Genetic Deletion of RHAMM Alleviates Hepatic Oxidative Stress, Reversing Thyroid Stimulating Hormone Elevation in Male Obese Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. RNA Extraction and Real-Time PCR
2.3. Protein Extraction and Western Blotting
2.4. Plasma Thyroid-Stimulating Hormone (TSH)
2.5. Quantification of Liver Triglycerides
2.6. Protein Oxidation Levels Measurement
2.7. Malondialdehyde (MDA) Level Measurement
2.8. Statistical Analysis
3. Results
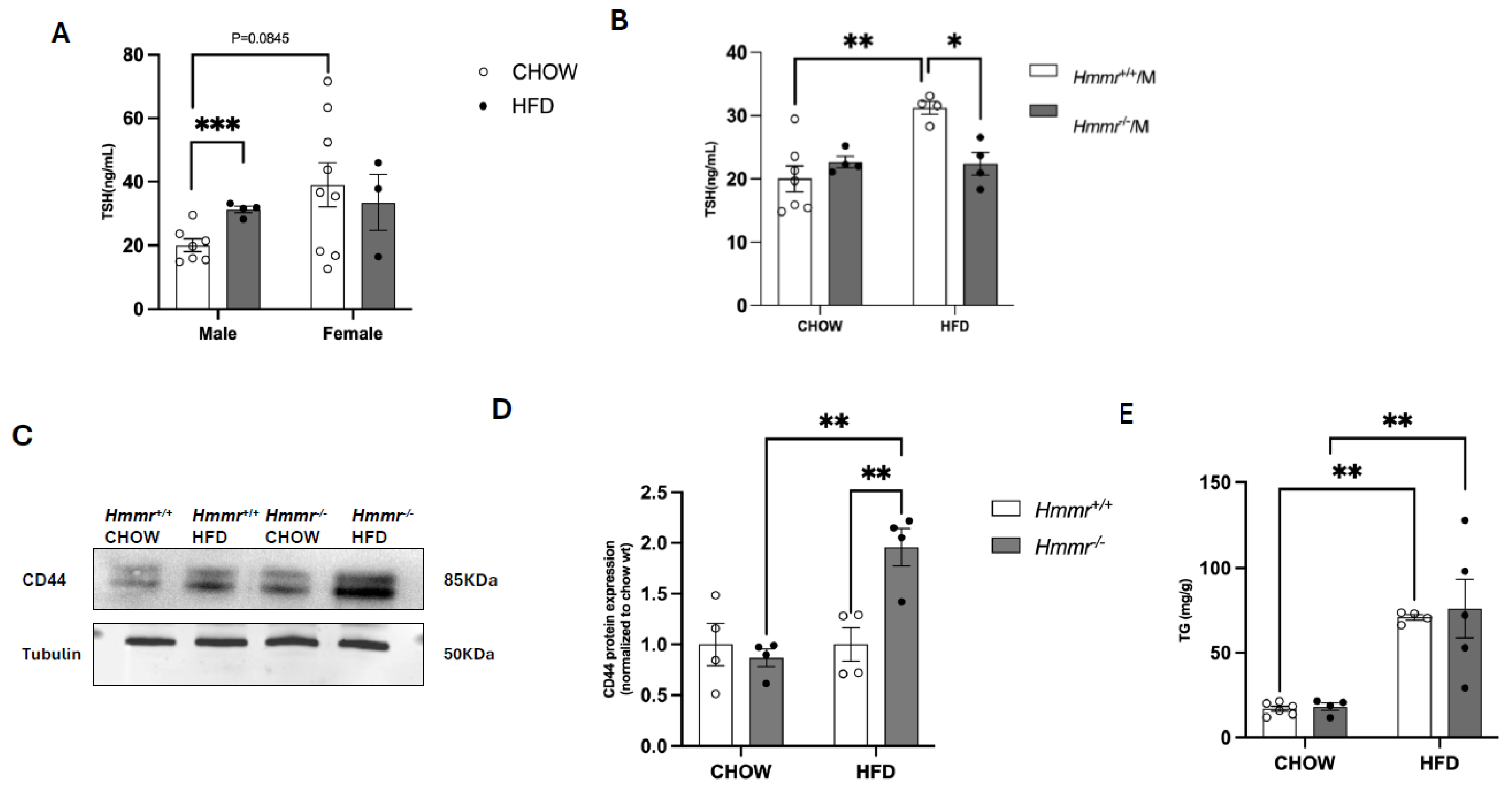
3.1. Global Hmmr Gene Deletion Mitigates Obesity-Induced Increases in Plasma TSH Concentrations in Male Mice
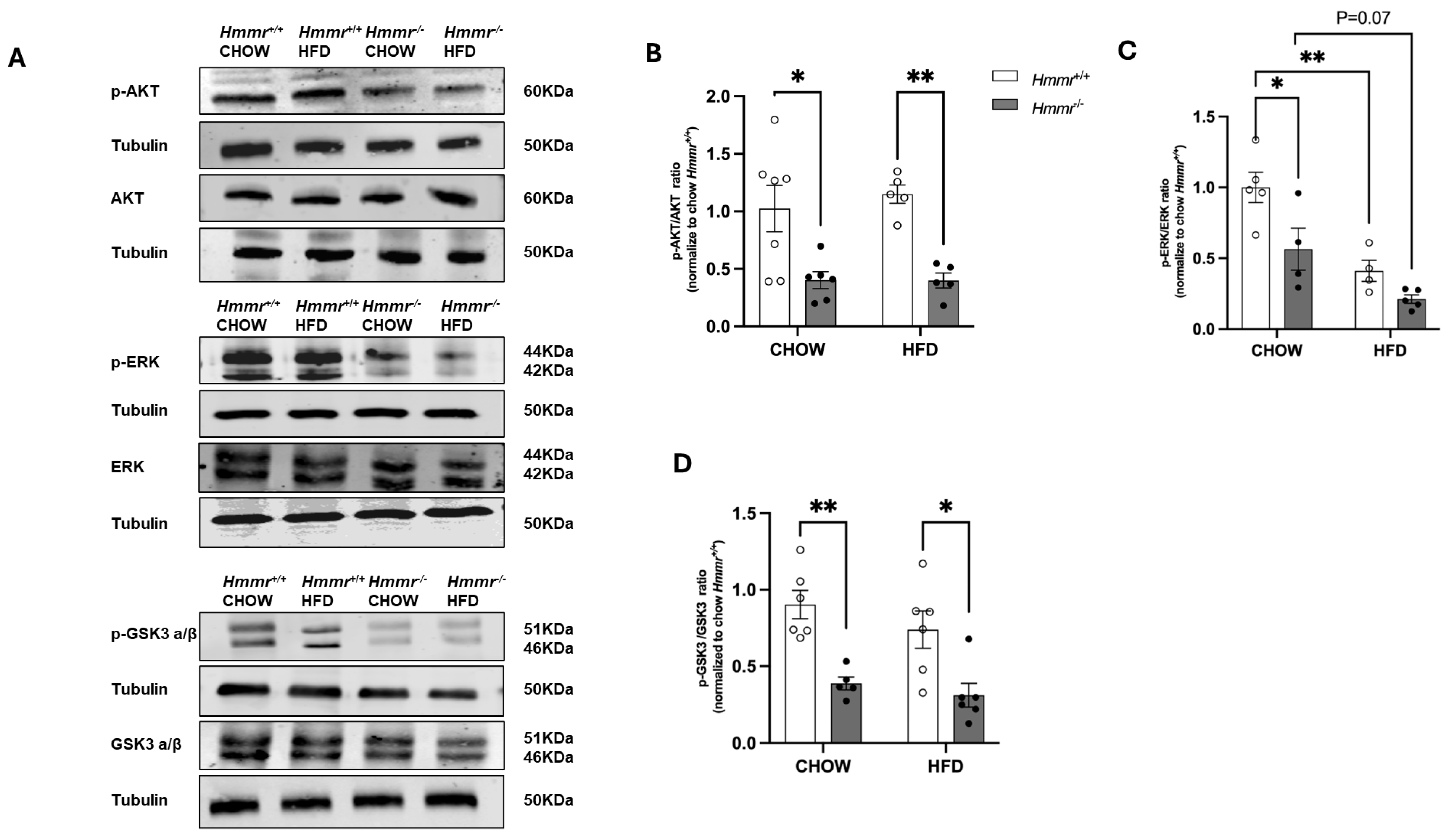
3.2. RHAMM Deletion Suppresses AKT/ERK and Activates GSK3 Signaling Pathways and Induces Nqo1 in Obese Mice
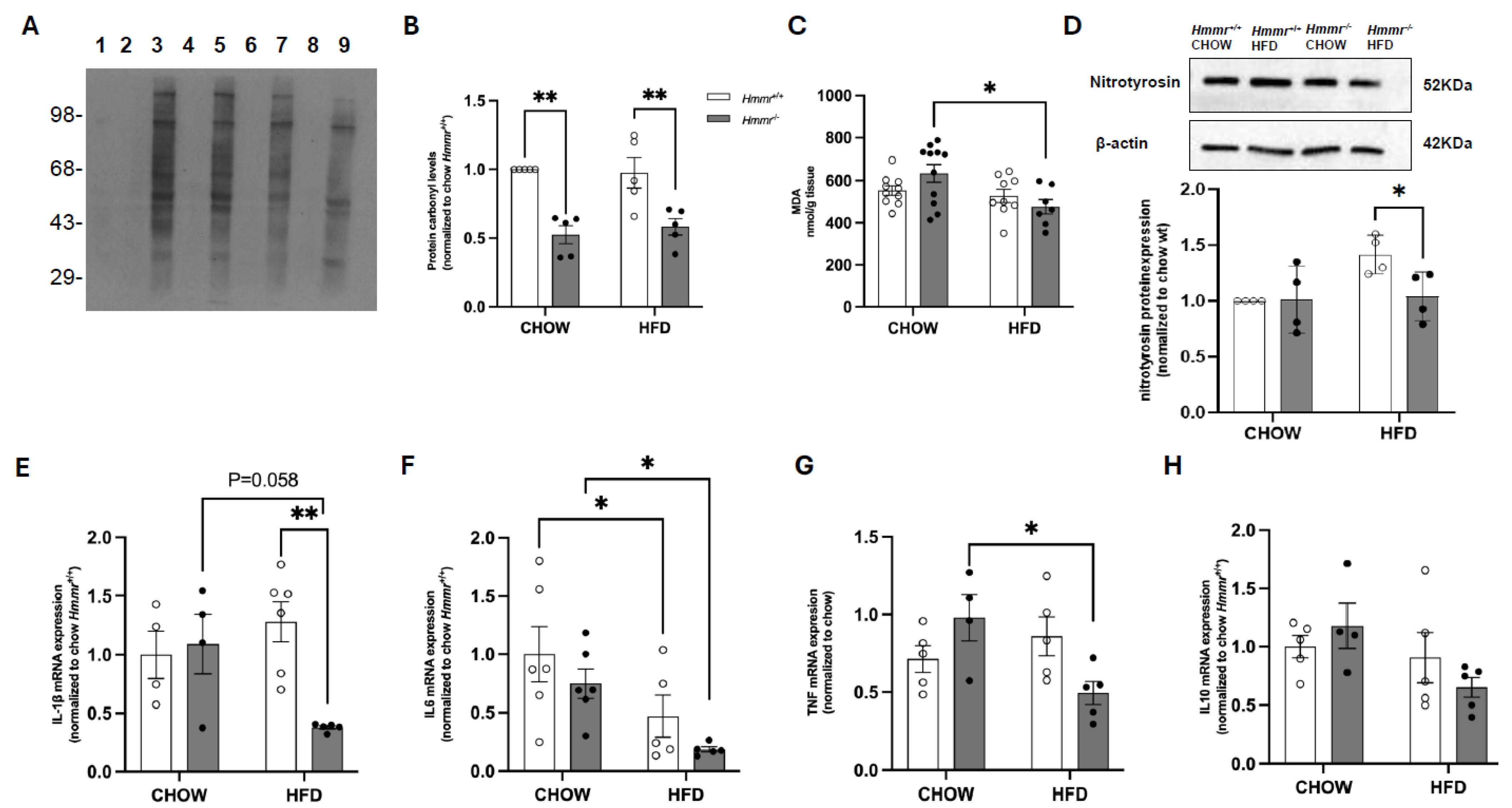
3.3. Global Hmmr Gene Deletion Decreases Hepatic Oxidative Stress and Inflammation in Obese Mice
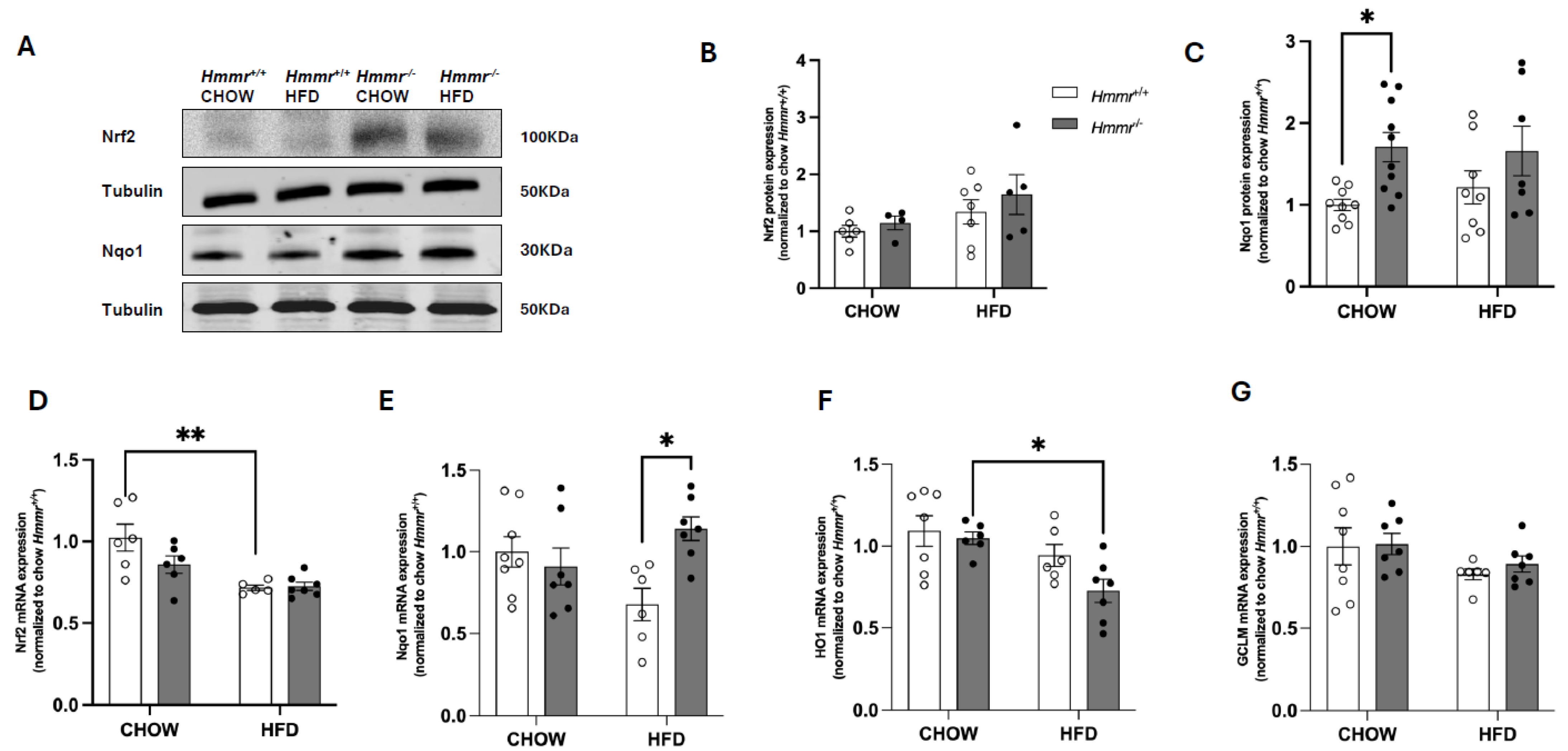
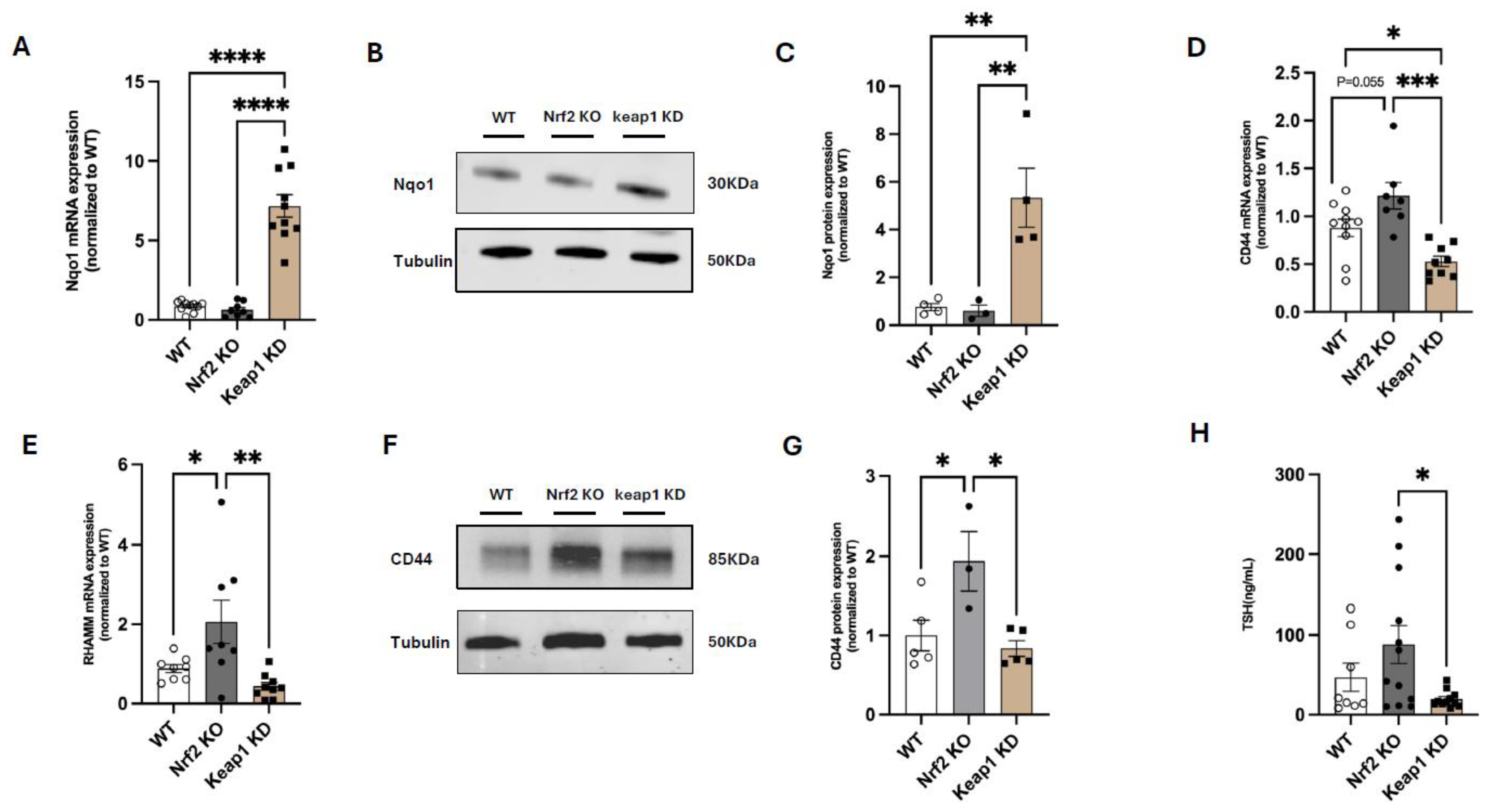
3.4. Nrf2 Suppresses CD44 and RHAMM Expression and Regulates Thyroid Function in Mice
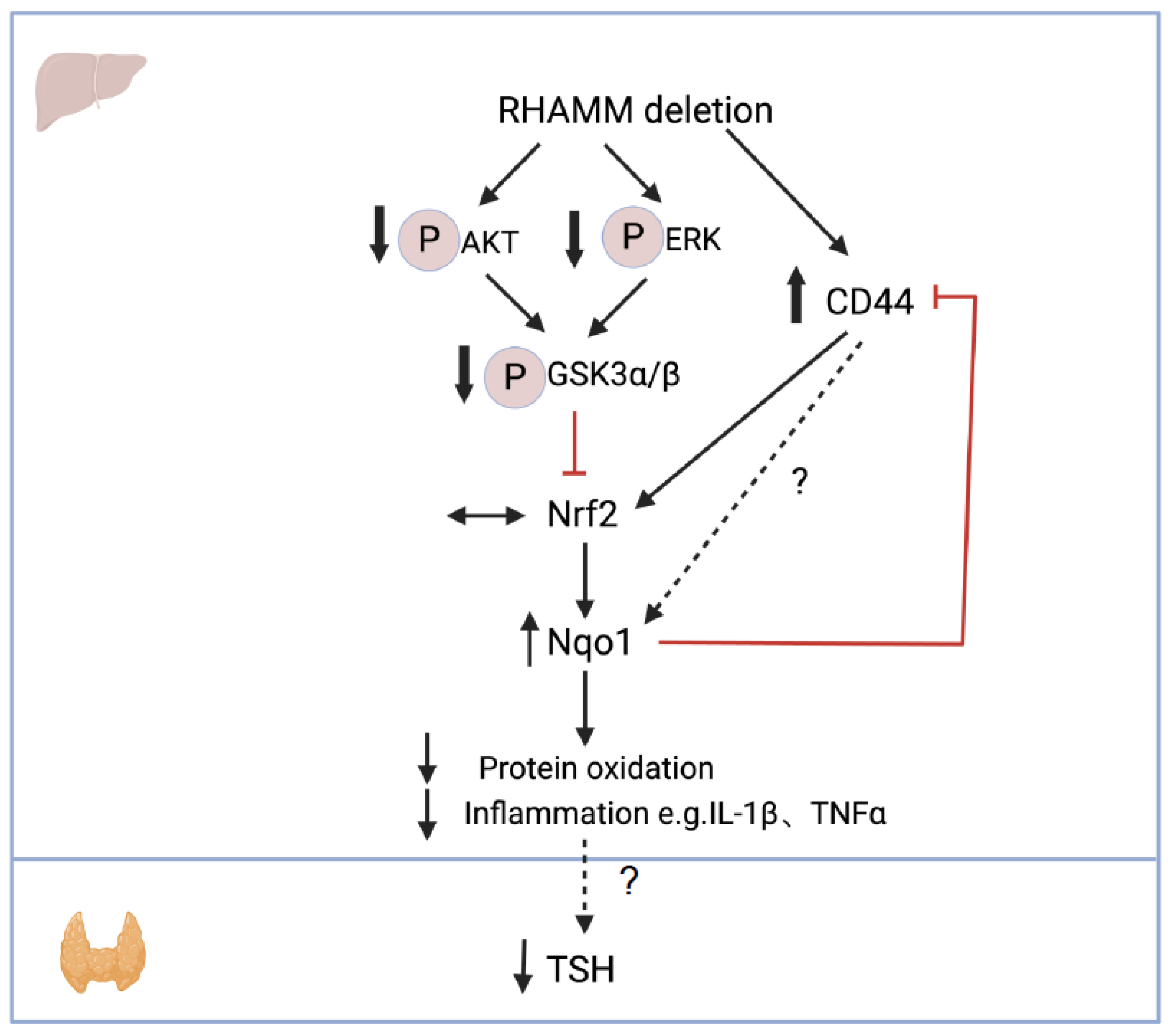
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef]
- Betry, C.; Challan-Belval, M.; Bernard, A.; Charrié, A.; Drai, J.; Laville, M.; Thivolet, C.; Disse, E. Increased TSH in obesity: Evidence for a BMI-independent association with leptin. Diabetes Metab. 2015, 41, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Nyrnes, A.; Jorde, R.; Sundsfjord, J. Serum TSH is positively associated with BMI. Int. J. Obes. 2006, 30, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Nabi, G.; Hao, Y.; Liu, X.; Sun, Y.; Wang, Y.; Jiang, C.; Li, J.; Wu, Y.; Li, D. Hypothalamic-Pituitary-Thyroid Axis Crosstalk with the Hypothalamic-Pituitary-Gonadal Axis and Metabolic Regulation in the Eurasian Tree Sparrow During Mating and Non-mating Periods. Front. Endocrinol. 2020, 11, 303. [Google Scholar] [CrossRef]
