Wings of Discovery: Using Drosophila to Decode Hereditary Spastic Paraplegia and Ataxias
Abstract
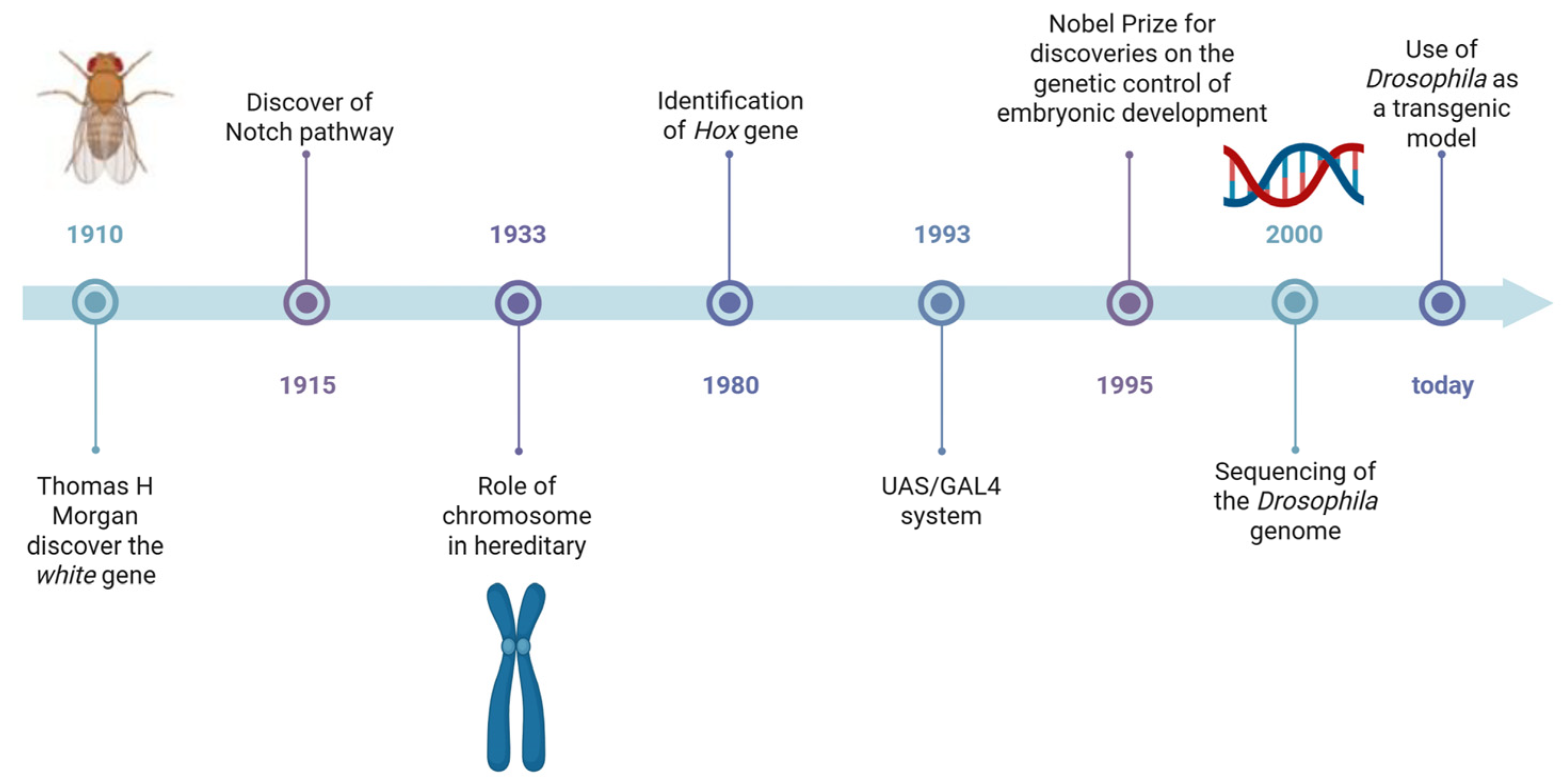
1. Introduction
2. Modelling Diseases in Drosophila
3. Investigating Visual Dysfunction in Drosophila Models of HA and HSP
4. Drosophila in the Study of HSP Disorders
| Human Genes | Protein Function | Identity and Similarity | Drosophila Genes | Type of Model | Behavior | Morphology | References |
|---|---|---|---|---|---|---|---|
| SPG3A/ATL1 | Membrane-anchored dynamin-like GTPase mediating GTP-dependent fusion of ER membranes; essential for maintaining a continuous tubular ER network and axonal homeostasis | 52–57% and 71–77% | atlastin | Loss of function | reduce climbing performance | muscular ER fragmentation at the NMJs, and progressive degeneration of dopaminergic neurons | [45,46,47,48] |
| SPG4/SPAST | ATP-dependent microtubule severing enzyme that preferentially cuts polyglutamylated microtubules; regulates axonal microtubule dynamics and transport | 44% and 55% | spas | Loss of function and gain of function | adults have severe movement defects, cannot fly, and have weak legs | larvae have altered NMJs in which presynaptic boutons are more numerous and smaller than in wild type | [36,49,50,51,52] |
| SPG7 | Catalytic component of the m-AAA protease, a protease that plays a key role in proteostasis of inner mitochondrial membrane proteins, and which is essential for axonal and neuron development | 58% and 75% | spg7 | Loss of function | shortened lifespan, climbing or flight defects, sensitivity to stressors | photoreceptor synaptic terminal disorganized, degeneration of indirect flight muscle and mitochondrial trafficking defects | [32] |
| SPG10/KIF5A | Microtubule-dependent motor required for slow axonal transport of neurofilament proteins | 60% and 76% | khc | Gain of function | complete paralysis, reduced lifespan, no stable flight | axons innervating posterior segments are considerably longer | [53,54,55] |
| SPG11 | play a role in neurite plasticity by maintaining cytoskeleton stability and regulating synaptic vesicle transport | spg11 | Loss of function | locomotor deficit | autophagosome accumulation, enlarged lysosomes, reduced free lysosomes, autophagic reformation defects | [43] | |
| SPG12/RTN2 | ER-shaping protein of the reticulon family; regulates curvature of ER tubules and ER network homeostasis | 30% and 46% | rtnl1/rtnl2 | Loss of function | locomotion impairment | ER stress response, abnormalities of ER marker, MT cytoskeleton and mitochondria | [43,44,56] |
| SPG15/ZFYVE26 | Phosphatidylinositol 3-phosphate-binding protein required for the abscission step in cytokinesis: recruited to the midbody during cytokinesis and acts as a regulator of abscission. May also be required for efficient homologous recombination DNA double-strand break repair | 30% and 50% | sptz | Loss of function | locomotor deficit | autophagosome accumulation, enlarged lysosomes, reduced free lysosomes, autophagic reformation defects | [57] |
| SPG17/BSCL2 | Plays a crucial role in the formation of lipid droplets (LDs) which are storage organelles at the center of lipid and energy homeostasis | 31% and 51% | seipin | Loss of function | reduced locomotor activity | mild triacylglycerol storage phenotype | [58] |
| SPG20/SPART | Lipophagy receptor that plays an important role in lipid droplet (LD) turnover in motor neurons | 25% and 40% | spartin | Loss of function | locomotion impairment | neurodegeneration, progressive vacuolization in adult brain, reduced neurotransmitter release | [59] |
| SPG30/KIF1A | Kinesin motor with a plus-end-directed microtubule motor activity | - | no orthologue | Loss of function | embryos are paralyzed and fail to hatch | nerve outgrowth fails, synaptic bouton defects, loss of SVs and AZs at NMJ | [60,61] |
| SPG31/REEP1 | ER membrane protein linking ER tubules to microtubules; required for ER shaping, remodeling, and axonal maintenance | 50% and 70% | reepA | Loss of function | locomotor dysfunction, shortened lifespan | expansion of ER sheet-like structures | [62,63] |
| SPG35/FA2H | Catalyzes the hydroxylation of free fatty acids at the C-2 position to produce 2-hydroxy fatty acids, which are building blocks of sphingolipids and glycosphingolipids common in neural tissue and epidermis | 60% and 75% | fa2h | Loss of function | flying disability, behavioral abnormalities | mitochondrial dynamics and autophagy alterations | [64] |
| SPG39/PNPLA6 | Catalyzes the hydrolysis of several naturally occurring membrane-associated lipids | 45% and 65% | sws | Loss of function | progressive behavioral defects | neurodegeneration with vacuole formation | [65,66,67] |
| SPG61/ARL6IP1 | Positively regulates SLC1A1/EAAC1-mediated glutamate transport by increasing its affinity for glutamate in a PKC activity-dependent manner | 29% and 61% | arl6IP1 | Loss of function | significant locomotor deficit | ER and mitochondrial disorganization, disrupted lipid droplets | [68,69] |
| SPG76/CAPN1 | Calcium-regulated non-lysosomal thiol-protease which catalyzes limited proteolysis of substrates involved in cytoskeletal remodeling and signal transduction | 45% and 64% | calpA/caplB | Loss of function | locomotor defects | axonal abnormalities | [70] |
| SPG77/FARS2 | Is responsible for the charging of tRNA (Phe) with phenylalanine in mitochondrial translation | 65% AND 80% | pheRS-m | knockout | developmental delay, seizures | mitochondrial tRNAphe and oxidative phosphorylation defects | [71,72] |
| SPG78/ATP13A2 | ATPase which acts as a lysosomal polyamine exporter with high affinity for spermine | - | no orthologue | knockdown | [73] | ||
| SPG92/FICD | Protein that can both mediate the addition of adenosine 5’-monophosphate (AMP) to specific residues of target proteins (AMPylation), and the removal of the same modification from target proteins (de-AMPylation), depending on the context | 35% and 60% | fic | knockout | [74] |
5. Genetic Modelling of HA in Drosophila
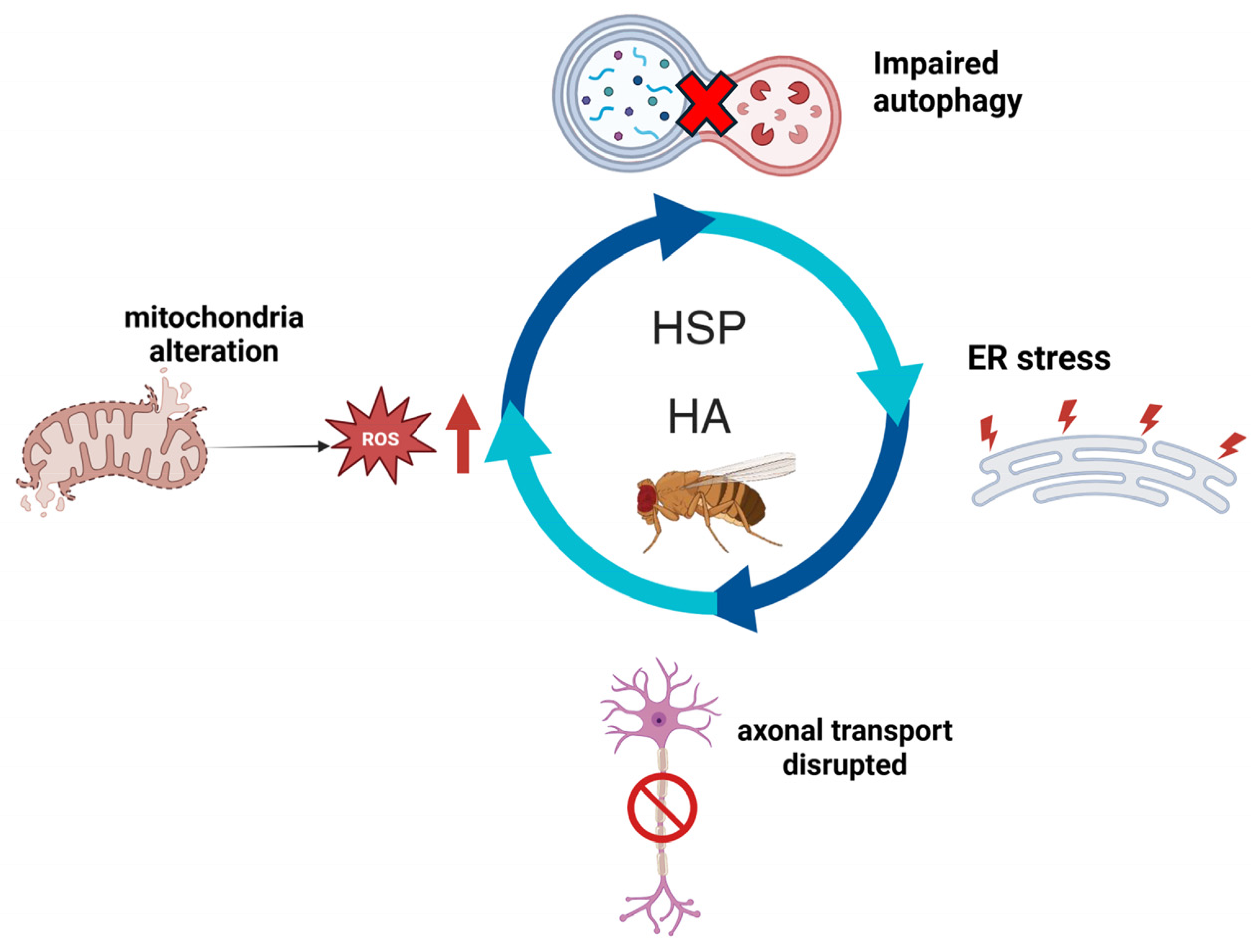
6. Commonalities and Differences
7. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lo Giudice, T.; Lombardi, F.; Santorelli, F.M.; Kawarai, T.; Orlacchio, A. Hereditary spastic paraplegia: Clinical-genetic characteristics and evolving molecular mechanisms. Exp. Neurol. 2014, 261, 518–539. [Google Scholar] [CrossRef]
