Curcumin Inhibits Protease Activated Receptor 2-Induced ERK Phosphorylation Calcium Mobilization and Anti-Apoptotic Signaling in Inflammation-Driven Colorectal Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics Considerations
2.3. Cell Line Selection
2.4. Cell Culture and Treatment
2.4.1. Cell Culture Methodology
2.4.2. Subculturing Cryopreservation of HT 29 and Caco-2 Cells
2.5. Assessment of Cytotoxicity Using the MTT Assay
2.6. Prepration of Curcumin Stock Solution
2.7. Induction of Inflammation in HT 29 and Caco-2 Cells Using LPS and Curcumin Treatment
2.8. RNA Extraction and cDNA Synthesis
Real-Time PCR for Quantification (qPCR)
2.9. Western Blot Analysis: Comprehensive Evaluation of PAR-2-Associated Signaling Axis and Apoptotic Pathways
2.9.1. Rationale for the Selection of Protease-Activated Receptors and Biochemical Markers in This Study
2.9.2. Protein Extraction and Quantification
2.9.3. Antibody Incubation and Detection
2.9.4. Densitometry and Statistical Analysis
2.10. Assessment of TNF-α Secretion via Quantitative ELISA Following Curcumin Treatment
2.11. Dual Fluorescence Staining and Quantification of Apoptotic Cells Using Acridine Orange/Ethidium Bromide (AO/EtBr)
2.12. Annexin V-FITC and Propidium Iodide (PI) Dual Staining for Apoptosis Detection
2.13. Calcium Signaling Assay
2.14. Computational Modeling of PAR-2
2.15. Molecular Docking of Curcumin with AlphaFold-Predicted PAR-2 Structure
3. Results
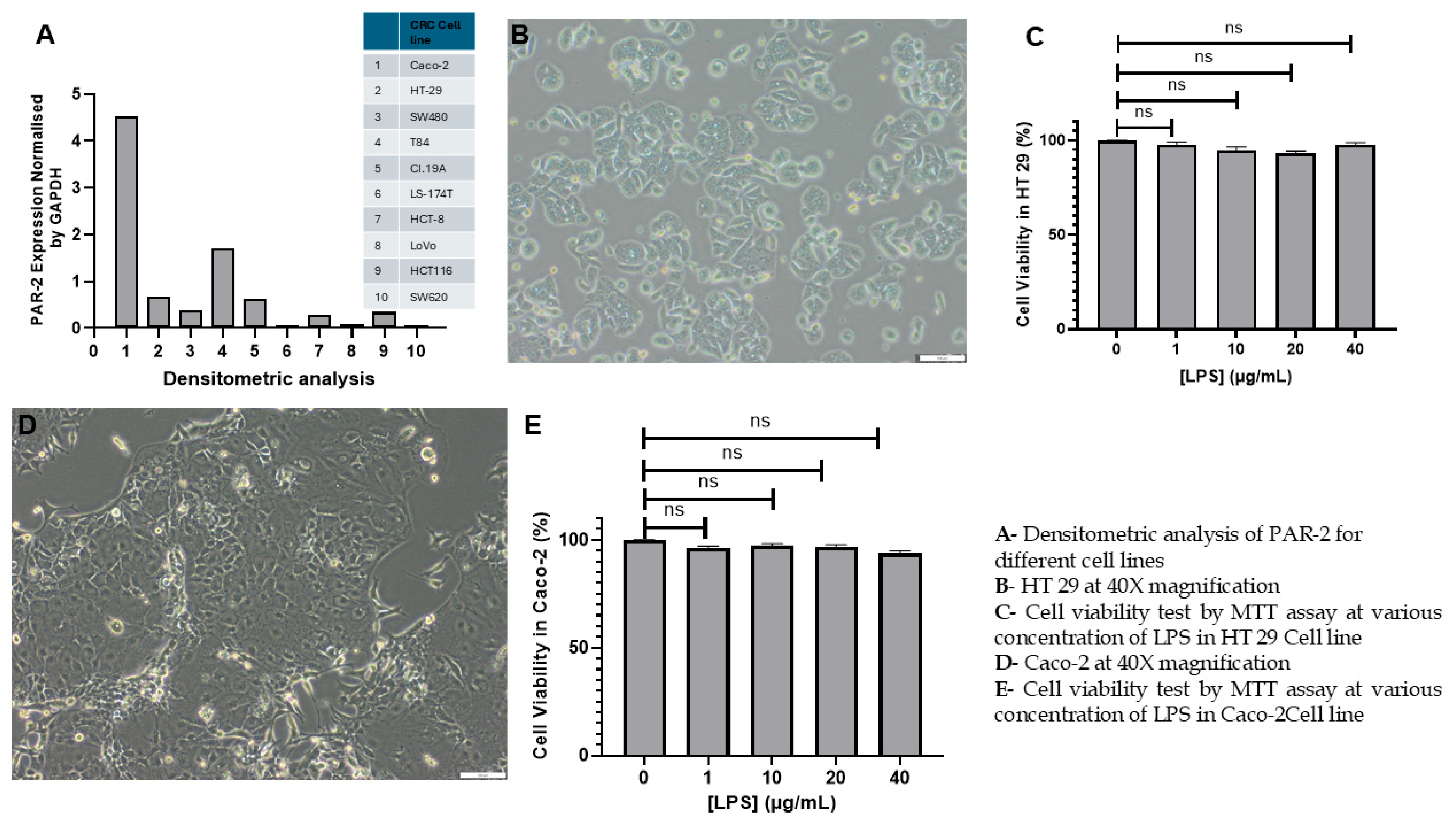
3.1. Assessment of LPS-Induced Cytotoxicity and Establishment of an Inflammatory CRC Model in HT 29 and Caco-2 Cell Lines
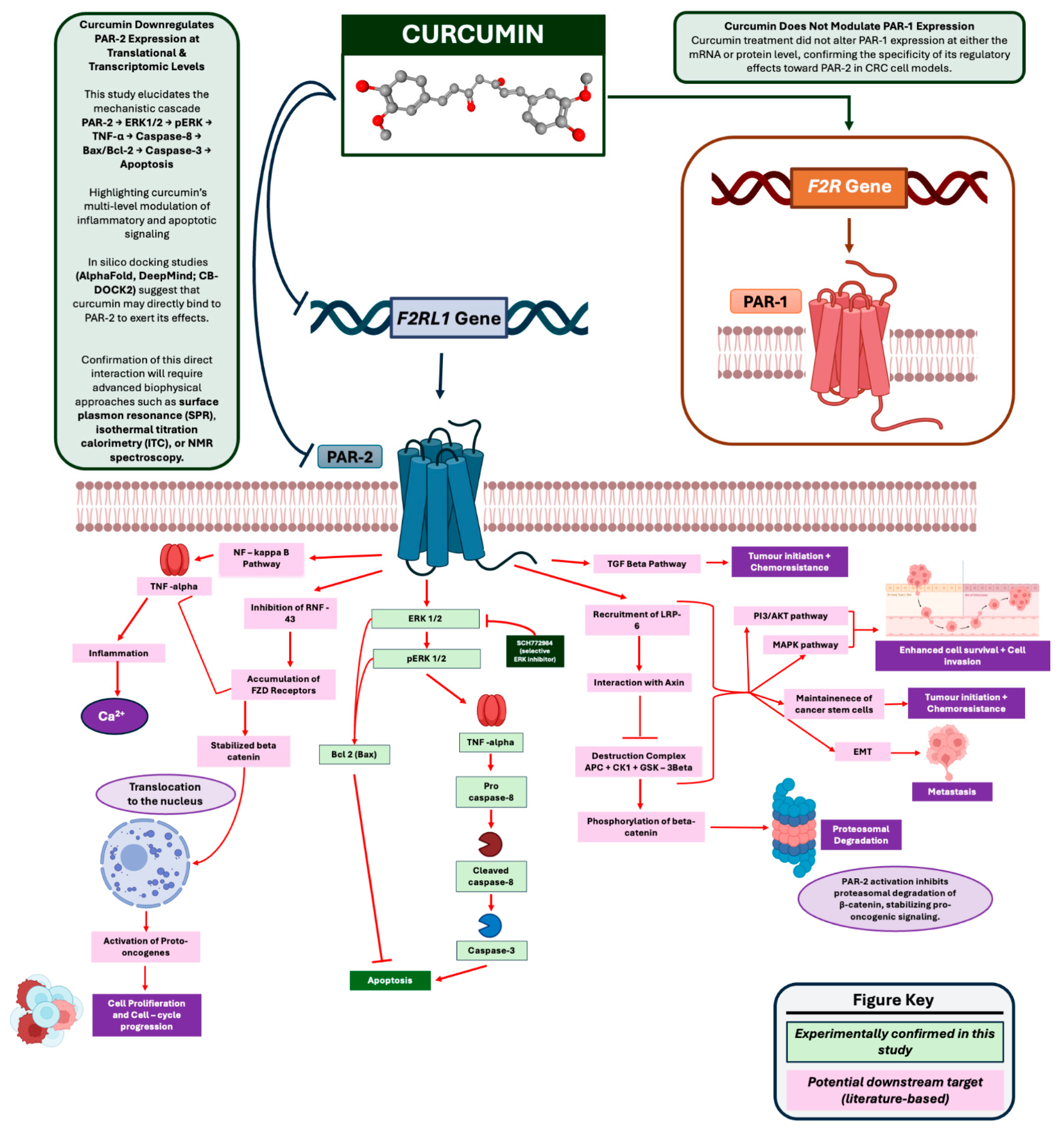
3.2. Curcumin Downregulates PAR-2 Expression at the Transcriptional and Translational Level in HT 29 and Caco-2 Cells
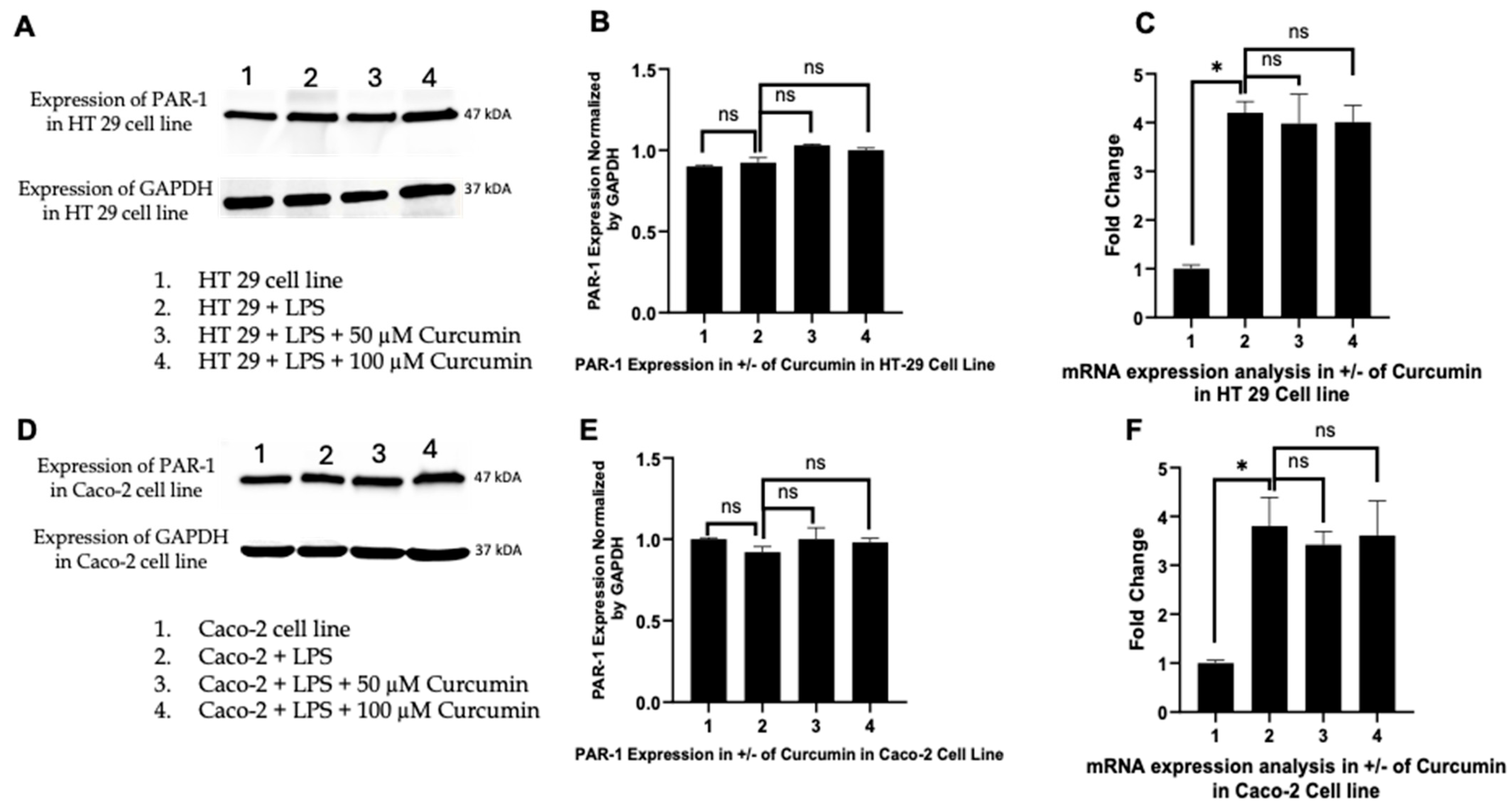
3.3. Curcumin Does Not Significantly Alter PAR-1 Expression in LPS-Stimulated HT 29 and Caco-2 Cells
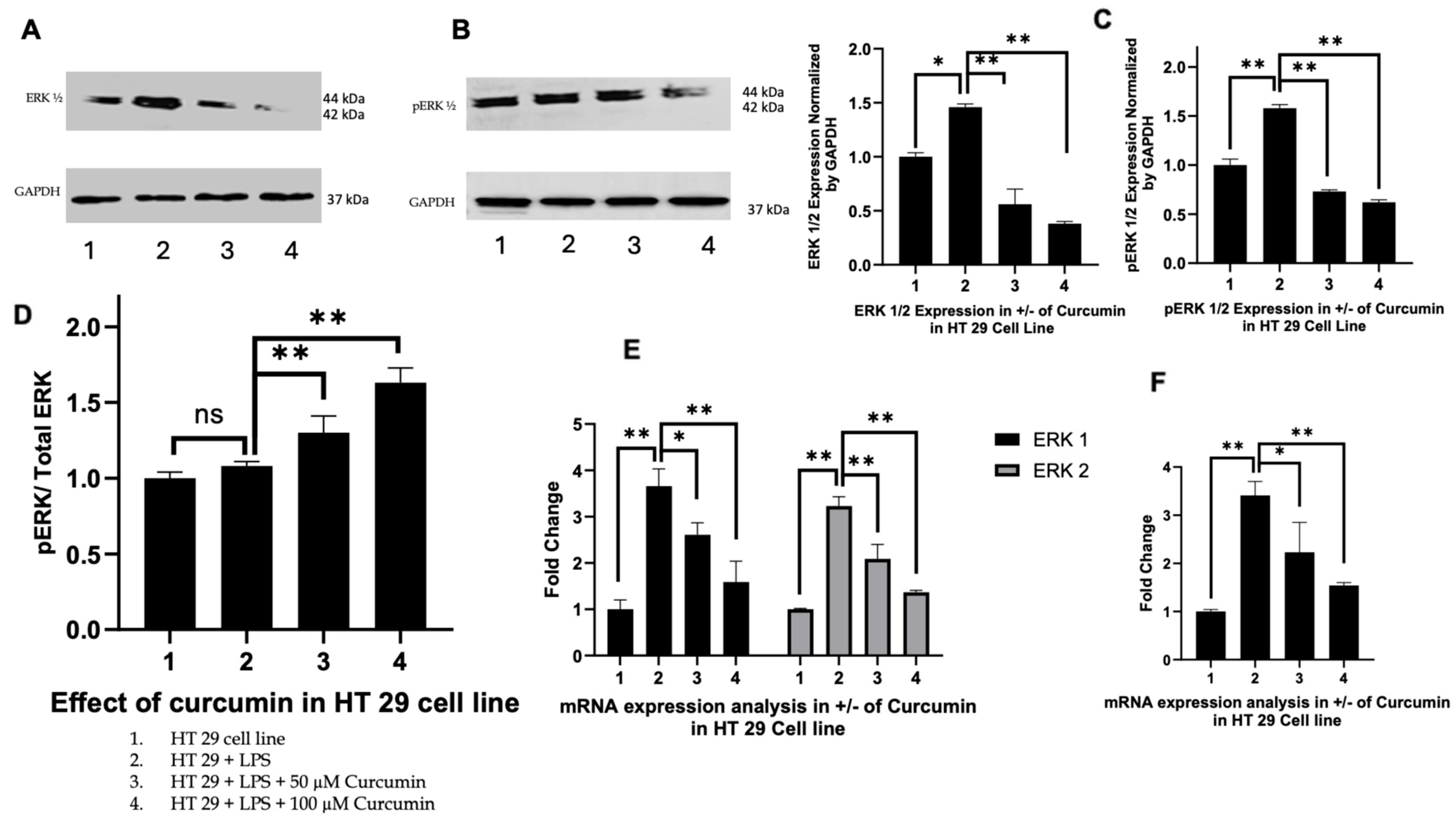
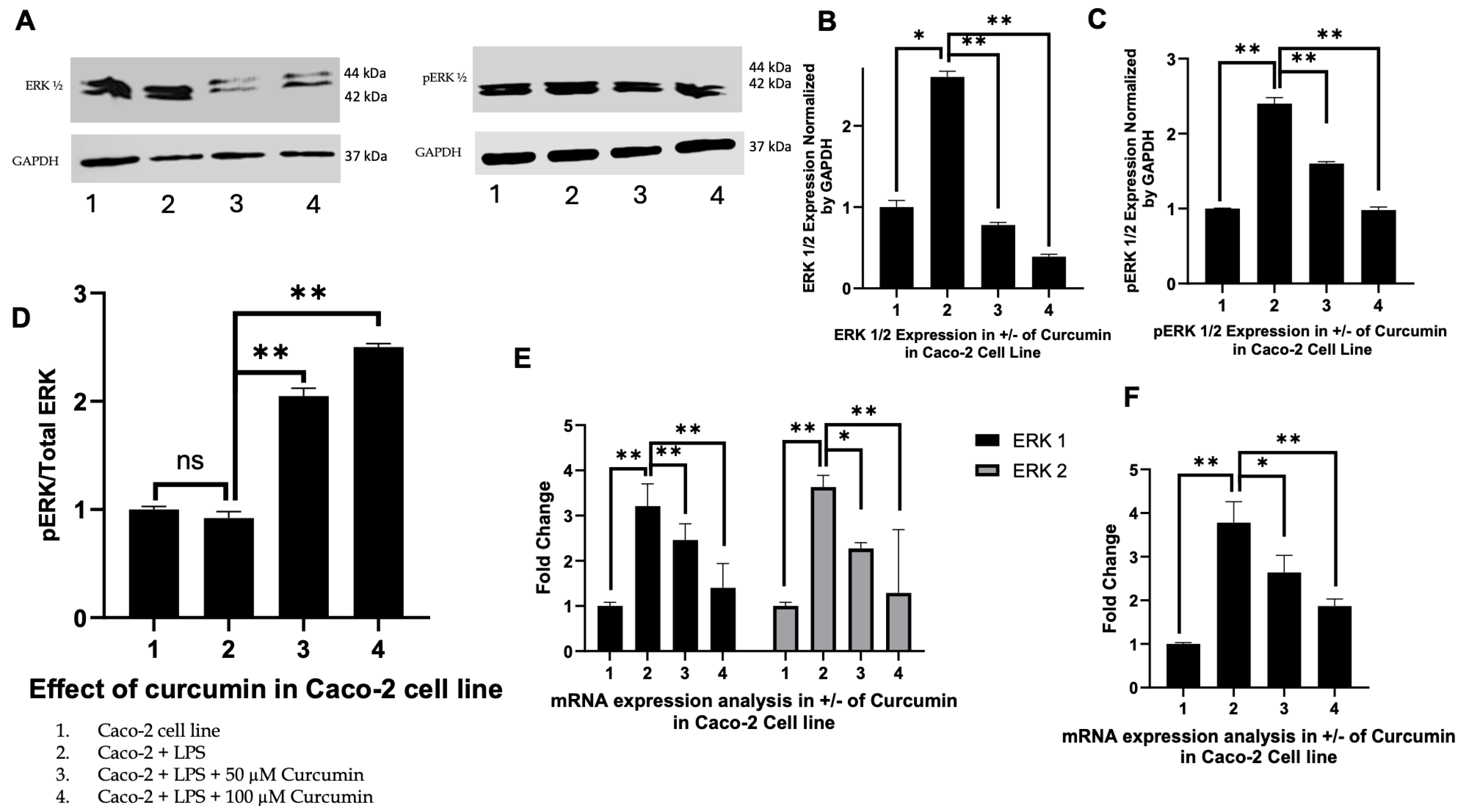
3.4. Curcumin Modulates ERK1/2 and Phosphorylated ERK Signaling in HT 29 and Caco-2 Cells
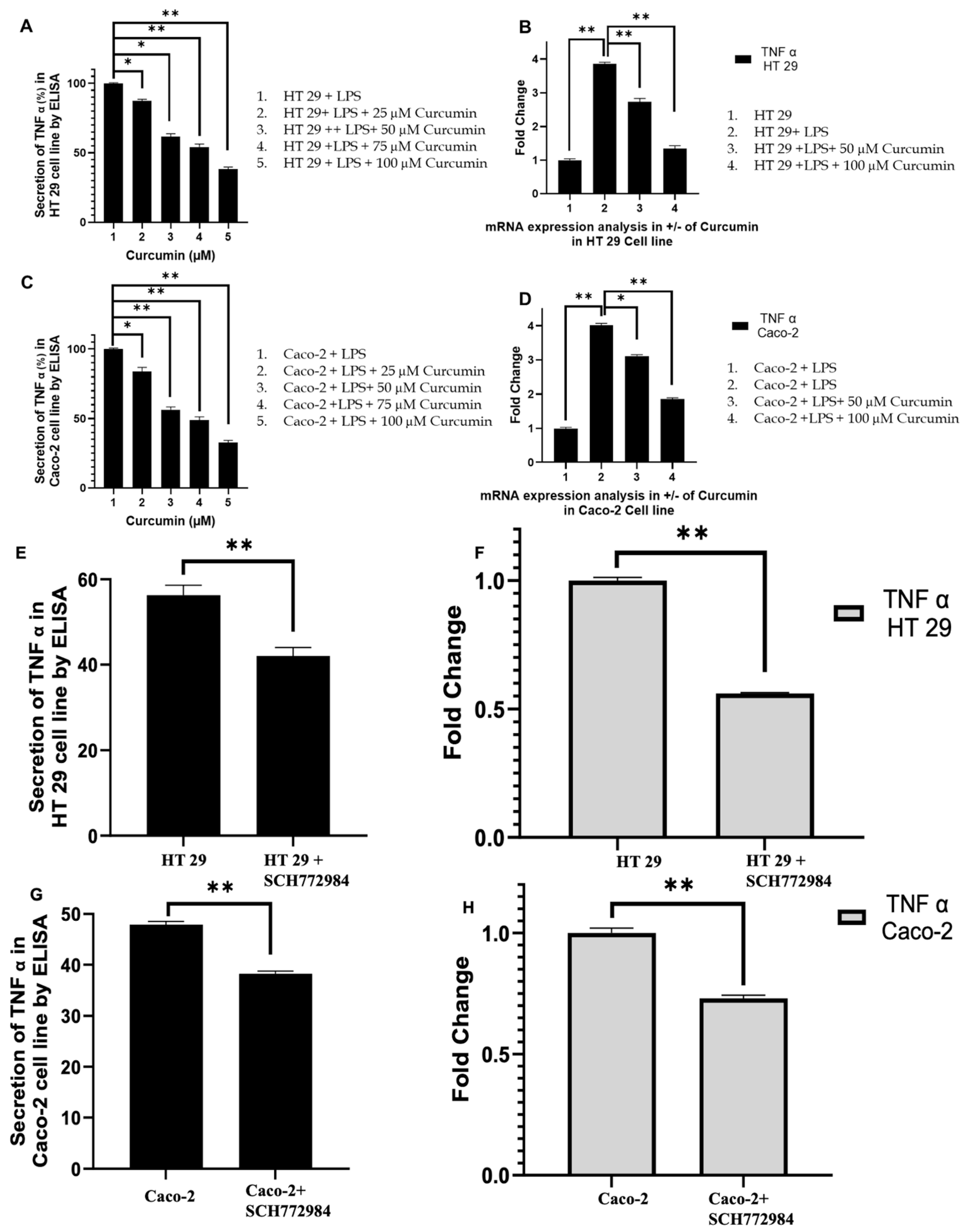
3.5. Curcumin Attenuates TNF-α Expression in LPS-Stimulated HT 29 and Caco-2 Cells
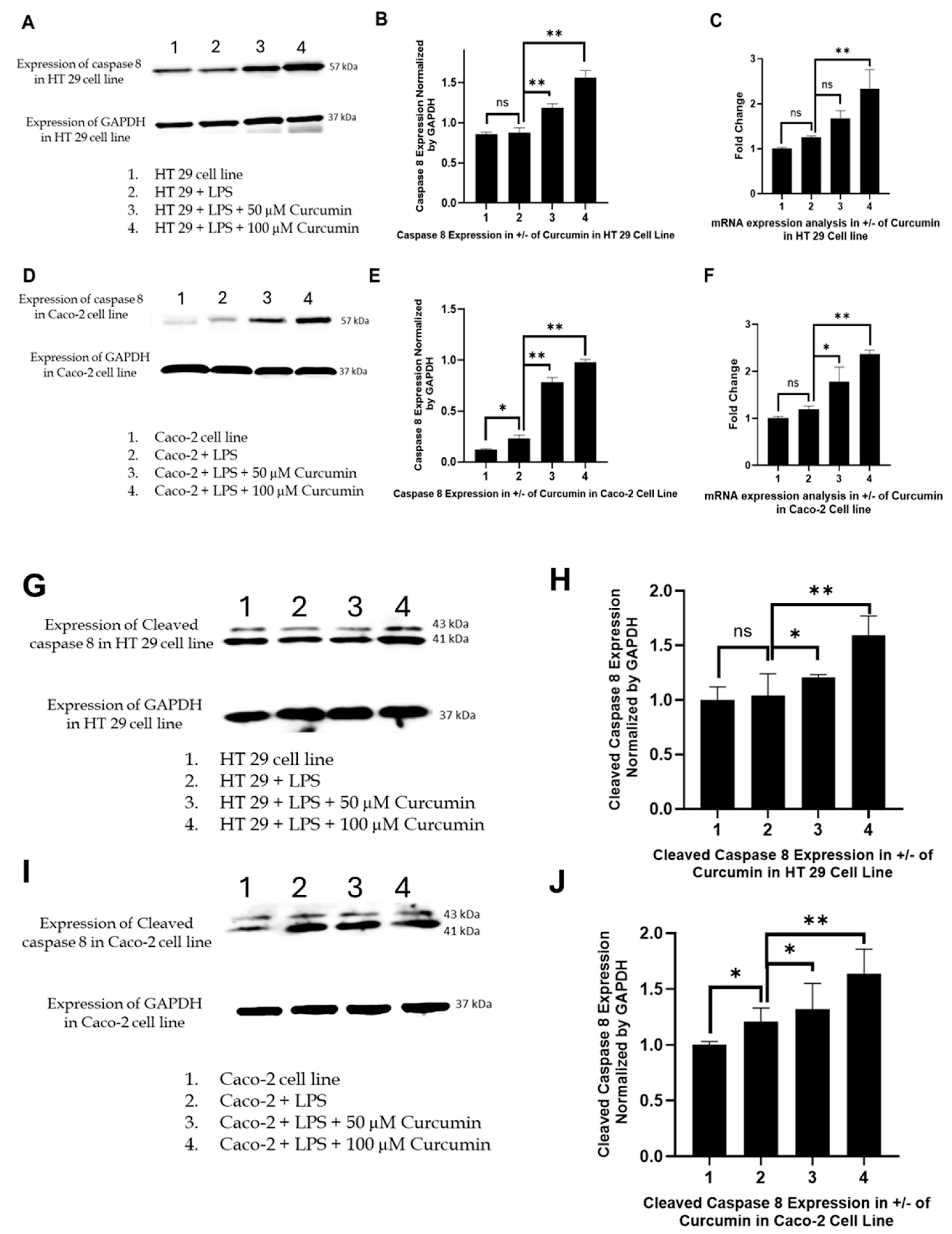
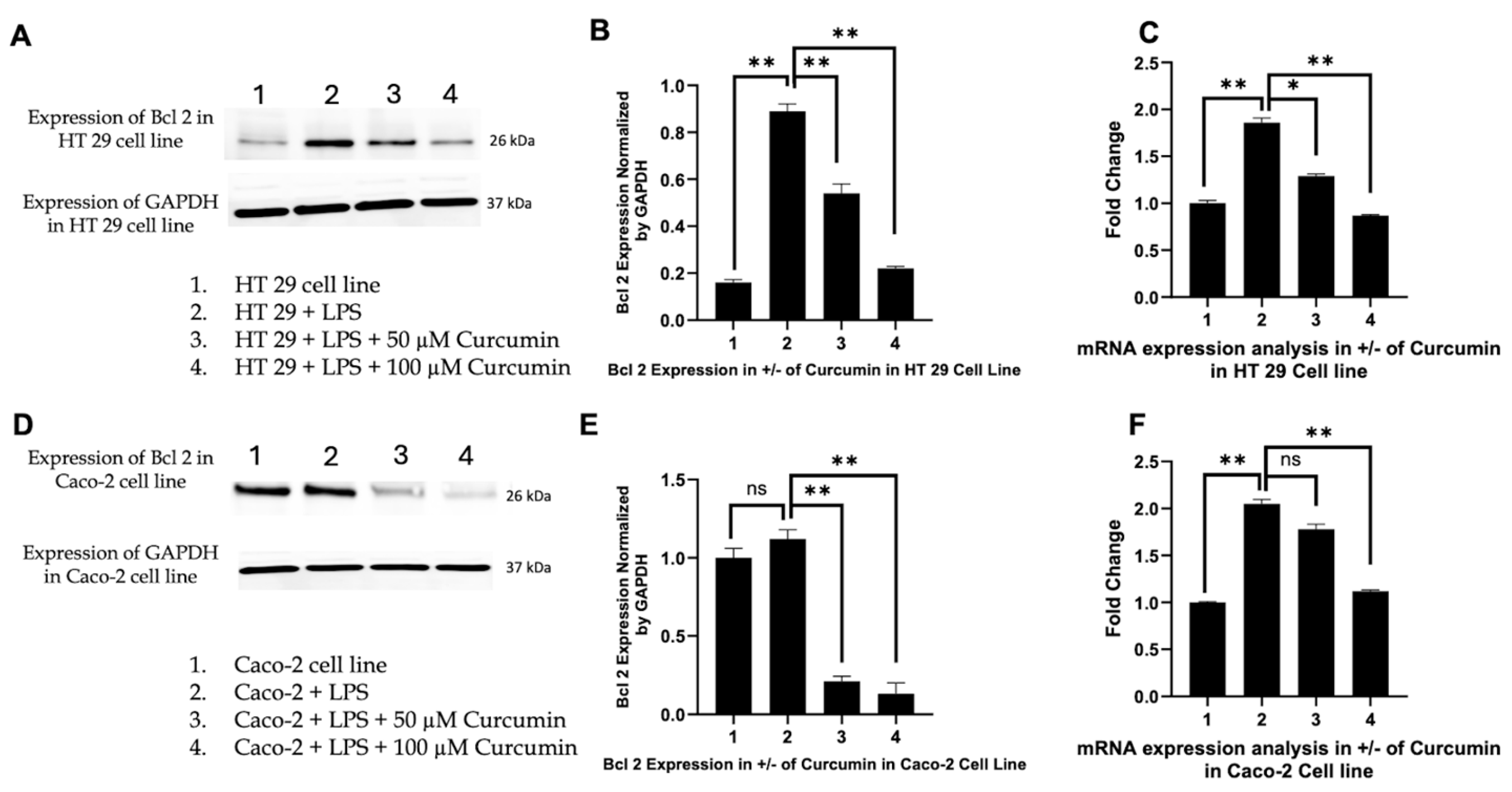
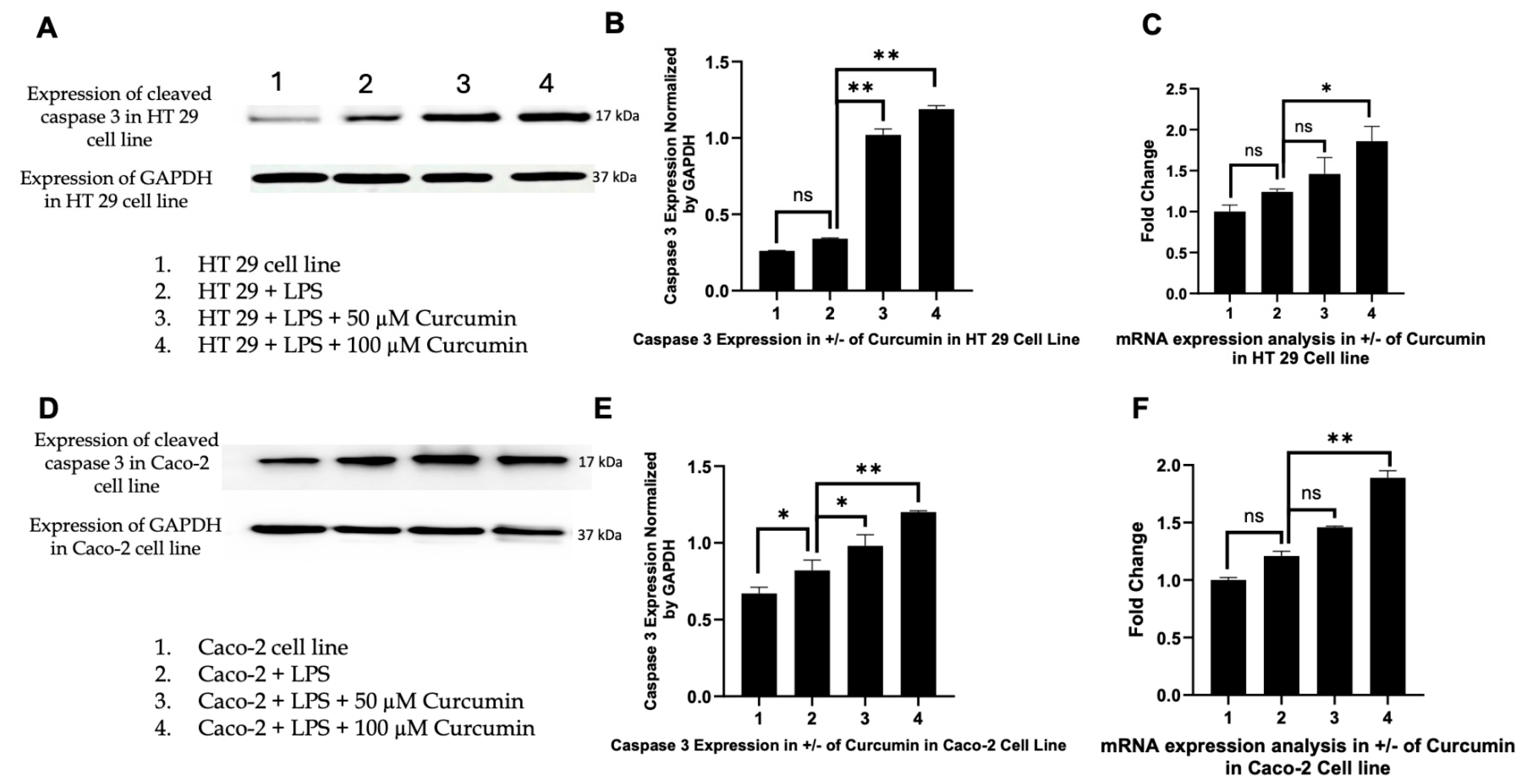
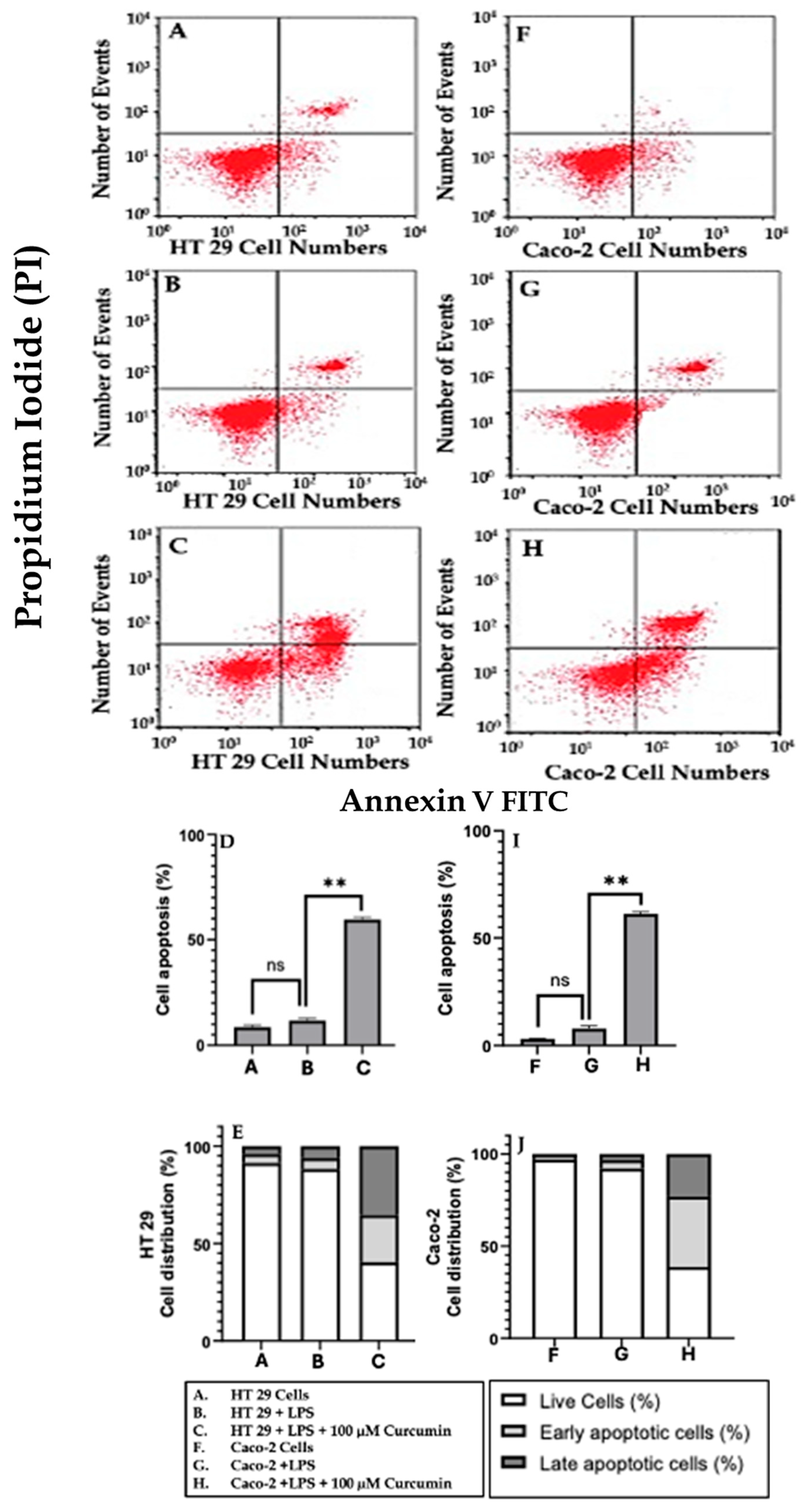
3.6. Curcumin Induces Apoptosis in HT 29 and Caco-2 Cells via PAR-2/ERK/TNF-α-Mediated Activation of Extrinsic and Intrinsic Pathways
3.7. Effect of Curcumin on Calcium Dynamics
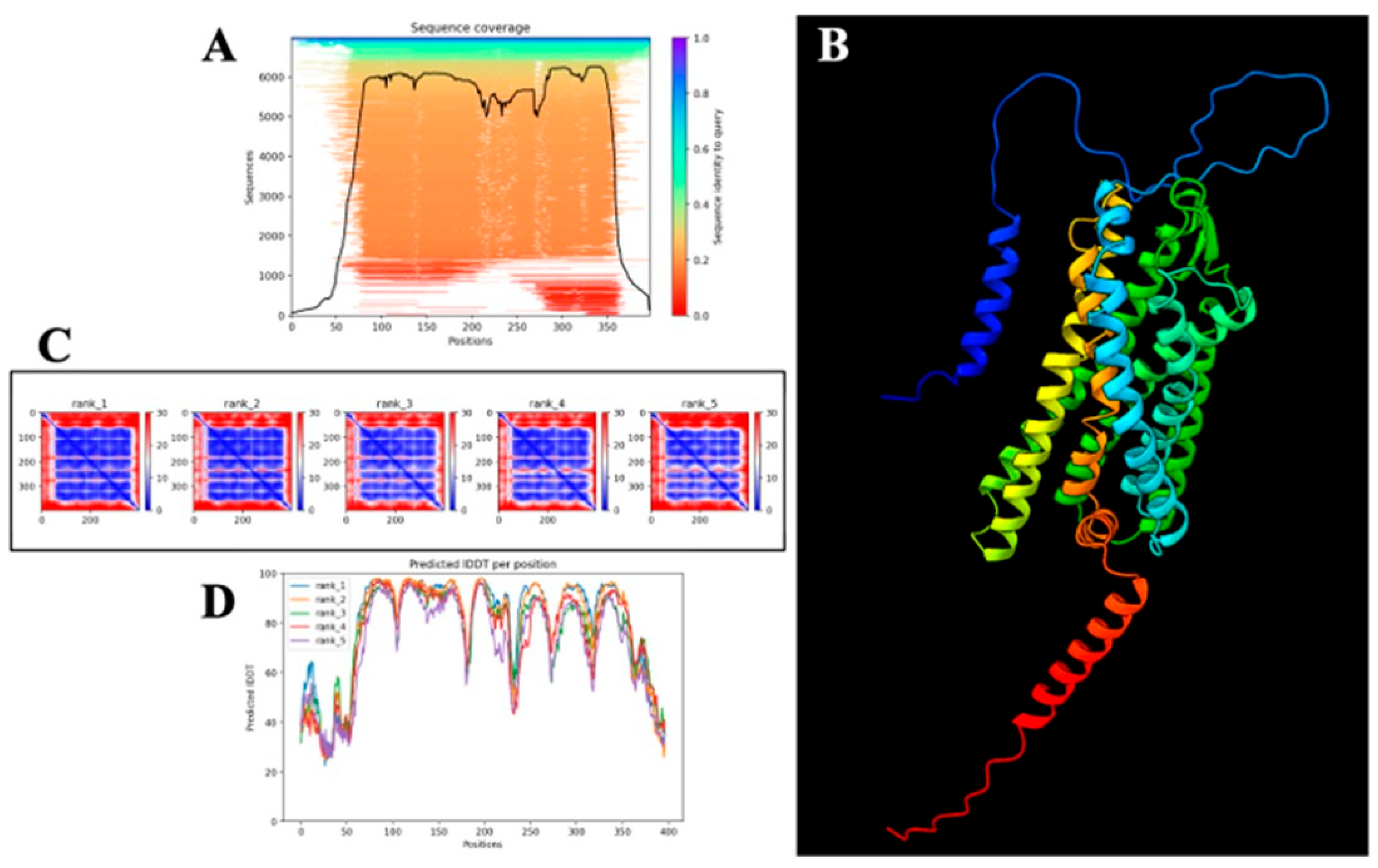
3.8. Structural Prediction of PAR-2 Using AlphaFold Confirms Canonical GPCR Topology and Identifies a Viable Ligand-Binding Pocket
3.9. Molecular Docking Suggests Direct Interaction of Curcumin with PAR-2 at a Deep Transmembrane Pocket
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| AO/EtBr | Acridine orange/ethidium bromide |
| AP-1 | Activator protein 1 |
| APC | Adenomatous polyposis coli |
| Bax | Bcl 2-associated X protein |
| Bcl 2 | B-cell lymphoma 2 |
| BIRC5 | Baculoviral IAP repeat-containing protein 5 (Survivin) |
| BRAF | v-Raf murine sarcoma viral oncogene homolog B |
| CAC | Colitis-associated cancer |
| CB-Dock2 | Cavity-detection guided protein–ligand docking tool |
| CIMP | CpG island methylator phenotype |
| CIN | Chromosomal instability |
| COX-1 | Cyclooxygenase-1 |
| COX-2 | Cyclooxygenase-2 |
| CRC | Colorectal cancer |
| CSCs | Cancer stem cells |
| DUSP6 | Dual Specificity Phosphatase 6. |
| Egr-1 | Early Growth Response 1. |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| F2R | Coagulation factor II (thrombin) receptor (gene encoding PAR-1) |
| F2RL1 | Coagulation factor II (thrombin) receptor-like 1 (gene encoding PAR-2) |
| F2RL2 | Coagulation factor II (thrombin) receptor-like 2 (gene encoding PAR-3) |
| F2RL3 | Coagulation factor II (thrombin) receptor-like 3 (gene encoding PAR-4) |
| GPCR | G-protein coupled receptor |
| IBD | Inflammatory bowel disease |
| IKK | IκB kinase |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MLH1 | MutL homolog 1 |
| MSI | Microsatellite instability |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-κB | Nuclear factor-kappa B |
| ns | Not significant |
| p-ERK | Phosphorylated extracellular signal-regulated kinase |
| PAR-1 | Protease-activated receptor-1 |
| PAR-2 | Protease-activated receptor-2 |
| PARP | Poly (ADP-ribose) polymerase |
| PI | Propidium iodide |
| PI3K | Phosphoinositide 3-kinase |
| ROS | Reactive oxygen species |
| STAT3 | Signal transducer and activator of transcription 3 |
| TME | Tumor microenvironment |
| TNF-α | Tumor necrosis factor-alpha |
| TP53 | Tumor protein p53 |
| UC | Ulcerative colitis |
| VEGF | Vascular endothelial growth factor |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Li, J.; Pan, J.; Wang, L.; Ji, G.; Dang, Y. Colorectal Cancer: Pathogenesis and Targeted Therapy. MedComm 2020, 6, 70127. [Google Scholar] [CrossRef]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.Q.; Luo, Q.; Wang, L.; Song, G.B.; Sheng, J.P.; Xu, B. Signaling Pathways Involved in Colorectal Cancer: Pathogenesis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef]
- Pierantoni, C.; Cosentino, L.; Ricciardiello, L. Molecular Pathways of Colorectal Cancer Development: Mechanisms of Action and Evolution of Main Systemic Therapy Compunds. Dig. Dis. 2024, 42, 319–324. [Google Scholar] [CrossRef]
- Wang, J.D.; Xu, G.S.; Hu, X.L.; Li, W.Q.; Yao, N.; Han, F.Z.; Zhang, Y.; Qu, J. The Histologic Features, Molecular Features, Detection and Management of Serrated Polyps: A Review. Front. Oncol. 2024, 14, 1356250. [Google Scholar] [CrossRef] [PubMed]
- Akkiz, H.; Simsek, H.; Balci, D.; Ulger, Y.; Onan, E.; Akcaer, N.; Delik, A. Inflammation and Cancer: Molecular Mechanisms and Clinical Consequences. Front. Oncol. 2025, 15, 1564572. [Google Scholar] [CrossRef]
- Olen, O.; Erichsen, R.; Sachs, M.C.; Pedersen, L.; Halfvarson, J.; Askling, J.; Ekbom, A.; Sorensen, H.T.; Ludvigsson, J.F. Colorectal cancer in ulcerative colitis: A Scandinavian population-based cohort study. Lancet 2020, 395, 123–131. [Google Scholar] [CrossRef]
- Ekbom, A.; Helmick, C.; Zack, M.; Adami, H.O. Ulcerative Colitis and Colorectal Cancer. A Population-Based Study. N. Engl. J. Med. 1990, 323, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Birch, R.J.; Burr, N.; Subramanian, V.; Tiernan, J.P.; Hull, M.A.; Finan, P.; Rose, A.; Rutter, M.; Valori, R.; Downing, A. Inflammatory Bowel Disease-Associated Colorectal Cancer Epidemiology and Outcomes: An English Population-Based Study. Am. J. Gastroenterol. 2022, 117, 1858–1870. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Tsujinaka, S.; Miura, T.; Kitamura, Y.; Suzuki, H.; Shibata, C. Inflammatory Bowel Disease and Colorectal Cancer: Epidemiology, Etiology, Surveillance, and Management. Cancers 2023, 15, 4154. [Google Scholar] [CrossRef]
