RNA Sequencing Identified Differentially Expressed Genes in the Mesocorticolimbic and Nigrostriatal Systems of Compulsive METH-Taking Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Intravenous Surgery
2.3. METH Self-Administration
2.4. Foot Shock Phase
2.5. Isolation of Prefrontal Cortex, Nucleus Accumbens, Dorsal Striatum, and Midbrain and RNA Extraction
2.6. RNA Sequencing and Data Analysis
2.7. Statistical Analyses
3. Results
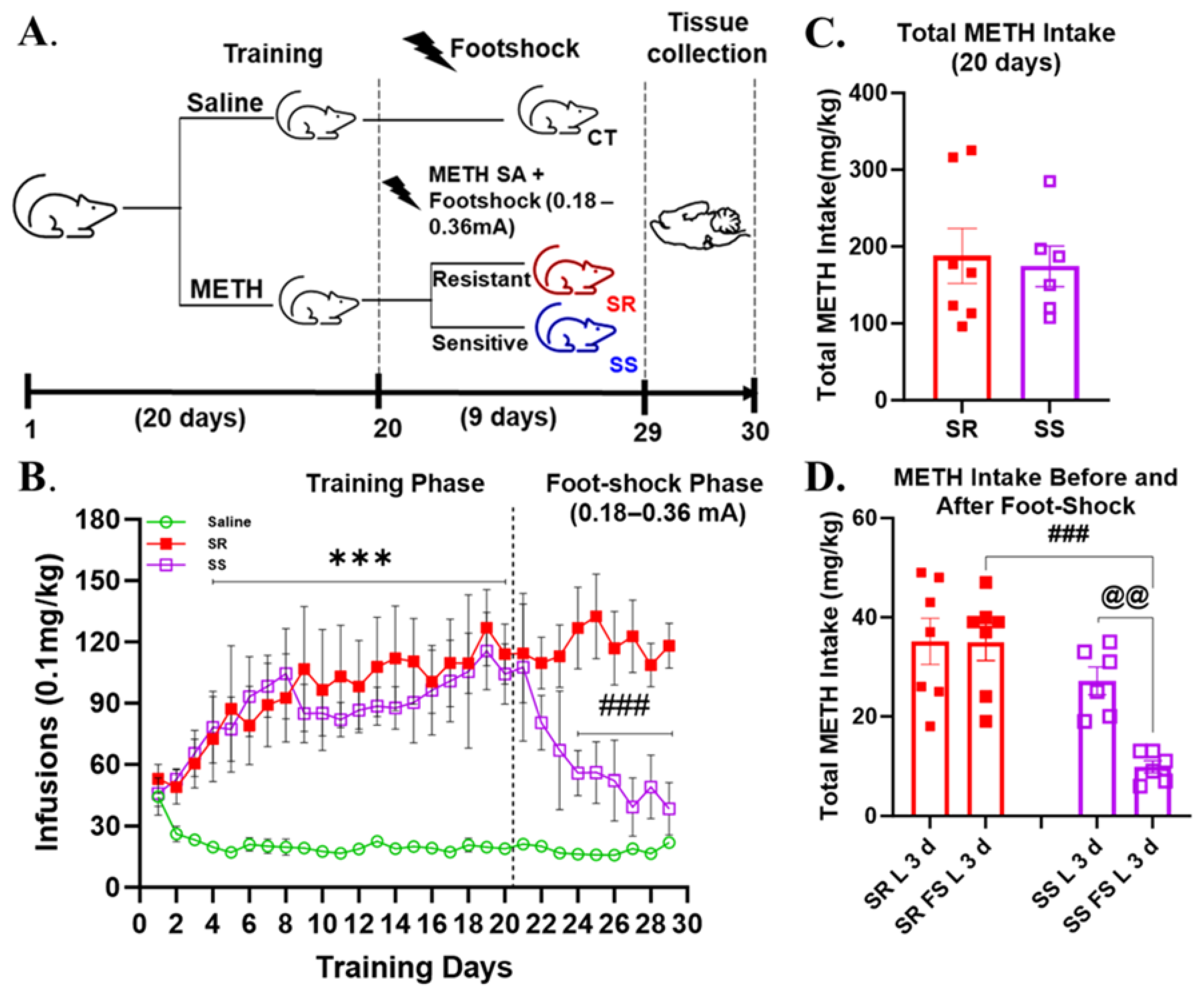
3.1. Rats That Self-Administered Methamphetamine Are Separated into Compulsive (Shock-Resistant) and Non-Compulsive (Shock-Sensitive) Behavioral Phenotypes When Introduced to Foot Shocks
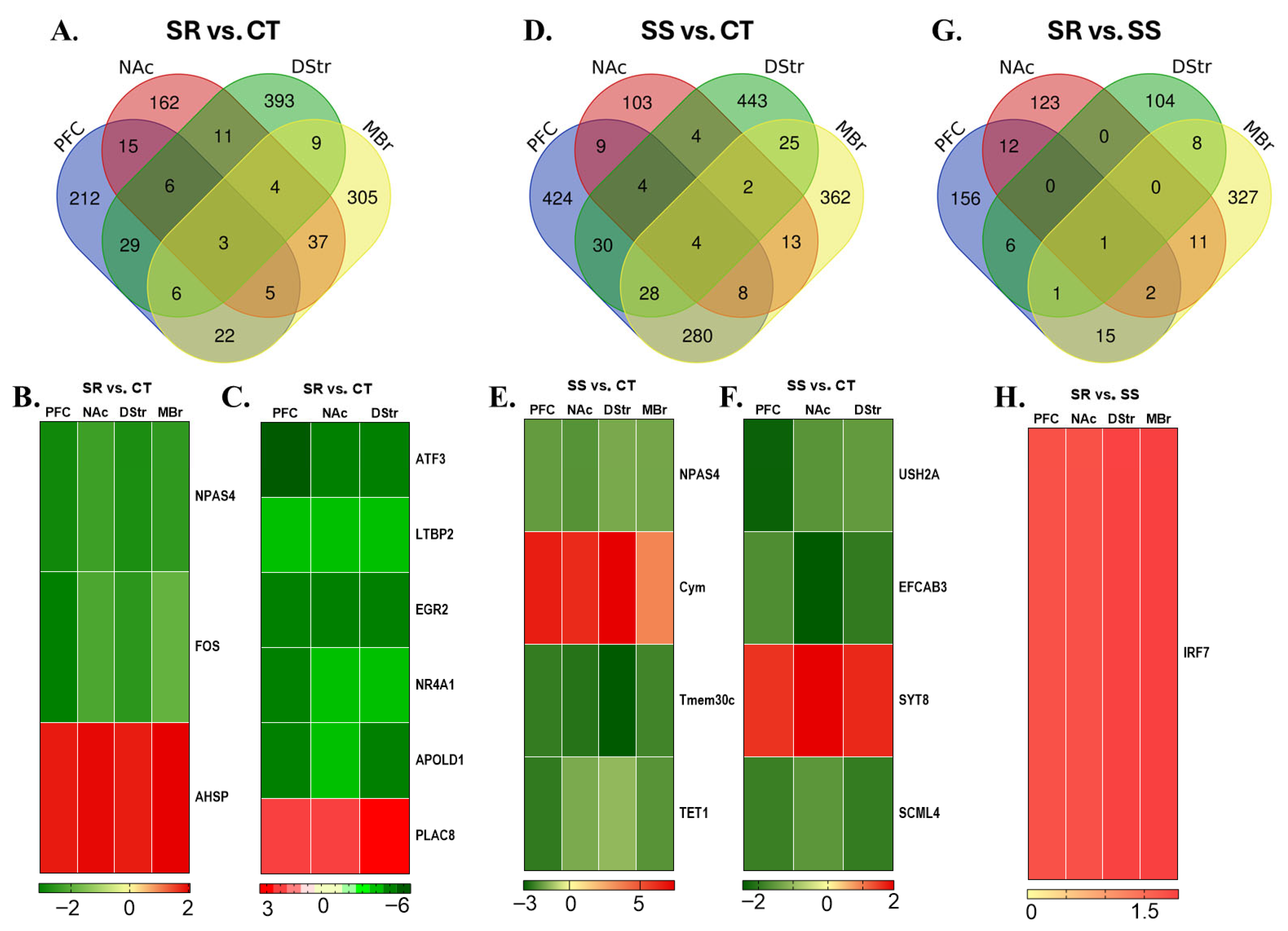
3.2. RNA Sequencing Revealed Dynamic Transcriptome Reprogramming in Both Compulsive and Non-Compulsive METH-Taking Rats
3.3. Differentially Expressed Genes in Prefrontal Cortex
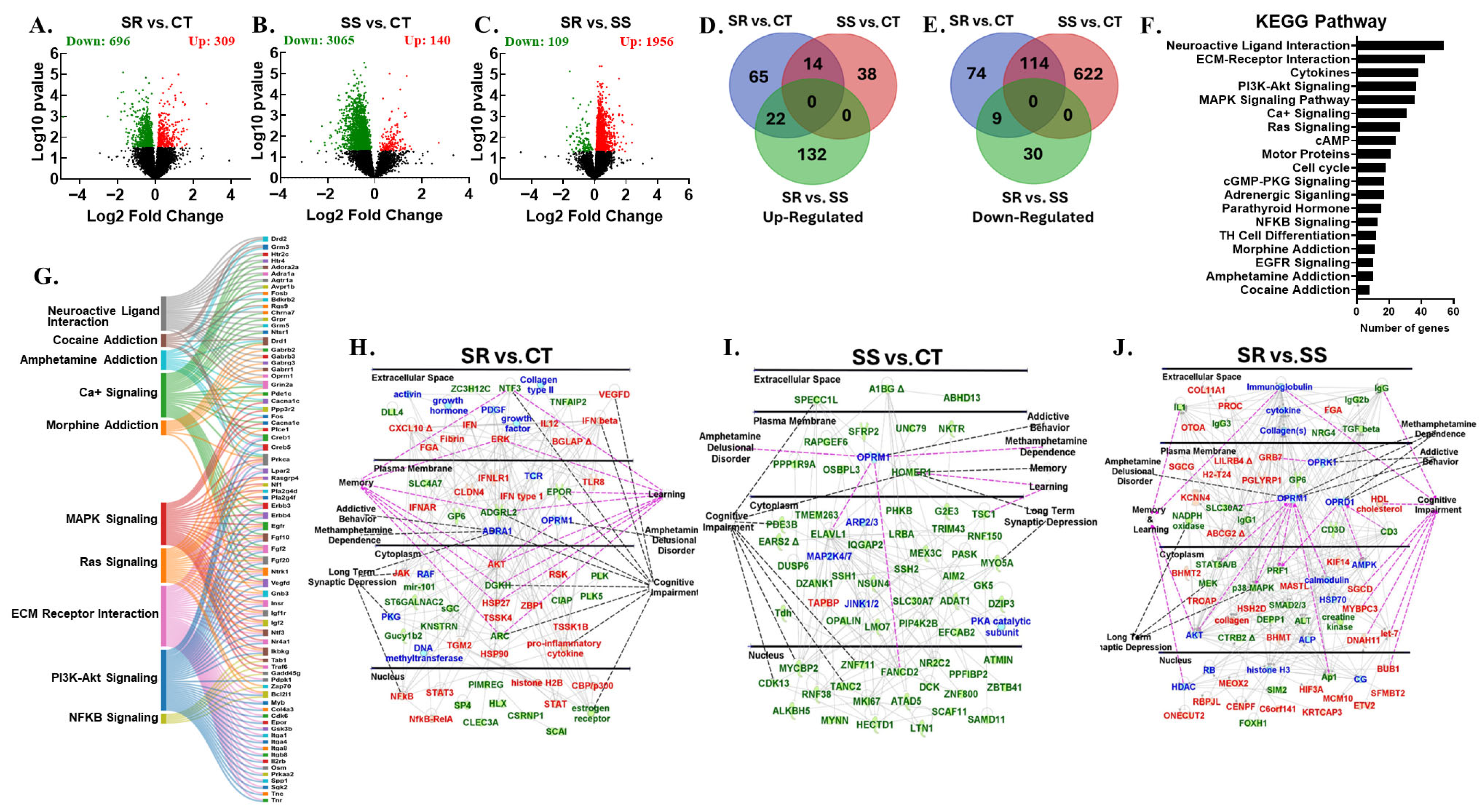
3.4. Differentially Expressed Genes in Nucleus Accumbens
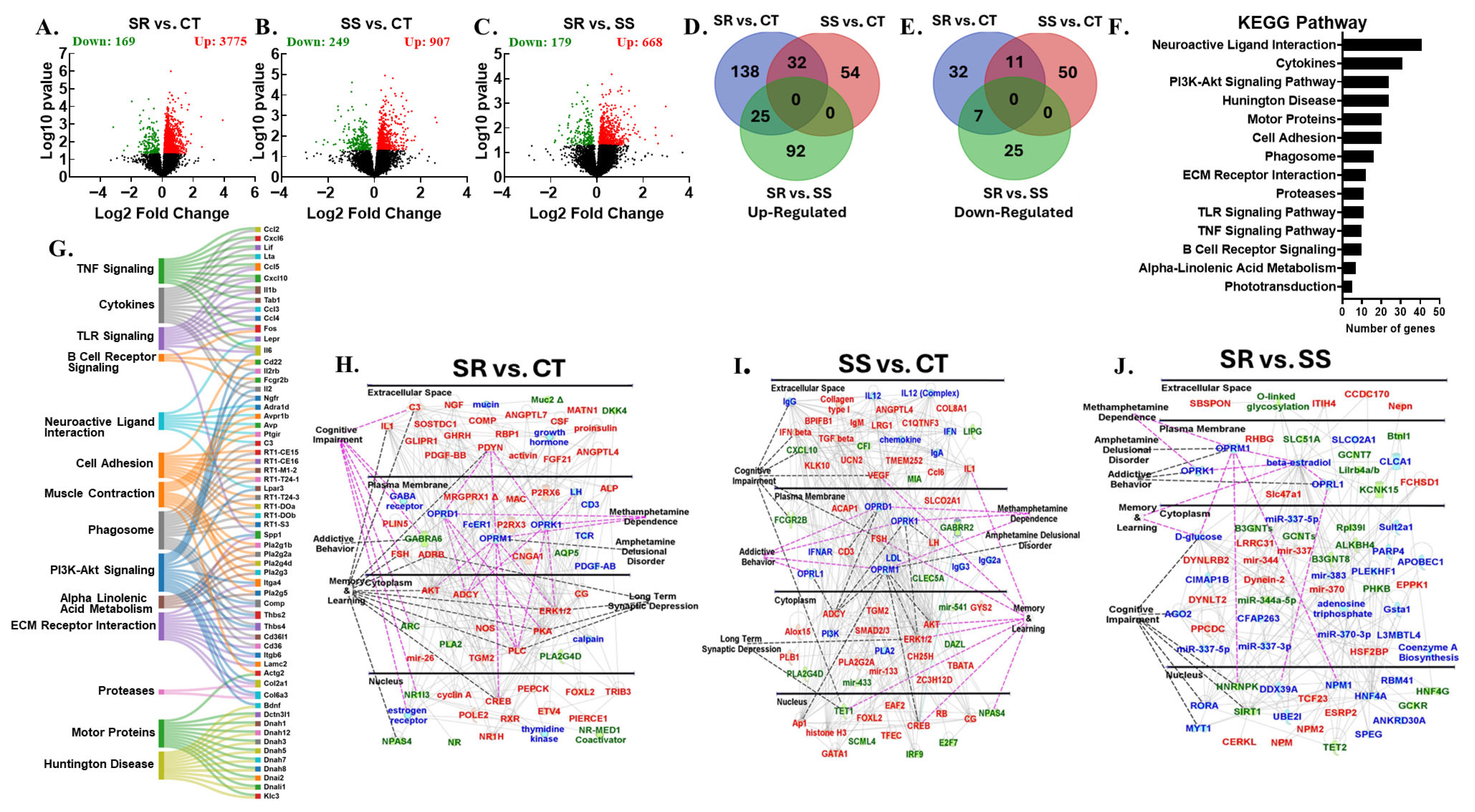
3.5. Differentially Expressed Genes in Dorsal Striatum
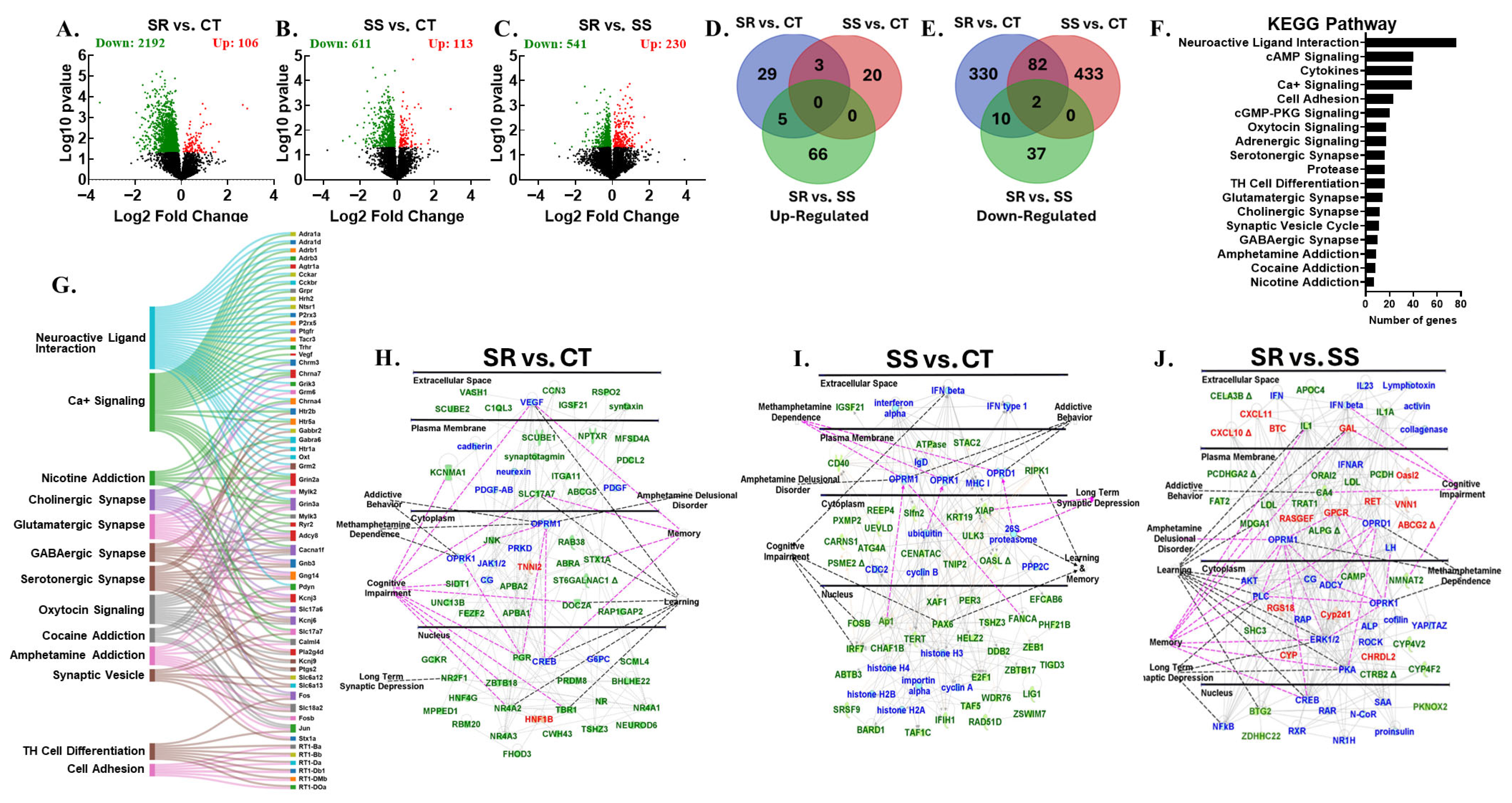
3.6. Differentially Expressed Genes in Midbrain
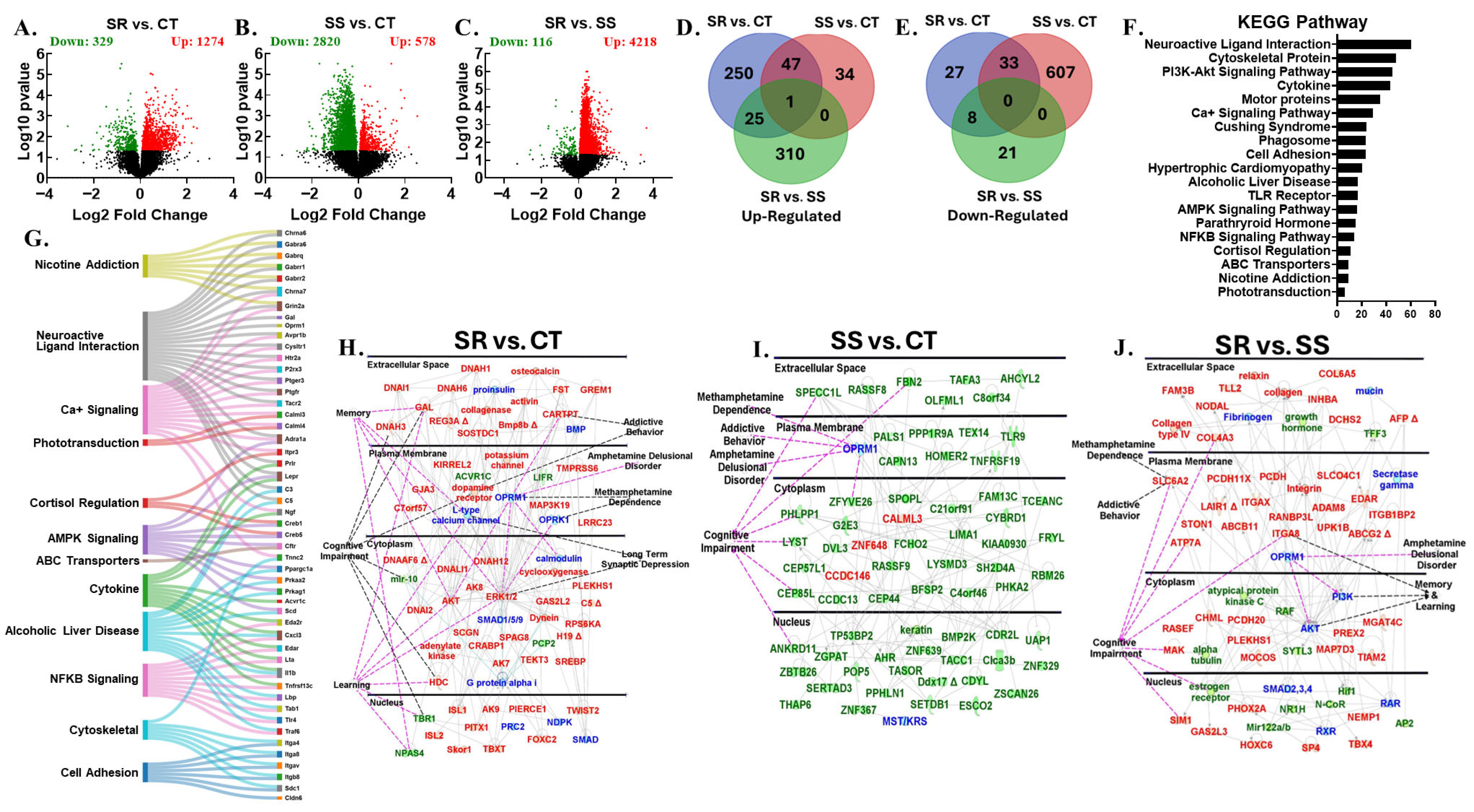
3.7. Shared Differentially Expressed Genes Across Multiple Brain Regions
4. Discussion
4.1. Molecular Mechanisms Associated with Compulsive METH Intake
4.2. Non-Compulsive Behavior and Differential Gene Expression
4.3. Potential Therapeutic Approaches for Methamphetamine Use Disorder
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MUD | Methamphetamine Use Disorder |
| METH | Methamphetamine |
| SR | Shock-Resistant |
| SS | Shock-Sensitive |
| CT | Control |
| PFC | Prefrontal Cortex |
| NAc | Nucleus Accumbens |
| DStr | Dorsal Striatum |
| MBr | Midbrain |
References
- Daiwile, A.P.; Sullivan, P.; Jayanthi, S.; Goldstein, D.S.; Cadet, J.L. Sex-Specific Alterations in Dopamine Metabolism in the Brain after Methamphetamine Self-Administration. Int. J. Mol. Sci. 2022, 23, 4353. [Google Scholar] [CrossRef]
- Jayanthi, S.; Daiwile, A.P.; Cadet, J.L. Neurotoxicity of Methamphetamine: Main Effects and Mechanisms. Exp. Neurol. 2021, 344, 113795. [Google Scholar] [CrossRef]
- Moszczynska, A.; Callan, S.P. Molecular, Behavioral, and Physiological Consequences of Methamphetamine Neurotoxicity: Implications for Treatment. J. Pharmacol. Exp. Ther. 2017, 362, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Schepers, R.J.; Oyler, J.M.; Joseph, R.E., Jr.; Cone, E.J.; Moolchan, E.T.; Huestis, M.A. Methamphetamine and Amphetamine Pharmacokinetics in Oral Fluid and Plasma after Controlled Oral Methamphetamine Administration to Human Volunteers. Clin. Chem. 2003, 49, 121–132. [Google Scholar] [CrossRef]
- Han, B.; Compton, W.M.; Jones, C.M.; Einstein, E.B.; Volkow, N.D. Methamphetamine Use, Methamphetamine Use Disorder, and Associated Overdose Deaths among US Adults. JAMA Psychiatry 2021, 78, 1329–1342. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Text Revision (DSM-5-TR); American Psychiatric Association Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Darke, S.; Kaye, S.; McKetin, R.; Duflou, J. Major Physical and Psychological Harms of Methamphetamine Use. Drug Alcohol Rev. 2008, 27, 253–262. [Google Scholar] [CrossRef]
- Perez, J.A., Jr.; Arsura, E.L.; Strategos, S. Methamphetamine-Related Stroke: Four Cases. J. Emerg. Med. 1999, 17, 469–471. [Google Scholar] [CrossRef]
