Tissue-Resident Memory T Cells in Cancer Metastasis Control
Abstract
1. Introduction
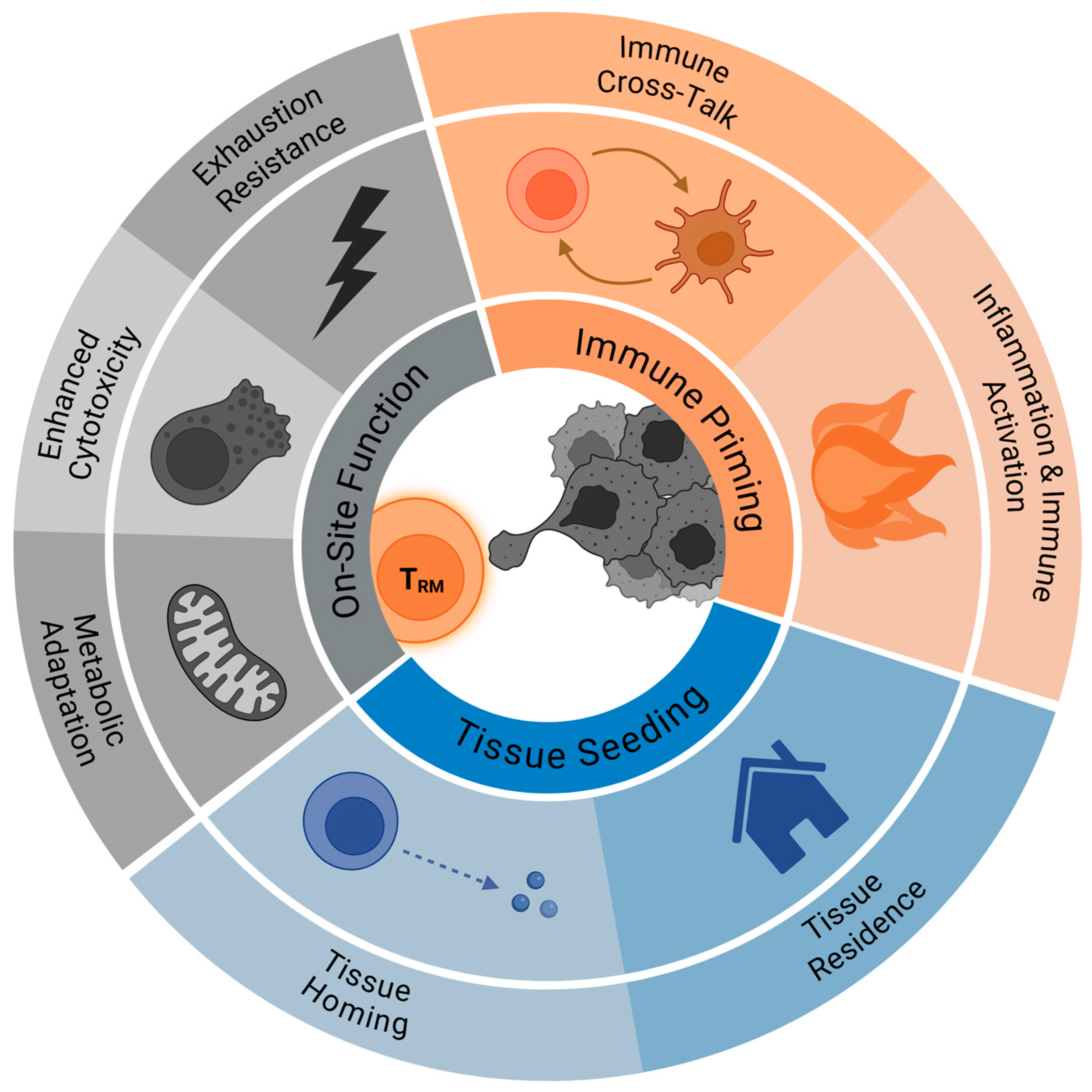
2. TRM Biology Relevant to Metastasis Control
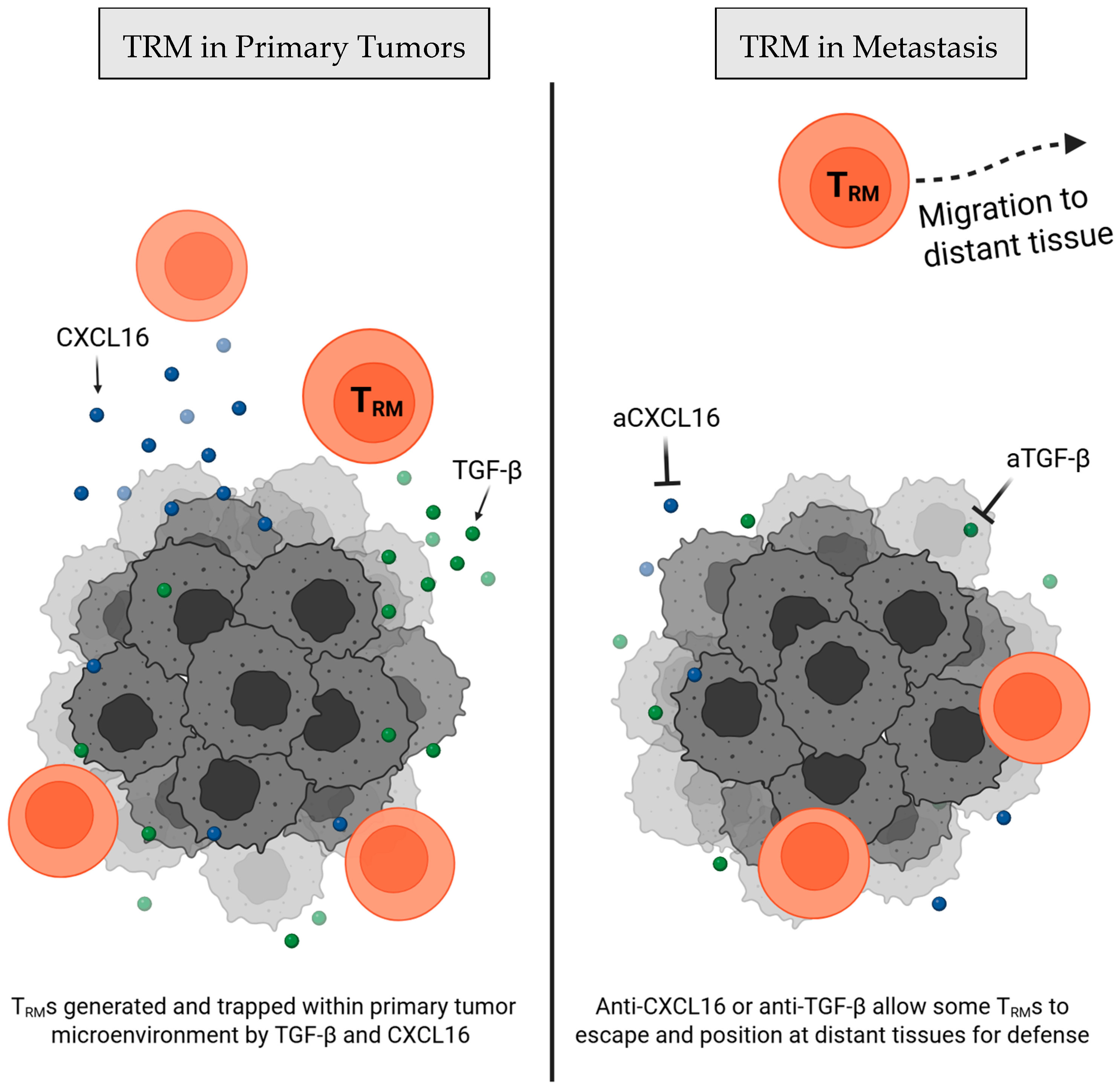
2.1. Tissue Seeding and Retention of TRM Cells
2.2. On-Site TRM Cell Effector Function
2.3. TRM Cell and Microenvironment Crosstalk
3. TRM Mechanistic and Phenotypic Insights
4. TRM Therapeutic and Biomarker Implications for Metastasis Control
4.1. Therapeutics Avenues Targeting TRM Cell Populations
| Therapy Approach | Techniques | Mechanism | Cancer Models | Key Outcome | Direct Metastasis Control Evidence |
|---|---|---|---|---|---|
| Vaccines | Prime-boost vaccination [35,44] | Intranasal attenuated influenza or boost following DNA vaccine priming promotes TRM migration and differentiation | Metastatic murine B16F10 (melanoma) and AB1 (mesothelioma) | ↑ TRM cell precursors to the lung; induce TRM cell differentiation; ↑ protection against lung metastasis | High (but studies currently limited to lung metastases) |
| Nasal mucosa vaccination [45,46] | Intranasal vaccination of an adenoviral vector vaccine with IL-1β adjuvant or tumor antigen containing CpG-coated nanoparticles | Metastatic mouse breast cancer (4T1) | ↑ TRM cell infiltration to existing lung metastasis; prevention of metastasis; ↓ primary tumor size (*CpG-coated nanoparticles only) | Moderate (in-depth metastatic mouse models, aligns with prime-boost vaccination findings. Studies limited to lung metastases) | |
| Chemokine & cytokine targeting | Anti-CXCL16 [15] | Neutralizes intratumoral CXCL16, allowing tumor-derived TRM cell migration to lung for metastasis protection | Murine triple negative breast cancer (4T1) | ↑ Tumor-specific TRM cells defending non-tumor tissues; ↓ metastatic tumor burden in the lung | Moderate (abundant conceptual support; but direct mechanistic study limited to murine metastasis models) |
| Immune checkpoint blockade | Neoadjuvant anti-PD-1 (± CTLA-4 or chemotherapy) [47,48,49] | Enhances TRM cell function and supports systemic tumor-specific immunity | Murine ESCC; Phase III ESCC (NCT01216527) & Phase II oral-cancer (NCT02919683) cohorts | ↑ CD8⁺CD103⁺ TRM cells; delayed progression; ↓ relapses; ↑ systemic anti-tumor immunity | High (abundant pre-clinical and clinical data) |
| Adoptive cell therapy | TGF-β-conditioned CAR-T cells [50] | Programs CAR-T into the TRM cell phenotype through exposure to TGF-β ex vivo | In vitro co-culture with pancreatic cancer cells (AsPC-1) | Proof-of-concept generation of CAR-TRM cells; ↑ primary tumor control; ↑ exhaustion resistance | Low (functional TRM cells; no direct metastasis data, only primary tumor control) |
| iPSC-derived TRM cells [51] | CRISPR-edited iPSCs showing increased TRM markers and behaviors | Human cervical cancer (SiHa) | Generation of iPSC-derived TRM-like cells; ↓ primary tumor growth | Low (functional TRM generation; efficacy shown against primary tumors only; no metastasis data) |
