Human CAR Tregs Targeting SOD1 and Expressing BDNF Reduce Inflammation and Delay Disease in G93A hSOD1-NSG Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. G93A hSOD1 Transgeninc NSG Mice
2.2. Human ALS and G93A hSOD1 Transgeninc Mouse Spinal Cord Samples
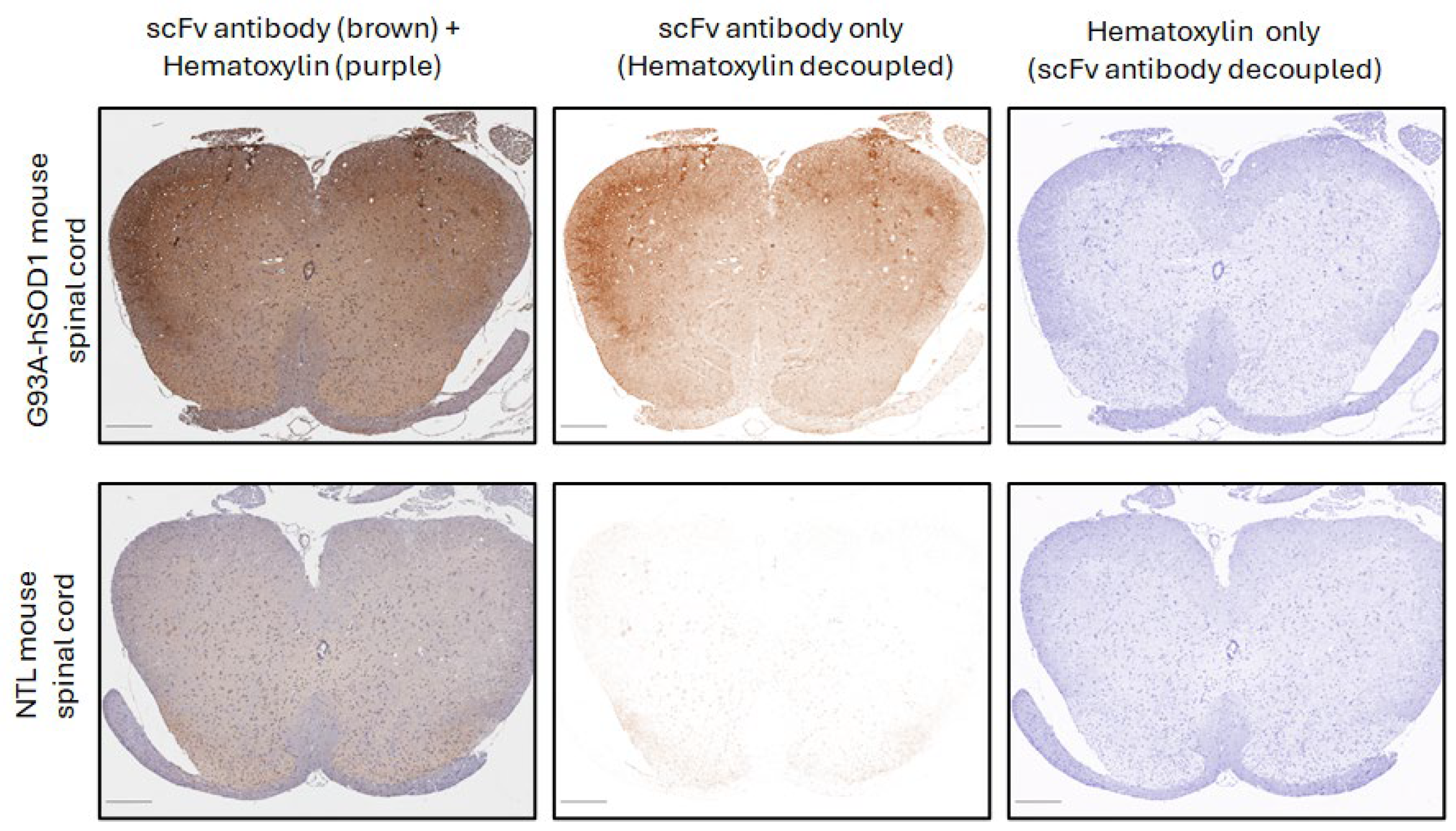
2.3. Immunohistochemistry (IHC) and Image Analysis
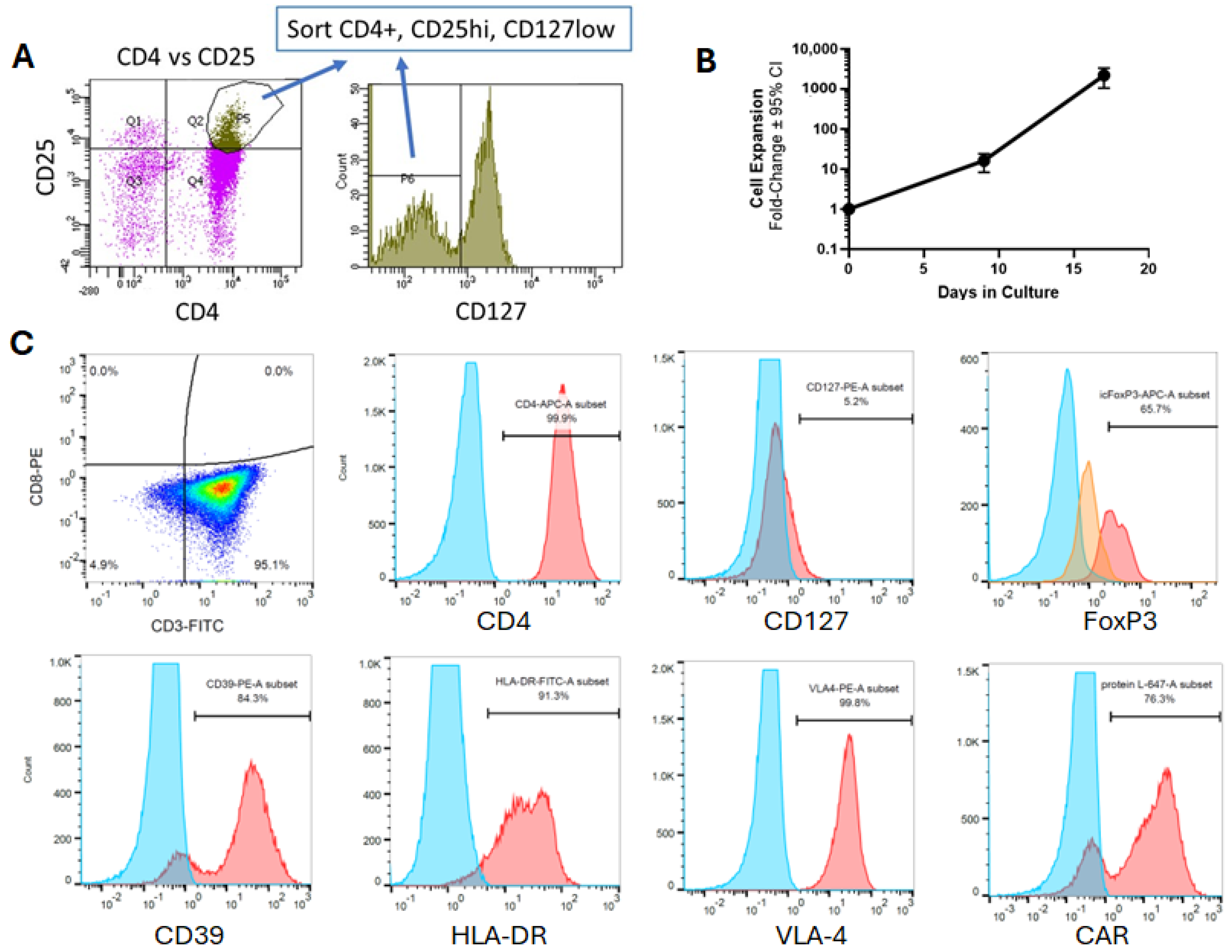
2.4. Expansion and Retroviral Transduction of Human Tregs
2.5. Retroviral Vector Production
2.6. Peroxide Toxicity in Neuronal SH-SY5Y Cells
2.7. Treatment with Human Tregs and Disease Evaluation in mSOD1-NSG Mice
2.8. Isolation of RNA and Real-Time qPCR of cDNA
2.9. Flow Cytometry
2.10. CAR Antigen Activations and Enzyme-Linked Immunosorbent Assays
2.11. Statistical Analysis
3. Results
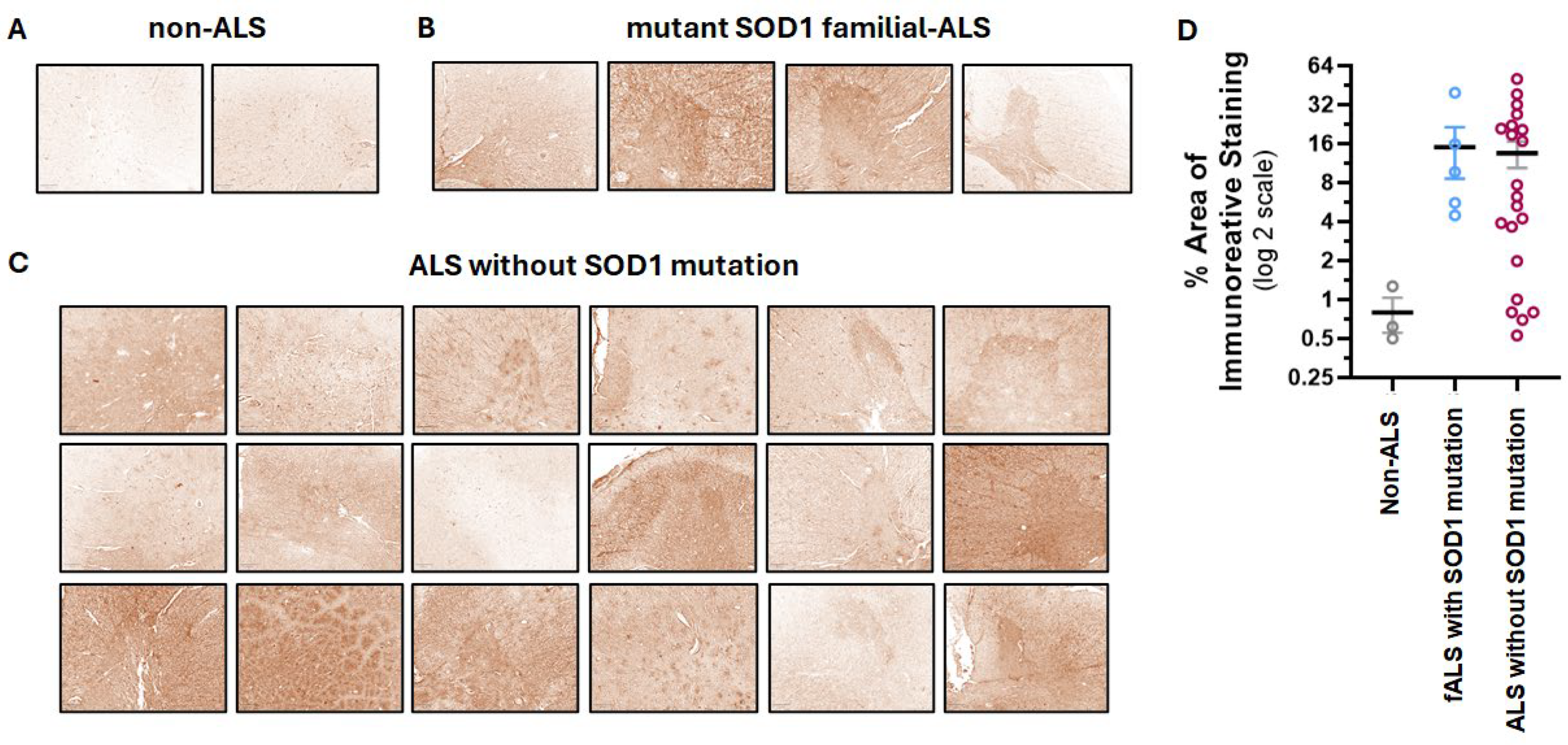
3.1. The Anti-SOD1 scFv Binds to Spinal Cord from Most ALS Patients
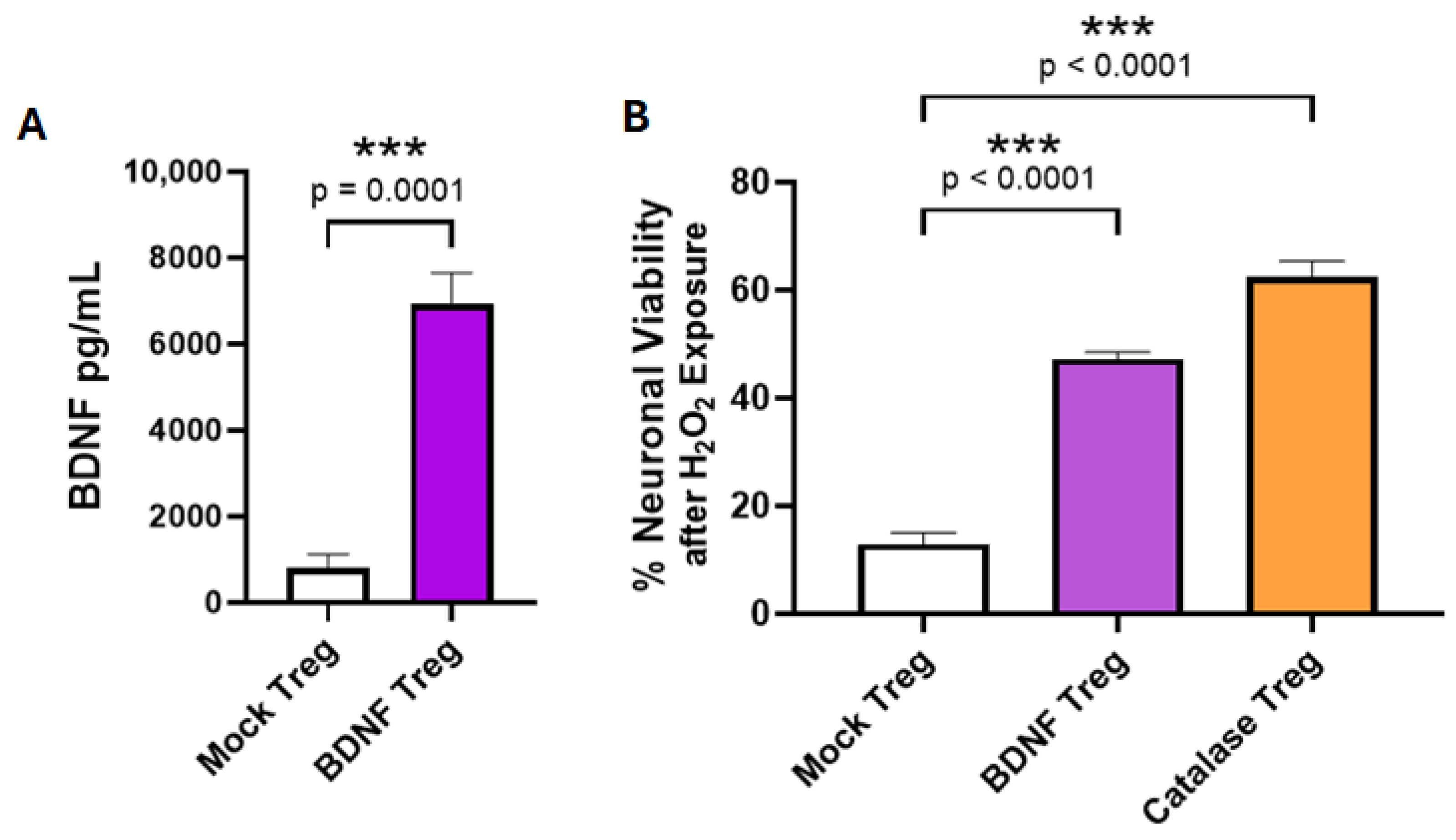
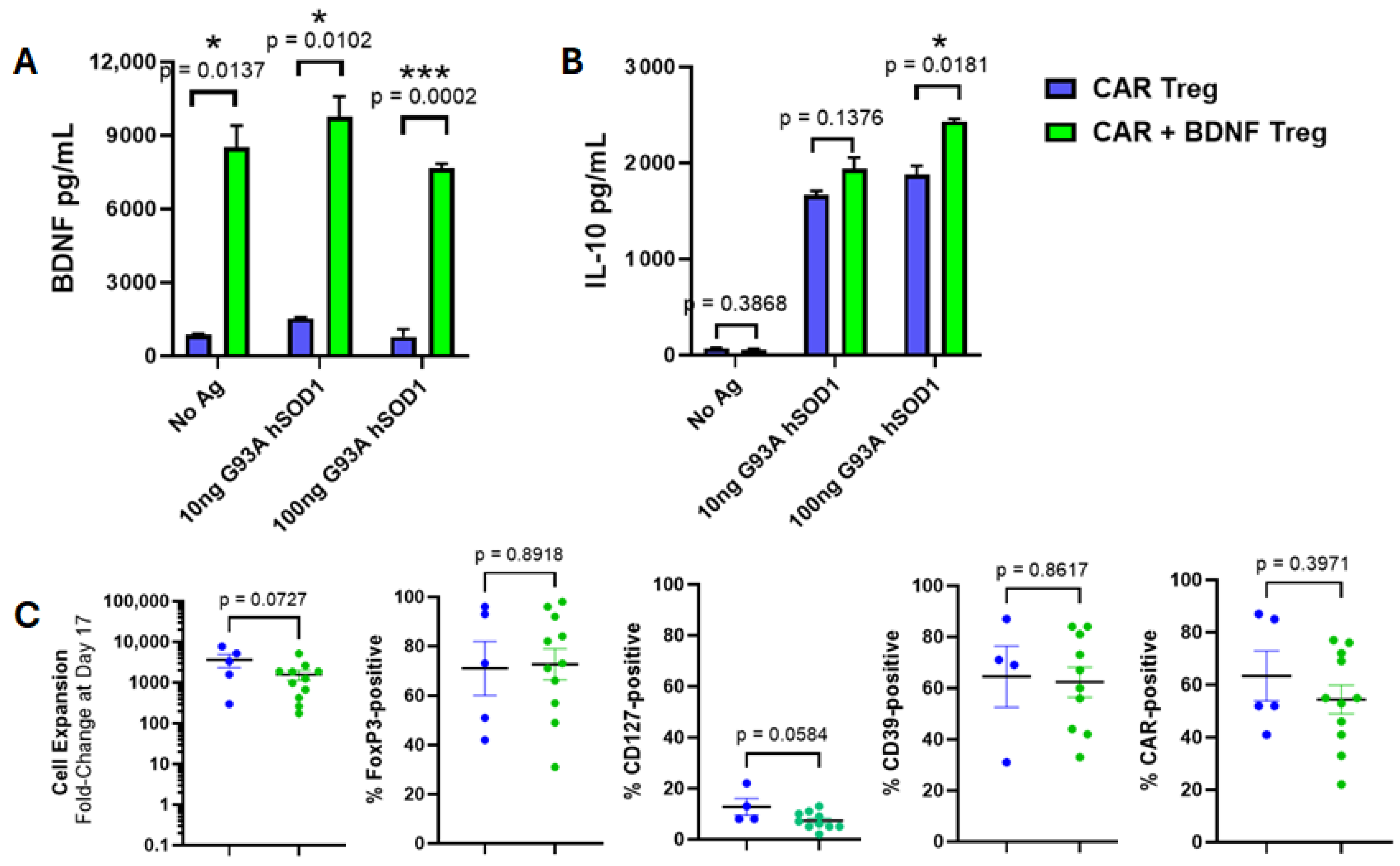
3.2. Co-Expression of BDNF and Anti-Misfolded Human SOD1 CAR in Human Tregs
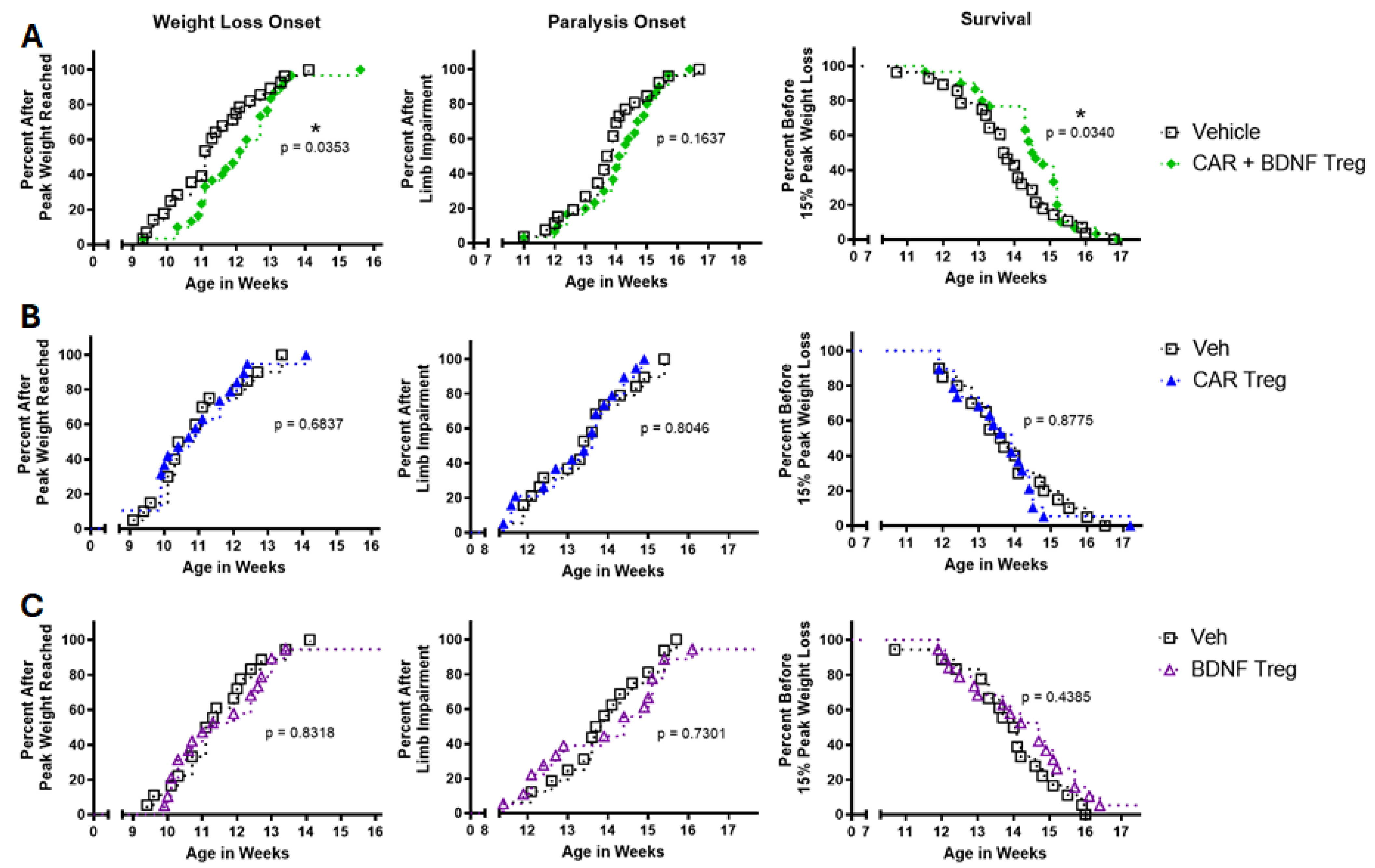
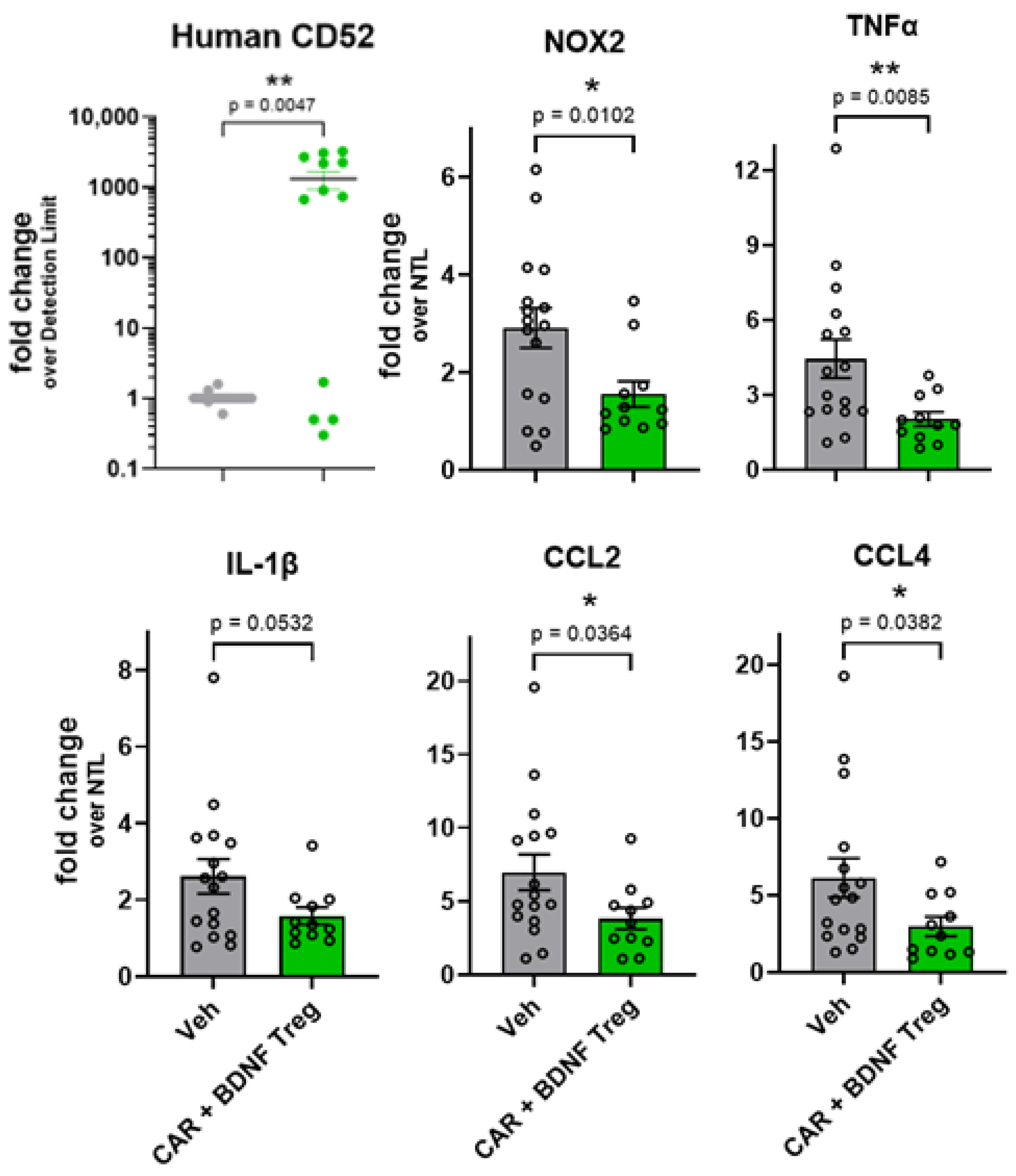
3.3. Human Tregs Expressing Both CAR and BDNF Delay Disease and Inhibit Neuroinflammation in mSOD1-NSG Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | amyotrophic lateral sclerosis |
| fALS | familial ALS |
| sALS | sporadic ALS |
| CNS | central nervous system |
| Tregs | regulatory T cells |
| DMM | disease-modifying molecule |
| BDNF | brain-derived neurotrophic factor |
| scFv | single-chain variable fragment |
| CAR | chimeric antigen receptor |
| SOD1 | superoxide dismutase-1 |
| hSOD1 | human SOD1 |
