VEGF-VEGFR Signaling Mechanism Directs the Migration of Newborn Hemocytes from the Hematopoietic Site of Oyster Crassostrea gigas
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Microbes
2.2. Immune Stimulation and Sample Collection
2.3. Bio-Layer Interferometry Assay
2.4. RNA Interference (RNAi) of CgVEGFR with siRNA
2.5. The Inhibition of CgVEGFR Expression by the Treatment with Inhibitors
2.6. Reverse Transcription Quantitative PCR (RT-qPCR) Analysis
2.7. Histochemical Staining and Immunohistochemistry Assay of Gill Sections
2.8. Western Blotting Analysis
2.9. The Flow Cytometry Analysis
2.10. Statistical Analysis
3. Results
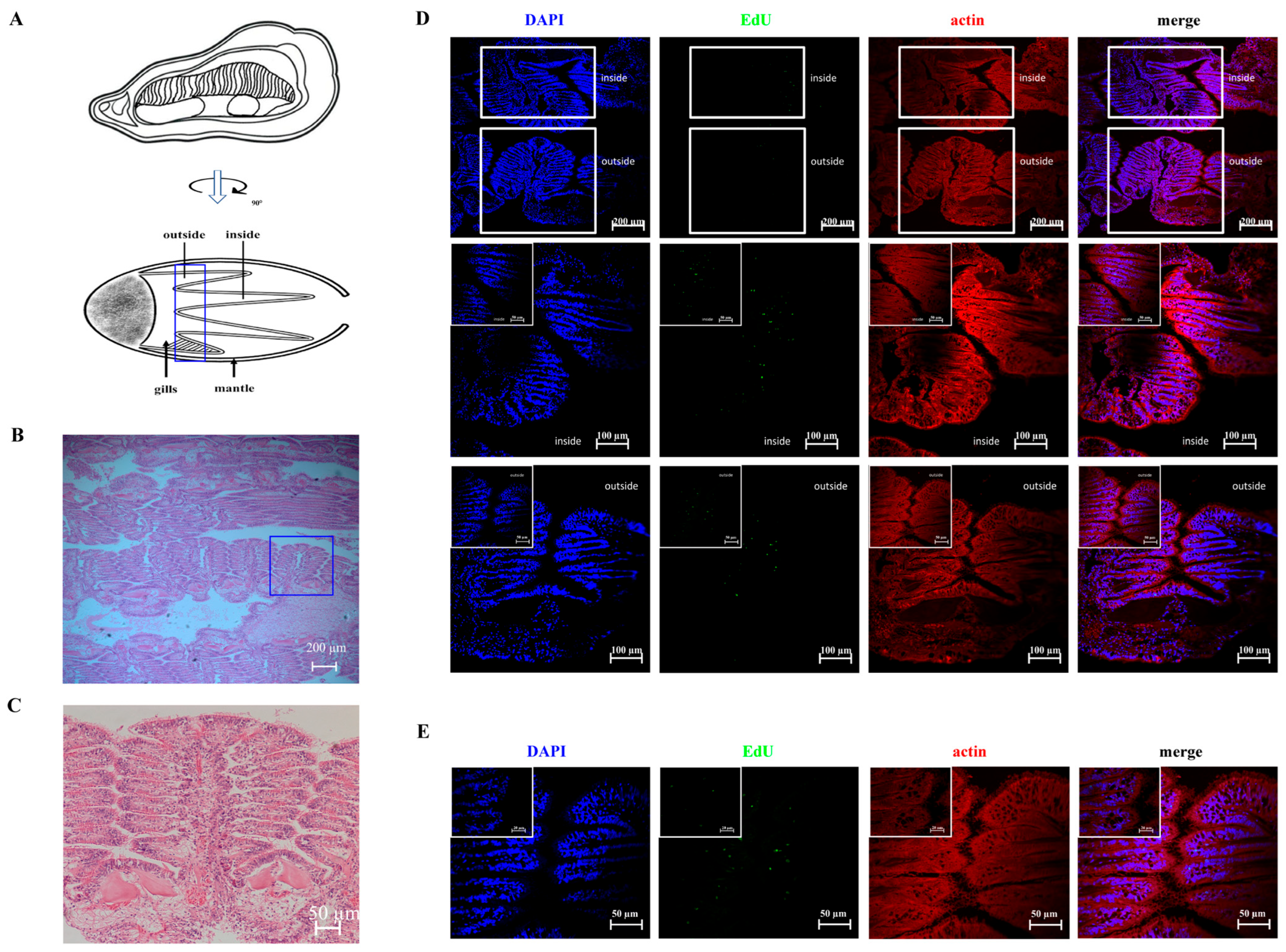
3.1. The Proximal Region of the Gill Hinge Is the Hematopoietic Site in Oysters
3.2. The Generation of Newborn Cells Occurs at the Edge of Normal Gill Filaments in the Inner Demi-Branch of G2–G3 Sector
3.3. The Green Signals of SOX2 Are Colocalized with Newborn Cells in the Hematopoietic Site
3.4. CgVEGF and CgVEGFR Are Highly Expressed in the G2–G3 Sector and Circulating Hemocytes, and Their Expression Levels Increase After V. splendidus Stimulation
3.5. CgVEGF Interacts with Its Specific Receptor CgVEGFR
3.6. VEGF Promotes the Proliferation of Hematopoietic Stem Cells and Circulating Hemocytes
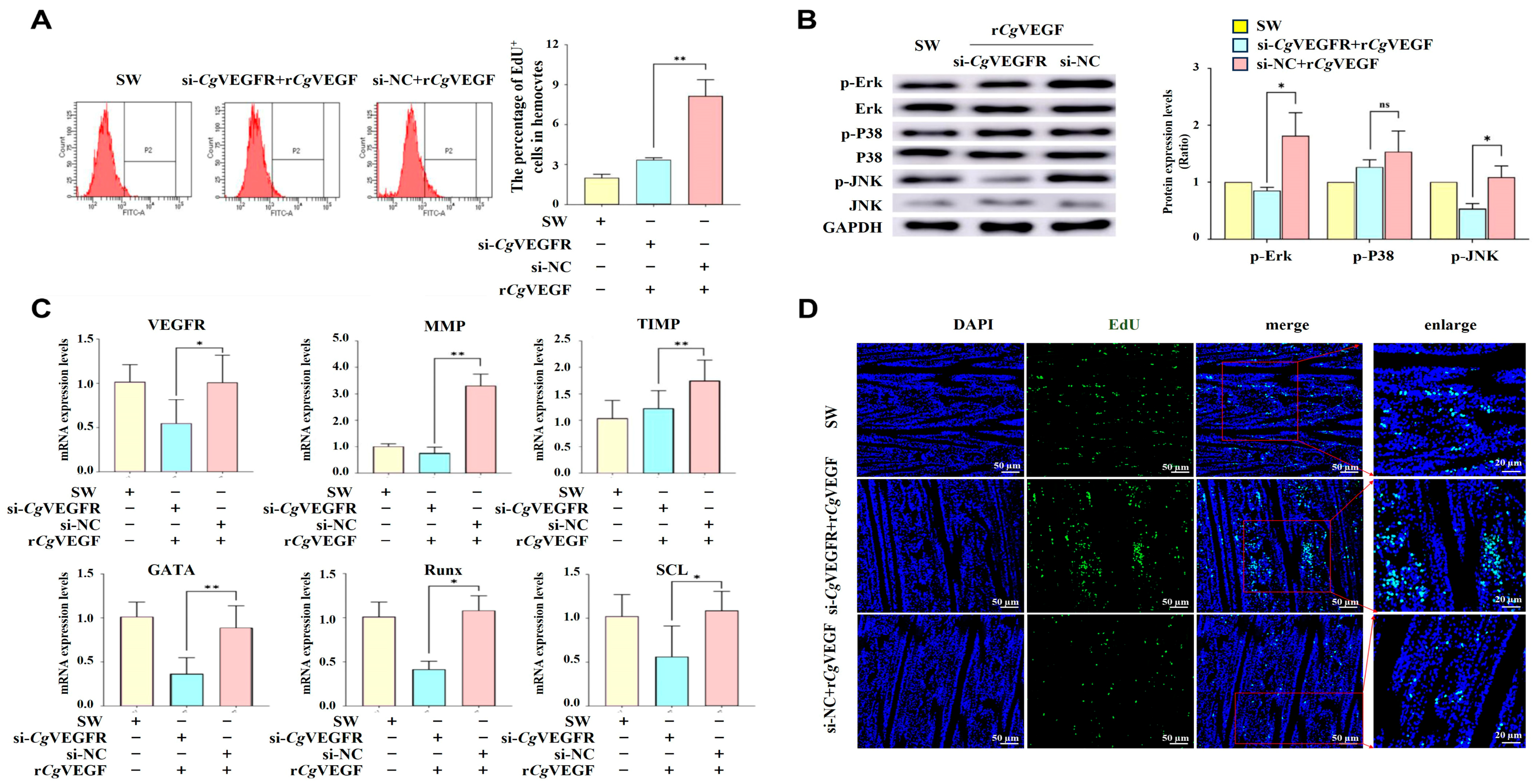
3.7. VEGF-VEGFR Promotes the Proliferation of Hematopoietic Stem Cells and Circulating Hemocytes Through the MAPK Pathway
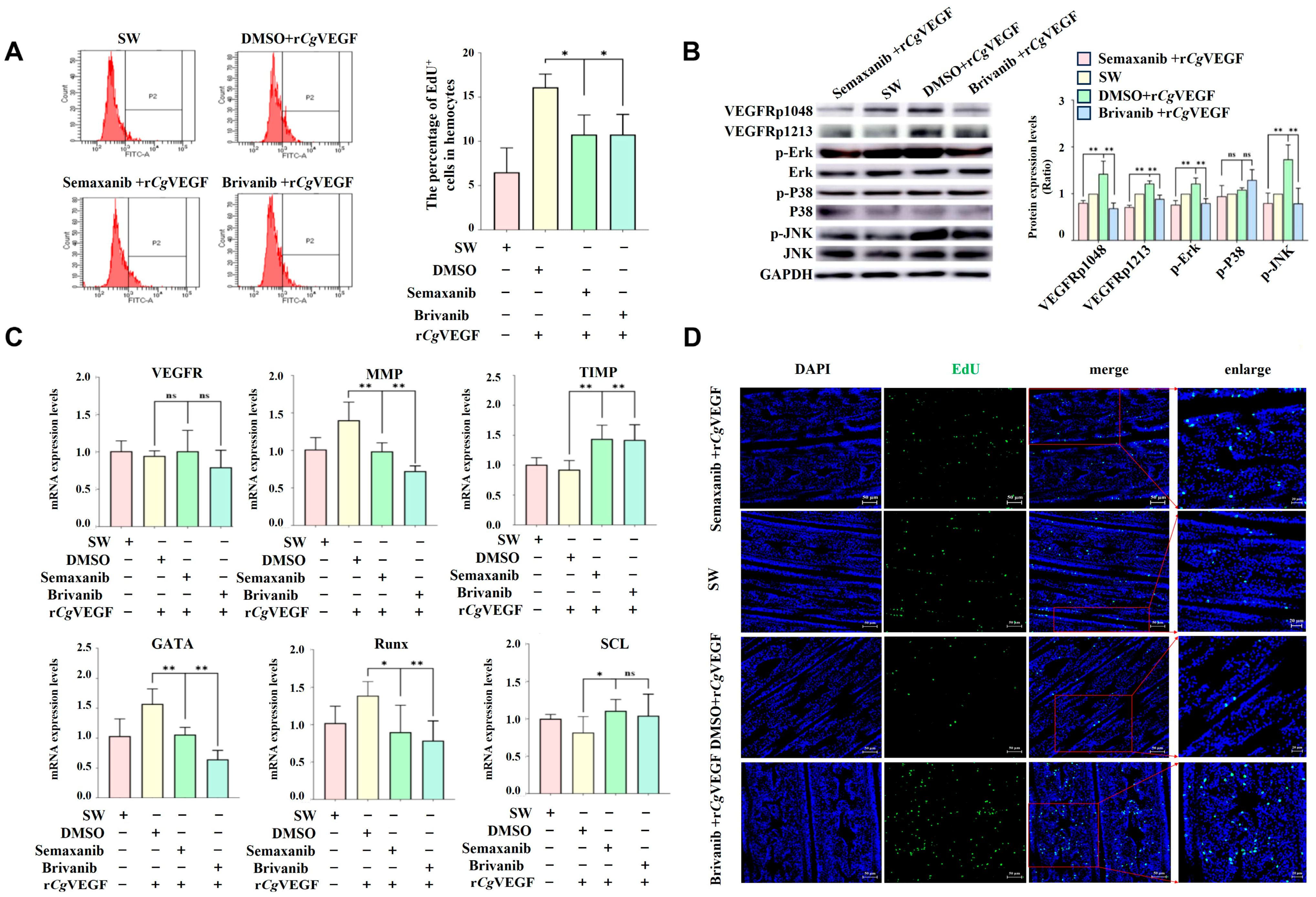
3.8. VEGF-VEGFR-MAPK Pathway Enhances the Proliferation of Hematopoietic Stem Cells and Circulating Hemocytes in a VEGF-Dependent Manner
3.9. The VEGF-VEGFR-MAPK Pathway Enhances the Proliferation and Migration of Hematopoietic Stem Cells and Circulating Hemocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HSCs | Hematopoietic stem cells. |
| G | Gill. |
| SW | Seawater. |
| VS | Vibrio splendidus. |
References
