Cardiac Development, Cellular Composition and Function: From Regulatory Mechanisms to Applications
Abstract
1. Introduction
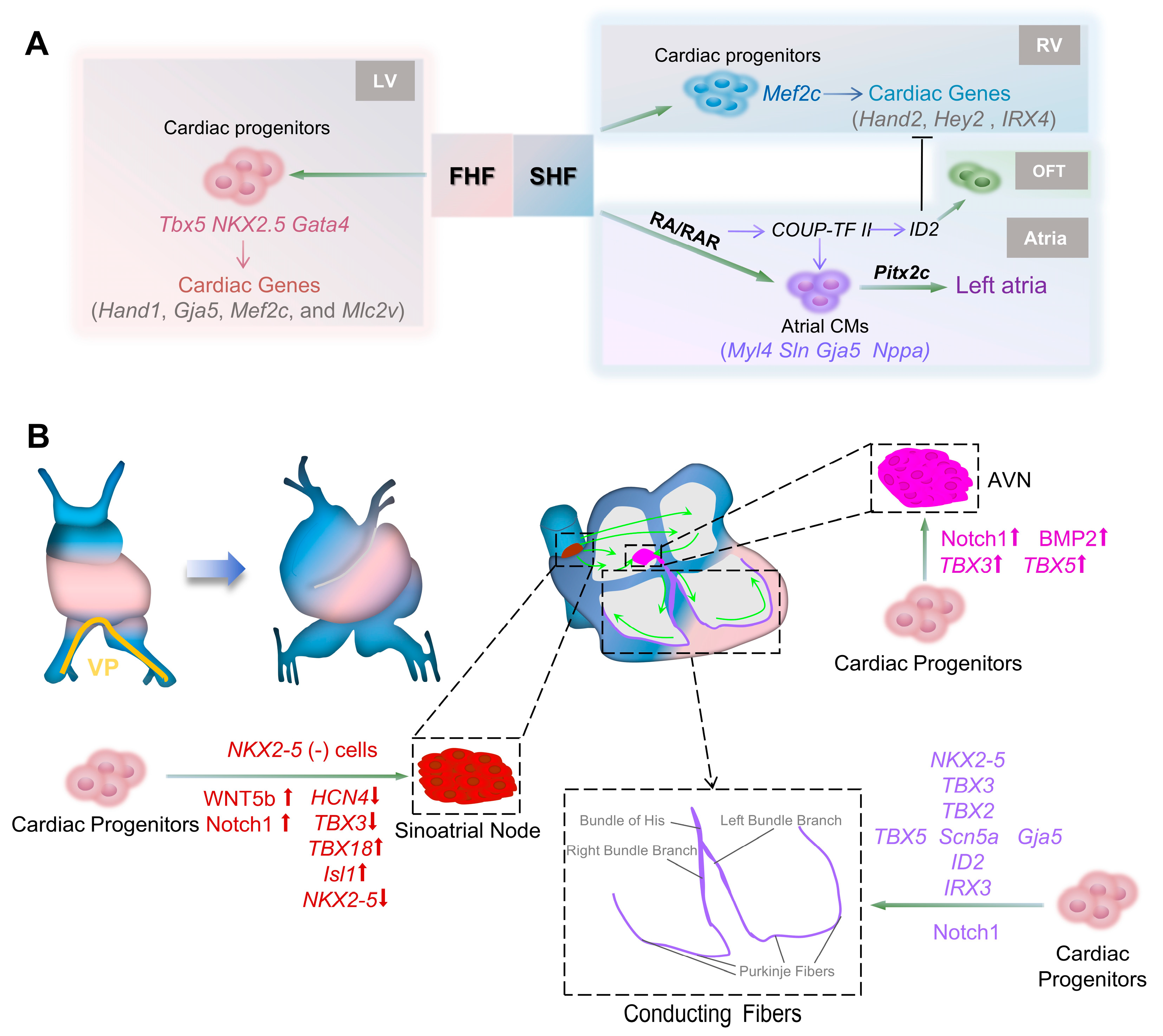
2. Heart Organogenesis
2.1. Primitive Heart Formation
2.2. Shape Establishment
2.3. Internal Compartmentalization
2.4. Conduction System Development
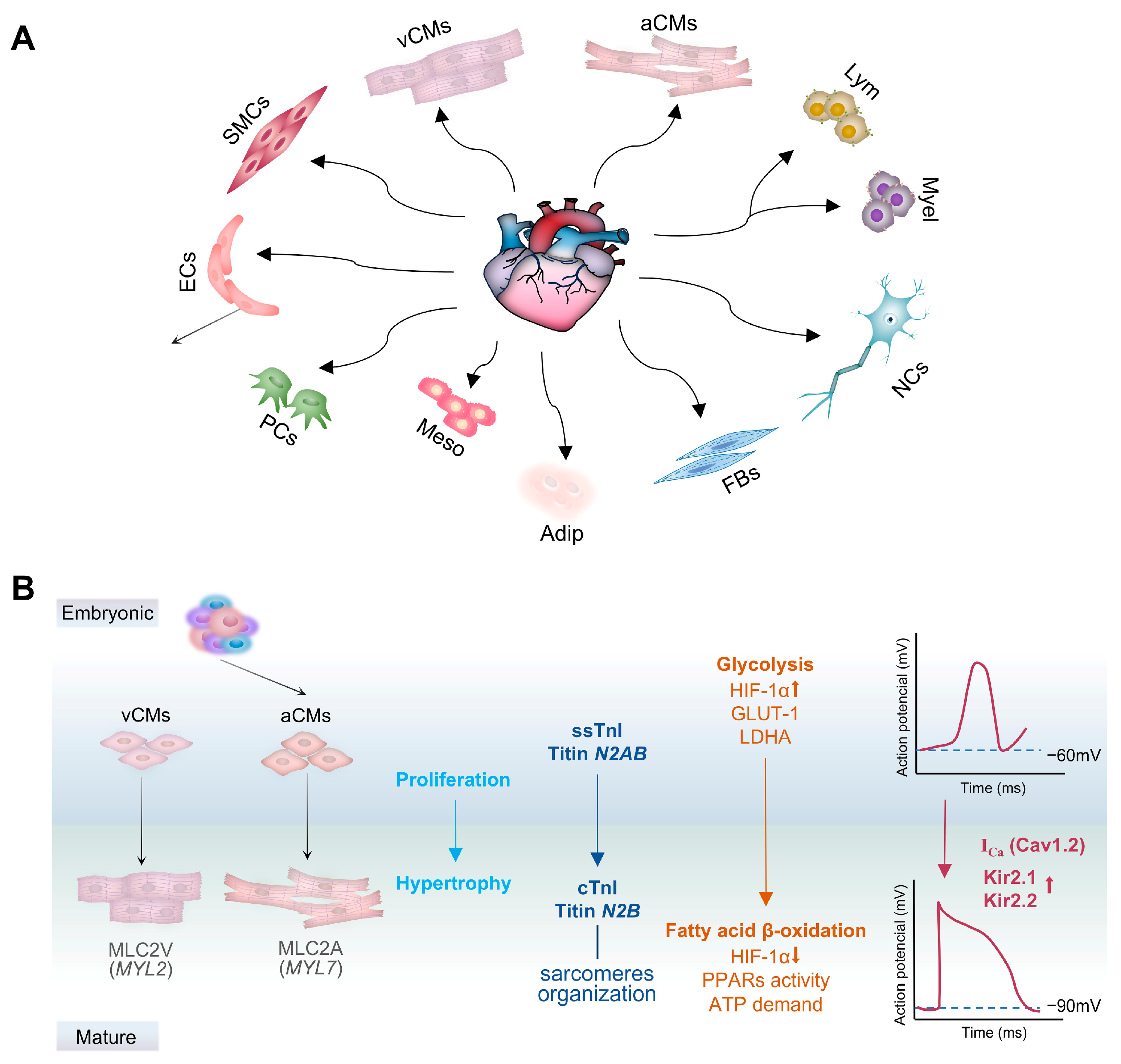
3. Cell Composition and Function
3.1. Cardiomyocytes
3.2. Fibroblasts
3.3. Endothelial Cells (ECs)
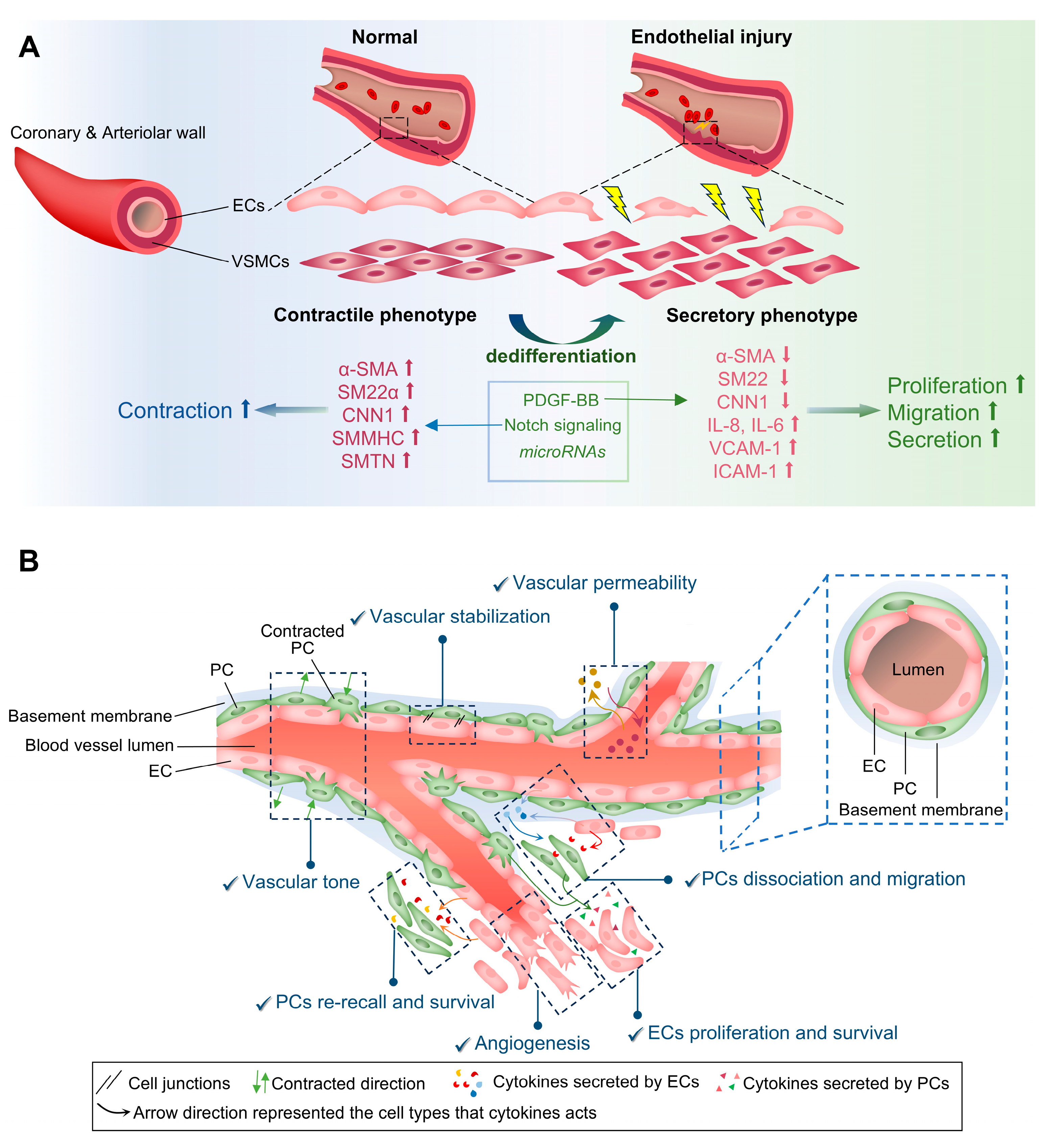
3.4. Smooth Muscle Cells (SMCs)
3.5. Pericytes
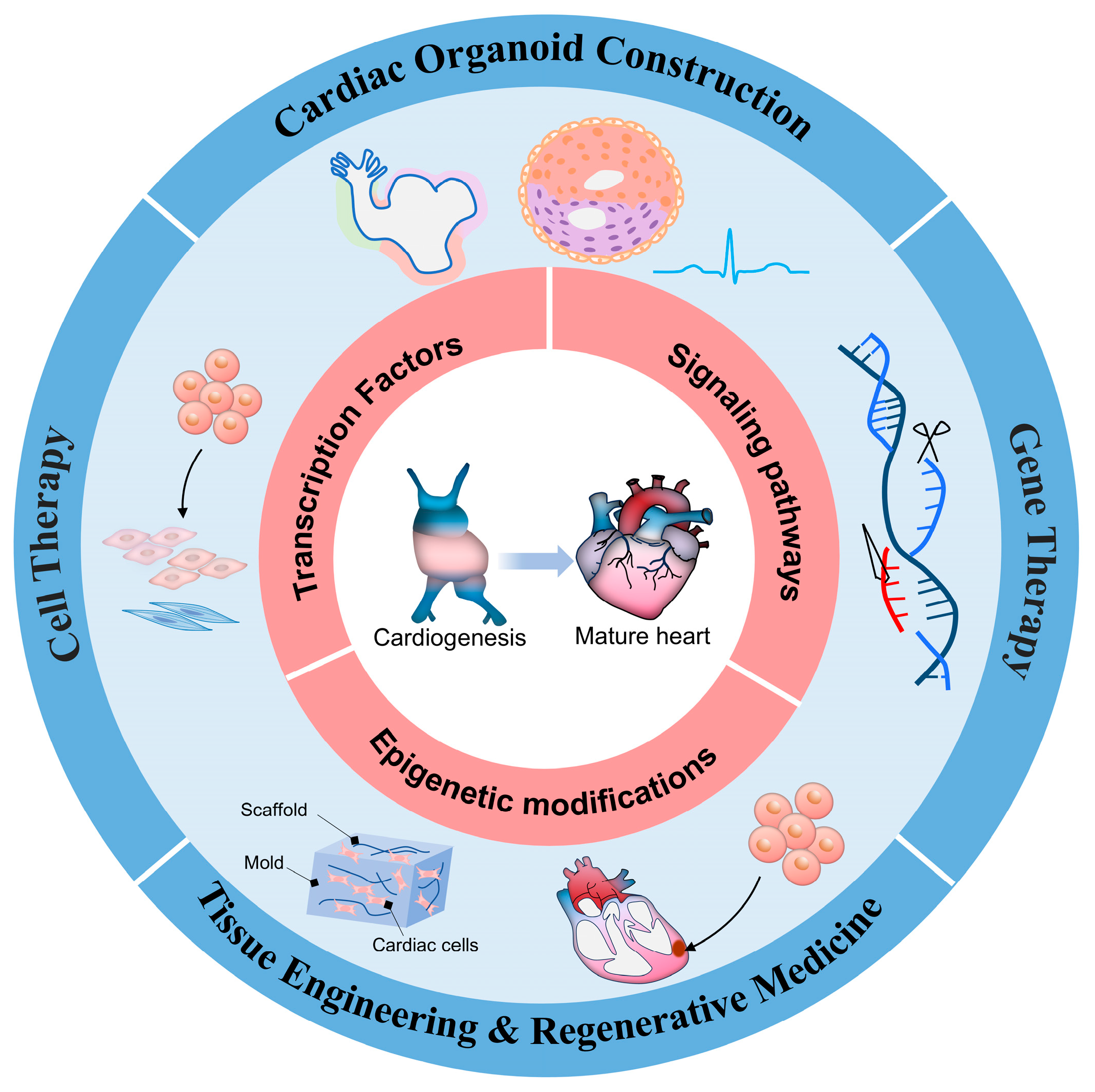
4. Breakthroughs in Treatment Strategies
4.1. Cell Therapy
4.2. Gene Therapy
4.3. Self-Organizing Cardiac Organoid Construction
4.4. Tissue Engineering and Regenerative Medicine
5. Conclusions and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buijtendijk, M.F.J.; Barnett, P.; van den Hoff, M.J.B. Development of the human heart. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 7–22. [Google Scholar] [CrossRef]
- Hikspoors, J.; Kruepunga, N.; Mommen, G.M.C.; Köhler, S.E.; Anderson, R.H.; Lamers, W.H. Human Cardiac Development. Adv. Exp. Med. Biol. 2024, 1441, 3–55. [Google Scholar] [CrossRef] [PubMed]
- Wilsdon, A.; Loughna, S. Human Genetics of Congenital Heart Defects. Adv. Exp. Med. Biol. 2024, 1441, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Karbassi, E.; Fenix, A.; Marchiano, S.; Muraoka, N.; Nakamura, K.; Yang, X.; Murry, C.E. Cardiomyocyte maturation: Advances in knowledge and implications for regenerative medicine. Nat. Rev. Cardiol. 2020, 17, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, J.T.; Hara, A.; Heckl, J.R.; Peña, B.; Bhutada, S.; DeMaris, R.; Ivey, M.J.; DeAngelo, L.P.; Liu, X.; Park, J.; et al. Regulation of extracellular matrix composition by fibroblasts during perinatal cardiac maturation. J. Mol. Cell. Cardiol. 2022, 169, 84–95. [Google Scholar] [CrossRef]
- Makkaoui, N.; Prasad, V.; Bagchi, P.; Carmona, T.; Li, K.; Latham, O.L.; Zhang, Y.; Lee, J.; Furdui, C.M.; Maxwell, J.T. Cell-based therapies reverse the heart failure-altered right ventricular proteome towards a pre-disease state. Stem. Cell Res. Ther. 2024, 15, 420. [Google Scholar] [CrossRef]
- Ali, S.A.; Mahmood, Z.; Mubarak, Z.; Asad, M.; Sarfraz Chaudhri, M.T.; Bilal, L.; Ashraf, T.; Khalifa, T.N.; Ashraf, T.; Saleem, F.; et al. Assessing the Potential Benefits of Stem Cell Therapy in Cardiac Regeneration for Patients with Ischemic Heart Disease. Cureus 2025, 17, e76770. [Google Scholar] [CrossRef]
- Ackerman, M.J.; Giudicessi, J.R. Top stories on gene therapy for genetic heart disease (2024). Heart Rhythm 2024, 21, 355–356. [Google Scholar] [CrossRef]
- Grisorio, L.; Bongianino, R.; Gianeselli, M.; Priori, S.G. Gene therapy for cardiac diseases: Methods, challenges, and future directions. Cardiovasc. Res. 2024, 120, 1664–1682. [Google Scholar] [CrossRef]
- Romeo, F.J.; Mavropoulos, S.A.; Ishikawa, K. Progress in Clinical Gene Therapy for Cardiac Disorders. Mol. Diagn. Ther. 2023, 27, 179–191. [Google Scholar] [CrossRef]
- Hofbauer, P.; Jahnel, S.M.; Papai, N.; Giesshammer, M.; Deyett, A.; Schmidt, C.; Penc, M.; Tavernini, K.; Grdseloff, N.; Meledeth, C.; et al. Cardioids reveal self-organizing principles of human cardiogenesis. Cell 2021, 184, 3299–3317.e3222. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Israeli, Y.R.; Wasserman, A.H.; Gabalski, M.A.; Volmert, B.D.; Ming, Y.; Ball, K.A.; Yang, W.; Zou, J.; Ni, G.; Pajares, N.; et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun. 2021, 12, 5142. [Google Scholar] [CrossRef] [PubMed]
- Kostina, A.; Kiselev, A.; Huang, A.; Lankerd, H.; Caywood, S.; Jurado-Fernandez, A.; Volmert, B.; O’Hern, C.; Juhong, A.; Liu, Y.; et al. Self-organizing human heart assembloids with autologous and developmentally relevant cardiac neural crest-derived tissues. bioRxiv 2024. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Han, M.; Long, M.; Li, T.; Hu, L.; Wang, L.; Huang, W.; Wu, Y. Engineering Cardiac Tissue for Advanced Heart-On-A-Chip Platforms. Adv. Healthc. Mater. 2024, 13, e2301338. [Google Scholar] [CrossRef]
- Edgar, L.; Pu, T.; Porter, B.; Aziz, J.M.; La Pointe, C.; Asthana, A.; Orlando, G. Regenerative medicine, organ bioengineering and transplantation. Br. J. Surg. 2020, 107, 793–800. [Google Scholar] [CrossRef]
- Shahbazi, M.N. Mechanisms of human embryo development: From cell fate to tissue shape and back. Development 2020, 147, dev190629. [Google Scholar] [CrossRef]
- Nakajima, Y. Retinoic acid signaling in heart development. Genesis 2019, 57, e23300. [Google Scholar] [CrossRef]
- Mendjan, S.; Mascetti, V.L.; Ortmann, D.; Ortiz, M.; Karjosukarso, D.W.; Ng, Y.; Moreau, T.; Pedersen, R.A. NANOG and CDX2 pattern distinct subtypes of human mesoderm during exit from pluripotency. Cell Stem Cell 2014, 15, 310–325. [Google Scholar] [CrossRef]
- Prummel, K.D.; Nieuwenhuize, S.; Mosimann, C. The lateral plate mesoderm. Development 2020, 147, dev175059. [Google Scholar] [CrossRef]
- Harvey, R.P. Patterning the vertebrate heart. Nat. Rev. Genet. 2002, 3, 544–556. [Google Scholar] [CrossRef]
- Yang, D.; Gomez-Garcia, J.; Funakoshi, S.; Tran, T.; Fernandes, I.; Bader, G.D.; Laflamme, M.A.; Keller, G.M. Modeling human multi-lineage heart field development with pluripotent stem cells. Cell Stem Cell 2022, 29, 1382–1401.e1388. [Google Scholar] [CrossRef]
- Buckingham, M.; Meilhac, S.; Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 2005, 6, 826–835. [Google Scholar] [CrossRef]
- Harris, I.S.; Black, B.L. Development of the endocardium. Pediatr. Cardiol. 2010, 31, 391–399. [Google Scholar] [CrossRef]
- Jain, R.; Li, D.; Gupta, M.; Manderfield, L.J.; Ifkovits, J.L.; Wang, Q.; Liu, F.; Liu, Y.; Poleshko, A.; Padmanabhan, A.; et al. HEART DEVELOPMENT. Integration of Bmp and Wnt signaling by Hopx specifies commitment of cardiomyoblasts. Science 2015, 348, aaa6071. [Google Scholar] [CrossRef]
- Sylva, M.; van den Hoff, M.J.; Moorman, A.F. Development of the human heart. Am. J. Med. Genet. A 2014, 164, 1347–1371. [Google Scholar] [CrossRef]
- Saba, R.; Kitajima, K.; Rainbow, L.; Engert, S.; Uemura, M.; Ishida, H.; Kokkinopoulos, I.; Shintani, Y.; Miyagawa, S.; Kanai, Y.; et al. Endocardium differentiation through Sox17 expression in endocardium precursor cells regulates heart development in mice. Sci. Rep. 2019, 9, 11953. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; Saga, Y.; Taketo, M.M.; Tzahor, E.; Birchmeier, W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18531–18536. [Google Scholar] [CrossRef] [PubMed]
- Briggs, L.E.; Phelps, A.L.; Brown, E.; Kakarla, J.; Anderson, R.H.; van den Hoff, M.J.; Wessels, A. Expression of the BMP receptor Alk3 in the second heart field is essential for development of the dorsal mesenchymal protrusion and atrioventricular septation. Circ. Res. 2013, 112, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- de Pater, E.; Ciampricotti, M.; Priller, F.; Veerkamp, J.; Strate, I.; Smith, K.; Lagendijk, A.K.; Schilling, T.F.; Herzog, W.; Abdelilah-Seyfried, S.; et al. Bmp signaling exerts opposite effects on cardiac differentiation. Circ. Res. 2012, 110, 578–587. [Google Scholar] [CrossRef]
- Tirosh-Finkel, L.; Zeisel, A.; Brodt-Ivenshitz, M.; Shamai, A.; Yao, Z.; Seger, R.; Domany, E.; Tzahor, E. BMP-mediated inhibition of FGF signaling promotes cardiomyocyte differentiation of anterior heart field progenitors. Development 2010, 137, 2989–3000. [Google Scholar] [CrossRef]