| NSG | NOD.Cg-Prkdc scid IL2rγ tm1Wjl |
| mSOD1-NSG | G93A hSOD1-NSG transgenic mice |
| NTLs | non-transgenic littermates |
| Luc-SH-SY5Y | luciferase-expressing SH-SY5Y cell line |
| IHC | immunohistochemistry |
| PBMCs | peripheral blood mononuclear cells |
| IP | intraperitoneal |
| IV | intravenous |
References
- Barberio, J.; Lally, C.; Kupelian, V.; Hardiman, O.; Flanders, W.D. Estimated familial amyotrophic lateral sclerosis proportion: A literature review and meta-analysis. Neurol. Genet. 2023, 9, e200109. [Google Scholar] [CrossRef]
- You, J.; Youssef, M.M.; Santos, J.R.; Lee, J.; Park, J. Microglia and astrocytes in amyotrophic lateral sclerosis: Disease-associated states, pathological roles, and therapeutic potential. Biology 2023, 12, 1307. [Google Scholar] [CrossRef]
- Cunha-Oliveira, T.; Montezinho, L.; Mendes, C.; Firuzi, O.; Saso, L.; Oliveira, P.J.; Silva, F.S. Oxidative stress in amyotrophic lateral sclerosis: Pathophysiology and opportunities for pharmacological intervention. Oxidative Med. Cell. Longev. 2020, 2020, 5021694. [Google Scholar] [CrossRef]
- Parakh, S.; Atkin, J.D. Protein folding alterations in amyotrophic lateral sclerosis. Brain Res. 2016, 1648, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Bruijn, L.I.; Houseweart, M.K.; Kato, S.; Anderson, K.L.; Anderson, S.D.; Ohama, E.; Reaume, A.G.; Scott, R.W.; Cleveland, D.W. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science 1998, 281, 1851–1854. [Google Scholar] [CrossRef] [PubMed]
- Urushitani, M.; Sik, A.; Sakurai, T.; Nukina, N.; Takahashi, R.; Julien, J.-P. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat. Neurosci. 2006, 9, 108–118. [Google Scholar] [CrossRef]