- Thanos, P.K.; Kim, R.; Delis, F.; Ananth, M.; Chachati, G.; Rocco, M.J.; Masad, I.; Muniz, J.A.; Grant, S.C.; Gold, M.S.; et al. Chronic Methamphetamine Effects on Brain Structure and Function in Rats. PLoS ONE 2016, 11, e0155457. [Google Scholar] [CrossRef] [PubMed]
- Belin, D.; Belin-Rauscent, A.; Murray, J.E.; Everitt, B.J. Addiction: Failure of Control over Maladaptive Incentive Habits. Curr. Opin. Neurobiol. 2013, 23, 564–572. [Google Scholar] [CrossRef]
- Jayanthi, S.; McCoy, M.T.; Chen, B.; Britt, J.P.; Kourrich, S.; Yau, H.J.; Ladenheim, B.; Krasnova, I.N.; Bonci, A.; Cadet, J.L. Methamphetamine Downregulates Striatal Glutamate Receptors via Diverse Epigenetic Mechanisms. Biol. Psychiatry 2014, 76, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.L.; Brannock, C.; Jayanthi, S.; Krasnova, I.N. Transcriptional and Epigenetic Substrates of Methamphetamine Addiction and Withdrawal: Evidence from a Long-Access Self-Administration Model in the Rat. Mol. Neurobiol. 2015, 51, 696–717. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of Addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef]
- Volkow, N.D.; Morales, M. The Brain on Drugs: From Reward to Addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef]
- Lipton, D.M.; Gonzales, B.J.; Citri, A. Dorsal Striatal Circuits for Habits, Compulsions and Addictions. Front. Syst. Neurosci. 2019, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Everitt, B.J.; Robbins, T.W. From the Ventral to the Dorsal Striatum: Devolving Views of Their Roles in Drug Addiction. Neurosci. Biobehav. Rev. 2013, 37, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.C.; Chapman, K.; Butler, P.; Mead, A.N. The Predictive Validity of the Rat Self-Administration Model for Abuse Liability. Neurosci. Biobehav. Rev. 2011, 35, 912–938. [Google Scholar] [CrossRef]
- Krasnova, I.N.; Chiflikyan, M.; Justinova, Z.; McCoy, M.T.; Ladenheim, B.; Jayanthi, S.; Quintero, C.; Brannock, C.; Barnes, C.; Adair, J.E.; et al. CREB Phosphorylation Regulates Striatal Transcriptional Responses in the Self-Administration Model of Methamphetamine Addiction in the Rat. Neurobiol. Dis. 2013, 58, 132–143. [Google Scholar] [CrossRef]
- Krasnova, I.N.; Justinova, Z.; Cadet, J.L. Methamphetamine Addiction: Involvement of CREB and Neuroinflammatory Signaling Pathways. Psychopharmacology 2016, 233, 1945–1962. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.L.; Brannock, C.; Krasnova, I.N.; Jayanthi, S.; Ladenheim, B.; McCoy, M.T.; Walther, D.; Godino, A.; Pirooznia, M.; Lee, R.S. Genome-Wide DNA Hydroxymethylation Identifies Potassium Channels in the Nucleus Accumbens as Discriminators of Methamphetamine Addiction and Abstinence. Mol. Psychiatry 2017, 22, 1196–1204. [Google Scholar] [CrossRef]
- Daiwile, A.P.; Jayanthi, S.; Ladenheim, B.; McCoy, M.T.; Brannock, C.; Schroeder, J.; Cadet, J.L. Sex Differences in Escalated Methamphetamine Self-Administration and Altered Gene Expression Associated with Incubation of Methamphetamine Seeking. Int. J. Neuropsychopharmacol. 2019, 22, 710–723. [Google Scholar] [CrossRef]
- Torres, O.V.; Jayanthi, S.; Ladenheim, B.; McCoy, M.T.; Krasnova, I.N.; Cadet, J.L. Compulsive Methamphetamine Taking under Punishment Is Associated with Greater Cue-Induced Drug Seeking in Rats. Behav. Brain Res. 2017, 326, 265–271. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2018 Update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 17 September 2025).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Daiwile, A.P.; McCoy, M.T.; Ladenheim, B.; Subramaniam, J.; Cadet, J.L. Incubation of Methamphetamine Craving in Punishment-Resistant Individuals Is Associated with Activation of Specific Gene Networks in the Rat Dorsal Striatum. Mol. Psychiatry 2024, 29, 1990–2000. [Google Scholar] [CrossRef]
