Pathologic and Therapeutic Schwann Cells
Abstract
1. Introduction
2. Schwann Cells in CNS Pathology
2.1. Schwann Cells for Treating Central Neurotrauma
2.2. Schwann Cells for Treating Non-Traumatic CNS Diseases
2.2.1. Multiple Sclerosis and Schwann Cells
2.2.2. Parkinson’s Disease and Schwann Cells
2.2.3. Alzheimer’s Disease and Schwann Cells
2.2.4. Huntington’s Disease
2.2.5. Leukodystrophies
2.2.6. Schwann Cells in Amyotrophic Lateral Sclerosis
3. Schwann Cells in PNS Pathology
3.1. Schwann Cells for Treating PNS Injury
3.1.1. Combinational Schwann Cells Transplantation for Treating PNS Injury
3.1.2. Modified Schwann Cells for Treating PNS Injury
3.2. Schwann Cells as Targets and Tools for Non-Traumatic Peripheral Neuropathy
Schwann Cells in Diabetic Neuropathy
3.3. Schwann Cells in Immune-Mediated Neuropathies
3.3.1. Schwann Cells in Guillain–Barré Syndrome
3.3.2. Schwann Cells in Chronic Inflammatory Neuropathies
3.3.3. Monoclonal Gammopathy-Associated Peripheral Neuropathies
3.4. Schwann Cells in Infection-Induced Demyelination and Neuropathy of the PNS
3.4.1. Schwann Cells in Infection-Associated Neuropathies of the PNS
3.4.2. Leprosy and Schwann Cells
3.5. Schwann Cells in Inherited Peripheral Neuropathies
3.5.1. Schwann Cells in Charcot–Marie–Tooth Disease
3.5.2. Schwann Cells and Hereditary Neuropathy with Liability to Pressure Palsies (HNPP)
3.6. Schwann Cells and Other Types of Peripheral Neuropathy
4. Peripheral Neuropathic Pain and Schwann Cells
5. Schwann Cells as a Therapeutic Target for Peripheral Neuropathies
6. Gene Therapy to Target and Modify Schwann Cells
6.1. Gene Therapeutic Targeting of Schwann Cells in CMT Disease
6.2. Genetic Engineering of Schwann Cells for Nerve Injury Treatment
6.3. Gene Therapeutic Targeting of Tumorigenic Schwann Cells
7. Schwann Cell-Derived Factors for Therapeutic Use
7.1. Schwann Cell Extracellular Vesicles and microRNA for Therapeutic Applications
7.2. Schwann Cell-Derived Extracellular Matrix Proteins and Factors
8. Schwann Cells in Wound Healing
9. Schwann Cells in Cancer
9.1. Schwann Cells in Tumorigenesis
9.2. Schwann Cell Alterations and Reprogramming in Cancer
9.3. Schwann Cells in Peripheral Neuropathies in Cancer Patients
9.3.1. Schwann Cells in Chemotherapy-Induced Peripheral Neuropathy
9.3.2. Paraneoplastic Neuropathies
9.4. Targeting Schwann Cells in Cancer
10. Tumorigenic Schwann Cells
10.1. Neurofibromatosis
10.2. Schwannomas
10.2.1. Schwannomas of Cranial Nerves and Spinal Cord
10.2.2. Peripheral Schwannomas
10.3. Schwannomatosis
10.4. Schwannosis
10.5. Transmissible Schwann Cell Cancers
10.6. Other Schwann Cell Tumors
11. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| AAV | adeno-associated virus/viral (vector) |
| AIDP | acute inflammatory demyelinating polyneuropathy |
| ALS | amyotrophic lateral sclerosis |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| ARAT | axon regeneration-associated transcript |
| BDNF | brain-derived neurotrophic factor |
| BMP5 | bone morphogenetic protein 5 |
| CAF(s) | cancer-associated fibroblast(s) |
| cAMP | cyclic adenosine 3′,5′-monophosphate |
| CB2 | cannabinoid receptor 2 |
| CGRP | calcitonin gene-related peptide |
| CIDP | chronic inflammatory demyelinating polyneuropathy |
| CMT | Charcot–Marie–Tooth Disease |
| CMV | cytomegalovirus |
| CNS | central nervous system |
| CNTF | ciliary neurotrophic factor |
| CSF | cerebrospinal fluid |
| CSF1 | colony-stimulating factor 1 |
| CSPG | chondroitin sulfate proteoglycan |
| DFT1 | Devil Facial Tumor 1 |
| DFT2 | Devil Facial Tumor 2 |
| DFTD | Devil facial tumor disease |
| DRG | dorsal root ganglion |
| EAE | experimental autoimmune encephalomyelitis |
| EBV | Epstein–Barr virus |
| ECM | extracellular matrix |
| EMT | epithelial–mesenchymal transition |
| ER | endoplasmic reticulum |
| ESC(s) | embryonic stem cell(s) |
| EV | extracellular vesicles |
| FGF | fibroblast growth factor |
| FOXM1 | Forkhead box M1 |
| GABA | gamma-aminobutyric acid |
| GBS | Guillain–Barré syndrome |
| GDNF | glial cell-derived neurotrophic factor |
| GFAP | glial fibrillary acidic protein |
| GLP-1 | glucagon-like peptide-1 |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| HDAC | histone deacetylases |
| HIF1α | hypoxia-inducible factor 1-alpha |
| HIV | human immunodeficiency virus |
| HNPP | hereditary neuropathy with liability to pressure palsies |
| HSC(s) | hematopoietic stem cell(s) |
| HSV | herpes simplex virus |
| IGFBP(s) | insulin-like growth factor binding protein(s) |
| iNOS | inducible nitric oxide synthase |
| iPSC(s) | induced pluripotent stem cell(s) |
| LFA-3 | lymphocyte function-associated antigen 3 |
| lncARAT | lncRNA axon regeneration-associated transcript |
| lncRNA | long non-coding RNA |
| LRP1 | low-density lipoprotein receptor-related protein 1 |
| LRP4 | low-density lipoprotein receptor-related protein 4 |
| mAChR | muscarinic acetylcholine receptor |
| MAG | myelin-associated glycoprotein |
| MALAT1 | metastasis-associated lung adenocarcinoma transcript 1 |
| MBP | myelin basic protein |
| MFG-E8 | milk fat globule-epidermal growth factor 8 |
| MGUS | monoclonal gammopathy of unknown significance |
| miRNA | microRNA |
| MMP | matrix metalloproteinase |
| MPZ | myelin protein zero |
| MSC(s) | mesenchymal stem cell(s) |
| MWCNT | multi-walled carbon nanotube |
| NCAM | neural cell adhesion molecule |
| NF1 | neurofibromatosis type 1 |
| NGF | nerve growth factor |
| NGFR | nerve growth factor receptor |
| NK1 | neurokinin 1 |
| NLR(s) | NOD-like receptor(s) |
| NMDA | N-methyl-D-aspartic acid |
| NO | nitric oxide |
| NOD | nucleotide-binding and oligomerization domain |
| NOS | nitric oxide synthase |
| NRG1 | neuregulin-1 |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| NSC(s) | neural stem cell(s) |
| NSCLC | non-small cell lung cancer |
| NT | neurotrophin |
| OPC(s) | oligodendrocyte progenitor cell(s) |
| P0 | myelin protein 0 |
| P2 | myelin protein 2 |
| PAK | p21-activated kinase |
| PLGA | high-molecular-weight copolymer of lactic and glycolic acid |
| PLLA | poly-L-lactic acid |
| PMP22 | peripheral myelin protein 22 |
| PNS | peripheral nervous system |
| POU6F1 | POU domain class 6 Homeobox 1 |
| PST | polysialyltransferase |
| PTEN | phosphatase and tensin homolog (pathway) |
| ROS | reactive oxygen species |
| SC(s) | Schwann cell(s) |
| SCF | stem cell factor |
| SCLC | small cell lung cancer |
| SCP(s) | Schwann cell precursor(s) |
| SDF1 | stromal cell-derived factor 1 |
| shRNA | small hairpin inhibitory RNA |
| SOCS-2 | suppressor of cytokine signaling |
| SOD1 | superoxide dismutase |
| SP | substance P |
| TAM | tumor-associated macrophage |
| TGF-β | transforming growth factor-β |
| TLR(s) | Toll-like receptor(s) |
| TNF-α | tumor necrosis factor-alpha |
| TREM2 | triggering receptor expressed on myeloid cells 2 |
| TrkB | tropomyosin receptor kinase B |
| TRPM7 | transient receptor potential melastatin M7 |
| TRPV1 | transient receptor potential cation channel subfamily V member 1 |
| TRPV4 | transient receptor potential cation channel subfamily V member 4 |
| VEGF | vascular endothelial growth factor |
| VZV | varicella zoster virus |
References
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef]
- Bosch-Queralt, M.; Fledrich, R.; Stassart, R.M. Schwann cell functions in peripheral nerve development and repair. Neurobiol. Dis. 2023, 176, 105952. [Google Scholar] [CrossRef] [PubMed]
- Whalley, K. Glia: Schwann cells provide life support for axons. Nat. Rev. Neurosci. 2014, 15, 698–699. [Google Scholar] [CrossRef]
- Li, J. Molecular regulators of nerve conduction—Lessons from inherited neuropathies and rodent genetic models. Exp. Neurol. 2015, 267, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Arcilla, C.K.; Tadi, P. Neuroanatomy, Unmyelinated Nerve Fibers. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Griffin, J.W.; Thompson, W.J. Biology and pathology of nonmyelinating Schwann cells. Glia 2008, 56, 1518–1531. [Google Scholar] [CrossRef]
- Ojeda-Alonso, J.; Calvo-Enrique, L.; Paricio-Montesinos, R.; Kumar, R.; Zhang, M.D.; Poulet, J.F.A.; Ernfors, P.; Lewin, G.R. Sensory Schwann cells set perceptual thresholds for touch and selectively regulate mechanical nociception. Nat. Commun. 2024, 15, 898. [Google Scholar] [CrossRef]
- Raut, N.G.; Maile, L.A.; Oswalt, L.M.; Mitxelena, I.; Adlakha, A.; Sprague, K.L.; Rupert, A.R.; Bokros, L.; Hofmann, M.C.; Patritti-Cram, J.; et al. Schwann cells modulate nociception in neurofibromatosis 1. JCI Insight 2024, 9, e171275. [Google Scholar] [CrossRef]
- Wei, Z.; Fei, Y.; Su, W.; Chen, G. Emerging Role of Schwann Cells in Neuropathic Pain: Receptors, Glial Mediators and Myelination. Front. Cell. Neurosci. 2019, 13, 116. [Google Scholar] [CrossRef]
- Abdo, H.; Calvo-Enrique, L.; Lopez, J.M.; Song, J.; Zhang, M.D.; Usoskin, D.; El Manira, A.; Adameyko, I.; Hjerling-Leffler, J.; Ernfors, P. Specialized cutaneous Schwann cells initiate pain sensation. Science 2019, 365, 695–699. [Google Scholar] [CrossRef]
- Octavian, I.; Emilia, M.; Mihaela, G.; Bogdan, O.P.; Laura Cristina, C. Non-Myelinating Schwann Cells in Health and Disease. In Demyelination Disorders; Stavros, J.B., Fabian, H.R., Welwin, L., Eds.; IntechOpen: Rijeka, Croatia, 2020; Chapter 4. [Google Scholar] [CrossRef]
- Tzekova, N.; Heinen, A.; Küry, P. Molecules involved in the crosstalk between immune- and peripheral nerve Schwann cells. J. Clin. Immunol. 2014, 34 (Suppl. S1), S86–S104. [Google Scholar] [CrossRef]
- Meyer Zu Horste, G.; Heidenreich, H.; Lehmann, H.C.; Ferrone, S.; Hartung, H.P.; Wiendl, H.; Kieseier, B.C. Expression of antigen processing and presenting molecules by Schwann cells in inflammatory neuropathies. Glia 2010, 58, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Nicholls, P.K.; Yin, C.; Kelman, K.; Yuan, Q.; Greene, W.K.; Shi, Z.; Ma, B. Immunofluorescent Localization of Non-myelinating Schwann Cells and Their Interactions with Immune Cells in Mouse Thymus. J. Histochem. Cytochem. 2018, 66, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Yin, C.; Hu, D.; Newman, M.; Nicholls, P.K.; Wu, Z.; Greene, W.K.; Shi, Z. Distribution of non-myelinating Schwann cells and their associations with leukocytes in mouse spleen revealed by immunofluorescence staining. Eur. J. Histochem. 2018, 62, 2890. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wen, X.; Kuang, R.; Lui, K.W.; He, B.; Li, G.; Zhu, Z. Roles of Macrophages and Their Interactions with Schwann Cells After Peripheral Nerve Injury. Cell. Mol. Neurobiol. 2023, 44, 11. [Google Scholar] [CrossRef]
- Berner, J.; Weiss, T.; Sorger, H.; Rifatbegovic, F.; Kauer, M.; Windhager, R.; Dohnal, A.; Ambros, P.F.; Ambros, I.M.; Boztug, K.; et al. Human repair-related Schwann cells adopt functions of antigen-presenting cells in vitro. Glia 2022, 70, 2361–2377. [Google Scholar] [CrossRef]
- Yamazaki, S.; Ema, H.; Karlsson, G.; Yamaguchi, T.; Miyoshi, H.; Shioda, S.; Taketo, M.M.; Karlsson, S.; Iwama, A.; Nakauchi, H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 2011, 147, 1146–1158. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Fornasari, B.E.; Zen, F.; Nato, G.; Fogli, M.; Luzzati, F.; Ronchi, G.; Raimondo, S.; Gambarotta, G. Blood Vessels: The Pathway Used by Schwann Cells to Colonize Nerve Conduits. Int. J. Mol. Sci. 2022, 23, 2254. [Google Scholar] [CrossRef]
- Trimarco, A.; Taveggia, C. Schwann cell energy to die for. Nat. Neurosci. 2020, 23, 1179–1181. [Google Scholar] [CrossRef]
- Qu, W.R.; Zhu, Z.; Liu, J.; Song, D.B.; Tian, H.; Chen, B.P.; Li, R.; Deng, L.X. Interaction between Schwann cells and other cells during repair of peripheral nerve injury. Neural Regen. Res. 2021, 16, 93–98. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Carty, L.; Iruarrizaga-Lejarreta, M.; Palomo-Irigoyen, M.; Varela-Rey, M.; Griffith, M.; Hantke, J.; Macias-Camara, N.; Azkargorta, M.; Aurrekoetxea, I.; et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J. Cell Biol. 2015, 210, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Stratton, J.A.; Holmes, A.; Rosin, N.L.; Sinha, S.; Vohra, M.; Burma, N.E.; Trang, T.; Midha, R.; Biernaskie, J. Macrophages Regulate Schwann Cell Maturation after Nerve Injury. Cell Rep. 2018, 24, 2561–2572.E6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Z.; Lin, H. Research progress on the reduced neural repair ability of aging Schwann cells. Front. Cell Neurosci. 2023, 17, 1228282. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.J.; Johnston, A.P.W. Schwann cells as drivers of tissue repair and regeneration. Curr. Opin. Neurobiol. 2017, 47, 52–57. [Google Scholar] [CrossRef]
- Silva, W.N.; Leonel, C.; Prazeres, P.; Sena, I.F.G.; Guerra, D.A.P.; Heller, D.; Diniz, I.M.A.; Fortuna, V.; Mintz, A.; Birbrair, A. Role of Schwann cells in cutaneous wound healing. Wound Repair. Regen. 2018, 26, 392–397. [Google Scholar] [CrossRef]
- Van Raamsdonk, C.D.; Deo, M. Links between Schwann cells and melanocytes in development and disease. Pigment. Cell Melanoma Res. 2013, 26, 634–645. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, Q.; Lin, W.; Wang, Q.; Zhang, D.; Xu, R.; Zhou, X.; Zhang, S.; Peng, L.; Yuan, Q. Schwann Cells Contribute to Alveolar Bone Regeneration by Promoting Cell Proliferation. J. Bone Miner. Res. 2023, 38, 119–130. [Google Scholar] [CrossRef]
- Jessen, K.R.; Arthur-Farraj, P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 2019, 67, 421–437. [Google Scholar] [CrossRef]
- Hertzog, N.; Jacob, C. Mechanisms and treatment strategies of demyelinating and dysmyelinating Charcot-Marie-Tooth disease. Neural Regen. Res. 2023, 18, 1931–1939. [Google Scholar] [CrossRef]
- Li, J.; Guan, R.; Pan, L. Mechanism of Schwann cells in diabetic peripheral neuropathy: A review. Medicine 2023, 102, e32653. [Google Scholar] [CrossRef]
- Park, H.T.; Kim, J.K.; Tricaud, N. The conceptual introduction of the “demyelinating Schwann cell” in peripheral demyelinating neuropathies. Glia 2019, 67, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Park, H.T.; Kim, Y.H.; Lee, K.E.; Kim, J.K. Behind the pathology of macrophage-associated demyelination in inflammatory neuropathies: Demyelinating Schwann cells. Cell Mol. Life Sci. 2020, 77, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.M.; Babetto, E.; Beirowski, B. Axon degeneration: Make the Schwann cell great again. Neural Regen. Res. 2017, 12, 518–524. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Wang, H.; Huo, R.; Jiang, S.-H.; Xue, J. Schwann cells and enteric glial cells: Emerging stars in colorectal cancer. Biochim. Biophys. Acta (BBA) Rev. Cancer 2024, 1879, 189160. [Google Scholar] [CrossRef]
- Lavdas, A.A.; Papastefanaki, F.; Thomaidou, D.; Matsas, R. Schwann cell transplantation for CNS repair. Curr. Med. Chem. 2008, 15, 151–160. [Google Scholar] [CrossRef]
- Kanno, H.; Pearse, D.D.; Ozawa, H.; Itoi, E.; Bunge, M.B. Schwann cell transplantation for spinal cord injury repair: Its significant therapeutic potential and prospectus. Rev. Neurosci. 2015, 26, 121–128. [Google Scholar] [CrossRef]
- Monje, P.V. Schwann Cell Cultures: Biology, Technology and Therapeutics. Cells 2020, 9, 1848. [Google Scholar] [CrossRef]
- Fu, H.; Hu, D.; Chen, J.; Wang, Q.; Zhang, Y.; Qi, C.; Yu, T. Repair of the Injured Spinal Cord by Schwann Cell Transplantation. Front. Neurosci. 2022, 16, 800513. [Google Scholar] [CrossRef]
- Duncan, I.D.; Aguayo, A.J.; Bunge, R.P.; Wood, P.M. Transplantation of rat Schwann cells grown in tissue culture into the mouse spinal cord. J. Neurol. Sci. 1981, 49, 241–252. [Google Scholar] [CrossRef]
- Berry, M.; Hall, S.; Follows, R.; Rees, L.; Gregson, N.; Sievers, J. Response of axons and glia at the site of anastomosis between the optic nerve and cellular or acellular sciatic nerve grafts. J. Neurocytol. 1988, 17, 727–744. [Google Scholar] [CrossRef]
- Duncan, I.D.; Hoffman, R.L. Schwann cell invasion of the central nervous system of the myelin mutants. J. Anat. 1997, 190 Pt 1, 35–49. [Google Scholar] [CrossRef]
- Guest, J.D.; Hiester, E.D.; Bunge, R.P. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp. Neurol. 2005, 192, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Sardella-Silva, G.; Mietto, B.S.; Ribeiro-Resende, V.T. Four Seasons for Schwann Cell Biology, Revisiting Key Periods: Development, Homeostasis, Repair, and Aging. Biomolecules 2021, 11, 1887. [Google Scholar] [CrossRef] [PubMed]
- Jasmin, L.; Janni, G.; Moallem, T.M.; Lappi, D.A.; Ohara, P.T. Schwann cells are removed from the spinal cord after effecting recovery from paraplegia. J. Neurosci. 2000, 20, 9215–9223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Huang, F.; Gates, M.; Holmberg, E.G. Role of endogenous Schwann cells in tissue repair after spinal cord injury. Neural Regen. Res. 2013, 8, 177–185. [Google Scholar] [CrossRef]
- Garcia-Diaz, B.; Bachelin, C.; Coulpier, F.; Gerschenfeld, G.; Deboux, C.; Zujovic, V.; Charnay, P.; Topilko, P.; Baron-Van Evercooren, A. Blood vessels guide Schwann cell migration in the adult demyelinated CNS through Eph/ephrin signaling. Acta Neuropathol. 2019, 138, 457–476. [Google Scholar] [CrossRef]
- Zawadzka, M.; Rivers, L.E.; Fancy, S.P.J.; Zhao, C.; Tripathi, R.; Jamen, F.; Young, K.; Goncharevich, A.; Pohl, H.; Rizzi, M.; et al. CNS-Resident Glial Progenitor/Stem Cells Produce Schwann Cells as well as Oligodendrocytes during Repair of CNS Demyelination. Cell Stem Cell 2010, 6, 578–590. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Plemel, J.R.; Lee, M.J.; Stratton, J.A.; Manesh, S.B.; Liu, J.; Ramer, L.M.; Kang, S.H.; Bergles, D.E.; et al. Myelinogenic Plasticity of Oligodendrocyte Precursor Cells following Spinal Cord Contusion Injury. J. Neurosci. 2017, 37, 8635–8654. [Google Scholar] [CrossRef]
- Chen, C.Z.; Neumann, B.; Förster, S.; Franklin, R.J.M. Schwann cell remyelination of the central nervous system: Why does it happen and what are the benefits? Open Biol. 2021, 11, 200352. [Google Scholar] [CrossRef]
- Pearse, D.D.; Sanchez, A.R.; Pereira, F.C.; Andrade, C.M.; Puzis, R.; Pressman, Y.; Golden, K.; Kitay, B.M.; Blits, B.; Wood, P.M.; et al. Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: Survival, migration, axon association, and functional recovery. Glia 2007, 55, 976–1000. [Google Scholar] [CrossRef]
- Maffei, L.; Carmignoto, G.; Perry, V.H.; Candeo, P.; Ferrari, G. Schwann cells promote the survival of rat retinal ganglion cells after optic nerve section. Proc. Natl. Acad. Sci. USA 1990, 87, 1855–1859. [Google Scholar] [CrossRef]
- Strauch, B.; Rodriguez, D.M.; Diaz, J.; Yu, H.L.; Kaplan, G.; Weinstein, D.E. Autologous Schwann cells drive regeneration through a 6-cm autogenous venous nerve conduit. J. Reconstr. Microsurg. 2001, 17, 589–595; discussion 596–597. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Raisman, G. Transplanted Schwann cells, not olfactory ensheathing cells, myelinate optic nerve fibres. Glia 2007, 55, 312–316. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, Y.; Zhang, W.; Wang, J.; Wu, J.; Li, J. Co-transplantation of neural stem cells and NT-3-overexpressing Schwann cells in transected spinal cord. J. Neurotrauma 2007, 24, 1863–1877. [Google Scholar] [CrossRef] [PubMed]
- Pearse, D.D.; Pereira, F.C.; Marcillo, A.E.; Bates, M.L.; Berrocal, Y.A.; Filbin, M.T.; Bunge, M.B. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004, 10, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, X.M. Long-term survival, axonal growth-promotion, and myelination of Schwann cells grafted into contused spinal cord in adult rats. Exp. Neurol. 2014, 261, 308–319. [Google Scholar] [CrossRef]
- Deng, L.X.; Walker, C.; Xu, X.M. Schwann cell transplantation and descending propriospinal regeneration after spinal cord injury. Brain Res. 2015, 1619, 104–114. [Google Scholar] [CrossRef]
- Sparling, J.S.; Bretzner, F.; Biernaskie, J.; Assinck, P.; Jiang, Y.; Arisato, H.; Plunet, W.T.; Borisoff, J.; Liu, J.; Miller, F.D.; et al. Schwann cells generated from neonatal skin-derived precursors or neonatal peripheral nerve improve functional recovery after acute transplantation into the partially injured cervical spinal cord of the rat. J. Neurosci. 2015, 35, 6714–6730. [Google Scholar] [CrossRef]
- Assinck, P.; Sparling, J.S.; Dworski, S.; Duncan, G.J.; Wu, D.L.; Liu, J.; Kwon, B.K.; Biernaskie, J.; Miller, F.D.; Tetzlaff, W. Transplantation of Skin Precursor-Derived Schwann Cells Yields Better Locomotor Outcomes and Reduces Bladder Pathology in Rats with Chronic Spinal Cord Injury. Stem Cell Rep. 2020, 15, 140–155. [Google Scholar] [CrossRef]
- Wan, H.; Zhang, S.D.; Li, J.H. Action of Schwann cells implanted in cerebral hemorrhage lesion. Biomed. Environ. Sci. 2007, 20, 47–51. [Google Scholar]
- Schaal, S.M.; Kitay, B.M.; Cho, K.S.; Lo, T.P., Jr.; Barakat, D.J.; Marcillo, A.E.; Sanchez, A.R.; Andrade, C.M.; Pearse, D.D. Schwann cell transplantation improves reticulospinal axon growth and forelimb strength after severe cervical spinal cord contusion. Cell Transplant. 2007, 16, 207–228. [Google Scholar] [CrossRef]
- Pearse, D.D.; Bastidas, J.; Izabel, S.S.; Ghosh, M. Schwann Cell Transplantation Subdues the Pro-Inflammatory Innate Immune Cell Response after Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 2550. [Google Scholar] [CrossRef]
- Mousavi, M.; Hedayatpour, A.; Mortezaee, K.; Mohamadi, Y.; Abolhassani, F.; Hassanzadeh, G. Schwann cell transplantation exerts neuroprotective roles in rat model of spinal cord injury by combating inflammasome activation and improving motor recovery and remyelination. Metab. Brain Dis. 2019, 34, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, J.; Athauda, G.; De La Cruz, G.; Chan, W.M.; Golshani, R.; Berrocal, Y.; Henao, M.; Lalwani, A.; Mannoji, C.; Assi, M.; et al. Human Schwann cells exhibit long-term cell survival, are not tumorigenic and promote repair when transplanted into the contused spinal cord. Glia 2017, 65, 1278–1301. [Google Scholar] [CrossRef] [PubMed]
- Saberi, H.; Moshayedi, P.; Aghayan, H.R.; Arjmand, B.; Hosseini, S.K.; Emami-Razavi, S.H.; Rahimi-Movaghar, V.; Raza, M.; Firouzi, M. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: An interim report on safety considerations and possible outcomes. Neurosci. Lett. 2008, 443, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.H.; Ning, G.Z.; Feng, S.Q.; Kong, X.H.; Chen, J.T.; Zheng, Y.F.; Ban, D.X.; Liu, T.; Li, H.; Wang, P. Transplantation of autologous activated Schwann cells in the treatment of spinal cord injury: Six cases, more than five years of follow-up. Cell Transplant. 2012, 21 (Suppl. S1), S39–S47. [Google Scholar] [CrossRef]
- Guest, J.; Santamaria, A.J.; Benavides, F.D. Clinical translation of autologous Schwann cell transplantation for the treatment of spinal cord injury. Curr. Opin. Organ. Transplant. 2013, 18, 682–689. [Google Scholar] [CrossRef]
- Chen, L.; Huang, H.; Xi, H.; Zhang, F.; Liu, Y.; Chen, D.; Xiao, J. A prospective randomized double-blind clinical trial using a combination of olfactory ensheathing cells and Schwann cells for the treatment of chronic complete spinal cord injuries. Cell Transplant. 2014, 23 (Suppl. S1), S35–S44. [Google Scholar] [CrossRef]
- Oraee-Yazdani, S.; Hafizi, M.; Atashi, A.; Ashrafi, F.; Seddighi, A.S.; Hashemi, S.M.; Seddighi, A.; Soleimani, M.; Zali, A. Co-transplantation of autologous bone marrow mesenchymal stem cells and Schwann cells through cerebral spinal fluid for the treatment of patients with chronic spinal cord injury: Safety and possible outcome. Spinal Cord. 2016, 54, 102–109. [Google Scholar] [CrossRef]
- Bunge, M.B.; Monje, P.V.; Khan, A.; Wood, P.M. From transplanting Schwann cells in experimental rat spinal cord injury to their transplantation into human injured spinal cord in clinical trials. Prog. Brain Res. 2017, 231, 107–133. [Google Scholar] [CrossRef]
- Monje, P.V.; Deng, L.; Xu, X.M. Human Schwann Cell Transplantation for Spinal Cord Injury: Prospects and Challenges in Translational Medicine. Front. Cell Neurosci. 2021, 15, 690894. [Google Scholar] [CrossRef]
- Anderson, K.D.; Guest, J.D.; Dietrich, W.D.; Bartlett Bunge, M.; Curiel, R.; Dididze, M.; Green, B.A.; Khan, A.; Pearse, D.D.; Saraf-Lavi, E.; et al. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J. Neurotrauma 2017, 34, 2950–2963. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.J.; Solano, J.P.; Benavides, F.D.; Guest, J.D. Intraspinal Delivery of Schwann Cells for Spinal Cord Injury. Methods Mol. Biol. 2018, 1739, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Gant, K.L.; Guest, J.D.; Palermo, A.E.; Vedantam, A.; Jimsheleishvili, G.; Bunge, M.B.; Brooks, A.E.; Anderson, K.D.; Thomas, C.K.; Santamaria, A.J.; et al. Phase 1 Safety Trial of Autologous Human Schwann Cell Transplantation in Chronic Spinal Cord Injury. J. Neurotrauma 2022, 39, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef]
- Iwashita, Y.; Blakemore, W.F. Areas of demyelination do not attract significant numbers of schwann cells transplanted into normal white matter. Glia 2000, 31, 232–240. [Google Scholar] [CrossRef]
- Iwashita, Y.; Fawcett, J.W.; Crang, A.J.; Franklin, R.J.; Blakemore, W.F. Schwann cells transplanted into normal and X-irradiated adult white matter do not migrate extensively and show poor long-term survival. Exp. Neurol. 2000, 164, 292–302. [Google Scholar] [CrossRef]