- Turner, B.J.; Atkin, J.D.; Farg, M.A.; Zang, D.W.; Rembach, A.; Lopes, E.C.; Patch, J.D.; Hill, A.F.; Cheema, S.S. Impaired extracellular secretion of mutant superoxide dismutase 1 associates with neurotoxicity in familial amyotrophic lateral sclerosis. J. Neurosci. 2005, 25, 108–117. [Google Scholar] [CrossRef]
- Rotunno, M.S.; Bosco, D.A. An emerging role for misfolded wild-type SOD1 in sporadic ALS pathogenesis. Front. Cell. Neurosci. 2013, 7, 253. [Google Scholar] [CrossRef]
- Maier, M.; Welt, T.; Wirth, F.; Montrasio, F.; Preisig, D.; McAfoose, J.; Vieira, F.G.; Kulic, L.; Späni, C.; Stehle, T. A human-derived antibody targets misfolded SOD1 and ameliorates motor symptoms in mouse models of amyotrophic lateral sclerosis. Sci. Transl. Med. 2018, 10, eaah3924. [Google Scholar] [CrossRef]
- Paré, B.; Lehmann, M.; Beaudin, M.; Nordström, U.; Saikali, S.; Julien, J.-P.; Gilthorpe, J.D.; Marklund, S.L.; Cashman, N.R.; Andersen, P.M. Misfolded SOD1 pathology in sporadic amyotrophic lateral sclerosis. Sci. Rep. 2018, 8, 14223. [Google Scholar] [CrossRef]
- Forsberg, K.; Graffmo, K.; Pakkenberg, B.; Weber, M.; Nielsen, M.; Marklund, S.; Brännström, T.; Andersen, P.M. Misfolded SOD1 inclusions in patients with mutations in C9orf72 and other ALS/FTD-associated genes. J. Neurol. Neurosurg. Psychiatry 2019, 90, 861–869. [Google Scholar] [CrossRef]
- Da Cruz, S.; Bui, A.; Saberi, S.; Lee, S.K.; Stauffer, J.; McAlonis-Downes, M.; Schulte, D.; Pizzo, D.P.; Parone, P.A.; Cleveland, D.W. Misfolded SOD1 is not a primary component of sporadic ALS. Acta Neuropathol. 2017, 134, 97–111. [Google Scholar] [CrossRef]
