Regional Expression of Dystrophin Gene Transcripts and Proteins in the Mouse Brain
Abstract
1. Introduction

2. Materials and Methods
2.1. Animals
2.2. Tissue Collection
2.3. Analysis of Dmd Transcripts via Quantitative Polymerase Chain Reaction
2.4. Protein Quantification via Simple Capillary Western Blot (WES)
2.5. Protein Quantification via Western Blot
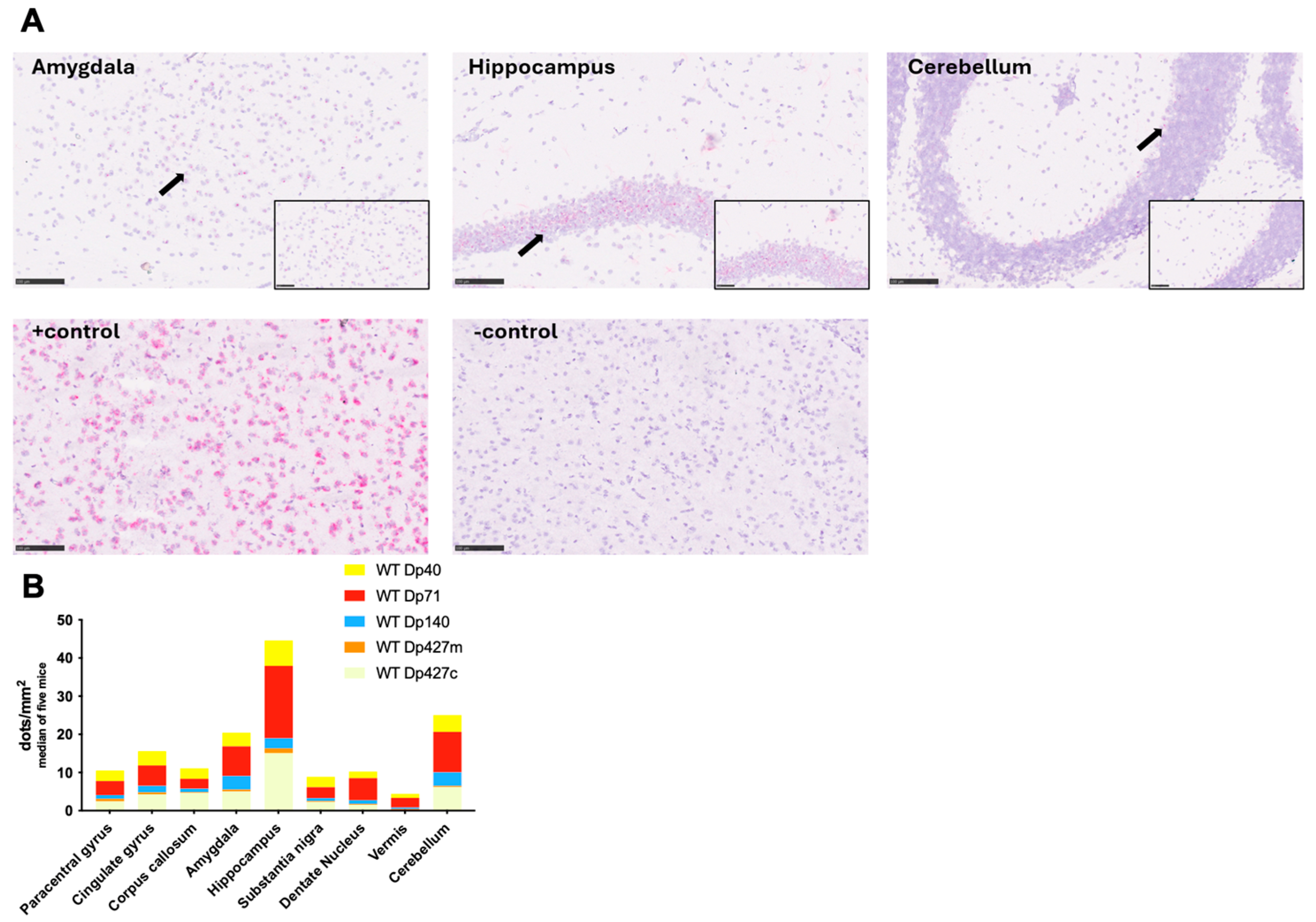
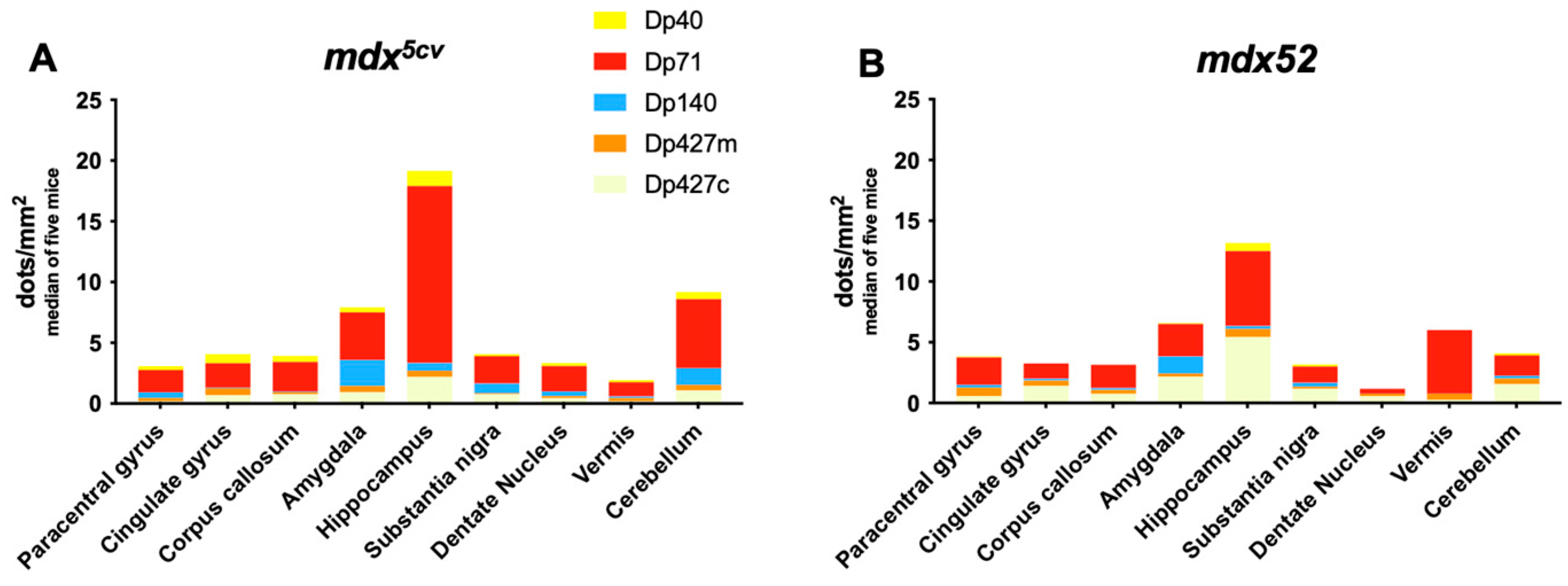
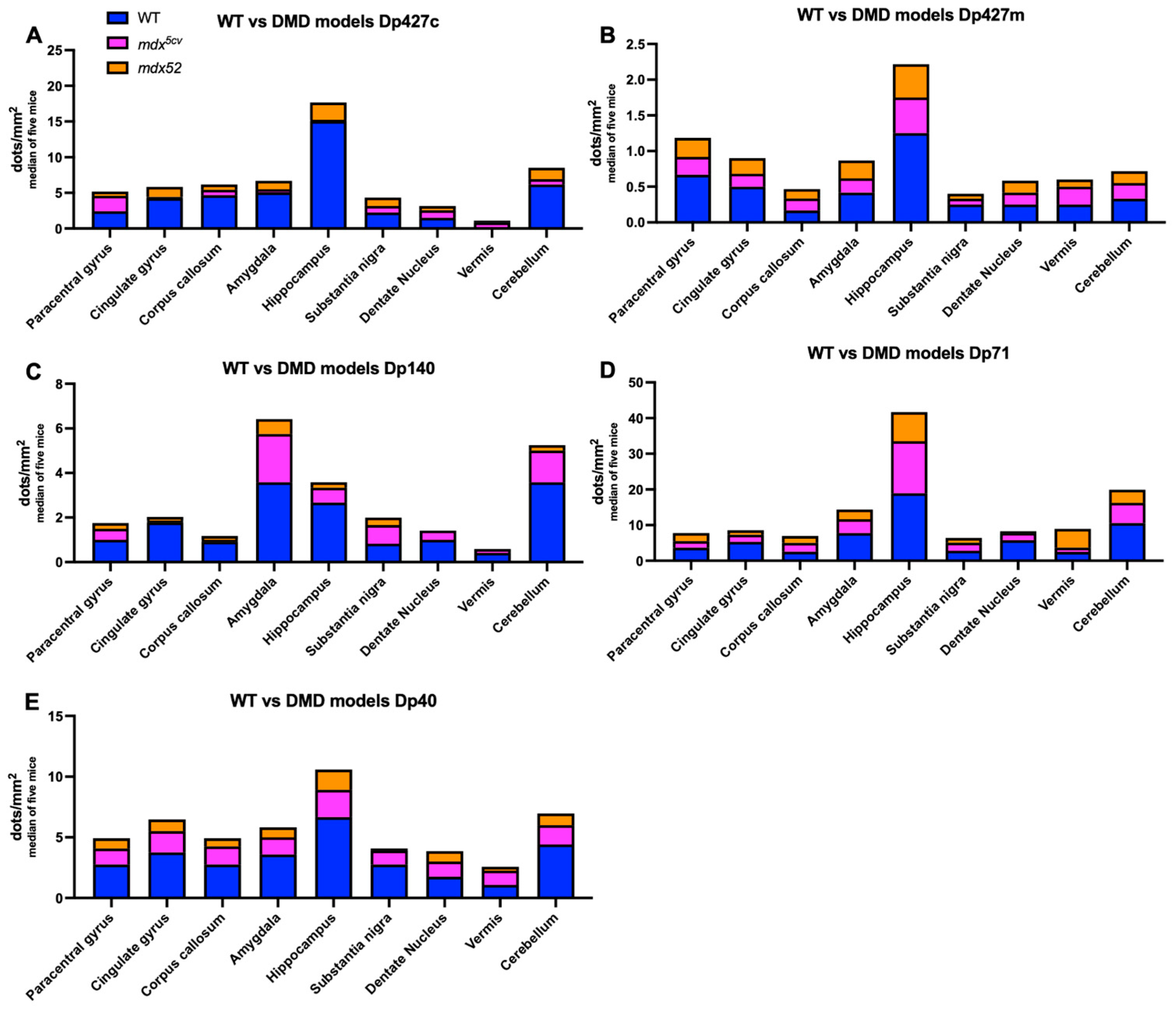
2.6. BaseScope Analysis of Dmd mRNA
2.7. Statistical Analysis
3. Results
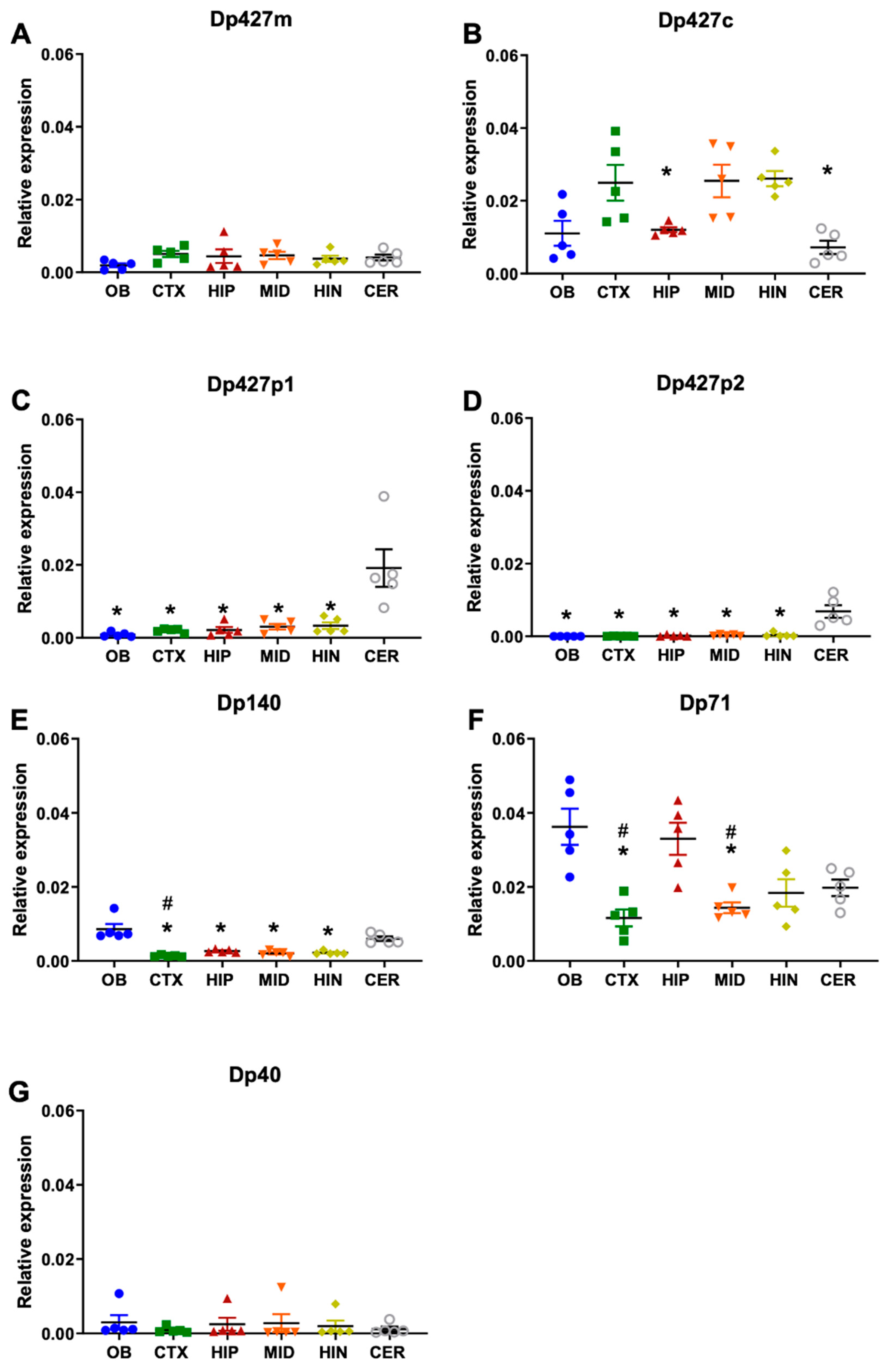
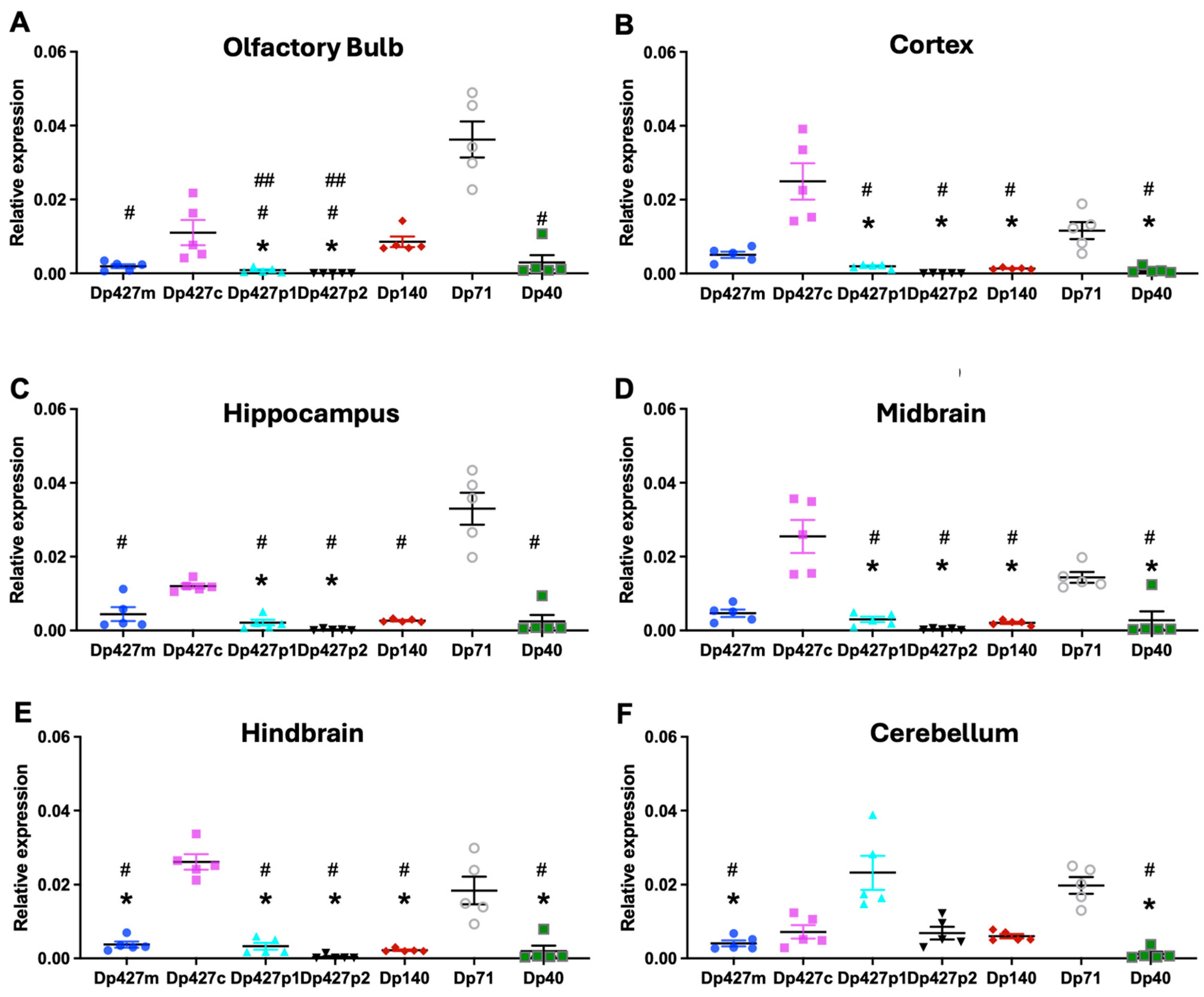
3.1. Regional Expression of Dystrophin mRNAs in the Adult Mouse Brain
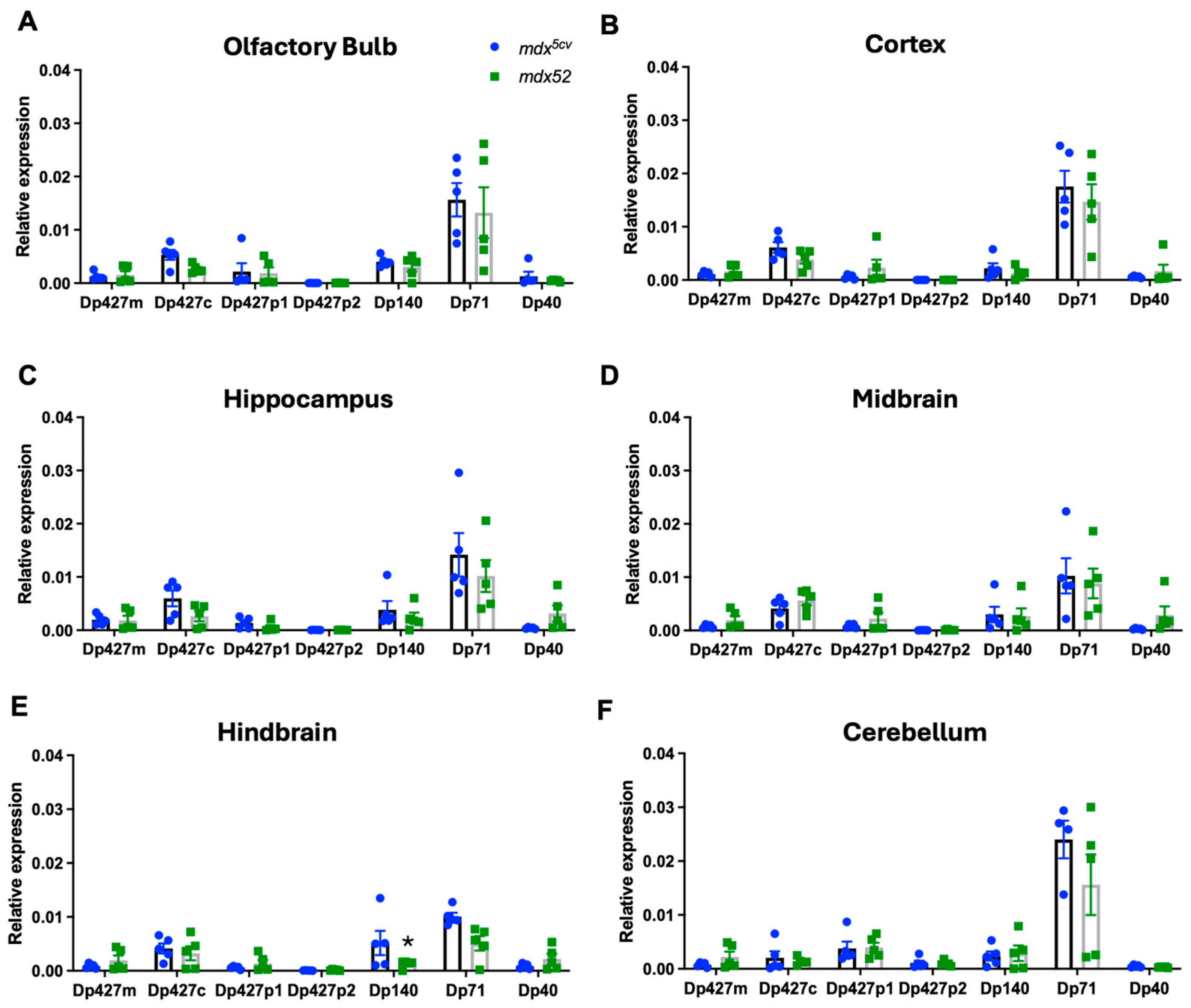
3.2. Regional Expression of Dmd mRNAs in Adult mdx5cv and mdx52 Mice
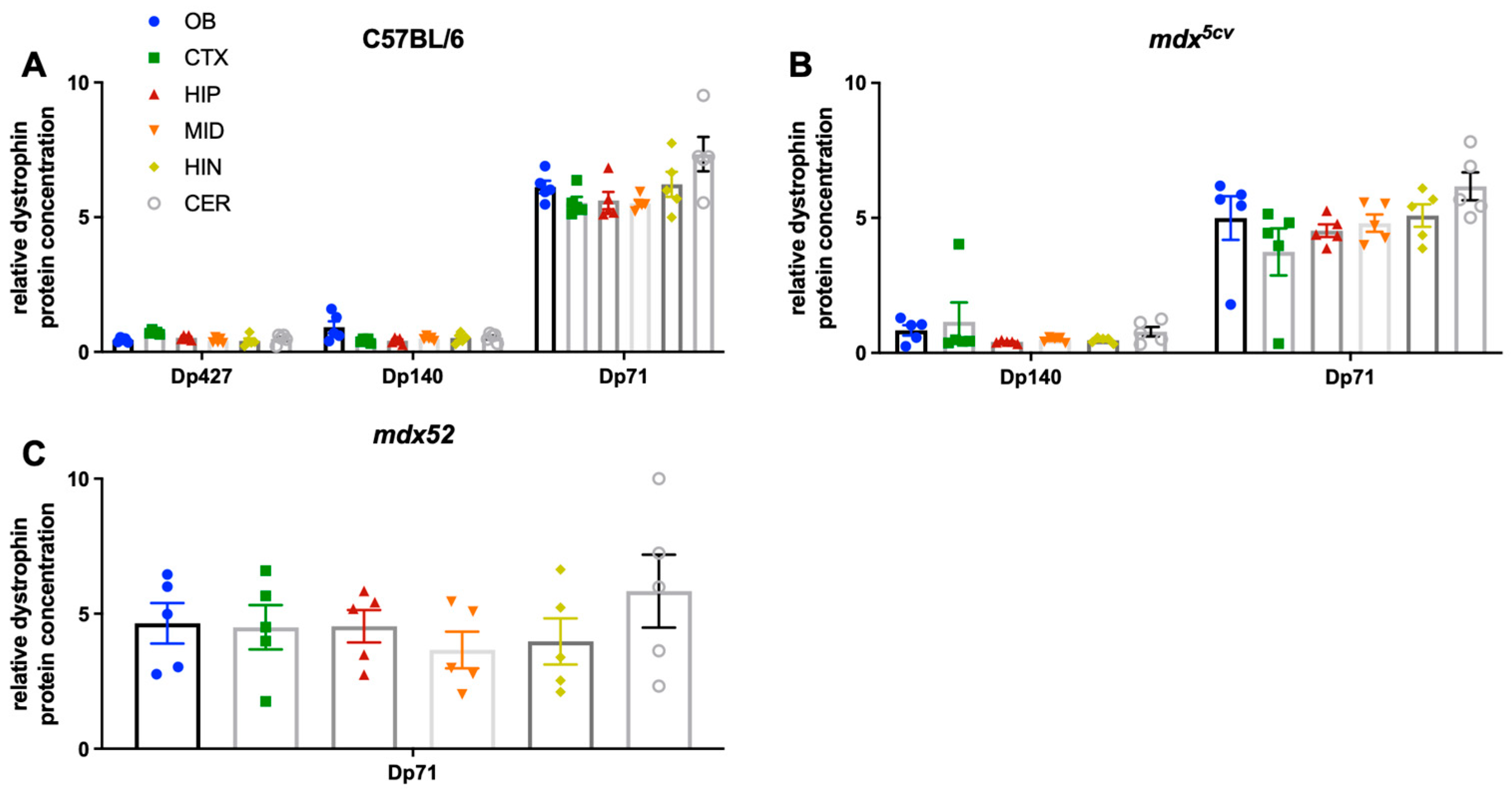
3.3. Regional Expression of Dystrophin Proteins in the Mouse Brain
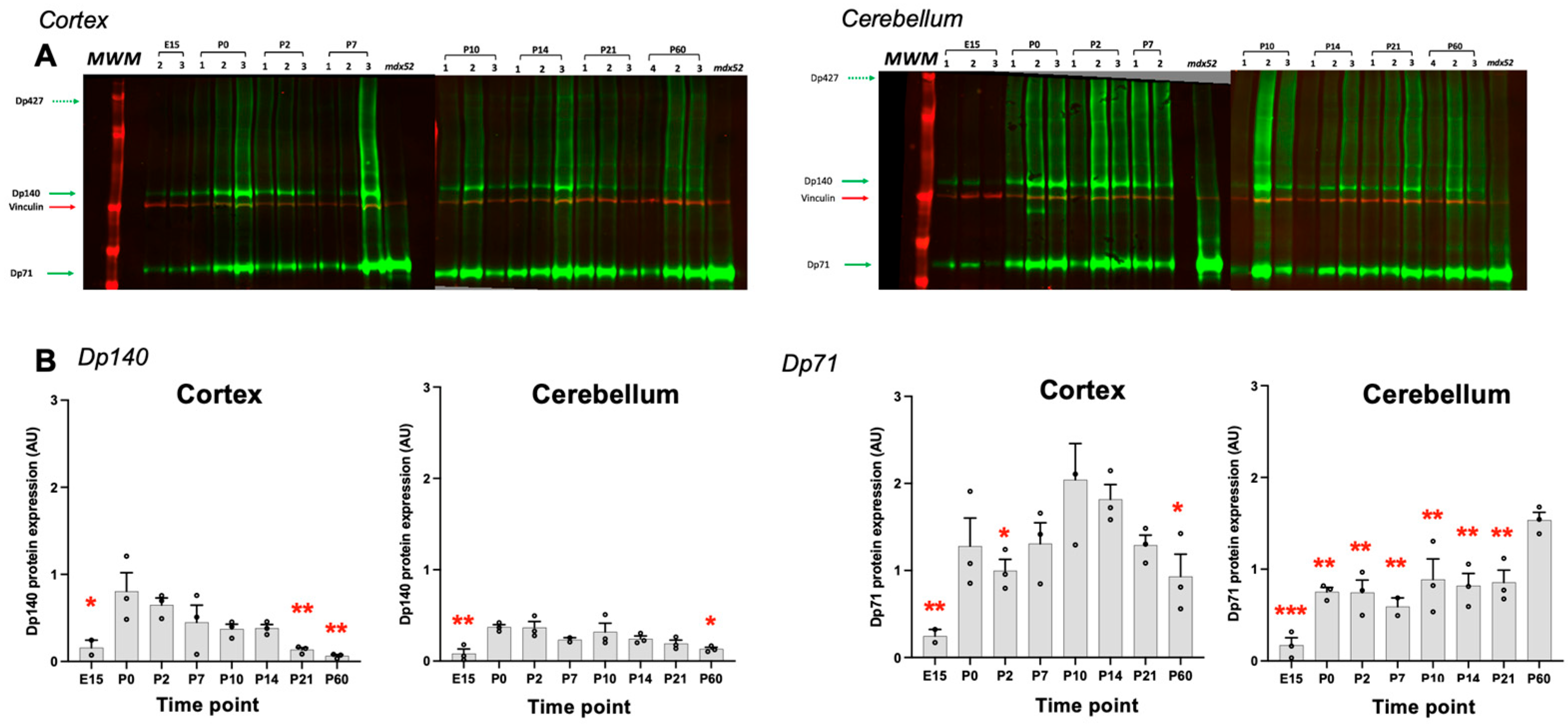
3.4. Expression Levels of Dystrophin Proteins During Development of C57BL/6J Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHD | Attention-deficit/hyperactivity disorder |
| ASD | Autism spectrum disorder |
| CC | Coiled-coil domain |
| CNS | Central nervous system |
| CR | Cysteine rich domain |
| DMD | Duchenne muscular dystrophy |
| EF/ZZ | Helix-loop-helix |
| GABAA | γ-Aminobutyric acid type A |
| ISH | In situ hybridisation |
| IVC | Individually ventilated cages |
| mdx52 | C57BL/6J mice lacking Dp427 and Dp140 isoform |
| mdx5cv | C57BL/6J mice lacking Dp427 isoform |
| NT | N-terminus |
| OCD | Obsessive-compulsive disorder |
| qPCR | Quantitative polymerase chain reaction |
| Ubc | Ubiquitin C |
| UTR | Untranslated region |
| WES | Western Blot capillary |
| WW | Double tryptophan |
| ZZ | Zinc-finger |
References
- Górecki, D.C.; Monaco, A.P.; Derry, J.M.J.; Walker, A.P.; Barnard, E.A.; Barnard, P.J. Expression of Four Alternative Dystrophin Transcripts in Brain Regions Regulated by Different Promoters. Hum. Mol. Genet. 1992, 1, 505–510. [Google Scholar] [CrossRef]
- Den Dunnen, J.T.; Grootscholten, R.M.; Bakker, E.; Blonden, L.A.J.; Ginjaar, H.B.; Wapenaar, M.C.; Van Paassen, H.M.B.; Van Broeckhoven, C.; Pearson, R.L.; Van Ommen, G.J.B. Topography of the Duchenne Muscular Dystrophy (DMD) Gene: FIGE and CDNA Analysis of 194 Cases Reveals 115 Deletions and 13 Duplications. Am. J. Hum. Genet. 1989, 45, 835–847. [Google Scholar] [PubMed]