- Itoyama, Y.; Ohnishi, A.; Tateishi, J.; Kuroiwa, Y.; Webster, H.D. Spinal cord multiple sclerosis lesions in Japanese patients: Schwann cell remyelination occurs in areas that lack glial fibrillary acidic protein (GFAP). Acta Neuropathol. 1985, 65, 217–223. [Google Scholar] [CrossRef]
- Blakemore, W.F.; Crang, A.J.; Curtis, R. The interaction of Schwann cells with CNS axons in regions containing normal astrocytes. Acta Neuropathol. 1986, 71, 295–300. [Google Scholar] [CrossRef]
- Chaudhry, N.; Bachelin, C.; Zujovic, V.; Hilaire, M.; Baldwin, K.T.; Follis, R.M.; Giger, R.; Carter, B.D.; Baron-Van Evercooren, A.; Filbin, M.T. Myelin-Associated Glycoprotein Inhibits Schwann Cell Migration and Induces Their Death. J. Neurosci. 2017, 37, 5885–5899. [Google Scholar] [CrossRef]
- Li, Q.; Ping, P.; Jiang, H.; Liu, K. Nerve conduit filled with GDNF gene-modified Schwann cells enhances regeneration of the peripheral nerve. Microsurgery 2006, 26, 116–121. [Google Scholar] [CrossRef]
- Deng, L.X.; Deng, P.; Ruan, Y.; Xu, Z.C.; Liu, N.K.; Wen, X.; Smith, G.M.; Xu, X.M. A novel growth-promoting pathway formed by GDNF-overexpressing Schwann cells promotes propriospinal axonal regeneration, synapse formation, and partial recovery of function after spinal cord injury. J. Neurosci. 2013, 33, 5655–5667. [Google Scholar] [CrossRef]
- Li, W.Y.; Li, Z.G.; Fu, X.M.; Wang, X.Y.; Lv, Z.X.; Sun, P.; Zhu, X.F.; Wang, Y. Transgenic Schwann cells overexpressing POU6F1 promote sciatic nerve regeneration within acellular nerve allografts. J. Neural Eng. 2022, 19, 066006. [Google Scholar] [CrossRef] [PubMed]
- Lavdas, A.A.; Franceschini, I.; Dubois-Dalcq, M.; Matsas, R. Schwann cells genetically engineered to express PSA show enhanced migratory potential without impairment of their myelinating ability in vitro. Glia 2006, 53, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Papastefanaki, F.; Chen, J.; Lavdas, A.A.; Thomaidou, D.; Schachner, M.; Matsas, R. Grafts of Schwann cells engineered to express PSA-NCAM promote functional recovery after spinal cord injury. Brain 2007, 130, 2159–2174. [Google Scholar] [CrossRef] [PubMed]
- Lavdas, A.A.; Chen, J.; Papastefanaki, F.; Chen, S.; Schachner, M.; Matsas, R.; Thomaidou, D. Schwann cells engineered to express the cell adhesion molecule L1 accelerate myelination and motor recovery after spinal cord injury. Exp. Neurol. 2010, 221, 206–216. [Google Scholar] [CrossRef]
- Oudega, M.; Xu, X.M. Schwann cell transplantation for repair of the adult spinal cord. J. Neurotrauma 2006, 23, 453–467. [Google Scholar] [CrossRef]
- Bunge, M.B. Novel combination strategies to repair the injured mammalian spinal cord. J. Spinal Cord. Med. 2008, 31, 262–269. [Google Scholar] [CrossRef]
- Bunge, M.B.; Wood, P.M. Realizing the maximum potential of Schwann cells to promote recovery from spinal cord injury. Handb. Clin. Neurol. 2012, 109, 523–540. [Google Scholar] [CrossRef]
- Bunge, M.B. Efficacy of Schwann cell transplantation for spinal cord repair is improved with combinatorial strategies. J. Physiol. 2016, 594, 3533–3538. [Google Scholar] [CrossRef]
- Golden, K.L.; Pearse, D.D.; Blits, B.; Garg, M.S.; Oudega, M.; Wood, P.M.; Bunge, M.B. Transduced Schwann cells promote axon growth and myelination after spinal cord injury. Exp. Neurol. 2007, 207, 203–217. [Google Scholar] [CrossRef]
- Kanno, H.; Pressman, Y.; Moody, A.; Berg, R.; Muir, E.M.; Rogers, J.H.; Ozawa, H.; Itoi, E.; Pearse, D.D.; Bunge, M.B. Combination of engineered Schwann cell grafts to secrete neurotrophin and chondroitinase promotes axonal regeneration and locomotion after spinal cord injury. J. Neurosci. 2014, 34, 1838–1855. [Google Scholar] [CrossRef]
- Lee, Y.S.; Wu, S.; Arinzeh, T.L.; Bunge, M.B. Enhanced noradrenergic axon regeneration into schwann cell-filled PVDF-TrFE conduits after complete spinal cord transection. Biotechnol. Bioeng. 2017, 114, 444–456. [Google Scholar] [CrossRef]
- Tan, C.; Yang, C.; Liu, H.; Tang, C.; Huang, S. Effect of Schwann cell transplantation combined with electroacupuncture on axonal regeneration and remyelination in rats with spinal cord injury. Anat. Rec. 2021, 304, 2506–2520. [Google Scholar] [CrossRef]
- Tan, C.F.; Huang, S.Q.; Tang, C.L.; Zhang, A.N.; Zhao, D.D.; Wu, M.J.; An, H.Y.; Qiu, L.; Dai, N.; Dai, P. Effect of electroacupuncture combined with Schwann cell transplantation on limb locomotor ability, regional remyelination and expression of spinal CD4 and CD8 proteins in compressive spinal injury rats. Zhen Ci Yan Jiu 2019, 44, 391–398. [Google Scholar] [CrossRef]
- Bernardo, A.; Visentin, S. Demyelinating Diseases: From Molecular Mechanisms to Therapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 4596. [Google Scholar] [CrossRef]
- Franklin, R.J.M.; Bodini, B.; Goldman, S.A. Remyelination in the Central Nervous System. Cold Spring Harb. Perspect. Biol. 2024, 16, a041371. [Google Scholar] [CrossRef] [PubMed]
- Cayre, M.; Falque, M.; Mercier, O.; Magalon, K.; Durbec, P. Myelin Repair: From Animal Models to Humans. Front. Cell Neurosci. 2021, 15, 604865. [Google Scholar] [CrossRef]
- Kegler, K.; Spitzbarth, I.; Imbschweiler, I.; Wewetzer, K.; Baumgärtner, W.; Seehusen, F. Contribution of Schwann Cells to Remyelination in a Naturally Occurring Canine Model of CNS Neuroinflammation. PLoS ONE 2015, 10, e0133916. [Google Scholar] [CrossRef] [PubMed]
- Imbschweiler, I.; Seehusen, F.; Peck, C.T.; Omar, M.; Baumgärtner, W.; Wewetzer, K. Increased p75 neurotrophin receptor expression in the canine distemper virus model of multiple sclerosis identifies aldynoglial Schwann cells that emerge in response to axonal damage. Glia 2012, 60, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, W.F. The case for a central nervous system (CNS) origin for the Schwann cells that remyelinate CNS axons following concurrent loss of oligodendrocytes and astrocytes. Neuropathol. Appl. Neurobiol. 2005, 31, 1–10. [Google Scholar] [CrossRef]
- Brambilla, R. The contribution of astrocytes to the neuroinflammatory response in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. 2019, 137, 757–783. [Google Scholar] [CrossRef]
- Voet, S.; Prinz, M.; van Loo, G. Microglia in Central Nervous System Inflammation and Multiple Sclerosis Pathology. Trends Mol. Med. 2019, 25, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Bhagavati, S. Autoimmune Disorders of the Nervous System: Pathophysiology, Clinical Features, and Therapy. Front. Neurol. 2021, 12, 664664. [Google Scholar] [CrossRef] [PubMed]
- Hart, B.A.; Hintzen, R.Q.; Laman, J.D. Multiple sclerosis—A response-to-damage model. Trends Mol. Med. 2009, 15, 235–244. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Lemprière, S. Epstein–Barr virus and MS—A causal link. Nat. Rev. Neurol. 2022, 18, 128. [Google Scholar] [CrossRef]
- Ward, M.; Goldman, M.D. Epidemiology and Pathophysiology of Multiple Sclerosis. Continuum 2022, 28, 988–1005. [Google Scholar] [CrossRef]
- Prineas, J.W.; Connell, F. Remyelination in multiple sclerosis. Ann. Neurol. 1979, 5, 22–31. [Google Scholar] [CrossRef]
- Prineas, J.W.; Kwon, E.E.; Cho, E.S.; Sharer, L.R. Continual breakdown and regeneration of myelin in progressive multiple sclerosis plaques. Ann. N. Y. Acad. Sci. 1984, 436, 11–32. [Google Scholar] [CrossRef]
- Bodini, B.; Veronese, M.; García-Lorenzo, D.; Battaglini, M.; Poirion, E.; Chardain, A.; Freeman, L.; Louapre, C.; Tchikviladze, M.; Papeix, C.; et al. Dynamic Imaging of Individual Remyelination Profiles in Multiple Sclerosis. Ann. Neurol. 2016, 79, 726–738. [Google Scholar] [CrossRef]
- Momenzadeh, S.; Jami, M.S. Remyelination in PNS and CNS: Current and upcoming cellular and molecular strategies to treat disabling neuropathies. Mol. Biol. Rep. 2021, 48, 8097–8110. [Google Scholar] [CrossRef]
- Li, H.; Lian, G.; Wang, G.; Yin, Q.; Su, Z. A review of possible therapies for multiple sclerosis. Mol. Cell. Biochem. 2021, 476, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Itoyama, Y.; Webster, H.D.; Richardson, E.P., Jr.; Trapp, B.D. Schwann cell remyelination of demyelinated axons in spinal cord multiple sclerosis lesions. Ann. Neurol. 1983, 14, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kawamura, J.; Hashimoto, S.; Nakamura, M. Extensive proliferation of peripheral type myelin in necrotic spinal cord lesions of multiple sclerosis. J. Neurol. Sci. 1991, 102, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, L.; Bollman, B.; De Feo, L.; Piccio, L.; Trapp, B.D.; Schmidt, R.E.; Cross, A.H. Schwann Cell Remyelination in the Multiple Sclerosis Central Nervous System. Lab. Investig. 2023, 103, 100128. [Google Scholar] [CrossRef]
- Ikota, H.; Iwasaki, A.; Kawarai, M.; Nakazato, Y. Neuromyelitis optica with intraspinal expansion of Schwann cell remyelination. Neuropathology 2010, 30, 427–433. [Google Scholar] [CrossRef]
- Plemel, J.R.; Liu, W.Q.; Yong, V.W. Remyelination therapies: A new direction and challenge in multiple sclerosis. Nat. Rev. Drug Discov. 2017, 16, 617–634. [Google Scholar] [CrossRef]
- Li, J.; Chen, W.; Li, Y.; Chen, Y.; Ding, Z.; Yang, D.; Zhang, X. Transplantation of olfactory ensheathing cells promotes partial recovery in rats with experimental autoimmune encephalomyelitis. Int. J. Clin. Exp. Pathol. 2015, 8, 11149–11156. [Google Scholar]
- Alamouti, M.A.; Bakhtiyari, M.; Moradi, F.; Mokhtari, T.; Hedayatpour, A.; Zafari, F.; Barbarestani, M. Remyelination of the corpus callosum by olfactory ensheathing cell in an experimental model of multiple sclerosis. Acta Medica Iran. 2015, 53, 533–539. [Google Scholar]
- Zujovic, V.; Doucerain, C.; Hidalgo, A.; Bachelin, C.; Lachapelle, F.; Weissert, R.; Stadelmann, C.; Linington, C.; Baron-Van Evercooren, A. Exogenous schwann cells migrate, remyelinate and promote clinical recovery in experimental auto-immune encephalomyelitis. PLoS ONE 2012, 7, e42667. [Google Scholar] [CrossRef]
- Raine, C.S.; Traugott, U.; Stone, S.H. Chronic relapsing experimental allergic encephalomyelitis: CNS plaque development in unsuppressed and suppressed animals. Acta Neuropathol. 1978, 43, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Lehmann-Horn, K.; Shen, Y.A.; Rankin, K.A.; Stebbins, K.J.; Lorrain, D.S.; Pekarek, K.; Sagan, S.A.; Xiao, L.; Teuscher, C.; et al. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. Elife 2016, 5, e18246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kyauk, R.V.; Shen, Y.A.; Xie, L.; Reichelt, M.; Lin, H.; Jiang, Z.; Ngu, H.; Shen, K.; Greene, J.J.; et al. TREM2-dependent microglial function is essential for remyelination and subsequent neuroprotection. Glia 2023, 71, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Cignarella, F.; Filipello, F.; Bollman, B.; Cantoni, C.; Locca, A.; Mikesell, R.; Manis, M.; Ibrahim, A.; Deng, L.; Benitez, B.A.; et al. TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathol. 2020, 140, 513–534. [Google Scholar] [CrossRef]
- Zhang, N.; Ji, Q.; Chen, Y.; Wen, X.; Shan, F. TREM2 deficiency impairs the energy metabolism of Schwann cells and exacerbates peripheral neurological deficits. Cell Death Dis. 2024, 15, 193. [Google Scholar] [CrossRef]
- Li, Z.Q.; Li, T.X.; Tian, M.; Ren, Z.S.; Yuan, C.Y.; Yang, R.K.; Shi, S.J.; Li, H.; Kou, Z.Z. Glial cells and neurologic autoimmune disorders. Front. Cell Neurosci. 2022, 16, 1028653. [Google Scholar] [CrossRef]
- Xie, H.; Shao, Y.; Du, J.; Song, Y.; Li, Y.; Duan, R.; Yao, Y.; Gong, Z.; Teng, J.; Jia, Y. Comparative analysis of clinical and imaging data between patients with myelin oligodendrocyte glycoprotein antibody disease and patients with aquaporin 4 antibody-positive neuromyelitis optica spectrum disorder. J. Neurol. 2022, 269, 1641–1650. [Google Scholar] [CrossRef]
- Garcia-Diaz, B.; Baron-Van Evercooren, A. Schwann cells: Rescuers of central demyelination. Glia 2020, 68, 1945–1956. [Google Scholar] [CrossRef]
- Dorszewska, J.; Kowalska, M.; Prendecki, M.; Piekut, T.; Kozłowska, J.; Kozubski, W. Oxidative stress factors in Parkinson’s disease. Neural Regen. Res. 2021, 16, 1383–1391. [Google Scholar] [CrossRef]
- Caproni, S.; Di Fonzo, A.; Colosimo, C. Oxidative Stress: A New Pathophysiological Pathway in Parkinson’s Disease and a Potential Target of the Brain-Sport Crosstalk. Park. Dis. 2025, 2025, 6691390. [Google Scholar] [CrossRef]
- Wal, P.; Dwivedi, J.; Wal, A.; Vig, H.; Singh, Y. Detailed insight into the pathophysiology and the behavioral complications associated with the Parkinson’s disease and its medications. Future J. Pharm. Sci. 2022, 8, 33. [Google Scholar] [CrossRef]
- Kanda, T.; Tsukagoshi, H.; Oda, M.; Miyamoto, K.; Tanabe, H. Changes of unmyelinated nerve fibers in sural nerve in amyotrophic lateral sclerosis, Parkinson’s disease and multiple system atrophy. Acta Neuropathol. 1996, 91, 145–154. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Shen, F.F.; Yuan, Y.S.; Li, X.; Ji, P.; Zhu, L.; Sun, L.; Ding, J.; Niu, Q.; et al. Activated Schwann cells and increased inflammatory cytokines IL-1β, IL-6, and TNF-α in patients’ sural nerve are lack of tight relationship with specific sensory disturbances in Parkinson’s disease. CNS Neurosci. Ther. 2020, 26, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, D.; Ewans, L.; Pham-Dinh, D.; Knott, J.; Reynolds, R. Upregulation of alpha-synuclein in neurons and glia in inflammatory demyelinating disease. Mol. Cell Neurosci. 2006, 31, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, L.; Sun, L.; Zhi, Y.; Ding, J.; Yuan, Y.S.; Shen, F.F.; Li, X.; Ji, P.; Wang, Z.; et al. Phosphorylated α-synuclein deposits in sural nerve deriving from Schwann cells: A biomarker for Parkinson’s disease. Park. Relat. Disord. 2019, 60, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Cheng, Y.; Wang, Y.; Wu, J.; Rong, Z.; Sun, L.; Zhou, Y.; Zhang, K. Involvement of Abnormal p-α-syn Accumulation and TLR2-Mediated Inflammation of Schwann Cells in Enteric Autonomic Nerve Dysfunction of Parkinson’s Disease: An Animal Model Study. Mol. Neurobiol. 2023, 60, 4738–4752. [Google Scholar] [CrossRef]
- Cheng, Y.; Tong, Q.; Yuan, Y.; Song, X.; Jiang, W.; Wang, Y.; Li, W.; Li, Y.; Zhang, K. α-Synuclein induces prodromal symptoms of Parkinson’s disease via activating TLR2/MyD88/NF-κB pathway in Schwann cells of vagus nerve in a rat model. J. Neuroinflamm. 2023, 20, 36. [Google Scholar] [CrossRef]
- Li, Y.; Tong, Q.; Wang, Y.; Cheng, Y.; Geng, Y.; Tian, T.; Yuan, Y.; Fan, Y.; Lu, M.; Zhang, K. Phosphorylated α-synuclein deposited in Schwann cells interacting with TLR2 mediates cell damage and induces Parkinson’s disease autonomic dysfunction. Cell Death Discov. 2024, 10, 52. [Google Scholar] [CrossRef]
- Hölscher, C. Central effects of GLP-1: New opportunities for treatments of neurodegenerative diseases. J. Endocrinol. 2014, 221, T31–T41. [Google Scholar] [CrossRef]
- Liu, W.J.; Jin, H.Y.; Lee, K.A.; Xie, S.H.; Baek, H.S.; Park, T.S. Neuroprotective effect of the glucagon-like peptide-1 receptor agonist, synthetic exendin-4, in streptozotocin-induced diabetic rats. Br. J. Pharmacol. 2011, 164, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Takaku, S.; Tsukamoto, M.; Niimi, N.; Yako, H.; Sango, K. Exendin-4 Promotes Schwann Cell Survival/Migration and Myelination In Vitro. Int. J. Mol. Sci. 2021, 22, 2971. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Olmos, I.; Dickson, J.; Kefalopoulou, Z.; Djamshidian, A.; Ell, P.; Soderlund, T.; Whitton, P.; Wyse, R.; Isaacs, T.; Lees, A.; et al. Exenatide and the treatment of patients with Parkinson’s disease. J. Clin. Investig. 2013, 123, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, W.; Cao, M. Role of the Peripheral Nervous System in PD Pathology, Diagnosis, and Treatment. Front. Neurosci. 2021, 15, 598457. [Google Scholar] [CrossRef]
- Date, I.; Felten, S.Y.; Felten, D.L. Cografts of adrenal medulla with peripheral nerve enhance the survivability of transplanted adrenal chromaffin cells and recovery of the host nigrostriatal dopaminergic system in MPTP-treated young adult mice. Brain Res. 1990, 537, 33–39. [Google Scholar] [CrossRef]
- van Horne, C.G.; Strömberg, I.; Young, D.; Olson, L.; Hoffer, B. Functional enhancement of intrastriatal dopamine-containing grafts by the co-transplantation of sciatic nerve tissue in 6-hydroxydopamine-lesioned rats. Exp. Neurol. 1991, 113, 143–154. [Google Scholar] [CrossRef]
- Wilby, M.J.; Sinclair, S.R.; Muir, E.M.; Zietlow, R.; Adcock, K.H.; Horellou, P.; Rogers, J.H.; Dunnett, S.B.; Fawcett, J.W. A glial cell line-derived neurotrophic factor-secreting clone of the Schwann cell line SCTM41 enhances survival and fiber outgrowth from embryonic nigral neurons grafted to the striatum and to the lesioned substantia nigra. J. Neurosci. 1999, 19, 2301–2312. [Google Scholar] [CrossRef]
- Timmer, M.; Müller-Ostermeyer, F.; Kloth, V.; Winkler, C.; Grothe, C.; Nikkhah, G. Enhanced survival, reinnervation, and functional recovery of intrastriatal dopamine grafts co-transplanted with Schwann cells overexpressing high molecular weight FGF-2 isoforms. Exp. Neurol. 2004, 187, 118–136. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.; Wang, W.; Shen, J.; Cai, K.; Liu, M.; Cao, M. SKP-SCs transplantation alleviates 6-OHDA-induced dopaminergic neuronal injury by modulating autophagy. Cell Death Dis. 2021, 12, 674. [Google Scholar] [CrossRef]
- Xia, Y.; Jiang, C.; Cao, Z.; Shi, K.; Wang, Y. Co–transplantation of macaque autologous Schwann cells and human embryonic nerve stem cells in treatment of macaque Parkinson’s disease. Asian Pac. J. Trop. Med. 2012, 5, 7–14. [Google Scholar] [CrossRef]
- Self, W.K.; Holtzman, D.M. Emerging diagnostics and therapeutics for Alzheimer disease. Nat. Med. 2023, 29, 2187–2199. [Google Scholar] [CrossRef]
- Bhatia, V.; Sharma, S. Role of mitochondrial dysfunction, oxidative stress and autophagy in progression of Alzheimer’s disease. J. Neurol. Sci. 2021, 421, 117253. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.-Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef] [PubMed]
- Zota, I.; Chanoumidou, K.; Gravanis, A.; Charalampopoulos, I. Stimulating myelin restoration with BDNF: A promising therapeutic approach for Alzheimer’s disease. Front. Cell. Neurosci. 2024, 18, 1422130. [Google Scholar] [CrossRef] [PubMed]
- McAleese, K.E.; Walker, L.; Graham, S.; Moya, E.L.J.; Johnson, M.; Erskine, D.; Colloby, S.J.; Dey, M.; Martin-Ruiz, C.; Taylor, J.P.; et al. Parietal white matter lesions in Alzheimer’s disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol. 2017, 134, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Wang, K.; Zhang, L.; Bai, L. Stem cell therapy for Alzheimer’s disease: An overview of experimental models and reality. Anim. Model. Exp. Med. 2022, 5, 15–26. [Google Scholar] [CrossRef]
- Ifediora, N.; Canoll, P.; Hargus, G. Human stem cell transplantation models of Alzheimer’s disease. Front. Aging Neurosci. 2024, 16, 1354164. [Google Scholar] [CrossRef]
- Zhan, Y.; Ma, D.; Zhang, Y. Effects of cotransplantated Schwann cells and neural stem cells in a rat model of Alzheimer’s disease. Neural Regen. Res. 2011, 6, 245–251. [Google Scholar]
- Yu, Z.; Men, Y.; Dong, P. Schwann cells promote the capability of neural stem cells to differentiate into neurons and secret neurotrophic factors. Exp. Ther. Med. 2017, 13, 2029–2035. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Y.; Grajales-Reyes, G.; Colonna, M. TREM2 dependent and independent functions of microglia in Alzheimer’s disease. Mol. Neurodegener. 2022, 17, 84. [Google Scholar] [CrossRef]
- Zhao, P.; Xu, Y.; Jiang, L.; Fan, X.; Li, L.; Li, X.; Arase, H.; Zhao, Y.; Cao, W.; Zheng, H.; et al. A tetravalent TREM2 agonistic antibody reduced amyloid pathology in a mouse model of Alzheimer’s disease. Sci. Transl. Med. 2022, 14, eabq0095. [Google Scholar] [CrossRef]
- Palaiogeorgou, A.M.; Papakonstantinou, E.; Golfinopoulou, R.; Sigala, M.; Mitsis, T.; Papageorgiou, L.; Diakou, I.; Pierouli, K.; Dragoumani, K.; Spandidos, D.A.; et al. Recent approaches on Huntington’s disease (Review). Biomed. Rep. 2023, 18, 5. [Google Scholar] [CrossRef]
- Conner, L.T.; Srinageshwar, B.; Bakke, J.L.; Dunbar, G.L.; Rossignol, J. Advances in stem cell and other therapies for Huntington’s disease: An update. Brain Res. Bull. 2023, 199, 110673. [Google Scholar] [CrossRef]
- Dhingra, H.; Gaidhane, S.A. Huntington’s Disease: Understanding Its Novel Drugs and Treatments. Cureus 2023, 15, e47526. [Google Scholar] [CrossRef]
- Zayed, M.A.; Sultan, S.; Alsaab, H.O.; Yousof, S.M.; Alrefaei, G.I.; Alsubhi, N.H.; Alkarim, S.; Al Ghamdi, K.S.; Bagabir, S.A.; Jana, A.; et al. Stem-Cell-Based Therapy: The Celestial Weapon against Neurological Disorders. Cells 2022, 11, 3476. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.W.; Kennedy, C.J.; Palpagama, T.H.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Current and Possible Future Therapeutic Options for Huntington’s Disease. J. Cent. Nerv. Syst. Dis. 2022, 14, 11795735221092517. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.M.; Hacker, J.L.; Sase, S.; Adang, L.; Almad, A. Glial cells in the driver seat of leukodystrophy pathogenesis. Neurobiol. Dis. 2020, 146, 105087. [Google Scholar] [CrossRef] [PubMed]
- van der Knaap, M.S.; Bugiani, M. Leukodystrophies: A proposed classification system based on pathological changes and pathogenetic mechanisms. Acta Neuropathol. 2017, 134, 351–382. [Google Scholar] [CrossRef]
- Aerts-Kaya, F.; van Til, N.P. Gene and Cellular Therapies for Leukodystrophies. Pharmaceutics 2023, 15, 2522. [Google Scholar] [CrossRef]
- van Rappard, D.F.; Boelens, J.J.; Wolf, N.I. Metachromatic leukodystrophy: Disease spectrum and approaches for treatment. Best. Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 261–273. [Google Scholar] [CrossRef]
- Beerepoot, S.; Nierkens, S.; Boelens, J.J.; Lindemans, C.; Bugiani, M.; Wolf, N.I. Peripheral neuropathy in metachromatic leukodystrophy: Current status and future perspective. Orphanet J. Rare Dis. 2019, 14, 240. [Google Scholar] [CrossRef] [PubMed]
- Argyrakis, A.; Pilz, H.; Goebel, H.H.; Müller, D. Ultrastructural findings of peripheral nerve in a preclinical case of adult metachromatic leukodystrophy. J. Neuropathol. Exp. Neurol. 1977, 36, 693–711. [Google Scholar] [CrossRef]
- Cravioto, H.; O’Brien, J.S.; Landing, B.H.; Finck, B. Ultrastructure of peripheral nerve in metachromatic leucodystrophy. Acta Neuropathol. 1966, 7, 111–124. [Google Scholar] [CrossRef]
- Ramakrishnan, H.; Hedayati, K.K.; Lüllmann-Rauch, R.; Wessig, C.; Fewou, S.N.; Maier, H.; Goebel, H.H.; Gieselmann, V.; Eckhardt, M. Increasing sulfatide synthesis in myelin-forming cells of arylsulfatase A-deficient mice causes demyelination and neurological symptoms reminiscent of human metachromatic leukodystrophy. J. Neurosci. 2007, 27, 9482–9490. [Google Scholar] [CrossRef]
- Sanchez-Álvarez, N.T.; Bautista-Niño, P.K.; Trejos-Suárez, J.; Serrano-Díaz, N.C. A model of metformin mitochondrial metabolism in metachromatic leukodystrophy: First description of human Schwann cells transfected with CRISPR-Cas9. Open Biol. 2022, 12, 210371. [Google Scholar] [CrossRef]
- Aguayo, A.J.; Kasarjian, J.; Skamene, E.; Kongshavn, P.; Bray, G.M. Myelination of mouse axons by Schwann cells transplanted from normal and abnormal human nerves. Nature 1977, 268, 753–755. [Google Scholar] [CrossRef]
- Sangalli, A.; Taveggia, C.; Salviati, A.; Wrabetz, L.; Bordignon, C.; Severini, G.M. Transduced fibroblasts and metachromatic leukodystrophy lymphocytes transfer arylsulfatase A to myelinating glia and deficient cells in vitro. Hum. Gene Ther. 1998, 9, 2111–2119. [Google Scholar] [CrossRef]
- Suzuki, K. Globoid cell leukodystrophy (Krabbe’s disease): Update. J. Child. Neurol. 2003, 18, 595–603. [Google Scholar] [CrossRef]
- Komiyama, A.; Suzuki, K. Progressive dysfunction of twitcher Schwann cells is evaluated better in vitro than in vivo. Brain Res. 1994, 637, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Suzuki, K. Responses to cyclic AMP is impaired in the twitcher Schwann cells in vitro. Brain Res. 1999, 816, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Shaimardanova, A.A.; Chulpanova, D.S.; Solovyeva, V.V.; Mullagulova, A.I.; Kitaeva, K.V.; Allegrucci, C.; Rizvanov, A.A. Metachromatic Leukodystrophy: Diagnosis, Modeling, and Treatment Approaches. Front. Med. 2020, 7, 576221. [Google Scholar] [CrossRef]
- Feltri, M.L.; Weinstock, N.I.; Favret, J.; Dhimal, N.; Wrabetz, L.; Shin, D. Mechanisms of demyelination and neurodegeneration in globoid cell leukodystrophy. Glia 2021, 69, 2309–2331. [Google Scholar] [CrossRef]
- Koç, O.N.; Day, J.; Nieder, M.; Gerson, S.L.; Lazarus, H.M.; Krivit, W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant. 2002, 30, 215–222. [Google Scholar] [CrossRef]
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Moss, K.R.; Saxena, S. Schwann Cells in Neuromuscular Disorders: A Spotlight on Amyotrophic Lateral Sclerosis. Cells 2025, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Scarlino, S.; Falzone, Y.M.; Lunetta, C.; Tremolizzo, L.; Quattrini, A.; Riva, N. The Peripheral Nervous System in Amyotrophic Lateral Sclerosis: Opportunities for Translational Research. Front. Neurosci. 2019, 13, 601. [Google Scholar] [CrossRef]