- Sheean, R.K.; McKay, F.C.; Cretney, E.; Bye, C.R.; Perera, N.D.; Tomas, D.; Weston, R.A.; Scheller, K.J.; Djouma, E.; Menon, P. Association of regulatory T-cell expansion with progression of amyotrophic lateral sclerosis: A study of humans and a transgenic mouse model. JAMA Neurol. 2018, 75, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Beers, D.R.; Henkel, J.S.; Zhao, W.; Wang, J.; Huang, A.; Wen, S.; Liao, B.; Appel, S.H. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain 2011, 134, 1293–1314. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Mosley, R.L.; Reynolds, A.D.; Dhar, A.; Jackson-Lewis, V.; Gordon, P.H.; Przedborski, S.; Gendelman, H.E. Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PLoS ONE 2008, 3, e2740. [Google Scholar] [CrossRef]
- Tiemessen, M.M.; Jagger, A.L.; Evans, H.G.; van Herwijnen, M.J.; John, S.; Taams, L.S. CD4+ CD25+ Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc. Natl. Acad. Sci. USA 2007, 104, 19446–19451. [Google Scholar] [CrossRef] [PubMed]
- Graber, D.J.; Cook, W.J.; Sentman, M.-L.; Murad-Mabaera, J.M.; Sentman, C.L. Human CD4+ CD25+ T cells expressing a chimeric antigen receptor against aberrant superoxide dismutase 1 trigger antigen-specific immunomodulation. Cytotherapy 2024, 26, 126–135. [Google Scholar] [CrossRef]
- Zhao, W.; Beers, D.R.; Liao, B.; Henkel, J.S.; Appel, S.H. Regulatory T lymphocytes from ALS mice suppress microglia and effector T lymphocytes through different cytokine-mediated mechanisms. Neurobiol. Dis. 2012, 48, 418–428. [Google Scholar] [CrossRef]
