Modulatory Action of Insulin-like Growth Factor I (IGF-I) on Cortical Activity: Entrainment of Metabolic and Brain Functions
Abstract
1. Introduction
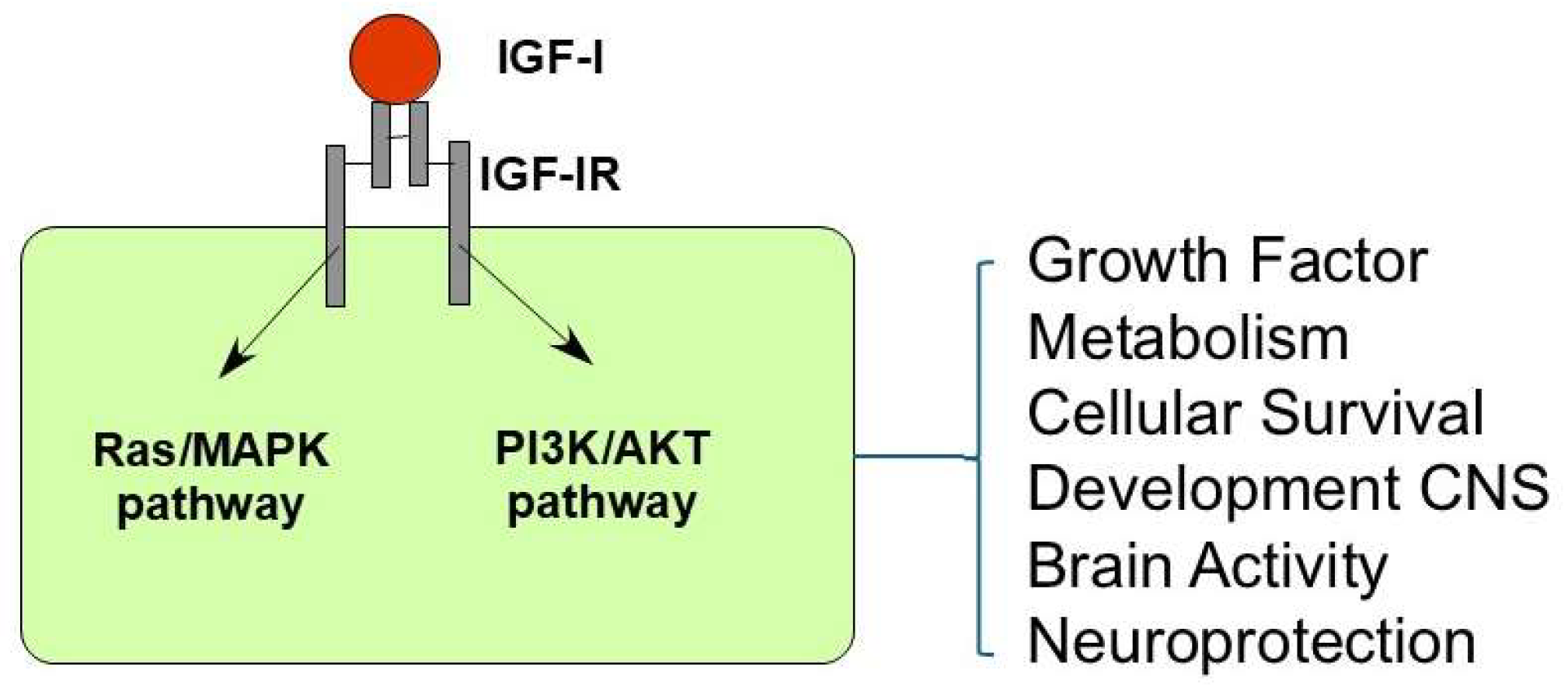
2. IGF-I Signal Pathways
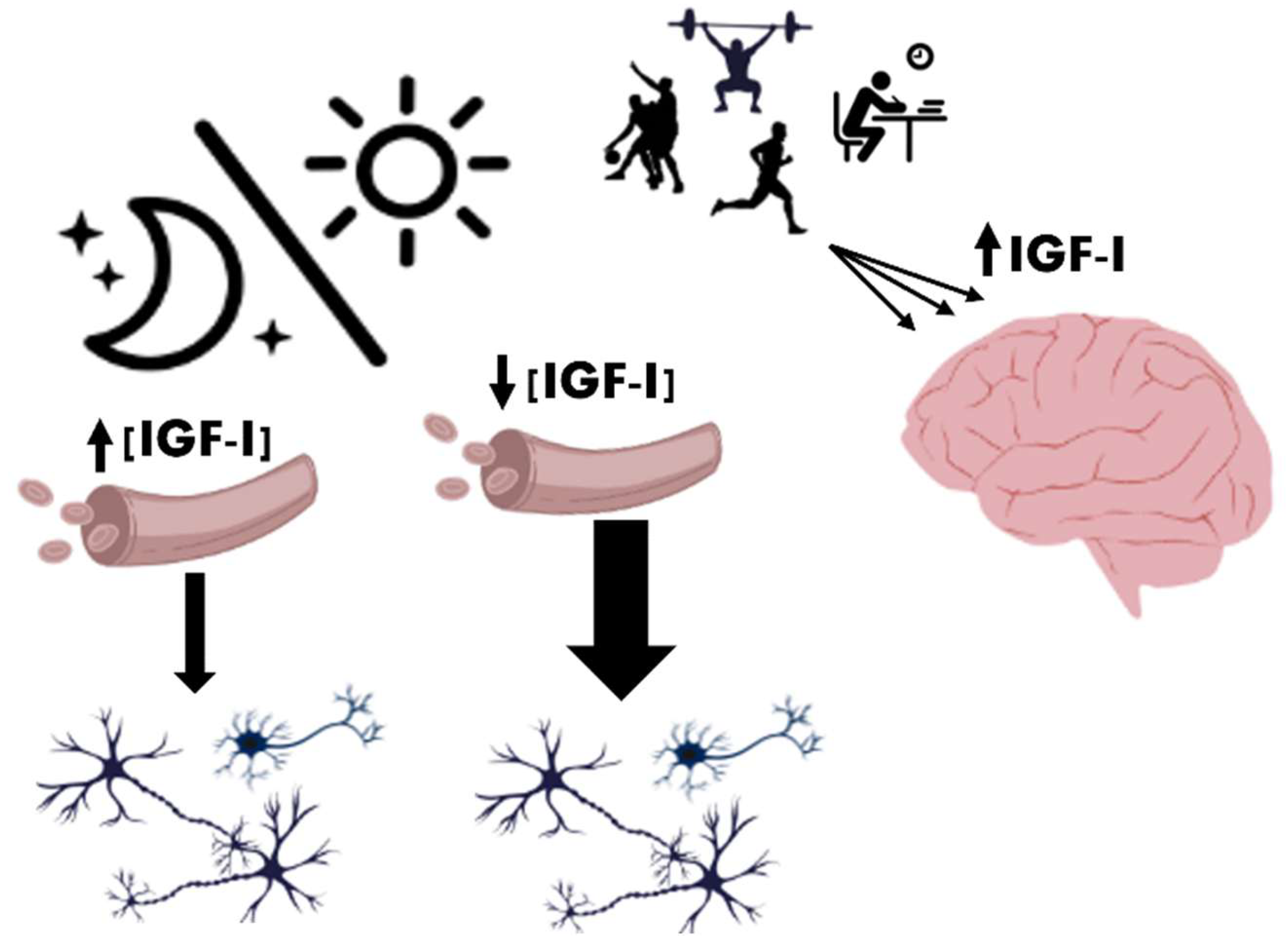
3. IGF-I and Its Role in Metabolism and Mitochondrial Function
4. IGF-I as a Modulator of Brain Activity
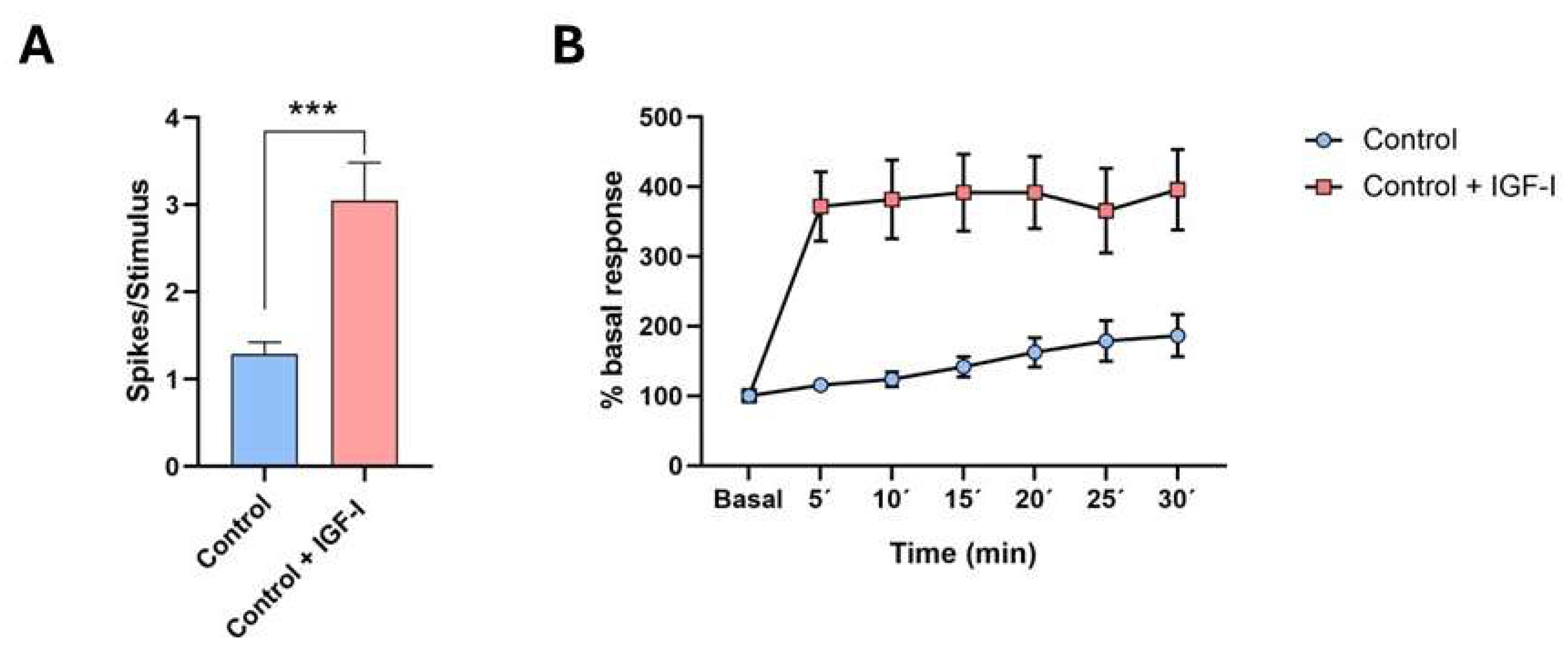
5. IGF-I and Cortical Activation
6. The Role of IGF-I in Cognitive Impairment and Neurodegenerative Disorders
6.1. The Role of IGF-I in the Pathophysiology of Alzheimer’s Disease
6.2. IGF-I in Parkinson’s Disease
7. IGF-I and the Regulation of the Sleep–Wake Cycle
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BF | Basal forebrain |
| CNS | Central nervous system |
| ECoG | Electrocorticogram |
| GH | Growth hormone |
| IGF-I | Insulin-like growth factor I |
| IGF-IR | IGF-I receptor |
| LRP2 | Lipoprotein receptor-related protein-2 |
| LDP | Long-term depression |
| LTP | Long-term potentiation |
| PeF | Perifornical |
| PI3K | Phosphatidylinositol-3-kinase |
| SCN | Suprachiasmatic nucleus |
References
- McCormick, D.A.; Nestvogel, D.B.; He, B.J. Neuromodulation of Brain State and Behavior. Annu. Rev. Neurosci. 2020, 43, 391–415. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Alzhanov, D.; Rotwein, P. Defining human insulin-like growth factor I gene regulation. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E519–E529. [Google Scholar] [CrossRef] [PubMed]
- Yakar, S.; Liu, J.L.; Stannard, B.; Butler, A.; Accili, D.; Sauer, B.; LeRoith, D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. USA 1999, 96, 7324–7329. [Google Scholar] [CrossRef]
- Marks, J.L.; King, M.G.; Baskin, D.G. Localization of insulin and type 1 IGF receptors in rat brain by in vitro autoradiography and in situ hybridization. Adv. Exp. Med. Biol. 1991, 293, 459–470. [Google Scholar] [CrossRef]
- Carro, E.; Nunez, A.; Busiguina, S.; Torres-Aleman, I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 2000, 20, 2926–2933. [Google Scholar] [CrossRef]
- Aleman, A.; Torres-Aleman, I. Circulating insulin-like growth factor I and cognitive function: Neuromodulation throughout the lifespan. Prog. Neurobiol. 2009, 89, 256–265. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Torres-Aleman, I. The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci. 2012, 13, 225–239. [Google Scholar] [CrossRef]
- Russo, V.C.; Gluckman, P.D.; Feldman, E.L.; Werther, G.A. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr. Rev. 2005, 26, 916–943. [Google Scholar] [CrossRef]
- Yamahara, K.; Yamamoto, N.; Kuwata, F.; Nakagawa, T. Neuroprotective role of insulin-like growth factor 1 in auditory and other nervous systems. Histol. Histopathol. 2022, 37, 609–619. [Google Scholar] [CrossRef]
- Nuñez, A.; Zegarra-Valdivia, J.; Fernandez de Sevilla, D.; Pignatelli, J.; Torres Aleman, I. The neurobiology of insulin-like growth factor I: From neuroprotection to modulation of brain states. Mol. Psychiatry 2023, 28, 3220–3230. [Google Scholar] [CrossRef]
- Ullrich, A.; Gray, A.; Tam, A.W.; Yang-Feng, T.; Tsubokawa, M.; Collins, C.; Henzel, W.; Le Bon, T.; Kathuria, S.; Chen, E.; et al. Insulin-like growth factor I receptor primary structure: Comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986, 5, 2503–2512. [Google Scholar] [CrossRef]
