H.; Kondakova, E.; Galova, E.A.; Whitworth, K.; Newby, M.L.; Bachert, C.; Hill, H.; Crispin, M.; Stamataki, Z.; et al. Severity of SARS-CoV-2 infection is associated with high numbers of alveolar mast cells and their degranulation. Front. Immunol. 2022, 13, 968981. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, C.R.; Cotugno, N.; Sardh, F.; Pou, C.; Amodio, D.; Rodriguez, L.; Tan, Z.; Zicari, S.; Ruggiero, A.; Pascucci, G.R.; et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020, 183, 968–981.e7. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Truong, C.; Vuchkovska, A.; Guo, W.; Good, J.; Liu, B.; Gang, A.; Infarinato, N.; Stewart, K.; Polak, L.; et al. CD80 on skin stem cells promotes local expansion of regulatory T cells upon injury to orchestrate repair within an inflammatory environment. Immunity 2024, 57, 1071–1086.e7. [Google Scholar] [CrossRef]
- Walker, J.A.; McKenzie, A.N.J. TH2 cell development and function. Nat. Rev. Immunol. 2017, 18, 121–133. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef]
- Lukassen, S.; Chua, R.L.; Trefzer, T.; Kahn, N.C.; Schneider, M.A.; Muley, T.; Winter, H.; Meister, M.; Veith, C.; Boots, A.W.; et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020, 39, e105114. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Anderson, E.M.; Goodwin, E.C.; Verma, A.; Arevalo, C.P.; Bolton, M.J.; Weirick, M.E.; Gouma, S.; McAllister, C.M.; Christensen, S.R.; Weaver, J.; et al. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell 2021, 184, 1858–1864.e10. [Google Scholar] [CrossRef]
- Pierce, C.A.; Preston-Hurlburt, P.; Dai, Y.; Aschner, C.B.; Cheshenko, N.; Galen, B.; Garforth, S.J.; Herrera, N.G.; Jangra, R.K.; Morano, N.C.; et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci. Transl. Med. 2020, 12, eabd5487. [Google Scholar] [CrossRef]
- Bartsch, Y.C.; Wang, C.; Zohar, T.; Fischinger, S.; Atyeo, C.; Burke, J.S.; Kang, J.; Edlow, A.G.; Fasano, A.; Baden, L.R.; et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat. Med. 2021, 27, 454–462. [Google Scholar] [CrossRef]
- Brodin, P. SARS-CoV-2 infections in children: Understanding diverse outcomes. Immunity 2022, 55, 201–209. [Google Scholar] [CrossRef]
- Peacock, T.P.; Goldhill, D.H.; Zhou, J.; Baillon, L.; Frise, R.; Swann, O.C.; Kugathasan, R.; Penn, R.; Brown, J.C.; Sanchez-David, R.Y.; et al. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nat. Microbiol. 2021, 6, 899–909. [Google Scholar] [CrossRef]
- Nehar-Belaid, D.; Mejías, A.; Xu, Z.; Marches, R.; Yerrabelli, R.; Chen, G.; Mertz, S.; Ye, F.; Sánchez, P.J.; Tsang, J.S.; et al. SARS-CoV-2 induced immune perturbations in infants vary with disease severity and differ from adults’ responses. Nat. Commun. 2025, 16, 4562. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, E.I.; Robertson, J.; Misaghian, S.; Brown, J.; Wang, M.; Stengelin, M.; Sigal, G.; Wohlstadter, J.; Gisslén, M.; Lindén, A. Systemic increase in IL-26 is associated with severe COVID-19 and comorbid obstructive lung disease. Front. Immunol. 2024, 15, 1434186. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, P.J.; Breman, J.G.; Djimde, A.A.; John, C.C.; Kamya, M.R.; Leke, R.G.F.; Moeti, M.R.; Nkengasong, J.; Bausch, D.G. COVID-19: Shining the Light on Africa. Am. J. Trop. Med. Hyg. 2020, 102, 1145–1148. [Google Scholar] [CrossRef]
- Sahli, W.; Vitte, J.; Desnues, B. Eosinophils and COVID-19: Insights into immune complexity and vaccine safety. Clin. Transl. Allergy 2025, 15, e70050. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, A.W.; Schwartz, J.T.; Rothenberg, M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J. Allergy Clin. Immunol. 2020, 146, 1–7. [Google Scholar] [CrossRef]
- Sungur, C.M.; Baker, J.C. Clinical images: Giant intraosseous synovial cyst with intraarticular connection at the elbow in rheumatoid arthritis. Arthritis Rheumatol. 2022, 74, 1927. [Google Scholar] [CrossRef]
- Woodruff, M.C.; Ramonell, R.P.; Nguyen, D.C.; Cashman, K.S.; Saini, A.S.; Haddad, N.S.; Ley, A.M.; Kyu, S.; Howell, J.C.; Ozturk, T.; et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 2020, 21, 1506–1516. [Google Scholar] [CrossRef]
- Zhou, S.; Butler-Laporte, G.; Nakanishi, T.; Morrison, D.R.; Afilalo, J.; Afilalo, M.; Laurent, L.; Pietzner, M.; Kerrison, N.; Zhao, K.; et al. A Neanderthal OAS1 isoform protects individuals of European ancestry against COVID-19 susceptibility and severity. Nat. Med. 2021, 27, 659–667. [Google Scholar] [CrossRef]
- Dhawan, M.; Rabaan, A.A.; Alwarthan, S.; Alhajri, M.; Halwani, M.A.; Alshengeti, A.; Najim, M.A.; Alwashmi, A.S.S.; Alshehri, A.A.; Alshamrani, S.A.; et al. Regulatory T Cells (Tregs) and COVID-19: Unveiling the Mechanisms, and Therapeutic Potentialities with a Special Focus on Long COVID. Vaccines 2023, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Wall, S.C.; Suryadevara, N.; Kim, C.; Shiakolas, A.R.; Holt, C.M.; Irbe, E.B.; Wasdin, P.T.; Suresh, Y.P.; Binshtein, E.; Chen, E.C.; et al. SARS-CoV-2 antibodies from children exhibit broad neutralization and belong to adult public clonotypes. Cell Rep. Med. 2023, 4, 101267. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Chen, Y.; Ouyang, Y.; Peng, Y.; Man, X. COVID-19 related epigenetic changes and atopic dermatitis: An exploratory analysis. World Allergy Organ. J. 2025, 18, 101022. [Google Scholar] [CrossRef] [PubMed]