- Chung, G.E.; Kim, D.; Kim, W.; Yim, J.Y.; Park, M.J.; Kim, Y.J.; Yoon, J.-H.; Lee, H.-S. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J. Hepatol. 2012, 57, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, L.; Babu, K.N.; Krishnamoorthy, S.; Behlen, J.; Muthusami, S.; Stanley, J.A. Oxidative Stress and Thyroid Disorders. In Handbook of Oxidative Stress in Cancer: Mechanistic Aspects; Chakraborti, S., Ray, B.K., Roychoudhury, S., Eds.; Springer Nature: Singapore, 2022; pp. 23–34. [Google Scholar]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda-Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef]
- Malik, R.; Hodgson, H. The relationship between the thyroid gland and the liver. QJM 2002, 95, 559–569. [Google Scholar] [CrossRef]
- Loganathan, R.; Rongish, B.J.; Smith, C.M.; Filla, M.B.; Czirok, A.; Bénazéraf, B.; Little, C.D. Extracellular matrix motion and early morphogenesis. Development 2016, 143, 2056–2065. [Google Scholar] [CrossRef]
- Musale, V.; Wasserman, D.H.; Kang, L. Extracellular matrix remodelling in obesity and metabolic disorders. Life Metab. 2023, 2, load021. [Google Scholar] [CrossRef]
- Hasib, A.; Hennayake, C.K.; Bracy, D.P.; Bugler-Lamb, A.R.; Lantier, L.; Khan, F.; Ashford, M.L.J.; McCrimmon, R.J.; Wasserman, D.H.; Kang, L. CD44 contributes to hyaluronan-mediated insulin resistance in skeletal muscle of high-fat-fed C57BL/6 mice. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E973–E983. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Lantier, L.; Kennedy, A.; Bonner, J.S.; Mayes, W.H.; Bracy, D.P.; Bookbinder, L.H.; Hasty, A.H.; Thompson, C.B.; Wasserman, D.H. Hyaluronan accumulates with high-fat feeding and contributes to insulin resistance. Diabetes 2013, 62, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Musale, V.; Murdoch, C.E.; Banah, A.K.; Hasib, A.; Hennayake, C.K.; Dong, B.; Lang, C.C.; Wasserman, D.H.; Kang, L. Limiting extracellular matrix expansion in diet-induced obese mice reduces cardiac insulin resistance and prevents myocardial remodelling. Mol. Metab. 2024, 86, 101970. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Musale, V.; Weng, X.; Banah, A.K.; Murdoch, C.E.; Lay, A.C.; Heesom, K.J.; Ju, W.; Bitzer, M.; Hills, C.; et al. A Novel Role of Hyaluronan and its Membrane Receptors, CD44 and RHAMM in Obesity-Related Glomerulopathy. bioRxiv 2024. [Google Scholar] [CrossRef]
- Berdiaki, A.; Neagu, M.; Spyridaki, I.; Kuskov, A.; Perez, S.; Nikitovic, D. Hyaluronan and Reactive Oxygen Species Signaling-Novel Cues from the Matrix? Antioxidants 2023, 12, 824. [Google Scholar] [CrossRef]
- Xu, D.; Xu, M.; Jeong, S.; Qian, Y.; Wu, H.; Xia, Q.; Kong, X. The Role of Nrf2 in Liver Disease: Novel Molecular Mechanisms and Therapeutic Approaches. Front. Pharmacol. 2018, 9, 1428. [Google Scholar] [CrossRef]
- Bardallo, R.G.; Panisello-Roselló, A.; Sanchez-Nuno, S.; Alva, N.; Roselló-Catafau, J.; Carbonell, T. Nrf2 and oxidative stress in liver ischemia/reperfusion injury. FEBS J. 2022, 289, 5463–5479. [Google Scholar] [CrossRef]
- Onodera, Y.; Teramura, T.; Takehara, T.; Fukuda, K. Hyaluronic acid regulates a key redox control factor Nrf2 via phosphorylation of Akt in bovine articular chondrocytes. FEBS Open Bio. 2015, 5, 476–484. [Google Scholar] [CrossRef]
- Tolg, C.; Hamilton, S.R.; Morningstar, L.; Zhang, J.; Zhang, S.; Esguerra, K.V.; Telmer, P.G.; Luyt, L.G.; Harrison, R.; McCarthy, J.B.; et al. RHAMM promotes interphase microtubule instability and mitotic spindle integrity through MEK1/ERK1/2 activity. J. Biol. Chem. 2010, 285, 26461–26474. [Google Scholar] [CrossRef]