- Molla, M.D.; Symonds, E.L.; Winter, J.M.; Debie, A.; Wassie, M.M. Metabolic Risk Factors of Colorectal Cancer: Umbrella Review. Crit. Rev. Oncol. Hematol. 2024, 204, 104502. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.; Wang, W.; Zhang, B.; Yu, X.; Shi, S. Epigenetic Regulation in the Tumor Microenvironment: Molecular Mechanisms and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 210. [Google Scholar] [CrossRef]
- Stayoussef, M.; Weili, X.; Habel, A.; Barbirou, M.; Bedoui, S.; Attia, A.; Omrani, Y.; Zouari, K.; Maghrebi, H.; Almawi, W.Y. Altered Expression of Cytokines, Chemokines, Growth Factors, and Soluble Receptors in Patients with Colorectal Cancer, and Correlation with Treatment Outcome. Cancer Immunol. Immunother. 2024, 73, 169. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Wu, C. The Role of the Tumor Microenvironment and Treatment Strategies in Colorectal Cancer. Front. Immunol. 2021, 12, 792691. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Sivani, B.M.; Azzeh, M.; Patnaik, R.; Pantea Stoian, A.; Rizzo, M.; Banerjee, Y. Reconnoitering the Therapeutic Role of Curcumin in Disease Prevention and Treatment: Lessons Learnt and Future Directions. Metabolites 2022, 12, 639. [Google Scholar] [CrossRef]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The Role of Curcumin in Cancer Treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.; Maldonado-Valderrama, J.; Galvez-Ruiz, M.J. Interactions Between Curcumin and Cell Membrane Models by Langmuir Monolayers. Colloids Surf. B Biointerfaces 2022, 217, 112636. [Google Scholar] [CrossRef]
- Jobin, C.; Bradham, C.A.; Russo, M.P.; Juma, B.; Narula, A.S.; Brenner, D.A.; Sartor, R.B. Curcumin Blocks Cytokine-Mediated NF-κB Activation and Proinflammatory Gene Expression by Inhibiting Inhibitory Factor I-κB Kinase Activity. J. Immunol. 1999, 163, 3474–3483. [Google Scholar] [CrossRef] [PubMed]
- Plummer, S.M.; Holloway, K.A.; Manson, M.M.; Munks, R.J.; Kaptein, A.; Farrow, S.; Howells, L. Inhibition of Cyclo-Oxygenase 2 Expression in Colon Cells by the Chemopreventive Agent Curcumin Involves Inhibition of NF-κB Activation via the NIK/IKK Signalling Complex. Oncogene 1999, 18, 6013–6020. [Google Scholar] [CrossRef]
- Kubota, M.; Shimizu, M.; Sakai, H.; Yasuda, Y.; Terakura, D.; Baba, A.; Ohno, T.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Preventive Effects of Curcumin on the Development of Azoxymethane-Induced Colonic Preneoplastic Lesions in Male C57BL/KsJ-Db/Db Obese Mice. Nutr. Cancer 2012, 64, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Villegas, I.; Sanchez-Fidalgo, S.; Lastra, C.A. Chemopreventive Effect of Dietary Curcumin on Inflammation-Induced Colorectal Carcinogenesis in Mice. Mol. Nutr. Food Res. 2011, 55, 259–267. [Google Scholar] [CrossRef]
- Goel, A.; Boland, C.R.; Chauhan, D.P. Specific Inhibition of Cyclooxygenase-2 (COX-2) Expression by Dietary Curcumin in HT-29 Human Colon Cancer Cells. Cancer Lett. 2001, 172, 111–118. [Google Scholar] [CrossRef]
- Ameer, S.F.; Mohamed, M.Y.; Elzubair, Q.A.; Sharif, E.A.M.; Ibrahim, W.N. Curcumin as a Novel Therapeutic Candidate for Cancer: Can This Natural Compound Revolutionize Cancer Treatment? Front. Oncol. 2024, 14, 1438040. [Google Scholar] [CrossRef]
- Li, Q.; Ding, Y.; Ou, Y.; Li, M.; Jithavech, P.; Buranasudja, V.; Sritularak, B.; Xu, Y.; Rojsitthisak, P.; Han, J. Curcuminoids Modulated the IL-6/JAK/STAT3 Signaling Pathway in LoVo and HT-29 Colorectal Cancer Cells. Curr. Pharm. Des. 2023, 29, 2867–2876. [Google Scholar] [CrossRef]
- Ham, I.H.; Wang, L.; Lee, D.; Woo, J.; Kim, T.H.; Jeong, H.Y.; Oh, H.J.; Choi, K.S.; Kim, T.M.; Hur, H. Curcumin Inhibits the Cancer-Associated Fibroblast-Derived Chemoresistance of Gastric Cancer Through the Suppression of the JAK/STAT3 Signaling Pathway. Int. J. Oncol. 2022, 61, 85. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.; Zhao, K.; Chen, B.; Sun, Z. Natural Products Modulating MAPK for CRC Treatment: A Promising Strategy. Front. Pharmacol. 2025, 16, 1514486. [Google Scholar] [CrossRef]
- Shinojima, N.; Yokoyama, T.; Kondo, Y.; Kondo, S. Roles of the Akt/mTOR/p70S6K and ERK1/2 Signaling Pathways in Curcumin-Induced Autophagy. Autophagy 2007, 3, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Tan, T.H. Inhibition of the C-Jun N-Terminal Kinase (JNK) Signaling Pathway by Curcumin. Oncogene 1998, 17, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.; Docena, G.; MacDonald, T.T.; Sanderson, I.R. Curcumin Suppresses P38 Mitogen-Activated Protein Kinase Activation, Reduces IL-1beta and Matrix Metalloproteinase-3 and Enhances IL-10 in the Mucosa of Children and Adults with Inflammatory Bowel Disease. Br. J. Nutr. 2010, 103, 824–832. [Google Scholar] [CrossRef]
- Jiang, Q.G.; Li, T.Y.; Liu, D.N.; Zhang, H.T. PI3K/Akt Pathway Involving into Apoptosis and Invasion in Human Colon Cancer Cells LoVo. Mol. Biol. Rep. 2014, 41, 3359–3367. [Google Scholar] [CrossRef]
- Hao, J.; Dai, X.; Gao, J.; Li, Y.; Hou, Z.; Chang, Z.; Wang, Y. Curcumin Suppresses Colorectal Tumorigenesis via the Wnt/Beta-Catenin Signaling Pathway by Downregulating Axin2. Oncol. Lett. 2021, 21, 186. [Google Scholar] [CrossRef]
- Ismail, N.I.; Othman, I.; Abas, F.; Lajis, H.N.; Naidu, R. Mechanism of Apoptosis Induced by Curcumin in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2454. [Google Scholar] [CrossRef]
- Gogada, R.; Amadori, M.; Zhang, H.; Jones, A.; Verone, A.; Pitarresi, J.; Jandhyam, S.; Prabhu, V.; Black, J.D.; Chandra, D. Curcumin Induces Apaf-1-Dependent, P21-Mediated Caspase Activation and Apoptosis. Cell Cycle 2011, 10, 4128–4137. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Lee, S.A.; Park, M.G.; Kim, J.S.; Yu, S.K.; Kim, C.S.; Kim, J.S.; Kim, S.G.; Oh, J.S.; Kim, H.J. Induction of Apoptosis by Diphenyldifluoroketone in Osteogenic Sarcoma Cells Is Associated with Activation of Caspases. Oncol. Rep. 2014, 31, 2286–2292. [Google Scholar] [CrossRef] [PubMed]
- Kuttikrishnan, S.; Siveen, K.S.; Prabhu, K.S.; Khan, A.Q.; Ahmed, E.I.; Akhtar, S.; Ali, T.A.; Merhi, M.; Dermime, S.; Steinhoff, M. Curcumin Induces Apoptotic Cell Death via Inhibition of PI3-Kinase/AKT Pathway in B-Precursor Acute Lymphoblastic Leukemia. Front. Oncol. 2019, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Lu, G.H.; Wu, Y.Q.; Zheng, Y.; Xu, K.; Wu, L.J.; Jiang, Z.Y.; Feng, R.; Zhou, J.Y. Curcumin Induces Mitochondria Pathway Mediated Cell Apoptosis in A549 Lung Adenocarcinoma Cells. Oncol. Rep. 2010, 23, 1285–1292. [Google Scholar] [CrossRef]
- Wang, J.B.; Qi, L.L.; Zheng, S.D.; Wu, T.X. Curcumin Induces Apoptosis Through the Mitochondria-Mediated Apoptotic Pathway in HT-29 Cells. J. Zhejiang Univ. Sci. B 2009, 10, 93–102. [Google Scholar] [CrossRef]