- Katsura, H.; Sontake, V.; Tata, A.; Kobayashi, Y.; Edwards, C.E.; Heaton, B.E.; Konkimalla, A.; Asakura, T.; Mikami, Y.; Fritch, E.J.; et al. Human Lung Stem Cell-Based Alveolospheres Provide Insights into SARS-CoV-2-Mediated Interferon Responses and Pneumocyte Dysfunction. Cell Stem Cell 2020, 27, 890–904.e8. [Google Scholar] [CrossRef]
- Edwards, M.R.; Strong, K.; Cameron, A.; Walton, R.P.; Jackson, D.J.; Johnston, S.L. Viral infections in allergy and immunology: How allergic inflammation influences viral infections and illness. J. Allergy Clin. Immunol. 2017, 140, 909–920. [Google Scholar] [CrossRef]
- Pouessel, G.; Petitpain, N.; Tanno, L.K.; Gautier, S.; Collaborators of the Anaphylaxis Working Group of the French Allergy Society. Adverse drug reactions from adrenaline auto-injectors: Analysis of the French pharmacovigilance database. Clin. Exp. Allergy 2023, 53, 955–958. [Google Scholar] [CrossRef]
- Silva-Avelar, I.; Santos-Silva, B.D.; Zheng, Y.; Ferreira, A.E.F.; Gonçalves, G.S.; Bain, V.; Matsuo, O.M.; Martins, F.; Suguita, P.; Fink, T.T.; et al. Elevated regulatory T cells in pediatric patients recovering from multisystem inflammatory syndrome (MIS-C) are evident five months post-COVID-19. Hum. Immunol. 2025, 86, 111342. [Google Scholar] [CrossRef]
- Donlan, A.N.; Sutherland, T.E.; Marie, C.; Preissner, S.; Bradley, B.T.; Carpenter, R.M.; Sturek, J.M.; Ma, J.Z.; Moreau, G.B.; Donowitz, J.R.; et al. IL-13 is a driver of COVID-19 severity. J. Clin. Investig. 2021, 6, e150107. [Google Scholar] [CrossRef] [PubMed]
- Contassot, E.; Brüggen, M. Sequential proteomic profiling of patients with Stevens–Johnson syndrome or toxic epidermal necrolysis. Allergy 2023, 78, 3289–3290. [Google Scholar] [CrossRef]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2021, 386, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kwak, B.O.; Cho, K.Y. Altered Gut Microbiota and Predicted Immune Dysregulation in Early Childhood SARS-CoV-2 Infection. Microorganisms 2025, 13, 1879. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Jayaram, A.; Shetty, U.; Varamballi, P.; Mukhopadhyay, C.; Jagadesh, A. Detection of H275Y oseltamivir resistance gene mutation among Influenza A(H1N1)pdm09 patients by allelic discrimination real-time RT-PCR. J. Med. Virol. 2023, 95, e28764. [Google Scholar] [CrossRef]
- Suskun, C.; Kilic, O.; Yilmaz Ciftdogan, D.; Guven, S.; Karbuz, A.; Ozkaya Parlakay, A.; Kara, Y.; Kacmaz, E.; Sahin, A.; Boga, A.; et al. Intestinal microbiota composition of children with infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and multisystem inflammatory syndrome (MIS-C). Eur. J. Pediatr. 2022, 181, 3175–3191. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Castells-Nobau, A.; Fernández-Real, J.M. “Indole-gence” for the mind. Cell Host Microbe 2024, 32, 151–153. [Google Scholar] [CrossRef]
- Vuong, V.; Kaye, L.; Barrett, M.; Stempel, D. Feasibility of capturing self-reported asthma exacerbations with a digital self-management platform. J. Allergy Clin. Immunol. 2022, 149, AB184. [Google Scholar] [CrossRef]
- Cappadona, C.; Rimoldi, V.; Paraboschi, E.M.; Asselta, R. Genetic susceptibility to severe COVID-19. Infect. Genet. Evol. 2023, 110, 105426. [Google Scholar] [CrossRef]
- COVID-19 Host Genetics Initiative. Mapping the human genetic architecture of COVID-19. Nature 2021, 600, 472–477. [Google Scholar] [CrossRef]
- Yang, X.; Sima, Y.; Zhao, J.; Zhang, J.; Wang, X.; Zhang, L. Risk and protective factors for coronavirus disease 2019 (COVID-19) in allergic rhinitis patients: A national survey in China. Front. Allergy. 2024, 5, 1479493. [Google Scholar] [CrossRef]
- Tangye, S.G.; COVID Human Genetic Effort consortium. Impact of SARS-CoV-2 infection and COVID-19 on patients with inborn errors of immunity. J. Allergy Clin. Immunol. 2023, 151, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, E.; Pellegrino, R.; Nascimento-Carvalho, C.M.; Galli, L. Recent Insights on Post-COVID in Pediatrics. Pediatr. Infect. Dis. J. 2023, 42, e304–e307. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Carballa, A.; Pischedda, S.; Pardo-Seco, J.; Gómez-Rial, J.; Martinón-Torres, F.; Salas, A. Interferon gene expression declines over time post-COVID infection and in long COVID patients. Infect. Dis. 2025, 57, 35–48. [Google Scholar] [CrossRef]
- Kortekaas Krohn, I.; Seys, S.F.; Lund, G.; Jonckheere, A.C.; Dierckx de Casterlé, I.; Ceuppens, J.L.; Steelant, B.; Hellings, P.W. Nasal epithelial barrier dysfunction increases sensitization and mast cell degranulation in the absence of allergic inflammation. Allergy 2020, 75, 1155–1164. [Google Scholar] [CrossRef]
- Griffin, D.E. Measles immunity and immunosuppression. Curr. Opin. Virol. 2021, 46, 9–14. [Google Scholar] [CrossRef]