- Liu, N.; Kawahira, N.; Nakashima, Y.; Nakano, H.; Iwase, A.; Uchijima, Y.; Wang, M.; Wu, S.M.; Minamisawa, S.; Kurihara, H.; et al. Notch and retinoic acid signals regulate macrophage formation from endocardium downstream of Nkx2-5. Nat. Commun. 2023, 14, 5398. [Google Scholar] [CrossRef]
- Morlanes-Gracia, P.; Antoniutti, G.; Alvarez-Rubio, J.; Torres-Juan, L.; Heine-Suñer, D.; Ripoll-Vera, T. Case Report: A Novel NKX2-5 Mutation in a Family with Congenital Heart Defects, Left Ventricular Non-compaction, Conduction Disease, and Sudden Cardiac Death. Front. Cardiovasc. Med. 2021, 8, 691203. [Google Scholar] [CrossRef] [PubMed]
- Schott, J.J.; Benson, D.W.; Basson, C.T.; Pease, W.; Silberbach, G.M.; Moak, J.P.; Maron, B.J.; Seidman, C.E.; Seidman, J.G. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science 1998, 281, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Bloomekatz, J.; Ren, J.; Zhang, R.; Grinstein, J.D.; Zhao, L.; Burns, C.G.; Burns, C.E.; Anderson, R.M.; Chi, N.C. Coordinating cardiomyocyte interactions to direct ventricular chamber morphogenesis. Nature 2016, 534, 700–704. [Google Scholar] [CrossRef]
- Liang, J.; Jiang, P.; Yan, S.; Cheng, T.; Chen, S.; Xian, K.; Xu, P.; Xiong, J.W.; He, A.; Li, J.; et al. Genetically encoded tension heterogeneity sculpts cardiac trabeculation. Sci. Adv. 2025, 11, eads2998. [Google Scholar] [CrossRef] [PubMed]
- Priya, R.; Allanki, S.; Gentile, A.; Mansingh, S.; Uribe, V.; Maischein, H.M.; Stainier, D.Y.R. Tension heterogeneity directs form and fate to pattern the myocardial wall. Nature 2020, 588, 130–134. [Google Scholar] [CrossRef]
- Del Monte-Nieto, G.; Ramialison, M.; Adam, A.A.S.; Wu, B.; Aharonov, A.; D’Uva, G.; Bourke, L.M.; Pitulescu, M.E.; Chen, H.; de la Pompa, J.L.; et al. Control of cardiac jelly dynamics by NOTCH1 and NRG1 defines the building plan for trabeculation. Nature 2018, 557, 439–445. [Google Scholar] [CrossRef]
- Grego-Bessa, J.; Gómez-Apiñaniz, P.; Prados, B.; Gómez, M.J.; MacGrogan, D.; de la Pompa, J.L. Nrg1 Regulates Cardiomyocyte Migration and Cell Cycle in Ventricular Development. Circ. Res. 2023, 133, 927–943. [Google Scholar] [CrossRef]
- Guan, J.; Del Re, D.P. Cell type specificity of Hippo-YAP signaling in cardiac development and disease. J. Mol. Cell. Cardiol. 2025, 207, 51–63. [Google Scholar] [CrossRef]
- Del Re, D.P.; Yang, Y.; Nakano, N.; Cho, J.; Zhai, P.; Yamamoto, T.; Zhang, N.; Yabuta, N.; Nojima, H.; Pan, D.; et al. Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury. J. Biol. Chem. 2013, 288, 3977–3988. [Google Scholar] [CrossRef]
- Red-Horse, K.; Ueno, H.; Weissman, I.L.; Krasnow, M.A. Coronary arteries form by developmental reprogramming of venous cells. Nature 2010, 464, 549–553. [Google Scholar] [CrossRef]
- Katz, T.C.; Singh, M.K.; Degenhardt, K.; Rivera-Feliciano, J.; Johnson, R.L.; Epstein, J.A.; Tabin, C.J. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev. Cell 2012, 22, 639–650. [Google Scholar] [CrossRef]
- von Gise, A.; Zhou, B.; Honor, L.B.; Ma, Q.; Petryk, A.; Pu, W.T. WT1 regulates epicardial epithelial to mesenchymal transition through β-catenin and retinoic acid signaling pathways. Dev. Biol. 2011, 356, 421–431. [Google Scholar] [CrossRef]
- Guadix, J.A.; Ruiz-Villalba, A.; Lettice, L.; Velecela, V.; Muñoz-Chápuli, R.; Hastie, N.D.; Pérez-Pomares, J.M.; Martínez-Estrada, O.M. Wt1 controls retinoic acid signalling in embryonic epicardium through transcriptional activation of Raldh2. Development 2011, 138, 1093–1097. [Google Scholar] [CrossRef]
- Cano, E.; Carmona, R.; Ruiz-Villalba, A.; Rojas, A.; Chau, Y.Y.; Wagner, K.D.; Wagner, N.; Hastie, N.D.; Muñoz-Chápuli, R.; Pérez-Pomares, J.M. Extracardiac septum transversum/proepicardial endothelial cells pattern embryonic coronary arterio-venous connections. Proc. Natl. Acad. Sci. USA 2016, 113, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Ramiro-Pareta, M.; Müller-Sánchez, C.; Portella-Fortuny, R.; Soler-Botija, C.; Torres-Cano, A.; Esteve-Codina, A.; Bayés-Genís, A.; Reina, M.; Soriano, F.X.; Montanez, E.; et al. Endothelial deletion of Wt1 disrupts coronary angiogenesis and myocardium development. Development 2023, 150, dev201147. [Google Scholar] [CrossRef] [PubMed]
- Théveniau-Ruissy, M.; Pérez-Pomares, J.M.; Parisot, P.; Baldini, A.; Miquerol, L.; Kelly, R.G. Coronary stem development in wild-type and Tbx1 null mouse hearts. Dev. Dyn. 2016, 245, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Martyniak, A.; Jeż, M.; Dulak, J.; Stępniewski, J. Adaptation of cardiomyogenesis to the generation and maturation of cardiomyocytes from human pluripotent stem cells. IUBMB Life 2023, 75, 8–29. [Google Scholar] [CrossRef]
- Bruneau, B.G. Signaling and transcriptional networks in heart development and regeneration. Cold Spring Harb. Perspect. Biol. 2013, 5, a008292. [Google Scholar] [CrossRef]
- Miquerol, L.; Kelly, R.G. Organogenesis of the vertebrate heart. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, S.; Zaffran, S. Mechanisms of retinoic acid signaling during cardiogenesis. Mech. Dev. 2017, 143, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Devalla, H.D.; Schwach, V.; Ford, J.W.; Milnes, J.T.; El-Haou, S.; Jackson, C.; Gkatzis, K.; Elliott, D.A.; Chuva de Sousa Lopes, S.M.; Mummery, C.L.; et al. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol. Med. 2015, 7, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.P.; Cheng, C.M.; Lanz, R.B.; Wang, T.; Respress, J.L.; Ather, S.; Chen, W.; Tsai, S.J.; Wehrens, X.H.; Tsai, M.J.; et al. Atrial identity is determined by a COUP-TFII regulatory network. Dev. Cell 2013, 25, 417–426. [Google Scholar] [CrossRef]
- Mommersteeg, M.T.; Hoogaars, W.M.; Prall, O.W.; de Gier-de Vries, C.; Wiese, C.; Clout, D.E.; Papaioannou, V.E.; Brown, N.A.; Harvey, R.P.; Moorman, A.F.; et al. Molecular pathway for the localized formation of the sinoatrial node. Circ. Res. 2007, 100, 354–362. [Google Scholar] [CrossRef]
- Markwald, R.R.; Fitzharris, T.P.; Manasek, F.J. Structural development of endocardial cushions. Am. J. Anat. 1977, 148, 85–119. [Google Scholar] [CrossRef]
- de Lange, F.J.; Moorman, A.F.; Anderson, R.H.; Männer, J.; Soufan, A.T.; de Gier-de Vries, C.; Schneider, M.D.; Webb, S.; van den Hoff, M.J.; Christoffels, V.M. Lineage and morphogenetic analysis of the cardiac valves. Circ. Res. 2004, 95, 645–654. [Google Scholar] [CrossRef]
- Wu, B.; Wu, B.; Benkaci, S.; Shi, L.; Lu, P.; Park, T.; Morrow, B.E.; Wang, Y.; Zhou, B. Crk and Crkl Are Required in the Endocardial Lineage for Heart Valve Development. J. Am. Heart Assoc. 2023, 12, e029683. [Google Scholar] [CrossRef]
- Dewing, J.M.; Saunders, V.; O’Kelly, I.; Wilson, D.I. Defining cardiac cell populations and relative cellular composition of the early fetal human heart. PLoS ONE 2022, 17, e0259477. [Google Scholar] [CrossRef]
- Sizarov, A.; Ya, J.; de Boer, B.A.; Lamers, W.H.; Christoffels, V.M.; Moorman, A.F. Formation of the building plan of the human heart: Morphogenesis, growth, and differentiation. Circulation 2011, 123, 1125–1135. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Munshi, N.V. Development of the Cardiac Conduction System. Cold Spring Harb. Perspect. Biol. 2020, 12, a037408. [Google Scholar] [CrossRef]
- Oh, Y.; Abid, R.; Dababneh, S.; Bakr, M.; Aslani, T.; Cook, D.P.; Vanderhyden, B.C.; Park, J.G.; Munshi, N.V.; Hui, C.C.; et al. Transcriptional regulation of the postnatal cardiac conduction system heterogeneity. Nat. Commun. 2024, 15, 6550. [Google Scholar] [CrossRef] [PubMed]