- Nolano, M.; Provitera, V.; Manganelli, F.; Iodice, R.; Caporaso, G.; Stancanelli, A.; Marinou, K.; Lanzillo, B.; Santoro, L.; Mora, G. Non-motor involvement in amyotrophic lateral sclerosis: New insight from nerve and vessel analysis in skin biopsy. Neuropathol. Appl. Neurobiol. 2017, 43, 119–132. [Google Scholar] [CrossRef]
- Vaughan, S.K.; Kemp, Z.; Hatzipetros, T.; Vieira, F.; Valdez, G. Degeneration of proprioceptive sensory nerve endings in mice harboring amyotrophic lateral sclerosis-causing mutations. J. Comp. Neurol. 2015, 523, 2477–2494. [Google Scholar] [CrossRef]
- Guo, Y.S.; Wu, D.X.; Wu, H.R.; Wu, S.Y.; Yang, C.; Li, B.; Bu, H.; Zhang, Y.S.; Li, C.Y. Sensory involvement in the SOD1-G93A mouse model of amyotrophic lateral sclerosis. Exp. Mol. Med. 2009, 41, 140–150. [Google Scholar] [CrossRef]
- Ki, S.M.; Jeong, H.S.; Lee, J.E. Primary Cilia in Glial Cells: An Oasis in the Journey to Overcoming Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 736888. [Google Scholar] [CrossRef]
- Fischer, L.R.; Culver, D.G.; Tennant, P.; Davis, A.A.; Wang, M.; Castellano-Sanchez, A.; Khan, J.; Polak, M.A.; Glass, J.D. Amyotrophic lateral sclerosis is a distal axonopathy: Evidence in mice and man. Exp. Neurol. 2004, 185, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Khurana, S.; Vats, A.; Sahu, B.; Ganguly, N.K.; Chakraborti, P.; Gourie-Devi, M.; Taneja, V. Neuromuscular Junction Dysfunction in Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2022, 59, 1502–1527. [Google Scholar] [CrossRef] [PubMed]
- Trias, E.; Kovacs, M.; King, P.H.; Si, Y.; Kwon, Y.; Varela, V.; Ibarburu, S.; Moura, I.C.; Hermine, O.; Beckman, J.S.; et al. Schwann cells orchestrate peripheral nerve inflammation through the expression of CSF1, IL-34, and SCF in amyotrophic lateral sclerosis. Glia 2020, 68, 1165–1181. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt-Clermont, P.J.; Khan, A.; Jimsheleishvili, G.; Graham, P.; Brooks, A.; Silvera, R.; Goldschmidt, A.J.P.; Pearse, D.D.; Dietrich, W.D.; Levi, A.D.; et al. Treating amyotrophic lateral sclerosis with allogeneic Schwann cell-derived exosomal vesicles: A case report. Neural Regen. Res. 2025, 20, 1207–1216. [Google Scholar] [CrossRef]
- Yim, A.K.Y.; Wang, P.L.; Bermingham, J.R., Jr.; Hackett, A.; Strickland, A.; Miller, T.M.; Ly, C.; Mitra, R.D.; Milbrandt, J. Disentangling glial diversity in peripheral nerves at single-nuclei resolution. Nat. Neurosci. 2022, 25, 238–251. [Google Scholar] [CrossRef]
- Kozlowski, M.M.; Strickland, A.; Benitez, A.M.; Schmidt, R.E.; Bloom, A.J.; Milbrandt, J.; DiAntonio, A. Pmp2+ Schwann Cells Maintain the Survival of Large-Caliber Motor Axons. J. Neurosci. 2025, 45, e1362242025. [Google Scholar] [CrossRef]
- Nagai, M.; Aoki, M.; Miyoshi, I.; Kato, M.; Pasinelli, P.; Kasai, N.; Brown, R.H., Jr.; Itoyama, Y. Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: Associated mutations develop motor neuron disease. J. Neurosci. 2001, 21, 9246–9254. [Google Scholar] [CrossRef]
- Ripps, M.E.; Huntley, G.W.; Hof, P.R.; Morrison, J.H.; Gordon, J.W. Transgenic mice expressing an altered murine superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 1995, 92, 689–693. [Google Scholar] [CrossRef]
- Reaume, A.G.; Elliott, J.L.; Hoffman, E.K.; Kowall, N.W.; Ferrante, R.J.; Siwek, D.F.; Wilcox, H.M.; Flood, D.G.; Beal, M.F.; Brown, R.H., Jr.; et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996, 13, 43–47. [Google Scholar] [CrossRef]
- Boillée, S.; Vande Velde, C.; Cleveland, D.W. ALS: A disease of motor neurons and their nonneuronal neighbors. Neuron 2006, 52, 39–59. [Google Scholar] [CrossRef]
- Ahtoniemi, T.; Jaronen, M.; Keksa-Goldsteine, V.; Goldsteins, G.; Koistinaho, J. Mutant SOD1 from spinal cord of G93A rats is destabilized and binds to inner mitochondrial membrane. Neurobiol. Dis. 2008, 32, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Peggion, C.; Scalcon, V.; Massimino, M.L.; Nies, K.; Lopreiato, R.; Rigobello, M.P.; Bertoli, A. SOD1 in ALS: Taking Stock in Pathogenic Mechanisms and the Role of Glial and Muscle Cells. Antioxidants 2022, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Louit, A.; Beaudet, M.J.; Gros-Louis, F.; Berthod, F. Tissue-engineered in vitro modeling of the impact of Schwann cells in amyotrophic lateral sclerosis. Biotechnol. Bioeng. 2022, 119, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.F.; Gravel, M.; Kriz, J. Live imaging of amyotrophic lateral sclerosis pathogenesis: Disease onset is characterized by marked induction of GFAP in Schwann cells. Glia 2009, 57, 1130–1142. [Google Scholar] [CrossRef]
- Chen, K.; Northington, F.J.; Martin, L.J. Inducible nitric oxide synthase is present in motor neuron mitochondria and Schwann cells and contributes to disease mechanisms in ALS mice. Brain Struct. Funct. 2010, 214, 219–234. [Google Scholar] [CrossRef]
- Lobsiger, C.S.; Boillee, S.; McAlonis-Downes, M.; Khan, A.M.; Feltri, M.L.; Yamanaka, K.; Cleveland, D.W. Schwann cells expressing dismutase active mutant SOD1 unexpectedly slow disease progression in ALS mice. Proc. Natl. Acad. Sci. USA 2009, 106, 4465–4470. [Google Scholar] [CrossRef]
- Yamamuro-Tanabe, A.; Kosuge, Y.; Ishimaru, Y.; Yoshioka, Y. Schwann cell derived-peroxiredoxin protects motor neurons against hydrogen peroxide-induced cell death in mouse motor neuron cell line NSC-34. J. Pharmacol. Sci. 2023, 153, 73–83. [Google Scholar] [CrossRef]
- Tang, B.; Ni, W.; Zhou, J.; Ling, Y.; Niu, D.; Lu, X.; Chen, T.; Ramalingam, M.; Hu, J. Peroxiredoxin 6 secreted by Schwann-like cells protects neuron against ischemic stroke in rats via PTEN/PI3K/AKT pathway. Tissue Cell 2021, 73, 101635. [Google Scholar] [CrossRef]
- Martineau, É.; Arbour, D.; Vallée, J.; Robitaille, R. Properties of Glial Cell at the Neuromuscular Junction Are Incompatible with Synaptic Repair in the SOD1(G37R) ALS Mouse Model. J. Neurosci. 2020, 40, 7759–7777. [Google Scholar] [CrossRef]
- Alhindi, A.; Shand, M.; Smith, H.L.; Leite, A.S.; Huang, Y.T.; van der Hoorn, D.; Ridgway, Z.; Faller, K.M.E.; Jones, R.A.; Gillingwater, T.H.; et al. Neuromuscular junction denervation and terminal Schwann cell loss in the hTDP-43 overexpression mouse model of amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 2023, 49, e12925. [Google Scholar] [CrossRef]
- Wang, L.; Pytel, P.; Feltri, M.L.; Wrabetz, L.; Roos, R.P. Selective knockdown of mutant SOD1 in Schwann cells ameliorates disease in G85R mutant SOD1 transgenic mice. Neurobiol. Dis. 2012, 48, 52–57. [Google Scholar] [CrossRef]
- Alhindi, A.; Boehm, I.; Forsythe, R.O.; Miller, J.; Skipworth, R.J.; Simpson, H.; Jones, R.A.; Gillingwater, T.H. Terminal Schwann cells at the human neuromuscular junction. Brain Commun. 2021, 3, fcab081. [Google Scholar] [CrossRef] [PubMed]
- Arbour, D.; Tremblay, E.; Martineau, É.; Julien, J.P.; Robitaille, R. Early and persistent abnormal decoding by glial cells at the neuromuscular junction in an ALS model. J. Neurosci. 2015, 35, 688–706. [Google Scholar] [CrossRef] [PubMed]
- Arbour, D.; Vande Velde, C.; Robitaille, R. New perspectives on amyotrophic lateral sclerosis: The role of glial cells at the neuromuscular junction. J. Physiol. 2017, 595, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, P.; Gawor, M.; Prószyński, T.J. Perisynaptic schwann cells—The multitasking cells at the developing neuromuscular junctions. Semin. Cell Dev. Biol. 2020, 104, 31–38. [Google Scholar] [CrossRef]
- Hastings, R.L.; Avila, M.F.; Suneby, E.; Juros, D.; O’Young, A.; Peres da Silva, J.; Valdez, G. Cellular and molecular evidence that synaptic Schwann cells contribute to aging of mouse neuromuscular junctions. Aging Cell 2023, 22, e13981. [Google Scholar] [CrossRef]
- Carrasco, D.I.; Seburn, K.L.; Pinter, M.J. Altered terminal Schwann cell morphology precedes denervation in SOD1 mice. Exp. Neurol. 2016, 275 Pt 1, 172–181. [Google Scholar] [CrossRef]
- Liu, J.X.; Brännström, T.; Andersen, P.M.; Pedrosa-Domellöf, F. Distinct changes in synaptic protein composition at neuromuscular junctions of extraocular muscles versus limb muscles of ALS donors. PLoS ONE 2013, 8, e57473. [Google Scholar] [CrossRef]
- Harrison, J.M.; Rafuse, V.F. Muscle fiber-type specific terminal Schwann cell pathology leads to sprouting deficits following partial denervation in SOD1(G93A) mice. Neurobiol. Dis. 2020, 145, 105052. [Google Scholar] [CrossRef]
- Chao, T.; Frump, D.; Lin, M.; Caiozzo, V.J.; Mozaffar, T.; Steward, O.; Gupta, R. Matrix metalloproteinase 3 deletion preserves denervated motor endplates after traumatic nerve injury. Ann. Neurol. 2013, 73, 210–223. [Google Scholar] [CrossRef]
- Negro, S.; Lessi, F.; Duregotti, E.; Aretini, P.; La Ferla, M.; Franceschi, S.; Menicagli, M.; Bergamin, E.; Radice, E.; Thelen, M.; et al. CXCL12alpha/SDF-1 from perisynaptic Schwann cells promotes regeneration of injured motor axon terminals. EMBO Mol. Med. 2017, 9, 1000–1010. [Google Scholar] [CrossRef]
- Negro, S.; Lauria, F.; Stazi, M.; Tebaldi, T.; D’Este, G.; Pirazzini, M.; Megighian, A.; Lessi, F.; Mazzanti, C.M.; Sales, G.; et al. Hydrogen peroxide induced by nerve injury promotes axon regeneration via connective tissue growth factor. Acta Neuropathol. Commun. 2022, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.A.; Amer, B.E.; Hawas, Y.; Mabrouk, M.A.; Meshref, M. Masitinib as a neuroprotective agent: A scoping review of preclinical and clinical evidence. Neurol. Sci. 2024, 45, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, A.; El-Hawary, H.; Efanov, J.I.; Xu, L. Peripheral Nerve Healing: So Near and Yet So Far. Semin. Plast. Surg. 2021, 35, 204–210. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, M.B.; Kozik, E.M.; Sakaguchi, D.S. Adult Stem Cell-Based Strategies for Peripheral Nerve Regeneration. Adv. Exp. Med. Biol. 2018, 1119, 41–71. [Google Scholar] [CrossRef]
- Leon-Andrino, A.; Noriega, D.C.; Lapuente, J.P.; Perez-Valdecantos, D.; Caballero-Garcia, A.; Herrero, A.J.; Cordova, A. Biological Approach in the Treatment of External Popliteal Sciatic Nerve (Epsn) Neurological Injury: Review. J. Clin. Med. 2022, 11, 2804. [Google Scholar] [CrossRef]
- Guenard, V.; Kleitman, N.; Morrissey, T.K.; Bunge, R.P.; Aebischer, P. Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J. Neurosci. 1992, 12, 3310–3320. [Google Scholar] [CrossRef]
- Cai, S.; Shea, G.K.; Tsui, A.Y.; Chan, Y.S.; Shum, D.K. Derivation of clinically applicable schwann cells from bone marrow stromal cells for neural repair and regeneration. CNS Neurol. Disord. Drug Targets 2011, 10, 500–508. [Google Scholar] [CrossRef]
- Wakao, S.; Matsuse, D.; Dezawa, M. Mesenchymal stem cells as a source of Schwann cells: Their anticipated use in peripheral nerve regeneration. Cells Tissues Organs 2014, 200, 31–41. [Google Scholar] [CrossRef]
- Levi, A.D.; Burks, S.S.; Anderson, K.D.; Dididze, M.; Khan, A.; Dietrich, W.D. The Use of Autologous Schwann Cells to Supplement Sciatic Nerve Repair with a Large Gap: First in Human Experience. Cell Transplant. 2016, 25, 1395–1403. [Google Scholar] [CrossRef]
- Gersey, Z.C.; Burks, S.S.; Anderson, K.D.; Dididze, M.; Khan, A.; Dietrich, W.D.; Levi, A.D. First human experience with autologous Schwann cells to supplement sciatic nerve repair: Report of 2 cases with long-term follow-up. Neurosurg. Focus. 2017, 42, E2. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, F.Y.; Ling, Z.M.; Su, W.F.; Zhao, Y.Y.; Chen, G.; Wei, Z.Y. The Effect of Schwann Cells/Schwann Cell-Like Cells on Cell Therapy for Peripheral Neuropathy. Front. Cell Neurosci. 2022, 16, 836931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, J.; Dai, W.; Lu, B.; Yi, S. Schwann cells in regeneration and cancer. Front. Pharmacol. 2025, 16, 1506552. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Belfiore, L.; Chu, T.H.; Fleming, T.; Midha, R.; Biernaskie, J.; Schuurmans, C. Insights Into the Role and Potential of Schwann Cells for Peripheral Nerve Repair From Studies of Development and Injury. Front. Mol. Neurosci. 2020, 13, 608442. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, M.; Liu, N. Interactions between Schwann cell and extracellular matrix in peripheral nerve regeneration. Front. Neurol. 2024, 15, 1372168. [Google Scholar] [CrossRef]
- Rao, Z.; Lin, Z.; Song, P.; Quan, D.; Bai, Y. Biomaterial-Based Schwann Cell Transplantation and Schwann Cell-Derived Biomaterials for Nerve Regeneration. Front. Cell Neurosci. 2022, 16, 926222. [Google Scholar] [CrossRef]
- Su, Q.; Nasser, M.I.; He, J.; Deng, G.; Ouyang, Q.; Zhuang, D.; Deng, Y.; Hu, H.; Liu, N.; Li, Z.; et al. Engineered Schwann Cell-Based Therapies for Injury Peripheral Nerve Reconstruction. Front. Cell Neurosci. 2022, 16, 865266. [Google Scholar] [CrossRef]
- Reed, C.B.; Frick, L.R.; Weaver, A.; Sidoli, M.; Schlant, E.; Feltri, M.L.; Wrabetz, L. Deletion of Calcineurin in Schwann Cells Does Not Affect Developmental Myelination, But Reduces Autophagy and Delays Myelin Clearance after Peripheral Nerve Injury. J. Neurosci. 2020, 40, 6165–6176. [Google Scholar] [CrossRef]
- Li, R.; Li, D.; Wu, C.; Ye, L.; Wu, Y.; Yuan, Y.; Yang, S.; Xie, L.; Mao, Y.; Jiang, T.; et al. Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics 2020, 10, 1649–1677. [Google Scholar] [CrossRef]
- Burks, S.S.; Diaz, A.; Haggerty, A.E.; Oliva, N.; Midha, R.; Levi, A.D. Schwann cell delivery via a novel 3D collagen matrix conduit improves outcomes in critical length nerve gap repairs. J. Neurosurg. 2021, 135, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Chen, Y.; Luo, Y.; Zhang, M.; Min, J. The phenotypic changes of Schwann cells promote the functional repair of nerve injury. Neuropeptides 2024, 106, 102438. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Golding, J.P.; Loughlin, A.J.; Kingham, P.J.; Phillips, J.B. Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials 2015, 37, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, F.; Hernández, J.; Heimann, C.; Phillips, J.B.; Udina, E.; Navarro, X. Schwann cells and mesenchymal stem cells in laminin- or fibronectin-aligned matrices and regeneration across a critical size defect of 15 mm in the rat sciatic nerve. J. Neurosurg. Spine 2018, 28, 109–118. [Google Scholar] [CrossRef]
- Berrocal, Y.A.; Almeida, V.W.; Gupta, R.; Levi, A.D. Transplantation of Schwann cells in a collagen tube for the repair of large, segmental peripheral nerve defects in rats. J. Neurosurg. 2013, 119, 720–732. [Google Scholar] [CrossRef]
- Petersen, M.A.; Ryu, J.K.; Akassoglou, K. Fibrinogen in neurological diseases: Mechanisms, imaging and therapeutics. Nat. Rev. Neurosci. 2018, 19, 283–301. [Google Scholar] [CrossRef]
- Akassoglou, K.; Yu, W.M.; Akpinar, P.; Strickland, S. Fibrin inhibits peripheral nerve remyelination by regulating Schwann cell differentiation. Neuron 2002, 33, 861–875. [Google Scholar] [CrossRef]
- Schuh, C.; Day, A.G.E.; Redl, H.; Phillips, J. An Optimized Collagen-Fibrin Blend Engineered Neural Tissue Promotes Peripheral Nerve Repair. Tissue Eng. Part A 2018, 24, 1332–1340. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Li, Q. Curcumin accelerates the repair of sciatic nerve injury in rats through reducing Schwann cells apoptosis and promoting myelinization. Biomed. Pharmacother. 2017, 92, 1103–1110. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Z.; Gao, S.; Wang, Y.; Li, Q. Curcumin enhances the proliferation and myelinization of Schwann cells through Runx2 to repair sciatic nerve injury. Neurosci. Lett. 2022, 770, 136391. [Google Scholar] [CrossRef]
- Jahromi, H.K.; Farzin, A.; Hasanzadeh, E.; Barough, S.E.; Mahmoodi, N.; Najafabadi, M.R.H.; Farahani, M.S.; Mansoori, K.; Shirian, S.; Ai, J. Enhanced sciatic nerve regeneration by poly-L-lactic acid/multi-wall carbon nanotube neural guidance conduit containing Schwann cells and curcumin encapsulated chitosan nanoparticles in rat. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110564. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, J.; Liu, Y.; Huang, D.; Lu, L. Resveratrol regulates the recovery of rat sciatic nerve crush injury by promoting the autophagy of Schwann cells. Life Sci. 2020, 256, 117959. [Google Scholar] [CrossRef]
- Ma, T.; Zhu, L.; Yang, Y.; Quan, X.; Huang, L.; Liu, Z.; Sun, Z.; Zhu, S.; Huang, J.; Luo, Z. Enhanced in vivo survival of Schwann cells by a synthetic oxygen carrier promotes sciatic nerve regeneration and functional recovery. J. Tissue Eng. Regen. Med. 2018, 12, e177–e189. [Google Scholar] [CrossRef]
- Hood, B.; Levene, H.B.; Levi, A.D. Transplantation of autologous Schwann cells for the repair of segmental peripheral nerve defects. Neurosurg. Focus. 2009, 26, E4. [Google Scholar] [CrossRef]
- Han, G.H.; Peng, J.; Liu, P.; Ding, X.; Wei, S.; Lu, S.; Wang, Y. Therapeutic strategies for peripheral nerve injury: Decellularized nerve conduits and Schwann cell transplantation. Neural Regen. Res. 2019, 14, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, F.A.; Diaz, A.; Errante, E.L.; Smartz, T.; Khan, A.; Silvera, R.; Brooks, A.E.; Lee, Y.S.; Burks, S.S.; Levi, A.D. Systematic review of the therapeutic use of Schwann cells in the repair of peripheral nerve injuries: Advancements from animal studies to clinical trials. Front. Cell Neurosci. 2022, 16, 929593. [Google Scholar] [CrossRef] [PubMed]
- McGrath, A.M.; Novikova, L.N.; Novikov, L.N.; Wiberg, M. BD™ PuraMatrix™ peptide hydrogel seeded with Schwann cells for peripheral nerve regeneration. Brain Res. Bull. 2010, 83, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Naseri-Nosar, M.; Ebrahimi-Barough, S.; Nourani, M.; Khojasteh, A.; Hamidieh, A.A.; Amani, A.; Farzamfar, S.; Ai, J. Sciatic nerve regeneration by transplantation of Schwann cells via erythropoietin controlled-releasing polylactic acid/multiwalled carbon nanotubes/gelatin nanofibrils neural guidance conduit. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1463–1476. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, K.; He, A.; Wan, S.; Wu, M.; Hu, D.; Xu, K.; Wei, P.; Yin, J. Dual-layer conduit containing VEGF-A—Transfected Schwann cells promotes peripheral nerve regeneration via angiogenesis. Acta Biomater. 2024, 180, 323–336. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Men, Y.; Chen, X.; Wang, B. Laminin-chitosan-PLGA conduit co-transplanted with Schwann and neural stem cells to repair the injured recurrent laryngeal nerve. Exp. Ther. Med. 2018, 16, 1250–1258. [Google Scholar] [CrossRef]
- Li, Y.; Men, Y.; Wang, B.; Chen, X.; Yu, Z. Co-transplantation of Schwann cells and neural stem cells in the laminin-chitosan-PLGA nerve conduit to repair the injured recurrent laryngeal nerve in SD rats. J. Mater. Sci. Mater. Med. 2020, 31, 99. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, T.; Sundback, C.; Hunter, D.; Cheney, M.; Vacanti, J.P. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000, 6, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Takeya, H.; Itai, S.; Kimura, H.; Kurashina, Y.; Amemiya, T.; Nagoshi, N.; Iwamoto, T.; Sato, K.; Shibata, S.; Matsumoto, M.; et al. Schwann cell-encapsulated chitosan-collagen hydrogel nerve conduit promotes peripheral nerve regeneration in rodent sciatic nerve defect models. Sci. Rep. 2023, 13, 11932. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Hsu, S.H. The effects of low-intensity ultrasound on peripheral nerve regeneration in poly(DL-lactic acid-co-glycolic acid) conduits seeded with Schwann cells. Ultrasound Med. Biol. 2004, 30, 1079–1084. [Google Scholar] [CrossRef]
- Huang, B.; Jiang, Y.; Zhang, L.; Yang, B.; Guo, Y.; Yang, X.; Gong, P. Low-intensity pulsed ultrasound promotes proliferation and myelinating genes expression of Schwann cells through NRG1/ErbB signaling pathway. Tissue Cell 2023, 80, 101985. [Google Scholar] [CrossRef]
- Ling, J.; He, C.; Zhang, S.; Zhao, Y.; Zhu, M.; Tang, X.; Li, Q.; Xu, L.; Yang, Y. Progress in methods for evaluating Schwann cell myelination and axonal growth in peripheral nerve regeneration via scaffolds. Front. Bioeng. Biotechnol. 2023, 11, 1308761. [Google Scholar] [CrossRef]
- Bolívar, S.; Navarro, X.; Udina, E. Schwann Cell Role in Selectivity of Nerve Regeneration. Cells 2020, 9, 2131. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Lu, L.; Liu, Y. SPIONs mediated magnetic actuation promotes nerve regeneration by inducing and maintaining repair-supportive phenotypes in Schwann cells. J. Nanobiotechnol. 2022, 20, 159. [Google Scholar] [CrossRef]
- Wan, L.; Xia, R.; Ding, W. Short-term low-frequency electrical stimulation enhanced remyelination of injured peripheral nerves by inducing the promyelination effect of brain-derived neurotrophic factor on Schwann cell polarization. J. Neurosci. Res. 2010, 88, 2578–2587. [Google Scholar] [CrossRef]
- Elzinga, K.; Tyreman, N.; Ladak, A.; Savaryn, B.; Olson, J.; Gordon, T. Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats. Exp. Neurol. 2015, 269, 142–153. [Google Scholar] [CrossRef]
- Kim, I.S.; Song, Y.M.; Cho, T.H.; Pan, H.; Lee, T.H.; Kim, S.J.; Hwang, S.J. Biphasic electrical targeting plays a significant role in schwann cell activation. Tissue Eng. Part. A 2011, 17, 1327–1340. [Google Scholar] [CrossRef]
- Xia, B.; Gao, J.; Li, S.; Huang, L.; Zhu, L.; Ma, T.; Zhao, L.; Yang, Y.; Luo, K.; Shi, X.; et al. Mechanical stimulation of Schwann cells promote peripheral nerve regeneration via extracellular vesicle-mediated transfer of microRNA 23b-3p. Theranostics 2020, 10, 8974–8995. [Google Scholar] [CrossRef]
- Chen, S.H.; Wang, H.W.; Yang, P.C.; Chen, S.S.; Ho, C.H.; Yang, P.C.; Kao, Y.C.; Liu, S.W.; Chiu, H.; Lin, Y.J.; et al. Schwann cells acquire a repair phenotype after assembling into spheroids and show enhanced in vivo therapeutic potential for promoting peripheral nerve repair. Bioeng. Transl. Med. 2024, 9, e10635. [Google Scholar] [CrossRef]
- Wang, J.; Lu, S.; Yuan, Y.; Huang, L.; Bian, M.; Yu, J.; Zou, J.; Jiang, L.; Meng, D.; Zhang, J. Inhibition of Schwann Cell Pyroptosis Promotes Nerve Regeneration in Peripheral Nerve Injury in Rats. Mediat. Inflamm. 2023, 2023, 9721375. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Z.; Wang, H.; Zhang, D.; Han, T.; Chen, H.; Chen, J.; Chen, Z.; Xie, Y.; Wang, L.; et al. Melatonin promotes peripheral nerve repair through Parkin-mediated mitophagy. Free Radic. Biol. Med. 2022, 185, 52–66. [Google Scholar] [CrossRef]
- Chang, H.M.; Liu, C.H.; Hsu, W.M.; Chen, L.Y.; Wang, H.P.; Wu, T.H.; Chen, K.Y.; Ho, W.H.; Liao, W.C. Proliferative effects of melatonin on Schwann cells: Implication for nerve regeneration following peripheral nerve injury. J. Pineal Res. 2014, 56, 322–332. [Google Scholar] [CrossRef]
- Pan, B.; Jing, L.; Cao, M.; Hu, Y.; Gao, X.; Bu, X.; Li, Z.; Feng, H.; Guo, K. Melatonin promotes Schwann cell proliferation and migration via the shh signalling pathway after peripheral nerve injury. Eur. J. Neurosci. 2021, 53, 720–731. [Google Scholar] [CrossRef]
- Klymenko, A.; Lutz, D. Melatonin signalling in Schwann cells during neuroregeneration. Front. Cell Dev. Biol. 2022, 10, 999322. [Google Scholar] [CrossRef]
- Xu, H.; Wen, L.; Luo, Y.; Zhou, J.; Yao, S.; Ding, W.; Feng, J. Cannabinoid receptor 2 facilitates the Schwann cells-dependent peripheral nerve regeneration. Clin. Transl. Med. 2025, 15, e70184. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Sun, Y.; Liu, X.; Chai, Y.; Wang, C.; Xu, J. Nerve Regeneration Potential of Antioxidant-Modified Black Phosphorus Quantum Dots in Peripheral Nerve Injury. ACS Nano 2024, 18, 23518–23536. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, J.D.; Seeher, K.M.; Schiess, N.; Nichols, E.; Cao, B.; Servili, C.; Cavallera, V.; Cousin, E.; Hagins, H.; Moberg, M.E.; et al. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Saleh, D.O.; Sedik, A.A. Novel drugs affecting diabetic peripheral neuropathy. Iran. J. Basic Med. Sci. 2024, 27, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sekido, H.; Kato, N.; Nakayama, Y.; Yabe-Nishimura, C. Neurotrophin-3-induced production of nerve growth factor is suppressed in Schwann cells exposed to high glucose: Involvement of the polyol pathway. J. Neurochem. 2004, 91, 1430–1438. [Google Scholar] [CrossRef]
- Sekido, H.; Suzuki, T.; Jomori, T.; Takeuchi, M.; Yabe-Nishimura, C.; Yagihashi, S. Reduced cell replication and induction of apoptosis by advanced glycation end products in rat Schwann cells. Biochem. Biophys. Res. Commun. 2004, 320, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Naruse, K. Schwann Cells as Crucial Players in Diabetic Neuropathy. Adv. Exp. Med. Biol. 2019, 1190, 345–356. [Google Scholar] [CrossRef]
- Mizisin, A.P. Mechanisms of diabetic neuropathy: Schwann cells. Handb. Clin. Neurol. 2014, 126, 401–428. [Google Scholar] [CrossRef]
- Chao, P.Y.; Lin, J.A.; Ye, J.C.; Hwang, J.M.; Ting, W.J.; Huang, C.Y.; Liu, J.Y. Attenuation of Oxidative Stress-Induced Cell Apoptosis in Schwann RSC96 Cells by Ocimum Gratissimum Aqueous Extract. Int. J. Med. Sci. 2017, 14, 764–771. [Google Scholar] [CrossRef]