- Yin, J.-X.; Agbana, Y.L.; Sun, Z.-S.; Fei, S.-W.; Zhao, H.-Q.; Zhou, X.-N.; Chen, J.-H.; Kassegne, K. Increased interleukin-6 is associated with long COVID-19: A systematic review and meta-analysis. Infect. Dis. Poverty 2023, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Author Correction: Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Jaxybayeva, I.; Boranbayeva, R.; Bulegenova, M.; Urazalieva, N.; Gerein, V.; Manzhuova, L. Long-term outcomes and immune profiling in children with multisystem inflammatory syndrome (MIS-C). Acta Biomed. 2023, 94, e2023233. [Google Scholar] [CrossRef]
- Abo-Haded, H.M.; Alshengeti, A.M.; Alawfi, A.D.; Khoshhal, S.Q.; Al-Harbi, K.M.; Allugmani, M.D.; El-Agamy, D.S. Cytokine Profiling among Children with Multisystem Inflammatory Syndrome versus Simple COVID-19 Infection: A Study from Northwest Saudi Arabia. Biology 2022, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Sacco, K.; Castagnoli, R.; Vakkilainen, S.; Liu, C.; Delmonte, O.; Oguz, C.; Kaplan, I.; Alehashemi, S.; Burbelo, P.; Bhuyan, F.; et al. Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19. Nat. Med. 2022, 28, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Gerla, L.; Moitra, S.; Pink, D.; Govindasamy, N.; Duchesne, M.; Reklow, E.; Hillaby, A.; May, A.; Lewis, J.D.; Melenka, L.; et al. SARS-CoV-2-Induced TSLP Is Associated with Duration of Hospital Stay in COVID-19 Patients. Viruses 2023, 15, 556. [Google Scholar] [CrossRef]
- Mohammed H Al-Mquter, L.F.; Abdul Azeez Atiayh, S. Evaluation of IL-6, IL-25 & IL-35 in the COVID 19 Patients and their Correlation to Demography Data in the Symptomatic Patients. Arch. Razi Inst. 2023, 78, 1049–1056. [Google Scholar] [CrossRef]
- Furci, F.; Murdaca, G.; Allegra, A.; Gammeri, L.; Senna, G.; Gangemi, S. IL-33 and the Cytokine Storm in COVID-19: From a Potential Immunological Relationship towards Precision Medicine. Int. J. Mol. Sci. 2022, 23, 14532. [Google Scholar] [CrossRef]
- Eto, D.; Lao, C.; DiToro, D.; Barnett, B.; Escobar, T.C.; Kageyama, R.; Yusuf, I.; Crotty, S.; Poh, L.N.F. IL-21 and IL-6 Are Critical for Different Aspects of B Cell Immunity and Redundantly Induce Optimal Follicular Helper CD4 T Cell (Tfh) Differentiation. PLoS ONE 2011, 6, e17739. [Google Scholar] [CrossRef]
- A Lin, A.; Freeman, A.F.; Nutman, T.B. IL-10 Indirectly Downregulates IL-4–Induced IgE Production by Human B Cells. Immuno Horizons 2018, 2, 398–406. [Google Scholar] [CrossRef]
- Portilho, A.I.; Silva, V.O.; Da Costa, H.H.M.; Yamashiro, R.; de Oliveira, I.P.; de Campos, I.B.; Prudencio, C.R.; Matsuda, E.M.; Brígido, L.F.d.M.; De Gaspari, E. An unexpected IgE anti-receptor binding domain response following natural infection and different types of SARS-CoV-2 vaccines. Sci. Rep. 2024, 14, 20003. [Google Scholar] [CrossRef]
- de la Poza, J.F.D.; Parés, A.R.; Aparicio-Calvente, I.; Blanco, I.B.; Masmitjà, J.G.; Berenguer-Llergo, A.; Fontova, J.C. Frequency of IgE antibody response to SARS-CoV-2 RBD protein across different disease severity COVID19 groups. Virol. J. 2025, 22, 58. [Google Scholar] [CrossRef]
- Benamar, M.; Chen, Q.; Martinez-Blanco, M.; Chatila, T.A. Regulatory T cells in allergic inflammation. Semin. Immunol. 2023, 70, 101847. [Google Scholar] [CrossRef] [PubMed]
- MacBeth, M.; Joetham, A.; Gelfand, E.W.; Schedel, M. Plasticity of Naturally Occurring Regulatory T Cells in Allergic Airway Disease Is Modulated by the Transcriptional Activity of Il-6. Int. J. Mol. Sci. 2021, 22, 4582. [Google Scholar] [CrossRef]
- Vella, L.A.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Diorio, C.; Kuri-Cervantes, L.; Alanio, C.; Pampena, M.B.; Wu, J.E.; Chen, Z.; et al. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci Immunol. 2021, 6, eabf7570. [Google Scholar] [CrossRef]
- Shouman, S.; El-Kholy, N.; Hussien, A.E.; El-Derby, A.M.; Magdy, S.; Abou-Shanab, A.M.; Elmehrath, A.O.; Abdelwaly, A.; Helal, M.; El-Badri, N. SARS-CoV-2-associated lymphopenia: Possible mechanisms and the role of CD147. Cell Commun. Signal. 2024, 22, 349. [Google Scholar] [CrossRef]
- Aquino, A.; Zaikova, E.; Kalinina, O.; Karonova, T.L.; Rubinstein, A.; Mikhaylova, A.A.; Kudryavtsev, I.; Golovkin, A.S. T Regulatory Cell Subsets Do Not Restore for One Year After Acute COVID-19. Int. J. Mol. Sci. 2024, 25, 11759. [Google Scholar] [CrossRef]
- Joudi, A.M.; Flores, C.P.R.; Singer, B.D. Epigenetic Control of Regulatory T Cell Stability and Function: Implications for Translation. Front. Immunol. 2022, 13, 861607. [Google Scholar] [CrossRef]
- Wang, S.; Gao, X.; Shen, G.; Wang, W.; Li, J.; Zhao, J.; Wei, Y.-Q.; Edwards, C.K. Interleukin-10 deficiency impairs regulatory T cell-derived neuropilin-1 functions and promotes Th1 and Th17 immunity. Sci. Rep. 2016, 6, 24249. [Google Scholar] [CrossRef] [PubMed]
- Goretzki, A.; Lin, Y.J.; Schülke, S. Immune metabolism in allergies, does it matter?-A review of immune metabolic basics and adaptations associated with the activation of innate immune cells in allergy. Allergy 2021, 76, 3314–3331. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Li, H. T-helper cells and their cytokines in pathogenesis and treatment of asthma. Front. Immunol. 2023, 14, 1149203. [Google Scholar] [CrossRef]