- Wiese, C.; Grieskamp, T.; Airik, R.; Mommersteeg, M.T.; Gardiwal, A.; de Gier-de Vries, C.; Schuster-Gossler, K.; Moorman, A.F.; Kispert, A.; Christoffels, V.M. Formation of the sinus node head and differentiation of sinus node myocardium are independently regulated by Tbx18 and Tbx3. Circ. Res. 2009, 104, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.H.; Kao, H.K.J.; Thai, P.N.; Smithers, R.; Chang, C.W.; Pretto, D.; Yechikov, S.; Oppenheimer, S.; Bedolla, A.; Chalker, B.A.; et al. The sinoatrial node extracellular matrix promotes pacemaker phenotype and protects automaticity in engineered heart tissues from cyclic strain. Cell Rep. 2023, 42, 113505. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Fang, Y.; Xiong, Q.; Luo, L.; Zhou, R.; Liao, B. Role of over-expression of TBX3 and TBX18 in the enrichment and differentiation of human induced pluripotent stem cells into sinoatrial node-like cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2019, 33, 497–506. [Google Scholar] [CrossRef]
- Ren, J.; Han, P.; Ma, X.; Farah, E.N.; Bloomekatz, J.; Zeng, X.I.; Zhang, R.; Swim, M.M.; Witty, A.D.; Knight, H.G.; et al. Canonical Wnt5b Signaling Directs Outlying Nkx2.5+ Mesoderm into Pacemaker Cardiomyocytes. Dev. Cell 2019, 50, 729–743.e725. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, P.; Jiang, L.; Wu, B.; Zhou, B. Control of sinus venous valve and sinoatrial node development by endocardial NOTCH1. Cardiovasc. Res. 2020, 116, 1473–1486. [Google Scholar] [CrossRef]
- Vicente-Steijn, R.; Kelder, T.P.; Tertoolen, L.G.; Wisse, L.J.; Pijnappels, D.A.; Poelmann, R.E.; Schalij, M.J.; deRuiter, M.C.; Gittenberger-de Groot, A.C.; Jongbloed, M.R.M. RHOA-ROCK signalling is necessary for lateralization and differentiation of the developing sinoatrial node. Cardiovasc. Res. 2017, 113, 1186–1197. [Google Scholar] [CrossRef]
- Hikspoors, J.; Macías, Y.; Tretter, J.T.; Anderson, R.H.; Lamers, W.H.; Mohun, T.J.; Sánchez-Quintana, D.; Farré, J.; Back Sternick, E. Miniseries 1-Part I: The Development of the atrioventricular conduction axis. Europace 2022, 24, 432–442. [Google Scholar] [CrossRef]
- Doménech-Mateu, J.M.; Arnó-Palau, A.; Martínez-Pozo, A. Study of the development of the atrioventricular conduction system as a consequence of observing an extra atrioventricular node in the normal heart of a human fetus. Anat. Rec. 1991, 230, 73–85. [Google Scholar] [CrossRef]
- Burnicka-Turek, O.; Broman, M.T.; Steimle, J.D.; Boukens, B.J.; Petrenko, N.B.; Ikegami, K.; Nadadur, R.D.; Qiao, Y.; Arnolds, D.E.; Yang, X.H.; et al. Transcriptional Patterning of the Ventricular Cardiac Conduction System. Circ. Res. 2020, 127, e94–e106. [Google Scholar] [CrossRef]
- Ma, L.; Lu, M.F.; Schwartz, R.J.; Martin, J.F. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 2005, 132, 5601–5611. [Google Scholar] [CrossRef]
- Singh, R.; Hoogaars, W.M.; Barnett, P.; Grieskamp, T.; Rana, M.S.; Buermans, H.; Farin, H.F.; Petry, M.; Heallen, T.; Martin, J.F.; et al. Tbx2 and Tbx3 induce atrioventricular myocardial development and endocardial cushion formation. Cell. Mol. Life Sci. 2012, 69, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.A.; Bosada, F.M.; van Weerd, J.H.; van Duijvenboden, K.; Wang, J.; Mommersteeg, M.T.M.; Hooijkaas, I.B.; Wakker, V.; de Gier-de Vries, C.; Coronel, R.; et al. T-box transcription factor 3 governs a transcriptional program for the function of the mouse atrioventricular conduction system. Proc. Natl. Acad. Sci. USA 2020, 117, 18617–18626. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, S.; Harris, B.S.; Kuznekoff, L.; Jain, R.; Manderfield, L.; Lu, M.M.; Morley, G.E.; Patel, V.V.; Epstein, J.A. Notch signaling regulates murine atrioventricular conduction and the formation of accessory pathways. J. Clin. Investig. 2011, 121, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Arnolds, D.E.; Liu, F.; Fahrenbach, J.P.; Kim, G.H.; Schillinger, K.J.; Smemo, S.; McNally, E.M.; Nobrega, M.A.; Patel, V.V.; Moskowitz, I.P. TBX5 drives Scn5a expression to regulate cardiac conduction system function. J. Clin. Investig. 2012, 122, 2509–2518. [Google Scholar] [CrossRef]
- Lee, C.; Xu, S.; Samad, T.; Goodyer, W.R.; Raissadati, A.; Heinrich, P.; Wu, S.M. The cardiac conduction system: History, development, and disease. Curr. Top. Dev. Biol. 2024, 156, 157–200. [Google Scholar] [CrossRef]
- Moskowitz, I.P.; Kim, J.B.; Moore, M.L.; Wolf, C.M.; Peterson, M.A.; Shendure, J.; Nobrega, M.A.; Yokota, Y.; Berul, C.; Izumo, S.; et al. A molecular pathway including Id2, Tbx5, and Nkx2-5 required for cardiac conduction system development. Cell 2007, 129, 1365–1376. [Google Scholar] [CrossRef]
- Kim, K.H.; Rosen, A.; Hussein, S.M.; Puviindran, V.; Korogyi, A.S.; Chiarello, C.; Nagy, A.; Hui, C.C.; Backx, P.H. Irx3 is required for postnatal maturation of the mouse ventricular conduction system. Sci. Rep. 2016, 6, 19197. [Google Scholar] [CrossRef]
- Rentschler, S.; Yen, A.H.; Lu, J.; Petrenko, N.B.; Lu, M.M.; Manderfield, L.J.; Patel, V.V.; Fishman, G.I.; Epstein, J.A. Myocardial Notch signaling reprograms cardiomyocytes to a conduction-like phenotype. Circulation 2012, 126, 1058–1066. [Google Scholar] [CrossRef]
- Ribeiro da Silva, A.; Neri, E.A.; Turaça, L.T.; Dariolli, R.; Fonseca-Alaniz, M.H.; Santos-Miranda, A.; Roman-Campos, D.; Venturini, G.; Krieger, J.E. NOTCH1 is critical for fibroblast-mediated induction of cardiomyocyte specialization into ventricular conduction system-like cells in vitro. Sci. Rep. 2020, 10, 16163. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G.; Luxán, G.; de la Pompa, J.L. Notch signalling in ventricular chamber development and cardiomyopathy. Febs. J. 2016, 283, 4223–4237. [Google Scholar] [CrossRef] [PubMed]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.P.; Zhou, Y.; Nakada, Y.; Zhang, J. Changes in Cardiomyocyte Cell Cycle and Hypertrophic Growth During Fetal to Adult in Mammals. J. Am. Heart Assoc. 2021, 10, e017839. [Google Scholar] [CrossRef]
- Zhang, Y.; Del Re, D.P. A growing role for the Hippo signaling pathway in the heart. J. Mol. Med. 2017, 95, 465–472. [Google Scholar] [CrossRef]
- Yang, Y.; Del Re, D.P.; Nakano, N.; Sciarretta, S.; Zhai, P.; Park, J.; Sayed, D.; Shirakabe, A.; Matsushima, S.; Park, Y.; et al. miR-206 Mediates YAP-Induced Cardiac Hypertrophy and Survival. Circ. Res. 2015, 117, 891–904. [Google Scholar] [CrossRef]
- Dainis, A.; Zaleta-Rivera, K.; Ribeiro, A.; Chang, A.C.H.; Shang, C.; Lan, F.; Burridge, P.W.; Liu, W.R.; Wu, J.C.; Chang, A.C.Y.; et al. Silencing of MYH7 ameliorates disease phenotypes in human iPSC-cardiomyocytes. Physiol. Genom. 2020, 52, 293–303. [Google Scholar] [CrossRef]
- Hinson, J.T.; Chopra, A.; Nafissi, N.; Polacheck, W.J.; Benson, C.C.; Swist, S.; Gorham, J.; Yang, L.; Schafer, S.; Sheng, C.C.; et al. HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science 2015, 349, 982–986. [Google Scholar] [CrossRef]
- Maroli, G.; Braun, T. The long and winding road of cardiomyocyte maturation. Cardiovasc. Res. 2021, 117, 712–726. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Jaswal, J.S. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J. Cardiovasc. Pharmacol. 2010, 56, 130–140. [Google Scholar] [CrossRef]
- Menendez-Montes, I.; Escobar, B.; Palacios, B.; Gómez, M.J.; Izquierdo-Garcia, J.L.; Flores, L.; Jiménez-Borreguero, L.J.; Aragones, J.; Ruiz-Cabello, J.; Torres, M.; et al. Myocardial VHL-HIF Signaling Controls an Embryonic Metabolic Switch Essential for Cardiac Maturation. Dev. Cell 2016, 39, 724–739. [Google Scholar] [CrossRef]