- Anto, R.J.; Mukhopadhyay, A.; Denning, K.; Aggarwal, B.B. Curcumin (Diferuloylmethane) Induces Apoptosis Through Activation of Caspase-8, BID Cleavage and Cytochrome c Release: Its Suppression by Ectopic Expression of Bcl-2 and Bcl-Xl. Carcinogenesis 2002, 23, 143–150. [Google Scholar] [CrossRef]
- Song, G.; Mao, Y.B.; Cai, Q.F.; Yao, L.M.; Ouyang, G.L.; Bao, S.D. Curcumin Induces Human HT-29 Colon Adenocarcinoma Cell Apoptosis by Activating P53 and Regulating Apoptosis-Related Protein Expression. Braz. J. Med. Biol. Res. 2005, 38, 1791–1798. [Google Scholar] [CrossRef]
- Huang, Y.F.; Zhu, D.J.; Chen, X.W.; Chen, Q.K.; Luo, Z.T.; Liu, C.C.; Wang, G.X.; Zhang, W.J.; Liao, N.Z. Curcumin Enhances the Effects of Irinotecan on Colorectal Cancer Cells Through the Generation of Reactive Oxygen Species and Activation of the Endoplasmic Reticulum Stress Pathway. Oncotarget 2017, 8, 40264–40275. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Huang, J.J.; Zhang, J.F.; Dai, W.J.; Li, R.L.; Lu, Z.Y.; Duan, J.L.; Li, J.P.; Chen, X.P.; Fan, J.P. Curcumin Induces G2/M Cell Cycle Arrest and Apoptosis of Head and Neck Squamous Cell Carcinoma In Vitro and In Vivo Through ATM/Chk2/P53-Dependent Pathway. Oncotarget 2017, 8, 50747–50760. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Xiong, X.; Lu, M.; Huang, S.; Luo, Y.; Wang, Y.; Ying, Y. Curcumin suppresses colorectal tumorigenesis through restoring the gut microbiota and metabolites. BMC Cancer 2024, 24, 1141. [Google Scholar] [CrossRef]
- Sordillo, P.P.; Helson, L. Curcumin and Cancer Stem Cells: Curcumin Has Asymmetrical Effects on Cancer and Normal Stem Cells. Anticancer Res. 2015, 35, 599–614. [Google Scholar] [PubMed]
- Ming, T.; Tao, Q.; Tang, S.; Zhao, H.; Yang, H.; Liu, M.; Ren, S.; Xu, H. Curcumin: An Epigenetic Regulator and Its Application in Cancer. Biomed. Pharmacother. 2022, 156, 113956. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, X.; Guan, S.; Yan, Y.; Lin, H.; Hua, Z.C. Curcumin Inhibits Angiogenesis and Improves Defective Hematopoiesis Induced by Tumor-Derived VEGF in Tumor Model Through Modulating VEGF-VEGFR2 Signaling Pathway. Oncotarget 2015, 6, 19469–19482. [Google Scholar] [CrossRef]
- Arora, C.; Matic, M.; Bisceglia, L.; Chiaro, P.; Oliveira Rosa, N.; Carli, F.; Clubb, L.; Nemati Fard, L.A.; Kargas, G.; Diaferia, G.R. The Landscape of Cancer-Rewired GPCR Signaling Axes. Cell Genomics 2024, 4, 100557. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, T.; Lu, X.; Lan, X.; Chen, Z.; Lu, S. G Protein-Coupled Receptors (GPCRs): Advances in Structures, Mechanisms, and Drug Discovery. Signal Transduct. Target. Ther. 2024, 9, 88. [Google Scholar] [CrossRef]
- Arang, N.; Gutkind, J.S. G Protein-Coupled Receptors and Heterotrimeric G Proteins as Cancer Drivers. FEBS Lett. 2020, 594, 4201–4232. [Google Scholar] [CrossRef]
- O’Hayre, M.; Vazquez-Prado, J.; Kufareva, I.; Stawiski, E.W.; Handel, T.M.; Seshagiri, S.; Gutkind, J.S. The Emerging Mutational Landscape of G Proteins and G-Protein-Coupled Receptors in Cancer. Nat. Rev. Cancer 2013, 13, 412–424. [Google Scholar] [CrossRef]
- Patnaik, R.; Riaz, S.; Sivani, B.M.; Faisal, S.; Naidoo, N.; Rizzo, M.; Banerjee, Y. Evaluating the Potential of Vitamin D and Curcumin to Alleviate Inflammation and Mitigate the Progression of Osteoarthritis Through Their Effects on Human Chondrocytes: A Proof-of-Concept Investigation. PLoS ONE 2023, 18, 0290739. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, R.; Varghese, R.L.; Khan, S.; Huda, B.; Bhurka, F.; Amiri, L.; Banerjee, Y. Targeting PAR-2-Driven Inflammatory Pathways in Colorectal Cancer: Mechanistic Insights from Atorvastatin and Rosuvastatin Treatment in Cell Line Models. Transl. Cancer Res. 2025, 14, 1531–1566. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, R.; Varghese, R.L.; Banerjee, Y. Selective Modulation of PAR-2-Driven Inflammatory Pathways by Oleocanthal: Attenuation of TNF-α and Calcium Dysregulation in Colorectal Cancer Models. Int. J. Mol. Sci. 2025, 26, 2934. [Google Scholar] [CrossRef]
- Jannati, S.; Patnaik, R.; Banerjee, Y. Beyond Anticoagulation: A Comprehensive Review of Non-Vitamin K Oral Anticoagulants (NOACs) in Inflammation and Protease-Activated Receptor Signaling. Int. J. Mol. Sci. 2024, 25, 8727. [Google Scholar] [CrossRef]
- Ma, Y.; He, L.; Zhao, X.; Li, W.; Lv, X.; Zhang, X.; Peng, J.; Yang, L.; Xu, Q.; Wang, H. Protease Activated Receptor 2 Signaling Promotes Self-Renewal and Metastasis in Colorectal Cancer Through Beta-Catenin and Periostin. Cancer Lett. 2021, 521, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Hill, T.A.; Lim, J.; Fairlie, D.P. Protease-Activated Receptor 2 Attenuates Doxorubicin-Induced Apoptosis in Colon Cancer Cells. J. Cell Commun. Signal 2023, 17, 1293–1307. [Google Scholar] [CrossRef]
- Jiang, P.; Li, S.; Li, Z.G.; Zhu, Y.C.; Yi, X.J.; Li, S.M. The Expression of Protease-Activated Receptors in Esophageal Carcinoma Cells: The Relationship Between Changes in Gene Expression and Cell Proliferation, Apoptosis In Vitro and Growing Ability In Vivo. Cancer Cell Int. 2018, 18, 81. [Google Scholar] [CrossRef]
- Vetvicka, D.; Suhaj, P.; Olejar, T.; Sivak, L.; Benes, J.; Pouckova, P. Proteinase-Activated Receptor 2: Springboard of Tumors. Anticancer Res. 2024, 44, 1–12. [Google Scholar] [CrossRef]
- Latorre, R.; Hegron, A.; Peach, C.J.; Teng, S.; Tonello, R.; Retamal, J.S.; Klein-Cloud, R.; Bok, D.; Jensen, D.D.; Gottesman-Katz, L. Mice Expressing Fluorescent PAR(2) Reveal That Endocytosis Mediates Colonic Inflammation and Pain. Proc. Natl. Acad. Sci. USA 2022, 119, e2112059119. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, G.R.; Lv, C.G.; Zhang, B.L.; Zhang, Z.L.; Zhang, X.F. Protease-Activated Receptor-2 Induces Expression of Vascular Endothelial Growth Factor and Cyclooxygenase-2 via the Mitogen-Activated Protein Kinase Pathway in Gastric Cancer Cells. Oncol. Rep. 2012, 28, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, J.; Zhou, H.; Yu, X.; Hu, L.; Meng, F.; Jiang, S. Effects of Calcium Signaling on Coagulation Factor VIIa-Induced Proliferation and Migration of the SW620 Colon Cancer Cell Line. Mol. Med. Rep. 2014, 10, 3021–3026. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Jin, K.; He, K.; Cao, J.; Teng, L. Protease-Activated Receptors in Cancer: A Systematic Review. Oncol. Lett. 2011, 2, 599–608. [Google Scholar] [CrossRef]
- Darmoul, D.; Marie, J.C.; Devaud, H.; Gratio, V.; Laburthe, M. Initiation of Human Colon Cancer Cell Proliferation by Trypsin Acting at Protease-Activated Receptor-2. Br. J. Cancer 2001, 85, 772–779. [Google Scholar] [CrossRef]
- Hu, X.; Fatima, S.; Chen, M.; Xu, K.; Huang, C.; Gong, R.H.; Su, T.; Wong, H.L.X.; Bian, Z.; Kwan, H.Y. Toll-like Receptor 4 Is a Master Regulator for Colorectal Cancer Growth Under High-Fat Diet by Programming Cancer Metabolism. Cell Death Dis. 2021, 12, 791. [Google Scholar] [CrossRef]
