Orlistat Confers Neuroprotection in Traumatic Brain Injury by Modulating Microglial Lipid Metabolism
Abstract
1. Introduction
2. Results
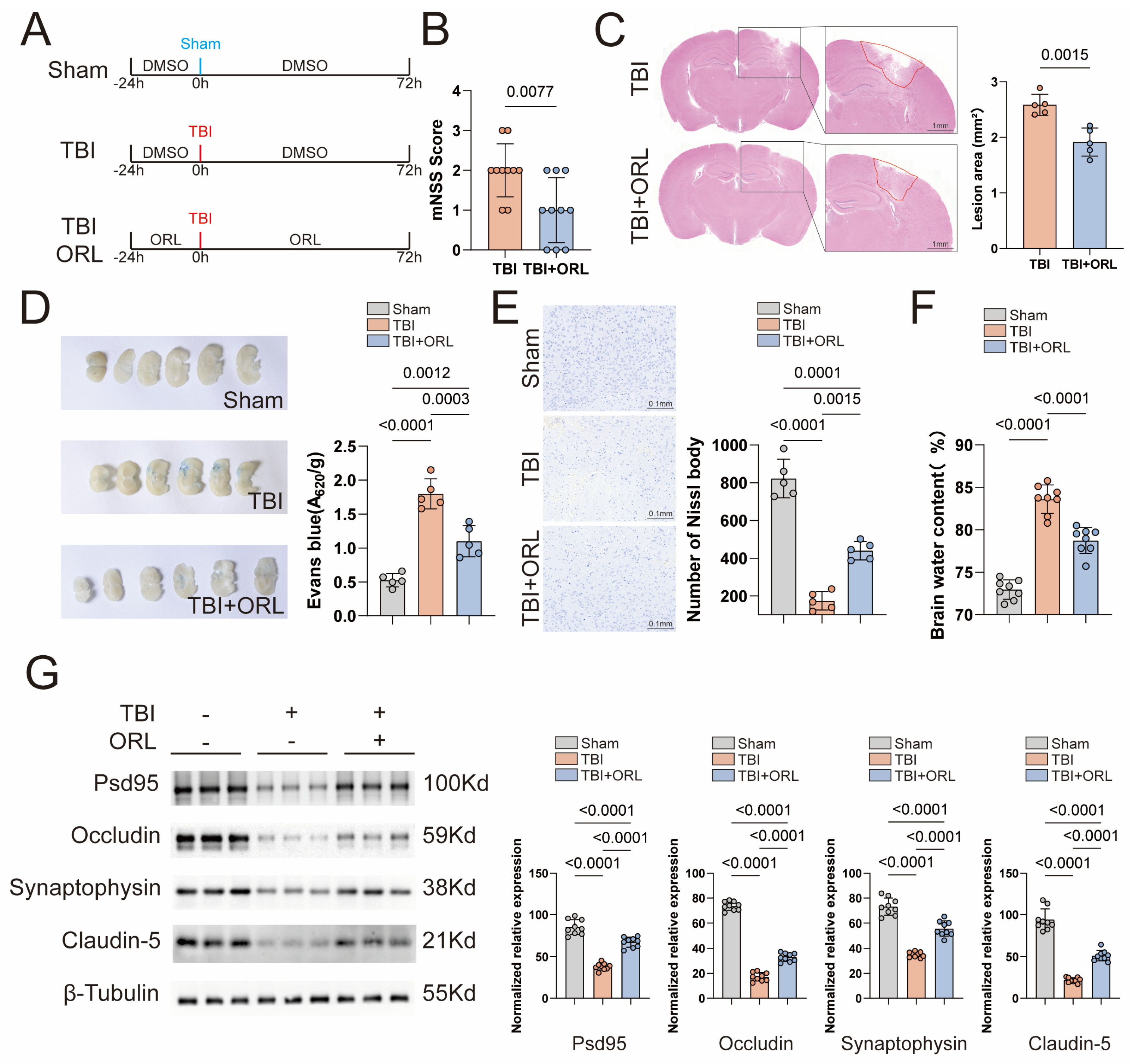
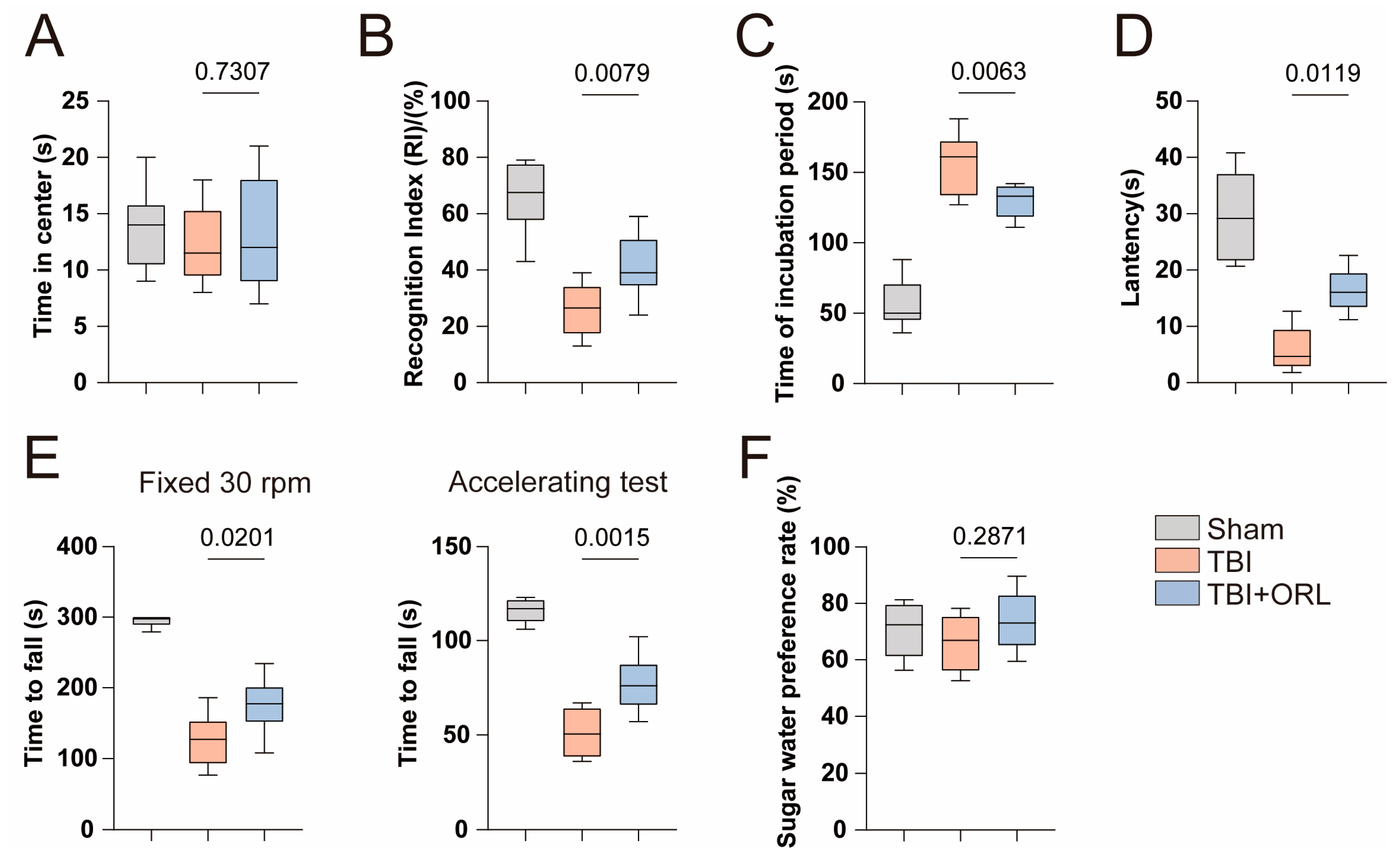
2.1. Orlistat Attenuates Neurological Impairments Following TBI in Mice
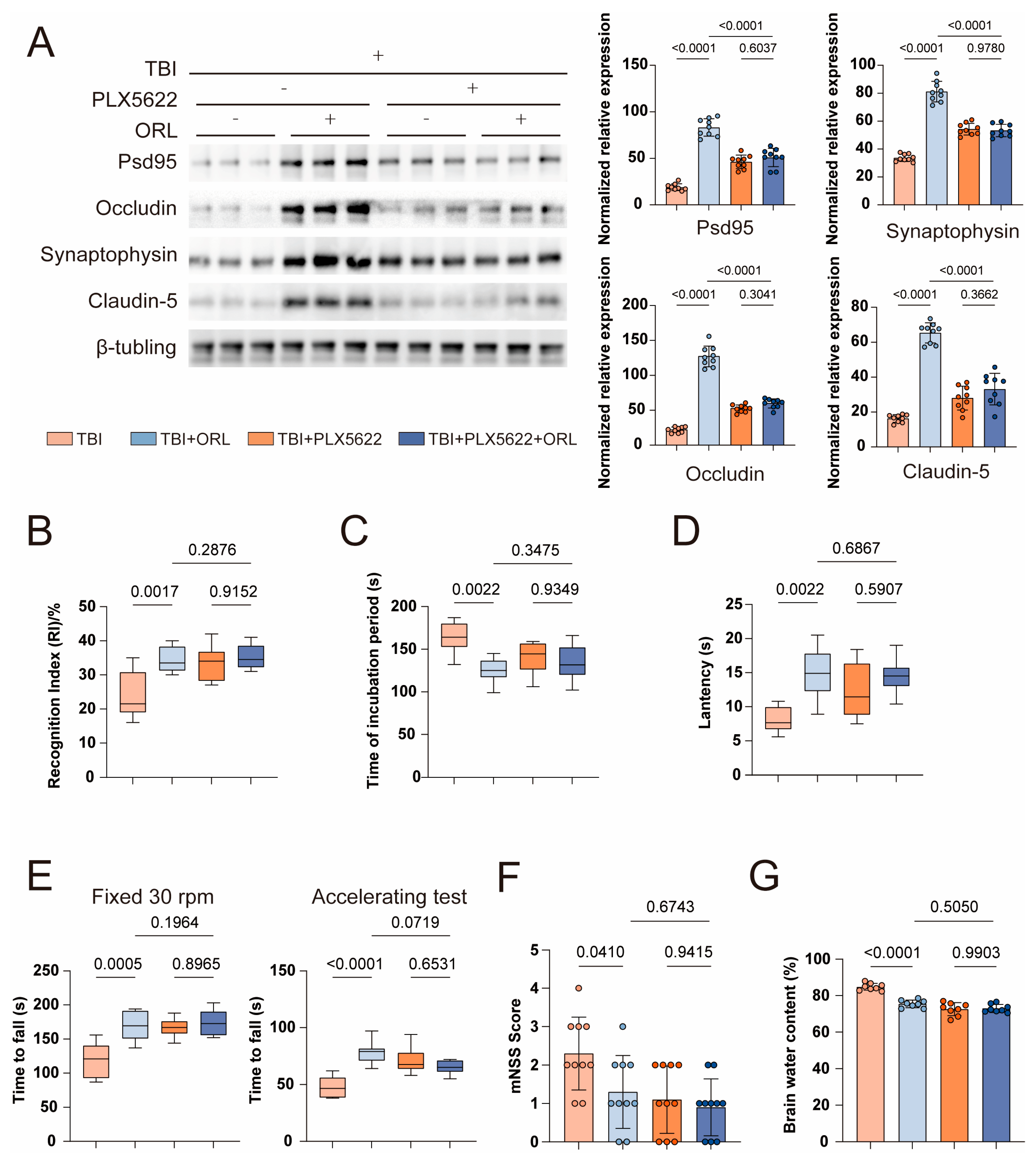
2.2. Microglia-Dependent Mechanisms Underlie Orlistat’s Neuroprotective Effects
2.3. Orlistat Suppresses Microglial Activation and Neuroinflammation Post-TBI
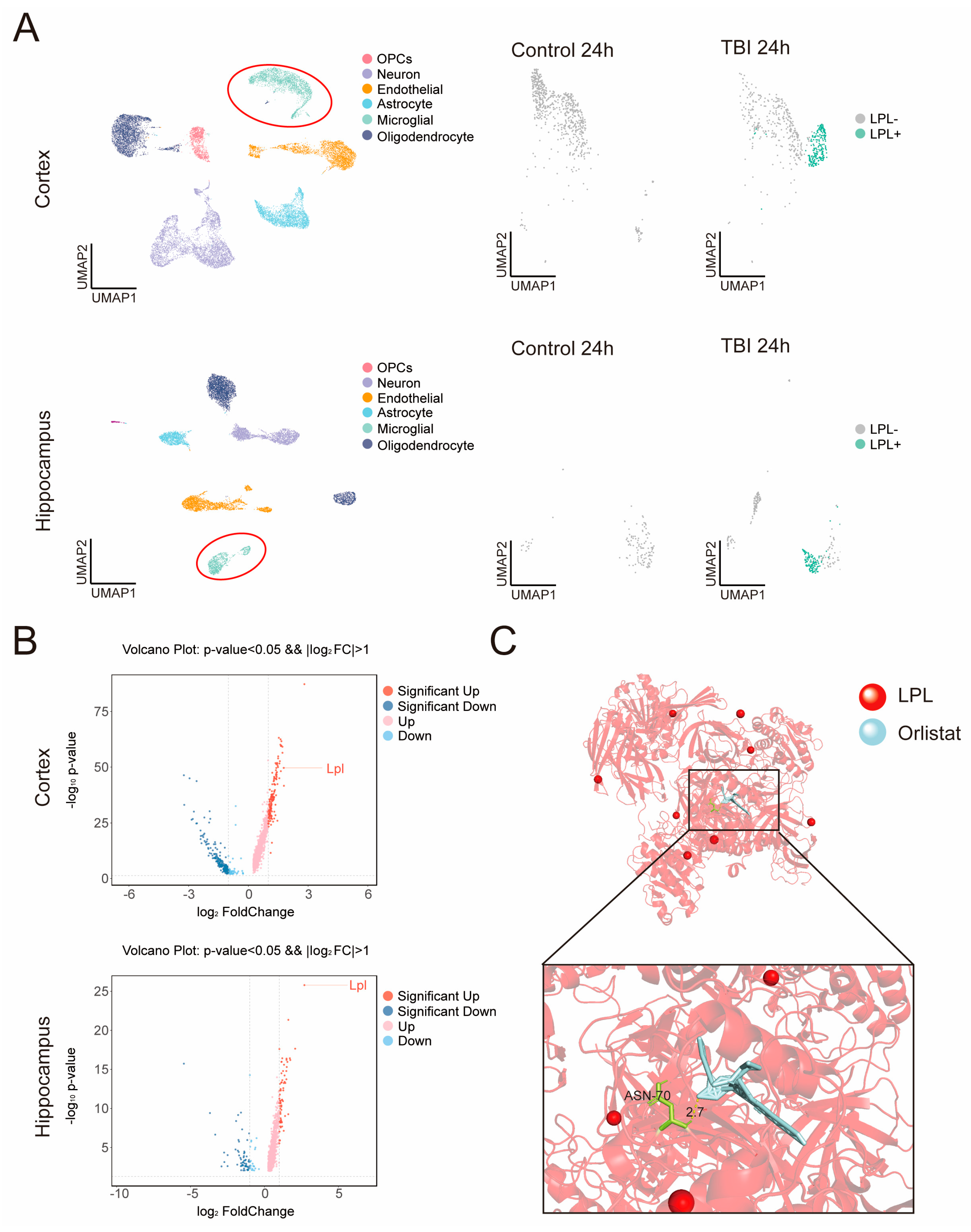
2.4. Lipoprotein Lipase (LPL) Emerges as a Key Target of Orlistat in Microglia
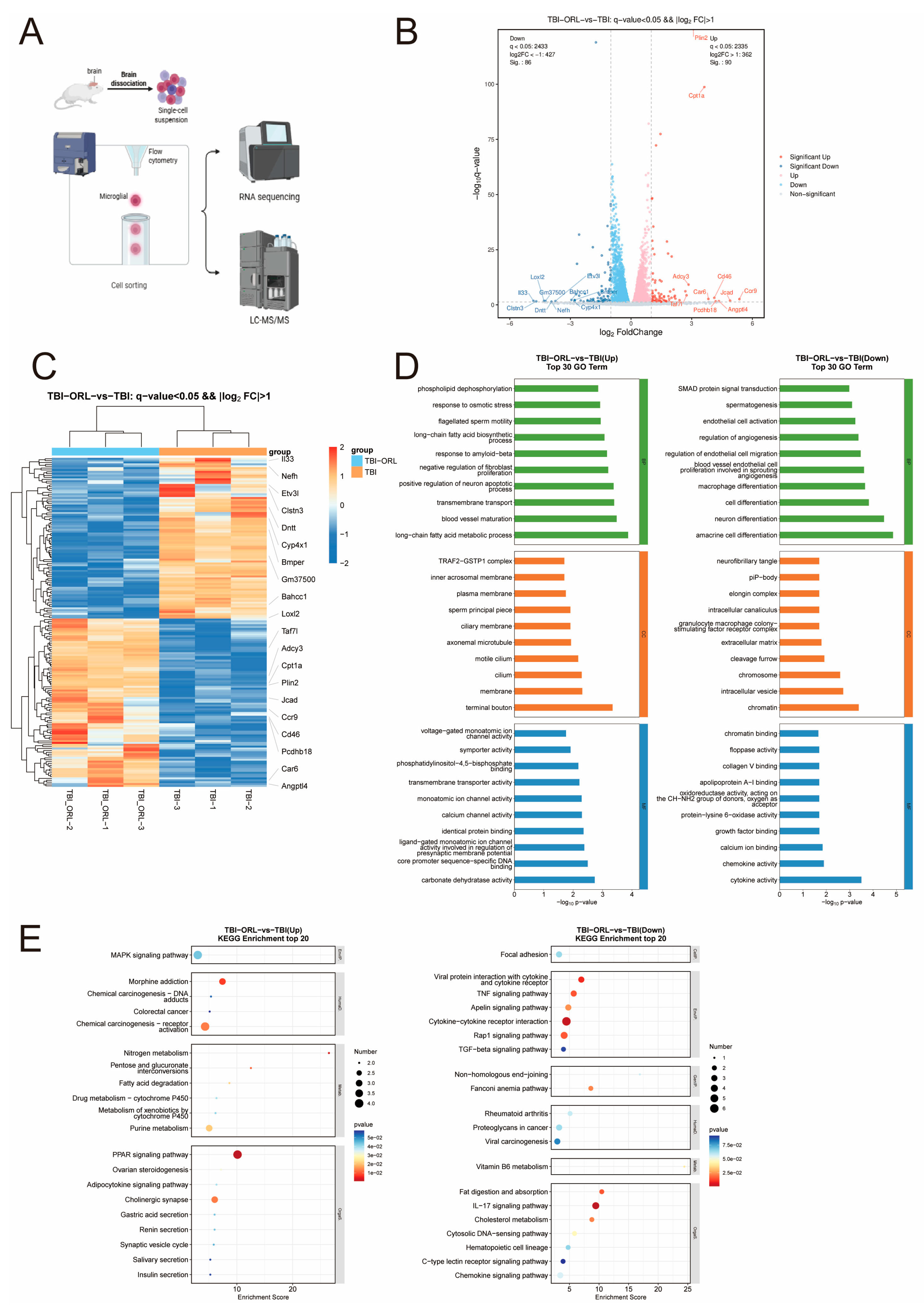
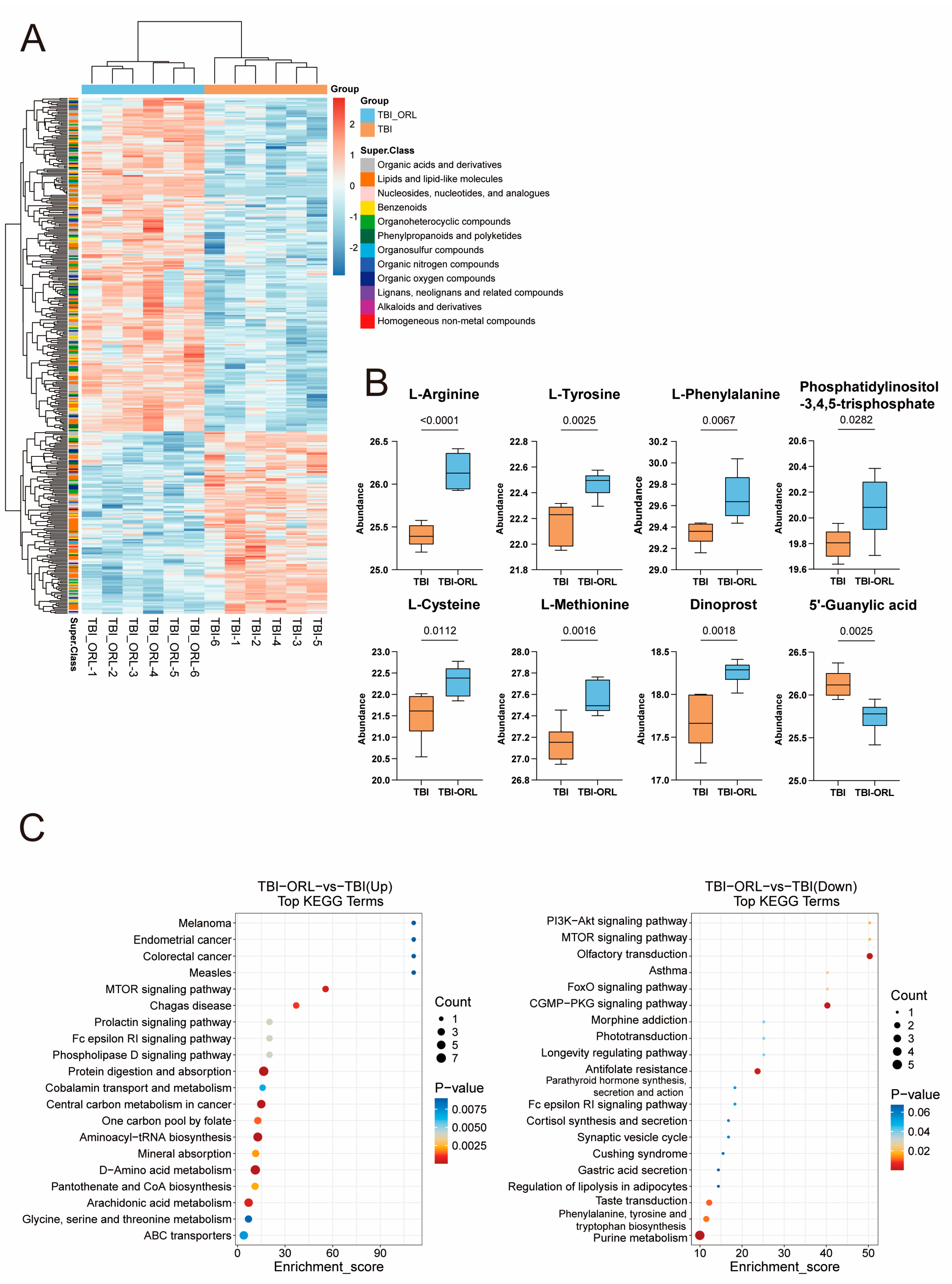
2.5. Transcriptomic and Metabolomic Profiling Reveals Orlistat’s Regulatory Effects on Microglial Function
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals
5.2. Animal Models
5.3. Microglia Clearance
5.4. Animal Sampling
5.5. The mNSS Score
| mNSS Score | |
| Exercise testing | |
| |
| Forelimb flexion | 1 |
| Hindlimb flexion | 1 |
| The head deviates from the vertical axis > 100° within 30 s | 1 |
| |
| Normal walking | 0 |
| Unable to walk in a straight line | 1 |
| Like hemiplegic lateral rotation | 2 |
| Like hemiplegic lateral tilt | 3 |
| Sensory experiment | |
| |
| Can mice avoid obstacles? | 1 |
| |
| Squeezing mouse claws to stimulate limb contraction | 1 |
| Balance beam experiment | |
| Stable balance posture | 0 |
| Grasp the edge of the balance beam tightly | 1 |
| Hold onto the balance beam tightly, with one limb hanging down from the balance beam | 2 |
| Hold onto the balance beam tightly, with both limbs hanging down from the balance beam or rotate on the balance beam > 60 s | 3 |
| Attempted to balance on the balance beam but fell >40 s | 4 |