- Torres-Aleman, I. Insulin-like growth factors as mediators of functional plasticity in the adult brain. Horm. Metab. Res. 1999, 31, 114–119. [Google Scholar] [CrossRef]
- Khan, M.Z.; Zugaza, J.L.; Torres Aleman, I. The signaling landscape of insulin-like growth factor 1. J. Biol. Chem. 2025, 301, 108047. [Google Scholar] [CrossRef]
- Hakuno, F.; Takahashi, S.I. IGF1 receptor signaling pathways. J. Mol. Endocrinol. 2018, 61, T69–T86. [Google Scholar] [CrossRef]
- Kavran, J.M.; McCabe, J.M.; Byrne, P.O.; Connacher, M.K.; Wang, Z.; Ramek, A.; Sarabipour, S.; Shan, Y.; Shaw, D.E.; Hristova, K.; et al. How IGF-1 activates its receptor. Elife 2014, 3, e03772. [Google Scholar] [CrossRef] [PubMed][Green Version]
- LeRoith, D.; Werner, H.; Beitner-Johnson, D.; Roberts, C.T., Jr. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr. Rev. 1995, 16, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Werner, H. The IGF1 Signaling Pathway: From Basic Concepts to Therapeutic Opportunities. Int. J. Mol. Sci. 2023, 24, 14882. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Clemmons, D.R. Role of IGF Binding Proteins in Regulating Metabolism. Trends Endocrinol. Metab. 2016, 27, 375–391. [Google Scholar] [CrossRef]
- Clemmons, D.R. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol. Metab. Clin. N. Am. 2012, 41, 425–443, vii–viii. [Google Scholar] [CrossRef]
- Nishijima, T.; Piriz, J.; Duflot, S.; Fernandez, A.M.; Gaitan, G.; Gomez-Pinedo, U.; Verdugo, J.M.; Leroy, F.; Soya, H.; Nunez, A.; et al. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron 2010, 67, 834–846. [Google Scholar] [CrossRef]
- LeRoith, D.; Yakar, S. Mechanisms of disease: Metabolic effects of growth hormone and insulin-like growth factor 1. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 302–310. [Google Scholar] [CrossRef]
- Rommel, C.; Bodine, S.C.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 2001, 3, 1009–1013. [Google Scholar] [CrossRef]
- Mysoet, J.; Canu, M.H.; Cieniewski-Bernard, C.; Bastide, B.; Dupont, E. Hypoactivity affects IGF-1 level and PI3K/AKT signaling pathway in cerebral structures implied in motor control. PLoS ONE 2014, 9, e107631. [Google Scholar] [CrossRef]
- Trejo, J.L.; Carro, E.; Torres-Alemán, I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 2001, 21, 1628–1634. [Google Scholar] [CrossRef]
- LeRoith, D.; Holly, J.M.P.; Forbes, B.E. Insulin-like growth factors: Ligands, binding proteins, and receptors. Mol. Metab. 2021, 52, 101245. [Google Scholar] [CrossRef] [PubMed]
- Baron-Van Evercooren, A.; Olichon-Berthe, C.; Kowalski, A.; Visciano, G.; Van Obberghen, E. Expression of IGF-I and insulin receptor genes in the rat central nervous system: A developmental, regional, and cellular analysis. J. Neurosci. Res. 1991, 28, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Bibollet-Bahena, O.; Almazan, G. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J. Neurochem. 2009, 109, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Sanna, P.P.; Cammalleri, M.; Berton, F.; Simpson, C.; Lutjens, R.; Bloom, F.E.; Francesconi, W. Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J. Neurosci. 2002, 22, 3359–3365. [Google Scholar] [CrossRef]
- Carro, E.; Spuch, C.; Trejo, J.L.; Antequera, D.; Torres-Aleman, I. Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. J. Neurosci. 2005, 25, 10884–10893. [Google Scholar] [CrossRef]