- Cerychova, R.; Pavlinkova, G. HIF-1, Metabolism, and Diabetes in the Embryonic and Adult Heart. Front. Endocrinol. 2018, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Dhillon-Richardson, R.M.; Haugan, A.K.; Lyons, L.W.; McKenna, J.K.; Bronner, M.E.; Martik, M.L. Reactivation of an embryonic cardiac neural crest transcriptional profile during zebrafish heart regeneration. Proc. Natl. Acad. Sci. USA 2025, 122, e2423697122. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.O. Cardiac ion channels. Circ. Arrhythm. Electrophysiol. 2009, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Han, J.L.; Entcheva, E. Syncytium cell growth increases Kir2.1 contribution in human iPSC-cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1112–H1122. [Google Scholar] [CrossRef]
- Vermij, S.H.; Abriel, H.; van Veen, T.A. Refining the molecular organization of the cardiac intercalated disc. Cardiovasc. Res. 2017, 113, 259–275. [Google Scholar] [CrossRef]
- Tallquist, M.D. Cardiac Fibroblast Diversity. Annu. Rev. Physiol. 2020, 82, 63–78. [Google Scholar] [CrossRef]
- Johansen, A.K.Z.; Kasam, R.K.; Vagnozzi, R.J.; Lin, S.J.; Gomez-Arroyo, J.; Shittu, A.; Bowers, S.L.K.; Kuwabara, Y.; Grimes, K.M.; Warrick, K.; et al. Transcription Factor 21 Regulates Cardiac Myofibroblast Formation and Fibrosis. Circ. Res. 2025, 136, 44–58. [Google Scholar] [CrossRef]
- Cai, C.L.; Martin, J.C.; Sun, Y.; Cui, L.; Wang, L.; Ouyang, K.; Yang, L.; Bu, L.; Liang, X.; Zhang, X.; et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature 2008, 454, 104–108. [Google Scholar] [CrossRef]
- Braitsch, C.M.; Kanisicak, O.; van Berlo, J.H.; Molkentin, J.D.; Yutzey, K.E. Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease. J. Mol. Cell. Cardiol. 2013, 65, 108–119. [Google Scholar] [CrossRef]
- Ivey, M.J.; Kuwabara, J.T.; Riggsbee, K.L.; Tallquist, M.D. Platelet-derived growth factor receptor-α is essential for cardiac fibroblast survival. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H330–H344. [Google Scholar] [CrossRef]
- Camelliti, P.; Borg, T.K.; Kohl, P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 2005, 65, 40–51. [Google Scholar] [CrossRef]
- Lodi, R.S.; Xia, L.; Zhang, Y.; Liu, F. Evolving roles of cardiac fibroblasts in cardiogenesis and immunology, electrophysiology, and aging. Rev. Cardiovasc. Med. 2021, 22, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Kamkin, A.; Kiseleva, I.; Isenberg, G.; Wagner, K.D.; Günther, J.; Theres, H.; Scholz, H. Cardiac fibroblasts and the mechano-electric feedback mechanism in healthy and diseased hearts. Prog. Biophys. Mol. Biol. 2003, 82, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Pesce, M.; Duda, G.N.; Forte, G.; Girao, H.; Raya, A.; Roca-Cusachs, P.; Sluijter, J.P.G.; Tschöpe, C.; Van Linthout, S. Cardiac fibroblasts and mechanosensation in heart development, health and disease. Nat. Rev. Cardiol. 2023, 20, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.A.; Camelliti, P.; Rog-Zielinska, E.A.; Siedlecka, U.; Poggioli, T.; O’Toole, E.T.; Knöpfel, T.; Kohl, P. Electrotonic coupling of excitable and nonexcitable cells in the heart revealed by optogenetics. Proc. Natl. Acad. Sci. USA 2016, 113, 14852–14857. [Google Scholar] [CrossRef]
- Porter, K.E.; Turner, N.A. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol. Ther. 2009, 123, 255–278. [Google Scholar] [CrossRef]
- Han, M.; Zhou, B. Role of Cardiac Fibroblasts in Cardiac Injury and Repair. Curr. Cardiol. Rep. 2022, 24, 295–304. [Google Scholar] [CrossRef]
- Venugopal, H.; Hanna, A.; Humeres, C.; Frangogiannis, N.G. Properties and Functions of Fibroblasts and Myofibroblasts in Myocardial Infarction. Cells 2022, 11, 1386. [Google Scholar] [CrossRef]
- Shinde, A.V.; Frangogiannis, N.G. Fibroblasts in myocardial infarction: A role in inflammation and repair. J. Mol. Cell. Cardiol. 2014, 70, 74–82. [Google Scholar] [CrossRef]
- Torimoto, K.; Elliott, K.; Nakayama, Y.; Yanagisawa, H.; Eguchi, S. Cardiac and perivascular myofibroblasts, matrifibrocytes, and immune fibrocytes in hypertension; commonalities and differences with other cardiovascular diseases. Cardiovasc. Res. 2024, 120, 567–580. [Google Scholar] [CrossRef]
- Weber, K.T.; Sun, Y.; Bhattacharya, S.K.; Ahokas, R.A.; Gerling, I.C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 2013, 10, 15–26. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Flinn, M.A.; Alvarez-Argote, S.; Knas, M.C.; Almeida, V.A.; Paddock, S.J.; Zhou, X.; Buddell, T.; Jamal, A.; Taylor, R.; Liu, P.; et al. Myofibroblast Ccn3 is regulated by Yap and Wwtr1 and contributes to adverse cardiac outcomes. Front. Cardiovasc. Med. 2023, 10, 1142612. [Google Scholar] [CrossRef] [PubMed]
- Francisco, J.; Zhang, Y.; Nakada, Y.; Jeong, J.I.; Huang, C.Y.; Ivessa, A.; Oka, S.; Babu, G.J.; Del Re, D.P. AAV-mediated YAP expression in cardiac fibroblasts promotes inflammation and increases fibrosis. Sci. Rep. 2021, 11, 10553. [Google Scholar] [CrossRef] [PubMed]
- Patrick, R.; Janbandhu, V.; Tallapragada, V.; Tan, S.S.M.; McKinna, E.E.; Contreras, O.; Ghazanfar, S.; Humphreys, D.T.; Murray, N.J.; Tran, Y.T.H.; et al. Integration mapping of cardiac fibroblast single-cell transcriptomes elucidates cellular principles of fibrosis in diverse pathologies. Sci. Adv. 2024, 10, eadk8501. [Google Scholar] [CrossRef]
- Mikryukov, A.A.; Mazine, A.; Wei, B.; Yang, D.; Miao, Y.; Gu, M.; Keller, G.M. BMP10 Signaling Promotes the Development of Endocardial Cells from Human Pluripotent Stem Cell-Derived Cardiovascular Progenitors. Cell Stem Cell 2021, 28, 96–111.e117. [Google Scholar] [CrossRef]
- Combs, M.D.; Yutzey, K.E. Heart valve development: Regulatory networks in development and disease. Circ. Res. 2009, 105, 408–421. [Google Scholar] [CrossRef]
- Person, A.D.; Klewer, S.E.; Runyan, R.B. Cell biology of cardiac cushion development. Int. Rev. Cytol. 2005, 243, 287–335. [Google Scholar] [CrossRef]
- Tian, X.; Hu, T.; Zhang, H.; He, L.; Huang, X.; Liu, Q.; Yu, W.; He, L.; Yang, Z.; Yan, Y.; et al. Vessel formation. De novo formation of a distinct coronary vascular population in neonatal heart. Science 2014, 345, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Franssen, C.; Chen, S.; Unger, A.; Korkmaz, H.I.; De Keulenaer, G.W.; Tschöpe, C.; Leite-Moreira, A.F.; Musters, R.; Niessen, H.W.; Linke, W.A.; et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2016, 4, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Adiarto, S.; Heiden, S.; Vignon-Zellweger, N.; Nakayama, K.; Yagi, K.; Yanagisawa, M.; Emoto, N. ET-1 from endothelial cells is required for complete angiotensin II-induced cardiac fibrosis and hypertrophy. Life Sci. 2012, 91, 651–657. [Google Scholar] [CrossRef] [PubMed]
- De Keulenaer, G.W.; Feyen, E.; Dugaucquier, L.; Shakeri, H.; Shchendrygina, A.; Belenkov, Y.N.; Brink, M.; Vermeulen, Z.; Segers, V.F.M. Mechanisms of the Multitasking Endothelial Protein NRG-1 as a Compensatory Factor During Chronic Heart Failure. Circ. Heart Fail. 2019, 12, e006288. [Google Scholar] [CrossRef]
- Sniegon, I.; Prieß, M.; Heger, J.; Schulz, R.; Euler, G. Endothelial Mesenchymal Transition in Hypoxic Microvascular Endothelial Cells and Paracrine Induction of Cardiomyocyte Apoptosis Are Mediated via TGFβ1/SMAD Signaling. Int. J. Mol. Sci. 2017, 18, 2290. [Google Scholar] [CrossRef]