| Attempted to balance on the balance beam but fell >20 s | 5 |
| Falling, not attempting to balance on the balance beam < 20 s | 6 |
| Loss of reflexes and abnormal movements | |
| Shake your head when in contact with the external auditory canal | 1 |
| Blink when cotton fibers lightly touch the cornea | 1 |
| Has a motion response to the noise of fast-moving cardboard | 1 |
| Epilepsy, myoclonus or dystonia | 1 |
5.6. Determination of Brain Water Content
5.7. Hematoxylin and Eosin (H&E) Staining
5.8. Rotarod Test
5.9. Open-Field Test
5.10. Novel Object Recognition (NOR) Test
5.11. Barnes Maze Test
5.12. Morris Water Maze (MWM)
5.13. Sucrose Preference Test
5.14. ELISA
5.15. Evans Blue Blood–Brain Barrier
5.16. Nissl Body Staining
5.17. Cell Culture
5.18. Transwell Assay
5.19. Flow Cytometry
5.20. RNA Isolation and Library Preparation
5.21. Western Blot
5.22. RT-qPCR
5.23. Immunofluorescence Staining
5.24. RNA Sequencing and Differentially Expressed Genes Analysis
5.25. LC-MS/MS
5.26. Molecular Docking
5.27. Statistical Method
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TBI | Traumatic brain injury |
| ORL | Orlistat |
| LPL | Lipoprotein lipase |
| mNSS | Mahmood’s method neurological severity score |
| BBB | Blood-brain barrier |
| WB | Western blot |
| RT-qPCR | Reverse Transcription Quantitative Polymerase Chain Reaction |
| LPS | Lipopolysaccharide |
| GEO | Gene Expression Omnibus |
| TLR4 | Toll-like receptor 4 |
| NF-κB | Nuclear factor kappa-B |
| NLRP3 | NOD-like receptor thermal protein domain associated protein 3 |
| PPAR | Peroxisome proliferators-activated receptors |
| MAPK | Mitogen-activated protein kinase |
| mTOR | Mammalian target of rapamycin |
| FoxO | Forkhead box O |
| RCT | Randomized controlled trial |
| AD | Alzheimer’s disease |
