Heart Failure Impacts Endothelial Cell Responses to Cardiac Surgery on Cardiopulmonary Bypass
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Blood Sample Collection
2.3. Circulating Plasma Marker Levels
2.4. Cell Culture
2.5. Ex Vivo EC Morphological Profiling
2.6. Mitochondrial Mass, Potential, Superoxide Production, and Respiration
2.7. Data and Statistical Analysis
3. Results
3.1. Patient Characteristics
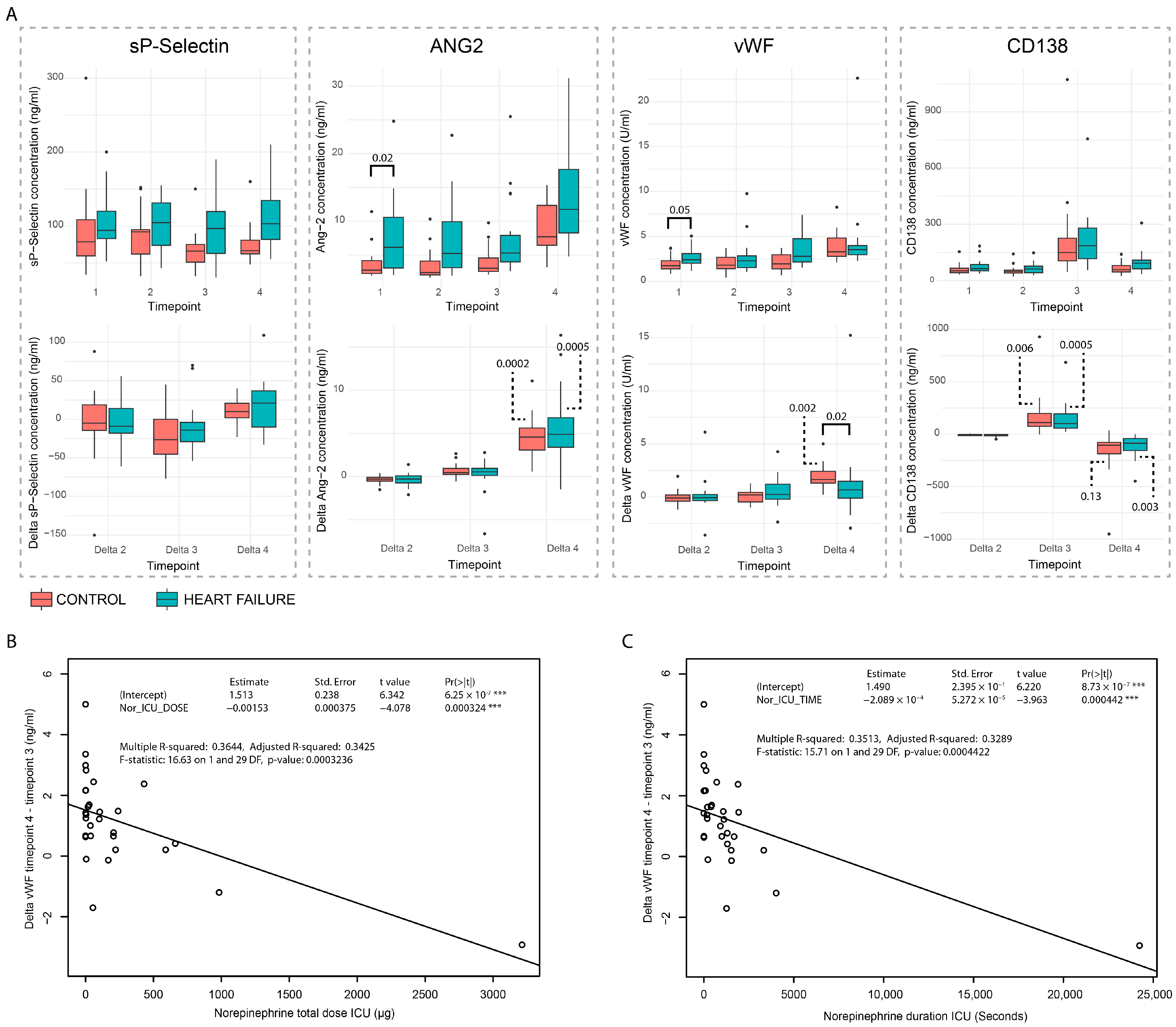
3.2. Circulating Plasma Markers
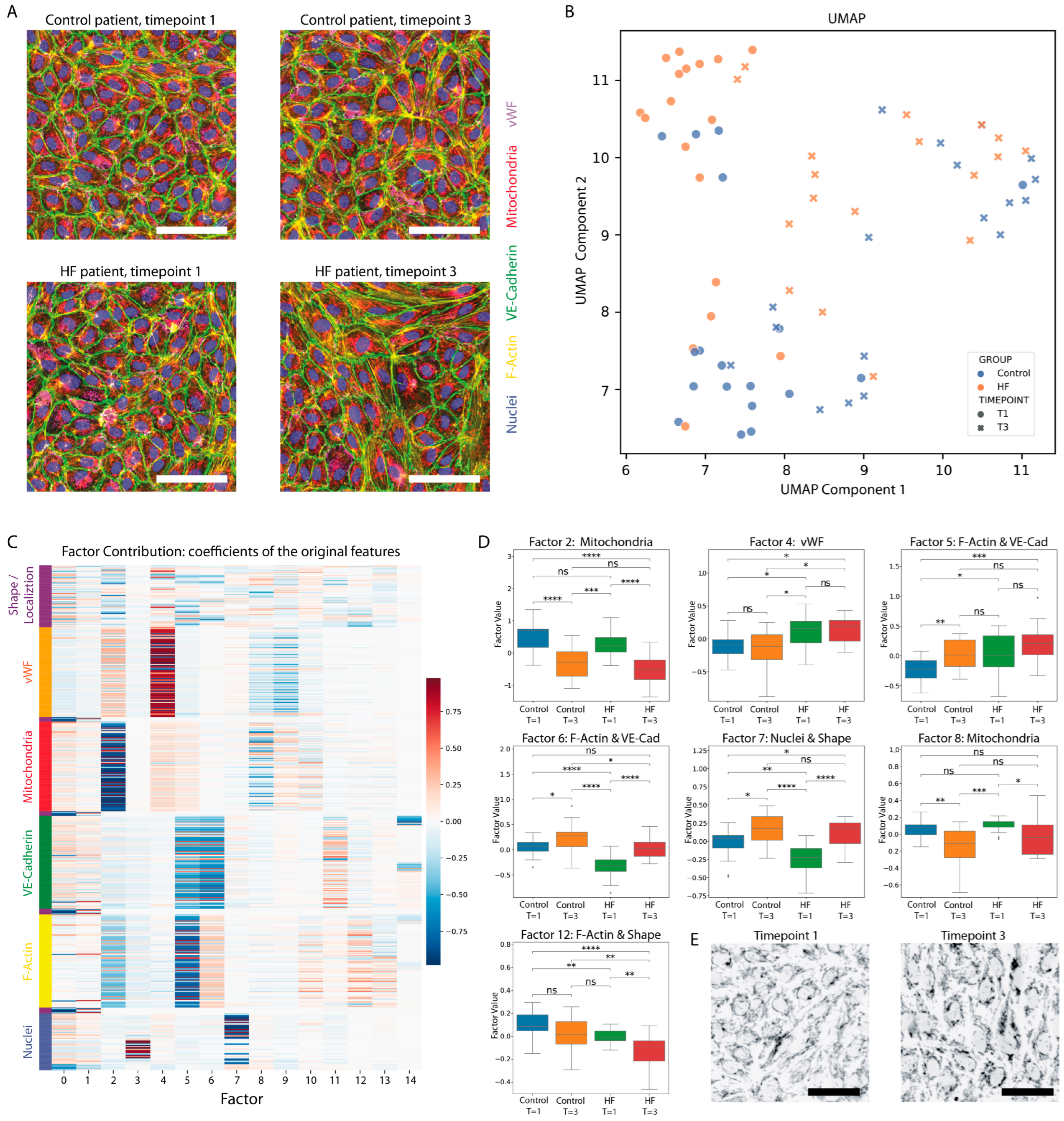
3.3. Morphological Profiling of EC Responses to Patient Plasma
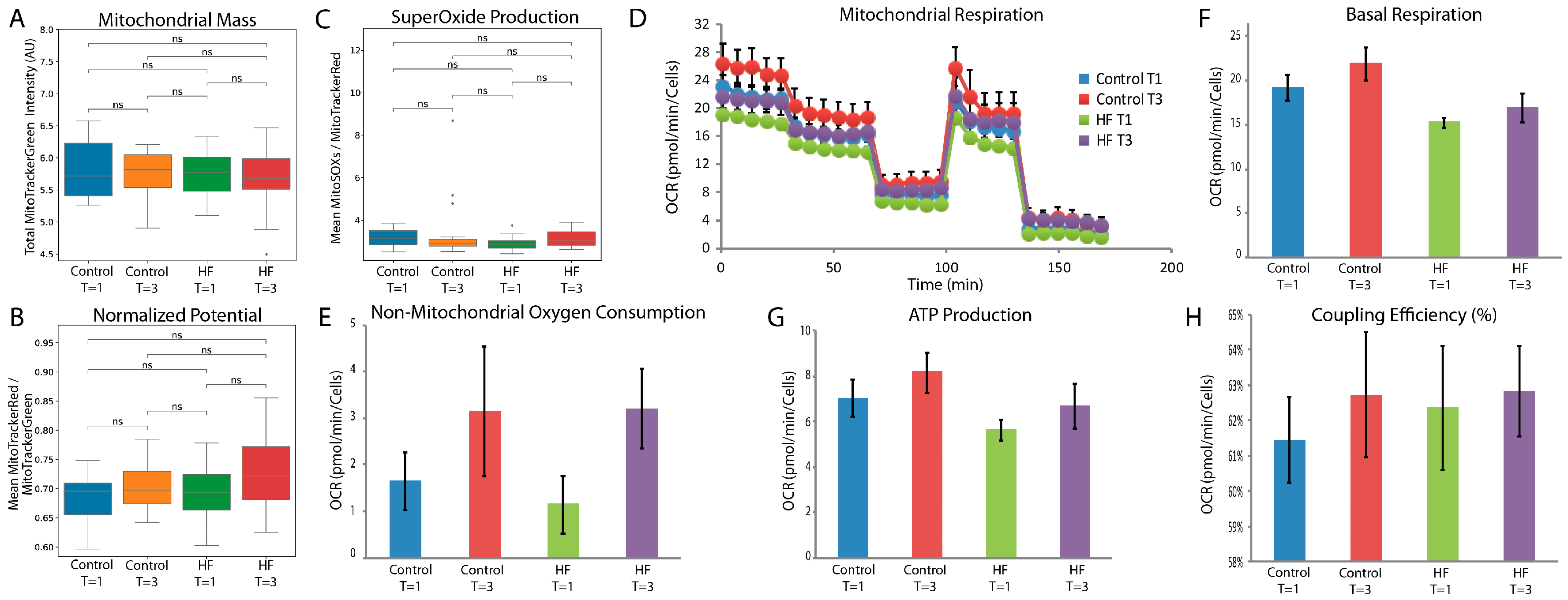
3.4. Mitochondrial Changes in ECs in Response to Patient Plasma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloom, M.W.; Greenberg, B.; Jaarsma, T.; Januzzi, J.L.; Lam, C.S.P.; Maggioni, A.P.; Trochu, J.-N.; Butler, J. Heart failure with reduced ejection fraction. Nat. Rev. Dis. Primers 2017, 3, 17058. [Google Scholar] [CrossRef]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2022, 118, 3272–3287. [Google Scholar] [CrossRef]
- Seferović, P.M.; Vardas, P.; Jankowska, E.A.; Maggioni, A.P.; Timmis, A.; Milinković, I.; Polovina, M.; Gale, C.P.; Lund, L.H.; Lopatin, Y.; et al. The Heart Failure Association Atlas: Heart Failure Epidemiology and Management Statistics 2019. Eur. J. Heart Fail. 2021, 23, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Ciarka, A.; Versteegh, M.I.M.; Delgado, V.; Boersma, E.; Verwey, H.F.; Schalij, M.J.; Bax, J.J.; Dion, R.A.E.; van de Veire, N.R.; et al. Cardiac support device, restrictive mitral valve annuloplasty, and optimized medical treatment: A multimodality approach to nonischemic cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2011, 142, e93–e100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haeck, M.L.A.; Hoogslag, G.E.; Rodrigo, S.F.; Atsma, D.E.; Klautz, R.J.; van der Wall, E.E.; Schalij, M.J.; Verwey, H.F. Treatment options in end-stage heart failure: Where to go from