- Quesada, A.; Romeo, H.E.; Micevych, P. Distribution and localization patterns of estrogen receptor-beta and insulin-like growth factor-1 receptors in neurons and glial cells of the female rat substantia nigra: Localization of ERbeta and IGF-1R in substantia nigra. J. Comp. Neurol. 2007, 503, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, A.I.; Borrajo, A.; Diaz-Ruiz, C.; Garrido-Gil, P.; Labandeira-Garcia, J.L. Crosstalk between insulin-like growth factor-1 and angiotensin-II in dopaminergic neurons and glial cells: Role in neuroinflammation and aging. Oncotarget 2016, 7, 30049–30067. [Google Scholar] [CrossRef] [PubMed]
- Davila, D.; Piriz, J.; Trejo, J.L.; Nunez, A.; Torres-Aleman, I. Insulin and insulin-like growth factor I signalling in neurons. Front. Biosci. 2007, 12, 3194–3202. [Google Scholar] [CrossRef] [PubMed]
- Ciucci, F.; Putignano, E.; Baroncelli, L.; Landi, S.; Berardi, N.; Maffei, L. Insulin-like growth factor 1 (IGF-1) mediates the effects of enriched environment (EE) on visual cortical development. PLoS ONE 2007, 2, e475. [Google Scholar] [CrossRef]
- Guzzetta, A.; Baldini, S.; Bancale, A.; Baroncelli, L.; Ciucci, F.; Ghirri, P.; Putignano, E.; Sale, A.; Viegi, A.; Berardi, N.; et al. Massage accelerates brain development and the maturation of visual function. J. Neurosci. 2009, 29, 6042–6051. [Google Scholar] [CrossRef]
- Arwert, L.I.; Deijen, J.B.; Drent, M.L. The relation between insulin-like growth factor I levels and cognition in healthy elderly: A meta-analysis. Growth Horm. IGF Res. 2005, 15, 416–422. [Google Scholar] [CrossRef]
- Ishii, D.N.; Lupien, S.B. Insulin-like growth factor replacement therapy for diabetic neuropathy: Experimental basis. Exp. Diabesity Res. 2003, 4, 257–269. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Santi, A.; Torres-Aleman, I. Insulin Peptides as Mediators of the Impact of Life Style in Alzheimer’s disease. Brain Plast. 2018, 4, 3–15. [Google Scholar] [CrossRef]
- Al-Samerria, S.; Radovick, S. The Role of Insulin-like Growth Factor-1 (IGF-1) in the Control of Neuroendocrine Regulation of Growth. Cells 2021, 10, 2664. [Google Scholar] [CrossRef]
- Dyer, A.H.; Vahdatpour, C.; Sanfeliu, A.; Tropea, D. The role of Insulin-Like Growth Factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience 2016, 325, 89–99. [Google Scholar] [CrossRef]
- Noriega-Prieto, J.A.; Maglio, L.E.; Perez-Domper, P.; Davila, J.C.; Gutierrez, A.; Torres-Aleman, I.; Fernandez de Sevilla, D. Bidirectional modulation of synaptic transmission by insulin-like growth factor-I. Front. Cell. Neurosci. 2024, 18, 1390663. [Google Scholar] [CrossRef]
- Noriega-Prieto, J.A.; Maglio, L.E.; Zegarra-Valdivia, J.A.; Pignatelli, J.; Fernandez, A.M.; Martinez-Rachadell, L.; Fernandes, J.; Nunez, A.; Araque, A.; Torres-Aleman, I.; et al. Astrocytic IGF-IRs Induce Adenosine-Mediated Inhibitory Downregulation and Improve Sensory Discrimination. J. Neurosci. 2021, 41, 4768–4781. [Google Scholar] [CrossRef] [PubMed]
- Barros-Zulaica, N.; Villa, A.E.P.; Nunez, A. Response Adaptation in Barrel Cortical Neurons Facilitates Stimulus Detection during Rhythmic Whisker Stimulation in Anesthetized Mice. eNeuro 2019, 6, 1–15. [Google Scholar] [CrossRef]