- Cohen, E.; Ophir, I.; Shaul, Y.B. Induced Differentiation in HT29, A Human Colon Adenocarcinoma Cell Line. J. Cell Sci. 1999, 112, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, J.; Kanwar, S.S.; Yu, Y.; Majumdar, A.P. Combination of dasatinib and curcumin eliminates chemo-resistant colon cancer cells. J. Mol. Signal. 2011, 6, 7. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. (Eds.) The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-15791-7. [Google Scholar]
- Fernando, E.H.; Gordon, M.H.; Beck, P.L.; MacNaughton, W.K. Inhibition of Intestinal Epithelial Wound Healing Through Protease-Activated Receptor-2 Activation in Caco2 Cells. J. Pharmacol. Exp. Ther. 2018, 367, 382–392. [Google Scholar] [CrossRef]
- Barrett, K.E.; Keely, S.J. Chloride Secretion by the Intestinal Epithelium: Molecular Basis and Regulatory Aspects. Annu. Rev. Physiol. 2000, 62, 535–572. [Google Scholar] [CrossRef]
- Leibovitz, A.; Stinson, J.C.; McCombs, W.B.; McCoy, C.E.; Mazur, K.C.; Mabry, N.D. Classification of Human Colorectal Adenocarcinoma Cell Lines. Cancer Res. 1976, 36, 4562–4569. [Google Scholar]
- Hsu, R.Y.; Chan, C.H.; Spicer, J.D.; Rousseau, M.C.; Giannias, B.; Rousseau, S.; Ferri, L.E. LPS-Induced TLR4 Signaling in Human Colorectal Cancer Cells Increases Beta1 Integrin-Mediated Cell Adhesion and Liver Metastasis. Cancer Res. 2011, 71, 1989–1998. [Google Scholar] [CrossRef]
- Chantret, I.; Barbat, A.; Dussaulx, E.; Brattain, M.G.; Zweibaum, A. Epithelial Polarity, Villin Expression, and Enterocytic Differentiation of Cultured Human Colon Carcinoma Cells: A Survey of Twenty Cell Lines. Cancer Res. 1988, 48, 1936–1942. [Google Scholar]
- Augeron, C.; Laboisse, C.L. Emergence of Permanently Differentiated Cell Clones in a Human Colonic Cancer Cell Line in Culture After Treatment with Sodium Butyrate. Cancer Res. 1984, 44, 3961–3969. [Google Scholar] [PubMed]
- Kolios, G.; Wright, K.L.; Jordan, N.J.; Leithead, J.B.; Robertson, D.A.; Westwick, J. C-X-C and C-C Chemokine Expression and Secretion by the Human Colonic Epithelial Cell Line, HT-29: Differential Effect of T Lymphocyte-Derived Cytokines. Eur. J. Immunol. 1999, 29, 530–536. [Google Scholar] [CrossRef]
- Househam, J.; Heide, T.; Cresswell, G.D.; Spiteri, I.; Kimberley, C.; Zapata, L.; Lynn, C.; James, C.; Mossner, M.; Fernandez-Mateos, J. Phenotypic Plasticity and Genetic Control in Colorectal Cancer Evolution. Nature 2022, 611, 744–753. [Google Scholar] [CrossRef]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKbeta Links Inflammation and Tumorigenesis in a Mouse Model of Colitis-Associated Cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef]
- Rousset, M. The Human Colon Carcinoma Cell Lines HT-29 and Caco-2: Two in Vitro Models for the Study of Intestinal Differentiation. Biochimie 1986, 68, 1035–1040. [Google Scholar] [CrossRef]
- Behrendt, I.; Röder, I.; Will, F.; Mostafa, H.; Gonzalez-Dominguez, R.; Meroño, T.; Andres-Lacueva, C.; Fasshauer, M.; Rudloff, S.; Kuntz, S. Influence of Plasma-Isolated Anthocyanins and Their Metabolites on Cancer Cell Migration (HT-29 and Caco-2) In Vitro: Results of the ATTACH Study. Antioxidants 2022, 11, 1341. [Google Scholar] [CrossRef]
- Gheytanchi, E.; Naseri, M.; Karimi-Busheri, F.; Atyabi, F.; Mirsharif, E.S.; Bozorgmehr, M.; Ghods, R.; Madjd, Z. Morphological and Molecular Characteristics of Spheroid Formation in HT-29 and Caco-2 Colorectal Cancer Cell Lines. Cancer Cell Int. 2021, 21, 204. [Google Scholar] [CrossRef]
- Patnaik, R.; Jannati, S.; Sivani, B.M.; Rizzo, M.; Naidoo, N.; Banerjee, Y. Efficient Generation of Chondrocytes from Bone Marrow-Derived Mesenchymal Stem Cells in a 3D Culture System: Protocol for a Practical Model for Assessing Anti-Inflammatory Therapies. JMIR Res. Protoc. 2023, 12, e42964. [Google Scholar] [CrossRef] [PubMed]
- Dery, O.; Corvera, C.U.; Steinhoff, M.; Bunnett, N.W. Proteinase-Activated Receptors: Novel Mechanisms of Signaling by Serine Proteases. Am. J. Physiol. 1998, 274, 1429–1452. [Google Scholar] [CrossRef]
- Kuwahara, I.; Lillehoj, E.P.; Lu, W.; Singh, I.S.; Isohama, Y.; Miyata, T.; Kim, K.C. Neutrophil Elastase Induces IL-8 Gene Transcription and Protein Release Through P38/NF-κB Activation via EGFR Transactivation in a Lung Epithelial Cell Line. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.G.; Shin, B.A.; Park, J.S.; Lee, K.H.; Chay, K.O.; Yang, S.Y.; Ahn, B.W.; Jung, Y.D. IL-1beta Induces MMP-9 via Reactive Oxygen Species and NF-κB in Murine Macrophage RAW 264.7 Cells. Biochem. Biophys. Res. Commun. 2002, 298, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Rallabhandi, P.; Nhu, Q.M.; Toshchakov, V.Y.; Piao, W.; Medvedev, A.E.; Hollenberg, M.D.; Fasano, A.; Vogel, S.N. Analysis of Proteinase-Activated Receptor 2 and TLR4 Signal Transduction: A Novel Paradigm for Receptor Cooperativity. J. Biol. Chem. 2008, 283, 24314–24325. [Google Scholar] [CrossRef]
- Nhu, Q.M.; Shirey, K.; Teijaro, J.R.; Farber, D.L.; Netzel-Arnett, S.; Antalis, T.M.; Fasano, A.; Vogel, S.N. Novel Signaling Interactions Between Proteinase-Activated Receptor 2 and Toll-like Receptors In Vitro and In Vivo. Mucosal Immunol. 2010, 3, 29–39. [Google Scholar] [CrossRef]
- Bohm, S.K.; Kong, W.; Bromme, D.; Smeekens, S.P.; Anderson, D.C.; Connolly, A.; Kahn, M.; Nelken, N.A.; Coughlin, S.R.; Payan, D.G. Molecular Cloning, Expression and Potential Functions of the Human Proteinase-Activated Receptor-2. Biochem. J. 1996, 314, 1009–1016. [Google Scholar] [CrossRef]
- Xu, W.F.; Andersen, H.; Whitmore, T.E.; Presnell, S.R.; Yee, D.P.; Ching, A.; Gilbert, T.; Davie, E.W.; Foster, D.C. Cloning and Characterization of Human Protease-Activated Receptor 4. Proc. Natl. Acad. Sci. USA 1998, 95, 6642–6646. [Google Scholar] [CrossRef]
- DeFea, K.A.; Zalevsky, J.; Thoma, M.S.; Dery, O.; Mullins, R.D.; Bunnett, N.W. Beta-Arrestin-Dependent Endocytosis of Proteinase-Activated Receptor 2 Is Required for Intracellular Targeting of Activated ERK1/2. J. Cell Biol. 2000, 148, 1267–1281. [Google Scholar] [CrossRef]
- Travis, J.; Salvesen, G.S. Human plasma proteinase inhibitors. Annu. Rev. Biochem. 1983, 52, 655–709. [Google Scholar] [CrossRef]
- Liu, Q.; Weng, H.J.; Patel, K.N.; Tang, Z.; Bai, H.; Steinhoff, M.; Dong, X. The Distinct Roles of Two GPCRs, MrgprC11 and PAR2, in Itch and Hyperalgesia. Sci. Signal. 2011, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, A.; Kuroda, R.; Nishikawa, H.; Kawai, K. Modulation by Protease-Activated Receptors of the Rat Duodenal Motility in Vitro: Possible Mechanisms Underlying the Evoked Contraction and Relaxation. Br. J. Pharmacol. 1999, 128, 865–872. [Google Scholar] [CrossRef]
- Ramachandran, R.; Mihara, K.; Chung, H.; Renaux, B.; Lau, C.S.; Muruve, D.A.; DeFea, K.A.; Bouvier, M.; Hollenberg, M.D. Neutrophil elastase acts as a biased agonist for proteinase-activated receptor-2 (PAR2). J. Biol. Chem. 2011, 286, 24638–24648. [Google Scholar] [CrossRef]