- Daiwile, A.P.; Ladenheim, B.; Jayanthi, S.; Cadet, J.L. Punishment-Induced Suppression of Methamphetamine Self-Administration Is Accompanied by the Activation of the CPEB4/GLD2 Polyadenylation Complex of the Translational Machinery. Int. J. Mol. Sci. 2025, 26, 2734. [Google Scholar] [CrossRef]
- Cadet, J.L.; McCoy, M.T.; Cai, N.S.; Krasnova, I.N.; Ladenheim, B.; Beauvais, G.; Wilson, N.; Wood, W.; Becker, K.G.; Hodges, A.B. Methamphetamine Preconditioning Alters Midbrain Transcriptional Responses to Methamphetamine-Induced Injury in the Rat Striatum. PLoS ONE 2009, 4, e7812. [Google Scholar] [CrossRef]
- Goldstein, R.Z.; Volkow, N.D. Dysfunction of the Prefrontal Cortex in Addiction: Neuroimaging Findings and Clinical Implications. Nat. Rev. Neurosci. 2011, 12, 652–669. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Tomasi, D. Addiction Circuitry in the Human Brain. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Munoz, C.; Jayanthi, S.; Ladenheim, B.; Cadet, J.L. Compulsive Methamphetamine Self-Administration in the Presence of Adverse Consequences Is Associated with Increased Hippocampal mRNA Expression of Cellular Adhesion Molecules. Front. Mol. Neurosci. 2023, 15, 1104657. [Google Scholar] [CrossRef]
- Miao, B.; Xing, X.; Bazylianska, V.; Madden, P.; Moszczynska, A.; Zhang, B. Methamphetamine-Induced Region-Specific Transcriptomic and Epigenetic Changes in the Brain of Male Rats. Commun. Biol. 2023, 6, 991. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, L.; Zhang, H.; Liu, J. Application of Omics-Based Biomarkers in Substance Use Disorders. Meta Radiol. 2023, 1, 100008. [Google Scholar] [CrossRef]
- Rosoff, D.B.; Wagner, J.; Bell, A.S.; Mavromatis, L.A.; Jung, J.; Lohoff, F.W. A Multi-Omics Mendelian Randomization Study Identifies New Therapeutic Targets for Alcohol Use Disorder and Problem Drinking. Nat. Hum. Behav. 2025, 9, 188–207. [Google Scholar] [CrossRef]
- Sullivan, K.A.; Kainer, D.; Lane, M.; Cashman, M.; Miller, J.I.; Garvin, M.R.; Townsend, A.; Quach, B.C.; Willis, C.; Kruse, P.; et al. Multiomic Network Analysis Identifies Dysregulated Neurobiological Pathways in Opioid Addiction. Biol. Psychiatry 2025, 98, 11–22. [Google Scholar] [CrossRef]
- Whalley, H.C.; Papmeyer, M.; Romaniuk, L.; Johnstone, E.C.; Hall, J.; Lawrie, S.M.; Sussmann, J.E.; McIntosh, A.M. Effect of Variation in Diacylglycerol Kinase η (DGKH) Gene on Brain Function in a Cohort at Familial Risk of Bipolar Disorder. Neuropsychopharmacology 2012, 37, 919–928. [Google Scholar] [CrossRef]
- Moya, P.R.; Murphy, D.L.; McMahon, F.J.; Wendland, J.R. Increased Gene Expression of Diacylglycerol Kinase η in Bipolar Disorder. Int. J. Neuropsychopharmacol. 2010, 13, 1127–1128. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Kittel-Schneider, S.; Gessner, A.; Domschke, K.; Neuner, M.; Jacob, C.P.; Buttenschon, H.N.; Boreatti-Hümmer, A.; Volkert, J.; Herterich, S.; et al. Cross-Disorder Analysis of Bipolar Risk Genes: Further Evidence of DGKH as a Risk Gene for Bipolar Disorder, but Also Unipolar Depression and Adult ADHD. Neuropsychopharmacology 2011, 36, 2076–2085. [Google Scholar] [CrossRef]
- Gregersen, N.O.; Lescai, F.; Liang, J.; Li, Q.; Als, T.; Buttenschøn, H.N.; Hedemand, A.; Biskopstø, M.; Wang, J.; Wang, A.G.; et al. Whole-Exome Sequencing Implicates DGKH as a Risk Gene for Panic Disorder in the Faroese Population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016, 171, 1013–1022. [Google Scholar] [CrossRef]