- Henriques, A.; Pitzer, C.; Schneider, A. Neurotrophic growth factors for the treatment of amyotrophic lateral sclerosis: Where do we stand? Front. Neurosci. 2010, 4, 1463. [Google Scholar] [CrossRef]
- Dittrich, F.; Ochs, G.; Große-Wilde, A.; Berweiler, U.; Yan, Q.; Miller, J.A.; Toyka, K.V.; Sendtner, M. Pharmacokinetics of intrathecally applied BDNF and effects on spinal motoneurons. Exp. Neurol. 1996, 141, 225–239. [Google Scholar] [CrossRef]
- Xie, L.; Choudhury, G.R.; Winters, A.; Yang, S.; Jin, K. Cerebral regulatory T cells restrain microglia/macrophage-mediated inflammatory responses via IL-10. Eur. J. Immunol. 2015, 45, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Glatigny, S.; Duhen, R.; Arbelaez, C.; Kumari, S.; Bettelli, E. Integrin alpha L controls the homing of regulatory T cells during CNS autoimmunity in the absence of integrin alpha 4. Sci. Rep. 2015, 5, 7834. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Yan, J.; Csurhes, P.; Greer, J.; McCombe, P. Circulating brain derived neurotrophic factor (BDNF) and frequency of BDNF positive T cells in peripheral blood in human ischemic stroke: Effect on outcome. J. Neuroimmunol. 2015, 286, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Shneider, N.A.; Nesta, A.V.; Rifai, O.M.; Yasek, J.; Elyaman, W.; Aziz-Zaman, S.; Lyu, M.-A.; Levy, S.H.; Hoover, B.N.; Vlad, G. Clinical safety and preliminary efficacy of regulatory T cells for ALS. NEJM Evid. 2025, 4, EVIDoa2400249. [Google Scholar] [CrossRef]
- Thonhoff, J.R.; Berry, J.D.; Macklin, E.A.; Beers, D.R.; Mendoza, P.A.; Zhao, W.; Thome, A.D.; Triolo, F.; Moon, J.J.; Paganoni, S. Combined regulatory T-lymphocyte and IL-2 treatment is safe, tolerable, and biologically active for 1 year in persons with amyotrophic lateral sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e200019. [Google Scholar] [CrossRef]