- Holt, P.G.; Sly, P.D. Viral infections and atopy in asthma pathogenesis: New rationales for asthma prevention and treatment. Nat. Med. 2012, 18, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Rotulo, G.A.; Palma, P. Understanding COVID-19 in children: Immune determinants and post-infection conditions. Pediatr. Res. 2023, 94, 434–442. [Google Scholar] [CrossRef]
- Sawaya, M.R.; Hughes, M.P.; Rodriguez, J.A.; Riek, R.; Eisenberg, D.S. The expanding amyloid family: Structure, stability, function, and pathogenesis. Cell 2021, 184, 4857–4873. [Google Scholar] [CrossRef]
- Gruber, C.N.; Patel, R.S.; Trachtman, R.; Lepow, L.; Amanat, F.; Krammer, F.; Wilson, K.M.; Onel, K.; Geanon, D.; Tuballes, K.; et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 2020, 183, 982–995.e14. [Google Scholar] [CrossRef]
- Hossain, F.M.A.; Choi, J.Y.; Uyangaa, E.; Park, S.O.; Eo, S.K. The Interplay between Host Immunity and Respiratory Viral Infection in Asthma Exacerbation. Immune Netw. 2019, 19, e31. [Google Scholar] [CrossRef]
- Guo, L.P.; Yan, M.; Niu, R.B.; Liu, L.; Yang, J.R.; Chen, R.L.; Duan, B.S.; Li, C.C.; Li, J.X. Role of Th2, Th17 and Treg Cells and relevant cytokines in pathogenesis of allergic rhinitis. Allergy Asthma Clin. Immunol. 2024, 20, 40. [Google Scholar] [CrossRef]
- Zou, X.L.; Chen, Z.G.; Zhang, T.T.; Feng, D.Y.; Li, H.T.; Yang, H.L. Th17/Treg homeostasis, but not Th1/Th2 homeostasis, is implicated in exacerbation of human bronchial asthma. Ther. Clin. Risk Manag. 2018, 14, 1627–1636. [Google Scholar] [CrossRef]
- Söderlund, S.; Boey, D.; van Midden, W.; Kjellander, M.; Ax, K.; Qian, H.; Dahlin, J.S.; Ungerstedt, J. Proteomic and transcriptomic screening demonstrates increased mast cell–derived CCL23 in systemic mastocytosis. J. Allergy Clin. Immunol. 2023, 152, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.M.; Dattilo, T.M.; Stutes, S.; Atkinson, D.; Carter, C.; MacDougall, J.; Virkud, Y.V.; Mullins, L.L.; Tackett, A.P. Experiences of caregivers of children with food allergy during the COVID-19 pandemic. Pediatr. Allergy Immunol. 2023, 34, e13946. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.A.; Jadhav, A.; Mondal, T.K.; Carson, K.; Lee, W.T.; Hogan, A.H.; Herbst, K.W.; Michelow, I.C.; Brimacombe, M.; Salazar, J.C.; et al. Inflammatory and Autoimmune Aspects of Multisystem Inflammatory Syndrome in Children (MIS-C): A Prospective Cohort Study. Viruses 2024, 16, 950. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Day-Lewis, M.; Henderson, L.A.; Friedman, K.G.; Lo, J.; Roberts, J.E.; Lo, M.S.; Platt, C.D.; Chou, J.; Hoyt, K.J.; et al. Distinct clinical and immunological features of SARS–CoV-2–induced multisystem inflammatory syndrome in children. J. Clin. Investig. 2020, 130, 5942–5950. [Google Scholar] [CrossRef]
- Ogulur, I.; Mitamura, Y.; Yazici, D.; Pat, Y.; Ardicli, S.; Li, M.; D’Avino, P.; Beha, C.; Babayev, H.; Zhao, B.; et al. Type 2 immunity in allergic diseases. Cell. Mol. Immunol. 2025, 22, 211–242. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an Interleukin-1-like Cytokine that Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Crooks, E.T.; Almanza, F.; D’aDdabbo, A.; Duggan, E.; Zhang, J.; Wagh, K.; Mou, H.; Allen, J.D.; Thomas, A.; Osawa, K.; et al. Engineering well-expressed, V2-immunofocusing HIV-1 envelope glycoprotein membrane trimers for use in heterologous prime-boost vaccine regimens. PLOS Pathog. 2021, 17, e1009807. [Google Scholar] [CrossRef]
- Ji, J.; Wu, M.; Zhong, L.; Liu, Z.; Wang, C.; Shao, Z.; Xie, Q.; Liu, Z. Early, low-dose, short-term methylprednisolone decreased the mortality in critical COVID-19 patients: A multicenter retrospective cohort study. J. Infect. 2021, 82, 84–123. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, J.R.; Lloyd, C.M. Chronic inflammation and asthma. Mutat. Res. 2010, 690, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cai, L.; Li, L.; Zhang, Y.; Li, J.; Luo, C.; Wang, Y.; Tao, L. Emerging Effects of IL-33 on COVID-19. Int. J. Mol. Sci. 2022, 23, 13656. [Google Scholar] [CrossRef]
- Lee, H.C.; Headley, M.B.; Loo, Y.M.; Berlin, A.; Gale, M., Jr.; Debley, J.S.; Lukacs, N.W.; Ziegler, S.F. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J. Allergy Clin. Immunol. 2012, 130, 1187–1196.e5. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Castillo, J.M.; Yoon, J.; Geha, R.S. IL-22 promotes allergic airway inflammation in epicutaneously sensitized mice. J. Allergy Clin. Immunol. 2019, 143, 619–630.e7. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, Å.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Engl. J. Med. 2021, 384, 1800–1809. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; O’BYrne, P.M.; Boulet, L.-P.; Wang, Y.; Cockcroft, D.; Bigler, J.; FitzGerald, J.M.; Boedigheimer, M.; Davis, B.E.; Dias, C.; et al. Effects of an Anti-TSLP Antibody on Allergen-Induced Asthmatic Responses. N. Engl. J. Med. 2014, 370, 2102–2110. [Google Scholar] [CrossRef]
- Gaffen, S.L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009, 9, 556–567, Erratum in Nat. Rev. Immunol. 2009, 9, 747. [Google Scholar] [CrossRef]