- Gladka, M.M.; Kohela, A.; Molenaar, B.; Versteeg, D.; Kooijman, L.; Monshouwer-Kloots, J.; Kremer, V.; Vos, H.R.; Huibers, M.M.H.; Haigh, J.J.; et al. Cardiomyocytes stimulate angiogenesis after ischemic injury in a ZEB2-dependent manner. Nat. Commun. 2021, 12, 84. [Google Scholar] [CrossRef]
- Liu, J.; Zhuang, T.; Pi, J.; Chen, X.; Zhang, Q.; Li, Y.; Wang, H.; Shen, Y.; Tomlinson, B.; Chan, P.; et al. Endothelial Forkhead Box Transcription Factor P1 Regulates Pathological Cardiac Remodeling Through Transforming Growth Factor-β1-Endothelin-1 Signal Pathway. Circulation 2019, 140, 665–680. [Google Scholar] [CrossRef]
- Sandner, P.; Stasch, J.P. Anti-fibrotic effects of soluble guanylate cyclase stimulators and activators: A review of the preclinical evidence. Respir. Med. 2017, 122 (Suppl. S1), S1–S9. [Google Scholar] [CrossRef]
- Liu, M.; Yan, M.; Lv, H.; Wang, B.; Lv, X.; Zhang, H.; Xiang, S.; Du, J.; Liu, T.; Tian, Y.; et al. Macrophage K63-Linked Ubiquitination of YAP Promotes Its Nuclear Localization and Exacerbates Atherosclerosis. Cell Rep. 2020, 32, 107990. [Google Scholar] [CrossRef]
- Lv, Y.; Kim, K.; Sheng, Y.; Cho, J.; Qian, Z.; Zhao, Y.Y.; Hu, G.; Pan, D.; Malik, A.B.; Hu, G. YAP Controls Endothelial Activation and Vascular Inflammation Through TRAF6. Circ. Res. 2018, 123, 43–56. [Google Scholar] [CrossRef]
- Duncker, D.J.; Bache, R.J. Regulation of coronary blood flow during exercise. Physiol. Rev. 2008, 88, 1009–1086. [Google Scholar] [CrossRef] [PubMed]
- Lother, A.; Kohl, P. The heterocellular heart: Identities, interactions, and implications for cardiology. Basic Res. Cardiol. 2023, 118, 30. [Google Scholar] [CrossRef] [PubMed]
- Farina, F.M.; Hall, I.F.; Serio, S.; Zani, S.; Climent, M.; Salvarani, N.; Carullo, P.; Civilini, E.; Condorelli, G.; Elia, L.; et al. miR-128-3p Is a Novel Regulator of Vascular Smooth Muscle Cell Phenotypic Switch and Vascular Diseases. Circ. Res. 2020, 126, e120–e135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, B.; Li, Y.; Li, Y.; Du, J.; Deng, S.; Jing, X.; She, Q. Autophagy is involved in the differentiation of epicardial progenitor cells into vascular smooth muscle cells in mice. Exp. Cell. Res. 2019, 375, 60–71. [Google Scholar] [CrossRef]
- Johnson, N.P.; Gould, K.L.; De Bruyne, B. Autoregulation of Coronary Blood Supply in Response to Demand: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 2335–2345. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, W.; Cui, J.; Yu, Y.; Zhao, Y.; Shi, J.; Wu, J.; Xia, Z.; Yu, B.; Liu, J. Arterial Wall Stress Induces Phenotypic Switching of Arterial Smooth Muscle Cells in Vascular Remodeling by Activating the YAP/TAZ Signaling Pathway. Cell. Physiol. Biochem. 2018, 51, 842–853. [Google Scholar] [CrossRef]
- Jensen, L.F.; Bentzon, J.F.; Albarrán-Juárez, J. The Phenotypic Responses of Vascular Smooth Muscle Cells Exposed to Mechanical Cues. Cells 2021, 10, 2209. [Google Scholar] [CrossRef]
- Cuthbertson, I.; Morrell, N.W.; Caruso, P. BMPR2 Mutation and Metabolic Reprogramming in Pulmonary Arterial Hypertension. Circ. Res. 2023, 132, 109–126. [Google Scholar] [CrossRef]
- Gorelova, A.; Berman, M.; Al Ghouleh, I. Endothelial-to-Mesenchymal Transition in Pulmonary Arterial Hypertension. Antioxid Redox Signal 2021, 34, 891–914. [Google Scholar] [CrossRef]
- Kurz, J.; Weiss, A.C.; Thiesler, H.; Qasrawi, F.; Deuper, L.; Kaur, J.; Rudat, C.; Lüdtke, T.H.; Wojahn, I.; Hildebrandt, H.; et al. Notch signaling is a novel regulator of visceral smooth muscle cell differentiation in the murine ureter. Development 2022, 149, dev199735. [Google Scholar] [CrossRef]
- Ragot, H.; Monfort, A.; Baudet, M.; Azibani, F.; Fazal, L.; Merval, R.; Polidano, E.; Cohen-Solal, A.; Delcayre, C.; Vodovar, N.; et al. Loss of Notch3 Signaling in Vascular Smooth Muscle Cells Promotes Severe Heart Failure Upon Hypertension. Hypertension 2016, 68, 392–400. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, G.; Yang, J.; Lu, R.; Hu, H. DLEU2 modulates proliferation, migration and invasion of platelet-derived growth factor-BB (PDGF-BB)-induced vascular smooth muscle cells (VSMCs) via miR-212-5p/YWHAZ axis. Cell Cycle 2022, 21, 2013–2026. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Park, H.S.; Lee, D.H.; Jo, J.H.; Heo, K.S.; Myung, C.S. Regulation of autophagy by controlling Erk1/2 and mTOR for platelet-derived growth factor-BB-mediated vascular smooth muscle cell phenotype shift. Life Sci. 2021, 267, 118978. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shao, L.; Yu, J.; Huang, J.; Feng, Q. PDGF-BB promotes vascular smooth muscle cell migration by enhancing Pim-1 expression via inhibiting miR-214. Ann. Transl. Med. 2021, 9, 1728. [Google Scholar] [CrossRef]
- Chou, C.C.; Wang, C.P.; Chen, J.H.; Lin, H.H. Anti-Atherosclerotic Effect of Hibiscus Leaf Polyphenols against Tumor Necrosis Factor-alpha-Induced Abnormal Vascular Smooth Muscle Cell Migration and Proliferation. Antioxidants 2019, 8, 620. [Google Scholar] [CrossRef]
- He, X.; Deng, J.; Yu, X.J.; Yang, S.; Yang, Y.; Zang, W.J. Activation of M3AChR (Type 3 Muscarinic Acetylcholine Receptor) and Nrf2 (Nuclear Factor Erythroid 2-Related Factor 2) Signaling by Choline Alleviates Vascular Smooth Muscle Cell Phenotypic Switching and Vascular Remodeling. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2649–2664. [Google Scholar] [CrossRef]
- Orr, A.W.; Lee, M.Y.; Lemmon, J.A.; Yurdagul, A., Jr.; Gomez, M.F.; Bortz, P.D.; Wamhoff, B.R. Molecular mechanisms of collagen isotype-specific modulation of smooth muscle cell phenotype. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 225–231. [Google Scholar] [CrossRef]
- Castro, M.M.; Cena, J.; Cho, W.J.; Walsh, M.P.; Schulz, R. Matrix metalloproteinase-2 proteolysis of calponin-1 contributes to vascular hypocontractility in endotoxemic rats. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 662–668. [Google Scholar] [CrossRef]
- Li, W.; Li, Q.; Qin, L.; Ali, R.; Qyang, Y.; Tassabehji, M.; Pober, B.R.; Sessa, W.C.; Giordano, F.J.; Tellides, G. Rapamycin inhibits smooth muscle cell proliferation and obstructive arteriopathy attributable to elastin deficiency. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1028–1035. [Google Scholar] [CrossRef]
- Wirka, R.C.; Wagh, D.; Paik, D.T.; Pjanic, M.; Nguyen, T.; Miller, C.L.; Kundu, R.; Nagao, M.; Coller, J.; Koyano, T.K.; et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat. Med. 2019, 25, 1280–1289. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Kwartler, C.S.; Kaw, K.; Li, Y.; Kaw, A.; Chen, J.; LeMaire, S.A.; Shen, Y.H.; Milewicz, D.M. Cholesterol-Induced Phenotypic Modulation of Smooth Muscle Cells to Macrophage/Fibroblast-like Cells Is Driven by an Unfolded Protein Response. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 302–316. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Wang, Y.; Shen, W. Transcriptome analysis revealed a two-step transformation of vascular smooth muscle cells to macrophage-like cells. Atherosclerosis 2022, 346, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.S.; Zhou, H.N.; He, S.S.; Xue, M.Y.; Li, T.; Liu, L.M. Research advances in pericyte function and their roles in diseases. Chin. J. Traumatol. 2020, 23, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.L.; Chintalgattu, V. Pericytes in the Heart. Adv. Exp. Med. Biol. 2019, 1122, 187–210. [Google Scholar] [CrossRef]