- Li, X.; Bedlack, R. Evaluating emerging drugs in phase II & III for the treatment of amyotrophic lateral sclerosis. Expert Opin. Emerg. Drugs 2024, 29, 93–102. [Google Scholar] [CrossRef]
- Graber, D.J.; Sentman, M.-L.; Cook, W.J.; Sentman, C.L. mSOD1-NSG mice: A new in vivo model to test human T cells in ALS. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Gacerez, A.T.; Sentman, C.L. T-bet promotes potent antitumor activity of CD4+ CAR T cells. Cancer Gene Ther. 2018, 25, 117–128. [Google Scholar] [CrossRef]
- Godbersen, C.; Coupet, T.A.; Huehls, A.M.; Zhang, T.; Battles, M.B.; Fisher, J.L.; Ernstoff, M.S.; Sentman, C.L. NKG2D Ligand–Targeted Bispecific T-Cell Engagers Lead to Robust Antitumor Activity against Diverse Human Tumors. Mol. Cancer Ther. 2017, 16, 1335–1346. [Google Scholar] [CrossRef]
- Broering, T.J.; Wang, H.; Boatright, N.K.; Wang, Y.; Baptista, K.; Shayan, G.; Garrity, K.A.; Kayatekin, C.; Bosco, D.A.; Matthews, C.R. Identification of human monoclonal antibodies specific for human SOD1 recognizing distinct epitopes and forms of SOD1. PLoS ONE 2013, 8, e61210. [Google Scholar] [CrossRef]
- Rangel-Peláez, C.; Martínez-Gutiérrez, L.; Tristán-Manzano, M.; Callejas, J.L.; Ortego-Centeno, N.; Martín, F.; Martín, J. CD19 CAR-T cell therapy: A new dawn for autoimmune rheumatic diseases? Front. Immunol. 2024, 15, 1502712. [Google Scholar] [CrossRef]