- Boutry, M.; Morais, S.; Stevanin, G. Update on the Genetics of Spastic Paraplegias. Curr. Neurol. Neurosci. Rep. 2019, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Deluca, G.C.; Ebers, G.C.; Esiri, M.M. The extent of axonal loss in the long tracts in hereditary spastic paraplegia. Neuropathol. Appl. Neurobiol. 2004, 30, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Hensiek, A.; Kirker, S.; Reid, E. Diagnosis, investigation and management of hereditary spastic paraplegias in the era of next-generation sequencing. J. Neurol. 2015, 262, 1601–1612. [Google Scholar] [CrossRef]
- Panza, E.; Meyyazhagan, A.; Orlacchio, A. Hereditary spastic paraplegia: Genetic heterogeneity and common pathways. Exp. Neurol. 2022, 357, 114203. [Google Scholar] [CrossRef]
- Elsayed, L.E.O.; Eltazi, I.Z.; Ahmed, A.E.; Stevanin, G. Insights into Clinical, Genetic, and Pathological Aspects of Hereditary Spastic Paraplegias: A Comprehensive Overview. Front. Mol. Biosci. 2021, 8, 690899. [Google Scholar] [CrossRef]
- Naef, V.; Mero, S.; Fichi, G.; D’Amore, A.; Ogi, A.; Gemignani, F.; Santorelli, F.M.; Marchese, M. Swimming in Deep Water: Zebrafish Modeling of Complicated Forms of Hereditary Spastic Paraplegia and Spastic Ataxia. Front. Neurosci. 2019, 13, 1311. [Google Scholar] [CrossRef] [PubMed]
- Synofzik, M.; Schüle, R. Overcoming the divide between ataxias and spastic paraplegias: Shared phenotypes, genes, and pathways. Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 332–345. [Google Scholar] [CrossRef]
- Pilotto, F.; Del Bondio, A.; Puccio, H. Hereditary Ataxias: From Bench to Clinic, Where Do We Stand? Cells 2024, 13, 319. [Google Scholar] [CrossRef]
- Finsterer, J. Ataxias with autosomal, X-chromosomal or maternal inheritance. The Canadian journal of neurological sciences. Le J. Can. Des Sci. Neurol. 2009, 36, 409–428. [Google Scholar] [CrossRef]
- Embiruçu, E.K.; Martyn, M.L.; Schlesinger, D.; Kok, F. Autosomal recessive ataxias: 20 types and counting. Arq. De Neuro-Psiquiatr. 2009, 67, 1143–1156. [Google Scholar] [CrossRef]
- Bustamante-Barrientos, F.A.; Luque-Campos, N.; Araya, M.J.; Lara-Barba, E.; de Solminihac, J.; Pradenas, C.; Molina, L.; Herrera-Luna, Y.; Utreras-Mendoza, Y.; Elizondo-Vega, R.; et al. Mitochondrial dysfunction in neurodegenerative disorders: Potential therapeutic application of mitochondrial transfer to central nervous system-residing cells. J. Transl. Med. 2023, 21, 613. [Google Scholar] [CrossRef]
- Oriel, C.; Lasko, P. Recent Developments in Using Drosophila as a Model for Human Genetic Disease. Int. J. Mol. Sci. 2018, 19, 2041. [Google Scholar] [CrossRef]
- Hales, K.G.; Korey, C.A.; Larracuente, A.M.; Roberts, D.M. Genetics on the Fly: A Primer on the Drosophila Model System. Genetics 2015, 201, 815–842. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yoshida, H. Drosophila as a Model Organism. Adv. Exp. Med. Biol. 2018, 1076, 1–10. [Google Scholar] [CrossRef]
- Ugur, B.; Chen, K.; Bellen, H.J. Drosophila tools and assays for the study of human diseases. Dis. Models Mech. 2016, 9, 235–244. [Google Scholar] [CrossRef]
- Lin, S.C.; Chang, Y.Y.; Chan, C.C. Strategies for gene disruption in Drosophila. Cell Biosci. 2014, 4, 63. [Google Scholar] [CrossRef]
- Trotta, N.; Orso, G.; Rossetto, M.G.; Daga, A.; Broadie, K. The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Curr. Biol. CB 2004, 14, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Sujkowski, A.; Ranxhi, B.; Bangash, Z.R.; Chbihi, Z.M.; Prifti, M.V.; Qadri, Z.; Alam, N.; Todi, S.V.; Tsou, W.L. Progressive degeneration in a new Drosophila model of spinocerebellar ataxia type 7. Sci. Rep. 2024, 14, 14332. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Martin, I.; Bhandari, P.; Grotewiel, M.S. Rapid iterative negative geotaxis (RING): A new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 2005, 40, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Post, Y.; Paululat, A. Muscle Function Assessment Using a Drosophila Larvae Crawling Assay. Bio-Protoc. 2018, 8, e2933. [Google Scholar] [CrossRef]
- Imlach, W.; McCabe, B.D. Electrophysiological methods for recording synaptic potentials from the NMJ of Drosophila larvae. J. Vis. Exp. JoVE 2009, 24, 1109. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.; Johnson, T.K.; Xie, W. Immunohistochemical Analysis of the Drosophila Larval Neuromuscular Junction. Methods Mol. Biol. 2024, 2746, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef]
- Jackson, S.M.; Whitworth, A.J.; Greene, J.C.; Libby, R.T.; Baccam, S.L.; Pallanck, L.J.; La Spada, A.R. A SCA7 CAG/CTG repeat expansion is stable in Drosophila melanogaster despite modulation of genomic context and gene dosage. Gene 2005, 347, 35–41. [Google Scholar] [CrossRef]
- Rodden, L.N.; McIntyre, K.; Keita, M.; Wells, M.; Park, C.; Profeta, V.; Waldman, A.; Rummey, C.; Balcer, L.J.; Lynch, D.R. Retinal hypoplasia and degeneration result in vision loss in Friedreich ataxia. Ann. Clin. Transl. Neurol. 2023, 10, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Paulk, A.; Millard, S.S.; van Swinderen, B. Vision in Drosophila: Seeing the world through a model’s eyes. Annu. Rev. Entomol. 2013, 58, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Prifti, M.V.; Nuga, O.; Dulay, R.O.; Patel, N.C.; Kula, T.; Libohova, K.; Jackson-Butler, A.; Tsou, W.L.; Richardson, K.; Todi, S.V. Insights into dentatorubral-pallidoluysian atrophy from a new Drosophila model of disease. Neurobiol. Dis. 2025, 207, 106834. [Google Scholar] [CrossRef]
- Sutton, J.R.; Blount, J.R.; Libohova, K.; Tsou, W.L.; Joshi, G.S.; Paulson, H.L.; Costa, M.D.C.; Scaglione, K.M.; Todi, S.V. Interaction of the polyglutamine protein ataxin-3 with Rad23 regulates toxicity in Drosophila models of Spinocerebellar Ataxia Type 3. Hum. Mol. Genet. 2017, 26, 1419–1431. [Google Scholar] [CrossRef]
- Ishiguro, T.; Sato, N.; Ueyama, M.; Fujikake, N.; Sellier, C.; Kanegami, A.; Tokuda, E.; Zamiri, B.; Gall-Duncan, T.; Mirceta, M.; et al. Regulatory Role of RNA Chaperone TDP-43 for RNA Misfolding and Repeat-Associated Translation in SCA31. Neuron 2017, 94, 108–124.e7. [Google Scholar] [CrossRef]