- Muntoni, F.; Torelli, S.; Ferlini, A. Dystrophin and Mutations: One Gene, Several Proteins, Multiple Phenotypes. Lancet Neurol. 2003, 2, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Coffey, A.J.; Roberts, R.G.; Green, E.D.; Cole, C.G.; Butler, R.; Anand, R.; Giannelli, F.; Bentley, D.R. Construction of a 2.6-Mb Contig in Yeast Artificial Chromosomes Spanning the Human Dystrophin Gene Using an STS-Based Approach. Genomics 1992, 12, 474–484. [Google Scholar] [CrossRef]
- D’souza, V.N.; Man, N.T.; Morris, G.E.; Karges, W.; Pillers, D.A.M.; Ray, P.N. A Novel Dystrophin Isoform Is Required for Normal Retinal Electrophysiology. Hum. Mol. Genet. 1995, 4, 837–842. [Google Scholar] [CrossRef]
- Hugnot, J.P.; Gilgenkrantz, H.; Vincent, N.; Chafey, P.; Morris, G.E.; Monaco, A.P.; Berwald-Netter, Y.; Koulakoff, A.; Kaplan, J.C.; Kahn, A.; et al. Distal Transcript of the Dystrophin Gene Initiated from an Alternative First Exon and Encoding a 75-KDa Protein Widely Distributed in Nonmuscle Tissues. Proc. Natl. Acad. Sci. USA 1992, 89, 7506. [Google Scholar] [CrossRef]
- Tinsley, J.M.; Blake, D.J.; Davies, K.E. Apo-Dystrophin-3: A 2.2kb Transcript from the DMD Locus Encoding the Dystrophin Glycoprotein Binding Site. Hum. Mol. Genet. 1993, 2, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Aragón, J.; Martínez-Herrera, A.; Bermúdez-Cruz, R.M.; Bazán, M.L.; Soid-Raggi, G.; Ceja, V.; Coy-Arechavaleta, A.S.; Alemán, V.; Depardón, F.; Montañez, C. EF-Hand Domains Are Involved in the Differential Cellular Distribution of Dystrophin Dp40. Neurosci. Lett. 2015, 600, 115–120. [Google Scholar] [CrossRef]
- Suzuki, A.; Yoshida, M.; Yamamoto, H.; Ozawa, E. Glycoprotein-Binding Site of Dystrophin Is Confined to the Cysteine-Rich Domain and the First Half of the Carboxy-Terminal Domain. FEBS Lett. 1992, 308, 154–160. [Google Scholar] [CrossRef]
- Warner, L.E.; DelloRusso, C.T.; Crawford, R.W.; Rybakova, I.N.; Patel, J.R.; Ervasti, J.M.; Chamberlain, J.S. Expression of Dp260 in Muscle Tethers the Actin Cytoskeleton to the Dystrophin–Glycoprotein Complex and Partially Prevents Dystrophy. Hum. Mol. Genet. 2002, 11, 1095–1105. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets—Update. Nucleic Acids Res. 2013, 41, D991. [Google Scholar] [CrossRef] [PubMed]
- Tetorou, K.; Aghaeipour, A.; Singh, S.; Morgan, J.E.; Muntoni, F. The Role of Dystrophin Isoforms and Interactors in the Brain. Brain 2012, 139, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Higuchi, S.; Aida, I.; Nakajima, T.; Nakada, T. Abnormal Distribution of GABAA Receptors in Brain of Duchenne Muscular Dystrophy Patients. Muscle Nerve 2017, 55, 591–595. [Google Scholar] [CrossRef]