- Tiong, Y.L.; Ng, K.Y.; Koh, R.Y.; Ponnudurai, G.; Chye, S.M. Melatonin Prevents Oxidative Stress-Induced Mitochondrial Dysfunction and Apoptosis in High Glucose-Treated Schwann Cells via Upregulation of Bcl2, NF-κB, mTOR, Wnt Signalling Pathways. Antioxidants 2019, 8, 198. [Google Scholar] [CrossRef]
- Li, H.; Jiang, L.; Zhang, S.; Miao, X.; Jiang, S.-H. Pan-cancer analysis reveals multifaceted roles of nonmyelinating Schwann cells in gastrointestinal cancers. Cancer Lett. 2025, in press. [Google Scholar]
- Kalichman, M.W.; Powell, H.C.; Mizisin, A.P. Reactive, degenerative, and proliferative Schwann cell responses in experimental galactose and human diabetic neuropathy. Acta Neuropathol. 1998, 95, 47–56. [Google Scholar] [CrossRef]
- Lennertz, R.C.; Medler, K.A.; Bain, J.L.; Wright, D.E.; Stucky, C.L. Impaired sensory nerve function and axon morphology in mice with diabetic neuropathy. J. Neurophysiol. 2011, 106, 905–914. [Google Scholar] [CrossRef]
- Thomas, P.K.; Lascelles, R.G. Schwann-cell abnormalities in diabetic neuropathy. Lancet 1965, 1, 1355–1357. [Google Scholar] [CrossRef] [PubMed]
- Mizisin, A.P.; Shelton, G.D.; Wagner, S.; Rusbridge, C.; Powell, H.C. Myelin splitting, Schwann cell injury and demyelination in feline diabetic neuropathy. Acta Neuropathol. 1998, 95, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Llorián-Salvador, M.; Cabeza-Fernández, S.; Gomez-Sanchez, J.A.; de la Fuente, A.G. Glial cell alterations in diabetes-induced neurodegeneration. Cell Mol. Life Sci. 2024, 81, 47. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, X.; Liu, H.; Lv, Q.; Zou, J.; Shi, Y.; Liu, Z. Expression of Nrf2 Promotes Schwann Cell-Mediated Sciatic Nerve Recovery in Diabetic Peripheral Neuropathy. Cell. Physiol. Biochem. 2018, 46, 1879–1894. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.A.; El Massry, M.; Hichor, M.; Haddad, M.; Grenier, J.; Dia, B.; Barakat, R.; Boutary, S.; Chanal, J.; Aractingi, S.; et al. Targeting the NADPH Oxidase-4 and Liver X Receptor Pathway Preserves Schwann Cell Integrity in Diabetic Mice. Diabetes 2020, 69, 448–464. [Google Scholar] [CrossRef]
- Yang, Y.M.; Ma, H.B.; Xiong, Y.; Wu, Q.; Gao, X.K. PEX11B palmitoylation couples peroxisomal dysfunction with Schwann cells fail in diabetic neuropathy. J. Biomed. Sci. 2025, 32, 20. [Google Scholar] [CrossRef]
- Eid, S.A.; Noureldein, M.; Kim, B.; Hinder, L.M.; Mendelson, F.E.; Hayes, J.M.; Hur, J.; Feldman, E.L. Single-cell RNA-seq uncovers novel metabolic functions of Schwann cells beyond myelination. J. Neurochem. 2023, 166, 367–388. [Google Scholar] [CrossRef]
- Eckersley, L. Role of the Schwann cell in diabetic neuropathy. Int. Rev. Neurobiol. 2002, 50, 293–321. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, S.; Yuan, Q.; Zhu, L.; Li, F.; Wang, H.; Kong, D.; Hao, J. TXNIP, a novel key factor to cause Schwann cell dysfunction in diabetic peripheral neuropathy, under the regulation of PI3K/Akt pathway inhibition-induced DNMT1 and DNMT3a overexpression. Cell Death Dis. 2021, 12, 642. [Google Scholar] [CrossRef]
- Fukunaga, M.; Miyata, S.; Liu, B.F.; Miyazaki, H.; Hirota, Y.; Higo, S.; Hamada, Y.; Ueyama, S.; Kasuga, M. Methylglyoxal induces apoptosis through activation of p38 MAPK in rat Schwann cells. Biochem. Biophys. Res. Commun. 2004, 320, 689–695. [Google Scholar] [CrossRef]
- Liu, Y.P.; Shao, S.J.; Guo, H.D. Schwann cells apoptosis is induced by high glucose in diabetic peripheral neuropathy. Life Sci. 2020, 248, 117459. [Google Scholar] [CrossRef]
- Wu, K.Y.; Deng, F.; Mao, X.Y.; Zhou, D.; Shen, W.G. Ferroptosis involves in Schwann cell death in diabetic peripheral neuropathy. Open Med. 2023, 18, 20230809. [Google Scholar] [CrossRef]
- Han, J.; Tan, P.; Li, Z.; Wu, Y.; Li, C.; Wang, Y.; Wang, B.; Zhao, S.; Liu, Y. Fuzi attenuates diabetic neuropathy in rats and protects schwann cells from apoptosis induced by high glucose. PLoS ONE 2014, 9, e86539. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Wang, J.L.; Yuan, J.; Quan, Q.H.; Ji, R.F.; Tan, P.; Han, J.; Liu, Y.G. Sugar Composition Analysis of Fuzi Polysaccharides by HPLC-MS(n) and Their Protective Effects on Schwann Cells Exposed to High Glucose. Molecules 2016, 21, 1496. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Gui, Z.; Ye, X.; Li, X. Jatrorrhizine ameliorates Schwann cell myelination via inhibiting HDAC3 ability to recruit Atxn2l for regulating the NRG1-ErbB2-PI3K-AKT pathway in diabetic peripheral neuropathy mice. Phytother. Res. 2023, 37, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Ding, Y.; Yan, N.; Nie, Y.; Li, M.; Tong, L. Trehalose prevents sciatic nerve damage to and apoptosis of Schwann cells of streptozotocin-induced diabetic C57BL/6J mice. Biomed. Pharmacother. 2018, 105, 907–914. [Google Scholar] [CrossRef]
- Syngle, A.; Verma, I.; Krishan, P.; Garg, N.; Syngle, V. Minocycline improves peripheral and autonomic neuropathy in type 2 diabetes: MIND study. Neurol. Sci. 2014, 35, 1067–1073. [Google Scholar] [CrossRef]
- Majd, H.; Amin, S.; Ghazizadeh, Z.; Cesiulis, A.; Arroyo, E.; Lankford, K.; Majd, A.; Farahvashi, S.; Chemel, A.K.; Okoye, M.; et al. Deriving Schwann cells from hPSCs enables disease modeling and drug discovery for diabetic peripheral neuropathy. Cell Stem Cell 2023, 30, 632–647.e610. [Google Scholar] [CrossRef]
- Lehmann, H.C.; Höke, A. Schwann cells as a therapeutic target for peripheral neuropathies. CNS Neurol. Disord. Drug Targets 2010, 9, 801–806. [Google Scholar] [CrossRef]
- Yu, T.; Li, L.; Bi, Y.; Liu, Z.; Liu, H.; Li, Z. Erythropoietin attenuates oxidative stress and apoptosis in Schwann cells isolated from streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2014, 66, 1150–1160. [Google Scholar] [CrossRef]
- Yu, T.; Li, L.; Chen, T.; Liu, Z.; Liu, H.; Li, Z. Erythropoietin attenuates advanced glycation endproducts-induced toxicity of Schwann cells in vitro. Neurochem. Res. 2015, 40, 698–712. [Google Scholar] [CrossRef]
- Li, R.; Wu, Y.; Zou, S.; Wang, X.; Li, Y.; Xu, K.; Gong, F.; Liu, Y.; Wang, J.; Liao, Y.; et al. NGF Attenuates High Glucose-Induced ER Stress, Preventing Schwann Cell Apoptosis by Activating the PI3K/Akt/GSK3β and ERK1/2 Pathways. Neurochem. Res. 2017, 42, 3005–3018. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Zhang, L.L.; Li, B.; Sun, F.X.; Wang, Z.D. BMP5 ameliorates diabetic peripheral neuropathy by augmenting mitochondrial function and inhibiting apoptosis in Schwann cells. Biochem. Biophys. Res. Commun. 2023, 643, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shi, X.; Luo, M.; Inam u, l.; Wu, P.; Zhang, M.; Zhang, C.; Li, Q.; Wang, Y.; Piao, F. Taurine protects against myelin damage of sciatic nerve in diabetic peripheral neuropathy rats by controlling apoptosis of schwann cells via NGF/Akt/GSK3β pathway. Exp. Cell Res. 2019, 383, 111557. [Google Scholar] [CrossRef]
- An, J.; Zhang, X.; Jia, K.; Zhang, C.; Zhu, L.; Cheng, M.; Li, F.; Zhao, S.; Hao, J. Trichostatin A increases BDNF protein expression by improving XBP-1s/ATF6/GRP78 axis in Schwann cells of diabetic peripheral neuropathy. Biomed. Pharmacother. 2021, 133, 111062. [Google Scholar] [CrossRef] [PubMed]
- Okawa, T.; Kamiya, H.; Himeno, T.; Kato, J.; Seino, Y.; Fujiya, A.; Kondo, M.; Tsunekawa, S.; Naruse, K.; Hamada, Y.; et al. Transplantation of neural crest-like cells derived from induced pluripotent stem cells improves diabetic polyneuropathy in mice. Cell Transplant. 2013, 22, 1767–1783. [Google Scholar] [CrossRef]
- Akter, S.; Choubey, M.; Mohib, M.M.; Arbee, S.; Sagor, M.A.T.; Mohiuddin, M.S. Stem Cell Therapy in Diabetic Polyneuropathy: Recent Advancements and Future Directions. Brain Sci. 2023, 13, 255. [Google Scholar] [CrossRef]
- Yum, Y.; Park, S.; Nam, Y.H.; Yoon, J.; Song, H.; Kim, H.J.; Lim, J.; Jung, S.C. Therapeutic Effect of Schwann Cell-Like Cells Differentiated from Human Tonsil-Derived Mesenchymal Stem Cells on Diabetic Neuropathy in db/db Mice. Tissue Eng. Regen. Med. 2024, 21, 761–776. [Google Scholar] [CrossRef]
- Abd Razak, N.H.; Idris, J.; Hassan, N.H.; Zaini, F.; Muhamad, N.; Daud, M.F. Unveiling the Role of Schwann Cell Plasticity in the Pathogenesis of Diabetic Peripheral Neuropathy. Int. J. Mol. Sci. 2024, 25, 785. [Google Scholar] [CrossRef]
- Wu, L.; Wang, X.J.; Luo, X.; Zhang, J.; Zhao, X.; Chen, Q. Diabetic peripheral neuropathy based on Schwann cell injury: Mechanisms of cell death regulation and therapeutic perspectives. Front. Endocrinol. 2024, 15, 1427679. [Google Scholar] [CrossRef]
- Sango, K.; Yako, H.; Niimi, N.; Takaku, S. Immortalized Schwann cell lines as useful tools for pathogenesis-based therapeutic approaches to diabetic peripheral neuropathy. Front. Endocrinol. 2024, 15, 1531209. [Google Scholar] [CrossRef]
- Ubogu, E.E. Inflammatory neuropathies: Pathology, molecular markers and targets for specific therapeutic intervention. Acta Neuropathol. 2015, 130, 445–468. [Google Scholar] [CrossRef] [PubMed]
- Meyer zu Hörste, G.; Hu, W.; Hartung, H.P.; Lehmann, H.C.; Kieseier, B.C. The immunocompetence of Schwann cells. Muscle Nerve 2008, 37, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ydens, E.; Lornet, G.; Smits, V.; Goethals, S.; Timmerman, V.; Janssens, S. The neuroinflammatory role of Schwann cells in disease. Neurobiol. Dis. 2013, 55, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.Y.; Yoon, B.A.; Shin, Y.K.; Yun, S.H.; Jo, Y.R.; Choi, Y.Y.; Ahn, M.; Shin, T.; Park, J.I.; Kim, J.K.; et al. Schwann cell dedifferentiation-associated demyelination leads to exocytotic myelin clearance in inflammatory segmental demyelination. Glia 2017, 65, 1848–1862. [Google Scholar] [CrossRef]
- Jo, Y.R.; Oh, Y.; Kim, Y.H.; Shin, Y.K.; Kim, H.R.; Go, H.; Shin, J.; Park, H.J.; Koh, H.; Kim, J.K.; et al. Adaptive autophagy reprogramming in Schwann cells during peripheral demyelination. Cell Mol. Life Sci. 2023, 80, 34. [Google Scholar] [CrossRef]
- Aya, F.; Ruiz-Esquide, V.; Viladot, M.; Font, C.; Prieto-González, S.; Prat, A.; Arance, A. Vasculitic neuropathy induced by pembrolizumab. Ann. Oncol. 2017, 28, 433–434. [Google Scholar] [CrossRef]
- de Maleissye, M.F.; Nicolas, G.; Saiag, P. Pembrolizumab-Induced Demyelinating Polyradiculoneuropathy. N. Engl. J. Med. 2016, 375, 296–297. [Google Scholar] [CrossRef]
- Voskens, C.J.; Goldinger, S.M.; Loquai, C.; Robert, C.; Kaehler, K.C.; Berking, C.; Bergmann, T.; Bockmeyer, C.L.; Eigentler, T.; Fluck, M.; et al. The price of tumor control: An analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS ONE 2013, 8, e53745. [Google Scholar] [CrossRef]
- Zoccarato, M.; Grisold, W.; Grisold, A.; Poretto, V.; Boso, F.; Giometto, B. Paraneoplastic Neuropathies: What’s New Since the 2004 Recommended Diagnostic Criteria. Front. Neurol. 2021, 12, 706169. [Google Scholar] [CrossRef] [PubMed]
- Berzero, G.; Picca, A.; Psimaras, D. Neurological complications of chimeric antigen receptor T cells and immune-checkpoint inhibitors: Ongoing challenges in daily practice. Curr. Opin. Oncol. 2020, 32, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Landry, K.; Thomas, A.A. Neurological Complications of CAR T Cell Therapy. Curr. Oncol. Rep. 2020, 22, 83. [Google Scholar] [CrossRef] [PubMed]
- Anthoney, D.A.; Bone, I.; Evans, T.R. Inflammatory demyelinating polyneuropathy: A complication of immunotherapy in malignant melanoma. Ann. Oncol. 2000, 11, 1197–1200. [Google Scholar] [CrossRef]
- Kamil, K.; Yazid, M.D.; Idrus, R.B.H.; Das, S.; Kumar, J. Peripheral Demyelinating Diseases: From Biology to Translational Medicine. Front. Neurol. 2019, 10, 87. [Google Scholar] [CrossRef]
- Bellanti, R.; Rinaldi, S. Guillain-Barré syndrome: A comprehensive review. Eur. J. Neurol. 2024, 31, e16365. [Google Scholar] [CrossRef]
- Querol, L.A.; Hartung, H.P.; Lewis, R.A.; van Doorn, P.A.; Hammond, T.R.; Atassi, N.; Alonso-Alonso, M.; Dalakas, M.C. The Role of the Complement System in Chronic Inflammatory Demyelinating Polyneuropathy: Implications for Complement-Targeted Therapies. Neurotherapeutics 2022, 19, 864–873. [Google Scholar] [CrossRef]
- Zhu, W.; Li, K.; Cui, T.; Yan, Y. Detection of anti-ganglioside antibodies in Guillain-Barré syndrome. Ann. Transl. Med. 2023, 11, 289. [Google Scholar] [CrossRef]
- Shastri, A.; Al Aiyan, A.; Kishore, U.; Farrugia, M.E. Immune-Mediated Neuropathies: Pathophysiology and Management. Int. J. Mol. Sci. 2023, 24, 7288. [Google Scholar] [CrossRef]
- Yuki, N.; Taki, T.; Takahashi, M.; Saito, K.; Yoshino, H.; Tai, T.; Handa, S.; Miyatake, T. Molecular mimicry between GQ1b ganglioside and lipopolysaccharides of Campylobacter jejuni isolated from patients with Fisher’s syndrome. Ann. Neurol. 1994, 36, 791–793. [Google Scholar] [CrossRef]
- Wanleenuwat, P.; Iwanowski, P.; Kozubski, W. Antiganglioside antibodies in neurological diseases. J. Neurol. Sci. 2020, 408, 116576. [Google Scholar] [CrossRef]
- Zhu, J.; Mix, E.; Olsson, T.; Link, H. Cellular mRNA expression of interferon-gamma, IL-4 and transforming growth factor-beta (TGF-beta) by rat mononuclear cells stimulated with peripheral nerve myelin antigens in experimental allergic neuritis. Clin. Exp. Immunol. 1994, 98, 306–312. [Google Scholar] [CrossRef]
- Súkeníková, L.; Mallone, A.; Schreiner, B.; Ripellino, P.; Nilsson, J.; Stoffel, M.; Ulbrich, S.E.; Sallusto, F.; Latorre, D. Autoreactive T cells target peripheral nerves in Guillain–Barré syndrome. Nature 2024, 626, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Kwa, M.S.G.; van Schaik, I.N.; De Jonge, R.R.; Brand, A.; Kalaydjieva, L.; van Belzen, N.; Vermeulen, M.; Baas, F. Autoimmunoreactivity to Schwann cells in patients with inflammatory neuropathies. Brain 2003, 126, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Lleixà, C.; Martín-Aguilar, L.; Pascual-Goñi, E.; Franco, T.; Caballero, M.; de Luna, N.; Gallardo, E.; Suárez-Calvet, X.; Martínez-Martínez, L.; Diaz-Manera, J.; et al. Autoantibody screening in Guillain–Barré syndrome. J. Neuroinflamm. 2021, 18, 251. [Google Scholar] [CrossRef] [PubMed]
- Hafer-Macko, C.E.; Sheikh, K.A.; Li, C.Y.; Ho, T.W.; Cornblath, D.R.; McKhann, G.M.; Asbury, A.K.; Griffin, J.W. Immune attack on the Schwann cell surface in acute inflammatory demyelinating polyneuropathy. Ann. Neurol. 1996, 39, 625–635. [Google Scholar] [CrossRef]
- Koski, C.L. Mechanisms of Schwann cell damage in inflammatory neuropathy. J. Infect. Dis. 1997, 176 (Suppl. S2), S169–S172. [Google Scholar] [CrossRef]
- Stoll, G.; Schmidt, B.; Jander, S.; Toyka, K.V.; Hartung, H.P. Presence of the terminal complement complex (C5b-9) precedes myelin degradation in immune-mediated demyelination of the rat peripheral nervous system. Ann. Neurol. 1991, 30, 147–155. [Google Scholar] [CrossRef]
- McGonigal, R.; Campbell, C.I.; Barrie, J.A.; Yao, D.; Cunningham, M.E.; Crawford, C.L.; Rinaldi, S.; Rowan, E.G.; Willison, H.J. Schwann cell nodal membrane disruption triggers bystander axonal degeneration in a Guillain-Barré syndrome mouse model. J. Clin. Investig. 2022, 132, e158524. [Google Scholar] [CrossRef]
- Koski, C.L.; Estep, A.E.; Sawant-Mane, S.; Shin, M.L.; Highbarger, L.; Hansch, G.M. Complement regulatory molecules on human myelin and glial cells: Differential expression affects the deposition of activated complement proteins. J. Neurochem. 1996, 66, 303–312. [Google Scholar] [CrossRef]
- Dashiell, S.M.; Vanguri, P.; Koski, C.L. Dibutyryl cyclic AMP and inflammatory cytokines mediate C3 expression in Schwann cells. Glia 1997, 20, 308–321. [Google Scholar] [CrossRef]
- Neal, J.W.; Gasque, P. The role of primary infection of Schwann cells in the aetiology of infective inflammatory neuropathies. J. Infect. 2016, 73, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Bedoui, Y.; De Larichaudy, D.; Daniel, M.; Ah-Pine, F.; Selambarom, J.; Guiraud, P.; Gasque, P. Deciphering the Role of Schwann Cells in Inflammatory Peripheral Neuropathies Post Alphavirus Infection. Cells 2022, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.E.; Meehan, G.R.; Robinson, S.; Yao, D.; McGonigal, R.; Willison, H.J. Perisynaptic Schwann cells phagocytose nerve terminal debris in a mouse model of Guillain-Barré syndrome. J. Peripher. Nerv. Syst. 2020, 25, 143–151. [Google Scholar] [CrossRef]
- Querol, L.; Devaux, J.; Rojas-Garcia, R.; Illa, I. Autoantibodies in chronic inflammatory neuropathies: Diagnostic and therapeutic implications. Nat. Rev. Neurol. 2017, 13, 533–547. [Google Scholar] [CrossRef]
- Mathey, E.K.; Park, S.B.; Hughes, R.A.; Pollard, J.D.; Armati, P.J.; Barnett, M.H.; Taylor, B.V.; Dyck, P.J.; Kiernan, M.C.; Lin, C.S. Chronic inflammatory demyelinating polyradiculoneuropathy: From pathology to phenotype. J. Neurol. Neurosurg. Psychiatry 2015, 86, 973–985. [Google Scholar] [CrossRef]
- Vallat, J.-M.; Mathis, S.; Vegezzi, E.; Richard, L.; Duchesne, M.; Gallouedec, G.; Corcia, P.; Magy, L.; Uncini, A.; Devaux, J. Antibody- and macrophage-mediated segmental demyelination in chronic inflammatory demyelinating polyneuropathy: Clinical, electrophysiological, immunological and pathological correlates. Eur. J. Neurol. 2020, 27, 692–701. [Google Scholar] [CrossRef]
- Fehmi, J.; Scherer, S.S.; Willison, H.J.; Rinaldi, S. Nodes, paranodes and neuropathies. J. Neurol. Neurosurg. Psychiatry 2018, 89, 61–71. [Google Scholar] [CrossRef]
- Budding, K.; Bos, J.W.; Dijkxhoorn, K.; de Zeeuw, E.; Bloemenkamp, L.M.; Zekveld, E.M.; Groen, E.J.N.; Jacobs, B.C.; Huizinga, R.; Goedee, H.S.; et al. IgM anti-GM2 antibodies in patients with multifocal motor neuropathy target Schwann cells and are associated with early onset. J. Neuroinflamm. 2024, 21, 100. [Google Scholar] [CrossRef]
- Aranami, T.; Yamamura, T. Pathogenesis of chronic inflammatory demyelinating polyneuropathy. Nihon Rinsho 2013, 71, 850–854. [Google Scholar]
- Murata, K.-y.; Dalakas, M.C. Expression of the co-stimulatory molecule BB-1, the ligands CTLA-4 and CD28 and their mRNAs in chronic inflammatory demyelinating polyneuropathy. Brain 2000, 123, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Van Rhijn, I.; Van den Berg, L.H.; Bosboom, W.M.; Otten, H.G.; Logtenberg, T. Expression of accessory molecules for T-cell activation in peripheral nerve of patients with CIDP and vasculitic neuropathy. Brain 2000, 123 Pt 10, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.R.; Holtmann, L.; Bobylev, I.; Schneider, C.; Ritter, C.; Weis, J.; Lehmann, H.C. Loss of Schwann cell plasticity in chronic inflammatory demyelinating polyneuropathy (CIDP). J. Neuroinflamm. 2016, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Katsuno, M. Paraproteinemia and neuropathy. Neurol. Sci. 2021, 42, 4489–4501. [Google Scholar] [CrossRef]
- Shelly, S.; Dubey, D.; Mills, J.R.; Klein, C.J. Chapter 15—Paraneoplastic neuropathies and peripheral nerve hyperexcitability disorders. In Handbook of Clinical Neurology; Giometto, B., Pittock, S.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 200, pp. 239–273. [Google Scholar]
- Lach, B.; Rippstein, P.; Atack, D.; Afar, D.E.; Gregor, A. Immunoelectron microscopic localization of monoclonal IgM antibodies in gammopathy associated with peripheral demyelinative neuropathy. Acta Neuropathol. 1993, 85, 298–307. [Google Scholar] [CrossRef]
- Rison, R.A.; Beydoun, S.R. Paraproteinemic neuropathy: A practical review. BMC Neurol. 2016, 16, 13. [Google Scholar] [CrossRef]
- Chaudhry, H.M.; Mauermann, M.L.; Rajkumar, S.V. Monoclonal Gammopathy-Associated Peripheral Neuropathy: Diagnosis and Management. Mayo Clin. Proc. 2017, 92, 838–850. [Google Scholar] [CrossRef]
- Maisonobe, T.; Léger, J.M.; Musset, L.; Cacoub, P. Neurological manifestations in cryoglobulinemia. Rev. Neurol. 2002, 158, 920–924. [Google Scholar]
- Dando, S.J.; Mackay-Sim, A.; Norton, R.; Currie, B.J.; St John, J.A.; Ekberg, J.A.; Batzloff, M.; Ulett, G.C.; Beacham, I.R. Pathogens penetrating the central nervous system: Infection pathways and the cellular and molecular mechanisms of invasion. Clin. Microbiol. Rev. 2014, 27, 691–726. [Google Scholar] [CrossRef]
- McGavern, D.B.; Kang, S.S. Illuminating viral infections in the nervous system. Nat. Rev. Immunol. 2011, 11, 318–329. [Google Scholar] [CrossRef]
- Bello-Morales, R.; Andreu, S.; López-Guerrero, J.A. The Role of Herpes Simplex Virus Type 1 Infection in Demyelination of the Central Nervous System. Int. J. Mol. Sci. 2020, 21, 5026. [Google Scholar] [CrossRef]
- Giraudon, P.; Bernard, A. Chronic viral infections of the central nervous system: Aspects specific to multiple sclerosis. Rev. Neurol. 2009, 165, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.B.; de Souza, F.M.A.; de Sá, L.B.M.; Pacheco, A.L.D.; Prado, M.R.; de Sousa Rodrigues, C.F.; Bassi, Ê.J.; Santana-Melo, I.; Silva-Júnior, A.; Sabino-Silva, R.; et al. Potential Mechanisms Underlying COVID-19-Mediated Central and Peripheral Demyelination: Roles of the RAAS and ADAM-17. Mol. Neurobiol. 2025, 62, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Tabanella, G.; Nowzari, H. Cytomegalovirus-associated periodontitis and Guillain-Barré syndrome. J. Periodontol. 2005, 76, 2306–2311. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kunishige, M.; Shinohara, M.; Kubo, K.; Inoue, H.; Yoshino, H.; Asano, A.; Honda, S.; Matsumoto, T.; Mitsui, T. Guillain-Barré syndrome and hemophagocytic lymphohistiocytosis in a patient with severe chronic active Epstein-Barr virus infection syndrome. Clin. Neurol. Neurosurg. 2005, 108, 80–83. [Google Scholar] [CrossRef]
- Brannagan, T.H., 3rd; Zhou, Y. HIV-associated Guillain-Barré syndrome. J. Neurol. Sci. 2003, 208, 39–42. [Google Scholar] [CrossRef]
- Roccatagliata, L.; Uccelli, A.; Murialdo, A. Guillain-Barré syndrome after reactivation of varicella-zoster virus. N. Engl. J. Med. 2001, 344, 65–66. [Google Scholar] [CrossRef]
- Sindic, C.J. Infectious neuropathies. Curr. Opin. Neurol. 2013, 26, 510–515. [Google Scholar] [CrossRef]
- Keswani, S.C.; Polley, M.; Pardo, C.A.; Griffin, J.W.; McArthur, J.C.; Hoke, A. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann. Neurol. 2003, 54, 287–296. [Google Scholar] [CrossRef]
- Pardo, C.A.; McArthur, J.C.; Griffin, J.W. HIV neuropathy: Insights in the pathology of HIV peripheral nerve disease. J. Peripher. Nerv. Syst. 2001, 6, 21–27. [Google Scholar] [CrossRef]
- Rambukkana, A.; Kunz, S.; Min, J.; Campbell, K.P.; Oldstone, M.B. Targeting Schwann cells by nonlytic arenaviral infection selectively inhibits myelination. Proc. Natl. Acad. Sci. USA 2003, 100, 16071–16076. [Google Scholar] [CrossRef]
- Weinkauf, C.; Salvador, R.; PereiraPerrin, M. Neurotrophin receptor TrkC is an entry receptor for Trypanosoma cruzi in neural, glial, and epithelial cells. Infect. Immun. 2011, 79, 4081–4087. [Google Scholar] [CrossRef]
- Hughes, D.; Narang, H.K.; Kelso, W. The effects of diphtheria toxin on developing peripheral myelin in culture. J. Neurol. Sci. 1972, 15, 457–470. [Google Scholar] [CrossRef]
- Pappenheimer, A.M., Jr.; Harper, A.A.; Moynihan, M.; Brockes, J.P. Diphtheria toxin and related proteins: Effect of route of injection on toxicity and the determination of cytotoxicity for various cultured cells. J. Infect. Dis. 1982, 145, 94–102. [Google Scholar] [CrossRef]
- Prasad, P.L.; Rai, P.L. Prospective Study of Diphtheria for Neurological Complications. J. Pediatr. Neurosci. 2018, 13, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.C.; Efstratiou, A.; Mokrousov, I.; Mutreja, A.; Das, B.; Ramamurthy, T. Diphtheria. Nat. Rev. Dis. Primers 2019, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Y.; Sizer, K.C.; Thompson, E.J.; Kabanja, J.; Li, J.; Hu, P.; Gómez-Valero, L.; Silva, F.J. Comparative sequence analysis of Mycobacterium leprae and the new leprosy-causing Mycobacterium lepromatosis. J. Bacteriol. 2009, 191, 6067–6074. [Google Scholar] [CrossRef] [PubMed]
- Scollard, D.M.; Truman, R.W.; Ebenezer, G.J. Mechanisms of nerve injury in leprosy. Clin. Dermatol. 2015, 33, 46–54. [Google Scholar] [CrossRef]
- Brügger, L.M.d.O.; dos Santos, M.M.L.; Lara, F.A.; Mietto, B.S. What happens when Schwann cells are exposed to Mycobacterium leprae–A systematic review. IBRO Neurosci. Rep. 2023, 15, 11–16. [Google Scholar] [CrossRef]
- Ebenezer, G.J.; Scollard, D.M. Treatment and Evaluation Advances in Leprosy Neuropathy. Neurotherapeutics 2021, 18, 2337–2350. [Google Scholar] [CrossRef]
- Medeiros, R.C.A.; Girardi, K.; Cardoso, F.K.L.; Mietto, B.S.; Pinto, T.G.T.; Gomez, L.S.; Rodrigues, L.S.; Gandini, M.; Amaral, J.J.; Antunes, S.L.G.; et al. Subversion of Schwann cell glucose metabolism by Mycobacterium leprae. J. Biol. Chem. 2016, 291, 24803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rosa, T.; Marques, M.A.M.; DeBoard, Z.; Hutchins, K.; Silva, C.A.A.; Montague, C.R.; Yuan, T.; Amaral, J.J.; Atella, G.C.; Rosa, P.S.; et al. Corrigendum: Reductive Power Generated by Mycobacterium leprae Through Cholesterol Oxidation Contributes to Lipid and ATP Synthesis. Front. Cell Infect. Microbiol. 2021, 11, 765326. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.F.; Medeiros, R.C.A.; Mietto, B.S.; Calvo, T.L.; Mendonça, A.P.M.; Rosa, T.; Silva, D.S.D.; Vasconcelos, K.; Pereira, A.M.R.; de Macedo, C.S.; et al. Reduction of host cell mitochondrial activity as Mycobacterium leprae’s strategy to evade host innate immunity. Immunol. Rev. 2021, 301, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.R.S.; Amôr, N.G.; Michellin, L.B.; Cury Filho, M.; Rosa, P.S.; Latini, A.C.P.; Rodrigues, L.S.; Lemes, R.M.R.; Lara, F.A.; Pessolani, M.C.V. Effect of Mycobacterium leprae on neurotrophins expression in human Schwann cells and mouse sciatic nerves. Mem. Do Inst. Oswaldo Cruz 2020, 115, e200075. [Google Scholar] [CrossRef]