- Mukherjee, S.; Lindell, D.M.; Berlin, A.A.; Morris, S.B.; Shanley, T.P.; Hershenson, M.B.; Lukacs, N.W. IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am. J. Pathol. 2011, 179, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Bédard-Matteau, J.; Soulé, A.; Liu, K.Y.; Fourcade, L.; Fraser, D.D.; Emad, A.; Rousseau, S. Circulating IL-17F, but not IL-17A, is elevated in severe COVID-19 and leads to an ERK1/2 and p38 MAPK-dependent increase in ICAM-1 cell surface expression and neutrophil adhesion on endothelial cells. Front. Immunol. 2024, 15, 1452788. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. Lung Biological Network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef]
- Ritter, M.; Göggel, R.; Chaudhary, N.; Wiedenmann, A.; Jung, B.; Weith, A.; Seither, P. Elevated expression of TARC (CCL17) and MDC (CCL22) in models of cigarette smoke-induced pulmonary inflammation. Biochem. Biophys. Res. Commun. 2005, 334, 254–262. [Google Scholar] [CrossRef]
- Doni Jayavelu, N.; Altman, M.C.; Benson, B.; Dufort, M.J.; Vanderwall, E.R.; Rich, L.M.; White, M.P.; Becker, P.M.; Togias, A.; Jackson, D.J.; et al. Type 2 inflammation reduces SARS-CoV-2 replication in the airway epithelium in allergic asthma through functional alteration of ciliated epithelial cells. J. Allergy Clin. Immunol. 2023, 152, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Björkander, S.; Du, L.; Zuo, F.; Ekström, S.; Wang, Y.; Wan, H.; Sherina, N.; Schoutens, L.; Andréll, J.; Andersson, N.; et al. SARS-CoV-2–specific B- and T-cell immunity in a population-based study of young Swedish adults. J. Allergy Clin. Immunol. 2021, 149, 65–75.e8. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.; Kale, S.L.; Ray, A. T1-T2 Interplay in the Complex Immune Landscape of Severe Asthma. Immunol. Rev. 2025, 330, e70011. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.G.; Chung, S.J.; Park, D.W.; Park, T.S.; Moon, J.Y.; Kim, T.H.; Sohn, J.W.; Yoon, H.J.; Kim, S.H. New-onset asthma following COVID-19 in adults. J. Allergy Clin. Immunol. Pract. 2023, 11, 2228–2231. [Google Scholar] [CrossRef]
- Cucè, F.; Visicaro, M. Adult-onset asthma induced by COVID-19: A case report. Heliyon 2024, 10, e36197. [Google Scholar] [CrossRef]
- Qin, R.; Feng, Y.; Zhang, H.; Zhao, B.; Lei, W.; Sun, H.; Zhi, L.; Zheng, Z.; Wang, S.; Yu, Y.; et al. Protective Effect of Allergen Immunotherapy in Patients With Allergic Rhinitis and Asthma Against COVID-19 Infection: Observational, Nationwide, and Multicenter Study. JMIR Public Health Surveill. 2024, 10, e50846. [Google Scholar] [CrossRef]
- Stanczak, M.A.; Sanin, D.E.; Apostolova, P.; Nerz, G.; Lampaki, D.; Hofmann, M.; Steinmann, D.; Krohn-Grimberghe, M.; Thimme, R.; Mittler, G.; et al. IL-33 expression in response to SARS-CoV-2 correlates with seropositivity in COVID-19 convalescent individuals. Nat. Commun. 2021, 12, 2133. [Google Scholar] [CrossRef] [PubMed]
- Vaz de Paula, C.B.; de Azevedo, M.L.V.; Nagashima, S.; Martins, A.P.C.; Malaquias, M.A.S.; Miggiolaro, A.F.R.D.S.; da Silva Motta Júnior, J.; Avelino, G.; do Carmo, L.A.P.; Carstens, L.B.; et al. IL-4/IL-13 remodeling pathway of COVID-19 lung injury. Sci. Rep. 2020, 10, 18689. [Google Scholar] [CrossRef]
- Gu, J.; Liu, Q.; Zhang, J.; Xu, S. COVID-19 and trained immunity: The inflammatory burden of long covid. Front. Immunol. 2023, 14, 1294959. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.C.; Ramonell, R.P.; Haddad, N.S.; Anam, F.A.; Rudolph, M.E.; Walker, T.A.; Truong, A.D.; Dixit, A.N.; Han, J.E.; Cabrera-Mora, M.; et al. Dysregulated naive B cells and de novo autoreactivity in severe COVID-19. Nature 2022, 611, 139–147. [Google Scholar] [CrossRef]
- Kamali, Z.; Vonk, J.M.; Thio, C.H.L.; Vaez, A.; Snieder, H. A Mendelian randomization cytokine screen reveals IL-13 as causal factor in risk of severe COVID-19. J. Infect. 2022, 85, 334–363. [Google Scholar] [CrossRef]
- Sadhu, S.; Dalal, R.; Dandotiya, J.; Binayke, A.; Singh, V.; Tripathy, M.R.; Das, V.; Goswami, S.; Kumar, S.; Rizvi, Z.A.; et al. IL-9 aggravates SARS-CoV-2 infection and exacerbates associated airway inflammation. Nat. Commun. 2023, 14, 4060. [Google Scholar] [CrossRef]
- Cai, G.; Du, M.; Bossé, Y.; Albrecht, H.; Qin, F.; Luo, X.; Androulakis, X.M.; Cheng, C.; Nagarkatti, M.; Nagarkatti, P.; et al. SARS-CoV-2 Impairs Dendritic Cells and Regulates DC-SIGN Gene Expression in Tissues. Int. J. Mol. Sci. 2021, 22, 9228. [Google Scholar] [CrossRef] [PubMed]
- van der Made, C.I.; Simons, A.; Schuurs-Hoeijmakers, J.; Heuvel, G.v.D.; Mantere, T.; Kersten, S.; van Deuren, R.C.; Steehouwer, M.; van Reijmersdal, S.V.; Jaeger, M.; et al. Presence of Genetic Variants Among Young Men With Severe COVID-19. JAMA 2020, 324, 663–673. [Google Scholar] [CrossRef]
- García, M.; Kokkinou, E.; Carrasco García, A.; Parrot, T.; Palma Medina, L.M.; Maleki, K.T.; Christ, W.; Varnaitė, R.; Filipovic, I.; Ljunggren, H.G.; et al. Innate lymphoid cell composition associates with COVID-19 disease severity. Clin. Transl. Immunol. 2020, 9, e1224. [Google Scholar] [CrossRef]