- Gazit, N.; Vertkin, I.; Shapira, I.; Helm, M.; Slomowitz, E.; Sheiba, M.; Mor, Y.; Rizzoli, S.; Slutsky, I. IGF-1 Receptor Differentially Regulates Spontaneous and Evoked Transmission via Mitochondria at Hippocampal Synapses. Neuron 2016, 89, 583–597. [Google Scholar] [CrossRef]
- Nuñez, A.; Carro, E.; Torres-Aleman, I. Insulin-like growth factor I modifies electrophysiological properties of rat brain stem neurons. J. Neurophysiol. 2003, 89, 3008–3017. [Google Scholar] [CrossRef]
- Zegarra-Valdivia, J.A.; Pignatelli, J.; Fernandez de Sevilla, M.E.; Fernandez, A.M.; Munive, V.; Martinez-Rachadell, L.; Nuñez, A.; Torres-Aleman, I. Insulin-like growth factor I modulates sleep through hypothalamic orexin neurons. FASEB J. 2020, 34, 15975–15990. [Google Scholar] [CrossRef]
- Zegarra-Valdivia, J.A.; Santi, A.; Fernandez de Sevilla, M.E.; Nuñez, A.; Torres-Aleman, I. Serum Insulin-Like Growth Factor I Deficiency Associates to Alzheimer’s Disease Co-Morbidities. J. Alzheimers Dis. 2019, 69, 979–987. [Google Scholar] [CrossRef]
- Mysoet, J.; Dupont, E.; Bastide, B.; Canu, M.H. Role of IGF-1 in cortical plasticity and functional deficit induced by sensorimotor restriction. Behav. Brain Res. 2015, 290, 117–123. [Google Scholar] [CrossRef]
- Xing, C.; Yin, Y.; Chang, R.; Gong, X.; He, X.; Xie, Z. Effects of insulin-like growth factor 1 on synaptic excitability in cultured rat hippocampal neurons. Exp. Neurol. 2007, 205, 222–229. [Google Scholar] [CrossRef]
- Ramsey, M.M.; Adams, M.M.; Ariwodola, O.J.; Sonntag, W.E.; Weiner, J.L. Functional characterization of des-IGF-1 action at excitatory synapses in the CA1 region of rat hippocampus. J. Neurophysiol. 2005, 94, 247–254. [Google Scholar] [CrossRef]
- Molina, D.P.; Ariwodola, O.J.; Weiner, J.L.; Brunso-Bechtold, J.K.; Adams, M.M. Growth hormone and insulin-like growth factor-I alter hippocampal excitatory synaptic transmission in young and old rats. Age (Dordr) 2013, 35, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Magro, N.; Zegarra-Valdivia, J.A.; Troyas-Martinez, S.; Torres-Aleman, I.; Nunez, A. Response Facilitation Induced by Insulin-like Growth Factor-I in the Primary Somatosensory Cortex of Mice Was Reduced in Aging. Cells 2022, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Maglio, L.E.; Noriega-Prieto, J.A.; Maroto, I.B.; Martin-Cortecero, J.; Munoz-Callejas, A.; Callejo-Mostoles, M.; Fernandez de Sevilla, D. IGF-1 facilitates extinction of conditioned fear. Elife 2021, 10, e67267. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Wang, Y.; Wang, Z.J. Insulin-like growth factor 1 regulates excitatory synaptic transmission in pyramidal neurons from adult prefrontal cortex. Neuropharmacology 2022, 217, 109204. [Google Scholar] [CrossRef]
- Feldman, D.E. Synaptic mechanisms for plasticity in neocortex. Annu. Rev. Neurosci. 2009, 32, 33–55. [Google Scholar] [CrossRef]
- Bliss, T.V.P.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Malenka, R.C.; Bear, M.F. LTP and LTD: An embarrassment of riches. Neuron 2004, 44, 5–21. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Shang, C.; Yan, F.; Shi, Y.; Zhang, J.; Qu, B.; Han, H.; Wang, Y.; Li, D.; et al. IGF1-Dependent Synaptic Plasticity of Mitral Cells in Olfactory Memory during Social Learning. Neuron 2017, 95, 106–122.e5. [Google Scholar] [CrossRef]