here? Neth. Heart J. 2011, 20, 167–175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klein, P.; Holman, E.R.; Versteegh, M.I.M.; Boersma, E.; Verwey, H.F.; Bax, J.J.; Dion, R.A.E.; Klautz, R.J.M. Wall motion score index predicts mortality and functional result after surgical ventricular restoration for advanced ischemic heart failure. Eur. J. Cardio-Thorac. Surg. 2009, 35, 847–853. [Google Scholar] [CrossRef]
- Argenziano, M.; Chen, J.M.; Choudhri, A.F.; Cullinane, S.; Garfein, E.; Weinberg, A.D.; Smith, C.R.; Rose, E.A.; Landry, D.W.; Oz, M.C. Management of vasodilatory shock after cardiac surgery: Identification of predisposing factors and use of a novel pressor agent. J. Thorac. Cardiovasc. Surg. 1998, 116, 973–980. [Google Scholar] [CrossRef]
- van Vessem, M.E.; Palmen, M.; Couperus, L.E.; Mertens, B.; Berendsen, R.R.; Tops, L.F.; Verwey, H.F.; de Jonge, E.; Klautz, R.J.; Schalij, M.J.; et al. Incidence and predictors of vasoplegia after heart failure surgery. Eur. J. Cardiothorac. Surg. 2017, 51, 532–538. [Google Scholar] [CrossRef]
- Kortekaas, K.A.; Lindeman, J.H.; Versteegh, M.I.; Stijnen, T.; Dion, R.A.; Klautz, R.J. Preexisting heart failure is an underestimated risk factor in cardiac surgery. Neth. Heart J. 2012, 20, 202–207. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, L.; Hill, P.C.; Lowery, R.; Lee, A.T.; Molyneaux, R.E.; Corso, P.J.; Boyce, S.W. Is incidence of postoperative vasoplegic syndrome different between off-pump and on-pump coronary artery bypass grafting surgery? Eur. J. Cardiothorac. Surg. 2008, 34, 820–825. [Google Scholar] [CrossRef]
- Omar, S.; Zedan, A.; Nugent, K. Cardiac Vasoplegia Syndrome: Pathophysiology, Risk Factors and Treatment. Am. J. Med. Sci. 2015, 349, 80–88. [Google Scholar] [CrossRef] [PubMed]
- van Vessem, M.E.; Petrus, A.H.J.; Palmen, M.; Braun, J.; Schalij, M.J.; Klautz, R.J.M.; Beeres, S. Vasoplegia After Restrictive Mitral Annuloplasty for Functional Mitral Regurgitation in Patients with Heart Failure. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3273–3280. [Google Scholar] [CrossRef]