- Khare, S.; Nick, J.A.; Zhang, Y.; Galeano, K.; Butler, B.; Khoshbouei, H.; Rayaprolu, S.; Hathorn, T.; Ranum, L.P.W.; Smithson, L.; et al. A KCNC3 mutation causes a neurodevelopmental, non-progressive SCA13 subtype associated with dominant negative effects and aberrant EGFR trafficking. PLoS ONE 2017, 12, e0173565. [Google Scholar] [CrossRef]
- Pareek, G.; Thomas, R.E.; Pallanck, L.J. Loss of the Drosophila m-AAA mitochondrial protease paraplegin results in mitochondrial dysfunction, shortened lifespan, and neuronal and muscular degeneration. Cell Death Dis. 2018, 9, 304. [Google Scholar] [CrossRef]
- Ozdowski, E.F.; Gayle, S.; Bao, H.; Zhang, B.; Sherwood, N.T. Loss of Drosophila melanogaster p21-activated kinase 3 suppresses defects in synapse structure and function caused by spastin mutations. Genetics 2011, 189, 123–135. [Google Scholar] [CrossRef]
- Awuah, W.A.; Tan, J.K.; Shkodina, A.D.; Ferreira, T.; Adebusoye, F.T.; Mazzoleni, A.; Wellington, J.; David, L.; Chilcott, E.; Huang, H.; et al. Hereditary spastic paraplegia: Novel insights into the pathogenesis and management. SAGE Open Med. 2023, 12, 20503121231221941. [Google Scholar] [CrossRef]
- Solowska, J.M.; Baas, P.W. Hereditary spastic paraplegia SPG4: What is known and not known about the disease. Brain A J. Neurol. 2015, 138 Pt 9, 2471–2484. [Google Scholar] [CrossRef]
- Orso, G.; Martinuzzi, A.; Rossetto, M.G.; Sartori, E.; Feany, M.; Daga, A. Disease-related phenotypes in a Drosophila model of hereditary spastic paraplegia are ameliorated by treatment with vinblastine. J. Clin. Investig. 2005, 115, 3026–3034. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Ozdowski, E.F.; Kotowski, I.K.; Marchuk, D.A.; Sherwood, N.T. Functional conservation of human Spastin in a Drosophila model of autosomal dominant-hereditary spastic paraplegia. Hum. Mol. Genet. 2010, 19, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Zhu, P.P.; Parker, R.L.; Blackstone, C. Hereditary spastic paraplegia proteins REEP1, spastin, and atlastin-1 coordinate microtubule interactions with the tubular ER network. J. Clin. Investig. 2010, 120, 1097–1110. [Google Scholar] [CrossRef]
- Orso, G.; Pendin, D.; Liu, S.; Tosetto, J.; Moss, T.J.; Faust, J.E.; Micaroni, M.; Egorova, A.; Martinuzzi, A.; McNew, J.A.; et al. Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature 2009, 460, 978–983. [Google Scholar] [CrossRef]
- De Gregorio, C.; Delgado, R.; Ibacache, A.; Sierralta, J.; Couve, A. Drosophila Atlastin in motor neurons is required for locomotion and presynaptic function. J. Cell Sci. 2017, 130, 3507–3516. [Google Scholar] [CrossRef] [PubMed]
- Casari, G.; Marconi, R.; Adam, M.P.; Feldman, J.; Mirzaa, G.M.; Pagon, R.A.; Wallace, S.E. Amemiya A Spastic Paraplegia 7. In GeneReviews®; University of Washington: Seattle, WA, USA, 2006. [Google Scholar]
- Vantaggiato, C.; Guarato, G.; Brivio, F.; Panzeri, E.; Speltoni, B.; Gumeni, S.; Orso, G.; Santorelli, F.M.; Bassi, M.T. Naringenin and SMER28 target lysosomal reformation and rescue SPG11 and SPG15 hereditary spastic paraplegia phenotypes. Pharmacol. Res. 2025, 218, 107836. [Google Scholar] [CrossRef]
- Espadas, J.; Pendin, D.; Bocanegra, R.; Escalada, A.; Misticoni, G.; Trevisan, T.; Velasco Del Olmo, A.; Montagna, A.; Bova, S.; Ibarra, B.; et al. Dynamic constriction and fission of endoplasmic reticulum membranes by reticulon. Nat. Commun. 2019, 10, 5327. [Google Scholar] [CrossRef]
- Pérez-Moreno, J.J.; Smith, R.C.; Oliva, M.K.; Gallo, F.; Ojha, S.; Müller, K.H.; O’Kane, C.J. Drosophila SPG12 ortholog, reticulon-like 1, governs presynaptic ER organization and Ca2+ dynamics. J. Cell Biol. 2023, 222, e202112101. [Google Scholar] [CrossRef] [PubMed]
- Candia, N.; Ibacache, A.; Medina-Yáñez, I.; Olivares, G.H.; Ramírez, M.; Vega-Macaya, F.; Couve, A.; Sierralta, J.; Olguín, P. Identification of atlastin genetic modifiers in a model of hereditary spastic paraplegia in Drosophila. Hum. Genet. 2023, 142, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Paik, D.; Bang, S.; Kang, J.; Chun, B.; Lee, S.; Bae, E.; Chung, J.; Kim, J. Loss of spastic paraplegia gene atlastin induces age-dependent death of dopaminergic neurons in Drosophila. Neurobiol. Aging 2008, 29, 84–94. [Google Scholar] [CrossRef]
- Xu, S.; Stern, M.; McNew, J.A. Beneficial effects of rapamycin in a Drosophila model for hereditary spastic paraplegia. J. Cell Sci. 2017, 130, 453–465. [Google Scholar] [CrossRef]
- Montagna, A.; Vajente, N.; Pendin, D.; Daga, A. In vivo Analysis of CRISPR/Cas9 Induced Atlastin Pathological Mutations in Drosophila. Front. Neurosci. 2020, 14, 547746. [Google Scholar] [CrossRef]
- Sardina, F.; Carsetti, C.; Giorgini, L.; Fattorini, G.; Cestra, G.; Rinaldo, C. Cul-4 inhibition rescues spastin levels and reduces defects in hereditary spastic paraplegia models. Brain: A J. Neurol. 2024, 147, 3534–3546. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, N.T.; Sun, Q.; Xue, M.; Zhang, B.; Zinn, K. Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS Biol. 2004, 2, e429. [Google Scholar] [CrossRef]
- Rao, K.; Stone, M.C.; Weiner, A.T.; Gheres, K.W.; Zhou, C.; Deitcher, D.L.; Levitan, E.S.; Rolls, M.M. Spastin, atlastin, and ER relocalization are involved in axon but not dendrite regeneration. Mol. Biol. Cell 2016, 27, 3245–3256. [Google Scholar] [CrossRef]
- Baxter, S.L.; Allard, D.E.; Crowl, C.; Sherwood, N.T. Cold temperature improves mobility and survival in Drosophila models of autosomal-dominant hereditary spastic paraplegia (AD-HSP). Dis. Models Mech. 2014, 7, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Soustelle, L.; Aimond, F.; López-Andrés, C.; Brugioti, V.; Raoul, C.; Layalle, S. ALS-Associated KIF5A Mutation Causes Locomotor Deficits Associated with Cytoplasmic Inclusions, Alterations of Neuromuscular Junctions, and Motor Neuron Loss. J. Neurosci. Off. J. Soc. Neurosci. 2023, 43, 8058–8072. [Google Scholar] [CrossRef]
- Füger, P.; Sreekumar, V.; Schüle, R.; Kern, J.V.; Stanchev, D.T.; Schneider, C.D.; Karle, K.N.; Daub, K.J.; Siegert, V.K.; Flötenmeyer, M.; et al. Spastic paraplegia mutation N256S in the neuronal microtubule motor KIF5A disrupts axonal transport in a Drosophila HSP model. PLoS Genet. 2012, 8, e1003066. [Google Scholar] [CrossRef]
- Djagaeva, I.; Rose, D.J.; Lim, A.; Venter, C.E.; Brendza, K.M.; Moua, P.; Saxton, W.M. Three routes to suppression of the neurodegenerative phenotypes caused by kinesin heavy chain mutations. Genetics 2012, 192, 173–183. [Google Scholar] [CrossRef]