- Barros-Zulaica, N.; Castejon, C.; Nunez, A. Frequency-specific response facilitation of supra and infragranular barrel cortical neurons depends on NMDA receptor activation in rats. Neuroscience 2014, 281, 178–194. [Google Scholar] [CrossRef]
- Garcia-Magro, N.; Mesa-Lombardo, A.; Barros-Zulaica, N.; Nunez, A. Impairment of synaptic plasticity in the primary somatosensory cortex in a model of diabetic mice. Front. Cell. Neurosci. 2024, 18, 1444395. [Google Scholar] [CrossRef]
- Herrero-Labrador, R.; Fernandez-Irigoyen, J.; Vecino, R.; Gonzalez-Arias, C.; Ausin, K.; Crespo, I.; Fernandez Acosta, F.J.; Nieto-Estevez, V.; Roman, M.J.; Perea, G.; et al. Brain IGF-I regulates LTP, spatial memory, and sexual dimorphic behavior. Life Sci. Alliance 2023, 6, e202201691. [Google Scholar] [CrossRef]
- Martin-Rodriguez, J.F.; Ramos-Herrero, V.D.; Parras, G.G.; Flores-Martinez, A.; Madrazo-Atutxa, A.; Cano, D.A.; Gruart, A.; Delgado-Garcia, J.M.; Leal-Cerro, A.; Leal-Campanario, R. Chronic adult-onset of growth hormone/IGF-I hypersecretion improves cognitive functions and LTP and promotes neuronal differentiation in adult rats. Acta Physiol. 2020, 229, e13293. [Google Scholar] [CrossRef]
- van Dam, P.S.; de Winter, C.F.; de Vries, R.; van der Grond, J.; Drent, M.L.; Lijffijt, M.; Kenemans, J.L.; Aleman, A.; de Haan, E.H.; Koppeschaar, H.P. Childhood-onset growth hormone deficiency, cognitive function and brain N-acetylaspartate. Psychoneuroendocrinology 2005, 30, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Lijffijt, M.; Van Dam, P.S.; Kenemans, J.L.; Koppeschaar, H.P.; de Vries, W.R.; Drent, M.L.; Wittenberg, A.; Kemner, C. Somatotropic-axis deficiency affects brain substrates of selective attention in childhood-onset growth hormone deficient patients. Neurosci Lett 2003, 353, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Bulow, B.; Hagmar, L.; Orbaek, P.; Osterberg, K.; Erfurth, E.M. High incidence of mental disorders, reduced mental well-being and cognitive function in hypopituitary women with GH deficiency treated for pituitary disease. Clin. Endocrinol. 2002, 56, 183–193. [Google Scholar] [CrossRef]
- Trejo, J.; Piriz, J.; Llorens-Martin, M.V.; Fernandez, A.M.; Bolos, M.; LeRoith, D.; Nunez, A.; Torres-Aleman, I. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol. Psychiatry 2007, 12, 1118–1128. [Google Scholar] [CrossRef]
- Carro, E.; Trejo, J.L.; Gerber, A.; Loetscher, H.; Torrado, J.; Metzger, F.; Torres-Aleman, I. Therapeutic actions of insulin-like growth factor I on APP/PS2 mice with severe brain amyloidosis. Neurobiol. Aging 2006, 27, 1250–1257. [Google Scholar] [CrossRef]
- Zegarra-Valdivia, J.A.; Fernandes, J.; Fernandez de Sevilla, M.E.; Trueba-Saiz, A.; Pignatelli, J.; Suda, K.; Martinez-Rachadell, L.; Fernandez, A.M.; Esparza, J.; Vega, M.; et al. Insulin-like growth factor I sensitization rejuvenates sleep patterns in old mice. Geroscience 2022, 44, 2243–2257. [Google Scholar] [CrossRef]
- Trueba-Saiz, A.; Cavada, C.; Fernandez, A.M.; Leon, T.; Gonzalez, D.A.; Fortea Ormaechea, J.; Lleo, A.; Del Ser, T.; Nunez, A.; Torres-Aleman, I. Loss of serum IGF-I input to the brain as an early biomarker of disease onset in Alzheimer mice. Transl. Psychiatry 2013, 3, e330. [Google Scholar] [CrossRef]