- Lohman, R.J.; Cotterell, A.J.; Barry, G.D.; Liu, L.; Suen, J.Y.; Vesey, D.A.; Fairlie, D.P. An Antagonist of Human Protease Activated Receptor-2 Attenuates PAR2 Signaling, Macrophage Activation, Mast Cell Degranulation, and Collagen-Induced Arthritis in Rats. FASEB J. 2012, 26, 2877–2887. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Gulhati, P.; Arrieta, I.; Wang, X.; Uchida, T.; Gao, T.; Evers, B.M. Curcumin Inhibits Proliferation of Colorectal Carcinoma by Modulating Akt/mTOR Signaling. Anticancer Res. 2009, 29, 3185–3190. [Google Scholar]
- Erk, M.J.; Teuling, E.; Staal, Y.C.; Huybers, S.; Bladeren, P.J.; Aarts, J.M.; Ommen, B. Time- and Dose-Dependent Effects of Curcumin on Gene Expression in Human Colon Cancer Cells. J. Carcinog. 2004, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Shrivastav, A.; Sharma, R.K. Curcumin Induces Caspase and Calpain-Dependent Apoptosis in HT29 Human Colon Cancer Cells. Mol. Med. Rep. 2009, 2, 627–631. [Google Scholar] [CrossRef]
- Chen, A.; Xu, J.; Johnson, A.C. Curcumin Inhibits Human Colon Cancer Cell Growth by Suppressing Gene Expression of Epidermal Growth Factor Receptor Through Reducing the Activity of the Transcription Factor Egr-1. Oncogene 2006, 25, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Conteas, C.N. Anti-Carcinogenic Properties of Curcumin on Colorectal Cancer. World J. Gastrointest. Oncol. 2010, 2, 169–176. [Google Scholar] [CrossRef]
- Herrero de la Parte, B.; Rodeño-Casado, M.; Iturrizaga Correcher, S.; Mar Medina, C.; García-Alonso, I. Curcumin Reduces Colorectal Cancer Cell Proliferation and Migration and Slows In Vivo Growth of Liver Metastases in Rats. Biomedicines 2021, 9, 1183. [Google Scholar] [CrossRef]
- Patel, V.B.; Misra, S.; Patel, B.B.; Majumdar, A.P. Colorectal cancer: Chemopreventive role of curcumin and resveratrol. Nutr. Cancer 2010, 62, 958–967. [Google Scholar] [CrossRef]
- Gunther, J.R.; Chadha, A.S.; Guha, S.; Raju, G.S.; Maru, D.M.; Munsell, M.F.; Jiang, Y.; Yang, P.; Felix, E.; Clemons, M. A Phase II Randomized Double Blinded Trial Evaluating the Efficacy of Curcumin with Pre-Operative Chemoradiation for Rectal Cancer. J. Gastrointest. Oncol. 2022, 13, 2938–2950. [Google Scholar] [CrossRef]
- Irving, G.R.; Howells, L.M.; Sale, S.; Kralj-Hans, I.; Atkin, W.S.; Clark, S.K.; Britton, R.G.; Jones, D.J.; Scott, E.N.; Berry, D.P. Prolonged Biologically Active Colonic Tissue Levels of Curcumin Achieved After Oral Administration—A Clinical Pilot Study Including Assessment of Patient Acceptability. Cancer Prev. Res. 2013, 6, 119–128. [Google Scholar] [CrossRef]
- Epstein, J.; Sanderson, I.R.; Macdonald, T.T. Curcumin as a Therapeutic Agent: The Evidence from in Vitro, Animal and Human Studies. Br. J. Nutr. 2010, 103, 1545–1557. [Google Scholar] [CrossRef]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of Curcumin Conjugate Metabolites in Healthy Human Subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef]
- Kim, H.-Y. Statistical Notes for Clinical Researchers: Post-Hoc Multiple Comparisons. Restor. Dent. Endod. 2015, 40, 172–176. [Google Scholar] [CrossRef]
- Ramey-Ward, A.N.; Walthall, H.P.; Smith, S.; Barrows, T.H. Human Keratin Matrices Promote Wound Healing by Modulating Skin Cell Expression of Cytokines and Growth Factors. Wound Repair. Regen. 2024, 32, 257–267. [Google Scholar] [CrossRef]
- Lin, H.; Liu, A.P.; Smith, T.H.; Trejo, J. Cofactoring and Dimerization of Proteinase-Activated Receptors. Pharmacol. Rev. 2013, 65, 1198–1213. [Google Scholar] [CrossRef] [PubMed]
- Procter, J.B.; Carstairs, G.M.; Soares, B.; Mourao, K.; Ofoegbu, T.C.; Barton, D.; Lui, L.; Menard, A.; Sherstnev, N.; Roldan-Martinez, D. Alignment of Biological Sequences with Jalview. Methods Mol. Biol. 2021, 2231, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, P.; Zhang, C.; Lee, S.; Wang, W.; Zou, H. PAR4 overexpression promotes colorectal cancer cell proliferation and migration. Oncol. Lett. 2018, 16, 5745–5752. [Google Scholar] [CrossRef] [PubMed]
- Kahsai, A.W.; Shah, K.S.; Shim, P.J.; Lee, M.A.; Shreiber, B.N.; Schwalb, A.M.; Zhang, X.; Kwon, H.Y.; Huang, L.Y.; Soderblom, E.J. Signal Transduction at GPCRs: Allosteric Activation of the ERK MAPK by Beta-Arrestin. Proc. Natl. Acad. Sci. USA 2023, 120, 2303794120. [Google Scholar] [CrossRef]
- Meloche, S.; Pouyssegur, J. The ERK1/2 Mitogen-Activated Protein Kinase Pathway as a Master Regulator of the G1- to S-Phase Transition. Oncogene 2007, 26, 3227–3239. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, X.; Li, Z.; Huang, Q.; Li, F.; Li, C.Y. Caspase-3 Regulates the Migration, Invasion and Metastasis of Colon Cancer Cells. Int. J. Cancer 2018, 143, 921–930. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, C.; Liao, Y.; Zhou, M.; Xu, W.; Zou, Z. Caspase-8 in Inflammatory Diseases: A Potential Therapeutic Target. Cell Mol. Biol. Lett. 2024, 29, 130. [Google Scholar] [CrossRef] [PubMed]
- Opferman, J.T.; Kothari, A. Anti-Apoptotic BCL-2 Family Members in Development. Cell Death Differ. 2018, 25, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Lavrik, I.N.; Krammer, P.H. Regulation of CD95/Fas Signaling at the DISC. Cell Death Differ. 2012, 19, 36–41. [Google Scholar] [CrossRef]
- Kluck, R.M.; Esposti, M.D.; Perkins, G.; Renken, C.; Kuwana, T.; Bossy-Wetzel, E.; Goldberg, M.; Allen, T.; Barber, M.J.; Green, D.R. The Pro-Apoptotic Proteins, Bid and Bax, Cause a Limited Permeabilization of the Mitochondrial Outer Membrane That Is Enhanced by Cytosol. J. Cell Biol. 1999, 147, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Chen, Q.; Sarva, K.; Siddiqui, I.; Srivastava, R.K. Curcumin Enhances the Apoptosis-Inducing Potential of TRAIL in Prostate Cancer Cells: Molecular Mechanisms of Apoptosis, Migration and Angiogenesis. J. Mol. Signal 2007, 2, 10. [Google Scholar] [CrossRef]
- Schroeder, A.B.; Dobson, E.T.A.; Rueden, C.T.; Tomancak, P.; Jug, F.; Eliceiri, K.W. The ImageJ Ecosystem: Open-Source Software for Image Visualization, Processing, and Analysis. Protein Sci. 2021, 30, 234–249. [Google Scholar] [CrossRef]
- Ribble, D.; Goldstein, N.B.; Norris, D.A.; Shellman, Y.G. A Simple Technique for Quantifying Apoptosis in 96-Well Plates. BMC Biotechnol. 2005, 5, 12. [Google Scholar] [CrossRef]
- Moon, D.O. Calcium’s Role in Orchestrating Cancer Apoptosis: Mitochondrial-Centric Perspective. Int. J. Mol. Sci. 2023, 24, 8982. [Google Scholar] [CrossRef]
- Macfarlane, S.R.; Sloss, C.M.; Cameron, P.; Kanke, T.; McKenzie, R.C.; Plevin, R. The Role of Intracellular Ca2+ in the Regulation of Proteinase-Activated Receptor-2 Mediated Nuclear Factor Kappa B Signalling in Keratinocytes. Br. J. Pharmacol. 2005, 145, 535–544. [Google Scholar] [CrossRef] [PubMed]
- UniProt, C. UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2025, 53, 609–617. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Mirdita, M.; Schutze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making Protein Folding Accessible to All. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Han, S.; Kwak, I.Y. Mastering Data Visualization with Python: Practical Tips for Researchers. J. Minim. Invasive Surg. 2023, 26, 167–175. [Google Scholar] [CrossRef]