- O’Sullivan, N.C.; Jahn, T.R.; Reid, E.; O’Kane, C.J. Reticulon-like-1, the Drosophila orthologue of the hereditary spastic paraplegia gene reticulon 2, is required for organization of endoplasmic reticulum and of distal motor axons. Hum. Mol. Genet. 2012, 21, 3356–3365. [Google Scholar] [CrossRef]
- Vantaggiato, C.; Orso, G.; Guarato, G.; Brivio, F.; Napoli, B.; Panzeri, E.; Masotti, S.; Santorelli, F.M.; Lamprou, M.; Gumeni, S.; et al. Rescue of lysosomal function as therapeutic strategy for SPG15 hereditary spastic paraplegia. Brain A J. Neurol. 2023, 146, 1103–1120. [Google Scholar] [CrossRef]
- Tian, Y.; Bi, J.; Shui, G.; Liu, Z.; Xiang, Y.; Liu, Y.; Wenk, M.R.; Yang, H.; Huang, X. Tissue-autonomous function of Drosophila seipin in preventing ectopic lipid droplet formation. PLoS Genet. 2011, 7, e1001364. [Google Scholar] [CrossRef] [PubMed]
- Nahm, M.; Lee, M.J.; Parkinson, W.; Lee, M.; Kim, H.; Kim, Y.J.; Kim, S.; Cho, Y.S.; Min, B.M.; Bae, Y.C.; et al. Spartin regulates synaptic growth and neuronal survival by inhibiting BMP-mediated microtubule stabilization. Neuron 2013, 77, 680–695. [Google Scholar] [CrossRef]
- Zhang, Y.V.; Hannan, S.B.; Stapper, Z.A.; Kern, J.V.; Jahn, T.R.; Rasse, T.M. The Drosophila KIF1A Homolog unc-104 Is Important for Site-Specific Synapse Maturation. Front. Cell. Neurosci. 2016, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hannan, S.; Kern, J.; Stanchev, D.T.; Koç, B.; Jahn, T.R.; Rasse, T.M. The KIF1A homolog Unc-104 is important for spontaneous release, postsynaptic density maturation and perisynaptic scaffold organization. Sci. Rep. 2017, 7, 38172. [Google Scholar] [CrossRef]
- Napoli, B.; Gumeni, S.; Forgiarini, A.; Fantin, M.; De Filippis, C.; Panzeri, E.; Vantaggiato, C.; Orso, G. Naringenin Ameliorates Drosophila ReepA Hereditary Spastic Paraplegia-Linked Phenotypes. Front. Neurosci. 2019, 13, 1202. [Google Scholar] [CrossRef]
- Oliva, M.K.; Pérez-Moreno, J.J.; O’Shaughnessy, J.; Wardill, T.J.; O’Kane, C.J. Endoplasmic Reticulum Lumenal Indicators in Drosophila Reveal Effects of HSP-Related Mutations on Endoplasmic Reticulum Calcium Dynamics. Front. Neurosci. 2020, 14, 816. [Google Scholar] [CrossRef]
- Mandik, F.; Kanana, Y.; Rody, J.; Misera, S.; Wilken, B.; Laabs von Holt, B.H.; Klein, C.; Vos, M. A new model for fatty acid hydroxylase-associated neurodegeneration reveals mitochondrial and autophagy abnormalities. Front. Cell Dev. Biol. 2022, 10, 1000553. [Google Scholar] [CrossRef]
- Sujkowski, A.; Rainier, S.; Fink, J.K.; Wessells, R.J. Delayed Induction of Human NTE (PNPLA6) Rescues Neurodegeneration and Mobility Defects of Drosophila swiss cheese (sws) Mutants. PLoS ONE 2015, 10, e0145356. [Google Scholar] [CrossRef]
- Sunderhaus, E.R.; Law, A.D.; Kretzschmar, D. Disease-Associated PNPLA6 Mutations Maintain Partial Functions When Analyzed in Drosophila. Front. Neurosci. 2019, 13, 1207. [Google Scholar] [CrossRef]
- Melentev, P.A.; Ryabova, E.V.; Surina, N.V.; Zhmujdina, D.R.; Komissarov, A.E.; Ivanova, E.A.; Boltneva, N.P.; Makhaeva, G.F.; Sliusarenko, M.I.; Yatsenko, A.S.; et al. Loss of swiss cheese in Neurons Contributes to Neurodegeneration with Mitochondria Abnormalities, Reactive Oxygen Species Acceleration and Accumulation of Lipid Droplets in Drosophila Brain. Int. J. Mol. Sci. 2021, 22, 8275. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.J.; Garcia-Pardo, M.E.; Cole, N.B.; Batnasan, B.; Heneghan, S.; Sohail, A.; Blackstone, C.; O’Sullivan, N.C. Liver X receptor-agonist treatment rescues degeneration in a Drosophila model of hereditary spastic paraplegia. Acta Neuropathol. Commun. 2022, 10, 40. [Google Scholar] [CrossRef]
- Fowler, P.C.; Byrne, D.J.; Blackstone, C.; O’Sullivan, N.C. Loss of the Mitochondrial Fission GTPase Drp1 Contributes to Neurodegeneration in a Drosophila Model of Hereditary Spastic Paraplegia. Brain Sci. 2020, 10, 646. [Google Scholar] [CrossRef]
- Gan-Or, Z.; Bouslam, N.; Birouk, N.; Lissouba, A.; Chambers, D.B.; Vérièpe, J.; Androschuk, A.; Laurent, S.B.; Rochefort, D.; Spiegelman, D.; et al. Mutations in CAPN1 Cause Autosomal-Recessive Hereditary Spastic Paraplegia. Am. J. Hum. Genet. 2016, 98, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Jin, X.; Xu, M.; Xi, Y.; Lu, W.; Yang, X.; Guan, M.X.; Ge, W. FARS2 deficiency in Drosophila reveals the developmental delay and seizure manifested by aberrant mitochondrial tRNA metabolism. Nucleic Acids Res. 2021, 49, 13108–13121. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Li, R.; He, C.; Chen, Q.; Xu, C.; Shen, L.; Chen, K.; Wu, Y. Hedgehog pathway is negatively regulated during the development of Drosophila melanogaster PheRS-m (Drosophila homologs gene of human FARS2) mutants. Hum. Cell 2023, 36, 121–131. [Google Scholar] [CrossRef]
- Schuurs-Hoeijmakers, J.H.; Geraghty, M.T.; Kamsteeg, E.J.; Ben-Salem, S.; de Bot, S.T.; Nijhof, B.; van de Vondervoort, I.I.; van der Graaf, M.; Nobau, A.C.; Otte-Höller, I.; et al. Mutations in DDHD2, encoding an intracellular phospholipase A (1), cause a recessive form of complex hereditary spastic paraplegia. Am. J. Hum. Genet. 2012, 91, 1073–1081. [Google Scholar] [CrossRef]
- Lobato, A.G.; Ortiz-Vega, N.N.; Canic, T.; Tao, X.; Bucan, N.; Ruan, K.; Rebelo, A.P.; Schule, R.; Zuchner, S.; Syed, S.; et al. Loss of Fic causes progressive neurodegeneration in a Drosophila model of hereditary spastic paraplegia. Biochimica et biophysica acta. Mol. Basis Dis. 2024, 1870, 167348. [Google Scholar] [CrossRef]
- Mundwiler, A.; Shakkottai, V.G. Autosomal-dominant cerebellar ataxias. Handb. Clin. Neurol. 2018, 147, 173–185. [Google Scholar] [CrossRef]
- Bellen, H.J.; Tong, C.; Tsuda, H. 100 years of Drosophila research and its impact on vertebrate neuroscience: A history lesson for the future. Nature reviews. Neuroscience 2010, 11, 514–522. [Google Scholar] [CrossRef]
- Lu, B.; Vogel, H. Drosophila models of neurodegenerative diseases. Annu. Rev. Pathol. 2009, 4, 315–342. [Google Scholar] [CrossRef]
- Bergeron, D.; Lapointe, C.; Bissonnette, C.; Tremblay, G.; Motard, J.; Roucou, X. An out-of-frame overlapping reading frame in the ataxin-1 coding sequence encodes a novel ataxin-1 interacting protein. J. Biol. Chem. 2013, 288, 21824–21835. [Google Scholar] [CrossRef]