- de Waal, E.E.C.; van Zaane, B.; van der Schoot, M.M.; Huisman, A.; Ramjankhan, F.; van Klei, W.A.; Marczin, N. Vasoplegia after implantation of a continuous flow left ventricular assist device: Incidence, outcomes and predictors. BMC Anesthesiol. 2018, 18, 185. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A Novel Paradigm for Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Zuchi, C.; Tritto, I.; Carluccio, E.; Mattei, C.; Cattadori, G.; Ambrosio, G. Role of endothelial dysfunction in heart failure. Heart Fail. Rev. 2019, 25, 21–30. [Google Scholar] [CrossRef]
- Torp, M.K.; Vaage, J.; Stenslokken, K.O. Mitochondria-derived damage-associated molecular patterns and inflammation in the ischemic-reperfused heart. Acta Physiol. 2023, 237, e13920. [Google Scholar] [CrossRef] [PubMed]
- Day, J.R.; Taylor, K.M. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int. J. Surg. 2005, 3, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Robich, M.; Ryzhov, S.; Kacer, D.; Palmeri, M.; Peterson, S.M.; Quinn, R.D.; Carter, D.; Sheppard, F.; Hayes, T.; Sawyer, D.B.; et al. Prolonged Cardiopulmonary Bypass is Associated with Endothelial Glycocalyx Degradation. J. Surg. Res. 2020, 251, 287–295. [Google Scholar] [CrossRef]
- Sandler, N.; Kaczmarek, E.; Itagaki, K.; Zheng, Y.; Otterbein, L.; Khabbaz, K.; Liu, D.; Senthilnathan, V.; Gruen, R.L.; Hauser, C.J. Mitochondrial DAMPs Are Released During Cardiopulmonary Bypass Surgery and Are Associated with Postoperative Atrial Fibrillation. Heart Lung Circ. 2018, 27, 122–129. [Google Scholar] [CrossRef]
- Eichler, W.; Bechtel, J.F.; Schumacher, J.; Wermelt, J.A.; Klotz, K.F.; Bartels, C. A rise of MMP-2 and MMP-9 in bronchoalveolar lavage fluid is associated with acute lung injury after cardiopulmonary bypass in a swine model. Perfusion 2003, 18, 107–113. [Google Scholar] [CrossRef]