- Ahn, H.J.; Hernandez, C.M.; Levenson, J.M.; Lubin, F.D.; Liou, H.C.; Sweatt, J.D. c-Rel, an NF-kappaB Family Transcription Factor, Is Required for Hippocampal Long-Term Synaptic Plasticity and Memory Formation. Learn. Mem. 2008, 15, 539–549. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Ndiaye, D.; Korte, M.; Pothion, S.; Arbibe, L.; Prüllage, M.; Pfeiffer, J.; Lindecke, A.; Staiger, V.; Israël, A.; et al. NF-kappaB Regulates Spatial Memory Formation and Synaptic Plasticity through Protein Kinase A/CREB Signaling. Mol. Cell. Biol. 2006, 26, 2936–2946. [Google Scholar] [CrossRef]
- Girault, J.A.; Valjent, E.; Caboche, J.; Hervé, D. ERK2: A Logical AND Gate Critical for Drug-Induced Plasticity? Curr. Opin. Pharmacol. 2007, 7, 77–85. [Google Scholar] [CrossRef]
- Sun, W.L.; Quizon, P.M.; Zhu, J. Molecular Mechanism: ERK Signaling, Drug Addiction, and Behavioral Effects. Prog. Mol. Biol. Transl. Sci. 2016, 137, 1–40. [Google Scholar] [CrossRef]
- Numan, S.; Lane-Ladd, S.B.; Zhang, L.; Lundgren, K.H.; Russell, D.S.; Seroogy, K.B.; Nestler, E.J. Differential Regulation of Neurotrophin and trk Receptor mRNAs in Catecholaminergic Nuclei during Chronic Opiate Treatment and Withdrawal. J. Neurosci. 1998, 18, 10700–10708. [Google Scholar] [CrossRef]
- Cadet, J.L.; Krasnova, I.N.; Walther, D.; Brannock, C.; Ladenheim, B.; McCoy, M.T.; Collector, D.; Torres, O.V.; Terry, N.; Jayanthi, S. Increased Expression of Proenkephalin and Prodynorphin mRNAs in the Nucleus Accumbens of Compulsive Methamphetamine Taking Rats. Sci. Rep. 2016, 6, 37002. [Google Scholar] [CrossRef]
- Bie, B.; Wang, Y.; Cai, Y.Q.; Zhang, Z.; Hou, Y.Y.; Pan, Z.Z. Upregulation of Nerve Growth Factor in Central Amygdala Increases Sensitivity to Opioid Reward. Neuropsychopharmacology 2012, 37, 2780–2788. [Google Scholar] [CrossRef]
- Meredith, A.L. BK Channelopathies and KCNMA1-Linked Disease Models. Annu. Rev. Physiol. 2024, 86, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Laumonnier, F.; Roger, S.; Guérin, P.; Molinari, F.; M’rad, R.; Cahard, D.; Belhadj, A.; Halayem, M.; Persico, A.M.; Elia, M.; et al. Association of a Functional Deficit of the BKCa Channel, a Synaptic Regulator of Neuronal Excitability, with Autism and Mental Retardation. Am. J. Psychiatry 2006, 163, 1622–1629. [Google Scholar] [CrossRef]
- McCoy, M.T.; Jayanthi, S.; Wulu, J.A.; Beauvais, G.; Ladenheim, B.; Martin, T.A.; Krasnova, I.N.; Hodges, A.B.; Cadet, J.L. Chronic Methamphetamine Exposure Suppresses the Striatal Expression of Members of Multiple Families of Immediate Early Genes (IEGs) in the Rat: Normalization by an Acute Methamphetamine Injection. Psychopharmacology 2011, 215, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Hawk, J.D.; Abel, T. The Role of NR4A Transcription Factors in Memory Formation. Brain Res. Bull. 2011, 85, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Krasnova, I.N.; Gerra, M.C.; Walther, D.; Jayanthi, S.; Ladenheim, B.; McCoy, M.T.; Brannock, C.; Cadet, J.L. Compulsive Methamphetamine Taking in the Presence of Punishment Is Associated with Increased Oxytocin Expression in the Nucleus Accumbens of Rats. Sci. Rep. 2017, 7, 8331. [Google Scholar] [CrossRef]
- Cadet, J.L.; Brannock, C.; Ladenheim, B.; McCoy, M.T.; Krasnova, I.N.; Lehrmann, E.; Becker, K.G.; Jayanthi, S. Enhanced Upregulation of CRH mRNA Expression in the Nucleus Accumbens of Male Rats after a Second Injection of Methamphetamine Given Thirty Days Later. PLoS ONE 2014, 9, e84665. [Google Scholar] [CrossRef]