- Fonseca, W.; Lukacs, N.W.; Elesela, S.; Malinczak, C.A. Role of ILC2 in Viral-Induced Lung Pathogenesis. Front. Immunol. 2021, 12, 675169. [Google Scholar] [CrossRef]
- Venter, C.; O’mahony, L. Immunonutrition: The importance of a new European Academy of Allergy and Clinical Immunology working group addressing a significant burden and unmet need. Allergy 2021, 76, 2303–2305. [Google Scholar] [CrossRef]
- Holstein, J.; Solimani, F.; Baum, C.; Meier, K.; Pollmann, R.; Didona, D.; Tekath, T.; Dugas, M.; Casadei, N.; Hudemann, C.; et al. Immunophenotyping in pemphigus reveals a TH17/TFH17 cell–dominated immune response promoting desmoglein1/3-specific autoantibody production. J. Allergy Clin. Immunol. 2021, 147, 2358–2369. [Google Scholar] [CrossRef] [PubMed]
- Meher-Homji, Z.; North, D.; Stewart, J.D.; Moir, D.; Kalff, A.; Fleming, S.; Ananda-Rajah, M.R.; Fuller, A.; Morrissey, C.O. A 40-Year-Old Man With Persistent Febrile Neutropenia and Subsequent Rash. Clin. Infect. Dis. 2020, 71, 3260–3262. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Masuho, I.; Balaji, S.; Muntean, B.S.; Skamangas, N.K.; Chavali, S.; Tesmer, J.J.; Babu, M.M.; Martemyanov, K.A. A Global Map of G Protein Signaling Regulation by RGS Proteins. Cell 2020, 183, 503–521.e19. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, H.; Pradana, F.R.; Adytia, G.J.; Ansharullah, B.A.; Waitupu, A.; Bramantono, B.; Fetarayani, D. Memory T Cells in Respiratory Virus Infections: Protective Potential and Persistent Vulnerabilities. Med. Sci. 2025, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- McLaren, J.E.; Clement, M.; Marsden, M.; Miners, K.L.; Llewellyn-Lacey, S.; Grant, E.J.; Rubina, A.; Gimeno Brias, S.; Gostick, E.; Stacey, M.A.; et al. IL-33 Augments Virus-Specific Memory T Cell Inflation and Potentiates the Efficacy of an Attenuated Cytomegalovirus-Based Vaccine. J. Immunol. 2019, 202, 943–955. [Google Scholar] [CrossRef]
- R Bonam, S.; Hu, H.; Bayry, J. Role of the PD-1 and PD-L1 axis in COVID-19. Future Microbiol. 2022, 17, 985–988. [Google Scholar] [CrossRef]
- Loske, J.; Röhmel, J.; Lukassen, S.; Stricker, S.; Magalhães, V.G.; Liebig, J.; Chua, R.L.; Thürmann, L.; Messingschlager, M.; Seegebarth, A.; et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat. Biotechnol. 2022, 40, 319–324. [Google Scholar] [CrossRef]
- Baekkevold, E.S.; Wurbel, M.-A.; Kivisäkk, P.; Wain, C.M.; Power, C.A.; Haraldsen, G.; Campbell, J.J. A role for CCR4 in development of mature circulating cutaneous T helper memory cell populations. J. Exp. Med. 2005, 201, 1045–1051. [Google Scholar] [CrossRef]
- Wurtz, O.; Bajénoff, M.; Guerder, S. IL-4-mediated inhibition of IFN-gamma production by CD4+ T cells proceeds by several developmentally regulated mechanisms. Int. Immunol. 2004, 16, 501–508. [Google Scholar] [CrossRef]
- Zabala-Peñafiel, A.; Gonzalez-Lombana, C.; Alameh, M.G.; Sacramento, L.A.; Mou, Z.; Phan, A.T.; Aunins, E.A.; Tam, Y.K.; Uzonna, J.E.; Weissman, D.; et al. IL-12 mRNA-LNP promotes dermal resident memory CD4+ T cell development. npj Vaccines 2025, 10, 154. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef] [PubMed]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for eight months following mild to mod-erate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Gebremeskel, S.; Schanin, J.; Coyle, K.M.; Butuci, M.; Luu, T.; Brock, E.C.; Xu, A.; Wong, A.; Leung, J.; Korver, W.; et al. Mast Cell and Eosinophil Activation Are Associated With COVID-19 and TLR-Mediated Viral Inflammation: Implications for an Anti-Siglec-8 Antibody. Front. Immunol. 2021, 12, 650331. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Zhang, M.; Yang, C.X.; Zhang, N.; Wang, X.C.; Yang, X.P.; Dong, X.Q.; Zheng, Y.T. Elevated exhaustion levels and reduced functional diversity of T cells predict severe COVID-19. Cell. Mol. Immunol. 2020, 17, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Filippatos, F.; Tzanoudaki, M.; Tatsi, E.B.; Dessypris, N.; Koukou, D.M.; Georgokosta, C.; Syriopoulou, V.; Michos, A. Comparison οf Immune Responses Through Multiparametric T-Cell Cytokine Expression Profile Between Children with Convalescent COVID-19 or Multisystem Inflammatory Syndrome. Children 2024, 11, 1278. [Google Scholar] [CrossRef]
- Lee, D.; Le Pen, J.; Yatim, A.; Dong, B.; Aquino, Y.; Ogishi, M.; Pescarmona, R.; Talouarn, E.; Rinchai, D.; Zhang, P.; et al. Inborn errors of OAS-RNase L in SARS-CoV-2-related multisystem inflammatory syndrome in children. Science 2023, 379, eabo3627. [Google Scholar] [CrossRef]
- Chang, S.E.; Feng, A.; Meng, W.; Apostolidis, S.A.; Mack, E.; Artandi, M.; Barman, L.; Bennett, K.; Chakraborty, S.; Chang, I.; et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat. Commun. 2021, 12, 5417. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef] [PubMed]
- Sezaki, M.; Hayashi, Y.; Wang, Y.; Johansson, A.; Umemoto, T.; Takizawa, H. Immuno-Modulation of Hematopoietic Stem and Progenitor Cells in Inflammation. Front. Immunol. 2020, 11, 585367. [Google Scholar] [CrossRef] [PubMed]