4.2. TRM Cells as a Biomarker for Metastatic Cancer
5. Future Directions
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Szabo, P.A.; Levitin, H.M.; Miron, M.; Snyder, M.E.; Senda, T.; Yuan, J.; Cheng, Y.L.; Bush, E.C.; Dogra, P.; Thapa, P.; et al. Single-Cell Transcriptomics of Human T Cells Reveals Tissue and Activation Signatures in Health and Disease. Nat. Commun. 2019, 10, 4706. [Google Scholar] [CrossRef]
- Crowl, J.T.; Heeg, M.; Ferry, A.; Milner, J.J.; Omilusik, K.D.; Toma, C.; He, Z.; Chang, J.T.; Goldrath, A.W. Tissue-Resident Memory CD8+ T Cells Possess Unique Transcriptional, Epigenetic and Functional Adaptations to Different Tissue Environments. Nat. Immunol. 2022, 23, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Ma, W.; Miron, M.; Granot, T.; Guyer, R.S.; Carpenter, D.J.; Senda, T.; Sun, X.; Ho, S.-H.; Lerner, H.; et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. 2017, 20, 2921–2934. [Google Scholar] [CrossRef]
- Mueller, S.N.; Mackay, L.K. Tissue-Resident Memory T Cells: Local Specialists in Immune Defence. Nat. Rev. Immunol. 2016, 16, 79–89. [Google Scholar] [CrossRef]
- Evrard, M.; Becht, E.; Fonseca, R.; Obers, A.; Park, S.L.; Ghabdan-Zanluqui, N.; Schroeder, J.; Christo, S.N.; Schienstock, D.; Lai, J.; et al. Single-Cell Protein Expression Profiling Resolves Circulating and Resident Memory T Cell Diversity across Tissues and Infection Contexts. Immunity 2023, 56, 1664–1680.e9. [Google Scholar] [CrossRef] [PubMed]
- Christo, S.N.; Park, S.L.; Mueller, S.N.; Mackay, L.K. The Multifaceted Role of Tissue-Resident Memory T Cells. Annu. Rev. Immunol. 2024, 42, 317–345. [Google Scholar] [CrossRef]
- Okła, K.; Farber, D.L.; Zou, W. Tissue-Resident Memory T Cells in Tumor Immunity and Immunotherapy. J. Exp. Med. 2021, 218, e20201605. [Google Scholar] [CrossRef] [PubMed]
- Gavil, N.V.; Cheng, K.; Masopust, D. Resident Memory T Cells and Cancer. Immunity 2024, 57, 1734–1751. [Google Scholar] [CrossRef]
- Min, D.; Fiedler, J.; Anandasabapathy, N. Tissue-Resident Memory Cells in Antitumoral Immunity and Cancer Immunotherapy. Curr. Opin. Immunol. 2024, 91, 102499. [Google Scholar] [CrossRef]
- Wein, A.N.; McMaster, S.R.; Takamura, S.; Dunbar, P.R.; Cartwright, E.K.; Hayward, S.L.; McManus, D.T.; Shimaoka, T.; Ueha, S.; Tsukui, T.; et al. CXCR6 Regulates Localization of Tissue-Resident Memory CD8 T Cells to the Airways. J. Exp. Med. 2019, 216, 2748–2762. [Google Scholar] [CrossRef]
- Mabrouk, N.; Tran, T.; Sam, I.; Pourmir, I.; Gruel, N.; Granier, C.; Pineau, J.; Gey, A.; Kobold, S.; Fabre, E.; et al. CXCR6 Expressing T Cells: Functions and Role in the Control of Tumors. Front. Immunol. 2022, 13, 1022136. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Kojder, K.; Kapczuk, P.; Kupnicka, P.; Gawrońska-Szklarz, B.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. The Effect of Hypoxia on the Expression of CXC Chemokines and CXC Chemokine Receptors-A Review of Literature. Int. J. Mol. Sci. 2021, 22, 843. [Google Scholar] [CrossRef]