- Gouel, F.; Rolland, A.-S.; Devedjian, J.-C.; Burnouf, T.; Devos, D. Past and future of neurotrophic growth factors therapies in ALS: From single neurotrophic growth factor to stem cells and human platelet lysates. Front. Neurol. 2019, 10, 835. [Google Scholar] [CrossRef]
- Ochs, G.; Penn, R.D.; York, M.; Giess, R.; Beck, M.; Tonn, J.; Haigh, J.; Malta, E.; Traub, M.; Sendtner, M. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000, 1, 201–206. [Google Scholar] [CrossRef]
- BDNF Study Group (Phase III). A controlled trial of recombinant methionyl human BDNF in ALS. Neurology 1999, 52, 1427. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef]
- Scott, S.; Kranz, J.E.; Cole, J.; Lincecum, J.M.; Thompson, K.; Kelly, N.; Bostrom, A.; Theodoss, J.; Al-Nakhala, B.M.; Vieira, F.G. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph. Lateral Scler. 2008, 9, 4–15. [Google Scholar] [CrossRef]
- Picher-Martel, V.; Valdmanis, P.N.; Gould, P.V.; Julien, J.-P.; Dupré, N. From animal models to human disease: A genetic approach for personalized medicine in ALS. Acta Neuropathol. Commun. 2016, 4, 70. [Google Scholar] [CrossRef]
- Heiman-Patterson, T.D.; Sher, R.B.; Blankenhorn, E.A.; Alexander, G.; Deitch, J.S.; Kunst, C.B.; Maragakis, N.; Cox, G. Effect of genetic background on phenotype variability in transgenic mouse models of amyotrophic lateral sclerosis: A window of opportunity in the search for genetic modifiers. Amyotroph. Lateral Scler. 2011, 12, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ciuro, M.; Sangiorgio, M.; Leanza, G.; Gulino, R. A meta-analysis study of SOD1-mutant mouse models of ALS to analyse the determinants of disease onset and progression. Int. J. Mol. Sci. 2022, 24, 216. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lian, D.; Wu, J.; Liu, Y.; Zhu, M.; Sun, J.; He, D.; Li, L. Brain-derived neurotrophic factor reduces inflammation and hippocampal apoptosis in experimental Streptococcus pneumoniae meningitis. J. Neuroinflamm. 2017, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wei, N.; Zhu, J.; Lu, T.; Chen, Z.; Xu, G.; Liu, X. Effects of brain-derived neurotrophic factor on local inflammation in experimental stroke of rat. Mediat. Inflamm. 2010, 2010, 372423. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graber, D.J.; Cook, W.J.; Sentman, M.-L.; Murad-Mabaera, J.M.; Stommel, E.W.; Sentman, C.L. Human CAR Tregs Targeting SOD1 and Expressing BDNF Reduce Inflammation and Delay Disease in G93A hSOD1-NSG Mice. Cells 2025, 14, 1318. https://doi.org/10.3390/cells14171318
Graber DJ, Cook WJ, Sentman M-L, Murad-Mabaera JM, Stommel EW, Sentman CL. Human CAR Tregs Targeting SOD1 and Expressing BDNF Reduce Inflammation and Delay Disease in G93A hSOD1-NSG Mice. Cells. 2025; 14(17):1318. https://doi.org/10.3390/cells14171318
Chicago/Turabian StyleGraber, David J., W. James Cook, Marie-Louise Sentman, Joana M. Murad-Mabaera, Elijah W. Stommel, and Charles L. Sentman. 2025. "Human CAR Tregs Targeting SOD1 and Expressing BDNF Reduce Inflammation and Delay Disease in G93A hSOD1-NSG Mice" Cells 14, no. 17: 1318. https://doi.org/10.3390/cells14171318
APA StyleGraber, D. J., Cook, W. J., Sentman, M.-L., Murad-Mabaera, J. M., Stommel, E. W., & Sentman, C. L. (2025). Human CAR Tregs Targeting SOD1 and Expressing BDNF Reduce Inflammation and Delay Disease in G93A hSOD1-NSG Mice. Cells, 14(17), 1318. https://doi.org/10.3390/cells14171318