- Fernandez-Funez, P.; Nino-Rosales, M.L.; de Gouyon, B.; She, W.C.; Luchak, J.M.; Martinez, P.; Turiegano, E.; Benito, J.; Capovilla, M.; Skinner, P.J.; et al. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature 2000, 408, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Lessing, D.; Bonini, N.M. Polyglutamine genes interact to modulate the severity and progression of neurodegeneration in Drosophila. PLoS Biol. 2008, 6, e29. [Google Scholar] [CrossRef] [PubMed]
- Warrick, J.M.; Paulson, H.L.; Gray-Board, G.L.; Bui, Q.T.; Fischbeck, K.H.; Pittman, R.N.; Bonini, N.M. Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell 1998, 93, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Warrick, J.M.; Morabito, L.M.; Bilen, J.; Gordesky-Gold, B.; Faust, L.Z.; Paulson, H.L.; Bonini, N.M. Ataxin-3 suppresses polyglutamine neurodegeneration in Drosophila by a ubiquitin-associated mechanism. Mol. Cell 2005, 18, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.R.; Kirby, K.; Hilliker, A.J.; Phillips, J.P. RNAi-mediated suppression of the mitochondrial iron chaperone, frataxin, in Drosophila. Hum. Mol. Genet. 2005, 14, 3397–3405. [Google Scholar] [CrossRef]
- Navarro, J.A.; Ohmann, E.; Sanchez, D.; Botella, J.A.; Liebisch, G.; Moltó, M.D.; Ganfornina, M.D.; Schmitz, G.; Schneuwly, S. Altered lipid metabolism in a Drosophila model of Friedreich’s ataxia. Hum. Mol. Genet. 2010, 19, 2828–2840. [Google Scholar] [CrossRef]
- Petersen, A.J.; Rimkus, S.A.; Wassarman, D.A. ATM kinase inhibition in glial cells activates the innate immune response and causes neurodegeneration in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, E656–E664. [Google Scholar] [CrossRef]
- Bolus, H.; Crocker, K.; Boekhoff-Falk, G.; Chtarbanova, S. Modeling Neurodegenerative Disorders in Drosophila melanogaster. Int. J. Mol. Sci. 2020, 21, 3055. [Google Scholar] [CrossRef]
- Al-Ramahi, I.; Lam, Y.C.; Chen, H.K.; de Gouyon, B.; Zhang, M.; Pérez, A.M.; Branco, J.; de Haro, M.; Patterson, C.; Zoghbi, H.Y.; et al. CHIP protects from the neurotoxicity of expanded and wild-type ataxin-1 and promotes their ubiquitination and degradation. J. Biol. Chem. 2006, 281, 26714–26724. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.M.; Snoddy, C.A.; York, P.M.; Davis, S.M.; Hunter, M.F.; Krishnan, N. Enhanced Age-Dependent Motor Impairment in Males of Drosophila melanogaster Modeling Spinocerebellar Ataxia Type 1 Is Linked to Dysregulation of a Matrix Metalloproteinase. Biology 2024, 13, 854. [Google Scholar] [CrossRef]
- Petrauskas, A.; Fortunati, D.L.; Kandi, A.R.; Pothapragada, S.S.; Agrawal, K.; Singh, A.; Huelsmeier, J.; Hillebrand, J.; Brown, G.; Chaturvedi, D.; et al. Structured and disordered regions of Ataxin-2 contribute differently to the specificity and efficiency of mRNP granule formation. PLoS Genet. 2024, 20, e1011251. [Google Scholar] [CrossRef]
- Del Castillo, U.; Norkett, R.; Lu, W.; Serpinskaya, A.; Gelfand, V.I. Ataxin-2 is essential for cytoskeletal dynamics and neurodevelopment in Drosophila. iScience 2021, 25, 103536. [Google Scholar] [CrossRef]
- Sujkowski, A.; Richardson, K.; Prifti, M.V.; Wessells, R.J.; Todi, S.V. Endurance exercise ameliorates phenotypes in Drosophila models of spinocerebellar ataxias. eLife 2022, 11, e75389. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; Prifti, M.V.; Sujkowski, A.; Libohova, K.; Blount, J.R.; Hong, L.; Tsou, W.L.; Todi, S.V. Drosophila as a Model of Unconventional Translation in Spinocerebellar Ataxia Type 3. Cells 2022, 11, 1223. [Google Scholar] [CrossRef]
- Denha, S.A.; Atang, A.E.; Hays, T.S.; Avery, A.W. β-III-spectrin N-terminus is required for high-affinity actin binding and SCA5 neurotoxicity. Sci. Rep. 2022, 12, 1726. [Google Scholar] [CrossRef]
- Avery, A.W.; Thomas, D.D.; Hays, T.S. β-III-spectrin spinocerebellar ataxia type 5 mutation reveals a dominant cytoskeletal mechanism that underlies dendritic arborization. Proc. Natl. Acad. Sci. USA 2017, 114, E9376–E9385. [Google Scholar] [CrossRef]
- Lorenzo, D.N.; Li, M.G.; Mische, S.E.; Armbrust, K.R.; Ranum, L.P.; Hays, T.S. Spectrin mutations that cause spinocerebellar ataxia type 5 impair axonal transport and induce neurodegeneration in Drosophila. J. Cell Biol. 2010, 189, 143–158. [Google Scholar] [CrossRef]
- Tsou, W.L.; Qiblawi, S.H.; Hosking, R.R.; Gomez, C.M.; Todi, S.V. Polyglutamine length-dependent toxicity from α1ACT in Drosophila models of spinocerebellar ataxia type 6. Biol. Open 2016, 5, 1770–1775. [Google Scholar] [CrossRef]
- Tsou, W.L.; Hosking, R.R.; Burr, A.A.; Sutton, J.R.; Ouyang, M.; Du, X.; Gomez, C.M.; Todi, S.V. DnaJ-1 and karyopherin α3 suppress degeneration in a new Drosophila model of Spinocerebellar Ataxia Type 6. Hum. Mol. Genet. 2015, 24, 4385–4396. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Rosenfeld, J.A.; Yamamoto, S.; Harel, T.; Zuo, Z.; Hall, M.; Wierenga, K.J.; Pastore, M.T.; Bartholomew, D.; Delgado, M.R.; et al. Members of the UDN Clinically severe CACNA1A alleles affect synaptic function and neurodegeneration differentially. PLoS Genet. 2017, 13, e1006905. [Google Scholar] [CrossRef]
- Nath, S.; Caron, N.S.; May, L.; Gluscencova, O.B.; Kolesar, J.; Brady, L.; Kaufman, B.A.; Boulianne, G.L.; Rodriguez, A.R.; Tarnopolsky, M.A.; et al. Functional characterization of variants of unknown significance in a spinocerebellar ataxia patient using an unsupervised machine learning pipeline. Hum. Genome Var. 2022, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Latouche, M.; Lasbleiz, C.; Martin, E.; Monnier, V.; Debeir, T.; Mouatt-Prigent, A.; Muriel, M.P.; Morel, L.; Ruberg, M.; Brice, A.; et al. A conditional pan-neuronal Drosophila model of spinocerebellar ataxia 7 with a reversible adult phenotype suitable for identifying modifier genes. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Mutsuddi, M.; Marshall, C.M.; Benzow, K.A.; Koob, M.D.; Rebay, I. The spinocerebellar ataxia 8 noncoding RNA causes neurodegeneration and associates with staufen in Drosophila. Curr. Biol. CB 2004, 14, 302–308. [Google Scholar] [CrossRef]