- van Vessem, M.E.; Beeres, S.; de Wilde, R.B.P.; de Vries, R.; Berendsen, R.R.; de Jonge, E.; Danser, A.H.J.; Klautz, R.J.M.; Schalij, M.J.; Palmen, M. Vasoresponsiveness in patients with heart failure (VASOR): Protocol for a prospective observational study. J. Cardiothorac. Surg. 2019, 14, 200. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- de Jong, A.; Dirven, R.J.; Oud, J.A.; Tio, D.; van Vlijmen, B.J.M.; Eikenboom, J. Correction of a dominant-negative von Willebrand factor multimerization defect by small interfering RNA-mediated allele-specific inhibition of mutant von Willebrand factor. J. Thromb. Haemost. 2018, 16, 1357–1368. [Google Scholar] [CrossRef]
- Vreeken, D.; Bruikman, C.S.; Cox, S.M.L.; Zhang, H.; Lalai, R.; Koudijs, A.; van Zonneveld, A.J.; Hovingh, G.K.; van Gils, J.M. EPH receptor B2 stimulates human monocyte adhesion and migration independently of its EphrinB ligands. J. Leukoc. Biol. 2020, 108, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Postma, R.J.; Broekhoven, A.G.C.; Verspaget, H.W.; de Boer, H.; Hankemeier, T.; Coenraad, M.J.; van Duinen, V.; van Zonneveld, A.J. Novel Morphological Profiling Assay Connects Ex Vivo Endothelial Cell Responses to Disease Severity in Liver Cirrhosis. Gastro Hep. Adv. 2024, 3, 238–249. [Google Scholar] [CrossRef]
- Junaid, A.; van Duinen, V.; Stam, W.; Dolleman, S.; Yang, W.; de Rijke, Y.; Endeman, H.; van Kooten, C.; Mashaghi, A.; de Boer, H.; et al. A Microfluidics-Based Screening Tool to Assess the Impact of Blood Plasma Factors on Microvascular Integrity. Adv. Biol. 2021, 5, e2100954. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; Version 4.0.0; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Pau, G.; Fuchs, F.; Sklyar, O.; Boutros, M.; Huber, W. EBImage—An R package for image processing with applications to cellular phenotypes. Bioinformatics 2010, 26, 979–981. [Google Scholar] [CrossRef]