- Guo, H.B.; Perminov, A.; Bekele, S.; Kedziora, G.; Farajollahi, S.; Varaljay, V.; Hinkle, K.; Molinero, V.; Meister, K.; Hung, C. AlphaFold2 Models Indicate That Protein Sequence Determines Both Structure and Dynamics. Sci. Rep. 2022, 12, 10696. [Google Scholar] [CrossRef]
- Wroblewski, K.; Kmiecik, S. Integrating AlphaFold pLDDT Scores into CABS-Flex for Enhanced Protein Flexibility Simulations. Comput. Struct. Biotechnol. J. 2024, 23, 4350–4356. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for Structure Building and Analysis. Protein Sci. 2023, 32, 4792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Q.; Li, Y.; Teng, A.; Hu, G.; Wuyun, Q.; Zheng, W. The Historical Evolution and Significance of Multiple Sequence Alignment in Molecular Structure and Function Prediction. Biomolecules 2024, 14, 1531. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.X.; Cao, Y. CB-Dock2: Improved Protein-Ligand Blind Docking by Integrating Cavity Detection, Docking and Homologous Template Fitting. Nucleic Acids Res. 2022, 50, 159–164. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y. Protein-Ligand Blind Docking Using CB-Dock2. Methods Mol. Biol. 2024, 2714, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.L.; Vegso, A.J.; Baggio, C.H.; MacNaughton, W.K. Protease-Activated Receptor 2 Drives Migration in a Colon Cancer Cell Line but Not in Noncancerous Human Epithelial Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2024, 326, 525–542. [Google Scholar] [CrossRef]
- Webster, J.D.; Vucic, D. The Balance of TNF Mediated Pathways Regulates Inflammatory Cell Death Signaling in Healthy and Diseased Tissues. Front. Cell Dev. Biol. 2020, 8, 365. [Google Scholar] [CrossRef]
- Sabio, G.; Davis, R.J. TNF and MAP Kinase Signalling Pathways. Semin. Immunol. 2014, 26, 237–245. [Google Scholar] [CrossRef]
- Serebrennikova, O.B.; Paraskevopoulou, M.D.; Aguado-Fraile, E.; Taraslia, V.; Ren, W.; Thapa, G.; Roper, J.; Du, K.; Croce, C.M.; Tsichlis, P.N. The Combination of TPL2 Knockdown and TNFα Causes Synthetic Lethality via Caspase-8 Activation in Human Carcinoma Cell Lines. Proc. Natl. Acad. Sci. USA 2019, 116, 14039–14048. [Google Scholar] [CrossRef] [PubMed]
- Khoon, L.; Piran, R. A New Strategy in Modulating the Protease-Activated Receptor 2 (Par2) in Autoimmune Diseases. Int. J. Mol. Sci. 2025, 26, 410. [Google Scholar] [CrossRef]
- Iablokov, V.; Hirota, C.L.; Peplowski, M.A.; Ramachandran, R.; Mihara, K.; Hollenberg, M.D.; MacNaughton, W.K. Proteinase-Activated Receptor 2 (PAR2) Decreases Apoptosis in Colonic Epithelial Cells. J. Biol. Chem. 2014, 289, 34366–34377. [Google Scholar] [CrossRef]
- Ma, G.; Wang, C.; Lv, B.; Jiang, Y.; Wang, L. Proteinase-Activated Receptor-2 Enhances Bcl2-like Protein-12 Expression in Lung Cancer Cells to Suppress P53 Expression. Arch. Med. Sci. 2019, 15, 1147–1153. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, J.; O’Neill, K.L.; Gurumurthy, C.B.; Quadros, R.M.; Tu, Y.; Luo, X. Cleavage by Caspase 8 and Mitochondrial Membrane Association Activate the BH3-Only Protein Bid During TRAIL-Induced Apoptosis. J. Biol. Chem. 2016, 291, 11843–11851. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. The Pathophysiology of Mitochondrial Cell Death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Lyu, Z.; Lyu, X.; Malyutin, A.G.; Xia, G.; Carney, D.; Alves, V.M.; Falk, M.; Arora, N.; Zou, H.; McGrath, A.P.; et al. Structural Basis for the Activation of Proteinase-Activated Receptors PAR1 and PAR2. Nat. Commun. 2025, 16, 3931. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Ding, H.; Peng, J.; Wang, X.; Pang, C.; Wei, J.; Wei, J.; Chen, H. Down-Regulation of Protease-Activated Receptor 2 Ameliorated Osteoarthritis in Rats Through Regulation of MAPK/NF-κB Signaling Pathway In Vivo and In Vitro. Biosci. Rep. 2020, 40, BSR20192620. [Google Scholar] [CrossRef]
- Li, W.; Ma, Y.; He, L.; Li, H.; Chu, Y.; Jiang, Z.; Zhao, X.; Nie, Y.; Wang, X.; Wang, H. Protease-Activated Receptor 2 Stabilizes Bcl-xL and Regulates EGFR-Targeted Therapy Response in Colorectal Cancer. Cancer Lett. 2021, 517, 14–23. [Google Scholar] [CrossRef]
- Sebert, M.; Sola-Tapias, N.; Mas, E.; Barreau, F.; Ferrand, A. Protease-Activated Receptors in the Intestine: Focus on Inflammation and Cancer. Front. Endocrinol. 2019, 10, 717. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, K.; Liu, J.; Yang, J.; Tian, Y.; Yang, C.; Li, Y.; Shao, M.; Su, W.; Song, N. Curcumin Regulates Cancer Progression: Focus on ncRNAs and Molecular Signaling Pathways. Front. Oncol. 2021, 11, 660712. [Google Scholar] [CrossRef] [PubMed]
- Baek, O.S.; Kang, O.H.; Choi, Y.A.; Choi, S.C.; Kim, T.H.; Nah, Y.H.; Kwon, D.Y.; Kim, Y.K.; Kim, Y.H.; Bae, K.H. Curcumin Inhibits Protease-Activated Receptor-2 and -4-Mediated Mast Cell Activation. Clin. Chim. Acta 2003, 338, 135–141. [Google Scholar] [CrossRef]
- Sugiura, R.; Satoh, R.; Takasaki, T. ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer. Cells 2021, 10, 2509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kobayashi, S.; Borczuk, A.C.; Leidner, R.S.; Laframboise, T.; Levine, A.D.; Halmos, B. Dual Specificity Phosphatase 6 (DUSP6) Is an ETS-Regulated Negative Feedback Mediator of Oncogenic ERK Signaling in Lung Cancer Cells. Carcinogenesis 2010, 31, 577–586. [Google Scholar] [CrossRef]
- Ahmad, M.K.; Abdollah, N.A.; Shafie, N.H.; Yusof, N.M.; Razak, S.R.A. Dual-Specificity Phosphatase 6 (DUSP6): A Review of Its Molecular Characteristics and Clinical Relevance in Cancer. Cancer Biol. Med. 2018, 15, 14–28. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, L.; Du, S.; Wang, K.; Liu, S. Antioxidant Curcumin Induces Oxidative Stress to Kill Tumor Cells (Review). Oncol. Lett. 2024, 27, 67. [Google Scholar] [CrossRef]
- Kubiniok, P.; Lavoie, H.; Therrien, M.; Thibault, P. Time-Resolved Phosphoproteome Analysis of Paradoxical RAF Activation Reveals Novel Targets of ERK. Mol. Cell Proteom. 2017, 16, 663–679. [Google Scholar] [CrossRef]
- Hayden, L.D.; Poss, K.D.; Simone, A.; Talia, S. Mathematical modeling of Erk activity waves in regenerating zebrafish scales. Biophys. J. 2021, 120, 4287–4297. [Google Scholar] [CrossRef]
- Vesela, K.; Kejik, Z.; Abramenko, N.; Kaplanek, R.; Jakubek, M.; Petrlova, J. Investigating Antibacterial and Anti-Inflammatory Properties of Synthetic Curcuminoids. Front. Med. 2024, 11, 1478122. [Google Scholar] [CrossRef]
- Esmaealzadeh, N.; Miri, M.S.; Mavaddat, H.; Peyrovinasab, A.; Ghasemi Zargar, S.; Sirous Kabiri, S.; Razavi, S.M.; Abdolghaffari, A.H. The Regulating Effect of Curcumin on NF-κB Pathway in Neurodegenerative Diseases: A Review of the Underlying Mechanisms. Inflammopharmacology 2024, 32, 2125–2151. [Google Scholar] [CrossRef] [PubMed]