- Orkin, S.H.; Zon, L.I. Hematopoiesis: An evolving paradigm for stem cell biology. Cell 2008, 132, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, A.N.; Dolgalev, I.; Hu, H.; Sivaraj, K.K.; Hoxha, E.; Cuesta-Domínguez, Á.; Pinho, S.; Akhmetzyanova, I.; Gao, J.; Witkowski, M.; et al. The bone marrow microenvironment at single-cell resolution. Nature 2019, 569, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Porteus, M.H. Genome editing of the blood: Opportunities and challenges. Curr. Stem Cell Rep. 2015, 1, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Rahman, A. In search of the origin of the thymus: The thymus and galt may be evolutionarily related. Scand. J. Immunol. 2001, 53, 1–6. [Google Scholar] [CrossRef]
- Golconda, P.; Buckley, K.M.; Reynolds, C.R.; Romanello, J.P.; Smith, L.C. The axial organ and the pharynx are sites of hematopoiesis in the sea urchin. Front. Immunol. 2019, 10, 870. [Google Scholar] [CrossRef]
- Ghosh, S.; Singh, A.; Mandal, S.; Mandal, L. Active hematopoietic hubs in drosophila adults generate hemocytes and contribute to immune response. Dev. Cell 2015, 33, 478–488. [Google Scholar] [CrossRef]
- Hartenstein, V. Blood cells and blood cell development in the animal kingdom. Annu. Rev. Cell Dev. Biol. 2006, 22, 677–712. [Google Scholar] [CrossRef]
- Ottaviani, E.; Caselgrandi, E.; Fontanili, P.; Franceschi, C. Evolution, immune responses and stress: Studies on molluscan cells. Acta Biol. Hung. 1992, 43, 293–298. [Google Scholar]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for hematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.; Dikov, M.M.; Novitskiy, S.V.; Mosse, C.A.; Yang, L.; Carbone, D.P. Distinct roles of vegfr-1 and vegfr-2 in the aberrant hematopoiesis associated with elevated levels of vegf. Blood 2007, 110, 624–631. [Google Scholar] [CrossRef]
- Munier, A.I.; Doucet, D.; Perrodou, E.; Zachary, D.; Meister, M.; Hoffmann, J.A.; Janeway, C.A., Jr.; Lagueux, M. Pvf2, a pdgf/vegf-like growth factor, induces hemocyte proliferation in Drosophila larvae. EMBO Rep. 2002, 3, 1195–1200. [Google Scholar] [CrossRef]