- García-Cruz, C.; Aragón, J.; Lourdel, S.; Annan, A.; Roger, J.E.; Montanez, C.; Vaillend, C. Tissue-and Cell-Specific Whole-Transcriptome Meta-Analysis from Brain and Retina Reveals Differential Expression of Dystrophin Complexes and New Dystrophin Spliced Isoforms. Hum. Mol. Genet. 2023, 32, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Doorenweerd, N.; Mahfouz, A.; Van Putten, M.; Kaliyaperumal, R.; T’Hoen, P.A.C.; Hendriksen, J.G.M.; Aartsma-Rus, A.M.; Verschuuren, J.J.G.M.; Niks, E.H.; Reinders, M.J.T.; et al. Timing and Localization of Human Dystrophin Isoform Expression Provide Insights into the Cognitive Phenotype of Duchenne Muscular Dystrophy. Sci. Rep. 2017, 7, 12575. [Google Scholar] [CrossRef]
- Catapano, F.; Alkharji, R.; Chambers, D.; Singh, S.; Aghaeipour, A.; Malhotra, J.; Ferretti, P.; Phadke, R.; Muntoni, F. A Comprehensive Spatiotemporal Map of Dystrophin Isoform Expression in the Developing and Adult Human Brain. Acta Neuropathol. Commun. 2025, 13, 110. [Google Scholar] [CrossRef]
- Aranmolate, A.; Tse, N.; Colognato, H. Myelination Is Delayed during Postnatal Brain Development in the Mdx Mouse Model of Duchenne Muscular Dystrophy. BMC Neurosci. 2017, 18, 63. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kuniishi, H.; Sakai, K.; Fukushima, Y.; Du, X.; Yamashiro, K.; Hori, K.; Imamura, M.; Hoshino, M.; Yamada, M.; et al. Brain Dp140 Alters Glutamatergic Transmission and Social Behaviour in the Mdx52 Mouse Model of Duchenne Muscular Dystrophy. Prog. Neurobiol. 2022, 216, 102288. [Google Scholar] [CrossRef]
- Belmaati Cherkaoui, M.; Vacca, O.; Izabelle, C.; Boulay, A.C.; Boulogne, C.; Gillet, C.; Barnier, J.V.; Rendon, A.; Cohen-Salmon, M.; Vaillend, C. Dp71 Contribution to the Molecular Scaffold Anchoring Aquaporine-4 Channels in Brain Macroglial Cells. Glia 2021, 69, 954–970. [Google Scholar] [CrossRef]
- Lange, J.; Gillham, O.; Alkharji, R.; Eaton, S.; Ferrari, G.; Madej, M.; Flower, M.; Tedesco, F.S.; Muntoni, F.; Ferretti, P. Dystrophin Deficiency Affects Human Astrocyte Properties and Response to Damage. Glia 2022, 70, 466–490. [Google Scholar] [CrossRef]
- Pascual-Morena, C.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Jiménez-López, E.; Saz-Lara, A.; Martínez-García, I.; Martínez-Vizcaíno, V. Global Prevalence of Intellectual Developmental Disorder in Dystrophinopathies: A Systematic Review and Meta-Analysis. Dev. Med. Child Neurol. 2023, 65, 734–744. [Google Scholar] [CrossRef]
- Ricotti, V.; Mandy, W.P.L.; Scoto, M.; Pane, M.; Deconinck, N.; Messina, S.; Mercuri, E.; Skuse, D.H.; Muntoni, F. Neurodevelopmental, Emotional, and Behavioural Problems in Duchenne Muscular Dystrophy in Relation to Underlying Dystrophin Gene Mutations. Dev. Med. Child Neurol. 2016, 58, 77–84. [Google Scholar] [CrossRef]
