1. Introduction
Osteoclasts are large, multinucleated cells specialized for bone resorption. They originate from the hematopoietic lineage and differentiate from myeloid precursors [
1,
2]. Protocadherin-7 (Pcdh7) is a member of the protocadherin subgroup within the cadherin superfamily, a group of calcium-dependent cell adhesion proteins [
3]. We previously demonstrated that Pcdh7 is essential for osteoclast differentiation, functioning as a signaling receptor. During RANKL-induced osteoclast differentiation, Pcdh7 ligation activates protein phosphatase 2 A (PP2A), which subsequently activates glycogen synthase kinase-3b (GSK3β). GSK3β then activates small GTPases, including RhoA, promoting osteoclast differentiation and multinucleation [
2,
4]. However, whether Pcdh7 also contributes to gene regulation during osteoclast differentiation remains unknown.
The type I interferon (IFN) family consists of pleiotropic cytokines that regulate cell-intrinsic immune responses, with IFN-α and IFN-β being the most extensively studied [
5,
6]. These cytokines signal through a shared heterodimeric receptor composed of interferon alpha and beta receptor 1 (IFNAR1) and interferon alpha and beta receptor 2 (IFNAR2) subunits, leading to the induction of interferon-stimulated genes (ISGs). In the context of bone metabolism, the role of type I IFNs has been well characterized [
7]. Upon stimulation with the osteoclast differentiation factor receptor activator of nuclear factor-kB ligand (RANKL), osteoclast precursors transiently produce low levels of IFN-β [
8]. IFN-β inhibits the expression of c-Fos, a key transcription factor essential for osteoclast differentiation, thereby suppressing the differentiation process [
8]. Consistently, genetic deficiency of either IFNAR1 or IFN-β results in enhanced osteoclast formation and reduced bone mass in mice [
8,