- Junkunlo, K.; Söderhäll, K.; Noonin, C.; Söderhäll, I. Pdgf/vegf-related receptor affects transglutaminase activity to control cell migration during crustacean hematopoiesis. Stem Cells Dev. 2017, 26, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, F.; Wegner, K.M.; Polz, M.F. Oysters and vibrios as a model for disease dynamics in wild animals. Trends Microbiol. 2016, 24, 568–580. [Google Scholar] [CrossRef]
- Jemaà, M.; Morin, N.; Cavelier, P.; Cau, J.; Strub, J.M.; Delsert, C. Adult somatic progenitor cells and hematopoiesis in oysters. J. Exp. Biol. 2014, 217, 3067–3077. [Google Scholar] [CrossRef]
- Chang, R.; Sun, J.; Leng, J.; Wang, Z.; Mu, S.; Li, Y.; Wang, J.; Song, L. A new type of Caspase-1 upon recognizing bacteria inhibits GSDME-dependent histone modification and NF-κB signaling. Commun. Biol. 2025, 8, 827. [Google Scholar] [CrossRef]
- Dong, M.; Xu, M.; Wu, W.; Wang, W.; Yang, C.; Liu, C.; Gao, X.; Wang, L.; Song, L. Transcription factor VBP specifically regulates granulocyte apoptosis during the immune response in oysters. Int. J. Biol. Macromol. 2025, 322, 146521. [Google Scholar] [CrossRef]
- Sawai, C.M.; Babovic, S.; Upadhaya, S.; Knapp, D.J.H.F.; Lavin, Y.; Lau, C.M.; Goloborodko, A.; Feng, J.; Fujisaki, J.; Ding, L.; et al. Hematopoietic stem cells are the major source of multilineage hematopoiesis in adult animals. Immunity 2016, 45, 597–609. [Google Scholar] [CrossRef]
- Li, Q.; Ren, Y.; Liang, C.; Qiao, G.; Wang, Y.; Ye, S.; Li, R. Regeneration of coelomocytes after evisceration in the sea cucumber, apostichopus japonicus. Fish Shellfish Immunol. 2018, 76, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Zhang, Y.; Xiang, Z.; Tong, Y.; Qu, F.; Yu, Z. Genomic characterization and expression analysis of five novel il-17 genes in the pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2014, 40, 455–465. [Google Scholar] [CrossRef]