- Taylor, P.J.; Betts, G.A.; Maroulis, S.; Gilissen, C.; Pedersen, R.L.; Mowat, D.R.; Johnston, H.M.; Buckley, M.F. Dystrophin Gene Mutation Location and the Risk of Cognitive Impairment in Duchenne Muscular Dystrophy. PLoS ONE 2010, 5, e8803. [Google Scholar] [CrossRef]
- Chamova, T.; Guergueltcheva, V.; Raycheva, M.; Todorov, T.; Genova, J.; Bichev, S.; Bojinova, V.; Mitev, V.; Tournev, I.; Todorova, A. Association Between Loss of Dp140 and Cognitive Impairment in Duchenne and Becker Dystrophies. Balkan J. Med. Genet. 2013, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Vaillend, C.; Aoki, Y.; Mercuri, E.; Hendriksen, J.; Tetorou, K.; Goyenvalle, A.; Muntoni, F. Duchenne Muscular Dystrophy: Recent Insights in Brain Related Comorbidities. Nat. Commun. 2025, 16, 1298. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, M.; Lloyd, E.M.; De Greef, J.C.; Raz, V.; Willmann, R.; Grounds, M.D. Mouse Models for Muscular Dystrophies: An Overview. Dis. Models Mech. 2020, 13, dmm043562. [Google Scholar] [CrossRef]
- Sicinski, P.; Geng, Y.; Ryder-Cook, A.S.; Barnard, E.A.; Darlison, M.G.; Barnard, P.J. The Molecular Basis of Muscular Dystrophy in the Mdx Mouse: A Point Mutation. Science (1979) 1989, 244, 1578–1580. [Google Scholar] [CrossRef]
- Phillips, R.G.; LeDoux, J.E. Differential Contribution of Amygdala and Hippocampus to Cued and Contextual Fear Conditioning. Behav. Neurosci. 1992, 106, 274–285. [Google Scholar] [CrossRef]
- Sekiguchi, M.; Zushida, K.; Yoshida, M.; Maekawa, M.; Kamichi, S.; Yoshida, M.; Sahara, Y.; Yuasa, S.; Takeda, S.; Wada, K. A Deficit of Brain Dystrophin Impairs Specific Amygdala GABAergic Transmission and Enhances Defensive Behaviour in Mice. Brain 2009, 132, 124–135. [Google Scholar] [CrossRef]
- Razzoli, M.; Lindsay, A.; Law, M.L.; Chamberlain, C.M.; Southern, W.M.; Berg, M.; Osborn, J.; Engeland, W.C.; Metzger, J.M.; Ervasti, J.M.; et al. Social Stress Is Lethal in the Mdx Model of Duchenne Muscular Dystrophy. eBioMedicine 2020, 55, 102700. [Google Scholar] [CrossRef]
- Vaillend, C.; Chaussenot, R. Relationships Linking Emotional, Motor, Cognitive and GABAergic Dysfunctions in Dystrophin-Deficient Mdx Mice. Hum. Mol. Genet. 2017, 26, 1041–1055. [Google Scholar] [CrossRef]