- Muthuswamy, R.; McGray, A.R.; Battaglia, S.; He, W.; Miliotto, A.; Eppolito, C.; Matsuzaki, J.; Takemasa, T.; Koya, R.; Chodon, T.; et al. CXCR6 by Increasing Retention of Memory CD8+ T Cells in the Ovarian Tumor Microenvironment Promotes Immunosurveillance and Control of Ovarian Cancer. J. Immunother. Cancer 2021, 9, e003329. [Google Scholar] [CrossRef] [PubMed]
- Christian, L.S.; Wang, L.; Lim, B.; Deng, D.; Wu, H.; Wang, X.-F.; Li, Q.-J. Resident Memory T Cells in Tumor-Distant Tissues Fortify against Metastasis Formation. Cell Rep. 2021, 35, 109118. [Google Scholar] [CrossRef] [PubMed]
- Karaki, S.; Blanc, C.; Tran, T.; Galy-Fauroux, I.; Mougel, A.; Dransart, E.; Anson, M.; Tanchot, C.; Paolini, L.; Gruel, N.; et al. CXCR6 Deficiency Impairs Cancer Vaccine Efficacy and CD8+ Resident Memory T-Cell Recruitment in Head and Neck and Lung Tumors. J. Immunother. Cancer 2021, 9, e001948. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Chu, T.H.; Sheridan, B.S. TGF-β: Many Paths to CD103+ CD8 T Cell Residency. Cells 2021, 10, 989. [Google Scholar] [CrossRef] [PubMed]
- Green, W.D.; Gomez, A.; Plotkin, A.L.; Pratt, B.M.; Merritt, E.F.; Mullins, G.N.; Kren, N.P.; Modliszewski, J.L.; Zhabotynsky, V.; Woodcock, M.G.; et al. Enhancer-Driven Gene Regulatory Networks Reveal Transcription Factors Governing T Cell Adaptation and Differentiation in the Tumor Microenvironment. Immunity 2025, 58, 1725–1741.e9. [Google Scholar] [CrossRef] [PubMed]
- Fay, M.; Sievers, C.; Robbins, Y.; Yang, X.; Huynh, A.; Redman, J.M.; Hodge, J.W.; Schlom, J.; Gulley, J.L.; Allen, C.T.; et al. TGF-β Neutralization Attenuates Tumor Residency of Activated T Cells to Enhance Systemic Immunity in Mice. iScience 2024, 27, 110520. [Google Scholar] [CrossRef]
- Vella, J.L.; Molodtsov, A.; Angeles, C.V.; Branchini, B.R.; Turk, M.J.; Huang, Y.H. Dendritic Cells Maintain Anti-Tumor Immunity by Positioning CD8 Skin-Resident Memory T Cells. Life Sci. Alliance 2021, 4, e202101056. [Google Scholar] [CrossRef]
- Pilato, M.D.; Kfuri-Rubens, R.; Pruessmann, J.N.; Ozga, A.J.; Messemaker, M.; Cadilha, B.L.; Sivakumar, R.; Cianciaruso, C.; Warner, R.D.; Marangoni, F.; et al. CXCR6 Positions Cytotoxic T Cells to Receive Critical Survival Signals in the Tumor Microenvironment. Cell 2021, 184, 4512–4530.e22. [Google Scholar] [CrossRef]
- Tieu, R.; Zeng, Q.; Zhao, D.; Zhang, G.; Feizi, N.; Manandhar, P.; Williams, A.L.; Popp, B.; Wood-Trageser, M.A.; Demetris, A.J.; et al. Tissue-Resident Memory T Cell Maintenance during Antigen Persistence Requires Both Cognate Antigen and Interleukin-15. Sci. Immunol. 2023, 8, eadd8454. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, M.; Uemura, M.; Hara, T.; Chijimatsu, R.; Motooka, D.; Hirai, T.; Konno, M.; Okuzaki, D.; Sekido, Y.; Hata, T.; et al. Cancer-Specific Tissue-Resident Memory T-Cells Express ZNF683 in Colorectal Cancer. Br. J. Cancer 2023, 128, 1828–1837. [Google Scholar] [CrossRef]