- Sorrentino, P.; Nieboer, D.; Twisk, J.W.R.; Stam, C.J.; Douw, L.; Hillebrand, A. The Hierarchy of Brain Networks Is Related to Insulin Growth Factor-1 in a Large, Middle-Aged, Healthy Cohort: An Exploratory Magnetoencephalography Study. Brain Connect. 2017, 7, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Metherate, R.; Cox, C.L.; Ashe, J.H. Cellular bases of neocortical activation—Modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J. Neurosci. 1992, 12, 4701–4711. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, A. Unit activity of rat basal forebrain neurons: Relationship to cortical activity. Neuroscience 1996, 72, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Duque, A.; Balatoni, B.; Detari, L.; Zaborszky, L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. J. Neurophysiol. 2000, 84, 1627–1635. [Google Scholar] [CrossRef]
- Celesia, G.G.; Jasper, H.H. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology 1966, 16, 1053–1063. [Google Scholar] [CrossRef]
- Jasper, H.H.; Tessier, J. Acetylcholine liberation from cerebral cortex during paradoxical REM sleep. Science 1971, 172, 601–602. [Google Scholar] [CrossRef]
- Zegarra-Valdivia, J.A.; Chaves-Coira, I.; Fernandez de Sevilla, M.E.; Martinez-Rachadell, L.; Esparza, J.; Torres-Aleman, I.; Nunez, A. Reduced Insulin-Like Growth Factor-I Effects in the Basal Forebrain of Aging Mouse. Front. Aging Neurosci. 2021, 13, 682388. [Google Scholar] [CrossRef]
- de Lecea, L.; Kilduff, T.S.; Peyron, C.; Gao, X.; Foye, P.E.; Danielson, P.E.; Fukuhara, C.; Battenberg, E.L.; Gautvik, V.T.; Bartlett, F.S., 2nd; et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA 1998, 95, 322–327. [Google Scholar] [CrossRef]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richarson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef]
- Sakurai, T. The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nat. Rev. Neurosci. 2007, 8, 171–181. [Google Scholar] [CrossRef]
- Kotz, C.M. Integration of feeding and spontaneous physical activity: Role for orexin. Physiol. Behav. 2006, 88, 294–301. [Google Scholar] [CrossRef]
- Sakurai, T. The role of orexin in motivated behaviours. Nat. Rev. Neurosci. 2014, 15, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; de Vries, W.R.; Koppeschaar, H.P.; Osman-Dualeh, M.; Verhaar, H.J.; Samson, M.M.; Bol, E.; de Haan, E.H. Relationship between circulating levels of sex hormones and insulin-like growth factor-1 and fluid intelligence in older men. Exp. Aging Res. 2001, 27, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef]
- Donohue, M.C.; Sperling, R.A.; Petersen, R.; Sun, C.K.; Weiner, M.W.; Aisen, P.S.; Alzheimer’s Disease Neuroimaging, I. Association Between Elevated Brain Amyloid and Subsequent Cognitive Decline Among Cognitively Normal Persons. JAMA 2017, 317, 2305–2316. [Google Scholar] [CrossRef]
- Insel, P.S.; Mattsson, N.; Donohue, M.C.; Mackin, R.S.; Aisen, P.S.; Jack, C.R., Jr.; Shaw, L.M.; Trojanowski, J.Q.; Weiner, M.W.; Alzheimer’s Disease Neuroimaging, I. The transitional association between beta-amyloid pathology and regional brain atrophy. Alzheimers Dement. 2015, 11, 1171–1179. [Google Scholar] [CrossRef]
- Lesne, S.E.; Sherman, M.A.; Grant, M.; Kuskowski, M.; Schneider, J.A.; Bennett, D.A.; Ashe, K.H. Brain amyloid-beta oligomers in ageing and Alzheimer’s disease. Brain 2013, 136 Pt 5, 1383–1398. [Google Scholar] [CrossRef]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef]