- Vaillend, C.; Billard, J.M.; Laroche, S. Impaired Long-Term Spatial and Recognition Memory and Enhanced CA1 Hippocampal LTP in the Dystrophin-Deficient Dmdmdx Mouse. Neurobiol. Dis. 2004, 17, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Bellissimo, C.A.; Castellani, L.N.; Finch, M.S.; Murugathasan, M.; Gandhi, S.; Sweeney, G.; Abdul-Sater, A.A.; MacPherson, R.E.K.; Perry, C.G.R. Memory Impairment in the D2.Mdx Mouse Model of Duchenne Muscular Dystrophy Is Prevented by the Adiponectin Receptor Agonist ALY688. Exp. Physiol. 2023, 108, 1108–1117. [Google Scholar] [CrossRef]
- Saoudi, A.; Mitsogiannis, M.D.; Zarrouki, F.; Fergus, C.; Stojek, E.; Talavera, S.; Moore-Frederick, D.; Kelly, V.P.; Goyenvalle, A.; Montanaro, F.; et al. Impact of Distinct Dystrophin Gene Mutations on Behavioral Phenotypes of Duchenne Muscular Dystrophy. Dis. Model. Mech. 2024, 17, dmm050707. [Google Scholar] [CrossRef] [PubMed]
- Im, W.B.; Phelps, S.F.; Copen, E.H.; Adams, E.G.; Slightom, J.L.; Chamberlain, J.S. Differential Expression of Dystrophin Isoforms in Strains of Mdx Mice with Different Mutations. Hum. Mol. Genet. 1996, 5, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Araki, E.; Nakamura, K.; Nakao, K.; Kameya, S.; Kobayashi, O.; Nonaka, I.; Kobayashi, T.; Katsuki, M. Targeted Disruption of Exon 52 in the Mouse Dystrophin Gene Induced Muscle Degeneration Similar to That Observed in Duchenne Muscular Dystrophy. Biochem. Biophys. Res. Commun. 1997, 238, 492–497. [Google Scholar] [CrossRef]
- Pomeroy, J.; Borczyk, M.; Kawalec, M.; Hajto, J.; Carlson, E.; Svärd, S.; Verma, S.; Bareke, E.; Boratyńska-Jasińska, A.; Dymkowska, D.; et al. Spatiotemporal Diversity in Molecular and Functional Abnormalities in the Mdx Dystrophic Brain. Mol. Med. 2025, 31, 108. [Google Scholar] [CrossRef]
- Chapman, V.M.; Miller, D.R.; Armstrong, D.; Caskey, C.T. Recovery of Induced Mutations for X Chromosome-Linked Muscular Dystrophy in Mice. Proc. Natl. Acad. Sci. USA 1989, 86, 1292. [Google Scholar] [CrossRef]
- Shin, J.H.; Hakim, C.H.; Zhang, K.; Duan, D. Genotyping Mdx, Mdx3cv, and Mdx4cv Mice by Primer Competition Polymerase Chain Reaction. Muscle Nerve 2011, 43, 283–286. [Google Scholar] [CrossRef]
- Untergasser, A.; Ruijter, J.M.; Benes, V.; van den Hoff, M.J.B. Web-Based LinRegPCR: Application for the Visualization and Analysis of (RT)-QPCR Amplification and Melting Data. BMC Bioinform. 2021, 22, 398. [Google Scholar] [CrossRef]
- Spitali, P.; Van Den Bergen, J.C.; Verhaart, I.E.C.; Wokke, B.; Janson, A.A.M.; Van Den Eijnde, R.; Den Dunnen, J.T.; Laros, J.F.J.; Verschuuren, J.J.G.M.; Peter, P.A.; et al. DMD Transcript Imbalance Determines Dystrophin Levels. FASEB J. 2013, 27, 4909–4916. [Google Scholar] [CrossRef]