- Singh, N.; Birdi, T.J.; Chandrashekar, S.; Antia, N.H. Schwann cell extracellular matrix protein production is modulated by Mycobacterium leprae and macrophage secretory products. J. Neurol. Sci. 1997, 151, 13–22. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Antunes, S.L.; Teles, R.M.; Costa da Silva, A.C.; Silva, T.P.; Brandão Teles, R.; Ferreira Medeiros, M.; Britto, C.; Jardim, M.R.; Pereira Sampaio, E.; et al. Schwann cells producing matrix metalloproteinases under Mycobacterium leprae stimulation may play a role in the outcome of leprous neuropathy. J. Neuropathol. Exp. Neurol. 2010, 69, 27–39. [Google Scholar] [CrossRef]
- Petito, R.B.; Amadeu, T.P.; Pascarelli, B.M.; Jardim, M.R.; Vital, R.T.; Antunes, S.L.; Sarno, E.N. Transforming growth factor-β1 may be a key mediator of the fibrogenic properties of neural cells in leprosy. J. Neuropathol. Exp. Neurol. 2013, 72, 351–366. [Google Scholar] [CrossRef]
- Oliveira, R.B.; Sampaio, E.P.; Aarestrup, F.; Teles, R.M.; Silva, T.P.; Oliveira, A.L.; Antas, P.R.; Sarno, E.N. Cytokines and Mycobacterium leprae induce apoptosis in human Schwann cells. J. Neuropathol. Exp. Neurol. 2005, 64, 882–890. [Google Scholar] [CrossRef]
- Souza, B.J.d.; Mendes, M.A.; Sperandio da Silva, G.M.; Sammarco-Rosa, P.; Moraes, M.O.d.; Jardim, M.R.; Sarno, E.N.; Pinheiro, R.O.; Mietto, B.S. Gene Expression Profile of Mycobacterium leprae Contribution in the Pathology of Leprosy Neuropathy. Front. Med. 2022, 9, 861586. [Google Scholar] [CrossRef]
- Mietto, B.; Andrade, P.; Jardim, M.; Antunes, S.; Sarno, E. Demyelination in peripheral nerves: Much to learn from leprosy neuropathy. J. Mult. Scler. 2016, 3, 174. [Google Scholar]
- Hess, S.; Rambukkana, A. Cell biology of intracellular adaptation of Mycobacterium leprae in the peripheral nervous system. In Bacteria and Intracellularity; Wiley: Hoboken, NJ, USA, 2019; pp. 227–245. [Google Scholar]
- Steinhoff, U.; Kaufmann, S.H. Specific lysis by CD8+ T cells of Schwann cells expressing Mycobacterium leprae antigens. Eur. J. Immunol. 1988, 18, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Spierings, E.; De Boer, T.; Zulianello, L.; Ottenhoff, T.H. Novel mechanisms in the immunopathogenesis of leprosy nerve damage: The role of Schwann cells, T cells and Mycobacterium leprae. Immunol. Cell Biol. 2000, 78, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Scollard, D.M. The biology of nerve injury in leprosy. Lepr. Rev. 2008, 79, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Su, S.B.; Chen, K.T. An update of the diagnosis, treatment, and prevention of leprosy: A narrative review. Medicine 2024, 103, e39006. [Google Scholar] [CrossRef]
- Fridman, V.; Reilly, M.M. Inherited Neuropathies. Semin. Neurol. 2015, 35, 407–423. [Google Scholar] [CrossRef]
- Van Lent, J.; Prior, R.; Pérez Siles, G.; Cutrupi, A.N.; Kennerson, M.L.; Vangansewinkel, T.; Wolfs, E.; Mukherjee-Clavin, B.; Nevin, Z.; Judge, L.; et al. Advances and challenges in modeling inherited peripheral neuropathies using iPSCs. Exp. Mol. Med. 2024, 56, 1348–1364. [Google Scholar] [CrossRef]
- Brennan, K.M.; Bai, Y.; Shy, M.E. Demyelinating CMT--what’s known, what’s new and what’s in store? Neurosci. Lett. 2015, 596, 14–26. [Google Scholar] [CrossRef]
- McLean, J.W.; Wilson, J.A.; Tian, T.; Watson, J.A.; VanHart, M.; Bean, A.J.; Scherer, S.S.; Crossman, D.K.; Ubogu, E.; Wilson, S.M. Disruption of Endosomal Sorting in Schwann Cells Leads to Defective Myelination and Endosomal Abnormalities Observed in Charcot-Marie-Tooth Disease. J. Neurosci. 2022, 42, 5085–5101. [Google Scholar] [CrossRef]
- Sahenk, Z.; Nagaraja, H.N.; McCracken, B.S.; King, W.M.; Freimer, M.L.; Cedarbaum, J.M.; Mendell, J.R. NT-3 promotes nerve regeneration and sensory improvement in CMT1A mouse models and in patients. Neurology 2005, 65, 681–689. [Google Scholar] [CrossRef]
- Berger, P.; Niemann, A.; Suter, U. Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease). Glia 2006, 54, 243–257. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhang, H.; Meng, L.B.; Tang, X.Y.; Gong, T.; Yin, J. Novel mutation in the periaxin gene causal to Charcot-Marie-Tooth disease type 4F. J. Int. Med. Res. 2020, 48, 300060519862064. [Google Scholar] [CrossRef]
- D’Urso, D.; Prior, R.; Greiner-Petter, R.; Gabreels-Festen, A.A.; Muller, H.W. Overloaded endoplasmic reticulum-Golgi compartments, a possible pathomechanism of peripheral neuropathies caused by mutations of the peripheral myelin protein PMP22. J. Neurosci. 1998, 18, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Hanemann, C.O.; D’Urso, D.; Gabreels-Festen, A.A.; Muller, H.W. Mutation-dependent alteration in cellular distribution of peripheral myelin protein 22 in nerve biopsies from Charcot-Marie-Tooth type 1A. Brain 2000, 123 Pt 5, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Fortun, J.; Dunn, W.A., Jr.; Joy, S.; Li, J.; Notterpek, L. Emerging role for autophagy in the removal of aggresomes in Schwann cells. J. Neurosci. 2003, 23, 10672–10680. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Sunada, Y. Schwann Cell and the Pathogenesis of Charcot-Marie-Tooth Disease. Adv. Exp. Med. Biol. 2019, 1190, 301–321. [Google Scholar] [CrossRef]
- Nobbio, L.; Vigo, T.; Abbruzzese, M.; Levi, G.; Brancolini, C.; Mantero, S.; Grandis, M.; Benedetti, L.; Mancardi, G.; Schenone, A. Impairment of PMP22 transgenic Schwann cells differentiation in culture: Implications for Charcot-Marie-Tooth type 1A disease. Neurobiol. Dis. 2004, 16, 263–273. [Google Scholar] [CrossRef]
- Wang, H.; Davison, M.; Wang, K.; Xia, T.H.; Kramer, M.; Call, K.; Luo, J.; Wu, X.; Zuccarino, R.; Bacon, C.; et al. Transmembrane protease serine 5: A novel Schwann cell plasma marker for CMT1A. Ann. Clin. Transl. Neurol. 2020, 7, 69–82. [Google Scholar] [CrossRef]
- Srinivasan, R.; Sun, G.; Keles, S.; Jones, E.A.; Jang, S.W.; Krueger, C.; Moran, J.J.; Svaren, J. Genome-wide analysis of EGR2/SOX10 binding in myelinating peripheral nerve. Nucleic Acids Res. 2012, 40, 6449–6460. [Google Scholar] [CrossRef]
- Prior, R.; Silva, A.; Vangansewinkel, T.; Idkowiak, J.; Tharkeshwar, A.K.; Hellings, T.P.; Michailidou, I.; Vreijling, J.; Loos, M.; Koopmans, B.; et al. PMP22 duplication dysregulates lipid homeostasis and plasma membrane organization in developing human Schwann cells. Brain 2024, 174, 3113–3130. [Google Scholar] [CrossRef]
- Melfi, S.; Montt Guevara, M.M.; Bonalume, V.; Ruscica, M.; Colciago, A.; Simoncini, T.; Magnaghi, V. Src and phospho-FAK kinases are activated by allopregnanolone promoting Schwann cell motility, morphology and myelination. J. Neurochem. 2017, 141, 165–178. [Google Scholar] [CrossRef]
- Castelnovo, L.F.; Thomas, P.; Magnaghi, V. Membrane progesterone receptors (mPRs/PAQRs) in Schwann cells represent a promising target for the promotion of neuroregeneration. Neural Regen. Res. 2021, 16, 281–282. [Google Scholar] [CrossRef]
- Nave, K.A.; Sereda, M.W.; Ehrenreich, H. Mechanisms of disease: Inherited demyelinating neuropathies--from basic to clinical research. Nat. Clin. Pract. Neurol. 2007, 3, 453–464. [Google Scholar] [CrossRef]
- Sereda, M.W.; Meyer zu Hörste, G.; Suter, U.; Uzma, N.; Nave, K.A. Therapeutic administration of progesterone antagonist in a model of Charcot-Marie-Tooth disease (CMT-1A). Nat. Med. 2003, 9, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, K.; Penno, A.; Schmidt, B.P.; Lee, H.J.; Frosch, M.P.; von Eckardstein, A.; Brown, R.H.; Hornemann, T.; Eichler, F.S. Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J. Clin. Investig. 2011, 121, 4735–4745. [Google Scholar] [CrossRef] [PubMed]
- Fridman, V.; Suriyanarayanan, S.; Novak, P.; David, W.; Macklin, E.A.; McKenna-Yasek, D.; Walsh, K.; Aziz-Bose, R.; Oaklander, A.L.; Brown, R. Randomized trial of l-serine in patients with hereditary sensory and autonomic neuropathy type 1. Neurology 2019, 92, e359–e370. [Google Scholar] [CrossRef] [PubMed]
- Patzko, A.; Bai, Y.; Saporta, M.A.; Katona, I.; Wu, X.; Vizzuso, D.; Feltri, M.L.; Wang, S.; Dillon, L.M.; Kamholz, J.; et al. Curcumin derivatives promote Schwann cell differentiation and improve neuropathy in R98C CMT1B mice. Brain 2012, 135, 3551–3566. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Pehlivan, D.; Wiszniewski, W.; Beck, C.R.; Snipes, G.J.; Lupski, J.R.; Khajavi, M. Curcumin facilitates a transitory cellular stress response in Trembler-J mice. Hum. Mol. Genet. 2013, 22, 4698–4705. [Google Scholar] [CrossRef]
- Passage, E.; Norreel, J.C.; Noack-Fraissignes, P.; Sanguedolce, V.; Pizant, J.; Thirion, X.; Robaglia-Schlupp, A.; Pellissier, J.F.; Fontés, M. Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot-Marie-Tooth disease. Nat. Med. 2004, 10, 396–401. [Google Scholar] [CrossRef]
- Lewis, R.A.; McDermott, M.P.; Herrmann, D.N.; Hoke, A.; Clawson, L.L.; Siskind, C.; Feely, S.M.; Miller, L.J.; Barohn, R.J.; Smith, P.; et al. High-dosage ascorbic acid treatment in Charcot-Marie-Tooth disease type 1A: Results of a randomized, double-masked, controlled trial. JAMA Neurol. 2013, 70, 981–987. [Google Scholar] [CrossRef]
- Pareyson, D.; Reilly, M.M.; Schenone, A.; Fabrizi, G.M.; Cavallaro, T.; Santoro, L.; Vita, G.; Quattrone, A.; Padua, L.; Gemignani, F.; et al. Ascorbic acid in Charcot-Marie-Tooth disease type 1A (CMT-TRIAAL and CMT-TRAUK): A double-blind randomised trial. Lancet Neurol. 2011, 10, 320–328. [Google Scholar] [CrossRef]
- Sahenk, Z.; Galloway, G.; Clark, K.R.; Malik, V.; Rodino-Klapac, L.R.; Kaspar, B.K.; Chen, L.; Braganza, C.; Montgomery, C.; Mendell, J.R. AAV1.NT-3 gene therapy for charcot-marie-tooth neuropathy. Mol. Ther. 2014, 22, 511–521. [Google Scholar] [CrossRef]
- Ozes, B.; Myers, M.; Moss, K.; McKinney, J.; Ridgley, A.; Chen, L.; Bai, S.; Abrams, C.K.; Freidin, M.M.; Mendell, J.R.; et al. AAV1.NT-3 gene therapy for X-linked Charcot-Marie-Tooth neuropathy type 1. Gene Ther. 2022, 29, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Sahenk, Z.; Oblinger, J.; Edwards, C. Neurotrophin-3 deficient Schwann cells impair nerve regeneration. Exp. Neurol. 2008, 212, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jung, N.; Myung, S.; Choi, Y.; Chung, K.W.; Choi, B.O.; Jung, S.C. Differentiation of Human Tonsil-Derived Mesenchymal Stem Cells into Schwann-Like Cells Improves Neuromuscular Function in a Mouse Model of Charcot-Marie-Tooth Disease Type 1A. Int. J. Mol. Sci. 2018, 19, 2393. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.H.; Park, S.; Yum, Y.; Jeong, S.; Park, H.E.; Kim, H.J.; Lim, J.; Choi, B.O.; Jung, S.C. Preclinical Efficacy of Peripheral Nerve Regeneration by Schwann Cell-like Cells Differentiated from Human Tonsil-Derived Mesenchymal Stem Cells in C22 Mice. Biomedicines 2023, 11, 3334. [Google Scholar] [CrossRef]
- Sahenk, Z.; Ozes, B. Gene therapy to promote regeneration in Charcot-Marie-Tooth disease. Brain Res. 2020, 1727, 146533. [Google Scholar] [CrossRef]
- Stavrou, M.; Sargiannidou, I.; Georgiou, E.; Kagiava, A.; Kleopa, K.A. Emerging Therapies for Charcot-Marie-Tooth Inherited Neuropathies. Int. J. Mol. Sci. 2021, 22, 6048. [Google Scholar] [CrossRef]
- Attarian, S.; Fatehi, F.; Rajabally, Y.A.; Pareyson, D. Hereditary neuropathy with liability to pressure palsies. J. Neurol. 2020, 267, 2198–2206. [Google Scholar] [CrossRef]
- Pantera, H.; Hu, B.; Moiseev, D.; Dunham, C.; Rashid, J.; Moran, J.J.; Krentz, K.; Rubinstein, C.D.; Won, S.; Li, J.; et al. Pmp22 super-enhancer deletion causes tomacula formation and conduction block in peripheral nerves. Hum. Mol. Genet. 2020, 29, 1689–1699. [Google Scholar] [CrossRef]
- Koike, H.; Furukawa, S.; Mouri, N.; Fukami, Y.; Iijima, M.; Katsuno, M. Dosage effects of PMP22 on nonmyelinating Schwann cells in hereditary neuropathy with liability to pressure palsies. Neuromuscul. Disord. 2022, 32, 503–511. [Google Scholar] [CrossRef]
- Spagnoli, C.; Pisani, F.; Di Mario, F.; Leandro, G.; Gaiani, F.; De’ Angelis, G.L.; Fusco, C. Peripheral neuropathy and gastroenterologic disorders: An overview on an underrecognized association. Acta Biomed. 2018, 89, 22–32. [Google Scholar] [CrossRef]
- Gondim, F.A.; Brannagan, T.H., 3rd; Sander, H.W.; Chin, R.L.; Latov, N. Peripheral neuropathy in patients with inflammatory bowel disease. Brain 2005, 128, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Skeen, M.B. Neurologic manifestations of gastrointestinal disease. Neurol. Clin. 2002, 20, 195–225. [Google Scholar] [CrossRef] [PubMed]
- Elsehety, A.; Bertorini, T.E. Neurologic and neuropsychiatric complications of Crohn’s disease. South. Med. J. 1997, 90, 606–610. [Google Scholar] [CrossRef]
- Thawani, S.P.; Brannagan, T.H., 3rd; Lebwohl, B.; Green, P.H.; Ludvigsson, J.F. Risk of Neuropathy Among 28,232 Patients with Biopsy-Verified Celiac Disease. JAMA Neurol. 2015, 72, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Goluba, K.; Kunrade, L.; Riekstina, U.; Parfejevs, V. Schwann Cells in Digestive System Disorders. Cells 2022, 11, 832. [Google Scholar] [CrossRef]
- Jonscher, R.; Belkind-Gerson, J. Concise Review: Cellular and Molecular Mechanisms of Postnatal Injury-Induced Enteric Neurogenesis. Stem Cells 2019, 37, 1136–1143. [Google Scholar] [CrossRef]
- Lyu, Y.; Xie, F.; Chen, B.; Shin, W.S.; Chen, W.; He, Y.; Leung, K.T.; Tse, G.M.K.; Yu, J.; To, K.F.; et al. The nerve cells in gastrointestinal cancers: From molecular mechanisms to clinical intervention. Oncogene 2024, 43, 77–91. [Google Scholar] [CrossRef]
- De Giorgio, R.; Guerrini, S.; Barbara, G.; Stanghellini, V.; De Ponti, F.; Corinaldesi, R.; Moses, P.L.; Sharkey, K.A.; Mawe, G.M. Inflammatory neuropathies of the enteric nervous system. Gastroenterology 2004, 126, 1872–1883. [Google Scholar] [CrossRef]
- Soret, R.; Schneider, S.; Bernas, G.; Christophers, B.; Souchkova, O.; Charrier, B.; Righini-Grunder, F.; Aspirot, A.; Landry, M.; Kembel, S.W.; et al. Glial Cell-Derived Neurotrophic Factor Induces Enteric Neurogenesis and Improves Colon Structure and Function in Mouse Models of Hirschsprung Disease. Gastroenterology 2020, 159, 1824–1838.e1817. [Google Scholar] [CrossRef]
- Pan, W.; Rahman, A.A.; Stavely, R.; Bhave, S.; Guyer, R.; Omer, M.; Picard, N.; Goldstein, A.M.; Hotta, R. Schwann Cells in the Aganglionic Colon of Hirschsprung Disease Can Generate Neurons for Regenerative Therapy. Stem Cells Transl. Med. 2022, 11, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Stenager, E.; Knudsen, L.; Jensen, K. Acute and chronic pain syndromes in multiple sclerosis. Acta Neurol. Scand. 1991, 84, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Adamian, L.; Fiocchi, J.; Hoyt, D.; Ernst, C.; Kaye, A.D.; Viswanath, O. Advances in the Understanding and Management of Chronic Pain in Multiple Sclerosis: A Comprehensive Review. Curr. Pain Headache Rep. 2019, 23, 59. [Google Scholar] [CrossRef] [PubMed]
- Solaro, C.; Trabucco, E.; Messmer Uccelli, M. Pain and multiple sclerosis: Pathophysiology and treatment. Curr. Neurol. Neurosci. Rep. 2013, 13, 320. [Google Scholar] [CrossRef]
- Mitsikostas, D.D.; Moka, E.; Orrillo, E.; Aurilio, C.; Vadalouca, A.; Paladini, A.; Varrassi, G. Neuropathic Pain in Neurologic Disorders: A Narrative Review. Cureus 2022, 14, e22419. [Google Scholar] [CrossRef]
- Schiffmann, R.; Moore, D.F. Neurological manifestations of Fabry disease. In Fabry Disease: Perspectives from 5 Years of FOS.; Mehta, A., Beck, M., Sunder-Plassmann, G., Eds.; Oxford PharmaGenesis: Oxford, UK, 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK11602/ (accessed on 8 June 2024).
- Waltz, T.B.; Chao, D.; Prodoehl, E.K.; Enders, J.D.; Ehlers, V.L.; Dharanikota, B.S.; Dahms, N.M.; Isaeva, E.; Hogan, Q.H.; Pan, B.; et al. Fabry disease Schwann cells release p11 to induce sensory neuron hyperactivity. JCI Insight 2024, 9, e172869. [Google Scholar] [CrossRef]
- Zhang, W.J.; Liu, S.C.; Ming, L.G.; Yu, J.W.; Zuo, C.; Hu, D.X.; Luo, H.L.; Zhang, Q. Potential role of Schwann cells in neuropathic pain. Eur. J. Pharmacol. 2023, 956, 175955. [Google Scholar] [CrossRef]
- Taveggia, C.; Feltri, M.L. Beyond Wrapping: Canonical and Noncanonical Functions of Schwann Cells. Annu. Rev. Neurosci. 2022, 45, 561–580. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Zhou, T.-H.; Chen, B.-K.; Liu, Z.-X. Schwann cells and trigeminal neuralgia. Mol. Pain 2020, 16, 1744806920963809. [Google Scholar] [CrossRef]
- Brum, E.S.; Fialho, M.F.P.; Souza Monteiro de Araújo, D.; Landini, L.; Marini, M.; Titiz, M.; Kuhn, B.L.; Frizzo, C.P.; Araújo, P.H.S.; Guimarães, R.M.; et al. Schwann cell TRPA1 elicits reserpine-induced fibromyalgia pain in mice. Br. J. Pharmacol. 2024, 181, 3445–3461. [Google Scholar] [CrossRef]
- Brum, E.S.; Landini, L.; Souza Monteiro de Araújo, D.; Marini, M.; Geppetti, P.; Nassini, R.; De Logu, F.; Oliveira, S.M. Characterisation of periorbital mechanical allodynia in the reserpine-induced fibromyalgia model in mice: The role of the Schwann cell TRPA1/NOX1 signalling pathway. Free Radic. Biol. Med. 2025, 229, 289–299. [Google Scholar] [CrossRef]
- Orita, S.; Henry, K.; Mantuano, E.; Yamauchi, K.; De Corato, A.; Ishikawa, T.; Feltri, M.L.; Wrabetz, L.; Gaultier, A.; Pollack, M.; et al. Schwann cell LRP1 regulates remak bundle ultrastructure and axonal interactions to prevent neuropathic pain. J. Neurosci. 2013, 33, 5590–5602. [Google Scholar] [CrossRef]
- Poplawski, G.; Ishikawa, T.; Brifault, C.; Lee-Kubli, C.; Regestam, R.; Henry, K.W.; Shiga, Y.; Kwon, H.; Ohtori, S.; Gonias, S.L.; et al. Schwann cells regulate sensory neuron gene expression before and after peripheral nerve injury. Glia 2018, 66, 1577–1590. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Liu, Y.G.; Chen, D.J.; Yang, B.L.; Liu, T.T.; Li, J.J.; Wang, X.Q.; Li, H.R.; Liu, Z.X. Microencapsulated Schwann cell transplantation inhibits P2X2/3 receptors overexpression in a sciatic nerve injury rat model with neuropathic pain. Neurosci. Lett. 2018, 676, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, H.Y.; Ling, Z.M.; Chen, G.; Wei, Z.Y. Inhibition of Schwann cell pannexin 1 attenuates neuropathic pain through the suppression of inflammatory responses. J. Neuroinflamm. 2022, 19, 244. [Google Scholar] [CrossRef]
- Brifault, C.; Romero, H.; Van-Enoo, A.; Pizzo, D.; Azmoon, P.; Kwon, H.; Nasamran, C.; Gonias, S.L.; Campana, W.M. Deletion of the Gene Encoding the NMDA Receptor GluN1 Subunit in Schwann Cells Causes Ultrastructural Changes in Remak Bundles and Hypersensitivity in Pain Processing. J. Neurosci. 2020, 40, 9121–9136. [Google Scholar] [CrossRef] [PubMed]
- Snijders, R.A.H.; Brom, L.; Theunissen, M.; van den Beuken-van Everdingen, M.H.J. Update on Prevalence of Pain in Patients with Cancer 2022: A Systematic Literature Review and Meta-Analysis. Cancers 2023, 15, 591. [Google Scholar] [CrossRef] [PubMed]
- Salvo, E.; Saraithong, P.; Curtin, J.G.; Janal, M.N.; Ye, Y. Reciprocal interactions between cancer and Schwann cells contribute to oral cancer progression and pain. Heliyon 2019, 5, e01223. [Google Scholar] [CrossRef]
- Salvo, E.; Tu, N.H.; Scheff, N.N.; Dubeykovskaya, Z.A.; Chavan, S.A.; Aouizerat, B.E.; Ye, Y. TNFα promotes oral cancer growth, pain, and Schwann cell activation. Sci. Rep. 2021, 11, 1840. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wu, C.L.; Liu, J.P. Schwann cells as a target cell for the treatment of cancer pain. Glia 2023, 71, 2309–2322. [Google Scholar] [CrossRef]
- Mulpuri, Y.; Tu, N.H.; Inoue, K.; Harden, G.; Nicholson, S.J.; Seenauth, A.; Huang, Y.; Escobar, K.G.; Moayedi, Y.; Bunnett, N.W.; et al. TRPV4 activation in Schwann cells mediates mechanically induced pain of oral cancer. Front. Pain. Res. 2025, 6, 1532885. [Google Scholar] [CrossRef]
- Itson-Zoske, B.; Gani, U.; Mikesell, A.; Qiu, C.; Fan, F.; Stucky, C.L.; Hogan, Q.H.; Shin, S.M.; Yu, H. Selective RNAi silencing of Schwann cell Piezo1 alleviates mechanical hypersensitization following peripheral nerve injury. Mol. Ther. Methods Clin. Dev. 2025, 33, 101433. [Google Scholar] [CrossRef] [PubMed]
- Magnaghi, V.; Procacci, P.; Tata, A.M. Chapter 15: Novel pharmacological approaches to Schwann cells as neuroprotective agents for peripheral nerve regeneration. Int. Rev. Neurobiol. 2009, 87, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Kim, M.; Jung, J.; Jeong, N.Y. Inhibition of Neuronal Nitric Oxide Synthase by Ethyl Pyruvate in Schwann Cells Protects Against Peripheral Nerve Degeneration. Neurochem. Res. 2019, 44, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, H.; Kim, D.; Kim, D.; Huh, Y.; Park, C.; Chung, H.J.; Jung, J.; Jeong, N.Y. Heme Oxygenase 1 in Schwann Cells Regulates Peripheral Nerve Degeneration Against Oxidative Stress. ASN Neuro 2019, 11, 1759091419838949. [Google Scholar] [CrossRef]
- Keilhoff, G.; Schild, L.; Fansa, H. Minocycline protects Schwann cells from ischemia-like injury and promotes axonal outgrowth in bioartificial nerve grafts lacking Wallerian degeneration. Exp. Neurol. 2008, 212, 189–200. [Google Scholar] [CrossRef]
- Piñero, G.; Berg, R.; Andersen, N.D.; Setton-Avruj, P.; Monje, P.V. Lithium Reversibly Inhibits Schwann Cell Proliferation and Differentiation Without Inducing Myelin Loss. Mol. Neurobiol. 2017, 54, 8287–8307. [Google Scholar] [CrossRef]
- Schepers, M.; Malheiro, A.; Gamardo, A.S.; Hellings, N.; Prickaerts, J.; Moroni, L.; Vanmierlo, T.; Wieringa, P. Phosphodiesterase (PDE) 4 inhibition boosts Schwann cell myelination in a 3D regeneration model. Eur. J. Pharm. Sci. 2023, 185, 106441. [Google Scholar] [CrossRef]
- Zhou, J.; Li, S.; Gao, J.; Hu, Y.; Chen, S.; Luo, X.; Zhang, H.; Luo, Z.; Huang, J. Epothilone B Facilitates Peripheral Nerve Regeneration by Promoting Autophagy and Migration in Schwann Cells. Front. Cell Neurosci. 2020, 14, 143. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, J.; Guan, Y.; Li, X.; Bai, J.; Song, X.; Jia, Z.; Chen, S.; Li, C.; Xu, Y.; et al. Deferoxamine Promotes Peripheral Nerve Regeneration by Enhancing Schwann Cell Function and Promoting Axon Regeneration of Dorsal Root Ganglion. Neuroscience 2023, 524, 149–157. [Google Scholar] [CrossRef]
- Nocera, G.; Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol. Life Sci. 2020, 77, 3977–3989. [Google Scholar] [CrossRef]
- Jacob, C.; Christen, C.N.; Pereira, J.A.; Somandin, C.; Baggiolini, A.; Lötscher, P.; Ozçelik, M.; Tricaud, N.; Meijer, D.; Yamaguchi, T.; et al. HDAC1 and HDAC2 control the transcriptional program of myelination and the survival of Schwann cells. Nat. Neurosci. 2011, 14, 429–436. [Google Scholar] [CrossRef]
- Ma, K.H.; Hung, H.A.; Svaren, J. Epigenomic Regulation of Schwann Cell Reprogramming in Peripheral Nerve Injury. J. Neurosci. 2016, 36, 9135. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Patel, N.; Martirena, F.; Fazal, S.V.; Mutschler, C.; Cabedo, H. Emerging Role of HDACs in Regeneration and Ageing in the Peripheral Nervous System: Repair Schwann Cells as Pivotal Targets. Int. J. Mol. Sci. 2022, 23, 2996. [Google Scholar] [CrossRef]
- Yadav, A.; Huang, T.C.; Chen, S.H.; Ramasamy, T.S.; Hsueh, Y.Y.; Lin, S.P.; Lu, F.I.; Liu, Y.H.; Wu, C.C. Sodium phenylbutyrate inhibits Schwann cell inflammation via HDAC and NFκB to promote axonal regeneration and remyelination. J. Neuroinflamm. 2021, 18, 238. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.; Choi, Y.I.; Jung, N.; Song, J.Y.; Bae, D.K.; Kim, M.C.; Lee, Y.J.; Song, H.; Kwak, G.; Jeong, S.; et al. A novel histone deacetylase 6 inhibitor improves myelination of Schwann cells in a model of Charcot-Marie-Tooth disease type 1A. Br. J. Pharmacol. 2020, 177, 5096–5113. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, N.; Duman, M.; Bochud, M.; Brügger-Verdon, V.; Gerhards, M.; Schön, F.; Dorndecker, F.; Meijer, D.; Fledrich, R.; Stassart, R.; et al. Hypoxia-induced conversion of sensory Schwann cells into repair cells is regulated by HDAC8. Nat. Commun. 2025, 16, 515. [Google Scholar] [CrossRef]
- Hui, T.K.; Lai, X.S.; Dong, X.; Jing, H.; Liu, Z.; Fei, E.; Chen, W.B.; Wang, S.; Ren, D.; Zou, S.; et al. Ablation of Lrp4 in Schwann Cells Promotes Peripheral Nerve Regeneration in Mice. Biology 2021, 10, 452. [Google Scholar] [CrossRef]
- Chun, Y.L.; Kim, M.; Kim, Y.H.; Kim, N.; Yang, H.; Park, C.; Huh, Y.; Jung, J. Carvacrol effectively protects demyelination by suppressing transient receptor potential melastatin 7 (TRPM7) in Schwann cells. Anat. Sci. Int. 2020, 95, 230–239. [Google Scholar] [CrossRef]
- Zhang, S.H.; Shurin, G.V.; Khosravi, H.; Kazi, R.; Kruglov, O.; Shurin, M.R.; Bunimovich, Y.L. Immunomodulation by Schwann cells in disease. Cancer Immunol. Immunother. 2020, 69, 245–253. [Google Scholar] [CrossRef]
- Saiwai, H.; Kumamaru, H.; Ohkawa, Y.; Kubota, K.; Kobayakawa, K.; Yamada, H.; Yokomizo, T.; Iwamoto, Y.; Okada, S. Ly6C+ Ly6G- Myeloid-derived suppressor cells play a critical role in the resolution of acute inflammation and the subsequent tissue repair process after spinal cord injury. J. Neurochem. 2013, 125, 74–88. [Google Scholar] [CrossRef]