- Citron, M.; Oltersdorf, T.; Haass, C.; McConlogue, L.; Hung, A.Y.; Seubert, P.; Vigo-Pelfrey, C.; Lieberburg, I.; Selkoe, D.J. Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature 1992, 360, 672–674. [Google Scholar] [CrossRef]
- Zegarra-Valdivia, J.A.; Pignatelli, J.; Nunez, A.; Torres Aleman, I. The Role of Insulin-like Growth Factor I in Mechanisms of Resilience and Vulnerability to Sporadic Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 16440. [Google Scholar] [CrossRef]
- Carro, E.; Torres-Aleman, I. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer’s disease. Eur. J. Pharmacol. 2004, 490, 127–133. [Google Scholar] [CrossRef]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef]
- O’Kusky, J.; Ye, P. Neurodevelopmental effects of insulin-like growth factor signaling. Front. Neuroendocrinol. 2012, 33, 230–251. [Google Scholar] [CrossRef] [PubMed]
- Vossel, K.A.; Ranasinghe, K.G.; Beagle, A.J.; Mizuiri, D.; Honma, S.M.; Dowling, A.F.; Darwish, S.M.; Van Berlo, V.; Barnes, D.E.; Mantle, M.; et al. Incidence and impact of subclinical epileptiform activity in Alzheimer’s disease. Ann. Neurol. 2016, 80, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Marin, K.E.; Zegarra-Valdivia, J.; Nuñez, A. Seizure susceptibility in Alzheimer’s disease. Arch. Med. Res. 2021, 9, 2–14. [Google Scholar] [CrossRef]
- Picillo, M.; Erro, R.; Santangelo, G.; Pivonello, R.; Longo, K.; Pivonello, C.; Vitale, C.; Amboni, M.; Moccia, M.; Colao, A.; et al. Insulin-like growth factor-1 and progression of motor symptoms in early, drug-naive Parkinson’s disease. J. Neurol. 2013, 260, 1724–1730. [Google Scholar] [CrossRef]
- Picillo, M.; Pivonello, R.; Santangelo, G.; Pivonello, C.; Savastano, R.; Auriemma, R.; Amboni, M.; Scannapieco, S.; Pierro, A.; Colao, A.; et al. Serum IGF-1 is associated with cognitive functions in early, drug-naive Parkinson’s disease. PLoS ONE 2017, 12, e0186508. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, J.; Ma, J.; Li, D.; Gu, Q.; Chen, S.; Wang, Z.; Sun, W.; Li, M. Correlation between serum IGF-1 and EGF levels and neuropsychiatric and cognitive in Parkinson’s disease patients. Neurol. Sci. 2023, 44, 881–887. [Google Scholar] [CrossRef]
- Kao, S.Y. Rescue of alpha-synuclein cytotoxicity by insulin-like growth factors. Biochem. Biophys. Res. Commun. 2009, 385, 434–438. [Google Scholar] [CrossRef]
- Henrich, M.T.; Oertel, W.H.; Surmeier, D.J.; Geibl, F.F. Mitochondrial dysfunction in Parkinson’s disease—A key disease hallmark with therapeutic potential. Mol. Neurodegener. 2023, 18, 83. [Google Scholar] [CrossRef]
- Cuttler, L. The regulation of growth hormone secretion. Endocrinol. Metab. Clin. N. Am. 1996, 25, 541–571. [Google Scholar] [CrossRef]
- Mitsugi, N.; Kimura, F. Simultaneous determination of blood levels of corticosterone and growth hormone in the male rat: Relation to sleep-wakefulness cycle. Neuroendocrinology 1985, 41, 125–130. [Google Scholar] [CrossRef]
- Van Cauter, E.; Plat, L.; Copinschi, G. Interrelations between sleep and the somatotropic axis. Sleep 1998, 21, 553–566. [Google Scholar] [CrossRef]
- Dardente, H.; Cermakian, N. Molecular circadian rhythms in central and peripheral clocks in mammals. Chronobiol. Int. 2007, 24, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Rutter, J.; Reick, M.; McKnight, S.L. Metabolism and the control of circadian rhythms. Annu. Rev. Biochem. 2002, 71, 307–331. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef]