| CCI | Controlled cortical impact |
| NOR | Novel object recognition |
| MWM | Morris water maze |
| ELISA | Enzyme-linked immunosorbnent assay |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GSEA | Gene Set Enrichment Analysis |
| LC-MS/MS | Liquid Chromatography-Tandem Mass Spectrometry |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
References
- Shao, F.; Wang, X.; Wu, H.; Wu, Q.; Zhang, J. Microglia and Neuroinflammation: Crucial Pathological Mechanisms in Traumatic Brain Injury-Induced Neurodegeneration. Front. Aging Neurosci. 2022, 14, 825086. [Google Scholar] [CrossRef]
- Bolte, A.C.; Lukens, J.R. Neuroimmune cleanup crews in brain injury. Trends Immunol. 2021, 42, 480–494. [Google Scholar] [CrossRef]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef]
- Corps, K.N.; Roth, T.L.; McGavern, D.B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015, 72, 355–362. [Google Scholar] [CrossRef]
- McNeely, W.; Benfield, P. Orlistat. Drugs 1998, 56, 241–249; discussion 250. [Google Scholar] [CrossRef]
- Wolf, S.A.; Boddeke, H.W.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Lattau, S.S.J.; Xin, W.; Pan, Y.; Tatenhorst, L.; Zhang, L.; Graf, I.; Kuang, Y.; Zheng, X.; Hao, Z.; et al. Dynamic Brain Lipid Profiles Modulate Microglial Lipid Droplet Accumulation and Inflammation Under Ischemic Conditions in Mice. Adv. Sci. 2024, 11, e2306863. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Huang, Y.; Liu, Y.; Zhang, X.; Wang, Q.; Liu, Y.; Ma, X.; Long, X.; Ruan, Y.; Lei, H.; et al. Astrocyte-derived exosomal lncRNA 4933431K23Rik modulates microglial phenotype and improves post-traumatic recovery via SMAD7 regulation. Mol. Ther. 2023, 31, 1313–1331. [Google Scholar] [CrossRef] [PubMed]