9]. These findings underscore the role of Type I IFN signaling as a negative feedback mechanism that regulates RANKL signaling and maintains osteoclast-mediated bone homeostasis.
Pyrin domain-only proteins (POPs) are a family of inflammasome regulators [
10,
11]. Three POPs—POP1, POP2, and POP3—have been identified in several species, including humans, but are absent in mice. These proteins regulate inflammasome assembly by binding to and sequestering the pyrin domain (PYD) in apoptosis-associated speck-like protein containing a CARD (ASC), a key adaptor required for the activation of downstream effectors such as caspase-1. POPs also bind to PYD-containing pattern recognition receptors, thereby preventing inflammasome formation. Among them, POP3 is particularly responsive to IFN-β signaling [
12]. It functions as an inhibitor of AIM2-like receptors (ALRs) inflammasome formation and promotes a type I interferon response. Pyrin domain-containing protein 3 (Pydc3), also known as interferon activated gene 208 (Ifi208), is considered a putative functional analog of human POP3 in mice [
13]. Pydc3 has been shown to be upregulated by IFN-β in mouse CD11b
+ macrophages and has been identified as a target of PGD
2/DP1 signaling in virus-induced central nervous system inflammation [
14].
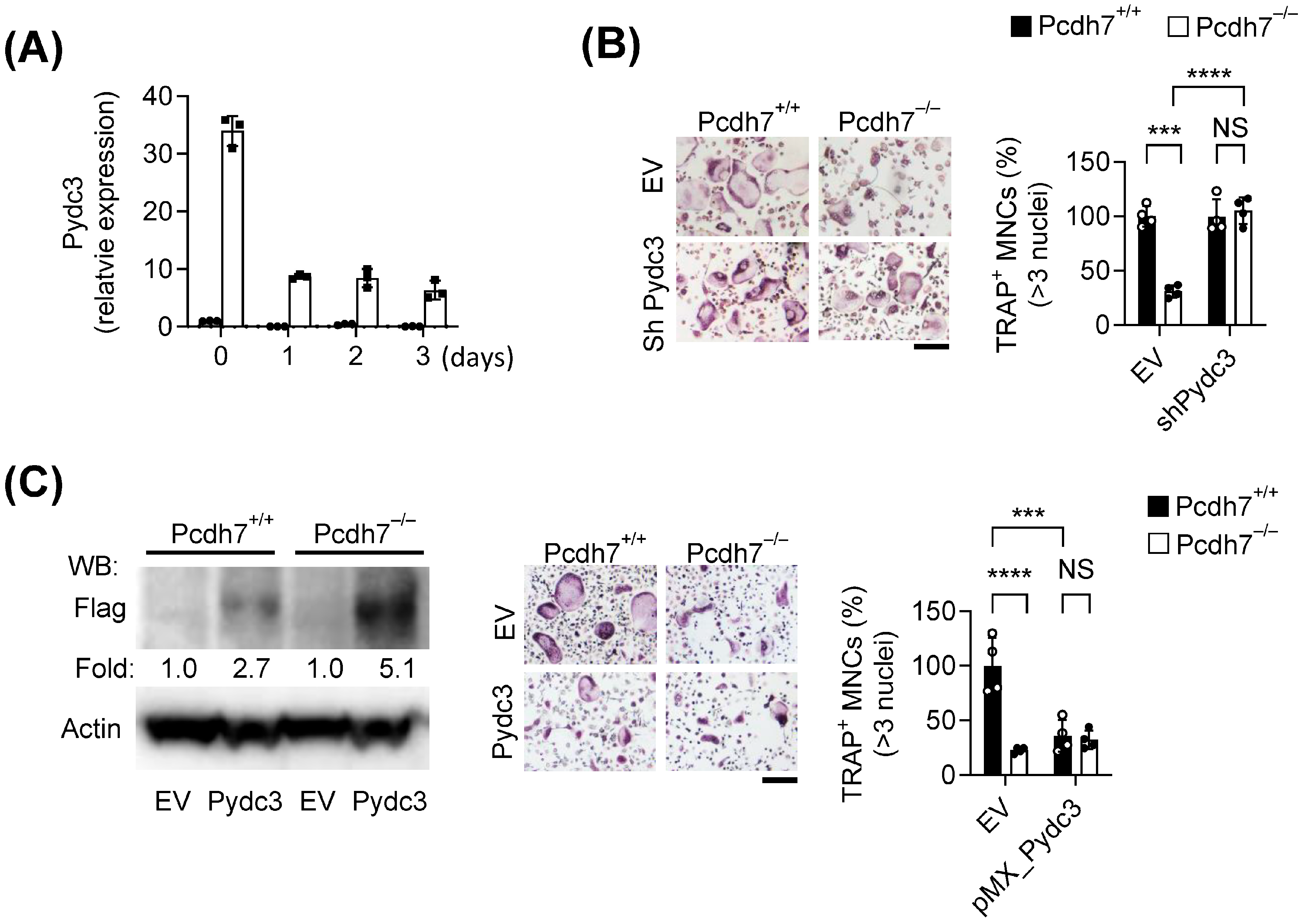
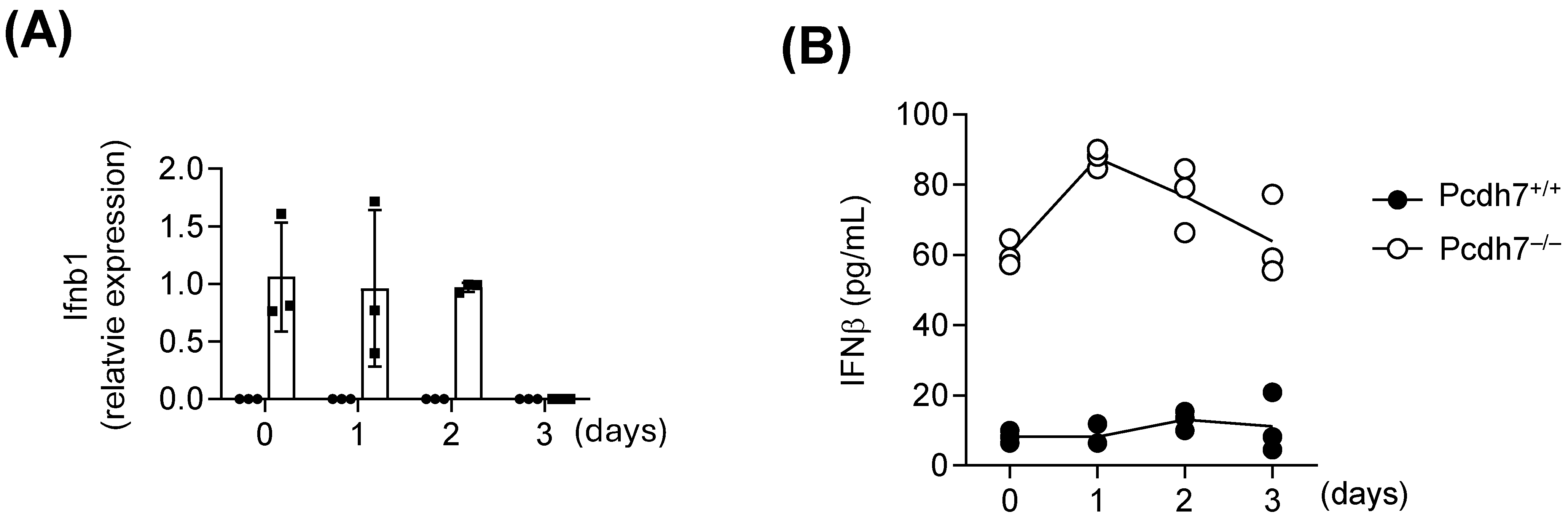
In this study, we identified a specific role for Pcdh7 in regulating Pydc3 expression and the IFN-β response during osteoclast differentiation. Transcriptomic analysis revealed significant upregulation of Pydc3 and ISGs in Pcdh7−/− cells. Through RNAi-mediated gene silencing and overexpression approaches, we demonstrated that Pydc3 negatively regulates osteoclast differentiation. Pcdh7 deficiency resulted in abnormal IFN-β production, and neutralization of IFN-β signaling restored the impaired osteoclast differentiation observed in Pcdh7−/− cells. Together, these findings uncover a previously unrecognized function of Pcdh7 during osteoclast differentiation.