- Boes, M.; Falter-Braun, P. Long-COVID-19: The persisting imprint of SARS-CoV-2 infections on the innate immune system. Signal Transduct. Target. Ther. 2023, 8, 460. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, Y.; Humaira Amanullah, F.; Saad, M.; Taleb, S.; Bradic, M.; Megarbane, A.; Ait Hssain, A.; Abi Khalil, C.; El Hajj, N. Epigenetic age acceleration in surviving versus deceased COVID-19 patients with acute respiratory distress syndrome following hospitalization. Clin. Epigenetics 2023, 15, 186. [Google Scholar] [CrossRef]
- Corley, M.J.; Pang, A.P.S.; Dody, K.; Mudd, P.A.; Patterson, B.K.; Seethamraju, H.; Bram, Y.; Peluso, M.J.; Torres, L.; Iyer, N.S.; et al. Genome-wide DNA methylation profiling of peripheral blood reveals an epigenetic signature associated with severe COVID-19. J. Leukoc. Biol. 2021, 110, 21–26. [Google Scholar] [CrossRef]
| Mediator | Allergy (Type 2 Asthma) | SARS-CoV-2 Infection | Effect on ACE2/TMPRSS2 | References |
|---|---|---|---|---|
| IL-4 | ↑ IL-4 (Th2 cytokine) | Lower in antiviral state | Reduces ACE2 expression; little effect on TMPRSS2. | [28,30,40] |
| IL-13 | ↑↑ IL-13 (Th2 cytokine) | Lower in antiviral state | Reduces ACE2, increases TMPRSS2. | [30,56,73,74] |
| IFN-γ | ↓ IFN-γ | ↑ in viral response | Increases ACE2; modest effect on TMPRSS2. | [58,59] |
| TSLP/IL-33/IL-25 | Produced by epithelium (alarmins) | ↑↑ on infection/repair | Induce ILC2→ ↑IL-5/IL-13; indirect ↓ACE2 via IL-13. | [9,10,11,140,146,147] |
| TGF-β | ↑ in chronic allergy remodeling | Varied (fibrosis role) | Indirect immunoregulatory; possibly ↑ACE2 via repair processes (speculative). | [127,144] |
| Alarmin Cytokine | Induction (Triggers) | Downstream Immune Effects | References |
|---|---|---|---|
| IL-33 | Released by necrotic epithelial/endothelial cells and damaged airway epithelium; upregulated in COVID-19. | Binds ST2 on ILC2 and Th2 cells, inducing IL-5 and IL-13 production and eosinophil recruitment; also activates mast cells. In SARS-CoV-2 infection, elevated IL-33 correlates with lung hyperinflammation. | [140,143,144,157] |
| IL-25
(IL-17E) | Produced by airway tuft and epithelial cells upon viral infection or allergen exposure. | Activates ILC2 to secrete IL-5, IL-13 (and some IL-4), promoting eosinophilia and mucus production. Synergizes with IL-33 and TSLP in driving type-2 immunity. | [9,12,147] |
| TSLP | Produced by airway and gut epithelia in response to viruses, allergens, or mechanical stress. | Primes myeloid DCs to express OX40L (and upregulate IL-4Rα), polarizing naive CD4^+ T cells into Th2 cells. Also acts directly on basophils, mast cells and ILC2 to augment type-2 cytokine release. | [146,147,148,149] |
| HMGB1 | Nuclear alarmin released by necrotic cells or secreted by activated macrophages. | Enhances dendritic cell maturation and Th2 priming (through RAGE/TLR4) and can promote airway hypersensitivity. | [150] |
| TSLP/IL-25/IL-33 (triad) | Often act in concert when epithelial damage occurs. | Jointly potently activate ILC2 and prime Th2 immunity even without antigen presentation. (ILC2 activated by any of these alarmins produce IL-5/IL-13, as in viral asthma models.) | [9,10,146] |
| Cell Type | Allergy Role | SARS-CoV-2 Role | References |
|---|---|---|---|
| CD4+ Th2 | Produce IL-4, IL-5, IL-13; help B cells class-switch to IgE; drive eosinophil/mast cell recruitment. | (Typically low in acute infection) Can arise if allergic bias; not major antiviral role. | [127,128,129] |
| CD4+ Th1 | (Baseline) Minor in allergy; secrete IFN-γ, IL-2 for macrophage activation. | Essential for viral clearance: produce IFN-γ, TNF-α; support CD8+ CTLs. | [128,155,181] |
| CD8+ T cells | (Baseline) Limited direct role in allergy. | Kill infected cells; produce IFN-γ/TNF; express granzyme B. | [37,176] |
| Tregs (CD25+FoxP3+) | Suppress allergic inflammation via IL-10, TGF-β; maintain tolerance to allergens. | Expand during COVID-19 (especially in children); can dampen immunopathology and aid resolution. | [120,122,124] |
| Th17 Cells | Produce IL-17; contribute to chronic airway inflammation and neutrophilia in severe asthma. | Contribute to hyperinflammation in MIS-C/COVID-19; secrete IL-17 (elevated in MIS-C). | [130,150,151] |
| Tfh Cells | Support IgE class switching by B cells in allergy; produce IL-4. | Support germinal center formation for anti–SARS-CoV-2 antibodies. | [176,177] |
| B cells/Plasma cells | Class-switch to IgE/IgG4 in allergy (IL-4 dependent); produce allergen-specific IgE. | Produce IgM/IgG/IgA against viral antigens; neutralizing antibodies. | [162,166,167] |
| Conventional DCs | Subsets: cDC2 present allergens to Th2 cells (via OX40L if TSLP-activated). | cDC1 cross-present viral antigens to CD8+ T cells; produce IL-12 to drive Th1. | [169,182] |
| Plasmacytoid DCs (pDCs) | Not involved in allergy. | Produce large amounts of type I/III IFN upon sensing SARS-CoV-2; crucial for antiviral defense. | [170,181] |
| Mast Cells | Express FcεRI for IgE; degranulate to release histamine, proteases, TNF-α, IL-4/IL-13; orchestrate allergic hypersensitivity. | Express TLRs; activated by viral RNA or complement; release histamine and pro-inflammatory cytokines in COVID-19. | [43,173] |