- Zeng, C.; Guo, M.; Xiao, K.; Li, C. Autophagy mediated by ROS-AKT-FoxO pathway is required for intestinal regeneration in echinoderms. Cell Commun. Signal 2025, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Wang, W.; Wu, W.; Cheng, X.; Cheng, J.; Wang, L.; Song, L. A novel surface marker CD49d promotes TNF expression in oyster agranulocytes by mediating the MAPK pathway. Fish Shellfish Immunol. 2024, 151, 109702. [Google Scholar]
- Van der Wath, R.C.; Wilson, A.; Laurenti, E.; Trumpp, A.; Liò, P. Estimating dormant and active hematopoietic stem cell kinetics through extensive modeling of bromodeoxyuridine label-retaining cell dynamics. PLoS ONE 2009, 4, e6972. [Google Scholar] [CrossRef]
- Lu, L.; Li, F.; Lu, J. Identification of functional tissue-resident cardiac stem/progenitor cells in adult mouse. Cell Biol. Int. Rep. 2012, 19, e00016. [Google Scholar] [CrossRef]
- Hall, C.J.; Flores, M.V.; Oehlers, S.H.; Sanderson, L.E.; Lam, E.Y.; Crosier, K.E.; Crosier, P.S. Infection-responsive expansion of the hematopoietic stem and progenitor cell compartment in zebrafish is dependent upon inducible nitric oxide. Cell Stem Cell 2012, 10, 198–209. [Google Scholar] [CrossRef]
- Ward, R.J.; McCrohan, C.R.; White, K.N. Influence of aqueous aluminium on the immune system of the freshwater crayfish Pacifastacus leniusculus. Aquat. Toxicol. 2006, 77, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Laurenti, E.; Oser, G.; van der Wath, R.C.; Blanco-Bose, W.; Jaworski, M.; Offner, S.; Dunant, C.F.; Eshkind, L.; Bockamp, E.; et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 2008, 135, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Omatsu, Y.; Sugiyama, T. Control of hematopoietic stem cells by the bone marrow stromal niche: The role of reticular cells. Trends Immunol. 2011, 32, 315–320. [Google Scholar] [CrossRef]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. Vegf receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar]