- Othman, Z.A.; Zakaria, Z.; Suleiman, J.B.; Ghazali, W.S.W.; Mohamed, M. Anti-Atherogenic Effects of Orlistat on Obesity-Induced Vascular Oxidative Stress Rat Model. Antioxidants 2021, 10, 251. [Google Scholar] [CrossRef]
- Xu, Y.; He, Q.; Wang, M.; Wang, X.; Gong, F.; Bai, L.; Zhang, J.; Wang, W. Quantifying blood-brain-barrier leakage using a combination of evans blue and high molecular weight FITC-Dextran. J. Neurosci. Methods 2019, 325, 108349. [Google Scholar] [CrossRef]
- Jamjoom, A.A.B.; Rhodes, J.; Andrews, P.J.D.; Grant, S.G.N. The synapse in traumatic brain injury. Brain 2021, 144, 18–31. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Badimon, A.; Strasburger, H.J.; Ayata, P.; Chen, X.; Nair, A.; Ikegami, A.; Hwang, P.; Chan, A.T.; Graves, S.M.; Uweru, J.O.; et al. Negative feedback control of neuronal activity by microglia. Nature 2020, 586, 417–423. [Google Scholar] [CrossRef]
- Alam, A.; Thelin, E.P.; Tajsic, T.; Khan, D.Z.; Khellaf, A.; Patani, R.; Helmy, A. Cellular infiltration in traumatic brain injury. J. Neuroinflamm. 2020, 17, 328. [Google Scholar] [CrossRef]
- Arneson, D.; Zhang, G.; Ahn, I.S.; Ying, Z.; Diamante, G.; Cely, I.; Palafox-Sanchez, V.; Gomez-Pinilla, F.; Yang, X. Systems spatiotemporal dynamics of traumatic brain injury at single-cell resolution reveals humanin as a therapeutic target. Cell. Mol. Life Sci. 2022, 79, 480. [Google Scholar] [CrossRef]
- Zhang, G.; Diamante, G.; Ahn, I.S.; Palafox-Sanchez, V.; Cheng, J.; Cheng, M.; Ying, Z.; Wang, S.S.; Abuhanna, K.D.; Phi, N.; et al. Thyroid hormone T4 mitigates traumatic brain injury in mice by dynamically remodeling cell type specific genes, pathways, and networks in hippocampus and frontal cortex. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167344. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, G.S.; Tatt, I.; Salanti, G.; Butterworth, A.S.; Sarwar, N.; van Maarle, M.; Jukema, J.W.; Wiman, B.; Kastelein, J.J.; Bennet, A.M.; et al. Seven lipoprotein lipase gene polymorphisms, lipid fractions, and coronary disease: A HuGE association review and meta-analysis. Am. J. Epidemiol. 2008, 168, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Chausse, B.; Kakimoto, P.A.; Kann, O. Microglia and lipids: How metabolism controls brain innate immunity. Semin. Cell Dev. Biol. 2021, 112, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.S.; Palovics, R.; Munson, C.N.; Long, C.; Johansson, P.K.; Yip, O.; Dong, W.; Rawat, E.; West, E.; Schlachetzki, J.C.M.; et al. APOE4/4 is linked to damaging lipid droplets in Alzheimer’s disease microglia. Nature 2024, 628, 154–161. [Google Scholar] [CrossRef]
- Yao, X.; Yang, C.; Jia, X.; Yu, Z.; Wang, C.; Zhao, J.; Chen, Y.; Xie, B.; Zhuang, H.; Sun, C.; et al. High-fat diet consumption promotes adolescent neurobehavioral abnormalities and hippocampal structural alterations via microglial overactivation accompanied by an elevated serum free fatty acid concentration. Brain Behav. Immun. 2024, 119, 236–250. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Terzioglu, G.; Young-Pearse, T.L. Microglial function, INPP5D/SHIP1 signaling, and NLRP3 inflammasome activation: Implications for Alzheimer’s disease. Mol. Neurodegener. 2023, 18, 89. [Google Scholar] [CrossRef]
- Han, X.; Xu, T.; Fang, Q.; Zhang, H.; Yue, L.; Hu, G.; Sun, L. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 2021, 44, 102010. [Google Scholar] [CrossRef]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef]
- Xu, D.; Zhuang, S.; Chen, H.; Jiang, M.; Jiang, P.; Wang, Q.; Wang, X.; Chen, R.; Tang, H.; Tang, L. IL-33 regulates adipogenesis via Wnt/beta-catenin/PPAR-gamma signaling pathway in preadipocytes. J. Transl. Med. 2024, 22, 363. [Google Scholar] [CrossRef]