- Mannangatti, P.; Arapulisamy, O.; Shippenberg, T.S.; Ramamoorthy, S.; Jayanthi, L.D. Cocaine Up-Regulation of the Norepinephrine Transporter Requires Threonine 30 Phosphorylation by p38 Mitogen-Activated Protein Kinase. J. Biol. Chem. 2011, 286, 20239–20250. [Google Scholar] [CrossRef]
- Macey, D.J.; Smith, H.R.; Nader, M.A.; Porrino, L.J. Chronic Cocaine Self-Administration Upregulates the Norepinephrine Transporter and Alters Functional Activity in the Bed Nucleus of the Stria Terminalis of the Rhesus Monkey. J. Neurosci. 2003, 23, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Barry, S.M.; Barry, G.M.; Martinez, D.; Penrod, R.D.; Cowan, C.W. The Activity-Regulated Cytoskeleton-Associated Protein, Arc, Functions in the Nucleus Accumbens Shell to Limit Multiple Triggers of Cocaine-Seeking Behaviour. Addict. Biol. 2023, 28, e13335. [Google Scholar] [CrossRef]
- Penrod, R.D.; Thomsen, M.; Taniguchi, M.; Guo, Y.; Cowan, C.W.; Smith, L.N. The Activity-Regulated Cytoskeleton-Associated Protein, Arc/Arg3.1, Influences Mouse Cocaine Self-Administration. Pharmacol. Biochem. Behav. 2020, 188, 172818. [Google Scholar] [CrossRef] [PubMed]
- Kodama, M.; Akiyama, K.; Ujike, H.; Shimizu, Y.; Tanaka, Y.; Kuroda, S. A Robust Increase in Expression of arc Gene, an Effector Immediate Early Gene, in the Rat Brain after Acute and Chronic Methamphetamine Administration. Brain Res. 1998, 796, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Piguel, N.H.; Khalatyan, N.; Dionisio, L.E.; Savas, J.N.; Penzes, P. Homer1 Promotes Dendritic Spine Growth through Ankyrin-G and Its Loss Reshapes the Synaptic Proteome. Mol. Psychiatry 2021, 26, 1775–1789. [Google Scholar] [CrossRef]
- Guo, H.; Bettella, E.; Marcogliese, P.C.; Zhao, R.; Andrews, J.C.; Nowakowski, T.J.; Gillentine, M.A.; Hoekzema, K.; Wang, T.; Wu, H.; et al. Disruptive Mutations in TANC2 Define a Neurodevelopmental Syndrome Associated with Psychiatric Disorders. Nat. Commun. 2019, 10, 4679. [Google Scholar] [CrossRef]
- Swanson, C.J.; Baker, D.A.; Carson, D.; Worley, P.F.; Kalivas, P.W. Repeated Cocaine Administration Attenuates Group I Metabotropic Glutamate Receptor-Mediated Glutamate Release and Behavioral Activation: A Potential Role for Homer. J. Neurosci. 2001, 21, 9043–9052. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.B.; Permenter, L.K.; Lake, R.; Worley, P.F.; Kalivas, P.W. Homer1 Proteins and AMPA Receptors Modulate Cocaine-Induced Behavioural Plasticity. Eur. J. Neurosci. 2003, 18, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Pacwa, A.; Machowicz, J.; Akhtar, S.; Rodak, P.; Liu, X.; Pietrucha-Dutczak, M.; Lewin-Kowalik, J.; Amadio, M.; Smedowski, A. Deficiency of the RNA-Binding Protein ELAVL1/HuR Leads to the Failure of Endogenous and Exogenous Neuroprotection of Retinal Ganglion Cells. Front. Cell. Neurosci. 2023, 17, 1131356. [Google Scholar] [CrossRef]
- Yin, W.; Clare, K.; Zhang, Q.; Volkow, N.D.; Du, C. Chronic Cocaine Induces HIF-VEGF Pathway Activation along with Angiogenesis in the Brain. PLoS ONE 2017, 12, e0175499. [Google Scholar] [CrossRef]
- Johnson, K.; Doucette, A.; Edwards, A.; Verdi, A.; McFarland, R.; Hulke, S.; Fowler, A.; Watts, V.J.; Klein, A.H. Reduced Activity of Adenylyl Cyclase 1 Attenuates Morphine Induced Hyperalgesia and Inflammatory Pain in Mice. Front. Pharmacol. 2022, 13, 937741. [Google Scholar] [CrossRef]
- Chan, P.; Lutfy, K. Molecular Changes in Opioid Addiction: The Role of Adenylyl Cyclase and cAMP/PKA System. Prog. Mol. Biol. Transl. Sci. 2016, 137, 203–227. [Google Scholar] [CrossRef]