- Caporali, A.; Martello, A.; Miscianinov, V.; Maselli, D.; Vono, R.; Spinetti, G. Contribution of pericyte paracrine regulation of the endothelium to angiogenesis. Pharmacol. Ther. 2017, 171, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Dessalles, C.A.; Babataheri, A.; Barakat, A.I. Pericyte mechanics and mechanobiology. J. Cell Sci. 2021, 134, jcs240226. [Google Scholar] [CrossRef]
- Avolio, E.; Campagnolo, P.; Katare, R.; Madeddu, P. The role of cardiac pericytes in health and disease: Therapeutic targets for myocardial infarction. Nat. Rev. Cardiol. 2024, 21, 106–118. [Google Scholar] [CrossRef]
- Kovacic, J.C.; Mercader, N.; Torres, M.; Boehm, M.; Fuster, V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: From cardiovascular development to disease. Circulation 2012, 125, 1795–1808. [Google Scholar] [CrossRef]
- Kumar, A.; D’Souza, S.S.; Moskvin, O.V.; Toh, H.; Wang, B.; Zhang, J.; Swanson, S.; Guo, L.W.; Thomson, J.A.; Slukvin, I.I. Specification and Diversification of Pericytes and Smooth Muscle Cells from Mesenchymoangioblasts. Cell Rep. 2017, 19, 1902–1916. [Google Scholar] [CrossRef]
- Nees, S.; Weiss, D.R.; Senftl, A.; Knott, M.; Förch, S.; Schnurr, M.; Weyrich, P.; Juchem, G. Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: The second most frequent myocardial cell type in vitro. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H69–H84. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, H.; Liu, Y.; Adams, S.; Eilken, H.; Stehling, M.; Corada, M.; Dejana, E.; Zhou, B.; Adams, R.H. Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells. Nat. Commun. 2016, 7, 12422. [Google Scholar] [CrossRef]
- Witt, R.; Weigand, A.; Boos, A.M.; Cai, A.; Dippold, D.; Boccaccini, A.R.; Schubert, D.W.; Hardt, M.; Lange, C.; Arkudas, A.; et al. Mesenchymal stem cells and myoblast differentiation under HGF and IGF-1 stimulation for 3D skeletal muscle tissue engineering. BMC Cell Biol. 2017, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Zhao, Y.; Guo, L.; Zhang, Z.; Yan, C.; Zhang, S.; Zhang, Y.; Shao, F.; Qi, Y.; Wang, X.; et al. Innovative approaches to boost mesenchymal stem cells efficacy in myocardial infarction therapy. Mater. Today Bio 2025, 31, 101476. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, M.; Sasaki, D.; Hibino, M.; Takeda, A.; Harashima, H.; Yamada, Y. Human cardiosphere-derived cells with activated mitochondria for better myocardial regenerative therapy. J. Control. Release 2024, 367, 486–499. [Google Scholar] [CrossRef]
- Cao, J.; Poss, K.D. The epicardium as a hub for heart regeneration. Nat. Rev. Cardiol. 2018, 15, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.A.; Atkins, B.Z.; Hungspreugs, P.; Jones, T.R.; Reedy, M.C.; Hutcheson, K.A.; Glower, D.D.; Kraus, W.E. Regenerating functional myocardium: Improved performance after skeletal myoblast transplantation. Nat. Med. 1998, 4, 929–933. [Google Scholar] [CrossRef]
- Zhang, J.J.; Pogwizd, S.M.; Fukuda, K.; Zimmermann, W.H.; Fan, C.; Hare, J.M.; Bolli, R.; Menasché, P. Trials and tribulations of cell therapy for heart failure: An update on ongoing trials. Nat. Rev. Cardiol. 2025, 22, 372–385. [Google Scholar] [CrossRef]
- Karantalis, V.; Schulman, I.H.; Balkan, W.; Hare, J.M. Allogeneic cell therapy: A new paradigm in therapeutics. Circ. Res. 2015, 116, 12–15. [Google Scholar] [CrossRef]
- Patel, A.N.; Henry, T.D.; Quyyumi, A.A.; Schaer, G.L.; Anderson, R.D.; Toma, C.; East, C.; Remmers, A.E.; Goodrich, J.; Desai, A.S.; et al. Ixmyelocel-T for patients with ischaemic heart failure: A prospective randomised double-blind trial. Lancet 2016, 387, 2412–2421. [Google Scholar] [CrossRef]
- Mathiasen, A.B.; Qayyum, A.A.; Jørgensen, E.; Helqvist, S.; Kofoed, K.F.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: Final 4-year follow-up of the MSC-HF trial. Eur. J. Heart Fail. 2020, 22, 884–892. [Google Scholar] [CrossRef]
- Bolli, R.; Mitrani, R.D.; Hare, J.M.; Pepine, C.J.; Perin, E.C.; Willerson, J.T.; Traverse, J.H.; Henry, T.D.; Yang, P.C.; Murphy, M.P.; et al. A Phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: The CCTRN CONCERT-HF trial. Eur. J. Heart Fail. 2021, 23, 661–674. [Google Scholar] [CrossRef]
- Perin, E.C.; Borow, K.M.; Henry, T.D.; Mendelsohn, F.O.; Miller, L.W.; Swiggum, E.; Adler, E.D.; Chang, D.H.; Fish, R.D.; Bouchard, A.; et al. Randomized Trial of Targeted Transendocardial Mesenchymal Precursor Cell Therapy in Patients with Heart Failure. J. Am. Coll. Cardiol. 2023, 81, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R.; Perin, E.C.; Willerson, J.T.; Yang, P.C.; Traverse, J.H.; Henry, T.D.; Pepine, C.J.; Mitrani, R.D.; Hare, J.M.; Murphy, M.P.; et al. Allogeneic Mesenchymal Cell Therapy in Anthracycline-Induced Cardiomyopathy Heart Failure Patients: The CCTRN SENECA Trial. JACC CardioOncology 2020, 2, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, J.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients with Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [CrossRef]
- Ni, Z.; Deng, J.; Potter, C.M.F.; Nowak, W.N.; Gu, W.; Zhang, Z.; Chen, T.; Chen, Q.; Hu, Y.; Zhou, B.; et al. Recipient c-Kit Lineage Cells Repopulate Smooth Muscle Cells of Transplant Arteriosclerosis in Mouse Models. Circ. Res. 2019, 125, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Messina, E.; De Angelis, L.; Frati, G.; Morrone, S.; Chimenti, S.; Fiordaliso, F.; Salio, M.; Battaglia, M.; Latronico, M.V.; Coletta, M.; et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004, 95, 911–921. [Google Scholar] [CrossRef]
- Li, T.S.; Cheng, K.; Malliaras, K.; Smith, R.R.; Zhang, Y.; Sun, B.; Matsushita, N.; Blusztajn, A.; Terrovitis, J.; Kusuoka, H.; et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J. Am. Coll. Cardiol. 2012, 59, 942–953. [Google Scholar] [CrossRef]
- Marbán, E.; Liao, K. On the cellular origin of cardiosphere-derived cells (CDCs). Basic Res Cardiol 2022, 117, 12. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, Q.; Rajagopal, S.; Wu, S.M.; Domian, I.; Rivera-Feliciano, J.; Jiang, D.; von Gise, A.; Ikeda, S.; Chien, K.R.; et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 2008, 454, 109–113. [Google Scholar] [CrossRef]
- Rao, K.S.; Spees, J.L. Harnessing Epicardial Progenitor Cells and Their Derivatives for Rescue and Repair of Cardiac Tissue After Myocardial Infarction. Curr. Mol. Biol. Rep. 2017, 3, 149–158. [Google Scholar] [CrossRef]
- Chen, T.; Chang, T.C.; Kang, J.O.; Choudhary, B.; Makita, T.; Tran, C.M.; Burch, J.B.; Eid, H.; Sucov, H.M. Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor. Dev. Biol. 2002, 250, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Foglia, M.J.; Poss, K.D. Building and re-building the heart by cardiomyocyte proliferation. Development 2016, 143, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Karra, R.; Poss, K.D. Redirecting cardiac growth mechanisms for therapeutic regeneration. J. Clin. Investig. 2017, 127, 427–436. [Google Scholar] [CrossRef]
- Smart, N.; Risebro, C.A.; Melville, A.A.; Moses, K.; Schwartz, R.J.; Chien, K.R.; Riley, P.R. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature 2007, 445, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.; Bollini, S.; Dubé, K.N.; Vieira, J.M.; Zhou, B.; Davidson, S.; Yellon, D.; Riegler, J.; Price, A.N.; Lythgoe, M.F.; et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011, 474, 640–644. [Google Scholar] [CrossRef]
- Wei, K.; Serpooshan, V.; Hurtado, C.; Diez-Cuñado, M.; Zhao, M.; Maruyama, S.; Zhu, W.; Fajardo, G.; Noseda, M.; Nakamura, K.; et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 2015, 525, 479–485. [Google Scholar] [CrossRef]