- Martyn, G.V.; Shurin, G.V.; Keskinov, A.A.; Bunimovich, Y.L.; Shurin, M.R. Schwann cells shape the neuro-immune environs and control cancer progression. Cancer Immunol. Immunother. 2019, 68, 1819–1829. [Google Scholar] [CrossRef]
- Shurin, G.V.; Vats, K.; Kruglov, O.; Bunimovich, Y.L.; Shurin, M.R. Tumor-Induced T Cell Polarization by Schwann Cells. Cells 2022, 11, 3541. [Google Scholar] [CrossRef] [PubMed]
- Drouyer, M.; Chu, T.H.; Labit, E.; Haase, F.; Navarro, R.G.; Nazareth, D.; Rosin, N.; Merjane, J.; Scott, S.; Cabanes-Creus, M.; et al. Novel AAV variants with improved tropism for human Schwann cells. Mol. Ther. Methods Clin. Dev. 2024, 32, 101234. [Google Scholar] [CrossRef]
- Sargiannidou, I.; Kagiava, A.; Kleopa, K.A. Gene therapy approaches targeting Schwann cells for demyelinating neuropathies. Brain Res. 2020, 1728, 146572. [Google Scholar] [CrossRef] [PubMed]
- Sargiannidou, I.; Kagiava, A.; Bashiardes, S.; Richter, J.; Christodoulou, C.; Scherer, S.S.; Kleopa, K.A. Intraneural GJB 1 gene delivery improves nerve pathology in a model of X-linked C harcot–M arie–T ooth disease. Ann. Neurol. 2015, 78, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Kagiava, A.; Sargiannidou, I.; Theophilidis, G.; Karaiskos, C.; Richter, J.; Bashiardes, S.; Schiza, N.; Nearchou, M.; Christodoulou, C.; Scherer, S.S.; et al. Intrathecal gene therapy rescues a model of demyelinating peripheral neuropathy. Proc. Natl. Acad. Sci. USA 2016, 113, E2421–E2429. [Google Scholar] [CrossRef]
- Kagiava, A.; Richter, J.; Tryfonos, C.; Karaiskos, C.; Heslegrave, A.J.; Sargiannidou, I.; Rossor, A.M.; Zetterberg, H.; Reilly, M.M.; Christodoulou, C.; et al. Gene replacement therapy after neuropathy onset provides therapeutic benefit in a model of CMT1X. Hum. Mol. Genet. 2019, 28, 3528–3542. [Google Scholar] [CrossRef]
- Kagiava, A.; Karaiskos, C.; Richter, J.; Tryfonos, C.; Lapathitis, G.; Sargiannidou, I.; Christodoulou, C.; Kleopa, K.A. Intrathecal gene therapy in mouse models expressing CMT1X mutations. Hum. Mol. Genet. 2018, 27, 1460–1473. [Google Scholar] [CrossRef]
- Schiza, N.; Georgiou, E.; Kagiava, A.; Médard, J.J.; Richter, J.; Tryfonos, C.; Sargiannidou, I.; Heslegrave, A.J.; Rossor, A.M.; Zetterberg, H.; et al. Gene replacement therapy in a model of Charcot-Marie-Tooth 4C neuropathy. Brain 2019, 142, 1227–1241. [Google Scholar] [CrossRef]
- Georgiou, E.; Kagiava, A.; Sargiannidou, I.; Schiza, N.; Stavrou, M.; Richter, J.; Tryfonos, C.; Heslegrave, A.; Zetterberg, H.; Christodoulou, C.; et al. AAV9-mediated <em>SH3TC2</em> gene replacement therapy targeted to Schwann cells for the treatment of CMT4C. Mol. Ther. 2023, 31, 3290–3307. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwak, G.; Kim, H.J.; Park, H.T.; Choi, B.O.; Hong, Y.B. miR-381 Attenuates Peripheral Neuropathic Phenotype Caused by Overexpression of PMP22. Exp. Neurobiol. 2019, 28, 279–288. [Google Scholar] [CrossRef]
- O’Carroll, S.J.; Cook, W.H.; Young, D. AAV Targeting of Glial Cell Types in the Central and Peripheral Nervous System and Relevance to Human Gene Therapy. Front. Mol. Neurosci. 2020, 13, 618020. [Google Scholar] [CrossRef] [PubMed]
- Kagiava, A.; Richter, J.; Tryfonos, C.; Leal-Julia, M.; Sargiannidou, I.; Christodoulou, C.; Bosch, A.; Kleopa, K.A. Efficacy of AAV serotypes to target Schwann cells after intrathecal and intravenous delivery. Sci. Rep. 2021, 11, 23358. [Google Scholar] [CrossRef] [PubMed]
- Kagiava, A.; Karaiskos, C.; Lapathitis, G.; Heslegrave, A.; Sargiannidou, I.; Zetterberg, H.; Bosch, A.; Kleopa, K.A. Gene replacement therapy in two Golgi-retained CMT1X mutants before and after the onset of demyelinating neuropathy. Mol. Ther. Methods Clin. Dev. 2023, 30, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Caballé, R.B.; Bortolozzi, M. New perspectives for gene therapy of the X-linked form of Charcot-Marie-Tooth disease. Mol. Ther. Methods Clin. Dev. 2024, 32, 101184. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Taniguchi, J.B.; Homma, H.; Tamura, T.; Fujita, K.; Inotsume, M.; Tagawa, K.; Misawa, K.; Matsumoto, N.; Nakagawa, M.; et al. AAV-mediated editing of PMP22 rescues Charcot-Marie-Tooth disease type 1A features in patient-derived iPS Schwann cells. Commun. Med. 2023, 3, 170. [Google Scholar] [CrossRef]
- Gautier, B.; Hajjar, H.; Soares, S.; Berthelot, J.; Deck, M.; Abbou, S.; Campbell, G.; Ceprian, M.; Gonzalez, S.; Fovet, C.M.; et al. AAV2/9-mediated silencing of PMP22 prevents the development of pathological features in a rat model of Charcot-Marie-Tooth disease 1 A. Nat. Commun. 2021, 12, 2356. [Google Scholar] [CrossRef]
- Stavrou, M.; Kagiava, A.; Choudury, S.G.; Jennings, M.J.; Wallace, L.M.; Fowler, A.M.; Heslegrave, A.; Richter, J.; Tryfonos, C.; Christodoulou, C.; et al. A translatable RNAi-driven gene therapy silences PMP22/Pmp22 genes and improves neuropathy in CMT1A mice. J. Clin. Investig. 2022, 132, e159814. [Google Scholar] [CrossRef]
- Lee, J.S.; Chang, E.H.; Koo, O.J.; Jwa, D.H.; Mo, W.M.; Kwak, G.; Moon, H.W.; Park, H.T.; Hong, Y.B.; Choi, B.O. Pmp22 mutant allele-specific siRNA alleviates demyelinating neuropathic phenotype in vivo. Neurobiol. Dis. 2017, 100, 99–107. [Google Scholar] [CrossRef]
- Boutary, S.; Caillaud, M.; El Madani, M.; Vallat, J.M.; Loisel-Duwattez, J.; Rouyer, A.; Richard, L.; Gracia, C.; Urbinati, G.; Desmaële, D.; et al. Squalenoyl siRNA PMP22 nanoparticles are effective in treating mouse models of Charcot-Marie-Tooth disease type 1 A. Commun. Biol. 2021, 4, 317. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.T.; Damle, S.; Ikeda-Lee, K.; Kuntz, S.; Li, J.; Mohan, A.; Kim, A.; Hung, G.; Scheideler, M.A.; Scherer, S.S.; et al. PMP22 antisense oligonucleotides reverse Charcot-Marie-Tooth disease type 1A features in rodent models. J. Clin. Investig. 2018, 128, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Lackington, W.A.; Raftery, R.M.; O’Brien, F.J. In vitro efficacy of a gene-activated nerve guidance conduit incorporating non-viral PEI-pDNA nanoparticles carrying genes encoding for NGF, GDNF and c-Jun. Acta Biomater. 2018, 75, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Allodi, I.; Mecollari, V.; González-Pérez, F.; Eggers, R.; Hoyng, S.; Verhaagen, J.; Navarro, X.; Udina, E. Schwann cells transduced with a lentiviral vector encoding Fgf-2 promote motor neuron regeneration following sciatic nerve injury. Glia 2014, 62, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Zhao, H.; Zhao, Y.; Jia, J.; Yang, L.; Ma, C.; Zhang, Y.; Dong, Y. Nanoparticles carrying neurotrophin-3-modified Schwann cells promote repair of sciatic nerve defects. Neural Regen. Res. 2013, 8, 1262–1268. [Google Scholar] [CrossRef]
- Huang, L.; Quan, X.; Liu, Z.; Ma, T.; Wu, Y.; Ge, J.; Zhu, S.; Yang, Y.; Liu, L.; Sun, Z.; et al. c-Jun gene-modified Schwann cells: Upregulating multiple neurotrophic factors and promoting neurite outgrowth. Tissue Eng. Part A 2015, 21, 1409–1421. [Google Scholar] [CrossRef]
- Huang, L.; Xia, B.; Shi, X.; Gao, J.; Yang, Y.; Xu, F.; Qi, F.; Liang, C.; Huang, J.; Luo, Z. Time-restricted release of multiple neurotrophic factors promotes axonal regeneration and functional recovery after peripheral nerve injury. Faseb J. 2019, 33, 8600–8613. [Google Scholar] [CrossRef]
- Homs, J.; Ariza, L.; Pagès, G.; Udina, E.; Navarro, X.; Chillón, M.; Bosch, A. Schwann cell targeting via intrasciatic injection of AAV8 as gene therapy strategy for peripheral nerve regeneration. Gene Ther. 2011, 18, 622–630. [Google Scholar] [CrossRef]
- Shin, S.M.; Moehring, F.; Itson-Zoske, B.; Fan, F.; Stucky, C.L.; Hogan, Q.H.; Yu, H. Piezo2 mechanosensitive ion channel is located to sensory neurons and nonneuronal cells in rat peripheral sensory pathway: Implications in pain. Pain 2021, 162, 2750–2768. [Google Scholar] [CrossRef]
- Acheta, J.; Bhatia, U.; Haley, J.; Hong, J.; Rich, K.; Close, R.; Bechler, M.E.; Belin, S.; Poitelon, Y. Piezo channels contribute to the regulation of myelination in Schwann cells. Glia 2022, 70, 2276–2289. [Google Scholar] [CrossRef]
- Wu, G.; Li, X.; Li, M.; Zhang, Z. Long non-coding RNA MALAT1 promotes the proliferation and migration of Schwann cells by elevating BDNF through sponging miR-129-5p. Exp. Cell Res. 2020, 390, 111937. [Google Scholar] [CrossRef]
- Yin, G.; Lin, Y.; Wang, P.; Zhou, J.; Lin, H. Upregulated lncARAT in Schwann cells promotes axonal regeneration by recruiting and activating proregenerative macrophages. Mol. Med. 2022, 28, 76. [Google Scholar] [CrossRef]
- Qiao, P.; Wu, W.; Wu, Y.; Wang, X. miR-328a-3p modulates the proliferative and migratory abilities of Schwann cells in peripheral nerves. Neurosci. Lett. 2022, 791, 136893. [Google Scholar] [CrossRef]
- Sun, J.; Liao, Z.; Li, Z.; Li, H.; Wu, Z.; Chen, C.; Wang, H. Down-regulation miR-146a-5p in Schwann cell-derived exosomes induced macrophage M1 polarization by impairing the inhibition on TRAF6/NF-kappaB pathway after peripheral nerve injury. Exp. Neurol. 2023, 362, 114295. [Google Scholar] [CrossRef] [PubMed]
- Staedtke, V.; Anstett, K.; Bedwell, D.; Giovannini, M.; Keeling, K.; Kesterson, R.; Kim, Y.; Korf, B.; Leier, A.; McManus, M.L.; et al. Gene-targeted therapy for neurofibromatosis and schwannomatosis: The path to clinical trials. Clin. Trials 2024, 21, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Wang, B.; Wang, Y.; Liu, P. Gene Therapy for Neurofibromatosis Type 2-Related Schwannomatosis: Recent Progress, Challenges, and Future Directions. Oncol. Ther. 2024, 12, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, S.; Messerli, S.M.; Stemmer-Rachamimov, A.O.; Liu, T.C.; Rabkin, S.; Martuza, R.; Breakefield, X.O. Treatment of implantable NF2 schwannoma tumor models with oncolytic herpes simplex virus G47Delta. Cancer Gene Ther. 2007, 14, 460–467. [Google Scholar] [CrossRef]
- Prabhakar, S.; Brenner, G.J.; Sung, B.; Messerli, S.M.; Mao, J.; Sena-Esteves, M.; Stemmer-Rachamimov, A.; Tannous, B.; Breakefield, X.O. Imaging and therapy of experimental schwannomas using HSV amplicon vector-encoding apoptotic protein under Schwann cell promoter. Cancer Gene Ther. 2010, 17, 266–274. [Google Scholar] [CrossRef]
- Prabhakar, S.; Taherian, M.; Gianni, D.; Conlon, T.J.; Fulci, G.; Brockmann, J.; Stemmer-Rachamimov, A.; Sena-Esteves, M.; Breakefield, X.O.; Brenner, G.J. Regression of schwannomas induced by adeno-associated virus-mediated delivery of caspase-1. Hum. Gene Ther. 2013, 24, 152–162. [Google Scholar] [CrossRef]
- Ahmed, S.G.; Abdelanabi, A.; Doha, M.; Brenner, G.J. Schwannoma gene therapy by adeno-associated virus delivery of the pore-forming protein Gasdermin-D. Cancer Gene Ther. 2019, 26, 259–267. [Google Scholar] [CrossRef]
- Bai, R.Y.; Esposito, D.; Tam, A.J.; McCormick, F.; Riggins, G.J.; Wade Clapp, D.; Staedtke, V. Feasibility of using NF1-GRD and AAV for gene replacement therapy in NF1-associated tumors. Gene Ther. 2019, 26, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.G.; Abdelnabi, A.; Maguire, C.A.; Doha, M.; Sagers, J.E.; Lewis, R.M.; Muzikansky, A.; Giovannini, M.; Stemmer-Rachamimov, A.; Stankovic, K.M.; et al. Gene therapy with apoptosis-associated speck-like protein, a newly described schwannoma tumor suppressor, inhibits schwannoma growth in vivo. Neuro-Oncology 2019, 21, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, S.; Beauchamp, R.L.; Cheah, P.S.; Yoshinaga, A.; Haidar, E.A.; Lule, S.; Mani, G.; Maalouf, K.; Stemmer-Rachamimov, A.; Jung, D.H.; et al. Gene replacement therapy in a schwannoma mouse model of neurofibromatosis type 2. Mol. Ther. Methods Clin. Dev. 2022, 26, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Aimaier, R.; Chung, M.-H.; Gu, Y.; Yu, Q.; Wei, C.; Li, H.; Guo, Z.; Long, M.; Li, Y.; Wang, W.; et al. FOXM1 promotes neurofibromatosis type 1-associated malignant peripheral nerve sheath tumor progression in a NUF2-dependent manner. Cancer Gene Ther. 2023, 30, 1390–1402. [Google Scholar] [CrossRef]
- Züchner, S. Schwann cell gene therapies in sight. Gene Ther. 2021, 28, 618–619. [Google Scholar] [CrossRef]
- Ferdoushi, A.; Li, X.; Jamaluddin, M.F.B.; Hondermarck, H. Proteomic Profile of Human Schwann Cells. Proteomics 2020, 20, e1900294. [Google Scholar] [CrossRef]
- Contreras, E.; Bolívar, S.; Navarro, X.; Udina, E. New insights into peripheral nerve regeneration: The role of secretomes. Exp. Neurol. 2022, 354, 114069. [Google Scholar] [CrossRef]
- Chan, J.R.; Rodriguez-Waitkus, P.M.; Ng, B.K.; Liang, P.; Glaser, M. Progesterone synthesized by Schwann cells during myelin formation regulates neuronal gene expression. Mol. Biol. Cell 2000, 11, 2283–2295. [Google Scholar] [CrossRef]
- Robert, F.; Guennoun, R.; Désarnaud, F.; Do-Thi, A.; Benmessahel, Y.; Baulieu, E.E.; Schumacher, M. Synthesis of progesterone in Schwann cells: Regulation by sensory neurons. Eur. J. Neurosci. 2001, 13, 916–924. [Google Scholar] [CrossRef]
- Schumacher, M.; Guennoun, R.; Robert, F.; Carelli, C.; Gago, N.; Ghoumari, A.; Gonzalez Deniselle, M.C.; Gonzalez, S.L.; Ibanez, C.; Labombarda, F.; et al. Local synthesis and dual actions of progesterone in the nervous system: Neuroprotection and myelination. Growth Horm. IGF Res. 2004, 14 (Suppl. SA), S18–S33. [Google Scholar] [CrossRef]
- Li, G.; Zhang, S.; Zou, Y.; Ai, H.; Zheng, X.; Qian, K.; Lei, C.; Fu, W. The therapeutic potential of exosomes in immunotherapy. Front. Immunol. 2024, 15, 1424081. [Google Scholar] [CrossRef] [PubMed]
- Putthanbut, N.; Lee, J.Y.; Borlongan, C.V. Extracellular vesicle therapy in neurological disorders. J. Biomed. Sci. 2024, 31, 85. [Google Scholar] [CrossRef] [PubMed]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lässer, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.V.; Hutchins, E.; Hamamoto, A.; et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Buller, B.A.; Zhang, Z.G.; Zhang, Y.; Lu, M.; Rosene, D.L.; Medalla, M.; Moore, T.L.; Chopp, M. Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system. Exp. Neurol. 2022, 347, 113895. [Google Scholar] [CrossRef]
- Fallahi, S.; Zangbar, H.S.; Farajdokht, F.; Rahbarghazi, R.; Mohaddes, G.; Ghiasi, F. Exosomes as a therapeutic tool to promote neurorestoration and cognitive function in neurological conditions: Achieve two ends with a single effort. CNS Neurosci. Ther. 2024, 30, e14752. [Google Scholar] [CrossRef]
- Xin, G.-D.; Liu, X.-Y.; Fan, X.-D.; Zhao, G.-J. Exosomes repairment for sciatic nerve injury: A cell-free therapy. Stem Cell Res. Ther. 2024, 15, 214. [Google Scholar] [CrossRef]
- López-Leal, R.; Díaz-Viraqué, F.; Catalán, R.; Saquel, C.; Enright, A.; Iraola, G.; Court, F. Schwann cell reprogramming into repair cells increases miRNA-21 expression in exosomes promoting axonal growth. J. Cell Sci. 2020, 133, jcs239004. [Google Scholar] [CrossRef]
- Wu, X.; Wang, L.; Cong, M.; Shen, M.; He, Q.; Ding, F.; Shi, H. Extracellular vesicles from skin precursor-derived Schwann cells promote axonal outgrowth and regeneration of motoneurons via Akt/mTOR/p70S6K pathway. Ann. Transl. Med. 2020, 8, 1640. [Google Scholar] [CrossRef]
- Lopez-Verrilli, M.A.; Picou, F.; Court, F.A. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 2013, 61, 1795–1806. [Google Scholar] [CrossRef]
- Wong, F.C.; Ye, L.; Demir, I.E.; Kahlert, C. Schwann cell-derived exosomes: Janus-faced mediators of regeneration and disease. Glia 2022, 70, 20–34. [Google Scholar] [CrossRef]
- Ghosh, M.; Pearse, D.D. Schwann Cell-Derived Exosomal Vesicles: A Promising Therapy for the Injured Spinal Cord. Int. J. Mol. Sci. 2023, 24, 7317. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Li, Y.; Yang, F.; Lv, Z.; Zhu, S.; Shao, Y.; Huang, Y.; Ning, G.; Feng, S. Increasing toll-like receptor 2 on astrocytes induced by Schwann cell-derived exosomes promotes recovery by inhibiting CSPGs deposition after spinal cord injury. J. Neuroinflamm. 2021, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Zhu, S.; Zhang, W.; Wei, Z.; Yang, F.; Guo, Z.; Ning, G.; Feng, S. Autophagy induced by Schwann cell-derived exosomes promotes recovery after spinal cord injury in rats. Biotechnol. Lett. 2022, 44, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhu, B.; Gu, G.; Zhang, W.; Li, J.; Wang, H.; Wang, M.; Song, X.; Wei, Z.; Feng, S. Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death Dis. 2023, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Chen, Y.N.; He, H.; Fu, C.H.; Xu, Z.Y.; Lin, F.Y. Schwann cells-derived exosomes promote functional recovery after spinal cord injury by promoting angiogenesis. Front. Cell Neurosci. 2023, 16, 1077071. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yu, M.; Chen, D.; Deng, C.; Zhang, Q.; Gu, X.; Ding, F. Chitosan Nerve Grafts Incorporated with SKP-SC-EVs Induce Peripheral Nerve Regeneration. Tissue Eng. Regen. Med. 2023, 20, 309–322. [Google Scholar] [CrossRef]
- Yu, M.; Gu, G.; Cong, M.; Du, M.; Wang, W.; Shen, M.; Zhang, Q.; Shi, H.; Gu, X.; Ding, F. Repair of peripheral nerve defects by nerve grafts incorporated with extracellular vesicles from skin-derived precursor Schwann cells. Acta Biomater. 2021, 134, 190–203. [Google Scholar] [CrossRef]
- Yu, M.; Shen, M.; Chen, D.; Li, Y.; Zhou, Q.; Deng, C.; Zhou, X.; Zhang, Q.; He, Q.; Wang, H.; et al. Chitosan/PLGA-based tissue engineered nerve grafts with SKP-SC-EVs enhance sciatic nerve regeneration in dogs through miR-30b-5p-mediated regulation of axon growth. Bioact. Mater. 2024, 40, 378–395. [Google Scholar] [CrossRef]
- Cong, M.; Shen, M.; Wu, X.; Li, Y.; Wang, L.; He, Q.; Shi, H.; Ding, F. Improvement of sensory neuron growth and survival via negatively regulating PTEN by miR-21-5p-contained small extracellular vesicles from skin precursor-derived Schwann cells. Stem Cell Res. Ther. 2021, 12, 80. [Google Scholar] [CrossRef]
- Wang, J.L.; Huang, Q.M.; Hu, D.X.; Zhang, W.J. Therapeutic effect of exosomes derived from Schwann cells in the repair of peripheral nerve injury. Life Sci. 2024, 357, 123086. [Google Scholar] [CrossRef]
- Nishimura, K.; Sanchez-Molano, J.; Kerr, N.; Pressman, Y.; Silvera, R.; Khan, A.; Gajavelli, S.; Bramlett, H.M.; Dietrich, W.D. Beneficial Effects of Human Schwann Cell-Derived Exosomes in Mitigating Secondary Damage After Penetrating Ballistic-Like Brain Injury. J. Neurotrauma 2024, 41, 2395–2412. [Google Scholar] [CrossRef]
- Blaya, M.O.; Pressman, Y.; Andreu, M.; Moreno, W.J.; Sanchez-Molano, J.; Kerr, N.A.; Umland, O.; Khan, A.; Bramlett, H.M.; Dietrich, W.D. Human Schwann cell exosome treatment attenuates secondary injury mechanisms, histopathological consequences, and behavioral deficits after traumatic brain injury. Neurotherapeutics 2025, 22, e00555. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Szalad, A.; Liu, X.S.; Zhang, Y.; Wang, X.; Golembieski, W.A.; Powell, B.; Mccann, M.; Lu, M.; et al. Schwann cell-derived exosomes ameliorate peripheral neuropathy induced by ablation of dicer in Schwann cells. Front. Cell. Neurosci. 2024, 18, 1462228. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chopp, M.; Szalad, A.; Lu, X.; Zhang, Y.; Wang, X.; Cepparulo, P.; Lu, M.; Li, C.; Zhang, Z.G. Exosomes Derived From Schwann Cells Ameliorate Peripheral Neuropathy in Type 2 Diabetic Mice. Diabetes 2020, 69, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Saquel, C.; Catalan, R.J.; Lopez-Leal, R.; Ramirez, R.A.; Necuñir, D.; Wyneken, U.; Lamaze, C.; Court, F.A. Neuronal activity-dependent ATP enhances the pro-growth effect of repair Schwann cell extracellular vesicles by increasing their miRNA-21 loading. Front. Cell Neurosci. 2022, 16, 943506. [Google Scholar] [CrossRef]
- You, M.; Xing, H.; Yan, M.; Zhang, J.; Chen, J.; Chen, Y.; Liu, X.; Zhu, J. Schwann Cell-Derived Exosomes Ameliorate Paclitaxel-Induced Peripheral Neuropathy Through the miR-21-Mediated PTEN Signaling Pathway. Mol. Neurobiol. 2023, 60, 6840–6851. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Oliveira, J.; Lee, Y.S.; Guest, J.D.; Silvera, R.; Pressman, Y.; Pearse, D.D.; Nettina, A.E.; Goldschmidt-Clermont, P.J.; Al-Ali, H.; et al. Human Schwann Cell-Derived Extracellular Vesicle Isolation, Bioactivity Assessment, and Omics Characterization. Int. J. Nanomed. 2025, 20, 4123–4144. [Google Scholar] [CrossRef]
- Chernousov, M.A.; Carey, D.J. Schwann cell extracellular matrix molecules and their receptors. Histol. Histopathol. 2000, 15, 593–601. [Google Scholar] [CrossRef]
- Bunge, R.P.; Bunge, M.B. Interrelationship between Schwann cell function and extracellular matrix production. Trends Neurosci. 1983, 6, 499–505. [Google Scholar] [CrossRef]
- Torres-Mejía, E.; Trümbach, D.; Kleeberger, C.; Dornseifer, U.; Orschmann, T.; Bäcker, T.; Brenke, J.K.; Hadian, K.; Wurst, W.; López-Schier, H.; et al. Sox2 controls Schwann cell self-organization through fibronectin fibrillogenesis. Sci. Rep. 2020, 10, 1984. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhu, J.; Xue, C.; Li, Z.; Ding, F.; Yang, Y.; Gu, X. Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials 2014, 35, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Khuong, H.T.; Kumar, R.; Senjaya, F.; Grochmal, J.; Ivanovic, A.; Shakhbazau, A.; Forden, J.; Webb, A.; Biernaskie, J.; Midha, R. Skin derived precursor Schwann cells improve behavioral recovery for acute and delayed nerve repair. Exp. Neurol. 2014, 254, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Huang, J.; Xue, C.; Wang, Y.; Wang, S.; Bao, S.; Chen, R.; Li, Y.; Gu, Y. Skin derived precursor Schwann cell-generated acellular matrix modified chitosan/silk scaffolds for bridging rat sciatic nerve gap. Neurosci. Res. 2018, 135, 21–31. [Google Scholar] [CrossRef]
- Xue, C.; Zhu, H.; Wang, H.; Wang, Y.; Xu, X.; Zhou, S.; Liu, D.; Zhao, Y.; Qian, T.; Guo, Q.; et al. Skin derived precursors induced Schwann cells mediated tissue engineering-aided neuroregeneration across sciatic nerve defect. Bioact. Mater. 2024, 33, 572–590. [Google Scholar] [CrossRef]
- Xu, Z.; Orkwis, J.A.; DeVine, B.M.; Harris, G.M. Extracellular matrix cues modulate Schwann cell morphology, proliferation, and protein expression. J. Tissue Eng. Regen. Med. 2020, 14, 229–242. [Google Scholar] [CrossRef]
- Lin, T.; Liu, S.; Chen, S.; Qiu, S.; Rao, Z.; Liu, J.; Zhu, S.; Yan, L.; Mao, H.; Zhu, Q.; et al. Hydrogel derived from porcine decellularized nerve tissue as a promising biomaterial for repairing peripheral nerve defects. Acta Biomater. 2018, 73, 326–338. [Google Scholar] [CrossRef]
- Liu, S.; Rao, Z.; Zou, J.; Chen, S.; Zhu, Q.; Liu, X.; Bai, Y.; Liu, Y.; Quan, D. Properties Regulation and Biological Applications of Decellularized Peripheral Nerve Matrix Hydrogel. ACS Appl. Bio Mater. 2021, 4, 6473–6487. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, Z.; Chen, S.; Zhang, F.; Rao, Z.; Zhao, C.; Quan, D.; Bai, Y.; Shen, J. Nanofibrous nerve guidance conduits decorated with decellularized matrix hydrogel facilitate peripheral nerve injury repair. Theranostics 2021, 11, 2917–2931. [Google Scholar] [CrossRef]
- Rao, Z.; Lin, T.; Qiu, S.; Zhou, J.; Liu, S.; Chen, S.; Wang, T.; Liu, X.; Zhu, Q.; Bai, Y.; et al. Decellularized nerve matrix hydrogel scaffolds with longitudinally oriented and size-tunable microchannels for peripheral nerve regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111791. [Google Scholar] [CrossRef]
- Noble, A.; Qubrosi, R.; Cariba, S.; Favaro, K.; Payne, S.L. Neural dependency in wound healing and regeneration. Dev. Dyn. 2024, 253, 181–203. [Google Scholar] [CrossRef] [PubMed]
- Basson, M.D.; Burney, R.E. Defective wound healing in patients with paraplegia and quadriplegia. Surg. Gynecol. Obstet. 1982, 155, 9–12. [Google Scholar] [PubMed]
- Nowak, N.C.; Menichella, D.M.; Miller, R.; Paller, A.S. Cutaneous innervation in impaired diabetic wound healing. Transl. Res. 2021, 236, 87–108. [Google Scholar] [CrossRef] [PubMed]
- McGlone, F.; Reilly, D. The cutaneous sensory system. Neurosci. Biobehav. Rev. 2010, 34, 148–159. [Google Scholar] [CrossRef]
- Rinwa, P.; Calvo-Enrique, L.; Zhang, M.D.; Nyengaard, J.R.; Karlsson, P.; Ernfors, P. Demise of nociceptive Schwann cells causes nerve retraction and pain hyperalgesia. Pain 2021, 162, 1816–1827. [Google Scholar] [CrossRef]
- Lebonvallet, N.; Laverdet, B.; Misery, L.; Desmoulière, A.; Girard, D. New insights into the roles of myofibroblasts and innervation during skin healing and innovative therapies to improve scar innervation. Exp. Dermatol. 2018, 27, 950–958. [Google Scholar] [CrossRef]
- Parfejevs, V.; Debbache, J.; Shakhova, O.; Schaefer, S.M.; Glausch, M.; Wegner, M.; Suter, U.; Riekstina, U.; Werner, S.; Sommer, L. Injury-activated glial cells promote wound healing of the adult skin in mice. Nat. Commun. 2018, 9, 236. [Google Scholar] [CrossRef]
- Zhou, S.; Wan, L.; Liu, X.; Hu, D.; Lu, F.; Chen, X.; Liang, F. Diminished schwann cell repair responses play a role in delayed diabetes-associated wound healing. Front. Physiol. 2022, 13, 814754. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Q.; Chen, Q.; Xu, L.; Yi, S. Transcriptional Control of Peripheral Nerve Regeneration. Mol. Neurobiol. 2023, 60, 329–341. [Google Scholar] [CrossRef]
- Ou, M.Y.; Tan, P.C.; Xie, Y.; Liu, K.; Gao, Y.M.; Yang, X.S.; Zhou, S.B.; Li, Q.F. Dedifferentiated Schwann cell-derived TGF-β3 is essential for the neural system to promote wound healing. Theranostics 2022, 12, 5470–5487. [Google Scholar] [CrossRef]