- Abdeljaoued, S.; Doussot, A.; Kroemer, M.; Laloy, E.; Pallandre, J.R.; El Kaddissi, A.; Spehner, L.; Ben Khelil, M.; Bouard, A.; Mougey, V.; et al. Liver Metastases of Colorectal Cancer Contain Different Subsets of Tissue-Resident Memory CD8 T Cells Correlated with a Distinct Risk of Relapse Following Surgery. Oncoimmunology 2025, 14, 2455176. [Google Scholar] [CrossRef]
- Liu, S.; Wang, P.; Wang, P.; Zhao, Z.; Zhang, X.; Pan, Y.; Pan, J. Tissue-Resident Memory CD103+CD8+ T Cells in Colorectal Cancer: Its Implication as a Prognostic and Predictive Liver Metastasis Biomarker. Cancer Immunol. Immunother. 2024, 73, 176. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, H.; Yuan, Y.; He, Q.; Zhou, J.; Li, S.; Sun, Y.; Li, D.Y.; Qiu, H.-B.; Wang, W.; et al. Fatty Acid Oxidation Controls CD8+ Tissue-Resident Memory T-Cell Survival in Gastric Adenocarcinoma. Cancer Immunol. Res. 2020, 8, 479–492. [Google Scholar] [CrossRef]
- Pan, Y.; Tian, T.; Park, C.O.; Lofftus, S.Y.; Mei, S.; Liu, X.; Luo, C.; O’Malley, J.T.; Gehad, A.; Teague, J.E.; et al. Survival of Tissue-Resident Memory T Cells Requires Exogenous Lipid Uptake and Metabolism. Nature 2017, 543, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Reina-Campos, M.; Heeg, M.; Kennewick, K.; Mathews, I.T.; Galletti, G.; Luna, V.; Nguyen, Q.; Huang, H.; Milner, J.J.; Hu, K.H.; et al. Metabolic Programs of T Cell Tissue Residency Empower Tumour Immunity. Nature 2023, 621, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Matsuzaki, J.; Odunsi, K. Tissue Residency of Memory CD8+ T Cells Matters in Shaping Immunogenicity of Ovarian Cancer. Cancer Cell 2022, 40, 452–454. [Google Scholar] [CrossRef]
- Menares, E.; Gálvez-Cancino, F.; Cáceres-Morgado, P.; Ghorani, E.; López, E.; Díaz, X.; Saavedra-Almarza, J.; Figueroa, D.A.; Roa, E.; Quezada, S.A.; et al. Tissue-Resident Memory CD8+ T Cells Amplify Anti-Tumor Immunity by Triggering Antigen Spreading through Dendritic Cells. Nat. Commun. 2019, 10, 4401. [Google Scholar] [CrossRef]
- Behr, F.M.; Parga-Vidal, L.; Kragten, N.A.M.; van Dam, T.J.P.; Wesselink, T.H.; Sheridan, B.S.; Arens, R.; van Lier, R.A.W.; Stark, R.; van Gisbergen, K.P.J.M. Tissue-Resident Memory CD8+ T Cells Shape Local and Systemic Secondary T Cell Responses. Nat. Immunol. 2020, 21, 1070–1081. [Google Scholar] [CrossRef]
- Olalekan, S.; Xie, B.; Back, R.; Eckart, H.; Basu, A. Characterizing the Tumor Microenvironment of Metastatic Ovarian Cancer by Single-Cell Transcriptomics. Cell Rep. 2021, 35, 109165. [Google Scholar] [CrossRef]
- Musial, S.C.; Kleist, S.A.; Degefu, H.N.; Ford, M.A.; Chen, T.; Isaacs, J.F.; Boussiotis, V.A.; Skorput, A.G.J.; Rosato, P.C. Alarm Functions of PD-1+ Brain-Resident Memory T Cells. J. Immunol. 2024, 213, 1585–1594. [Google Scholar] [CrossRef]
- Molodtsov, A.K.; Khatwani, N.; Vella, J.L.; Lewis, K.A.; Zhao, Y.; Han, J.; Sullivan, D.E.; Searles, T.G.; Preiss, N.K.; Shabaneh, T.B.; et al. Resident Memory CD8+ T Cells in Regional Lymph Nodes Mediate Immunity to Metastatic Melanoma. Immunity 2021, 54, 2117–2132.e7. [Google Scholar] [CrossRef]