- Xue, X.; Flury-Wetherill, L.; Dick, D.; Goate, A.; Tischfield, J.; Nurnberger, J., Jr.; Schuckit, M.; Kramer, J.; Kuperman, S.; Hesselbrock, V.; et al. GABRR1 and GABRR2, Encoding the GABA-A Receptor Subunits Rho1 and Rho2, Are Associated with Alcohol Dependence. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010, 153B, 418–427. [Google Scholar] [CrossRef]
- Flora, G.; Lee, Y.W.; Nath, A.; Maragos, W.; Hennig, B.; Toborek, M. Methamphetamine-Induced TNF-Alpha Gene Expression and Activation of AP-1 in Discrete Regions of Mouse Brain: Potential Role of Reactive Oxygen Intermediates and Lipid Peroxidation. Neuromolecular Med. 2002, 2, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Sheng, P.; Wang, X.B.; Ladenheim, B.; Epstein, C.; Cadet, J.L. AP-1 DNA-Binding Activation by Methamphetamine Involves Oxidative Stress. Synapse 1996, 24, 213–217. [Google Scholar] [CrossRef]
- Lee, Y.W.; Hennig, B.; Yao, J.; Toborek, M. Methamphetamine Induces AP-1 and NF-KappaB Binding and Transactivation in Human Brain Endothelial Cells. J. Neurosci. Res. 2001, 66, 583–591. [Google Scholar] [CrossRef]
- Heyman, I.; Frampton, I.; van Heyningen, V.; Hanson, I.; Teague, P.; Taylor, A.; Simonoff, E. Psychiatric Disorder and Cognitive Function in a Family with an Inherited Novel Mutation of the Developmental Control Gene PAX6. Psychiatr. Genet. 1999, 9, 85–90. [Google Scholar] [CrossRef]
- Kikkawa, T.; Casingal, C.R.; Chun, S.H.; Shinohara, H.; Hiraoka, K.; Osumi, N. The Role of Pax6 in Brain Development and Its Impact on Pathogenesis of Autism Spectrum Disorder. Brain Res. 2019, 1705, 95–103. [Google Scholar] [CrossRef]
- Ochi, S.; Manabe, S.; Kikkawa, T.; Osumi, N. Thirty Years’ History since the Discovery of Pax6: From Central Nervous System Development to Neurodevelopmental Disorders. Int. J. Mol. Sci. 2022, 23, 6115. [Google Scholar] [CrossRef]
- Smaga, I.; Gawlińska, K.; Frankowska, M.; Wydra, K.; Sadakierska-Chudy, A.; Suder, A.; Piechota, M.; Filip, M. Extinction Training after Cocaine Self-Administration Influences the Epigenetic and Genetic Machinery Responsible for Glutamatergic Transporter Gene Expression in Male Rat Brain. Neuroscience 2020, 451, 99–110. [Google Scholar] [CrossRef]
- Xu, W.; Yang, T.; Lou, X.; Chen, J.; Wang, X.; Hu, M.; An, D.; Gao, R.; Wang, J.; Chen, X. Role of the Peli1-RIPK1 Signaling Axis in Methamphetamine-Induced Neuroinflammation. ACS Chem. Neurosci. 2023, 14, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, Q.; Wang, Q.; Tan, X.; Pang, K.; Liu, X.; Zhu, S.; Xi, K.; Zhang, J.; Gao, Q.; et al. Profiling Circular RNA in Methamphetamine-Treated Primary Cortical Neurons Identified Novel circRNAs Related to Methamphetamine Addiction. Neurosci. Lett. 2019, 701, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Ka, M.; Kim, W.Y. ANKRD11 Associated with Intellectual Disability and Autism Regulates Dendrite Differentiation via the BDNF/TrkB Signaling Pathway. Neurobiol. Dis. 2018, 111, 138–152. [Google Scholar] [CrossRef]
- Sirmaci, A.; Spiliopoulos, M.; Brancati, F.; Powell, E.; Duman, D.; Abrams, A.; Bademci, G.; Agolini, E.; Guo, S.; Konuk, B.; et al. Mutations in ANKRD11 Cause KBG Syndrome, Characterized by Intellectual Disability, Skeletal Malformations, and Macrodontia. Am. J. Hum. Genet. 2011, 89, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Rudelius, M.; Osanger, A.; Kohlmann, S.; Augustin, M.; Piontek, G.; Heinzmann, U.; Jennen, G.; Russ, A.; Matiasek, K.; Stumm, G.; et al. A missense mutation in the WD40 domain of murine Lyst is linked to severe progressive Purkinje cell degeneration. Acta Neuropathol. 2006, 112, 267–276. [Google Scholar] [CrossRef]