- Sharma, R.S.; Harrison, D.J.; Kisielewski, D.; Cassidy, D.M.; McNeilly, A.D.; Gallagher, J.R.; Walsh, S.V.; Honda, T.; McCrimmon, R.J.; Dinkova-Kostova, A.T.; et al. Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 p45-Related Factor 2). Cell Mol. Gastroenterol. Hepatol. 2018, 5, 367–398. [Google Scholar] [CrossRef]
- Knatko, E.V.; Castro, C.; Higgins, M.; Zhang, Y.; Honda, T.; Henderson, C.J.; Wolf, C.R.; Griffin, J.L.; Dinkova-Kostova, A.T. Nrf2 activation does not affect adenoma development in a mouse model of colorectal cancer. Commun. Biol. 2021, 4, 1081. [Google Scholar] [CrossRef] [PubMed]
- Meakin, P.J.; Chowdhry, S.; Sharma, R.S.; Ashford, F.B.; Walsh, S.V.; McCrimmon, R.J.; Dinkova-Kostova, A.T.; Dillon, J.F.; Hayes, J.D.; Ashford, M.L.J. Susceptibility of Nrf2-null mice to steatohepatitis and cirrhosis upon consumption of a high-fat diet is associated with oxidative stress, perturbation of the unfolded protein response, and disturbance in the expression of metabolic enzymes but not with insulin resistance. Mol. Cell Biol. 2014, 34, 3305–3320. [Google Scholar] [PubMed]
- More, V.R.; Xu, J.; Shimpi, P.C.; Belgrave, C.; Luyendyk, J.P.; Yamamoto, M.; Slitt, A.L. Keap1 knockdown increases markers of metabolic syndrome after long-term high fat diet feeding. Free Radic. Biol. Med. 2013, 61, 85–94. [Google Scholar] [CrossRef]
- Hinneh, J.A.; Gillis, J.L.; Moore, N.L.; Butler, L.M.; Centenera, M.M. The role of RHAMM in cancer: Exposing novel therapeutic vulnerabilities. Front. Oncol. 2022, 12, 982231. [Google Scholar] [CrossRef]
- Robertson, H.; Hayes, J.D.; Sutherland, C. A partnership with the proteasome; the destructive nature of GSK3. Biochem. Pharmacol. 2018, 147, 77–92. [Google Scholar] [CrossRef]