- Bott, R.C.; McFee, R.M.; Clopton, D.T.; Toombs, C.; Cupp, A.S. Vascular endothelial growth factor and kinase domain region receptor are involved in both seminiferous cord formation and vascular development during testis morphogenesis in the rat. Biol. Reprod. 2006, 75, 56–67. [Google Scholar] [CrossRef]
- Yu, Y.A.; Zhang, G.S.; Zhang, J.; Ju, L.; Zhu, Q.D.; Song, Y.L.; Wang, J.W.; Niu, N.; Ma, S.C. Molecular cloning and characterization of a proliferating cell nuclear antigen gene by chemically induced male sterility in wheat (Triticum aestivum L.). Genet. Mol. Res. 2015, 14, 12030–12042. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, L.; Gai, X.; Zhou, Z.; Wang, L.; Zhang, H.; Gai, Y.; Song, L.; Yu, J.; Liang, C. The involvement of pdgf/vegf related factor in regulation of immune and neuroendocrine in Chinese mitten crab eriocheir sinensis. Fish Shellfish Immunol. 2013, 35, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.C.; Metzendorf, C.; Soderhall, K. Microarray analysis of immune challenged drosophila hemocytes. Exp. Cell Res. 2005, 305, 145–155. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Lian, J.; Ma, N.; Huang, Z.; Li, J.; Wen, Z.; Zhang, W.; Zhang, Y. Slc20a1b is essential for hematopoietic stem/progenitor cell expansion in zebrafish. Sci. China Life Sci. 2021, 64, 2186–2201. [Google Scholar] [CrossRef]
- Krishnapati, L.S.; Ghaskadbi, S. Identification and characterization of vegf and fgf from hydra. Int. J. Dev. Biol. 2013, 57, 897–906. [Google Scholar] [CrossRef]
- Pascual-Anaya, J.; Albuixech-Crespo, B.; Somorjai, I.M.; Carmona, R.; Oisi, Y.; Alvarez, S.; Kuratani, S.; Muñoz-Chápuli, R.; Garcia-Fernàndez, J. The evolutionary origins of chordate hematopoiesis and vertebrate endothelia. Dev. Biol. 2013, 375, 182–192. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, M.; Li, C.; Shao, Y.; Zhao, X.; Zhang, W. Vegf-like protein from apostichopus japonicus promotes cell proliferation and migration. Dev. Comp. Immunol. 2019, 92, 230–237. [Google Scholar] [CrossRef]