- Weisfeld-Adams, J.D.; Mehta, L.; Rucker, J.C.; Dembitzer, F.R.; Szporn, A.; Lublin, F.D.; Introne, W.J.; Bhambhani, V.; Chicka, M.C.; Cho, C. Atypical Chédiak-Higashi syndrome with attenuated phenotype: Three adult siblings homozygous for a novel LYST deletion and with neurodegenerative disease. Orphanet J. Rare Dis. 2013, 8, 46. [Google Scholar] [CrossRef]
- Vantaggiato, C.; Crimella, C.; Airoldi, G.; Polishchuk, R.; Bonato, S.; Brighina, E.; Scarlato, M.; Musumeci, O.; Toscano, A.; Martinuzzi, A.; et al. Defective autophagy in spastizin mutated patients with hereditary spastic paraparesis type 15. Brain 2013, 136, 3119–3139. [Google Scholar] [CrossRef]
- Garg, V.; André, S.; Heyer, L.; Kracht, G.; Ruhwedel, T.; Scholz, P.; Ischebeck, T.; Werner, H.B.; Dullin, C.; Engelmann, J.; et al. Axon demyelination and degeneration in a zebrafish spastizin model of hereditary spastic paraplegia. Open Biol. 2024, 14, 240100. [Google Scholar] [CrossRef]
- Han, G.; Cao, C.; Yang, X.; Zhao, G.W.; Hu, X.J.; Yu, D.L.; Yang, R.F.; Yang, K.; Zhang, Y.Y.; Wang, W.T.; et al. Nrf2 expands the intracellular pool of the chaperone AHSP in a cellular model of β-thalassemia. Redox Biol. 2022, 50, 102239. [Google Scholar] [CrossRef]
- Cao, C.; Zhao, G.; Yu, W.; Xie, X.; Wang, W.; Yang, R.; Lv, X.; Liu, D. Activation of STAT3 stimulates AHSP expression in K562 cells. Sci. China Life Sci. 2014, 57, 488–494. [Google Scholar] [CrossRef]
- Mu, Q.; Zhang, Y.; Gu, L.; Gerner, S.T.; Qiu, X.; Tao, Q.; Pang, J.; Dipritu, G.; Zhang, L.; Yin, S.; et al. Transcriptomic profiling reveals the antiapoptosis and antioxidant stress effects of Fos in ischemic stroke. Front. Neurol. 2021, 12, 728984. [Google Scholar] [CrossRef]
- Zhou, H.; Gao, J.; Lu, Z.Y.; Lu, L.; Dai, W.; Xu, M. Role of c-Fos/JunD in protecting stress-induced cell death. Cell Prolif. 2007, 40, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Yun, J.; Park, B. Methamphetamine-induced neuronal damage: Neurotoxicity and neuroinflammation. Biomol. Ther. 2020, 28, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, S.; Gonzalez, B.; McCoy, M.T.; Ladenheim, B.; Bisagno, V.; Cadet, J.L. Methamphetamine induces TET1- and TET3-dependent DNA hydroxymethylation of Crh and Avp genes in the rat nucleus accumbens. Mol. Neurobiol. 2018, 55, 5154–5166. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shao, N.; Szulwach, K.E.; Vialou, V.; Huynh, J.; Zhong, C.; Le, T.; Ferguson, D.; Cahill, M.E.; Li, Y.; et al. Role of Tet1 and 5-hydroxymethylcytosine in cocaine action. Nat. Neurosci. 2015, 18, 536–544. [Google Scholar] [CrossRef]
- Yang, X.; Shui, X.; Dai, X.; Hao, S.; Ke, F.; Zhu, L.; Chen, X. PLAC8 promotes EV71 infected inflammatory lesion by disturbing Th-cell-related cytokines release in neonatal mouse. Virology 2021, 564, 39–45. [Google Scholar] [CrossRef]
- Zhao, C.; Zou, T.; Tang, R.; Zhu, C. Placenta-specific 8 (PLAC8) mediates inflammation and mobility of the hPDLCs via MEK/ERK signaling pathway. Int. Immunopharmacol. 2022, 103, 108459. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Q.; Li, F.; Pang, R.; Chen, C.; Li, P.; Wang, X.; Xuan, W.; Yu, W. The multifaceted roles of activating transcription factor 3 (ATF3) in inflammatory responses—Potential target to regulate neuroinflammation in acute brain injury. J. Cereb. Blood Flow Metab. 2023, 43, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, R.; Aronheim, A.; Cheng, Y.C.; Perlman, I. Editorial: ATF3: A crucial stress-responsive gene of glia and neurons in CNS. Front. Mol. Neurosci. 2024, 17, 1484487. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.C.; Hsu, S.H.; Chen, C.H. Chronic methamphetamine treatment reduces the expression of synaptic plasticity genes and changes their DNA methylation status in the mouse brain. Brain Res. 2015, 1629, 126–134. [Google Scholar] [CrossRef]