- Weitzenböck, H.P.; Gschwendtner, A.; Wiesner, C.; Depke, M.; Schmidt, F.; Trautinger, F.; Hengstschläger, M.; Hundsberger, H.; Mikula, M. Proteome analysis of NRF2 inhibition in melanoma reveals CD44 up-regulation and increased apoptosis resistance upon vemurafenib treatment. Cancer Med. 2022, 11, 956–967. [Google Scholar] [CrossRef]
- Nedvetzki, S.; Gonen, E.; Assayag, N.; Reich, R.; Williams, R.O.; Thurmond, R.L.; Huang, J.-F.; Neudecker, B.A.; Wang, F.-S.; Turley, E.A.; et al. RHAMM, a receptor for hyaluronan-mediated motility, compensates for CD44 in inflamed CD44-knockout mice: A different interpretation of redundancy. Proc. Natl. Acad. Sci. USA 2004, 101, 18081–18086. [Google Scholar] [CrossRef]
- Kang, H.S.; Liao, G.; DeGraff, L.M.; Gerrish, K.; Bortner, C.D.; Garantziotis, S.; Jetten, A.M.; Brennan, L. CD44 plays a critical role in regulating diet-induced adipose inflammation, hepatic steatosis, and insulin resistance. PLoS ONE 2013, 8, e58417. [Google Scholar] [CrossRef]
- Liu, X.; Yao, Z. Chronic over-nutrition and dysregulation of GSK3 in diseases. Nutr. Metab. 2016, 13, 49. [Google Scholar] [CrossRef]
- Messam, B.J.; Tolg, C.; McCarthy, J.B.; Nelson, A.C.; Turley, E.A. RHAMM Is a Multifunctional Protein That Regulates Cancer Progression. Int. J. Mol. Sci. 2021, 22, 10313. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.A.; Alessi, D.R.; Vandenheede, J.R.; McDowell, H.; Hundal, H.S.; Cohen, P. The inhibition of glycogen synthase kinase-3 by insulin or insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin, but not by rapamycin: Evidence that wortmannin blocks activation of the mitogen-activated protein kinase pathway in L6 cells between Ras and Raf. Biochem. J. 1994, 303 Pt 1, 21–26. [Google Scholar]
- Jin, J.; Yang, H.; Hu, L.; Wang, Y.; Wu, W.; Hu, C.; Wu, K.; Wu, Z.; Cheng, W.; Huang, Y. Inonotsuoxide B suppresses hepatic stellate cell activation and proliferation via the PI3K/AKT and ERK1/2 pathway. Exp. Ther. Med. 2022, 23, 417. [Google Scholar] [CrossRef]