- Gille, H.; Kowalski, J.; Yu, L.; Chen, H.; Pisabarro, M.T.; Davis-Smyth, T.; Ferrara, N. A repressor sequence in the juxtamembrane domain of flt-1 (vegfr-1) constitutively inhibits vascular endothelial growth factor-dependent phosphatidylinositol 3′-kinase activation and endothelial cell migration. EMBO J. 2000, 19, 4064–4073. [Google Scholar] [CrossRef] [PubMed]
- Duloquin, L.; Lhomond, G.; Gache, C. Localized vegf signaling from ectoderm to mesenchyme cells controls morphogenesis of the sea urchin embryo skeleton. Development 2007, 134, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.; Foley, E. Autocrine platelet-derived growth factor-vascular endothelial growth factor receptor-related (pvr) pathway activity controls intestinal stem cell proliferation in the adult drosophila midgut. J. Biol. Chem. 2012, 287, 27359–27370. [Google Scholar] [CrossRef]
- Vinals, F.; Pouyssegur, J. Confluence of vascular endothelial cells induces cell cycle exit by inhibiting p42/p44 mitogen-activated protein kinase activity. Mol. Cell Biol. 1999, 19, 2763–2772. [Google Scholar] [CrossRef]
- Bond, D.; Foley, E. A quantitative rnai screen for jnk modifiers identifies pvr as a novel regulator of drosophila immune signaling. PLoS Pathog. 2009, 5, e1000655. [Google Scholar]
- Yoshida, M.A.; Shigeno, S.; Tsuneki, K.; Furuya, H. Squid vascular endothelial growth factor receptor: A shared molecular signature in the convergent evolution of closed circulatory systems. Evol. Dev. 2010, 12, 25–33. [Google Scholar] [CrossRef]
- Seipel, K.; Eberhardt, M.; Müller, P.; Pescia, E.; Yanze, N.; Schmid, V. Homologs of vascular endothelial growth factor and receptor, VEGF and VEGFR, in the jellyfish Podocoryne carnea. Dev. Dyn. 2004, 231, 303–312. [Google Scholar] [CrossRef]
- Deng, J.; Jing, S.; Zhang, M.; Quan, X.; Hu, X.; Yun, J.; Geng, T.; Zhang, Y.; Zhang, L. Jiao-Ai Decoction and active ingredients promoted angiogenesis to alleviate threatened abortion through miR-16 mediated VEGF/VEGFR signaling pathway. J. Ethnopharmacol. 2025, 353, 120385. [Google Scholar]