- Andrade-Talavera, Y.; Pérez-Rodríguez, M.; Prius-Mengual, J.; Rodríguez-Moreno, A. Neuronal and Astrocyte Determinants of Critical Periods of Plasticity. Trends Neurosci. 2023, 46, 566–580. [Google Scholar] [CrossRef]
- Morris, G.E.; Simmons, C.; Man, N.T. Apo-Dystrophins (DP140 and DP71) and Dystrophin-Splicing Isoforms in Developing Brain. Biochem. Biophys. Res. Commun. 1995, 215, 361–367. [Google Scholar] [CrossRef]
- Fujimoto, T.; Yaoi, T.; Nakano, K.; Arai, T.; Okamura, T.; Itoh, K. Generation of Dystrophin Short Product-Specific Tag-Insertion Mouse: Distinct Dp71 Glycoprotein Complexes at Inhibitory Postsynapse and Glia Limitans. Cell. Mol. Life Sci. 2022, 79, 109. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Stam, K.; Yaoi, T.; Nakano, K.; Arai, T.; Okamura, T.; Itoh, K. Dystrophin Short Product, Dp71, Interacts with AQP4 and Kir4.1 Channels in the Mouse Cerebellar Glial Cells in Contrast to Dp427 at Inhibitory Postsynapses in the Purkinje Neurons. Mol. Neurobiol. 2023, 60, 3664–3677. [Google Scholar] [CrossRef]
- Zarrouki, F.; Goutal, S.; Vacca, O.; Garcia, L.; Tournier, N.; Goyenvalle, A.; Vaillend, C. Abnormal Expression of Synaptic and Extrasynaptic GABAA Receptor Subunits in the Dystrophin-Deficient Mdx Mouse. Int. J. Mol. Sci. 2022, 23, 12617. [Google Scholar] [CrossRef]
- Ressler, K.J. Amygdala Activity, Fear, and Anxiety: Modulation by Stress. Biol. Psychiatry 2010, 67, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- LaBar, K.S.; Gatenby, J.C.; Gore, J.C.; LeDoux, J.E.; Phelps, E.A. Human Amygdala Activation during Conditioned Fear Acquisition and Extinction: A Mixed-Trial FMRI Study. Neuron 1998, 20, 937–945. [Google Scholar] [CrossRef]
- Whalen, P.J.; Shin, L.M.; McInerney, S.C.; Fischer, H.; Wright, C.I.; Rauch, S.L. A Functional MRI Study of Human Amygdala Responses to Facial Expressions of Fear versus Anger. Emotion 2001, 1, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Tennyson, C.N.; Klamut, H.J.; Worton, R.G. The Human Dystrophin Gene Requires 16 Hours to Be Transcribed and Is Cotranscriptionally Spliced. Nat. Genet. 1995, 9, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Mori, M.; Tonosaki, M.; Yaoi, T.; Nakano, K.; Okamura, T.; Itoh, K. Characterization of Dystrophin Dp71 Expression and Interaction Partners in Embryonic Brain Development: Implications for Duchenne/Becker Muscular Dystrophy. Mol. Neurobiol. 2025, 62, 6256–6272. [Google Scholar] [CrossRef]