- Tripathi, B.K.; Das, R.; Mukherjee, A.; Mutsuddi, M. Interaction of Spoonbill with Prospero in Drosophila: Implications in neuroblast development. Genesis 2017, 55, e23049. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lee, C.M.; Lee, L.C.; Tung, L.C.; Hsieh-Li, H.M.; Lee-Chen, G.J.; Su, M.T. Mitochondrial dysfunction and oxidative stress contribute to the pathogenesis of spinocerebellar ataxia type 12 (SCA12). J. Biol. Chem. 2011, 286, 21742–21754. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Iradi, C.; Bickford, J.S.; Khare, S.; Hall, A.; Nick, J.A.; Salmasinia, D.; Wawrowsky, K.; Bannykh, S.; Huynh, D.P.; Rincon-Limas, D.E.; et al. KCNC3(R420H), a K(+) channel mutation causative in spinocerebellar ataxia 13 displays aberrant intracellular trafficking. Neurobiol. Dis. 2014, 71, 270–279. [Google Scholar] [CrossRef]
- Chakraborty, S.; Hasan, G. Functional complementation of Drosophila itpr mutants by rat Itpr1. J. Neurogenet. 2012, 26, 328–337. [Google Scholar] [CrossRef]
- Montalvo-Méndez, R.J.; Cárdenas-Tueme, M.; Reséndez-Pérez, D. Drosophila in the study of hTBP protein interactions in the development and modeling of SCA17. Drosophila en el estudio de las interacciones proteicas de hTBP en el desarrollo y modelaje de SCA17. Gac. Medica De Mex. 2024, 160, 1–8. [Google Scholar] [CrossRef]
- Patel, N.; Alam, N.; Libohova, K.; Dulay, R.; Todi, S.V.; Sujkowski, A. Phenotypic defects from the expression of wild-type and pathogenic TATA-binding proteins in new Drosophila models of Spinocerebellar Ataxia Type 17. G3: Genes Genomes Genet. 2023, 13, jkad180. [Google Scholar] [CrossRef]
- Hsu, T.C.; Wang, C.K.; Yang, C.Y.; Lee, L.C.; Hsieh-Li, H.M.; Ro, L.S.; Chen, C.M.; Lee-Chen, G.J.; Su, M.T. Deactivation of TBP contributes to SCA17 pathogenesis. Hum. Mol. Genet. 2014, 23, 6878–6893. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ren, J.; Jegga, A.G.; Zhang, M.; Deng, J.; Liu, J.; Gordon, C.B.; Aronow, B.J.; Lu, L.J.; Zhang, B.; Ma, J. A Drosophila model of the neurodegenerative disease SCA17 reveals a role of RBP-J/Su(H) in modulating the pathological outcome. Hum. Mol. Genet. 2011, 20, 3424–3436. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pareek, G.; Pallanck, L.J. Inactivation of the mitochondrial protease Afg3l2 results in severely diminished respiratory chain activity and widespread defects in mitochondrial gene expression. PLoS Genet. 2020, 16, e1009118. [Google Scholar] [CrossRef]
- Shibata, T.; Nagano, K.; Ueyama, M.; Ninomiya, K.; Hirose, T.; Nagai, Y.; Ishikawa, K.; Kawai, G.; Nakatani, K. Small molecule targeting r(UGGAA)n disrupts RNA foci and alleviates disease phenotype in Drosophila model. Nat. Commun. 2021, 12, 236. [Google Scholar] [CrossRef]
- Tripathy, D.; Vignoli, B.; Ramesh, N.; Polanco, M.J.; Coutelier, M.; Stephen, C.D.; Canossa, M.; Monin, M.L.; Aeschlimann, P.; Turberville, S.; et al. Mutations in TGM6 induce the unfolded protein response in SCA35. Hum. Mol. Genet. 2017, 26, 3749–3762. [Google Scholar] [CrossRef]
- Nisoli, I.; Chauvin, J.P.; Napoletano, F.; Calamita, P.; Zanin, V.; Fanto, M.; Charroux, B. Neurodegeneration by polyglutamine Atrophin is not rescued by induction of autophagy. Cell Death Differ. 2010, 17, 1577–1587. [Google Scholar] [CrossRef]
- Charroux, B.; Fanto, M. The fine line between waste disposal and recycling: DRPLA fly models illustrate the importance of completing the autophagy cycle for rescuing neurodegeneration. Autophagy 2010, 6, 667–669. [Google Scholar] [CrossRef]
- Navarro, J.A.; Llorens, J.V.; Soriano, S.; Botella, J.A.; Schneuwly, S.; Martínez-Sebastián, M.J.; Moltó, M.D. Overexpression of human and fly frataxins in Drosophila provokes deleterious effects at biochemical, physiological and developmental levels. PLoS ONE 2011, 6, e21017. [Google Scholar] [CrossRef]
- Russi, M.; Martin, E.; D’Autréaux, B.; Tixier, L.; Tricoire, H.; Monnier, V. A Drosophila model of Friedreich ataxia with CRISPR/Cas9 insertion of GAA repeats in the frataxin gene reveals in vivo protection by N-acetyl cysteine. Hum. Mol. Genet. 2020, 29, 2831–2844. [Google Scholar] [CrossRef] [PubMed]
- Rimkus, S.A.; Katzenberger, R.J.; Trinh, A.T.; Dodson, G.E.; Tibbetts, R.S.; Wassarman, D.A. Mutations in String/CDC25 inhibit cell cycle re-entry and neurodegeneration in a Drosophila model of Ataxia telangiectasia. Genes Dev. 2008, 22, 1205–1220. [Google Scholar] [CrossRef]
- Petersen, A.J.; Katzenberger, R.J.; Wassarman, D.A. The innate immune response transcription factor relish is necessary for neurodegeneration in a Drosophila model of ataxia-telangiectasia. Genetics 2013, 194, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Seong, E.; Insolera, R.; Dulovic, M.; Kamsteeg, E.J.; Trinh, J.; Brüggemann, N.; Sandford, E.; Li, S.; Ozel, A.B.; Li, J.Z.; et al. Mutations in VPS13D lead to a new recessive ataxia with spasticity and mitochondrial defects. Ann. Neurol. 2018, 83, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Sone, M.; Uchida, A.; Komatsu, A.; Suzuki, E.; Ibuki, I.; Asada, M.; Shiwaku, H.; Tamura, T.; Hoshino, M.; Okazawa, H.; et al. Loss of yata, a novel gene regulating the subcellular localization of APPL, induces deterioration of neural tissues and lifespan shortening. PLoS ONE 2009, 4, e4466. [Google Scholar] [CrossRef]
- Duan, R.; Shi, Y.; Yu, L.; Zhang, G.; Li, J.; Lin, Y.; Guo, J.; Wang, J.; Shen, L.; Jiang, H.; et al. UBA5 Mutations Cause a New Form of Autosomal Recessive Cerebellar Ataxia. PLoS ONE 2016, 11, e0149039. [Google Scholar] [CrossRef]
- Pan, X.; Alvarez, A.N.; Ma, M.; Lu, S.; Crawford, M.W.; Briere, L.C.; Kanca, O.; Yamamoto, S.; Sweetser, D.A.; Wilson, J.L.; et al. Allelic strengths of encephalopathy-associated UBA5 variants correlate between in vivo and in vitro assays. eLife 2023, 12, RP89891. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sandford, E.; Gatica, D.; Qiu, Y.; Liu, X.; Zheng, Y.; Schulman, B.A.; Xu, J.; Semple, I.; Ro, S.H.; et al. Mutation in ATG5 reduces autophagy and leads to ataxia with developmental delay. eLife 2016, 5, e12245. [Google Scholar] [CrossRef]
- Eidhof, I.; Baets, J.; Kamsteeg, E.J.; Deconinck, T.; van Ninhuijs, L.; Martin, J.J.; Schüle, R.; Züchner, S.; De Jonghe, P.; Schenck, A.; et al. GDAP2 mutations implicate susceptibility to cellular stress in a new form of cerebellar ataxia. Brain A J. Neurol. 2018, 141, 2592–2604. [Google Scholar] [CrossRef]
- Rebelo, A.P.; Eidhof, I.; Cintra, V.P.; Guillot-Noel, L.; Pereira, C.V.; Timmann, D.; Traschütz, A.; Schöls, L.; Coarelli, G.; Durr, A.; et al. Biallelic loss-of-function variations in PRDX3 cause cerebellar ataxia. Brain A J. Neurol. 2021, 144, 1467–1481. [Google Scholar] [CrossRef]