- Vainchtein, I.D.; Chin, G.; Cho, F.S.; Kelley, K.W.; Miller, J.G.; Chien, E.C.; Liddelow, S.A.; Nguyen, P.T.; Nakao-Inoue, H.; Dorman, L.C.; et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 2018, 359, 1269–1273. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Dorman, L.C.; Pan, S.; Vainchtein, I.D.; Han, R.T.; Nakao-Inoue, H.; Taloma, S.E.; Barron, J.J.; Molofsky, A.B.; Kheirbek, M.A.; et al. Microglial Remodeling of the Extracellular Matrix Promotes Synapse Plasticity. Cell 2020, 182, 388–403.e315. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Xu, H.; Zhang, H.; Tang, R.; Lan, Y.; Xing, R.; Li, S.; Christian, E.; Hou, Y.; Lorello, P.; et al. Disruption of the IL-33-ST2-AKT signaling axis impairs neurodevelopment by inhibiting microglial metabolic adaptation and phagocytic function. Immunity 2022, 55, 159–173.e159. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Dufort, C.; Du, Z.; Shi, R.; Xu, F.; Huang, Z.; Sigler, A.R.; Leak, R.K.; Hu, X. IL-33/ST2 signaling in monocyte-derived macrophages maintains blood-brain barrier integrity and restricts infarctions early after ischemic stroke. J. Neuroinflamm. 2024, 21, 274. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Blackburn, J.K.; Elsworth, J.D. PPARgamma/PGC1alpha signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders. Pharmacol. Ther. 2021, 219, 107705. [Google Scholar] [CrossRef] [PubMed]
- Iroegbu, J.D.; Ijomone, O.K.; Femi-Akinlosotu, O.M.; Ijomone, O.M. ERK/MAPK signalling in the developing brain: Perturbations and consequences. Neurosci. Biobehav. Rev. 2021, 131, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Waisman, A.; Hauptmann, J.; Regen, T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015, 129, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, X.; Li, J.; Hu, B.; Yang, W.; Zhan, M.; Ma, T.; Xu, S. IL-17 crosses the blood-brain barrier to trigger neuroinflammation: A novel mechanism in nitroglycerin-induced chronic migraine. J. Headache Pain. 2022, 23, 1. [Google Scholar] [CrossRef]
- Tcw, J.; Qian, L.; Pipalia, N.H.; Chao, M.J.; Liang, S.A.; Shi, Y.; Jain, B.R.; Bertelsen, S.E.; Kapoor, M.; Marcora, E.; et al. Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell 2022, 185, 2213–2233.e2225. [Google Scholar] [CrossRef] [PubMed]
- Saher, G. Cholesterol Metabolism in Aging and Age-Related Disorders. Annu. Rev. Neurosci. 2023, 46, 59–78. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, S.Y. Long-Term Efficacy and Safety of Anti-Obesity Treatment: Where Do We Stand? Curr. Obes. Rep. 2021, 10, 14–30. [Google Scholar] [CrossRef]
- Foxcroft, D.R.; Milne, R. Orlistat for the treatment of obesity: Rapid review and cost-effectiveness model. Obes. Rev. 2000, 1, 121–126. [Google Scholar] [CrossRef]
- Feng, X.; Lin, Y.; Zhuo, S.; Dong, Z.; Shao, C.; Ye, J.; Zhong, B. Treatment of obesity and metabolic-associated fatty liver disease with a diet or orlistat: A randomized controlled trial. Am. J. Clin. Nutr. 2023, 117, 691–700. [Google Scholar] [CrossRef]
- Torgerson, J.S.; Hauptman, J.; Boldrin, M.N.; Sjostrom, L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004, 27, 155–161. [Google Scholar] [CrossRef]
- Park, Y.J.; Gil, T.Y.; Jin, B.R.; Cha, Y.Y.; An, H.J. Apocynin alleviates weight gain and obesity-induced adipose tissue inflammation in high-fat diet-fed C57BL/6 mice. Phytother. Res. 2023, 37, 3481–3494. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Kumar, A.; Singh, S.M. Myelopoietic efficacy of orlistat in murine hosts bearing T cell lymphoma: Implication in macrophage differentiation and activation. PLoS ONE 2013, 8, e82396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization-new prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef]