- Xu, H.; Yue, M.; Zhou, R.; Wang, P.; Wong, M.Y.-C.; Wang, J.; Huang, H.; Chen, B.; Mo, Y.; Tam, R.C.-Y.; et al. A Prime-Boost Vaccination Approach Induces Lung Resident Memory CD8+ T Cells Derived from Central Memory T Cells That Prevent Tumor Lung Metastasis. Cancer Res. 2024, 84, 3173–3188. [Google Scholar] [CrossRef]
- Devi, K.S.P.; Wang, E.; Jaiswal, A.; Konieczny, P.; Kim, T.-G.; Nirschl, C.J.; Verma, A.; Liu, Y.; Milczanowski, J.; Christo, S.N.; et al. PD-1 Is Requisite for Skin TRM Cell Formation and Specification by TGFβ. Nat. Immunol. 2025, 26, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Schøller, A.S.; Nazerai, L.; Christensen, J.P.; Thomsen, A.R. Functionally Competent, PD-1+ CD8+ Trm Cells Populate the Brain Following Local Antigen Encounter. Front. Immunol. 2020, 11, 595707. [Google Scholar] [CrossRef]
- Beura, L.K.; Wijeyesinghe, S.; Thompson, E.A.; Macchietto, M.G.; Rosato, P.C.; Pierson, M.J.; Schenkel, J.M.; Mitchell, J.S.; Vezys, V.; Fife, B.T.; et al. T Cells in Nonlymphoid Tissues Give Rise to Lymph-Node-Resident Memory T Cells. Immunity 2018, 48, 327–338.e5. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.M.; Van Braeckel-Budimir, N.; Moioffer, S.J.; van de Wall, S.; Shan, Q.; Vijay, R.; Sompallae, R.; Hartwig, S.M.; Jensen, I.J.; Varga, S.M.; et al. Protective Function and Durability of Mouse Lymph Node-Resident Memory CD8+ T Cells. eLife 2021, 10, e68662. [Google Scholar] [CrossRef] [PubMed]
- Park, S.L.; Buzzai, A.; Rautela, J.; Hor, J.L.; Hochheiser, K.; Effern, M.; McBain, N.; Wagner, T.; Edwards, J.; McConville, R.; et al. Tissue-Resident Memory CD8+ T Cells Promote Melanoma-Immune Equilibrium in Skin. Nature 2019, 565, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Hochheiser, K.; Aw Yeang, H.X.; Wagner, T.; Tutuka, C.; Behren, A.; Waithman, J.; Angel, C.; Neeson, P.J.; Gebhardt, T.; Gyorki, D.E. Accumulation of CD103+ CD8+ T Cells in a Cutaneous Melanoma Micrometastasis. Clin. Transl. Immunol. 2019, 8, e1100. [Google Scholar] [CrossRef]
- Pizzolla, A.; Keam, S.P.; Vergara, I.A.; Caramia, F.; Thio, N.; Wang, M.; Kocovski, N.; Tantalo, D.; Jabbari, J.; Au-Yeung, G.; et al. Tissue-Resident Memory T Cells from a Metastatic Vaginal Melanoma Patient Are Tumor-Responsive T Cells and Increase after Anti-PD-1 Treatment. J. Immunother. Cancer 2022, 10, e004574. [Google Scholar] [CrossRef]
- Nizard, M.; Roussel, H.; Diniz, M.O.; Karaki, S.; Tran, T.; Voron, T.; Dransart, E.; Sandoval, F.; Riquet, M.; Rance, B.; et al. Induction of Resident Memory T Cells Enhances the Efficacy of Cancer Vaccine. Nat. Commun. 2017, 8, 15221. [Google Scholar] [CrossRef]
- MacKerracher, A.; Sommershof, A.; Groettrup, M. PLGA Particle Vaccination Elicits Resident Memory CD8 T Cells Protecting from Tumors and Infection. Eur. J. Pharm. Sci. 2022, 175, 106209. [Google Scholar] [CrossRef]