- Manders, E.M.M.; Verbeek, F.J.; Aten, J.A. Measurement of co-localization of objects in dual-colour confocal images. J. Microsc. 1993, 169, 375–382. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Bray, M.A.; Singh, S.; Han, H.; Davis, C.T.; Borgeson, B.; Hartland, C.; Kost-Alimova, M.; Gustafsdottir, S.M.; Gibson, C.C.; Carpenter, A.E. Cell Painting, a high-content image-based assay for morphological profiling using multiplexed fluorescent dyes. Nat. Protoc. 2016, 11, 1757–1774. [Google Scholar] [CrossRef] [PubMed]
- Young, D.W.; Bender, A.; Hoyt, J.; McWhinnie, E.; Chirn, G.W.; Tao, C.Y.; Tallarico, J.A.; Labow, M.; Jenkins, J.L.; Mitchison, T.J.; et al. Integrating high-content screening and ligand-target prediction to identify mechanism of action. Nat. Chem. Biol. 2008, 4, 59–68. [Google Scholar] [CrossRef]
- Peplinski, B.S.; Houston, B.A.; Bluemke, D.A.; Kawut, S.M.; Kolb, T.M.; Kronmal, R.A.; Lima, J.A.C.; Ralph, D.D.; Rayner, S.G.; Steinberg, Z.L.; et al. Associations of Angiopoietins with Heart Failure Incidence and Severity. J. Card. Fail 2021, 27, 786–795. [Google Scholar] [CrossRef]
- Lip, G.Y.; Blann, A. von Willebrand factor: A marker of endothelial dysfunction in vascular disorders? Cardiovasc. Res. 1997, 34, 255–265. [Google Scholar] [CrossRef]
- Baidildinova, G.; Nagy, M.; Jurk, K.; Wild, P.S.; ten Cate, H.; van der Meijden, P.E.J. Soluble Platelet Release Factors as Biomarkers for Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 684920. [Google Scholar] [CrossRef]
- Gurney, D.; Lip, G.Y.H.; Blann, A.D. A reliable plasma marker of platelet activation: Does it exist? Am. J. Hematol. 2002, 70, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, K.A.; Lindeman, J.H.N.; Reinders, M.E.J.; Palmen, M.; Klautz, R.J.M.; de Groot, P.G.; Roest, M. Pre-existing endothelial cell activation predicts vasoplegia after mitral valve surgery†. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Richter, R.P.; Ashtekar, A.R.; Zheng, L.; Pretorius, D.; Kaushlendra, T.; Sanderson, R.D.; Gaggar, A.; Richter, J.R. Glycocalyx heparan sulfate cleavage promotes endothelial cell angiopoietin-2 expression by impairing shear stress-related AMPK/FoxO1 signaling. JCI Insight 2022, 7, e155010. [Google Scholar] [CrossRef]