- Mukherjee, D.; Gonzales, B.J.; Ashwal-Fluss, R.; Turm, H.; Groysman, M.; Citri, A. Egr2 induction in spiny projection neurons of the ventrolateral striatum contributes to cocaine place preference in mice. Elife 2021, 10, e65228. [Google Scholar] [CrossRef]
- Carpenter, M.D.; Hu, Q.; Bond, A.M.; Lombroso, S.I.; Czarnecki, K.S.; Lim, C.J.; Song, H.; Wimmer, M.E.; Pierce, R.C.; Heller, E.A. Nr4a1 suppresses cocaine-induced behavior via epigenetic regulation of homeostatic target genes. Nat. Commun. 2020, 11, 504. [Google Scholar] [CrossRef]
- Heath, A.C.; Whitfield, J.B.; Martin, N.G.; Pergadia, M.L.; Goate, A.M.; Lind, P.A.; McEvoy, B.P.; Schrage, A.J.; Grant, J.D.; Chou, Y.L.; et al. A quantitative-trait genome-wide association study of alcoholism risk in the community: Findings and implications. Biol. Psychiatry 2011, 70, 513–518. [Google Scholar] [CrossRef]
- Johnson, C.; Drgon, T.; Liu, Q.R.; Walther, D.; Edenberg, H.; Rice, J.; Foroud, T.; Uhl, G.R. Pooled association genome scanning for alcohol dependence using 104,268 SNPs: Validation and use to identify alcoholism vulnerability loci in unrelated individuals from the collaborative study on the genetics of alcoholism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141B, 844–853. [Google Scholar] [CrossRef]
- Lilue, J.; Doran, A.G.; Fiddes, I.T.; Abrudan, M.; Armstrong, J.; Bennett, R.; Chow, W.; Collins, J.; Collins, S.; Czechanski, A.; et al. Sixteen diverse laboratory mouse reference genomes define strain-specific haplotypes and novel functional loci. Nat. Genet. 2018, 50, 1574–1583. [Google Scholar] [CrossRef]
- Monterrat, C.; Boal, F.; Grise, F.; Hémar, A.; Lang, J. Synaptotagmin 8 is expressed both as a calcium-insensitive soluble and membrane protein in neurons, neuroendocrine and endocrine cells. Biochim. Biophys. Acta 2006, 1763, 73–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adjei, N.; Ladenheim, B.; McCoy, M.T.; Palande, V.; Cadet, J.L.; Daiwile, A.P. RNA Sequencing Identified Differentially Expressed Genes in the Mesocorticolimbic and Nigrostriatal Systems of Compulsive METH-Taking Rats. Cells 2025, 14, 1472. https://doi.org/10.3390/cells14181472
Adjei N, Ladenheim B, McCoy MT, Palande V, Cadet JL, Daiwile AP. RNA Sequencing Identified Differentially Expressed Genes in the Mesocorticolimbic and Nigrostriatal Systems of Compulsive METH-Taking Rats. Cells. 2025; 14(18):1472. https://doi.org/10.3390/cells14181472
Chicago/Turabian StyleAdjei, Nasser, Bruce Ladenheim, Michael T. McCoy, Vikrant Palande, Jean Lud Cadet, and Atul P. Daiwile. 2025. "RNA Sequencing Identified Differentially Expressed Genes in the Mesocorticolimbic and Nigrostriatal Systems of Compulsive METH-Taking Rats" Cells 14, no. 18: 1472. https://doi.org/10.3390/cells14181472
APA StyleAdjei, N., Ladenheim, B., McCoy, M. T., Palande, V., Cadet, J. L., & Daiwile, A. P. (2025). RNA Sequencing Identified Differentially Expressed Genes in the Mesocorticolimbic and Nigrostriatal Systems of Compulsive METH-Taking Rats. Cells, 14(18), 1472. https://doi.org/10.3390/cells14181472