- Oltmanns, F.; Vieira Antão, A.; Irrgang, P.; Viherlehto, V.; Jörg, L.; Schmidt, A.; Wagner, J.T.; Rückert, M.; Flohr, A.-S.; Geppert, C.I.; et al. Mucosal Tumor Vaccination Delivering Endogenous Tumor Antigens Protects against Pulmonary Breast Cancer Metastases. J. Immunother. Cancer 2024, 12, e008652. [Google Scholar] [CrossRef]
- Donkor, M.; Choe, J.; Reid, D.M.; Quinn, B.; Pulse, M.; Ranjan, A.; Chaudhary, P.; Jones, H.P. Nasal Tumor Vaccination Protects against Lung Tumor Development by Induction of Resident Effector and Memory Anti-Tumor Immune Responses. Pharmaceutics 2023, 15, 445. [Google Scholar] [CrossRef]
- Liu, J.; Blake, S.J.; Yong, M.C.R.; Harjunpää, H.; Ngiow, S.F.; Takeda, K.; Young, A.; O’Donnell, J.S.; Allen, S.; Smyth, M.J.; et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov. 2016, 6, 1382–1399. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.; Chen, Y.; Zhu, C.; Fang, W.; Yu, Z.; Mao, W.; Xiang, J.; Han, Y.; Chen, Z.; et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J. Clin. Oncol. 2018, 36, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Luoma, A.M.; Suo, S.; Wang, Y.; Gunasti, L.; Porter, C.B.M.; Nabilsi, N.; Tadros, J.; Ferretti, A.P.; Liao, S.; Gurer, C.; et al. Tissue-Resident Memory and Circulating T Cells Are Early Responders to Pre-Surgical Cancer Immunotherapy. Cell 2022, 185, 2918–2935.e29. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.-Y.; Noguera-Ortega, E.; Bartoszek, R.; Collins, S.M.; Williams, E.; Davis, M.; Jadlowsky, J.K.; Plesa, G.; Siegel, D.L.; Chew, A.; et al. Tissue-Resident Memory CAR T Cells with Stem-like Characteristics Display Enhanced Efficacy against Solid and Liquid Tumors. Cell Rep. Med. 2023, 4, 101053. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Ishii, M.; Ando, J.; Ikeda, K.; Igarashi, K.J.; Kinoshita, S.; Azusawa, Y.; Toyota, T.; Honda, T.; Nakanishi, M.; et al. iPSC-Derived Hypoimmunogenic Tissue Resident Memory T Cells Mediate Robust Anti-Tumor Activity against Cervical Cancer. Cell Rep. Med. 2023, 4, 101327. [Google Scholar] [CrossRef] [PubMed]
- Virassamy, B.; Caramia, F.; Savas, P.; Sant, S.; Wang, J.; Christo, S.N.; Byrne, A.; Clarke, K.; Brown, E.; Teo, Z.L.; et al. Intratumoral CD8+ T Cells with a Tissue-Resident Memory Phenotype Mediate Local Immunity and Immune Checkpoint Responses in Breast Cancer. Cancer Cell 2023, 41, 585–601.e8. [Google Scholar] [CrossRef]
- Zhao, Y.; Wucherpfennig, K.W. Tissue-Resident T Cells in Clinical Response and Immune-Related Adverse Events of Immune Checkpoint Blockade. Clin. Cancer Res. 2024, 30, 5527–5534. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.-Y.; Zhang, X.; Cao, T.-Y.; Li, D.-D.; Jiang, W.; Kong, L.-H.; Tang, J.-H.; Han, K.; Zhang, C.-Z.; Mei, W.-J.; et al. Neoadjuvant Immunotherapy Leads to Major Response and Low Recurrence in Localized Mismatch Repair-Deficient Colorectal Cancer. J. Natl. Compr. Cancer Netw. 2023, 21, 60–66.e5. [Google Scholar] [CrossRef]