- Dekker, N.A.M.; Veerhoek, D.; Koning, N.J.; van Leeuwen, A.L.I.; Elbers, P.W.G.; van den Brom, C.E.; Vonk, A.B.A.; Boer, C. Postoperative microcirculatory perfusion and endothelial glycocalyx shedding following cardiac surgery with cardiopulmonary bypass. Anaesthesia 2019, 74, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Bruegger, D.; Rehm, M.; Abicht, J.; Paul, J.O.; Stoeckelhuber, M.; Pfirrmann, M.; Reichart, B.; Becker, B.F.; Christ, F. Shedding of the endothelial glycocalyx during cardiac surgery: On-pump versus off-pump coronary artery bypass graft surgery. J. Thorac. Cardiovasc. Surg. 2009, 138, 1445–1447. [Google Scholar] [CrossRef]
- Higdon, A.N.; Benavides, G.A.; Chacko, B.K.; Ouyang, X.; Johnson, M.S.; Landar, A.; Zhang, J.; Darley-Usmar, V.M. Hemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: The protective role of autophagy. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1394–H1409. [Google Scholar] [CrossRef]
| Heart Failure (n = 18) | Control (n = 18) | p Value | |
|---|---|---|---|
| Age (years) (IQR) | 68 (62–71) | 64 (59–69) | 0.279 |
| Male sex (%) | 67 | 89 | 0.228 |
| Body mass index (kg/m2) (±SD) | 27 ± 4 | 24 ± 3 | 0.024 |
| Diabetes (%) | 28 | 6 | 0.177 |
| Prior hypertension (%) | 39 | 17 | 0.264 |
| Pulmonary hypertension (%) | 28 | 11 | 0.402 |
| Previous cardiac surgery (%) | 22 | 6 | 0.338 |
| Hemoglobin (mmol/L) (±SD) | 8.5 ± 1.0 | 9.1 ± 1.0 | 0.093 |
| Creatinine clearance (mL/min/1.73 m2) (±SD) | 67 ± 20 | 78 ± 16 | 0.090 |
| EuroSCORE II (%) (IQR) | 9.76 (6.59–15.49) | 1.45 (1.07–2.71) | <0.001 |
| Left ventricular ejection fraction (%) (±SD) | 24.9 ± 6.4 | 60.3 ± 6.9 | <0.001 |
| Medication use | |||
| Beta blocker (%) | 89 | 33 | 0.002 |
| Angiotensin-converting-enzyme inhibitor/angiotensin receptor blockers (%) | 61 | 50 | 0.738 |