- Rafa, H.; Benkhelifa, S.; AitYounes, S.; Saoula, H.; Belhadef, S.; Belkhelfa, M.; Boukercha, A.; Toumi, R.; Soufli, I.; Moralès, O.; et al. All-Trans Retinoic Acid Modulates TLR4/NF-κB Signaling Pathway Targeting TNF-α and Nitric Oxide Synthase 2 Expression in Colonic Mucosa During Ulcerative Colitis and Colitis Associated Cancer. Mediat. Inflamm. 2017, 2017, 7353252. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An Orally Bioavailable Blocker of TNF and Other Pro-Inflammatory Biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.R.; Leite, F.R.M.; Spolidorio, L.C.; Kirkwood, K.L.; Rossa, C. Curcumin Abrogates LPS-Induced pro-Inflammatory Cytokines in RAW 264.7 Macrophages. Evidence for Novel Mechanisms Involving SOCS-1, -3 and P38 MAPK. Arch. Oral Biol. 2013, 58, 1309–1317. [Google Scholar] [CrossRef]
- Ma, F.; Liu, F.; Ding, L.; You, M.; Yue, H.; Zhou, Y.; Hou, Y. Anti-Inflammatory Effects of Curcumin Are Associated with down Regulating microRNA-155 in LPS-Treated Macrophages and Mice. Pharm. Biol. 2017, 55, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Y. Tumor Necrosis Factor and Cancer, Buddies or Foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Kokolakis, G.; Sabat, R.; Kruger-Krasagakis, S.; Eberle, J. Ambivalent Effects of Tumor Necrosis Factor Alpha on Apoptosis of Malignant and Normal Human Keratinocytes. Ski. Skin. Pharmacol. Physiol. 2021, 34, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.D.; Liu, X.P.; Zhao, W.J.; Dong, Q.; Li, F.N.; Wang, H.B.; Kong, B. Curcumin Induces Apoptosis in Breast Cancer Cells and Inhibits Tumor Growth In Vitro and In Vivo. Int. J. Clin. Exp. Pathol. 2014, 7, 2818–2824. [Google Scholar]
- Chiu, T.L.; Su, C.C. Curcumin Inhibits Proliferation and Migration by Increasing the Bax to Bcl-2 Ratio and Decreasing NF-κBp65 Expression in Breast Cancer MDA-MB-231 Cells. Int. J. Mol. Med. 2009, 23, 469–475. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Y.; Yang, J.; Li, H.; Zhang, H.; Zheng, P. Curcumin Induces Apoptotic Cell Death and Protective Autophagy in Human Gastric Cancer Cells. Oncol. Rep. 2017, 37, 3459–3466. [Google Scholar] [CrossRef]
- Catz, S.D.; Johnson, J.L. Transcriptional Regulation of Bcl-2 by Nuclear Factor Kappa B and Its Significance in Prostate Cancer. Oncogene 2001, 20, 7342–7351. [Google Scholar] [CrossRef]
- Paul, M.; Murphy, S.F.; Hall, C.; Schaeffer, A.J.; Thumbikat, P. Protease-Activated Receptor 2 Activates CRAC-Mediated Ca2+ Influx to Cause Prostate Smooth Muscle Contraction. FASEB Bioadv. 2019, 1, 255–264. [Google Scholar] [CrossRef]
- Koller, H.; Thiem, K.; Siebler, M. Tumour Necrosis Factor-Alpha Increases Intracellular Ca2+ and Induces a Depolarization in Cultured Astroglial Cells. Brain 1996, 119, 2021–2027. [Google Scholar] [CrossRef]
- Rose, A.J.; Hargreaves, M. Exercise Increases Ca2+-Calmodulin-Dependent Protein Kinase II Activity in Human Skeletal Muscle. J. Physiol. 2003, 553, 303–309. [Google Scholar] [CrossRef]
- Chin, E.R. Role of Ca2+/Calmodulin-Dependent Kinases in Skeletal Muscle Plasticity. J. Appl. Physiol. 1985, 99, 414–423. [Google Scholar] [CrossRef]
- Smedt, H.; Verkhratsky, A.; Muallem, S. Ca2+ Signaling Mechanisms of Cell Survival and Cell Death: An Introduction. Cell Calcium 2011, 50, 207–210. [Google Scholar] [CrossRef]
- Gueguinou, M.; Ibrahim, S.; Bourgeais, J.; Robert, A.; Pathak, T.; Zhang, X.; Crottes, D.; Dupuy, J.; Ternant, D.; Monbet, V. Curcumin and NCLX Inhibitors Share Anti-Tumoral Mechanisms in Microsatellite-Instability-Driven Colorectal Cancer. Cell Mol. Life Sci. 2022, 79, 284. [Google Scholar] [CrossRef]
- Xu, X.; Chen, D.; Ye, B.; Zhong, F.; Chen, G. Curcumin Induces the Apoptosis of Non-Small Cell Lung Cancer Cells Through a Calcium Signaling Pathway. Int. J. Mol. Med. 2015, 35, 1610–1616. [Google Scholar] [CrossRef]
- Lilienbaum, A.; Israel, A. From Calcium to NF-κB Signaling Pathways in Neurons. Mol. Cell Biol. 2003, 23, 2680–2698. [Google Scholar] [CrossRef]
- Peterson, M.D.; Mooseker, M.S. Characterization of the Enterocyte-like Brush Border Cytoskeleton of the C2BBe Clones of the Human Intestinal Cell Line, Caco-2. J. Cell Sci. 1992, 102 Pt 3, 581–600. [Google Scholar] [CrossRef]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the Human Colon Carcinoma Cell Line (Caco-2) as a Model System for Intestinal Epithelial Permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [PubMed]
- Zheng, H.; Liu, H.; Li, H.; Dou, W.; Wang, J.; Zhang, J.; Liu, T.; Wu, Y.; Liu, Y.; Wang, X. Characterization of Stem Cell Landscape and Identification of Stemness-Relevant Prognostic Gene Signature to Aid Immunotherapy in Colorectal Cancer. Stem Cell Res. Ther. 2022, 13, 244. [Google Scholar] [CrossRef]
- Mariadason, J.M.; Nicholas, C.; L’Italien, K.E.; Zhuang, M.; Smartt, H.J.M.; Heerdt, B.G.; Yang, W.; Corner, G.A.; Wilson, A.J.; Klampfer, L.; et al. Gene Expression Profiling of Intestinal Epithelial Cell Maturation along the Crypt-Villus Axis. Gastroenterology 2005, 128, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.J.; Sundstrom, L.; Geschwindner, S.; Poon, E.K.Y.; Jiang, Y.; Chen, R.; Cooke, R.; Johnstone, S.; Madin, A.; Lim, J. Protease-Activated Receptor-2 Ligands Reveal Orthosteric and Allosteric Mechanisms of Receptor Inhibition. Commun. Biol. 2020, 3, 782. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.J.; Baxter, N.J.; Khan, M.L.; Moir, A.J.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/Peptide Binding and Precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.V.; Banerjee, Y.; Fernandez, J.A.; Deguchi, H.; Xu, X.; Mosnier, L.O.; Urbanus, R.T.; Groot, P.G.; White-Adams, T.C.; McCarty, O.J. Activated Protein C Ligation of ApoER2 (LRP8) Causes Dab1-Dependent Signaling in U937 Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, Y.; Lakshminarayanan, R.; Vivekanandan, S.; Anand, G.S.; Valiyaveettil, S.; Kini, R.M. Biophysical characterization of anticoagulant hemextin AB complex from the venom of snake Hemachatus haemachatus. Biophys. J. 2007, 93, 3963–3976. [Google Scholar] [CrossRef] [PubMed]
- Langelaan, D.N.; Ngweniform, P.; Rainey, J.K. Biophysical Characterization of G-Protein Coupled Receptor-Peptide Ligand Binding. Biochem. Cell Biol. 2011, 89, 98–105. [Google Scholar] [CrossRef]
- Yeagle, P.L.; Choi, G.; Albert, A.D. Studies on the Structure of the G-Protein-Coupled Receptor Rhodopsin Including the Putative G-Protein Binding Site in Unactivated and Activated Forms. Biochemistry 2001, 40, 11932–11937. [Google Scholar] [CrossRef]
- Du, B.; Jiang, L.; Xia, Q.; Zhong, L. Synergistic Inhibitory Effects of Curcumin and 5-Fluorouracil on the Growth of the Human Colon Cancer Cell Line HT-29. Chemotherapy 2006, 52, 23–28. [Google Scholar] [CrossRef]