- Patibandla, C.; van Aalten, L.; Dinkova-Kostova, A.T.; Honda, T.; Cuadrado, A.; Fernández-Ginés, R.; McNeilly, A.D.; Hayes, J.D.; Cantley, J.; Sutherland, C. Inhibition of glycogen synthase kinase-3 enhances NRF2 protein stability, nuclear localisation and target gene transcription in pancreatic beta cells. Redox Biol. 2024, 71, 103117. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, I.G.; Choi, B.-H.; Ku, S.-K.; Kwak, M.-K. High CD44 expression mediates p62-associated NFE2L2/NRF2 activation in breast cancer stem cell-like cells: Implications for cancer stem cell resistance. Redox Biol. 2018, 17, 246–258. [Google Scholar] [CrossRef]
- Piantanida, E.; Ippolito, S.; Gallo, D.; Masiello, E.; Premoli, P.; Cusini, C.; Rosetti, S.; Sabatino, J.; Segato, S.; Trimarchi, F.; et al. The interplay between thyroid and liver: Implications for clinical practice. J. Endocrinol. Investig. 2020, 43, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Bruinstroop, E.; van der Spek, A.H.; Boelen, A. Role of hepatic deiodinases in thyroid hormone homeostasis and liver metabolism, inflammation, and fibrosis. Eur. Thyroid. J. 2023, 12, e220211. [Google Scholar] [CrossRef] [PubMed]
- Marschner, R.A.; Roginski, A.C.; Ribeiro, R.T.; Longo, L.; Álvares-Da-Silva, M.R.; Wajner, S.M. Uncovering Actions of Type 3 Deiodinase in the Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Cells 2023, 12, 1022. [Google Scholar] [CrossRef]
- Chen, K.; Yan, B.; Wang, F.; Wen, F.; Xing, X.; Tang, X.; Shi, Y.; Le, G. Type 1 5′-deiodinase activity is inhibited by oxidative stress and restored by alpha-lipoic acid in HepG2 cells. Biochem. Biophys. Res. Commun. 2016, 472, 496–501. [Google Scholar] [CrossRef]