- Fairfax, B.P.; Taylor, C.A.; Watson, R.A.; Nassiri, I.; Danielli, S.; Fang, H.; Mahé, E.A.; Cooper, R.; Woodcock, V.; Traill, Z.; et al. Peripheral CD8+ T Cell Characteristics Associated with Durable Responses to Immune Checkpoint Blockade in Patients with Metastatic Melanoma. Nat. Med. 2020, 26, 193–199. [Google Scholar] [CrossRef]
- Liu, A.; Liu, D.; Liu, X.; Chi, Y.; Guo, L.; Li, D.; Wang, Q.; Li, Y.; Li, Y.; Zheng, G.; et al. The Prognosis Prediction Value of CD69+ CD8+ Tissue-Resident Memory T Cell as a Novel Indicator of Pathologic Complete Response Heterogeneity Following Different Neoadjuvant Therapy Regimen in Esophageal Squamous Cell Carcinoma. Cancer Immunol. Immunother. 2025, 74, 147. [Google Scholar] [CrossRef]
- Beumer-Chuwonpad, A.; Taggenbrock, R.L.R.E.; Ngo, T.A.; van Gisbergen, K.P.J.M. The Potential of Tissue-Resident Memory T Cells for Adoptive Immunotherapy against Cancer. Cells 2021, 10, 2234. [Google Scholar] [CrossRef]
- Brummel, K.; Eerkens, A.L.; de Bruyn, M.; Nijman, H.W. Tumour-Infiltrating Lymphocytes: From Prognosis to Treatment Selection. Br. J. Cancer 2023, 128, 451–458. [Google Scholar] [CrossRef]
- Anadon, C.M.; Yu, X.; Hänggi, K.; Biswas, S.; Chaurio, R.A.; Martin, A.; Payne, K.K.; Mandal, G.; Innamarato, P.; Harro, C.M.; et al. Ovarian Cancer Immunogenicity Is Governed by a Narrow Subset of Progenitor Tissue-Resident Memory T Cells. Cancer Cell 2022, 40, 545–557.e13. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Younas, K.; Khatoon, A.; Murtaza, B.; Ji, Z.; Akbar, K.; Tanveer, Q.; Bahadur, S.U.K.; Su, Z. Immune Watchdogs: Tissue-Resident Lymphocytes as Key Players in Cancer Defense. Crit. Rev. Oncol. Hematol. 2025, 208, 104644. [Google Scholar] [CrossRef]
- Ohno, M.; Kuramitsu, S.; Yamashita, K.; Nagasaka, T.; Haimoto, S.; Fujita, M. Tumor-Infiltrating B Cells and Tissue-Resident Memory T Cells as Prognostic Indicators in Brain Metastases Derived from Gastrointestinal Cancers. Cancers 2024, 16, 3765. [Google Scholar] [CrossRef]
- Natsuki, S.; Tanaka, H.; Nishiyama, M.; Mori, T.; Deguchi, S.; Miki, Y.; Yoshii, M.; Tamura, T.; Toyokawa, T.; Lee, S.; et al. Prognostic Relevance of Tumor-Resident Memory T Cells in Metastatic Lymph Nodes of Esophageal Squamous Cell Carcinoma. Cancer Sci. 2023, 114, 1846–1858. [Google Scholar] [CrossRef]
- Nishiyama, M.; Miki, Y.; Tanaka, H.; Natsuki, S.; Kuroda, K.; Yoshii, M.; Tamura, T.; Toyokawa, T.; Lee, S.; Maeda, K. Resident Memory T Cell in Metastatic Lymph Nodes Is Associated with Favorable Prognosis in Gastric Cancer Patients. Cancer Sci. 2025. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Verma, A.; Dannenfelser, R.; Melssen, M.; Tirosh, I.; Izar, B.; Kim, T.-G.; Nirschl, C.J.; Devi, K.S.P.; Olson, W.C.; et al. An Activation to Memory Differentiation Trajectory of Tumor-Infiltrating Lymphocytes Informs Metastatic Melanoma Outcomes. Cancer Cell 2022, 40, 524–544.e5. [Google Scholar] [CrossRef] [PubMed]
- von Witzleben, A.; Ellis, M.; Thomas, G.J.; Hoffmann, T.K.; Jackson, R.; Laban, S.; Ottensmeier, C.H. Tumor-Infiltrating CD103+ Tissue-Resident Memory T Cells and CD103-CD8+ T Cells in HNSCC Are Linked to Outcome in Primary but Not Metastatic Disease. Clin. Cancer Res. 2024, 30, 224–234. [Google Scholar] [CrossRef]