- Ménard, C.; Hagège, A.A.; Agbulut, O.; Barro, M.; Morichetti, M.C.; Brasselet, C.; Bel, A.; Messas, E.; Bissery, A.; Bruneval, P.; et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: A preclinical study. Lancet 2005, 366, 1005–1012. [Google Scholar] [CrossRef]
- Sartiani, L.; Bettiol, E.; Stillitano, F.; Mugelli, A.; Cerbai, E.; Jaconi, M.E. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: A molecular and electrophysiological approach. Stem Cells 2007, 25, 1136–1144. [Google Scholar] [CrossRef]
- Nussbaum, J.; Minami, E.; Laflamme, M.A.; Virag, J.A.; Ware, C.B.; Masino, A.; Muskheli, V.; Pabon, L.; Reinecke, H.; Murry, C.E. Transplantation of undifferentiated murine embryonic stem cells in the heart: Teratoma formation and immune response. FASEB J. 2007, 21, 1345–1357. [Google Scholar] [CrossRef]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar] [CrossRef]
- Menasché, P.; Renault, N.K.; Hagège, A.; Puscas, T.; Bellamy, V.; Humbert, C.; Le, L.; Blons, H.; Granier, C.; Benhamouda, N.; et al. First-in-man use of a cardiovascular cell-derived secretome in heart failure. Case report. eBioMedicine 2024, 103, 105145. [Google Scholar] [CrossRef]
- Nouspikel, T. Genetic instability in human embryonic stem cells: Prospects and caveats. Future Oncol. 2013, 9, 867–877. [Google Scholar] [CrossRef]
- Ilic, D.; Ogilvie, C. Concise Review: Human Embryonic Stem Cells-What Have We Done? What Are We Doing? Where Are We Going? Stem Cells 2017, 35, 17–25. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, L.L.; Zhang, F.; Bi, W.; Zhang, P.; Yu, X.J.; Rao, S.L.; Wang, S.H.; Li, Q.; Ding, C.; et al. Dual human iPSC-derived cardiac lineage cell-seeding extracellular matrix patches promote regeneration and long-term repair of infarcted hearts. Bioact. Mater. 2023, 28, 206–226. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Wu, Q.; Cao, N.; Yang, H.T. Perspective on human pluripotent stem cell-derived cardiomyocytes in heart disease modeling and repair. Stem Cells Transl. Med. 2020, 9, 1121–1128. [Google Scholar] [CrossRef]
- Miyagawa, S.; Kainuma, S.; Kawamura, T.; Suzuki, K.; Ito, Y.; Iseoka, H.; Ito, E.; Takeda, M.; Sasai, M.; Mochizuki-Oda, N.; et al. Case report: Transplantation of human induced pluripotent stem cell-derived cardiomyocyte patches for ischemic cardiomyopathy. Front. Cardiovasc. Med. 2022, 9, 950829. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Wang, G.; Song, Y.; An, Y. Novel association of LBX1 mutation with tetralogy of Fallot and hypertrophic cardiomyopathy: Implications for cardiac development. Sci. Rep. 2024, 14, 26179. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.F.; Yu, T. CRISPR/Cas9 therapeutics: Progress and prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef]
- Wang, N.; Wang, W.; Wang, X.; Mang, G.; Chen, J.; Yan, X.; Tong, Z.; Yang, Q.; Wang, M.; Chen, L.; et al. Histone Lactylation Boosts Reparative Gene Activation Post-Myocardial Infarction. Circ. Res. 2022, 131, 893–908. [Google Scholar] [CrossRef]
- Wu, X.; Reboll, M.R.; Korf-Klingebiel, M.; Wollert, K.C. Angiogenesis after acute myocardial infarction. Cardiovasc. Res. 2021, 117, 1257–1273. [Google Scholar] [CrossRef]
- Wagner, N.; Vukolic, A.; Baudouy, D.; Pagnotta, S.; Michiels, J.F.; Wagner, K.D. Enhanced neoangiogenesis and balance of the immune response mediated by the Wilms’ tumor suppressor WT1 favor repair after myocardial infarction. Theranostics 2025, 15, 6593–6614. [Google Scholar] [CrossRef] [PubMed]
- Moya, I.M.; Halder, G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 2019, 20, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Musolino, P.L.; Rosser, S.J.; Brittan, M.; Newby, D.E.; Berry, C.; Riley, P.R.; Giacca, M.; Hajjar, R.J.; Baker, A.H. Gene therapy in cardiac and vascular diseases: A review of approaches to treat genetic and common cardiovascular diseases with novel gene-based therapeutics. Cardiovasc. Res. 2025, cvaf109. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; von Gise, A.; Zhou, P.; Gu, F.; Ma, Q.; Jiang, J.; Yau, A.L.; Buck, J.N.; Gouin, K.A.; van Gorp, P.R.; et al. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ. Res. 2014, 115, 354–363. [Google Scholar] [CrossRef]
- Morikawa, Y.; Heallen, T.; Leach, J.; Xiao, Y.; Martin, J.F. Dystrophin-glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation. Nature 2017, 547, 227–231. [Google Scholar] [CrossRef]
- Liu, S.; Li, K.; Wagner Florencio, L.; Tang, L.; Heallen, T.R.; Leach, J.P.; Wang, Y.; Grisanti, F.; Willerson, J.T.; Perin, E.C.; et al. Gene therapy knockdown of Hippo signaling induces cardiomyocyte renewal in pigs after myocardial infarction. Sci. Transl. Med. 2021, 13, eabd6892. [Google Scholar] [CrossRef]
- Li, X.; Le, Y.; Zhang, Z.; Nian, X.; Liu, B.; Yang, X. Viral Vector-Based Gene Therapy. Int. J. Mol. Sci. 2023, 24, 7736. [Google Scholar] [CrossRef]
- Silva, A.C.; Matthys, O.B.; Joy, D.A.; Kauss, M.A.; Natarajan, V.; Lai, M.H.; Turaga, D.; Blair, A.P.; Alexanian, M.; Bruneau, B.G.; et al. Co-emergence of cardiac and gut tissues promotes cardiomyocyte maturation within human iPSC-derived organoids. Cell Stem Cell 2021, 28, 2137–2152.e2136. [Google Scholar] [CrossRef]
- Rossi, G.; Broguiere, N.; Miyamoto, M.; Boni, A.; Guiet, R.; Girgin, M.; Kelly, R.G.; Kwon, C.; Lutolf, M.P. Capturing Cardiogenesis in Gastruloids. Cell Stem Cell 2021, 28, 230–240.e236. [Google Scholar] [CrossRef]
- Dardano, M.; Kleemiß, F.; Kosanke, M.; Lang, D.; Wilson, L.; Franke, A.; Teske, J.; Shivaraj, A.; de la Roche, J.; Fischer, M.; et al. Blood-generating heart-forming organoids recapitulate co-development of the human haematopoietic system and the embryonic heart. Nat. Cell Biol. 2024, 26, 1984–1996. [Google Scholar] [CrossRef]
- Schmidt, C.; Deyett, A.; Ilmer, T.; Haendeler, S.; Torres Caballero, A.; Novatchkova, M.; Netzer, M.A.; Ceci Ginistrelli, L.; Mancheno Juncosa, E.; Bhattacharya, T.; et al. Multi-chamber cardioids unravel human heart development and cardiac defects. Cell 2023, 186, 5587–5605.e5527. [Google Scholar] [CrossRef]
- Wang, F.; Zou, X.; Zheng, H.; Kong, T.; Pei, D. Human epicardial organoids from pluripotent stem cells resemble fetal stage with potential cardiomyocyte- transdifferentiation. Cell Biosci. 2025, 15, 4. [Google Scholar] [CrossRef]
- Feng, W.; Schriever, H.; Jiang, S.; Bais, A.; Wu, H.; Kostka, D.; Li, G. Computational profiling of hiPSC-derived heart organoids reveals chamber defects associated with NKX2-5 deficiency. Commun. Biol. 2022, 5, 399. [Google Scholar] [CrossRef]
- Voges, H.K.; Foster, S.R.; Reynolds, L.; Parker, B.L.; Devilée, L.; Quaife-Ryan, G.A.; Fortuna, P.R.J.; Mathieson, E.; Fitzsimmons, R.; Lor, M.; et al. Vascular cells improve functionality of human cardiac organoids. Cell Rep. 2023, 42, 112322. [Google Scholar] [CrossRef]
- Ergir, E.; Oliver-De La Cruz, J.; Fernandes, S.; Cassani, M.; Niro, F.; Pereira-Sousa, D.; Vrbský, J.; Vinarský, V.; Perestrelo, A.R.; Debellis, D.; et al. Generation and maturation of human iPSC-derived 3D organotypic cardiac microtissues in long-term culture. Sci. Rep. 2022, 12, 17409. [Google Scholar] [CrossRef]
- Yang, J.; Lei, W.; Xiao, Y.; Tan, S.; Yang, J.; Lin, Y.; Yang, Z.; Zhao, D.; Zhang, C.; Shen, Z.; et al. Generation of human vascularized and chambered cardiac organoids for cardiac disease modelling and drug evaluation. Cell Prolif. 2024, 57, e13631. [Google Scholar] [CrossRef] [PubMed]