- Thanas, C.; Ziros, P.G.; Chartoumpekis, D.V.; Renaud, C.O.; Sykiotis, G.P. The Keap1/Nrf2 Signaling Pathway in the Thyroid-2020 Update. Antioxidants 2020, 9, 1082. [Google Scholar] [CrossRef]
- Ziros, P.G.; Habeos, I.G.; Chartoumpekis, D.V.; Ntalampyra, E.; Somm, E.; Renaud, C.O.; Bongiovanni, M.; Trougakos, I.P.; Yamamoto, M.; Kensler, T.W.; et al. NFE2-Related Transcription Factor 2 Coordinates Antioxidant Defense with Thyroglobulin Production and Iodination in the Thyroid Gland. Thyroid 2018, 28, 780–798. [Google Scholar] [CrossRef]
- Ziros, P.G.; Renaud, C.O.; Chartoumpekis, D.V.; Bongiovanni, M.; Habeos, I.G.; Liao, X.-H.; Refetoff, S.; Kopp, P.A.; Brix, K.; Sykiotis, G.P. Mice Hypomorphic for Keap1, a Negative Regulator of the Nrf2 Antioxidant Response, Show Age-Dependent Diffuse Goiter with Elevated Thyrotropin Levels. Thyroid 2021, 31, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Ziros, P.G.; Chartoumpekis, D.V.; Georgakopoulos-Soares, I.; Psarias, G.; Sykiotis, G.P. Transcriptomic profiling of the response to excess iodide in Keap1 hypomorphic mice reveals new gene-environment interactions in thyroid homeostasis. Redox Biol. 2024, 69, 102978. [Google Scholar] [CrossRef] [PubMed]
- Slevin, M.; Krupinski, J.; Gaffney, J.; Matou, S.; West, D.; Delisser, H.; Savani, R.C.; Kumar, S. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007, 26, 58–68. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Mohan, S. Role and Mechanisms of Actions of Thyroid Hormone on the Skeletal Development. Bone Res. 2013, 1, 146–161. [Google Scholar] [CrossRef]
- De Los Santos, E.T.; Mazzaferri, E.L. Sensitive thyroid-stimulating hormone assays: Clinical applications and limitations. Compr. Ther. 1988, 14, 26–33. [Google Scholar]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef]