- Direder, M.; Weiss, T.; Copic, D.; Vorstandlechner, V.; Laggner, M.; Pfisterer, K.; Mildner, C.S.; Klas, K.; Bormann, D.; Haslik, W.; et al. Schwann cells contribute to keloid formation. Matrix Biol. 2022, 108, 55–76. [Google Scholar] [CrossRef]
- Direder, M.; Wielscher, M.; Weiss, T.; Laggner, M.; Copic, D.; Klas, K.; Bormann, D.; Vorstandlechner, V.; Tschachler, E.; Jan Ankersmit, H.; et al. The transcriptional profile of keloidal Schwann cells. Exp. Mol. Med. 2022, 54, 1886–1900. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, X.; He, X.; Zhou, X.; Wang, X.; Lin, K.; Mao, L. Schwann cell-derived exosomes accelerate periodontal bone regeneration with osteogenesis, angiogenesis, and neurogenesis. J. Mater. Chem. B 2025, 13, 4020–4029. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.P.; Naska, S.; Jones, K.; Jinno, H.; Kaplan, D.R.; Miller, F.D. Sox2-mediated regulation of adult neural crest precursors and skin repair. Stem Cell Rep. 2013, 1, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.P.W.; Miller, F.D. The Contribution of Innervation to Tissue Repair and Regeneration. Cold Spring Harb. Perspect. Biol. 2022, 14, a041233. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, X.; Jiang, S.; Cai, X.; Yu, S.; Pei, G. Schwann cells promote prevascularization and osteogenesis of tissue-engineered bone via bone marrow mesenchymal stem cell-derived endothelial cells. Stem Cell Res. Ther. 2021, 12, 382. [Google Scholar] [CrossRef]
- Hao, Z.; Ren, L.; Zhang, Z.; Yang, Z.; Wu, S.; Liu, G.; Cheng, B.; Wu, J.; Xia, J. A multifunctional neuromodulation platform utilizing Schwann cell-derived exosomes orchestrates bone microenvironment via immunomodulation, angiogenesis and osteogenesis. Bioact. Mater. 2023, 23, 206–222. [Google Scholar] [CrossRef]
- Wu, Z.; Pu, P.; Su, Z.; Zhang, X.; Nie, L.; Chang, Y. Schwann Cell-derived exosomes promote bone regeneration and repair by enhancing the biological activity of porous Ti6Al4V scaffolds. Biochem. Biophys. Res. Commun. 2020, 531, 559–565. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Montoro, D.T.; Muhonen, E.; Walmsley, G.G.; Lo, D.; Hasegawa, M.; Januszyk, M.; Connolly, A.J.; Weissman, I.L.; Longaker, M.T. Clonal analysis reveals nerve-dependent and independent roles on mammalian hind limb tissue maintenance and regeneration. Proc. Natl. Acad. Sci. USA 2014, 111, 9846–9851. [Google Scholar] [CrossRef]
- Yu, X.; Gong, Z.; Lin, Q.; Wang, W.; Liu, S.; Li, S. Denervation effectively aggravates rat experimental periodontitis. J. Periodontal Res. 2017, 52, 1011–1020. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Zhou, H.; Chai, Y.; Li, Z.; Chen, M.; Yang, Z.; Xu, C.; Lei, C.; He, Y.; et al. Dental pulp stem cells alleviate Schwann cell pyroptosis via mitochondrial transfer to enhance facial nerve regeneration. Bioact. Mater. 2025, 47, 313–326. [Google Scholar] [CrossRef]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef]
- Demicheli, R.; Retsky, M.W.; Hrushesky, W.J.; Baum, M. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: Learning from failures. Nat. Clin. Pract. Oncol. 2007, 4, 699–710. [Google Scholar] [CrossRef]
- Demicheli, R.; Retsky, M.W.; Hrushesky, W.J.; Baum, M.; Gukas, I.D. The effects of surgery on tumor growth: A century of investigations. Ann. Oncol. 2008, 19, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Alieva, M.; van Rheenen, J.; Broekman, M.L.D. Potential impact of invasive surgical procedures on primary tumor growth and metastasis. Clin. Exp. Metastasis 2018, 35, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Salanti, A.; Gögenur, I. Premetastatic niches, exosomes and circulating tumor cells: Early mechanisms of tumor dissemination and the relation to surgery. Int. J. Cancer 2020, 146, 3244–3255. [Google Scholar] [CrossRef] [PubMed]
- Shurin, M.R.; Baraldi, J.H.; Shurin, G.V. Neuroimmune Regulation of Surgery-Associated Metastases. Cells 2021, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Bordonaro, M.; Lazarova, D. Hypothesis: Cancer Hormesis and Its Potential for Cancer Therapeutics. Life 2024, 14, 401. [Google Scholar] [CrossRef]
- Chiarella, P.; Bruzzo, J.; Meiss, R.P.; Ruggiero, R.A. Concomitant tumor resistance. Cancer Lett. 2012, 324, 133–141. [Google Scholar] [CrossRef]
- Bruzzo, J.; Chiarella, P.; Meiss, R.P.; Ruggiero, R.A. Biphasic effect of a primary tumor on the growth of secondary tumor implants. J. Cancer Res. Clin. Oncol. 2010, 136, 1605–1615. [Google Scholar] [CrossRef]
- Guba, M.; Cernaianu, G.; Koehl, G.; Geissler, E.K.; Jauch, K.W.; Anthuber, M.; Falk, W.; Steinbauer, M. A primary tumor promotes dormancy of solitary tumor cells before inhibiting angiogenesis. Cancer Res. 2001, 61, 5575–5579. [Google Scholar]
- Gorelik, E.; Segal, S.; Feldman, M. On the mechanism of tumor “concomitant immunity”. Int. J. Cancer 1981, 27, 847–856. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Takagi, Y.; Aoki, S.; Futamura, M.; Saji, S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann. Surg. 2000, 232, 58–65. [Google Scholar] [CrossRef]
- Onuma, A.E.; Zhang, H.; Gil, L.; Huang, H.; Tsung, A. Surgical Stress Promotes Tumor Progression: A Focus on the Impact of the Immune Response. J. Clin. Med. 2020, 9, 4096. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhang, H.; Hamad, A.; Huang, H.; Tsung, A. Surgery-mediated tumor-promoting effects on the immune microenvironment. Semin. Cancer Biol. 2022, 86, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, H.; Sun, Y. Effect of acute inflammatory reaction induced by biopsy on tumor microenvironment. J. Cancer Res. Clin. Oncol. 2024, 150, 177. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, J.H.; Martyn, G.V.; Shurin, G.V.; Shurin, M.R. Tumor Innervation: History, Methodologies, and Significance. Cancers 2022, 14, 1979. [Google Scholar] [CrossRef]
- Reavis, H.D.; Chen, H.I.; Drapkin, R. Tumor Innervation: Cancer Has Some Nerve. Trends Cancer 2020, 6, 1059–1067. [Google Scholar] [CrossRef]
- Zahalka, A.H.; Frenette, P.S. Nerves in cancer. Nat. Rev. Cancer 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Gysler, S.M.; Drapkin, R. Tumor innervation: Peripheral nerves take control of the tumor microenvironment. J. Clin. Investig. 2021, 131, e147276. [Google Scholar] [CrossRef]
- Erin, N.; Shurin, G.V.; Baraldi, J.H.; Shurin, M.R. Regulation of Carcinogenesis by Sensory Neurons and Neuromediators. Cancers 2022, 14, 2333. [Google Scholar] [CrossRef]
- Deborde, S.; Wong, R.J. How Schwann cells facilitate cancer progression in nerves. Cell Mol. Life Sci. 2017, 74, 4405–4420. [Google Scholar] [CrossRef]
- Shurin, M.R.; Shurin, G.V.; Zlotnikov, S.B.; Bunimovich, Y.L. The Neuroimmune Axis in the Tumor Microenvironment. J. Immunol. 2020, 204, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Khanmammadova, N.; Islam, S.; Sharma, P.; Amit, M. Neuro-immune interactions and immuno-oncology. Trends Cancer 2023, 9, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Yurteri, Ü.; Çifcibaşı, K.; Friess, H.; Ceyhan, G.O.; Istvanffy, R.; Demir, I.E. Schwann Cells in Peripheral Cancers: Bystanders or Promoters? Adv. Biol. 2022, 6, e2200033. [Google Scholar] [CrossRef] [PubMed]
- Fujii-Nishimura, Y.; Yamazaki, K.; Masugi, Y.; Douguchi, J.; Kurebayashi, Y.; Kubota, N.; Ojima, H.; Kitago, M.; Shinoda, M.; Hashiguchi, A.; et al. Mesenchymal-epithelial transition of pancreatic cancer cells at perineural invasion sites is induced by Schwann cells. Pathol. Int. 2018, 68, 214–223. [Google Scholar] [CrossRef]
- Zhou, Y.; Shurin, G.V.; Zhong, H.; Bunimovich, Y.L.; Han, B.; Shurin, M.R. Schwann Cells Augment Cell Spreading and Metastasis of Lung Cancer. Cancer Res. 2018, 78, 5927–5939. [Google Scholar] [CrossRef]
- Han, S.; Wang, D.; Huang, Y.; Zeng, Z.; Xu, P.; Xiong, H.; Ke, Z.; Zhang, Y.; Hu, Y.; Wang, F.; et al. A reciprocal feedback between colon cancer cells and Schwann cells promotes the proliferation and metastasis of colon cancer. J. Exp. Clin. Cancer Res. 2022, 41, 348. [Google Scholar] [CrossRef]
- Chen, S.; Chen, M. Schwann cells promote the migration and invasion of colorectal cancer cells via the activated NF-κB/IL-8 axis in the tumor microenvironment. Front. Oncol. 2022, 12, 1026670. [Google Scholar] [CrossRef]
- Ferdoushi, A.; Li, X.; Griffin, N.; Faulkner, S.; Jamaluddin, M.F.B.; Gao, F.; Jiang, C.C.; van Helden, D.F.; Tanwar, P.S.; Jobling, P.; et al. Schwann Cell Stimulation of Pancreatic Cancer Cells: A Proteomic Analysis. Front. Oncol. 2020, 10, 1601. [Google Scholar] [CrossRef]
- Su, D.; Guo, X.; Huang, L.; Ye, H.; Li, Z.; Lin, L.; Chen, R.; Zhou, Q. Tumor-neuroglia interaction promotes pancreatic cancer metastasis. Theranostics 2020, 10, 5029–5047. [Google Scholar] [CrossRef]
- Sroka, I.C.; Chopra, H.; Das, L.; Gard, J.M.; Nagle, R.B.; Cress, A.E. Schwann Cells Increase Prostate and Pancreatic Tumor Cell Invasion Using Laminin Binding A6 Integrin. J. Cell Biochem. 2016, 117, 491–499. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Xu, J.; Wang, Y.; Yang, Y.; Wang, W.; Gu, A.; Han, B.; Shurin, G.V.; Zhong, R.; et al. Schwann cell-derived exosomes promote lung cancer progression via miRNA-21-5p. Glia 2024, 72, 692–707. [Google Scholar] [CrossRef]
- Cao, S.; Wang, Y.; Zhou, Y.; Zhang, Y.; Ling, X.; Zhang, L.; Li, J.; Yang, Y.; Wang, W.; Shurin, M.R.; et al. A Novel Therapeutic Target for Small-Cell Lung Cancer: Tumor-Associated Repair-like Schwann Cells. Cancers 2022, 14, 6132. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Han, B.; Zhong, R.; Zhong, H. Schwann cells promote lung cancer proliferation by promoting the M2 polarization of macrophages. Cell. Immunol. 2020, 357, 104211. [Google Scholar] [CrossRef]
- Shan, C.; Wei, J.; Hou, R.; Wu, B.; Yang, Z.; Wang, L.; Lei, D.; Yang, X. Schwann cells promote EMT and the Schwann-like differentiation of salivary adenoid cystic carcinoma cells via the BDNF/TrkB axis. Oncol. Rep. 2016, 35, 427–435. [Google Scholar] [CrossRef]
- García-Reyes, B.; Kuzmanov, I.; Schneider, R.; Schneiker, B.; Efferz, P.; Kalff, J.C.; Wehner, S. Glial cell-derived soluble factors increase the metastatic potential of pancreatic adenocarcinoma cells and induce epithelial-to-mesenchymal transition. J. Cancer Res. Clin. Oncol. 2023, 149, 14315–14327. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Liu, E.; Zhang, C.; Feng, R.; Tran, N.; Zhai, W.; Wang, F.; Qin, Z. Promotion of perineural invasion of cholangiocarcinoma by Schwann cells via nerve growth factor. J. Gastrointestig. Oncol. 2024, 15, 1198–1213. [Google Scholar] [CrossRef] [PubMed]
- Allgood, J.; Roe, A.; Pullan, J.E. How Schwann Cells Are Involved in Brain Metastasis. Neuroglia 2024, 5, 155–164. [Google Scholar] [CrossRef]
- Demir, I.E.; Boldis, A.; Pfitzinger, P.L.; Teller, S.; Brunner, E.; Klose, N.; Kehl, T.; Maak, M.; Lesina, M.; Laschinger, M.; et al. Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J. Natl. Cancer Inst. 2014, 106, dju184. [Google Scholar] [CrossRef]
- Deborde, S.; Omelchenko, T.; Lyubchik, A.; Zhou, Y.; He, S.; McNamara, W.F.; Chernichenko, N.; Lee, S.-Y.; Barajas, F.; Chen, C.-H.; et al. Schwann cells induce cancer cell dispersion and invasion. J. Clin. Investig. 2016, 126, 1538–1554. [Google Scholar] [CrossRef]
- Azam, S.H.; Pecot, C.V. Cancer’s got nerve: Schwann cells drive perineural invasion. J. Clin. Investig. 2016, 126, 1242–1244. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Maitra, A. The boring Schwann cells: Tumor Me-TAST-asis along nerves. Cancer Discov. 2022, 12, 2240–2243. [Google Scholar] [CrossRef] [PubMed]
- Deborde, S.; Gusain, L.; Powers, A.; Marcadis, A.; Yu, Y.; Chen, C.H.; Frants, A.; Kao, E.; Tang, L.H.; Vakiani, E.; et al. Reprogrammed Schwann Cells Organize into Dynamic Tracks that Promote Pancreatic Cancer Invasion. Cancer Discov. 2022, 12, 2454–2473. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, Q.; Huang, T.; Xu, C.; Yang, X.; Zhang, L.; Wang, J.; Yang, L.; Zheng, X.; Fan, Q.; et al. Cervical cancer-produced neuromedin-B reprograms Schwann cells to initiate perineural invasion. Cell Death Dis. 2024, 15, 636. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, Z.; Sun, H.; You, J.; Chu, J.; Gao, J.; Qiu, L.; Liu, X. Dedifferentiated Schwann cells promote perineural invasion mediated by the PACAP paracrine signalling in cervical cancer. J. Cell Mol. Med. 2023, 27, 3692–3705. [Google Scholar] [CrossRef]
- Silva, S.L.; Neta, G.C.d.O.; Rocha, R.M.L.; Duarte, A.K.d.S.F.; Fraga, C.A.d.C. Perineural invasion in prostate cancer is associated with Schwann cells and disruption of circadian rhythm-related gene expression: A bioinformatics approach. GPD 2024, 3, 3146. [Google Scholar] [CrossRef]
- de Franchis, V.; Petrungaro, S.; Pizzichini, E.; Camerini, S.; Casella, M.; Somma, F.; Mandolini, E.; Carpino, G.; Overi, D.; Cardinale, V.; et al. Cholangiocarcinoma Malignant Traits Are Promoted by Schwann Cells through TGFβ Signaling in a Model of Perineural Invasion. Cells 2024, 13, 366. [Google Scholar] [CrossRef]
- Santi, M.D.; Zhang, M.; Salvo, E.; Asam, K.; Viet, C.T.; Xie, T.; Amit, M.; Aouizerat, B.; Ye, Y. Schwann Cells Induce Phenotypic Changes in Oral Cancer Cells. Adv. Biol. 2022, 6, e2200187. [Google Scholar] [CrossRef]
- Rocha, B.G.S.; Picoli, C.C.; Gonçalves, B.O.P.; Silva, W.N.; Costa, A.C.; Moraes, M.M.; Costa, P.A.C.; Santos, G.S.P.; Almeida, M.R.; Silva, L.M.; et al. Tissue-resident glial cells associate with tumoral vasculature and promote cancer progression. Angiogenesis 2023, 26, 129–166. [Google Scholar] [CrossRef]
- Yu, J.; Ye, K.; Li, J.; Wei, Y.; Zhou, J.; Ni, W.; Zhang, L.; Chen, T.; Tang, B.; Xu, H.; et al. Schwann-like cell conditioned medium promotes angiogenesis and nerve regeneration. Cell Tissue Bank. 2022, 23, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zeng, Q.; Wu, Z.; Li, Z.; Gao, Q.; Liao, Z.; Li, H.; Ling, C.; Chen, C.; Wang, H.; et al. Enhancing intraneural revascularization following peripheral nerve injury through hypoxic Schwann-cell-derived exosomes: An insight into endothelial glycolysis. J. Nanobiotechnol. 2024, 22, 283. [Google Scholar] [CrossRef] [PubMed]
- Kruglov, O.; Vats, K.; Soman, V.; Tyurin, V.A.; Tyurina, Y.Y.; Wang, J.; Williams, L.; Zhang, J.; Donahue Carey, C.; Jaklitsch, E.; et al. Melanoma-associated repair-like Schwann cells suppress anti-tumor T-cells via 12/15-LOX/COX2-associated eicosanoid production. Oncoimmunology 2023, 12, 2192098. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, J.; Cheng, F.; Zheng, F. The Emerging Role of Schwann Cells in the Tumor Immune Microenvironment and Its Potential Clinical Application. Int. J. Mol. Sci. 2024, 25, 13722. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-P.; Booker, R.C.; Brosseau, J.-P.; Chen, Z.; Mo, J.; Tchegnon, E.; Wang, Y.; Clapp, D.W.; Le, L.Q. Contributions of inflammation and tumor microenvironment to neurofibroma tumorigenesis. J. Clin. Investig. 2018, 128, 2848–2861. [Google Scholar] [CrossRef]
- Xue, M.; Zhu, Y.; Jiang, Y.; Han, L.; Shi, M.; Su, R.; Wang, L.; Xiong, C.; Wang, C.; Wang, T.; et al. Schwann cells regulate tumor cells and cancer-associated fibroblasts in the pancreatic ductal adenocarcinoma microenvironment. Nat. Commun. 2023, 14, 4600. [Google Scholar] [CrossRef]
- Tian, Z.; Du, Z.; Bai, G.; Gong, Q.; You, Y.; Xu, G.; Liu, J.; Xiao, M.; Wang, Y.; He, Y. Schwann cell derived pleiotrophin stimulates fibroblast for proliferation and excessive collagen deposition in plexiform neurofibroma. Cancer Gene Ther. 2024, 31, 627–640. [Google Scholar] [CrossRef]
- Parrinello, S.; Napoli, I.; Ribeiro, S.; Wingfield Digby, P.; Fedorova, M.; Parkinson, D.B.; Doddrell, R.D.; Nakayama, M.; Adams, R.H.; Lloyd, A.C. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 2010, 143, 145–155. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, B.; Gu, Y.; Zhou, S.; Qian, T.; Wang, Y.; Ding, G.; Ding, F.; Gu, X. Fibroblast-derived tenascin-C promotes Schwann cell migration through β1-integrin dependent pathway during peripheral nerve regeneration. Glia 2016, 64, 374–385. [Google Scholar] [CrossRef]
- Fornasari, B.E.; El Soury, M.; Nato, G.; Fucini, A.; Carta, G.; Ronchi, G.; Crosio, A.; Perroteau, I.; Geuna, S.; Raimondo, S.; et al. Fibroblasts Colonizing Nerve Conduits Express High Levels of Soluble Neuregulin1, a Factor Promoting Schwann Cell Dedifferentiation. Cells 2020, 9, 1366. [Google Scholar] [CrossRef]
- Bunimovich, Y.L.; Keskinov, A.A.; Shurin, G.V.; Shurin, M.R. Schwann cells: A new player in the tumor microenvironment. Cancer Immunol. Immunother. 2017, 66, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, S.; Chen, M. Schwann Cells in the Tumor Microenvironment: Need More Attention. J. Oncol. 2022, 2022, 1058667. [Google Scholar] [CrossRef] [PubMed]
- Deborde, S.; Wong, R.J. The Role of Schwann Cells in Cancer. Adv. Biol. 2022, 6, e2200089. [Google Scholar] [CrossRef] [PubMed]
- Shurin, G.V.; Kruglov, O.; Ding, F.; Lin, Y.; Hao, X.; Keskinov, A.A.; You, Z.; Lokshin, A.E.; LaFramboise, W.A.; Falo, L.D., Jr.; et al. Melanoma-Induced Reprogramming of Schwann Cell Signaling Aids Tumor Growth. Cancer Res. 2019, 79, 2736–2747. [Google Scholar] [CrossRef]
- Shurin, M.R.; Wheeler, S.E.; Shurin, G.V.; Zhong, H.; Zhou, Y. Schwann cells in the normal and pathological lung microenvironment. Front. Mol. Biosci. 2024, 11, 1365760. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, H.; Han, B. Schwann Cells Are Overexpressed and Inversely Correlated with Survival of Non-Small Cell Lung Cancer Patients. J. Thorac. Oncol. 2019, 14, S673–S674. [Google Scholar] [CrossRef]
- Sun, C.; Ye, Y.; Tan, Z.; Liu, Y.; Li, Y.; Hu, W.; Liang, K.; Egranov, S.D.; Huang, L.A.; Zhang, Z.; et al. Tumor-associated nonmyelinating Schwann cell-expressed PVT1 promotes pancreatic cancer kynurenine pathway and tumor immune exclusion. Sci. Adv. 2023, 9, eadd6995. [Google Scholar] [CrossRef]
- Otani, Y.; Katayama, H.; Zhu, Y.; Huang, R.; Shigehira, T.; Shien, K.; Suzawa, K.; Yamamoto, H.; Shien, T.; Toyooka, S.; et al. Adrenergic microenvironment driven by cancer-associated Schwann cells contributes to chemoresistance in patients with lung cancer. Cancer Sci. 2024, 115, 2333–2345. [Google Scholar] [CrossRef]
- Silva, V.M.; Gomes, J.A.; Tenorio, L.P.G.; de Omena Neta, G.C.; da Costa Paixao, K.; Duarte, A.K.F.; da Silva, G.C.B.; Ferreira, R.J.S.; Koike, B.D.V.; de Sales Marques, C.; et al. Schwann cell reprogramming and lung cancer progression: A meta-analysis of transcriptome data. Oncotarget 2019, 10, 7288–7307. [Google Scholar] [CrossRef]
- Wong, F.C.; Merker, S.R.; Bauer, L.; Han, Y.; Le, V.M.H.; Wenzel, C.; Böthig, L.; Heiduk, M.; Drobisch, P.; Rao, V.S.; et al. Extracellular vesicles from pancreatic cancer and its tumour microenvironment promote increased Schwann cell migration. Br. J. Cancer 2025, 132, 326–339. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, X.; Huang, L.; Zhang, Y.; Li, Z.; Su, D.; Lin, L.; Zhou, P.; Ye, H.; Lu, Y. Tumour-associated macrophages and Schwann cells promote perineural invasion via paracrine loop in pancreatic ductal adenocarcinoma. Br. J. Cancer 2024, 130, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Deininger, S.; Schumacher, J.; Blechschmidt, A.; Song, J.; Klugmann, C.; Antoniadis, G.; Pedro, M.; Knöll, B.; Meyer Zu Reckendorf, S. Nerve injury converts Schwann cells in a long-term repair-like state in human neuroma tissue. Exp. Neurol. 2024, 382, 114981. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.; Li, Y.; Deng, M.; Hua, T.; Yang, M.; Li, J.; Feng, X.; Yuan, H. Glycometabolism Reprogramming of Glial Cells in Central Nervous System: Novel Target for Neuropathic Pain. Front. Immunol. 2022, 13, 861290. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Domínguez, D.; Elosúa-Bayes, M.; Beckedorff, F.; Laudanna, C.; Bigas, C.; Douillet, D.; Greco, C.; Symeonidi, A.; Hernández, I.; et al. Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature 2021, 599, 485–490. [Google Scholar] [CrossRef]
- Rangel-Sosa, M.M.; Mann, F.; Chauvet, S. Pancreatic Schwann cell reprogramming supports cancer-associated neuronal remodeling. Glia 2024, 72, 1840–1861. [Google Scholar] [CrossRef]
- Edwards, H.L.; Mulvey, M.R.; Bennett, M.I. Cancer-Related Neuropathic Pain. Cancers 2019, 11, 373. [Google Scholar] [CrossRef]
- Ganaraja, V.H.; Rezk, M.; Dubey, D. Paraneoplastic neurological syndrome: Growing spectrum and relevance. Neurol. Sci. 2022, 43, 3583–3594. [Google Scholar] [CrossRef]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef]
- Bao, T.; Basal, C.; Seluzicki, C.; Li, S.Q.; Seidman, A.D.; Mao, J.J. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: Prevalence, risk factors, and fall risk. Breast Cancer Res. Treat. 2016, 159, 327–333. [Google Scholar] [CrossRef]
- Tonyan, S.; Pospelova, M.; Krasnikova, V.; Fionik, O.; Alekseeva, T.; Samochernykh, K.; Ivanova, N.; Vavilova, T.; Vasilieva, E.; Makhanova, A.; et al. Neurotrophin-3 (NT-3) as a Potential Biomarker of the Peripheral Nervous System Damage Following Breast Cancer Treatment. Pathophysiology 2023, 30, 110–122. [Google Scholar] [CrossRef]
- Martino, M.A.; Miller, E.; Grendys, E.C., Jr. The administration of chemotherapy in a patient with Charcot-Marie-Tooth and ovarian cancer. Gynecol. Oncol. 2005, 97, 710–712. [Google Scholar] [CrossRef]
- Chauvenet, A.R.; Shashi, V.; Selsky, C.; Morgan, E.; Kurtzberg, J.; Bell, B. Vincristine-induced neuropathy as the initial presentation of charcot-marie-tooth disease in acute lymphoblastic leukemia: A Pediatric Oncology Group study. J. Pediatr. Hematol. Oncol. 2003, 25, 316–320. [Google Scholar] [CrossRef]
- Burgess, J.; Ferdousi, M.; Gosal, D.; Boon, C.; Matsumoto, K.; Marshall, A.; Mak, T.; Marshall, A.; Frank, B.; Malik, R.A.; et al. Chemotherapy-Induced Peripheral Neuropathy: Epidemiology, Pathomechanisms and Treatment. Oncol. Ther. 2021, 9, 385–450. [Google Scholar] [CrossRef]
- Desforges, A.D.; Hebert, C.M.; Spence, A.L.; Reid, B.; Dhaibar, H.A.; Cruz-Topete, D.; Cornett, E.M.; Kaye, A.D.; Urits, I.; Viswanath, O. Treatment and diagnosis of chemotherapy-induced peripheral neuropathy: An update. Biomed. Pharmacother. 2022, 147, 112671. [Google Scholar] [CrossRef]
- Wu, Z.; Yan, W.; Wang, K.; Xu, G.; Zhu, D.; Li, X.; Wang, H.; Yang, M.; Zhang, X.; Wu, J. Lysosomal dysfunction in Schwann cells is involved in bortezomib-induced peripheral neurotoxicity. Arch. Toxicol. 2023, 97, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Nagayasu, K.; Shirakawa, H.; Kaneko, S. Specific Cellular Effects of Low Bortezomib Concentrations on Purified Cultures of Schwann Cells, Satellite Glial Cells, Macrophages, and Dorsal Root Ganglion Neurons. Biol. Pharm. Bull. 2023, 46, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Jang, S.Y.; Lee, H.K.; Jung, J.; Suh, D.J.; Seo, S.Y.; Park, H.T. Pathological adaptive responses of Schwann cells to endoplasmic reticulum stress in bortezomib-induced peripheral neuropathy. Glia 2010, 58, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.M.; Jimenez-Andrade, J.M.; Jonas, B.M.; Sevcik, M.A.; Koewler, N.J.; Ghilardi, J.R.; Wong, G.Y.; Mantyh, P.W. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp. Neurol. 2007, 203, 42–54. [Google Scholar] [CrossRef]
- Imai, S.; Koyanagi, M.; Azimi, Z.; Nakazato, Y.; Matsumoto, M.; Ogihara, T.; Yonezawa, A.; Omura, T.; Nakagawa, S.; Wakatsuki, S.; et al. Taxanes and platinum derivatives impair Schwann cells via distinct mechanisms. Sci. Rep. 2017, 7, 5947. [Google Scholar] [CrossRef]
- Wang, X.; Yang, W.; Wang, L.; Zheng, L.; Choi, W.S. Platinum-based chemotherapy induces demyelination of Schwann cells in oral squamous cell carcinoma treatment. Toxicol. Appl. Pharmacol. 2023, 481, 116751. [Google Scholar] [CrossRef]
- Matta, C.; Meyer, L.; Mensah-Nyagan, A.G.; Taleb, O. Behavioral, Electrophysiological, and Histological Characterization of a New Rat Model for Neoadjuvant Chemotherapy-Induced Neuropathic Pain: Therapeutic Potential of Duloxetine and Allopregnanolone Concomitant Treatment. Neurotox. Res. 2020, 38, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, M.; Imai, S.; Matsumoto, M.; Iguma, Y.; Kawaguchi-Sakita, N.; Kotake, T.; Iwamitsu, Y.; Ntogwa, M.; Hiraiwa, R.; Nagayasu, K. Pronociceptive roles of schwann cell–derived galectin-3 in taxane-induced peripheral neuropathy. Cancer Res. 2021, 81, 2207–2219. [Google Scholar] [CrossRef]
- Nakano, A.; Abe, M.; Oda, A.; Amou, H.; Hiasa, M.; Nakamura, S.; Miki, H.; Harada, T.; Fujii, S.; Kagawa, K.; et al. Delayed treatment with vitamin C and N-acetyl-L-cysteine protects Schwann cells without compromising the anti-myeloma activity of bortezomib. Int. J. Hematol. 2011, 93, 727–735. [Google Scholar] [CrossRef]
- Huff, T.C.; Sant, D.W.; Camarena, V.; Van Booven, D.; Andrade, N.S.; Mustafi, S.; Monje, P.V.; Wang, G. Vitamin C regulates Schwann cell myelination by promoting DNA demethylation of pro-myelinating genes. J. Neurochem. 2021, 157, 1759–1773. [Google Scholar] [CrossRef]