- Daoud, F.; Candelario-Martínez, A.; Billard, J.M.; Avital, A.; Khelfaoui, M.; Rozenvald, Y.; Guegan, M.; Mornet, D.; Jaillard, D.; Nudel, U.; et al. Role of Mental Retardation-Associated Dystrophin-Gene Product Dp71 in Excitatory Synapse Organization, Synaptic Plasticity and Behavioral Functions. PLoS ONE 2009, 4, e6574. [Google Scholar] [CrossRef]
- González-Reyes, M.; Aragón, J.; Sánchez-Trujillo, A.; Rodríguez-Martínez, G.; Duarte, K.; Eleftheriou, E.; Barnier, J.V.; Naquin, D.; Thermes, C.; Romo-Yáñez, J.; et al. Expression of Dystrophin Dp71 Splice Variants Is Temporally Regulated During Rodent Brain Development. Mol. Neurobiol. 2024, 61, 10883–10900. [Google Scholar] [CrossRef]
- Aragón, J.; González-Reyes, M.; Romo-Yáñez, J.; Vacca, O.; Aguilar-González, G.; Rendón, A.; Vaillend, C.; Montañez, C. Dystrophin Dp71 Isoforms Are Differentially Expressed in the Mouse Brain and Retina: Report of New Alternative Splicing and a Novel Nomenclature for Dp71 Isoforms. Mol. Neurobiol. 2018, 55, 1376–1386. [Google Scholar] [CrossRef]
- Rusilowicz-Jones, E.V.; Urbé, S.; Clague, M.J. Protein Degradation on the Global Scale. Mol. Cell 2022, 82, 1414–1423. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The Blood–brain barrier: An Overview: Structure, Regulation, and Clinical Implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Namihira, M.; Nakashima, K. Mechanisms of Astrocytogenesis in the Mammalian Brain. Curr. Opin. Neurobiol. 2013, 23, 921–927. [Google Scholar] [CrossRef]
- Mancuso, M.R.; Kuhnert, F.; Kuo, C.J. Developmental Angiogenesis of the Central Nervous System. Lymphat. Res. Biol. 2008, 6, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Sillitoe, R.V.; Joyner, A.L. Morphology, Molecular Codes, and Circuitry Produce the Three-Dimensional Complexity of the Cerebellum. Annu. Rev. Cell Dev. Biol. 2007, 23, 549–577. [Google Scholar] [CrossRef] [PubMed]
- Ohtaka-Maruyama, C.; Okado, H. Molecular Pathways Underlying Projection Neuron Production and Migration during Cerebral Cortical Development. Front. Neurosci. 2015, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Leto, K.; Arancillo, M.; Becker, E.B.E.; Buffo, A.; Chiang, C.; Ding, B.; Dobyns, W.B.; Dusart, I.; Haldipur, P.; Hatten, M.E.; et al. Consensus Paper: Cerebellar Development. Cerebellum 2016, 15, 789–828. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Gaiarsa, J.L.; Tyzio, R.; Khazipov, R. GABA: A Pioneer Transmitter That Excites Immature Neurons and Generates Primitive Oscillations. Physiol. Rev. 2007, 87, 1215–1284. [Google Scholar] [CrossRef]