- Volmert, B.; Kiselev, A.; Juhong, A.; Wang, F.; Riggs, A.; Kostina, A.; O’Hern, C.; Muniyandi, P.; Wasserman, A.; Huang, A.; et al. A patterned human primitive heart organoid model generated by pluripotent stem cell self-organization. Nat. Commun. 2023, 14, 8245. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.Y.; Chang, Y.; Jin, G.; Lian, X.; Bao, X. Wnt signaling directs human pluripotent stem cells into vascularized cardiac organoids with chamber-like structures. Front. Bioeng. Biotechnol. 2022, 10, 1059243. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, Y.J.; Son, M.Y.; Oh, M.S.; Kim, J.; Ryu, B.; Kang, K.R.; Baek, J.; Chung, G.; Woo, D.H.; et al. Generation of human iPSCs derived heart organoids structurally and functionally similar to heart. Biomaterials 2022, 290, 121860. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, L.; Li, T.; Liu, S.; Guo, B.; Huang, W.; Wu, Y. 3D bioprinting in cardiac tissue engineering. Theranostics 2021, 11, 7948–7969. [Google Scholar] [CrossRef]
- De Santis, M.M.; Alsafadi, H.N.; Tas, S.; Bölükbas, D.A.; Prithiviraj, S.; Da Silva, I.A.N.; Mittendorfer, M.; Ota, C.; Stegmayr, J.; Daoud, F.; et al. Extracellular-Matrix-Reinforced Bioinks for 3D Bioprinting Human Tissue. Adv. Mater. 2021, 33, e2005476. [Google Scholar] [CrossRef]
- Roshanbinfar, K.; Evans, A.D.; Samanta, S.; Kolesnik-Gray, M.; Fiedler, M.; Krstic, V.; Engel, F.B.; Oommen, O.P. Enhancing biofabrication: Shrink-resistant collagen-hyaluronan composite hydrogel for tissue engineering and 3D bioprinting applications. Biomaterials 2025, 318, 123174. [Google Scholar] [CrossRef]
- Ng, X.J.; Esser, T.U.; Trossmann, V.T.; Rudisch, C.; Fiedler, M.; Roshanbinfar, K.; Lamberger, Z.; Stahlhut, P.; Lang, G.; Scheibel, T.; et al. Enhancing Form Stability: Shrink-Resistant Hydrogels Made of Interpenetrating Networks of Recombinant Spider Silk and Collagen-I. Adv. Healthc. Mater. 2025, 14, e2500311. [Google Scholar] [CrossRef]
- Dave, R.; Luraghi, G.; Sierad, L.; Migliavacca, F.; Kung, E. Shear Stress Quantification in Tissue Engineering Bioreactor Heart Valves: A Computational Approach. J. Funct. Biomater. 2024, 15, 76. [Google Scholar] [CrossRef]
- Tani, H.; Kobayashi, E.; Yagi, S.; Tanaka, K.; Kameda-Haga, K.; Shibata, S.; Moritoki, N.; Takatsuna, K.; Moriwaki, T.; Sekine, O.; et al. Heart-derived collagen promotes maturation of engineered heart tissue. Biomaterials 2023, 299, 122174. [Google Scholar] [CrossRef] [PubMed]
- McKinley, K.L.; Longaker, M.T.; Naik, S. Emerging frontiers in regenerative medicine. Science 2023, 380, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hao, Y.; Zhang, Z.; Zhou, H.; Peng, S.; Zhang, D.; Li, K.; Chen, Y.; Chen, M. Advanced Cardiac Patches for the Treatment of Myocardial Infarction. Circulation 2024, 149, 2002–2020. [Google Scholar] [CrossRef] [PubMed]
- Thej, C.; Kishore, R. Unfathomed Nanomessages to the Heart: Translational Implications of Stem Cell-Derived, Progenitor Cell Exosomes in Cardiac Repair and Regeneration. Cells 2021, 10, 1811. [Google Scholar] [CrossRef]
- Wang, R.; Liu, L.; Han, F.; Ma, Q.; He, H. Exosomes derived from human umbilical cord mesenchymal stem cells can reverse ventricular remodeling and improve long-term cardiac function after acute myocardial infarction. Biochem. Biophys. Res. Commun. 2025, 768, 151920. [Google Scholar] [CrossRef]
- Qiao, S.; Wu, B.; Chen, L.; Ma, L.; Wang, Y.; Xu, B.; Gu, R. Lymph Node Exosomes Delivery Attenuates Myocardial Ischemia-Reperfusion Injury via Regulating PTEN-PI3K/Akt Pathway Mediated Myocardiocyte Apoptosis. Int. J. Nanomed. 2025, 20, 4967–4981. [Google Scholar] [CrossRef]
- Cheng, N.; Luo, Q.; Yang, Y.; Shao, N.; Nie, T.; Deng, X.; Chen, J.; Zhang, S.; Huang, Y.; Hu, K.; et al. Injectable pH Responsive Conductive Hydrogel for Intelligent Delivery of Metformin and Exosomes to Enhance Cardiac Repair after Myocardial Ischemia-Reperfusion Injury. Adv. Sci. 2025, 12, e2410590. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, H.; Liu, C.; Shao, L.; Zhang, H.; Lu, K.; Wang, J.; Wang, Y.; Yu, Q.; Zhang, Y.; et al. Microneedle Patch Loaded with Exosomes Containing MicroRNA-29b Prevents Cardiac Fibrosis after Myocardial Infarction. Adv. Healthc. Mater. 2023, 12, e2202959. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, Z.; Zheng, F.; Yang, D.; Xu, K.; Ke, M.; Zhou, X.; Zhang, X.; Tan, J.; Liu, Y.; et al. Cardiac Organoid Model Inspired Micro-Robot Smart Patch to Treat Myocardial Infarction. Adv. Mater. 2025, 37, e2417327. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, X.; Wu, S.X.; Chang, Q.; Zheng, Z.K.; Xu, J. Cardioprotective effects of hUCMSCs-Exosi-EGR1 MN patch in MI/RI by modulating oxidative stress and mitophagy. Mater. Today Bio 2025, 31, 101500. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.T.; Zhu, J.; Wang, Y.C.; Shao, C.L.; Li, X.Y.; Lu, P.P.; Huang, M.Y.; Mou, F.F.; Guo, H.D.; Ji, G. Targeting delivery of miR-146a via IMTP modified milk exosomes exerted cardioprotective effects by inhibiting NF-κB signaling pathway after myocardial ischemia-reperfusion injury. J. Nanobiotechnology 2024, 22, 382. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zhao, Z.; Meng, Q.; Yu, Y.; Sun, J.; Yang, Z.; Chen, Y.; Li, J.; Ma, T.; et al. Engineered Exosomes with Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J. Am. Heart Assoc. 2018, 7, e008737. [Google Scholar] [CrossRef]
- Sun, A.R.; Ramli, M.F.H.; Shen, X.; Kannivadi Ramakanth, K.; Chen, D.; Hu, Y.; Vidyasekar, P.; Foo, R.S.; Long, Y.; Zhu, J.; et al. Hybrid hydrogel-extracellular matrix scaffolds identify biochemical and mechanical signatures of cardiac ageing. Nat. Mater. 2025, 24, 1489–1501. [Google Scholar] [CrossRef]
- Li, X.; Wu, F.; Günther, S.; Looso, M.; Kuenne, C.; Zhang, T.; Wiesnet, M.; Klatt, S.; Zukunft, S.; Fleming, I.; et al. Inhibition of fatty acid oxidation enables heart regeneration in adult mice. Nature 2023, 622, 619–626. [Google Scholar] [CrossRef]
- Holubarsch, C.J.; Rohrbach, M.; Karrasch, M.; Boehm, E.; Polonski, L.; Ponikowski, P.; Rhein, S. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: The ERGO (etomoxir for the recovery of glucose oxidation) study. Clin. Sci. 2007, 113, 205–212. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Jin, Y.; Chen, K.; Zhang, L.; Gao, X.; Li, M.; Yuan, Z.; Jia, J.; Sun, A.; et al. Mitochondrial Transplantation Augments the Reparative Capacity of Macrophages Following Myocardial Injury. Adv. Sci. 2025, 29, e06337. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.-Y.; Jiang, J.-B.; Wang, S.-N.; Miao, C.-Y. Cardiac Development, Cellular Composition and Function: From Regulatory Mechanisms to Applications. Cells 2025, 14, 1390. https://doi.org/10.3390/cells14171390
Zhao H-Y, Jiang J-B, Wang S-N, Miao C-Y. Cardiac Development, Cellular Composition and Function: From Regulatory Mechanisms to Applications. Cells. 2025; 14(17):1390. https://doi.org/10.3390/cells14171390
Chicago/Turabian StyleZhao, Huan-Yu, Jie-Bing Jiang, Shu-Na Wang, and Chao-Yu Miao. 2025. "Cardiac Development, Cellular Composition and Function: From Regulatory Mechanisms to Applications" Cells 14, no. 17: 1390. https://doi.org/10.3390/cells14171390
APA StyleZhao, H.-Y., Jiang, J.-B., Wang, S.-N., & Miao, C.-Y. (2025). Cardiac Development, Cellular Composition and Function: From Regulatory Mechanisms to Applications. Cells, 14(17), 1390. https://doi.org/10.3390/cells14171390