- Morgulis, M.; Gildor, T.; Roopin, M.; Sher, N.; Malik, A.; Lalzar, M.; Dines, M.; Ben-Tabou de-Leon, S.; Khalaily, L.; Ben-Tabou de-Leon, S. Possible cooption of a VEGF-driven tubulogenesis program for biomineralization in echinoderms. Proc. Natl. Acad. Sci. USA 2019, 116, 12353–12362. [Google Scholar] [CrossRef]
- Duchek, P.; Somogyi, K.; Jékely, G.; Beccari, S.; Rørth, P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 2001, 107, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Ragab, A.; Buechling, T.; Gesellchen, V.; Spirohn, K.; Boettcher, A.L.; Boutros, M. Drosophila Ras/MAPK signalling regulates innate immune responses in immune and intestinal stem cells. EMBO J. 2014, 30, 1123–1136. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Dong, M.; Qiao, X.; Jin, Y.; Liu, X.; He, M.; Wang, L.; Song, L. VEGF-VEGFR Signaling Mechanism Directs the Migration of Newborn Hemocytes from the Hematopoietic Site of Oyster Crassostrea gigas. Cells 2025, 14, 1446. https://doi.org/10.3390/cells14181446
Yu S, Dong M, Qiao X, Jin Y, Liu X, He M, Wang L, Song L. VEGF-VEGFR Signaling Mechanism Directs the Migration of Newborn Hemocytes from the Hematopoietic Site of Oyster Crassostrea gigas. Cells. 2025; 14(18):1446. https://doi.org/10.3390/cells14181446
Chicago/Turabian StyleYu, Simiao, Miren Dong, Xue Qiao, Yuhao Jin, Xiyang Liu, Muchun He, Lingling Wang, and Linsheng Song. 2025. "VEGF-VEGFR Signaling Mechanism Directs the Migration of Newborn Hemocytes from the Hematopoietic Site of Oyster Crassostrea gigas" Cells 14, no. 18: 1446. https://doi.org/10.3390/cells14181446
APA StyleYu, S., Dong, M., Qiao, X., Jin, Y., Liu, X., He, M., Wang, L., & Song, L. (2025). VEGF-VEGFR Signaling Mechanism Directs the Migration of Newborn Hemocytes from the Hematopoietic Site of Oyster Crassostrea gigas. Cells, 14(18), 1446. https://doi.org/10.3390/cells14181446