- Koyanagi, M.; Imai, S.; Iwamitsu, Y.; Matsumoto, M.; Saigo, M.; Moriya, A.; Ogihara, T.; Nakazato, Y.; Yonezawa, A.; Nakagawa, S.; et al. Cilostazol is an effective causal therapy for preventing paclitaxel-induced peripheral neuropathy by suppression of Schwann cell dedifferentiation. Neuropharmacology 2021, 188, 108514. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Li, X.; Zhang, C.; Cai, H.; Qi, J.; Wang, K.; Li, X.; Wu, X.; Ye, Z.; et al. Dihydromyricetin restores lysosomal function in Schwann cells to alleviate bortezomib-induced peripheral neuropathy via ERK/TFEB signaling. Arch. Toxicol. 2025, 99, 2639–2653. [Google Scholar] [CrossRef]
- Al-Massri, K.F.; Ahmed, L.A.; El-Abhar, H.S. Mesenchymal stem cells in chemotherapy-induced peripheral neuropathy: A new challenging approach that requires further investigations. J. Tissue Eng. Regen. Med. 2020, 14, 108–122. [Google Scholar] [CrossRef]
- Chiu, D.; Rhee, J.; Gonzalez Castro, L.N. Diagnosis and Treatment of Paraneoplastic Neurologic Syndromes. Antibodies 2023, 12, 50. [Google Scholar] [CrossRef]
- Giometto, B.; Grisold, W.; Vitaliani, R.; Graus, F.; Honnorat, J.; Bertolini, G. Paraneoplastic neurologic syndrome in the PNS Euronetwork database: A European study from 20 centers. Arch. Neurol. 2010, 67, 330–335. [Google Scholar] [CrossRef]
- Siles, A.M.; Martínez-Hernández, E.; Araque, J.; Diaz-Manera, J.; Rojas-Garcia, R.; Gallardo, E.; Illa, I.; Graus, F.; Querol, L. Antibodies against cell adhesion molecules and neural structures in paraneoplastic neuropathies. Ann. Clin. Transl. Neurol. 2018, 5, 559–569. [Google Scholar] [CrossRef]
- Tomimoto, H.; Brengman, J.M.; Yanagihara, T. Paraneoplastic cerebellar degeneration with a circulating antibody against neurons and non-neuronal cells. Acta Neuropathol. 1993, 86, 206–211. [Google Scholar] [CrossRef]
- Weiss, T.; Taschner-Mandl, S.; Janker, L.; Bileck, A.; Rifatbegovic, F.; Kromp, F.; Sorger, H.; Kauer, M.O.; Frech, C.; Windhager, R.; et al. Schwann cell plasticity regulates neuroblastic tumor cell differentiation via epidermal growth factor-like protein 8. Nat. Commun. 2021, 12, 1624. [Google Scholar] [CrossRef]
- Bakst, R.L.; Barajas, F.; He, S.; Chernichenko, N.; Chen, C.; He, S.; McNamara, W.; Lee, S.; Deborde, S.; Wong, R.J. Are Schwann Cells a Target in Radiation for Perineural Invasion? Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, S629–S630. [Google Scholar] [CrossRef]
- Zhang, W.; He, R.; Yang, W.; Zhang, Y.; Yuan, Q.; Wang, J.; Liu, Y.; Chen, S.; Zhang, S.; Zhang, W. Autophagic Schwann cells promote perineural invasion mediated by the NGF/ATG7 paracrine pathway in pancreatic cancer. J. Exp. Clin. Cancer Res. 2022, 41, 48. [Google Scholar] [CrossRef]
- He, Z.; Liu, F.; Lin, L.; Huang, Z.; Wang, Y. Interplay Between Schwann Cells and Peripheral Cancers: Mechanisms and Therapeutic Targets in Cancer Progression. Glia 2025, 73, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.L.; Ratner, N. How does the Schwann cell lineage form tumors in NF1? Glia 2008, 56, 1590–1605. [Google Scholar] [CrossRef] [PubMed]
- Rahrmann, E.P.; Moriarity, B.S.; Otto, G.M.; Watson, A.L.; Choi, K.; Collins, M.H.; Wallace, M.; Webber, B.R.; Forster, C.L.; Rizzardi, A.E.; et al. Trp53 haploinsufficiency modifies EGFR-driven peripheral nerve sheath tumorigenesis. Am. J. Pathol. 2014, 184, 2082–2098. [Google Scholar] [CrossRef]
- Keng, V.W.; Rahrmann, E.P.; Watson, A.L.; Tschida, B.R.; Moertel, C.L.; Jessen, W.J.; Rizvi, T.A.; Collins, M.H.; Ratner, N.; Largaespada, D.A. PTEN and NF1 inactivation in Schwann cells produces a severe phenotype in the peripheral nervous system that promotes the development and malignant progression of peripheral nerve sheath tumors. Cancer Res. 2012, 72, 3405–3413. [Google Scholar] [CrossRef]
- Kazmi, S.J.; Byer, S.J.; Eckert, J.M.; Turk, A.N.; Huijbregts, R.P.; Brossier, N.M.; Grizzle, W.E.; Mikhail, F.M.; Roth, K.A.; Carroll, S.L. Transgenic mice overexpressing neuregulin-1 model neurofibroma-malignant peripheral nerve sheath tumor progression and implicate specific chromosomal copy number variations in tumorigenesis. Am. J. Pathol. 2013, 182, 646–667. [Google Scholar] [CrossRef]
- Jiang, C.; McKay, R.M.; Le, L.Q. Tumorigenesis in neurofibromatosis type 1: Role of the microenvironment. Oncogene 2021, 40, 5781–5787. [Google Scholar] [CrossRef]
- Macarenco, R.S.; Ellinger, F.; Oliveira, A.M. Perineurioma: A distinctive and underrecognized peripheral nerve sheath neoplasm. Arch. Pathol. Lab. Med. 2007, 131, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.; Pedro, M.T.; Pala, A.; Heinen, C.; Scheuerle, A.; Braun, M.; Antoniadis, G. Perineurioma: A Rare Entity of Peripheral Nerve Sheath Tumors. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2022, 83, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lenartowicz, K.A.; Smith, B.W.; Jack, M.M.; Wilson, T.J.; Klein, C.J.; Amrami, K.K.; Spinner, R.J. What is new in intraneural perineurioma? Acta Neurochir. 2023, 165, 3539–3547. [Google Scholar] [CrossRef]
- Furlan, A.; Dyachuk, V.; Kastriti, M.E.; Calvo-Enrique, L.; Abdo, H.; Hadjab, S.; Chontorotzea, T.; Akkuratova, N.; Usoskin, D.; Kamenev, D.; et al. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science 2017, 357, eaal3753. [Google Scholar] [CrossRef]
- Abramowicz, A.; Gos, M. Neurofibromin in neurofibromatosis type 1—Mutations in NF1gene as a cause of disease. Dev. Period. Med. 2014, 18, 297–306. [Google Scholar]
- Plotkin, S.R.; Wick, A. Neurofibromatosis and Schwannomatosis. Semin. Neurol. 2018, 38, 73–85. [Google Scholar] [CrossRef]
- Rosenfeld, A.; Listernick, R.; Charrow, J.; Goldman, S. Neurofibromatosis type 1 and high-grade tumors of the central nervous system. Childs Nerv. Syst. 2010, 26, 663–667. [Google Scholar] [CrossRef]
- Ferner, R.E.; Gutmann, D.H. Neurofibromatosis type 1 (NF1): Diagnosis and management. Handb. Clin. Neurol. 2013, 115, 939–955. [Google Scholar] [CrossRef]
- Staedtke, V.; Bai, R.Y.; Blakeley, J.O. Cancer of the Peripheral Nerve in Neurofibromatosis Type 1. Neurotherapeutics 2017, 14, 298–306. [Google Scholar] [CrossRef]
- Zhu, Y.; Ghosh, P.; Charnay, P.; Burns, D.K.; Parada, L.F. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science 2002, 296, 920–922. [Google Scholar] [CrossRef]
- Parrinello, S.; Lloyd, A.C. Neurofibroma development in NF1—Insights into tumour initiation. Trends Cell Biol. 2009, 19, 395–403. [Google Scholar] [CrossRef]
- Muir, D.; Neubauer, D.; Lim, I.T.; Yachnis, A.T.; Wallace, M.R. Tumorigenic properties of neurofibromin-deficient neurofibroma Schwann cells. Am. J. Pathol. 2001, 158, 501–513. [Google Scholar] [CrossRef]
- White, E.E.; Rhodes, S.D. The NF1+/- Immune Microenvironment: Dueling Roles in Neurofibroma Development and Malignant Transformation. Cancers 2024, 16, 994. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.; Napoli, I.; White, I.J.; Parrinello, S.; Flanagan, A.M.; Suter, U.; Parada, L.F.; Lloyd, A.C. Injury signals cooperate with Nf1 loss to relieve the tumor-suppressive environment of adult peripheral nerve. Cell Rep. 2013, 5, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Somatilaka, B.N.; Sadek, A.; McKay, R.M.; Le, L.Q. Malignant peripheral nerve sheath tumor: Models, biology, and translation. Oncogene 2022, 41, 2405–2421. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.M.N.; Deng, Y.; Wang, J.; Zhao, C.; Wang, J.; Rao, R.; Xu, L.; Zhou, W.; Choi, K.; Rizvi, T.A.; et al. Programming of Schwann Cells by Lats1/2-TAZ/YAP Signaling Drives Malignant Peripheral Nerve Sheath Tumorigenesis. Cancer Cell 2018, 33, 292–308.e297. [Google Scholar] [CrossRef] [PubMed]
- Ferdoushi, A.; Jamaluddin, M.F.B.; Li, X.; Pundavela, J.; Faulkner, S.; Hondermarck, H. Secretome analysis of human schwann cells derived from malignant peripheral nerve sheath tumor. Proteomics 2022, 22, e2100063. [Google Scholar] [CrossRef]
- Sanchez, L.D.; Bui, A.; Klesse, L.J. Targeted Therapies for the Neurofibromatoses. Cancers 2021, 13, 6032. [Google Scholar] [CrossRef]
- Na, B.; Shah, S.R.; Vasudevan, H.N. Past, Present, and Future Therapeutic Strategies for NF-1-Associated Tumors. Curr. Oncol. Rep. 2024, 26, 706–713. [Google Scholar] [CrossRef]
- Williams, K.B.; Largaespada, D.A. New Model Systems and the Development of Targeted Therapies for the Treatment of Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors. Genes 2020, 11, 477. [Google Scholar] [CrossRef]
- Mazuelas, H.; Magallón-Lorenz, M.; Fernández-Rodríguez, J.; Uriarte-Arrazola, I.; Richaud-Patin, Y.; Terribas, E.; Villanueva, A.; Castellanos, E.; Blanco, I.; Raya, Á.; et al. Modeling iPSC-derived human neurofibroma-like tumors in mice uncovers the heterogeneity of Schwann cells within plexiform neurofibromas. Cell Rep. 2022, 38, 110385. [Google Scholar] [CrossRef]
- Rambo, M.; Agarwala, I.; Vanek, C.; Xiao, Y.; Brown, E.; Mills, K.L. Schwann Cells Deficient in Neurofibromin Lack Sensitivity to Their Biomechanical Microenvironment. Genes Chromosomes Cancer 2025, 64, e70036. [Google Scholar] [CrossRef] [PubMed]
- Fay, C.X.; Zunica, E.R.M.; Awad, E.; Bradley, W.; Church, C.; Liu, J.; Liu, H.; Crossman, D.K.; Mobley, J.A.; Kirwan, J.P.; et al. Global proteomics and affinity mass spectrometry analysis of human Schwann cells indicates that variation in and loss of neurofibromin (NF1) alters protein expression and cellular and mitochondrial metabolism. Sci. Rep. 2025, 15, 3883. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.; Robanus-Maandag, E.; Niwa-Kawakita, M.; van der Valk, M.; Woodruff, J.M.; Goutebroze, L.; Mérel, P.; Berns, A.; Thomas, G. Schwann cell hyperplasia and tumors in transgenic mice expressing a naturally occurring mutant NF2 protein. Genes. Dev. 1999, 13, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, H.N.; Payne, E.; Delley, C.L.; John Liu, S.; Mirchia, K.; Sale, M.J.; Lastella, S.; Nunez, M.S.; Lucas, C.-H.G.; Eaton, C.D.; et al. Functional interactions between neurofibromatosis tumor suppressors underlie Schwann cell tumor de-differentiation and treatment resistance. Nat. Commun. 2024, 15, 477. [Google Scholar] [CrossRef]
- Vasudevan, H.N.; Lucas, C.G.; Villanueva-Meyer, J.E.; Theodosopoulos, P.V.; Raleigh, D.R. Genetic Events and Signaling Mechanisms Underlying Schwann Cell Fate in Development and Cancer. Neurosurgery 2021, 88, 234–245. [Google Scholar] [CrossRef]
- Helbing, D.-L.; Schulz, A.; Morrison, H. Pathomechanisms in schwannoma development and progression. Oncogene 2020, 39, 5421–5429. [Google Scholar] [CrossRef]
- Pećina-Šlaus, N. Merlin, the NF2 gene product. Pathol. Oncol. Res. 2013, 19, 365–373. [Google Scholar] [CrossRef]
- Chiasson-MacKenzie, C.; Vitte, J.; Liu, C.H.; Wright, E.A.; Flynn, E.A.; Stott, S.L.; Giovannini, M.; McClatchey, A.I. Cellular mechanisms of heterogeneity in NF2-mutant schwannoma. Nat. Commun. 2023, 14, 1559. [Google Scholar] [CrossRef]
- Goutagny, S.; Raymond, E.; Esposito-Farese, M.; Trunet, S.; Mawrin, C.; Bernardeschi, D.; Larroque, B.; Sterkers, O.; Giovannini, M.; Kalamarides, M. Phase II study of mTORC1 inhibition by everolimus in neurofibromatosis type 2 patients with growing vestibular schwannomas. J. Neurooncol. 2015, 122, 313–320. [Google Scholar] [CrossRef]
- Mautner, V.F.; Lindenau, M.; Baser, M.E.; Hazim, W.; Tatagiba, M.; Haase, W.; Samii, M.; Wais, R.; Pulst, S.M. The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery 1996, 38, 880–885; discussion 885–886. [Google Scholar] [CrossRef] [PubMed]
- Gerganov, V.; Petrov, M.; Sakelarova, T. Schwannomas of Brain and Spinal Cord. Adv. Exp. Med. Biol. 2023, 1405, 331–362. [Google Scholar] [CrossRef]
- Roosli, C.; Linthicum, J.; Fred, H.; Cureoglu, S.; Merchant, S.N. What Is the Site of Origin of Cochleovestibular Schwannomas? Audiol. Neurotol. 2011, 17, 121–125. [Google Scholar] [CrossRef]
- Skovronsky, D.M.; Oberholtzer, J.C. Pathologic classification of peripheral nerve tumors. Neurosurg. Clin. N. Am. 2004, 15, 157–166. [Google Scholar] [CrossRef]
- Mohamed, T.; Melfi, V.; Colciago, A.; Magnaghi, V. Hearing loss and vestibular schwannoma: New insights into Schwann cells implication. Cell Death Dis. 2023, 14, 629. [Google Scholar] [CrossRef]
- Barrett, T.F.; Patel, B.; Khan, S.M.; Mullins, R.D.Z.; Yim, A.K.Y.; Pugazenthi, S.; Mahlokozera, T.; Zipfel, G.J.; Herzog, J.A.; Chicoine, M.R.; et al. Single-cell multi-omic analysis of the vestibular schwannoma ecosystem uncovers a nerve injury-like state. Nat. Commun. 2024, 15, 478. [Google Scholar] [CrossRef]
- Hansen, M.R.; Roehm, P.C.; Chatterjee, P.; Green, S.H. Constitutive neuregulin-1/ErbB signaling contributes to human vestibular schwannoma proliferation. Glia 2006, 53, 593–600. [Google Scholar] [CrossRef]
- Stonecypher, M.S.; Byer, S.J.; Grizzle, W.E.; Carroll, S.L. Activation of the neuregulin-1/ErbB signaling pathway promotes the proliferation of neoplastic Schwann cells in human malignant peripheral nerve sheath tumors. Oncogene 2005, 24, 5589–5605. [Google Scholar] [CrossRef]
- Black, L.E.; Longo, J.F.; Anderson, J.C.; Carroll, S.L. Inhibition of Erb-B2 Receptor Tyrosine Kinase 3 and Associated Regulatory Pathways Potently Impairs Malignant Peripheral Nerve Sheath Tumor Proliferation and Survival. Am. J. Pathol. 2023, 193, 1298–1318. [Google Scholar] [CrossRef]
- Wu, L.; Vasilijic, S.; Sun, Y.; Chen, J.; Landegger, L.D.; Zhang, Y.; Zhou, W.; Ren, J.; Early, S.; Yin, Z.; et al. Losartan prevents tumor-induced hearing loss and augments radiation efficacy in NF2 schwannoma rodent models. Sci. Transl. Med. 2021, 13, eabd4816. [Google Scholar] [CrossRef]
- Koeller, K.K.; Shih, R.Y. Intradural Extramedullary Spinal Neoplasms: Radiologic-Pathologic Correlation. Radiographics 2019, 39, 468–490. [Google Scholar] [CrossRef] [PubMed]
- Gelabert-González, M.; Castro-Bouzas, D.; Serramito-García, R.; Santín-Amo, J.M.; Arán-Echabe, E.; Prieto-González, Á.; Allut, A.G. Tumours of the nerve root sheath in the spine. Rev. Neurol. 2011, 53, 390–396. [Google Scholar]
- Lenzi, J.; Anichini, G.; Landi, A.; Piciocchi, A.; Passacantilli, E.; Pedace, F.; Delfini, R.; Santoro, A. Spinal Nerves Schwannomas: Experience on 367 Cases-Historic Overview on How Clinical, Radiological, and Surgical Practices Have Changed over a Course of 60 Years. Neurol. Res. Int. 2017, 2017, 3568359. [Google Scholar] [CrossRef]
- Celli, P.; Trillò, G.; Ferrante, L. Spinal extradural schwannoma. J. Neurosurg. Spine 2005, 2, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Ahuja, A.; Chauhan, D.S.; Paliwal, P.; Bhardwaj, M. A clinicopathological study of peripheral schwannomas. Med. Pharm. Rep. 2021, 94, 191–196. [Google Scholar] [CrossRef]
- Young, E.D.; Ingram, D.; Metcalf-Doetsch, W.; Khan, D.; Al Sannaa, G.; Le Loarer, F.; Lazar, A.J.F.; Slopis, J.; Torres, K.E.; Lev, D.; et al. Clinicopathological variables of sporadic schwannomas of peripheral nerve in 291 patients and expression of biologically relevant markers. J. Neurosurg. 2018, 129, 805–814. [Google Scholar] [CrossRef]
- Albert, P.; Patel, J.; Badawy, K.; Weissinger, W.; Brenner, M.; Bourhill, I.; Parnell, J. Peripheral Nerve Schwannoma: A Review of Varying Clinical Presentations and Imaging Findings. J. Foot Ankle Surg. 2017, 56, 632–637. [Google Scholar] [CrossRef]
- Pennington, Z.; Lubelski, D.; Medikonda, R.; Belzberg, A.J. Schwannomatosis: Review of Diagnosis and Management. In Diagnostic Assessment and Treatment of Peripheral Nerve Tumors; Guedes, F., Zager, E.L., Garozzo, D., Rasulic, L., Socolovsky, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 371–382. [Google Scholar] [CrossRef]
- Evans, G.R.; Lloyd, S.K.W.; Ramsden, R.T. Neurofibromatosis type 2. Adv. Otorhinolaryngol. 2011, 70, 91–98. [Google Scholar] [CrossRef]
- Evans, D.G. Neurofibromatosis type 2 (NF2): A clinical and molecular review. Orphanet J. Rare Dis. 2009, 4, 16. [Google Scholar] [CrossRef]
- Plotkin, S.R.; Messiaen, L.; Legius, E.; Pancza, P.; Avery, R.A.; Blakeley, J.O.; Babovic-Vuksanovic, D.; Ferner, R.; Fisher, M.J.; Friedman, J.M.; et al. Updated diagnostic criteria and nomenclature for neurofibromatosis type 2 and schwannomatosis: An international consensus recommendation. Genet. Med. 2022, 24, 1967–1977. [Google Scholar] [CrossRef]
- Smith, M.J.; Bowers, N.L.; Bulman, M.; Gokhale, C.; Wallace, A.J.; King, A.T.; Lloyd, S.K.; Rutherford, S.A.; Hammerbeck-Ward, C.L.; Freeman, S.R.; et al. Revisiting neurofibromatosis type 2 diagnostic criteria to exclude LZTR1-related schwannomatosis. Neurology 2017, 88, 87–92. [Google Scholar] [CrossRef]
- Melfi, V.; Mohamed, T.; Colciago, A.; Fasciani, A.; De Francesco, R.; Bettio, D.; Cerqua, C.; Boaretto, F.; Basso, E.; Ferraresi, S.; et al. Typical NF2 and LTZR1 Mutations Are Retained in an Immortalized Human Schwann Cell Model of Schwannomatosis. Heliyon 2024, 10, e38957. [Google Scholar] [CrossRef]
- Miranda, I.C.; Taylor, K.R.; Castleman, W.; de Lahunta, A.; Summers, B.A.; Miller, A.D. Schwannosis in Three Foals and a Calf. Vet. Pathol. 2019, 56, 783–788. [Google Scholar] [CrossRef]
- Wallace, M.C.; Tator, C.H.; Lewis, A.J. Chronic regenerative changes in the spinal cord after cord compression injury in rats. Surg. Neurol. 1987, 27, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Walter, G.F.; Gerhard, L. The expression of nerve growth factor receptor on Schwann cells and the effect of these cells on the regeneration of axons in traumatically injured human spinal cord. Acta Neuropathol. 1996, 91, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.H.; Norenberg, M.D.; Kraydieh, S.; Puckett, W.; Marcillo, A.; Dietrich, D. Schwannosis: Role of gliosis and proteoglycan in human spinal cord injury. J. Neurotrauma 2000, 17, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, D.N.; Kamphausen, T.; Van de Nes, J. A 32-year-old male with brainstem lesions. Brain Pathol. 2013, 23, 101–104. [Google Scholar] [CrossRef]
- Adelman, L.S.; Aronson, S.M. Intramedullary nerve fiber and Schwann cell proliferation within the spinal cord (schwannosis). Neurology 1972, 22, 726–731. [Google Scholar] [CrossRef]
- Hori, A. Intraspinal schwannosis of Lissauer’s zone terminalis (formes frustes of Recklinghausen’s neurofibromatosis or reactive?). Acta Neuropathol. 1973, 25, 89–94. [Google Scholar] [CrossRef]
- Rubinstein, L.J. The malformative central nervous system lesions in the central and peripheral forms of neurofibromatosis. A neuropathological study of 22 cases. Ann. N. Y. Acad. Sci. 1986, 486, 14–29. [Google Scholar] [CrossRef]
- Owen, R.S.; Ramarathinam, S.H.; Bailey, A.; Gastaldello, A.; Hussey, K.; Skipp, P.J.; Purcell, A.W.; Siddle, H.V. The differentiation state of the Schwann cell progenitor drives phenotypic variation between two contagious cancers. PLoS Pathog. 2021, 17, e1010033. [Google Scholar] [CrossRef] [PubMed]
- Stammnitz, M.R.; Coorens, T.H.H.; Gori, K.C.; Hayes, D.; Fu, B.; Wang, J.; Martin-Herranz, D.E.; Alexandrov, L.B.; Baez-Ortega, A.; Barthorpe, S.; et al. The Origins and Vulnerabilities of Two Transmissible Cancers in Tasmanian Devils. Cancer Cell 2018, 33, 607–619.e615. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, A.; Coleby, R.; Tovar, C.; Stammnitz, M.R.; Kwon, Y.M.; Owen, R.S.; Tringides, M.; Murchison, E.P.; Skjødt, K.; Thomas, G.J.; et al. The newly-arisen Devil facial tumour disease 2 (DFT2) reveals a mechanism for the emergence of a contagious cancer. eLife 2018, 7, e35314. [Google Scholar] [CrossRef] [PubMed]
- Patchett, A.L.; Flies, A.S.; Lyons, A.B.; Woods, G.M. Curse of the devil: Molecular insights into the emergence of transmissible cancers in the Tasmanian devil (Sarcophilus harrisii). Cell Mol. Life Sci. 2020, 77, 2507–2525. [Google Scholar] [CrossRef]
- Murchison, E.P.; Tovar, C.; Hsu, A.; Bender, H.S.; Kheradpour, P.; Rebbeck, C.A.; Obendorf, D.; Conlan, C.; Bahlo, M.; Blizzard, C.A.; et al. The Tasmanian devil transcriptome reveals Schwann cell origins of a clonally transmissible cancer. Science 2010, 327, 84–87. [Google Scholar] [CrossRef]
- Patchett, A.L.; Coorens, T.H.H.; Darby, J.; Wilson, R.; McKay, M.J.; Kamath, K.S.; Rubin, A.; Wakefield, M.; McIntosh, L.; Mangiola, S.; et al. Two of a kind: Transmissible Schwann cell cancers in the endangered Tasmanian devil (Sarcophilus harrisii). Cell Mol. Life Sci. 2020, 77, 1847–1858. [Google Scholar] [CrossRef]
- Ong, C.E.B.; Cheng, Y.; Siddle, H.V.; Lyons, A.B.; Woods, G.M.; Flies, A.S. Class II transactivator induces expression of MHC-I and MHC-II in transmissible Tasmanian devil facial tumours. Open Biol. 2022, 12, 220208. [Google Scholar] [CrossRef]
- Kreiss, A.; Brown, G.K.; Tovar, C.; Lyons, A.B.; Woods, G.M. Evidence for induction of humoral and cytotoxic immune responses against devil facial tumor disease cells in Tasmanian devils (Sarcophilus harrisii) immunized with killed cell preparations. Vaccine 2015, 33, 3016–3025. [Google Scholar] [CrossRef]
- Flies, A.S.; Flies, E.J.; Fox, S.; Gilbert, A.; Johnson, S.R.; Liu, G.S.; Lyons, A.B.; Patchett, A.L.; Pemberton, D.; Pye, R.J. An oral bait vaccination approach for the Tasmanian devil facial tumor diseases. Expert. Rev. Vaccines 2020, 19, 1–10. [Google Scholar] [CrossRef]
- Pasquini, P.; Baiocchini, A.; Falasca, L.; Annibali, D.; Gimbo, G.; Pace, F.; Del Nonno, F. Mucosal Schwann cell “Hamartoma”: A new entity? World J. Gastroenterol. 2009, 15, 2287–2289. [Google Scholar] [CrossRef]
- Mauriz Barreiro, V.; Ramos Alonso, M.; Fernández López, M.; Rivera Castillo, D.A.; Durana Tonder, C.; Pradera Cibreiro, C. Mucosal Schwann cell hamartoma: A benign and little-known entity. Rev. Esp. Enferm. Dig. 2024, 116, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.A.; Hornick, J.L. Mucosal Schwann cell “hamartoma”: Clinicopathologic study of 26 neural colorectal polyps distinct from neurofibromas and mucosal neuromas. Am. J. Surg. Pathol. 2009, 33, 781–787. [Google Scholar] [CrossRef]
- Han, J.; Chong, Y.; Kim, T.J.; Lee, E.J.; Kang, C.S. Mucosal Schwann Cell Hamartoma in Colorectal Mucosa: A Rare Benign Lesion That Resembles Gastrointestinal Neuroma. J. Pathol. Transl. Med. 2017, 51, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Altaf, F.; Javed, N.; Ghazanfar, H.; Dev, A. Schwann Cell Hamartoma Presenting as a Colonic Polyp: A Rare Case Report with a Literature Review. Cureus 2024, 16, e57674. [Google Scholar] [CrossRef]
- Weiss, T.; Taschner-Mandl, S.; Bileck, A.; Slany, A.; Kromp, F.; Rifatbegovic, F.; Frech, C.; Windhager, R.; Kitzinger, H.; Tzou, C.H.; et al. Proteomics and transcriptomics of peripheral nerve tissue and cells unravel new aspects of the human Schwann cell repair phenotype. Glia 2016, 64, 2133–2153. [Google Scholar] [CrossRef]
- Faroni, A.; Smith, R.J.; Lu, L.; Reid, A.J. Human Schwann-like cells derived from adipose-derived mesenchymal stem cells rapidly de-differentiate in the absence of stimulating medium. Eur. J. Neurosci. 2016, 43, 417–430. [Google Scholar] [CrossRef]
- Horner, S.J.; Couturier, N.; Gueiber, D.C.; Hafner, M.; Rudolf, R. Development and In Vitro Differentiation of Schwann Cells. Cells 2022, 11, 3753. [Google Scholar] [CrossRef]
- Hopf, A.; Schaefer, D.J.; Kalbermatten, D.F.; Guzman, R.; Madduri, S. Schwann Cell-Like Cells: Origin and Usability for Repair and Regeneration of the Peripheral and Central Nervous System. Cells 2020, 9, 1990. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, M.H.; Kim, D.W.; Ahn, W.; Chung, E.; Son, Y.; Chi, G. Comparative Analysis of the Cell Fates of Induced Schwann Cells from Subcutaneous Fat Tissue and Naive Schwann Cells in the Sciatic Nerve Injury Model. BioMed Res. Int. 2017, 2017, 1252851. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.; Lee, D.Y.; Kim, Y.D.; Kim, J.Y.; Lim, H.J.; Lim, S.; Cho, Y.S. Schwann Cell Precursors from Human Pluripotent Stem Cells as a Potential Therapeutic Target for Myelin Repair. Stem Cell Rep. 2017, 8, 1714–1726. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.Y.; Song, C.L.; Jeong, J.E.; Cho, Y.S. Directly induced human Schwann cell precursors as a valuable source of Schwann cells. Stem Cell Res. Ther. 2020, 11, 257. [Google Scholar] [CrossRef]
- Yun, W.; Kim, Y.J.; Lee, G. Direct Conversion to Achieve Glial Cell Fates: Oligodendrocytes and Schwann Cells. Int. J. Stem Cells 2022, 15, 14–25. [Google Scholar] [CrossRef]
- Painter, M.W.; Brosius Lutz, A.; Cheng, Y.C.; Latremoliere, A.; Duong, K.; Miller, C.M.; Posada, S.; Cobos, E.J.; Zhang, A.X.; Wagers, A.J.; et al. Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron 2014, 83, 331–343. [Google Scholar] [CrossRef]
- Painter, M.W. Aging Schwann cells: Mechanisms, implications, future directions. Curr. Opin. Neurobiol. 2017, 47, 203–208. [Google Scholar] [CrossRef]
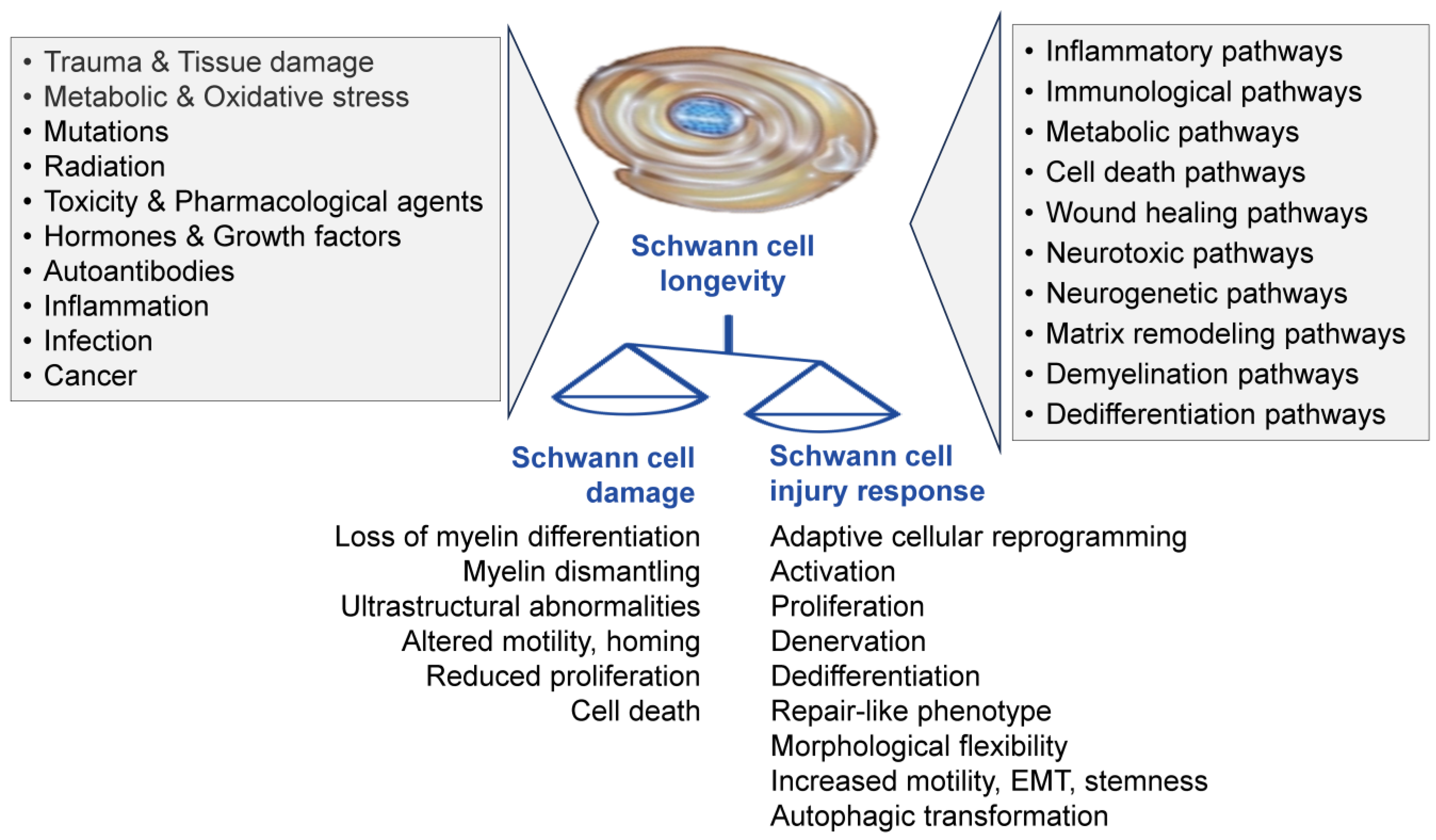
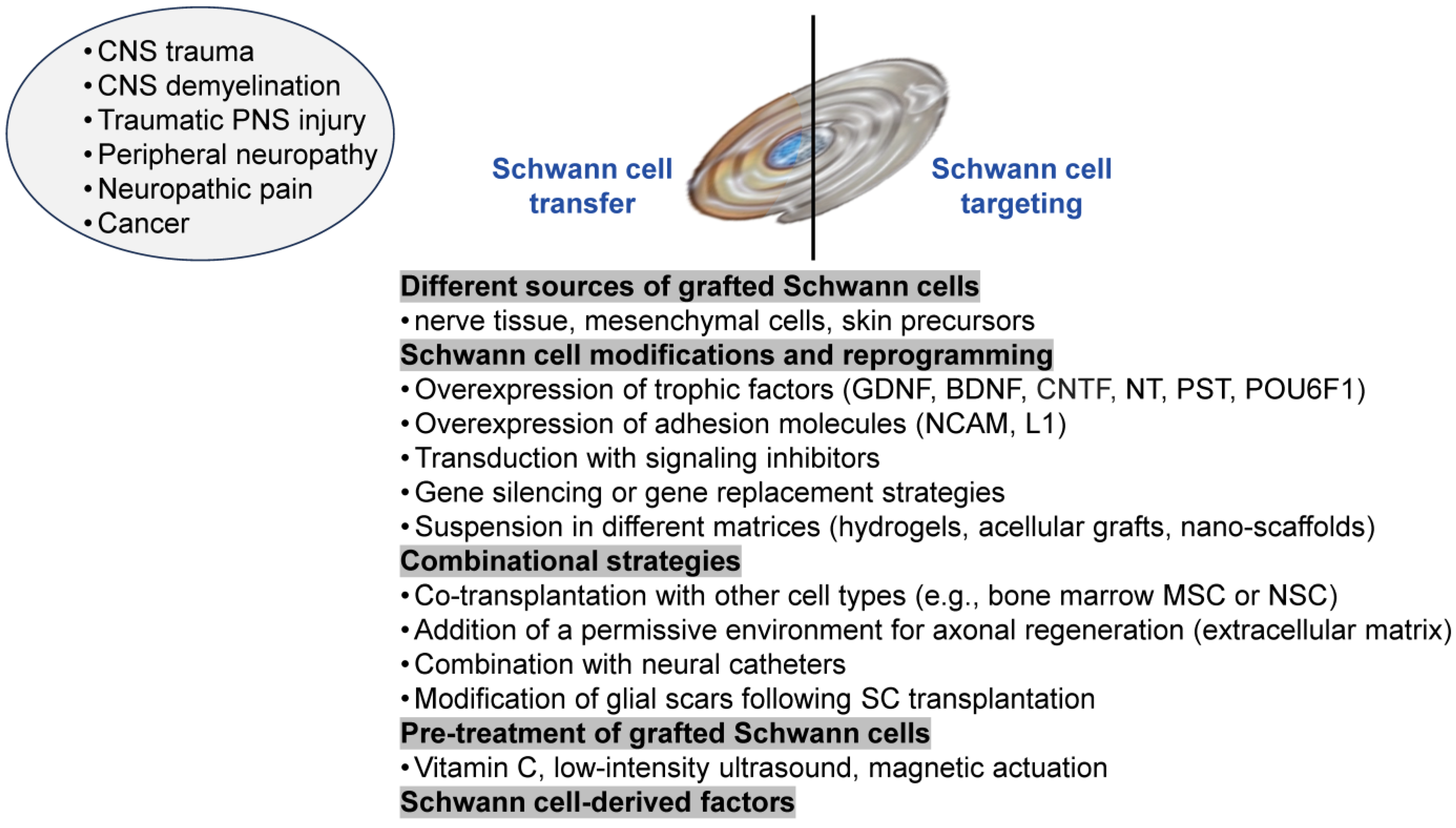
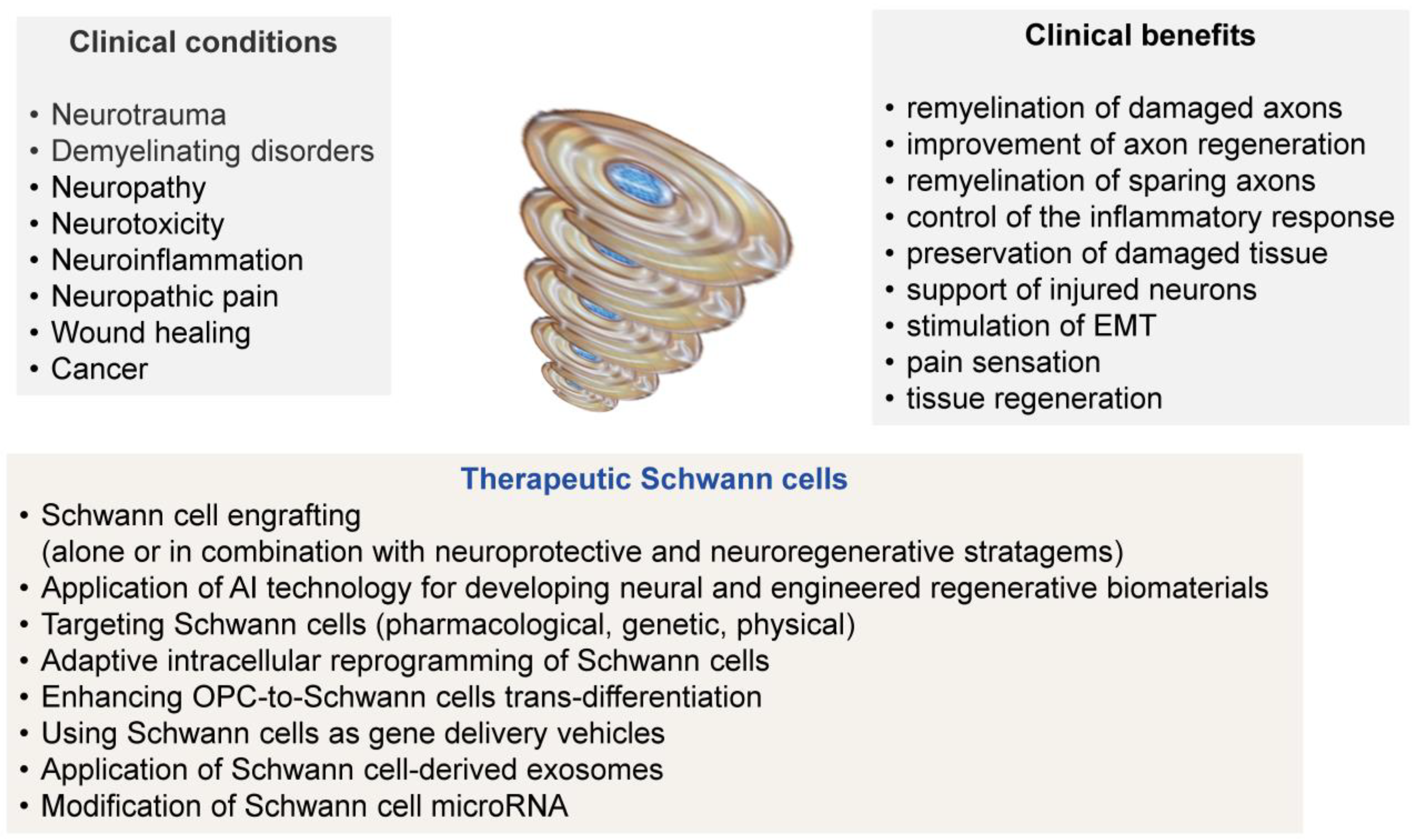
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shurin, M.R.; Wheeler, S.E.; Zhong, H.; Zhou, Y. Pathologic and Therapeutic Schwann Cells. Cells 2025, 14, 1336. https://doi.org/10.3390/cells14171336
Shurin MR, Wheeler SE, Zhong H, Zhou Y. Pathologic and Therapeutic Schwann Cells. Cells. 2025; 14(17):1336. https://doi.org/10.3390/cells14171336
Chicago/Turabian StyleShurin, Michael R., Sarah E. Wheeler, Hua Zhong, and Yan Zhou. 2025. "Pathologic and Therapeutic Schwann Cells" Cells 14, no. 17: 1336. https://doi.org/10.3390/cells14171336
APA StyleShurin, M. R., Wheeler, S. E., Zhong, H., & Zhou, Y. (2025). Pathologic and Therapeutic Schwann Cells. Cells, 14(17), 1336. https://doi.org/10.3390/cells14171336