- Wang, M.J.; Zhang, H.-L.; Chen, F.; Guo, X.-J.; Liu, Q.-G.; Hou, J. The double-edged effects of IL-6 in liver regeneration, aging, inflammation, and diseases. Exp. Hematol. Oncol. 2024, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Shriki, A.; Lanton, T.; Sonnenblick, A.; Levkovitch-Siany, O.; Eidelshtein, D.; Abramovitch, R.; Rosenberg, N.; Pappo, O.; Elgavish, S.; Nevo, Y.; et al. Multiple Roles of IL6 in Hepatic Injury, Steatosis, and Senescence Aggregate to Suppress Tumorigenesis. Cancer Res. 2021, 81, 4766–4777. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Sun, H.; Banah, A.K.; Weng, X.; Dayalan Naidu, S.; Kisielewski, D.; Ang, A.; Hayes, J.D.; Dinkova-Kostova, A.T.; Kang, L. Genetic Deletion of RHAMM Alleviates Hepatic Oxidative Stress, Reversing Thyroid Stimulating Hormone Elevation in Male Obese Mice. Cells 2025, 14, 1448. https://doi.org/10.3390/cells14181448
Wang T, Sun H, Banah AK, Weng X, Dayalan Naidu S, Kisielewski D, Ang A, Hayes JD, Dinkova-Kostova AT, Kang L. Genetic Deletion of RHAMM Alleviates Hepatic Oxidative Stress, Reversing Thyroid Stimulating Hormone Elevation in Male Obese Mice. Cells. 2025; 14(18):1448. https://doi.org/10.3390/cells14181448
Chicago/Turabian StyleWang, Tianzhen, Helin Sun, Ayman K. Banah, Xiong Weng, Sharadha Dayalan Naidu, Dot Kisielewski, Abel Ang, John D. Hayes, Albena T. Dinkova-Kostova, and Li Kang. 2025. "Genetic Deletion of RHAMM Alleviates Hepatic Oxidative Stress, Reversing Thyroid Stimulating Hormone Elevation in Male Obese Mice" Cells 14, no. 18: 1448. https://doi.org/10.3390/cells14181448
APA StyleWang, T., Sun, H., Banah, A. K., Weng, X., Dayalan Naidu, S., Kisielewski, D., Ang, A., Hayes, J. D., Dinkova-Kostova, A. T., & Kang, L. (2025). Genetic Deletion of RHAMM Alleviates Hepatic Oxidative Stress, Reversing Thyroid Stimulating Hormone Elevation in Male Obese Mice. Cells, 14(18), 1448. https://doi.org/10.3390/cells14181448