- Srimuangwong, K.; Tocharus, C.; Yoysungnoen Chintana, P.; Suksamrarn, A.; Tocharus, J. Hexahydrocurcumin enhances inhibitory effect of 5-fluorouracil on HT-29 human colon cancer cells. World J. Gastroenterol. 2012, 18, 2383–2389. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Diagaradjane, P.; Anand, P.; Harikumar, K.B.; Deorukhkar, A.; Gelovani, J.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Curcumin Sensitizes Human Colorectal Cancer to Capecitabine by Modulation of Cyclin D1, COX-2, MMP-9, VEGF and CXCR4 Expression in an Orthotopic Mouse Model. Int. J. Cancer 2009, 125, 2187–2197. [Google Scholar] [CrossRef]
- Patel, B.B.; Sengupta, R.; Qazi, S.; Vachhani, H.; Yu, Y.; Rishi, A.K.; Majumdar, A.P. Curcumin Enhances the Effects of 5-Fluorouracil and Oxaliplatin in Mediating Growth Inhibition of Colon Cancer Cells by Modulating EGFR and IGF-1R. Int. J. Cancer 2008, 122, 267–273. [Google Scholar] [CrossRef]
- James, M.I.; Iwuji, C.; Irving, G.; Karmokar, A.; Higgins, J.A.; Griffin-Teal, N.; Thomas, A.; Greaves, P.; Cai, H.; Patel, S.R. Curcumin Inhibits Cancer Stem Cell Phenotypes in Ex Vivo Models of Colorectal Liver Metastases, and Is Clinically Safe and Tolerable in Combination with FOLFOX Chemotherapy. Cancer Lett. 2015, 364, 135–141. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, X.; Ke, Z.; Wang, W.; Tang, J.; Dai, Y. PAR2 Deficiency Impairs Antitumor Immunity and Attenuates Anti-PD1 Efficacy in Colorectal Cancer. Pharmacol. Res. 2025, 215, 107721. [Google Scholar] [CrossRef]
- Bahadori, F.; Demiray, M. A Realistic View on “The Essential Medicinal Chemistry of Curcumin”. ACS Med. Chem. Lett. 2017, 8, 893–896. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Baell, J.B. Feeling Nature’s PAINS: Natural Products, Natural Product Drugs, and Pan Assay Interference Compounds (PAINS). J. Nat. Prod. 2016, 79, 616–628. [Google Scholar] [CrossRef]
- Tsai, Y.M.; Chien, C.F.; Lin, L.C.; Tsai, T.H. Curcumin and Its Nano-Formulation: The Kinetics of Tissue Distribution and Blood-Brain Barrier Penetration. Int. J. Pharm. 2011, 416, 331–338. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanoformulations: A future nanomedicine for cancer. Drug Discov. Today 2012, 17, 71–80. [Google Scholar] [CrossRef]
- Sadia, H.; Qureshi, I.Z.; Naveed, M.; Aziz, T.; Alharbi, M.; Alasmari, A.F.; Albekairi, T.H. Natural AI-Based Drug Designing by Modification of Ascorbic Acid and Curcumin to Combat Buprofezin Toxicity by Using Molecular Dynamics Study. Sci. Rep. 2024, 14, 28445. [Google Scholar] [CrossRef]
- Gangwal, A.; Lavecchia, A. Artificial Intelligence in Natural Product Drug Discovery: Current Applications and Future Perspectives. J. Med. Chem. 2025, 68, 3948–3969. [Google Scholar] [CrossRef]
- Maagdenberg, H.W.; Otterloo, J.; Hasselt, J.G.C.; Graaf, P.H.; Westen, G.J.P. Integrating Pharmacokinetics and Quantitative Systems Pharmacology Approaches in Generative Drug Design. J. Chem. Inf. Model. 2025, 65, 4783–4796. [Google Scholar] [CrossRef]
- Cui, M.; Cheng, C.; Zhang, L. High-Throughput Proteomics: A Methodological Mini-Review. Lab. Investig. 2022, 102, 1170–1181. [Google Scholar] [CrossRef]






| Genes for (Proteins) | Primer Type | Sequence | Accession | E-Value | Bit Score |
|---|---|---|---|---|---|
| Bax | Forward (5′-3′) Reverse (5′-3′) | TCAGGATGCGTCCACCAAGAAG TGTGTCCACGGCGGCAATCATC | NM_004324 | 0.0 | 1412 |
| Bcl 2 | Forward (5′-3′) Reverse (5′-3′) | ATCGCCCTGTGGATGACTGAGT GCCAGGAGAAATCAAACAGAGGC | BC027258 | 0.0 | 2704 |
| CASP3 | Forward (5′-3′) Reverse (5′-3′) | GGAAGCGAATCAATGGACTCTGG GCATCGACATCTGTACCAGACC | NM_004346 | 0.0 | 2645 |
| CASP8 | Forward (5′-3′) Reverse (5′-3′) | AGAAGAGGGTCATCCTGGGAGA TCAGGACTTCCTTCAAGGCTGC | NM_001080125 | 0.0 | 2929 |
| ERK 1 | Forward (5′-3′) Reverse (5′-3′) | TGGCAAGCACTACCTGGATCAG GCAGAGACTGTAGGTAGTTTCGG | NM_002746 | 0.0 | 1787 |
| ERK 2 | Forward (5′-3′) Reverse (5′-3′) | ACACCAACCTCTCGTACATCGG TGGCAGTAGGTCTGGTGCTCAA | NM_002745 | 0.0 | 5881 |
| GAPDH | Forward (5′-3′) Reverse (5′-3′) | GTCTCCTCTGACTTCAACAGCG ACCACCCTGTTGCTGTAGCCAA | NM_002046 | 0.0 | 2374 |
| PAR-1 | Forward (5′-3′) Reverse (5′-3′) | GCTGTCCTACTGCTTGGAAGAC CTGCATCAGCACATACTCCTCC | NM_022002 | 0.0 | 2745 |
| PAR-2 | Forward (5′-3′) Reverse (5′-3′) | CTCCTCTCTGTCATCTGGTTCC TGCACACTGAGGCAGGTCATGA | NM_005242 | 0.0 | 2861 |
| pERK | Forward (5′-3′) Reverse (5′-3′) | GTCCCAAGGCTTTGGAATCTGTC CCTACCAAGACAGGAGTTCTGG | NM_004836 | 0.0 | 4536 |
| TNF-α | Forward (5′-3′) Reverse (5′-3′) | CTCTTCTGCCTGCTGCACTTTG ATGGGCTACAGGCTTGTCACTC | NM_000594 | 0.0 | 2449 |
| Pocket ID | Vina Score (Kcal/Mol) | Cavity Volume (Å3) | Center Coordinates (x, y, z) | Docking Box Size (x, y, z) |
|---|---|---|---|---|
| C4 | −6.9 | 324 | (13, 2, 11) | (26, 26, 26) |
| C1 | −6.5 | 698 | (−14, 10, 19) | (26, 26, 26) |
| C3 | −6.1 | 353 | (−6, 16, 1) | (26, 26, 26) |
| C2 | −6.0 | 580 | (−25, −6, 12) | (26, 26, 26) |
| C5 | −3.5 | 283 | (9, −8, −7) | (26, 26, 26) |
| Pocket ID | Vina Score (kcal/mol) | Cavity Volume (Å3) | Center Coordinates (x, y, z) | Docking Box Size (x, y, z) |
| C4 | −6.9 | 324 | (13, 2, 11) | (26, 26, 26) |
| C1 | −6.5 | 698 | (−14, 10, 19) | (26, 26, 26) |
| C3 | −6.1 | 353 | (−6, 16, 1) | (26, 26, 26) |
| C2 | −6.0 | 580 | (−25, −6, 12) | (26, 26, 26) |
| C5 | −3.5 | 283 | (9, −8, −7) | (26, 26, 26) |
| Pocket ID | Vina Score (kcal/mol) | Cavity Volume (Å3) | Center Coordinates (x, y, z) | Docking Box Size (x, y, z) |
| C4 | −6.9 | 324 | (13, 2, 11) | (26, 26, 26) |
| C1 | −6.5 | 698 | (−14, 10, 19) | (26, 26, 26) |
| C3 | −6.1 | 353 | (−6, 16, 1) | (26, 26, 26) |
| C2 | −6.0 | 580 | (−25, −6, 12) | (26, 26, 26) |
| C5 | −3.5 | 283 | (9, −8, −7) | (26, 26, 26) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patnaik, R.; Varghese, R.; Al-Kabani, A.; Jannati, S.; Banerjee, Y. Curcumin Inhibits Protease Activated Receptor 2-Induced ERK Phosphorylation Calcium Mobilization and Anti-Apoptotic Signaling in Inflammation-Driven Colorectal Cancer Cells. Cells 2025, 14, 1451. https://doi.org/10.3390/cells14181451
Patnaik R, Varghese R, Al-Kabani A, Jannati S, Banerjee Y. Curcumin Inhibits Protease Activated Receptor 2-Induced ERK Phosphorylation Calcium Mobilization and Anti-Apoptotic Signaling in Inflammation-Driven Colorectal Cancer Cells. Cells. 2025; 14(18):1451. https://doi.org/10.3390/cells14181451
Chicago/Turabian StylePatnaik, Rajashree, Riah Varghese, Ahad Al-Kabani, Shirin Jannati, and Yajnavalka Banerjee. 2025. "Curcumin Inhibits Protease Activated Receptor 2-Induced ERK Phosphorylation Calcium Mobilization and Anti-Apoptotic Signaling in Inflammation-Driven Colorectal Cancer Cells" Cells 14, no. 18: 1451. https://doi.org/10.3390/cells14181451
APA StylePatnaik, R., Varghese, R., Al-Kabani, A., Jannati, S., & Banerjee, Y. (2025). Curcumin Inhibits Protease Activated Receptor 2-Induced ERK Phosphorylation Calcium Mobilization and Anti-Apoptotic Signaling in Inflammation-Driven Colorectal Cancer Cells. Cells, 14(18), 1451. https://doi.org/10.3390/cells14181451