| Antiarrhythmics (%) | 28 | 11 | 0.402 |
| Mineralocorticoid receptor antagonists (%) | 44 | 11 | 0.060 |
| Diuretics (%) | 89 | 28 | <0.001 |
| Inotropes (%) | 11 | 0 | 0.486 |
| Procedure type | |||
| Mitral valve plasty (%) | 56 | 94 | 0.018 |
| Tricuspid valve plasty (%) | 33 | 50 | 0.500 |
| Surgical left ventricular restoration (%) | 39 | 0 | 0.008 |
| Left ventricular assist device implantation (%) | 22 | 0 | 0.104 |
| Coronary artery bypass grafting (%) | 39 | 6 | 0.041 |
| Aortic valve replacement (%) | 17 | 0 | 0.229 |
| Aorta surgery (%) | 11 | 6 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papazisi, O.; Postma, R.J.; Dirven, R.J.; Beeres, S.L.M.A.; Berendsen, R.R.; Arbous, S.M.; Klautz, R.J.M.; van Vessem, M.E.; Bijkerk, R.; Lindeman, J.H.N.; et al. Heart Failure Impacts Endothelial Cell Responses to Cardiac Surgery on Cardiopulmonary Bypass. Cells 2025, 14, 1357. https://doi.org/10.3390/cells14171357
Papazisi O, Postma RJ, Dirven RJ, Beeres SLMA, Berendsen RR, Arbous SM, Klautz RJM, van Vessem ME, Bijkerk R, Lindeman JHN, et al. Heart Failure Impacts Endothelial Cell Responses to Cardiac Surgery on Cardiopulmonary Bypass. Cells. 2025; 14(17):1357. https://doi.org/10.3390/cells14171357
Chicago/Turabian StylePapazisi, Olga, Rudmer J. Postma, Richard J. Dirven, Saskia L. M. A. Beeres, Remco R. Berendsen, Sesmu M. Arbous, Robert J. M. Klautz, Marieke E. van Vessem, Roel Bijkerk, Jan H. N. Lindeman, and et al. 2025. "Heart Failure Impacts Endothelial Cell Responses to Cardiac Surgery on Cardiopulmonary Bypass" Cells 14, no. 17: 1357. https://doi.org/10.3390/cells14171357
APA StylePapazisi, O., Postma, R. J., Dirven, R. J., Beeres, S. L. M. A., Berendsen, R. R., Arbous, S. M., Klautz, R. J. M., van Vessem, M. E., Bijkerk, R., Lindeman, J. H. N., Palmen, M., & van Zonneveld, A. J. (2025). Heart Failure Impacts Endothelial Cell Responses to Cardiac Surgery on Cardiopulmonary Bypass. Cells, 14(17), 1357. https://doi.org/10.3390/cells14171357