- Yu, L.; Li, G.; Deng, J.; Jiang, X.; Xue, J.; Zhu, Y.; Huang, W.; Tang, B.; Duan, R. The UFM1 cascade times mitosis entry associated with microcephaly. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 1319–1330. [Google Scholar] [CrossRef]
- Choudhury, S.D.; Vs, A.; Mushtaq, Z.; Kumar, V. Altered translational repression of an RNA-binding protein, Elav by AOA2-causative Senataxin mutation. Synapse 2017, 71, e21969. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Choudhury, S.D.; Gangwar, S.K.; Orso, G.; Kumar, V. Human Senataxin Modulates Structural Plasticity of the Neuromuscular Junction in Drosophila through a Neuronally Conserved TGFβ Signalling Pathway. Neuro-Degener. Dis. 2016, 16, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y.; Okunushi, R.; Hara, T.; Hashiguchi, A.; Yuan, J.; Yoshimura, A.; Murayama, K.; Ohtake, A.; Ando, M.; Hiramatsu, Y.; et al. Mutations in COA7 cause spinocerebellar ataxia with axonal neuropathy. Brain A J. Neurol. 2018, 141, 1622–1636. [Google Scholar] [CrossRef]
- Casas-Tintó, S. Drosophila as a Model for Human Disease: Insights into Rare and Ultra-Rare Diseases. Insects 2024, 15, 870. [Google Scholar] [CrossRef] [PubMed]
- Azkona, G.; Sanchez-Pernaute, R. Mice in translational neuroscience: What R we doing? Prog. Neurobiol. 2022, 217, 102330. [Google Scholar] [CrossRef]
- Chen-Tsai, R.Y. Integrase-Mediated Targeted Transgenics Through Pronuclear Microinjection. Methods Mol. Biol. 2020, 2066, 35–46. [Google Scholar] [CrossRef]
- Schilit, S.L.P.; Ohtsuka, M.; Quadros, R.M.; Gurumurthy, C.B. Pronuclear Injection-Based Targeted Transgenesis. Curr. Protoc. Hum. Genet. 2016, 91, 15.10.1–15.10.28. [Google Scholar] [CrossRef] [PubMed]
- Bellen, H.J.; Wangler, M.F.; Yamamoto, S. The fruit fly at the interface of diagnosis and pathogenic mechanisms of rare and common human diseases. Hum. Mol. Genet. 2019, 28, R207–R214. [Google Scholar] [CrossRef]
- Ma, M.; Moulton, M.J.; Lu, S.; Bellen, H.J. ‘Fly-ing’ from rare to common neurodegenerative disease mechanisms. Trends Genet. TIG 2022, 38, 972–984. [Google Scholar] [CrossRef]
- Kottmeier, R.; Bittern, J.; Schoofs, A.; Scheiwe, F.; Matzat, T.; Pankratz, M.; Klämbt, C. Wrapping glia regulates neuronal signaling speed and precision in the peripheral nervous system of Drosophila. Nat. Commun. 2020, 11, 4491. [Google Scholar] [CrossRef]
- Heiduschka, S.; Prigione, A. iPSC models of mitochondrial diseases. Neurobiol. Dis. 2025, 207, 106822. [Google Scholar] [CrossRef]
- Seguin, A.; Monnier, V.; Palandri, A.; Bihel, F.; Rera, M.; Schmitt, M.; Camadro, J.M.; Tricoire, H.; Lesuisse, E. A Yeast/Drosophila Screen to Identify New Compounds Overcoming Frataxin Deficiency. Oxidative Med. Cell. Longev. 2015, 2015, 565140. [Google Scholar] [CrossRef]
- Verheyen, E.M. The power of Drosophila in modeling human disease mechanisms. Dis. Models Mech. 2022, 15, dmm049549. [Google Scholar] [CrossRef] [PubMed]
- Rudrapatna, V.A.; Cagan, R.L.; Das, T.K. Drosophila cancer models. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2012, 241, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Bangi, E.; Murgia, C.; Teague, A.G.; Sansom, O.J.; Cagan, R.L. Functional exploration of colorectal cancer genomes using Drosophila. Nat. Commun. 2016, 7, 13615. [Google Scholar] [CrossRef]
- Zhang, B.; Burke, R. Copper homeostasis and the ubiquitin proteasome system. Met. Integr. Biometal Sci. 2023, 15, mfad010. [Google Scholar] [CrossRef] [PubMed]
- Rohde, P.D.; Østergaard, S.; Kristensen, T.N.; Sørensen, P.; Loeschcke, V.; Mackay, T.F.C.; Sarup, P. Functional Validation of Candidate Genes Detected by Genomic Feature Models. G3 Genes Genomes Genet. 2018, 8, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Giacomotto, J.; Ségalat, L. High-throughput screening and small animal models where are we? Br. J. Pharmacol. 2010, 160, 204–216. [Google Scholar] [CrossRef] [PubMed]

| Human Genes | Protein Function | Identity and Similarity | Drosophila Genes | Behavior | Morphology | References |
|---|---|---|---|---|---|---|
| SCA1/ATXN1 | Chromatin-binding protein acting as a transcriptional regulator; represses Notch signaling in absence of NICD by serving as CBF1 co-repressor | 38% and 58% | atx-1 | shortened lifespan and worsened motor function | degenerations in the eye, and visible phenotypes in the wing and bristles | [87,88] |
| SCA2/ ATXN2 | RNA-binding protein regulating endocytosis and trafficking of EGFR; modulates actin cytoskeleton and organelle transport | 24% and 36% | atx-2 | decreased locomotion of larvae | morphological defects in the nervous system, altered cytoskeletal network, Impaired organelle transport | [89,90] |
| SCA3/ATXN3 | Deubiquitinating enzyme involved in protein homeostasis maintenance, transcription, cytoskeleton regulation, myogenesis and degradation of misfolded chaperone substrates | - | no orthologue | shortened lifespan | severe and progressive adult-onset neural degeneration, compromise in vision | [29,91,92,81] |
| SCA5/ SPTBN2 | protein (beta-III spectrin) is a crucial structural component of the neuronal plasma membrane, especially in Purkinje cells, where it helps stabilize glutamate transporters (EAAT4) | 56% and 72% | β-Spec | posterior paralysis | reduced synaptic transmission, reduced synaptic terminal growth, and axonal transport deficits | [93,94,95] |
| SCA6/ CACNA1A | P/Q-type voltage-gated calcium channel α1 subunit; controls Ca2+ entry into neurons and Ca2+-dependent processes including neurotransmitter release and gene expression | 43% and 54% | cac | shorter life span | retinal disruption | [96,97,98] |
| SCA7/ ATXN7 | Acts as a component of the SAGA (also known as STAGA) transcription coactivator-HAT complex | - | no orthologue | Impaired movement, and early lethality | neural and retinal degeneration | [19,25,99,100] |
| SCA8/ ATXN8 | encodes a nearly pure polyglutamine expansion protein in the CAG direction | - | no orthologue | shortened lifespan | progressive neurodegeneration in the adult retina | [101,102] |
| SCA12/PPP2R2B | The B regulatory subunit might modulate substrate selectivity and catalytic activity and might also direct the localization of the catalytic enzyme to a particular subcellular compartment. Within the PP2A holoenzyme complex, isoform 2 is required to promote proapoptotic activity | 68% and 78% | tws | fail to hatch, shortened life span | neurodegeneration, apoptosis, mitochondrial abnormalities | [103] |