- Thal, S.C.; Heinemann, M.; Luh, C.; Pieter, D.; Werner, C.; Engelhard, K. Pioglitazone reduces secondary brain damage after experimental brain trauma by PPAR-gamma-independent mechanisms. J. Neurotrauma 2011, 28, 983–993. [Google Scholar] [CrossRef]
- Wang, H.; Eckel, R.H. Lipoprotein lipase in the brain and nervous system. Annu. Rev. Nutr. 2012, 32, 147–160. [Google Scholar] [CrossRef]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Ni, Y.; Xiong, Y.; Kang, H.; Jiang, Z.; Liu, Y.; Zhang, X.; Liu, Y.; Zhao, K.; Wang, S.; et al. Orlistat Confers Neuroprotection in Traumatic Brain Injury by Modulating Microglial Lipid Metabolism. Cells 2025, 14, 1469. https://doi.org/10.3390/cells14181469
Yu C, Ni Y, Xiong Y, Kang H, Jiang Z, Liu Y, Zhang X, Liu Y, Zhao K, Wang S, et al. Orlistat Confers Neuroprotection in Traumatic Brain Injury by Modulating Microglial Lipid Metabolism. Cells. 2025; 14(18):1469. https://doi.org/10.3390/cells14181469
Chicago/Turabian StyleYu, Chenxuan, Yu Ni, Yuxuan Xiong, Huayu Kang, Zhengqiao Jiang, Yuan Liu, Xincheng Zhang, Yanchao Liu, Kai Zhao, Sheng Wang, and et al. 2025. "Orlistat Confers Neuroprotection in Traumatic Brain Injury by Modulating Microglial Lipid Metabolism" Cells 14, no. 18: 1469. https://doi.org/10.3390/cells14181469
APA StyleYu, C., Ni, Y., Xiong, Y., Kang, H., Jiang, Z., Liu, Y., Zhang, X., Liu, Y., Zhao, K., Wang, S., Gan, C., & Zhang, H. (2025). Orlistat Confers Neuroprotection in Traumatic Brain Injury by Modulating Microglial Lipid Metabolism. Cells, 14(18), 1469. https://doi.org/10.3390/cells14181469