- Wijesinghe, S.K.M.; Rausch, L.; Gabriel, S.S.; Galletti, G.; De Luca, M.; Qin, L.; Wen, L.; Tsui, C.; Man, K.; Heyden, L.; et al. Lymph-Node-Derived Stem-like but Not Tumor-Tissue-Resident CD8+ T Cells Fuel Anticancer Immunity. Nat. Immunol. 2025, 26, 1367–1383. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, H.; Zhao, M.; Yuan, H.; Geng, J.; Yan, Y.; Wu, L.; Xing, L.; Yu, J.; Sun, X. Stage-Dependent Spatial Distribution and Prognostic Value of CD8+ Tissue-Resident Memory T Cells in NSCLC. NPJ Precis. Oncol. 2025, 9, 51. [Google Scholar] [CrossRef]
- Natsuki, S.; Tanaka, H.; Nishiyama, M.; Deguchi, S.; Miki, Y.; Yoshii, M.; Tamura, T.; Toyokawa, T.; Lee, S.; Maeda, K. Significance of CD103+ Tissue-Resident Memory T Cells for Predicting the Effectiveness of Immune Checkpoint Inhibitors in Esophageal Cancer. BMC Cancer 2023, 23, 1011. [Google Scholar] [CrossRef]
- Greenlee, J.D.; King, M.R. A Syngeneic MC38 Orthotopic Mouse Model of Colorectal Cancer Metastasis. Biol. Methods Protoc. 2022, 7, bpac024. [Google Scholar] [CrossRef]
- Breuer, C.B.; Xiong, Z.; Wang, A.; Rodriguez, G.E.; Abhiraman, G.C.; Garcia, K.C.; Reticker-Flynn, N.E. Spontaneous and Experimental Models of Lymph Node Metastasis. Nat. Protoc. 2025, 1–18. [Google Scholar] [CrossRef]
- Reina-Campos, M.; Monell, A.; Ferry, A.; Luna, V.; Cheung, K.P.; Galletti, G.; Scharping, N.E.; Takehara, K.K.; Quon, S.; Challita, P.P.; et al. Tissue-Resident Memory CD8 T Cell Diversity Is Spatiotemporally Imprinted. Nature 2025, 639, 483–492. [Google Scholar] [CrossRef] [PubMed]
- van de Wall, S.; Anthony, S.M.; Hancox, L.S.; Pewe, L.L.; Langlois, R.A.; Zehn, D.; Badovinac, V.P.; Harty, J.T. Dynamic Landscapes and Protective Immunity Coordinated by Influenza-Specific Lung-Resident Memory CD8+ T Cells Revealed by Intravital Imaging. Immunity 2024, 57, 1878–1892.e5. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montgomery, T.H.; Master, A.P.; Jin, Z.; Shi, Q.; Lai, Q.; Desai, R.; Zhang, W.; Maharjan, C.K.; Kolb, R. Tissue-Resident Memory T Cells in Cancer Metastasis Control. Cells 2025, 14, 1297. https://doi.org/10.3390/cells14161297
Montgomery TH, Master AP, Jin Z, Shi Q, Lai Q, Desai R, Zhang W, Maharjan CK, Kolb R. Tissue-Resident Memory T Cells in Cancer Metastasis Control. Cells. 2025; 14(16):1297. https://doi.org/10.3390/cells14161297
Chicago/Turabian StyleMontgomery, Tyler H., Anuj P. Master, Zeng Jin, Qiongyu Shi, Qin Lai, Rohan Desai, Weizhou Zhang, Chandra K. Maharjan, and Ryan Kolb. 2025. "Tissue-Resident Memory T Cells in Cancer Metastasis Control" Cells 14, no. 16: 1297. https://doi.org/10.3390/cells14161297
APA StyleMontgomery, T. H., Master, A. P., Jin, Z., Shi, Q., Lai, Q., Desai, R., Zhang, W., Maharjan, C. K., & Kolb, R. (2025). Tissue-Resident Memory T Cells in Cancer Metastasis Control. Cells, 14(16), 1297. https://doi.org/10.3390/cells14161297