- Chaudhari, A.; Gupta, R.; Patel, S.; Velingkaar, N.; Kondratov, R. Cryptochromes regulate IGF-1 production and signaling through control JAK2 dependent STAT5B phosphorylation. Mol. Biol. Cell. 2017, 28, 834–842. [Google Scholar] [CrossRef]
- Chennaoui, M.; Leger, D.; Gomez-Merino, D. Sleep and the GH/IGF-1 axis: Consequences and countermeasures of sleep loss/disorders. Sleep Med. Rev. 2020, 49, 101223. [Google Scholar] [CrossRef]
- Chennaoui, M.; Arnal, P.J.; Dorey, R.; Sauvet, F.; Ciret, S.; Gallopin, T.; Leger, D.; Drogou, C.; Gomez-Merino, D. Changes of Cerebral and/or Peripheral Adenosine A(1) Receptor and IGF-I Concentrations under Extended Sleep Duration in Rats. Int. J. Mol. Sci. 2017, 18, 2439. [Google Scholar] [CrossRef]
- Oscarsson, J.; Johannsson, G.; Johansson, J.O.; Lundberg, P.A.; Lindstedt, G.; Bengtsson, B.A. Diurnal variation in serum insulin-like growth factor (IGF)-I and IGF binding protein-3 concentrations during daily subcutaneous injections of recombinant human growth hormone in GH-deficient adults. Clin. Endocrinol. 1997, 46, 63–68. [Google Scholar] [CrossRef]
- Copinschi, G. Metabolic and endocrine effects of sleep deprivation. Essent. Psychopharmacol. 2005, 6, 341–347. [Google Scholar] [PubMed]
- Crosby, P.; Hamnett, R.; Putker, M.; Hoyle, N.P.; Reed, M.; Karam, C.J.; Maywood, E.S.; Stangherlin, A.; Chesham, J.E.; Hayter, E.A.; et al. Insulin/IGF-1 Drives PERIOD Synthesis to Entrain Circadian Rhythms with Feeding Time. Cell 2019, 177, 896–909. [Google Scholar] [CrossRef]
- Szymusiak, R.; McGinty, D. Hypothalamic regulation of sleep and arousal. Ann. N. Y. Acad. Sci. 2008, 1129, 275–286. [Google Scholar] [CrossRef]
- de Lecea, L.; Sutcliffe, J.G. The hypocretins and sleep. Febs. J. 2005, 272, 5675–5688. [Google Scholar] [CrossRef]
- Sakurai, T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med. Rev. 2005, 9, 231–241. [Google Scholar] [CrossRef]
- Pignatelli, J.; de Sevilla, M.E.F.; Sperber, J.; Horrillo, D.; Medina-Gomez, G.; Aleman, I.T. Insulin-like Growth Factor I Couples Metabolism with Circadian Activity through Hypothalamic Orexin Neurons. Int. J. Mol. Sci. 2022, 23, 4679. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Magro, N.; Mesa-Lombardo, A.; Nuñez, Á. Modulatory Action of Insulin-like Growth Factor I (IGF-I) on Cortical Activity: Entrainment of Metabolic and Brain Functions. Cells 2025, 14, 1325. https://doi.org/10.3390/cells14171325
García-Magro N, Mesa-Lombardo A, Nuñez Á. Modulatory Action of Insulin-like Growth Factor I (IGF-I) on Cortical Activity: Entrainment of Metabolic and Brain Functions. Cells. 2025; 14(17):1325. https://doi.org/10.3390/cells14171325
Chicago/Turabian StyleGarcía-Magro, Nuria, Alberto Mesa-Lombardo, and Ángel Nuñez. 2025. "Modulatory Action of Insulin-like Growth Factor I (IGF-I) on Cortical Activity: Entrainment of Metabolic and Brain Functions" Cells 14, no. 17: 1325. https://doi.org/10.3390/cells14171325
APA StyleGarcía-Magro, N., Mesa-Lombardo, A., & Nuñez, Á. (2025). Modulatory Action of Insulin-like Growth Factor I (IGF-I) on Cortical Activity: Entrainment of Metabolic and Brain Functions. Cells, 14(17), 1325. https://doi.org/10.3390/cells14171325