- Zeiss, C.J. Comparative Milestones in Rodent and Human Postnatal Central Nervous System Development. Toxicol. Pathol. 2021, 49, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- McKay, B.E.; Turner, R.W. Physiological and Morphological Development of the Rat Cerebellar Purkinje Cell. J. Physiol. 2005, 567, 829. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yamamoto, Y.; Tanaka-Yamamoto, K. Refinement of Cerebellar Network Organization by Extracellular Signaling During Development. Neuroscience 2021, 462, 44–55. [Google Scholar] [CrossRef] [PubMed]
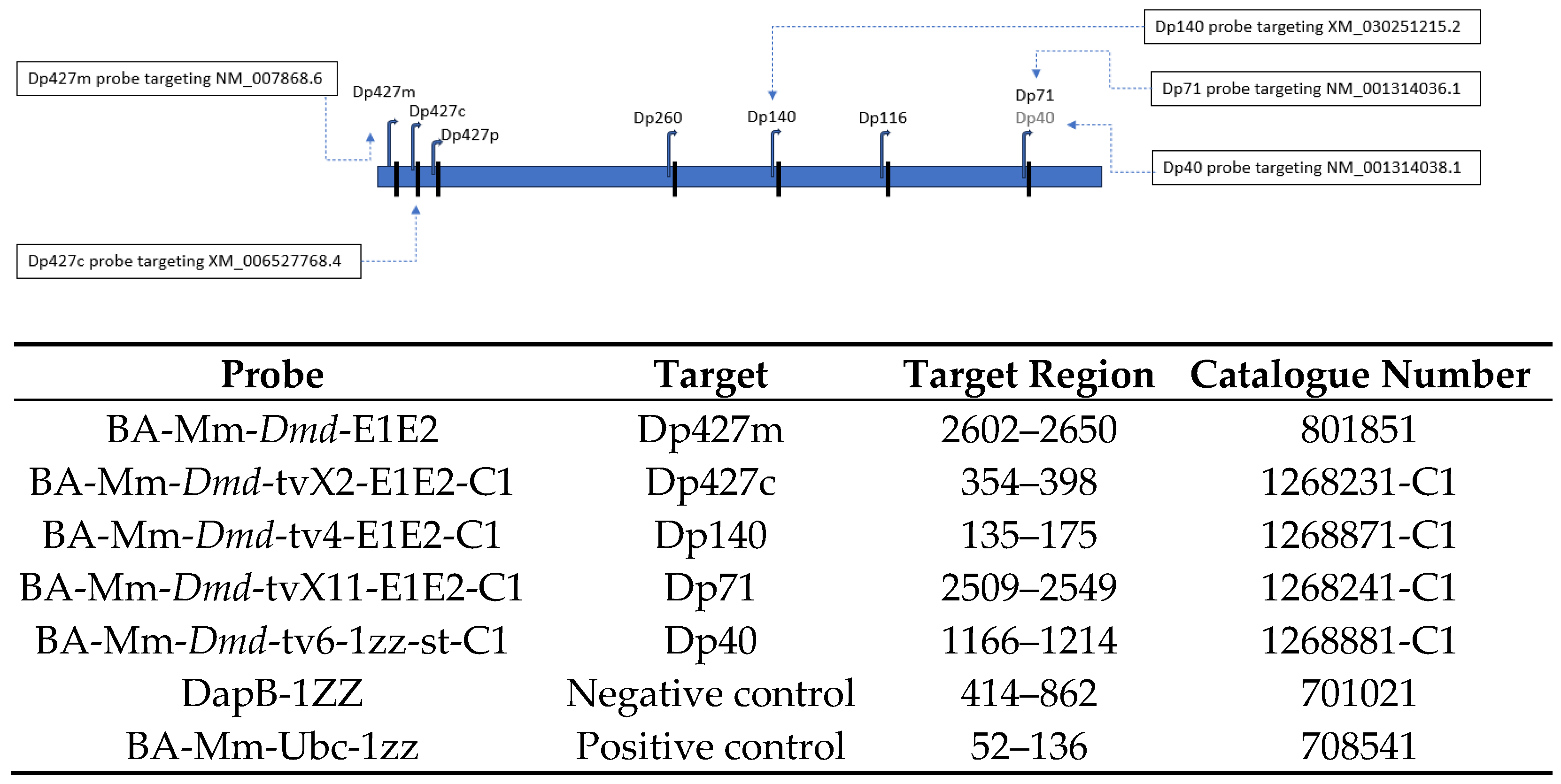

| Probe | Forward Primer 5′-3′ | Reverse Primer 3′-5′ | Probe | Location |
|---|---|---|---|---|
| Dp427m | GGACTGTTATGAAAGAGAAGATGTTCA | TGGCAGTTTTTGCCCTGTAAG | ACCTGCAGGATGGAAAACGCCTCC | Exon 1–2 |
| Dp427c | GGCATGGAAGATGAAAGAGAAGA | GGCAGTTTTTGCCCTGTAAGG | ACCTGCAGGATGGAAAACGCCTCC | Exon 1–2 |
| Dp427p1 | CTTTCATCAGAAGAAACCTCAGACAT | CAGCCAAATGCTTTCCTATGAAG | AAATTCTGCGGAGGCTG | Exon 1–2 |
| Dp427p2 | CAGGCTTCCCTAAAGATGAAAGAG | GGCAGTTTTTGCCCTGTAAGG | ACCTGCAGGATGGAAAACGCCTCC | Exon 1–2 |
| Dp140 | GCTGACTGTTCTGAGCTAAAATCG | GCCATCCTGGAGTTCCTTAATAAG | ACCAGAAGGGGGTTTTG | Exon 1–2 of Dp140 |
| Dp71 | CACTGCCTGTGAAACCCTTACA | TGGGTCTCGTGGCCTTTG | CCATGAGGGAACACC | Exon 1–2 of Dp71 |
| Dp40 | AACGTGAGTAGTGGCAGAAGCA | TTTTGGCTGGGAGGAGTTCA | AGCAAACTTGCATTTGATA | Exon 9 of Dp40 absent on Dp71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetorou, K.; Aghaeipour, A.; Ma, S.; Gileadi, T.; Saoudi, A.; Perdomo Quinteiro, P.; Aragón, J.; van Putten, M.; Spitali, P.; Montanez, C.; et al. Regional Expression of Dystrophin Gene Transcripts and Proteins in the Mouse Brain. Cells 2025, 14, 1441. https://doi.org/10.3390/cells14181441
Tetorou K, Aghaeipour A, Ma S, Gileadi T, Saoudi A, Perdomo Quinteiro P, Aragón J, van Putten M, Spitali P, Montanez C, et al. Regional Expression of Dystrophin Gene Transcripts and Proteins in the Mouse Brain. Cells. 2025; 14(18):1441. https://doi.org/10.3390/cells14181441
Chicago/Turabian StyleTetorou, Konstantina, Artadokht Aghaeipour, Shunyi Ma, Talia Gileadi, Amel Saoudi, Pablo Perdomo Quinteiro, Jorge Aragón, Maaike van Putten, Pietro Spitali, Cecilia Montanez, and et al. 2025. "Regional Expression of Dystrophin Gene Transcripts and Proteins in the Mouse Brain" Cells 14, no. 18: 1441. https://doi.org/10.3390/cells14181441
APA StyleTetorou, K., Aghaeipour, A., Ma, S., Gileadi, T., Saoudi, A., Perdomo Quinteiro, P., Aragón, J., van Putten, M., Spitali, P., Montanez, C., Vaillend, C., Morgan, J. E., Montanaro, F., & Muntoni, F. (2025). Regional Expression of Dystrophin Gene Transcripts and Proteins in the Mouse Brain. Cells, 14(18), 1441. https://doi.org/10.3390/cells14181441