| Basophils | Circulate with FcεRI-bound IgE; release IL-4 and IL-13 upon allergen encounter; support Th2 polarization. | Can be activated during viral infection; possibly produce IL-4. | [174,180] |
| Eosinophils | Recruited by IL-5; release major basic protein and eosinophil peroxidase, contributing to tissue damage in allergy. | Decreased in acute COVID-19 (eosinopenia); but COVID patients can have eosinophil activation markers. | [32,61,175] |
| Neutrophils | Attracted by IL-8; contribute to late-phase allergic inflammation (release ROS, proteases). | First responders in COVID-19; release NETs and ROS, contribute to lung damage in ARDS. | [150,155] |
| NK cells | (Minor) Produce IFN-γ; may help contain low-level viral infections in allergic airways. | Kill virus-infected cells early; produce IFN-γ/TNF-α; their activity is often blunted by SARS-CoV-2. | [37,181] |
| ILC2 (Innate Lymphoid 2) | Produce IL-5, IL-13, IL-9 (after IL-25/IL-33/TSLP stimulation); potentiate Th2 immunity and tissue eosinophilia. | Activated by virus-induced epithelial alarmins; contribute to airway hyperreactivity even without allergen. | [60,171,172] |
| Macrophages | M2-type macrophages (IL-4/IL-13-driven) produce IL-10, TGF-β; support tissue remodeling in chronic allergy. | M1-type macrophages produce IL-1β, IL-6, TNF-α in viral infection; essential for pathogen clearance (but can be pathological if overactive). | [164,187] |
| Cytokine | Pediatric (Acute) | Pediatric (Post-Acute) | Adult (Acute) | Adult (Post-Acute) | References |
|---|---|---|---|---|---|
| IL-6 | ↔ ~normal (low inflammation) | ↔ (typically normal or slight ↑) | ↑↑ (elevated, especially if severe) | ↔ (return toward baseline unless PASC) | [108,111,155] |
| IL-1β | ↔ (mild) | ↔ | ↑ (elevated in severe cases) | ↔ (usually normal post-recovery) | [111,155] |
| TNF-α | ↔ (mild) | ↔ | ↑ (elevated) | ↔/↑ (can remain modestly ↑ in PASC) | [155,157] |
| IFN-γ | ↑ (robust innate response) | ↔ | ↑ (T cell response) | ↔ (normalizing) | [37,181] |
| IL-2 | ↔ (baseline) | ↔ | ↔ (baseline) | ↑ (often elevated in long COVID) | [130,188] |
| IL-10 | ↔ (low in mild, ↑ if MIS-C) | ↔ (normalizing) | ↑ (feedback in acute) | ↓ (often low in long COVID) | [111,134] |
| IL-4 | ↔ (no major change) | ↔ | ↔ (not prominently induced) | ↓ (low in long COVID) | [155,164] |
| IL-5, IL-13 | ↔ (baseline) | ↔ | ↔ (baseline) | ↔ (baseline or low) | [30,168] |
| IL-17 | ↔ (slight ↑ in MIS-C) | ↔ | ↑ (elevated in severe) | ↑ (markedly ↑ in PASC) | [130,151,152] |
| CCL11 (eotaxin) | – | – | ↑ (inflammation) | – | [156] |
| Epigenetic Marker/Change | Affected Genes/Pathways | Potential Impact on Immune Dysregulation | References |
|---|---|---|---|
| DNA methylation (monocytes) | Hypomethylation of interferon-related genes; changes at antigen-presenting genes. | Alters innate antiviral response (dampened IFN signaling) and antigen presentation, skewing subsequent adaptive responses. | [195,196] |
| Chromatin accessibility (monocytes) | Increased open chromatin at IL10 and IFNG loci. | Durable “memory” in monocytes leading to altered cytokine production (e.g., heightened IFN-γ/IL-10 potential) long after infection. Could bias balance of Th1/regulatory signals in tissues. | [189,195] |
| CpG methylation at LMAN2 (cg04543273) | cg04543273 (LMAN2 intron) hypomethylation suppresses LMAN2 expression; LMAN2 is upregulated in COVID-19. | Linked to increased Th2-cell abundance; suggests SARS-CoV-2–induced methylation may promote Th2 polarization and atopic dermatitis. | [94,198] |
| Histone modifications (general) | Reported global increase in H3K4me1, H3K27ac at inflammatory loci in myeloid cells (unpublished/analogous to sepsis models). | Facilitates rapid re-expression of pro-inflammatory genes upon stimulus, potentially exacerbating allergic inflammation. | [195,196] |
| HSPC transcriptional reprogramming | IL-6–driven upregulation of myelopoiesis genes in HSPCs. | Skews hematopoiesis toward inflammatory myeloid cells for months, embedding a pro-allergic innate bias in new monocytes/macrophages. | [195,197] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippatos, F.; Matara, D.-I.; Michos, A.; Kakleas, K. Immunological Mechanisms Underlying Allergy Predisposition After SARS-CoV-2 Infection in Children. Cells 2025, 14, 1511. https://doi.org/10.3390/cells14191511
Filippatos F, Matara D-I, Michos A, Kakleas K. Immunological Mechanisms Underlying Allergy Predisposition After SARS-CoV-2 Infection in Children. Cells. 2025; 14(19):1511. https://doi.org/10.3390/cells14191511
Chicago/Turabian StyleFilippatos, Filippos, Dimitra-Ifigeneia Matara, Athanasios Michos, and Konstantinos Kakleas. 2025. "Immunological Mechanisms Underlying Allergy Predisposition After SARS-CoV-2 Infection in Children" Cells 14, no. 19: 1511. https://doi.org/10.3390/cells14191511
APA StyleFilippatos, F., Matara, D.-I., Michos, A., & Kakleas, K. (2025). Immunological Mechanisms Underlying Allergy Predisposition After SARS-CoV-2 Infection in Children. Cells, 14(19), 1511. https://doi.org/10.3390/cells14191511