| SCA13/ KCNC3 | Voltage-gated potassium channel that plays an important role in the rapid repolarization of fast-firing brain neurons | 60% and 75% | shaw/shawl | shortened lifespan | aberrant differentiation and polarity of photoreceptor cells, aberrant wing vein | [31,104] |
| SCA15/ ITPR1 | Inositol 1,4,5-trisphosphate-gated calcium channel that, upon inositol 1,4,5-trisphosphate binding, mediates calcium release from the ER | 57% and 70% | itpr | flight defective, locomotor behavior defective, and feeding behavior defective | neurophysiology and neuroanatomy defects | [105] |
| SCA17/ TBP | The TFIID basal transcription factor complex plays a major role in the initiation of RNA polymerase II (Pol II)-dependent transcription | 58% and 63% | tbp | late-onset locomotor impairment and shortened lifespan | progressive neurodegeneration | [106,107,108,109] |
| SCA28/AFG3L2 | Catalytic subunit of the mitochondrial m-AAA protease; regulates proteostasis of inner mitochondrial membrane proteins, essential for axonal development and neuronal survival | 63% and 74% | afg3l2 | behavioral defects, shortened lifespan, locomotor deficits | mitochondrial functional deficits and neurodegeneration | [110] |
| SCA31/BEAN1 | is one of several proteins that interact with NEDD4, a member of a family of ubiquitin–protein ligases | - | no orthologue | shorter lifespan, progressive locomotor defects | severe degenerative eye morphologies, neurodegeneration progresses with age | [28,111] |
| SCA35/ TGM6 | Catalyzes the cross-linking of proteins and the conjugation of polyamines to proteins | 30% and 55% | tg | reduced the survival of models | death of primary neurons | [112] |
| DRPLA/ATN1 | Transcriptional corepressor. Recruits NR2E1 to repress transcription. Promotes vascular smooth cell (VSMC) migration and orientation | 23% and 31% | gug | shortened lifespan | eye depigmentation and retinal collapse, neural defects | [28,113,114] |
| FRDA/ FXN | mitochondrial protein which belongs to the FRATAXIN family. The protein functions in regulating mitochondrial iron transport and respiration | 42% and 57% | fh | shortened life span, reduced climbing abilities, enhanced sensitivity to oxidative stress, cardiac defects | neuroanatomy and neurophysiology defect | [83,84,115,116] |
| ATM | Serine/threonine protein kinase which activates checkpoint signaling upon double strand breaks (DSBs), apoptosis and genotoxic stresses such as ionizing ultraviolet A light (UVA), thereby acting as a DNA damage sensor | 22% and 40% | tefu | rough eyes, notched wings, and shorter or missing bristles | neuron and glial cell death in the adult brain, neuroblast display mitotic abnormalities | [117,118] |
| SCAR4/VPS13D | Mediates the transfer of lipids between membranes at organelle contact sites | 29% and 47% | vps13D | reduced survival to adulthood | changes in mitochondrial morphology and impairment in mitochondrial distribution along axons | [119] |
| SCAR21/SCYL1 | Regulates COPI-mediated retrograde protein traffic at the interface between the Golgi apparatus and the endoplasmic reticulum | 44% and 57% | yata | shortened lifespan | developmental abnormalities, progressive eye vacuolization | [120] |
| SCAR24/UBA5 | 1-like enzyme which specifically catalyzes the first step in ufmylation | 63% and 75% | uba5 | locomotor defects, and shortened lifespan | neuroanatomical defects in larval neuromuscular junctions | [121,122] |
| SCAR25/ATG5 | an E1-like activating enzyme in a ubiquitin-like conjugating system | 48% and 67% | atg5 | significant mobility defects | disruption in autophagy activity | [123] |
| SCAR27/GDAP2 | Ganglioside Induced Differentiation Associated Protein 2 | 40% and 60% | gdap2 | shortened lifespan, locomotor defects, and increased sensitivity to stressors | progressive neurodegeneration | [124] |
| SCAR32/PRDX3 | Thiol-specific peroxidase catalyzes the reduction of hydrogen peroxide and organic hydroperoxides to water and alcohols, respectively. Plays a role in cell protection against oxidative stress by detoxifying peroxides | 64% and 78% | prx3 | reduces motor behavior | [125] | |
| UFM1 | Ubiquitin-like modifier which can be covalently attached via an isopeptide bond to lysine residues of substrate proteins as a monomer or a lysine-linked polymer | 89% and 95% | ufm1 | locomotor defects, and shortened lifespan | neuroanatomical defects in larval neuromuscular junctions | [126] |
| SCAN2/SETX | Probable RNA/DNA helicase involved in diverse aspects of RNA metabolism and genomic integrity. Plays a role in transcription regulation by its ability to modulate RNA Polymerase II (Pol II) binding to chromatin and through its interaction with proteins involved in transcription | 21% and 36% | setx | affects motor behavior | morphological plasticity at neuromuscular junction (NMJ) synapses | [127,128] |
| SCAN3/COA7 | required for assembly of mitochondrial respiratory chain complex I and complex IV | 32% and 50% | coa7 | reduced adult lifespan, progressive impairment in locomotive ability | shortened synaptic branches of motor neurons in larval neuromuscular junctions | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivarelli, R.; Vantaggiato, C.; Bassi, M.T.; Santorelli, F.M.; Marchese, M. Wings of Discovery: Using Drosophila to Decode Hereditary Spastic Paraplegia and Ataxias. Cells 2025, 14, 1466. https://doi.org/10.3390/cells14181466
Vivarelli R, Vantaggiato C, Bassi MT, Santorelli FM, Marchese M. Wings of Discovery: Using Drosophila to Decode Hereditary Spastic Paraplegia and Ataxias. Cells. 2025; 14(18):1466. https://doi.org/10.3390/cells14181466
Chicago/Turabian StyleVivarelli, Rachele, Chiara Vantaggiato, Maria Teresa Bassi, Filippo Maria Santorelli, and Maria Marchese. 2025. "Wings of Discovery: Using Drosophila to Decode Hereditary Spastic Paraplegia and Ataxias" Cells 14, no. 18: 1466. https://doi.org/10.3390/cells14181466
APA StyleVivarelli, R., Vantaggiato, C., Bassi, M. T., Santorelli, F. M., & Marchese, M. (2025). Wings of Discovery: Using Drosophila to Decode Hereditary Spastic Paraplegia and Ataxias. Cells, 14(18), 1466. https://doi.org/10.3390/cells14181466