2. Materials and Methods
2.1. RNA Sequencing
Total RNA was extracted from the cultured cells using the RNeasy Plus Mini Kit (QIAGEN) according to the manufacturer’s protocol. The purified RNA samples were carefully packed on dry ice and shipped to Azenta Life Sciences for further processing. At Azenta, cDNA library preparation, RNA sequencing, and comprehensive data analysis were performed. The Standard RNA sequencing service was employed, enabling the construction of libraries suitable for both coding and long non-coding RNA profiling. mRNA was isolated using poly (A) selection. Sequencing was conducted on the Illumina platform. Sequence reads were trimmed to remove possible adapter sequences and mapped to the Mus musculus GRCm38 reference genome available on ENSEMBL. Unique gene hit counts were calculated. Gene hit counts were extracted from the sequencing data and analyzed to assess differences in expression between Pcdh7+/+ and Pcdh7−/− cell culture groups using the DESeq2 package. Statistical analysis was performed with the Wald test, which was used to calculate p-values and log2 fold changes for each gene. Genes meeting the criteria of an adjusted p-value less than 0.05 and an absolute log2 fold change greater than 1 were classified as differentially expressed. This approach ensured that only statistically significant and biologically relevant changes in gene expression were considered. The experiment was conducted with three biological replicates.
2.2. Osteoclast Differentiation
Osteoclast differentiation was performed based on a well-established and previously described protocol [
15]. Initially, bone marrow cells were harvested and cultured under specific conditions to generate bone marrow-derived macrophages (BMMs). This was achieved by incubating the bone marrow cells in a culture medium supplemented with macrophage colony-stimulating factor (M-CSF) at a concentration of 60 ng/mL for a duration of three days. This step promotes the proliferation and survival of macrophage precursor cells. After the initial culture period, the resulting BMMs were carefully seeded into 96-well culture plates at a consistent density of 1 × 10
4 cells per well, then briefly centrifuged at 200×
g for 3 min to promote uniform cell attachment and facilitate subsequent growth and differentiation. The cells were then cultured for an additional three days in the presence of both M-CSF (60 ng/mL) and receptor activator of nuclear factor κB ligand (RANKL) at 150 ng/mL. The combined presence of these two factors is essential, as M-CSF supports macrophage viability and proliferation, while RANKL drives the differentiation process toward mature osteoclasts. Following the culture period, the formation of differentiated osteoclasts was confirmed by staining and visualization using the Leukocyte Acid Phosphatase Kit (Sigma-Aldrich, St. Louis, MO, USA; Cat.#387A-1KT). This kit specifically detects acid phosphatase activity, a hallmark enzymatic function of osteoclasts. The staining procedure was carried out strictly in accordance with the manufacturer’s guidelines to ensure accurate identification and assessment of osteoclast differentiation. BMMs and osteoclasts were used for the following experiments. Experiments were conducted at least three times.
2.3. Retroviral Transduction
For Pydc3 silencing, retroviral particles were produced using Plat-E packaging cells transfected with pSuper vectors encoding shRNAs targeting Pydc3 (5′-GCCTAAGTTTCCATTACTTTC-3′). For Pydc3 overexpression, mouse Pydc3 cDNA (OriGene technology, Rockville, MD, USA Cat.#MR217014) was subcloned into the pMX vector. Empty pSuper or pMX vectors were used as negative controls. Retroviral supernatants were collected, filtered through a 0.45 μm syringe filter, and used to transduce BMMs overnight in the presence of hexadimethrine bromide (6–8 μg/mL) and M-CSF (120 ng/mL).
2.4. Immunoblotting
Cells were subjected to lysis using RIPA lysis and extraction buffer (Thermo Scientific™, Waltham, MA, USA, Cat.#89901), which was supplemented with a cocktail of protease and phosphatase inhibitors (Roche, Indianapolis, IN, USA). The lysates were quantified and equal amounts of proteins from samples were separated by electrophoresis on a 4–15% SDS-polyacrylamide gradient gel. Following electrophoretic separation, the proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane, and the membrane was then incubated with specific primary antibodies. For protein visualization, the blots were scanned and analyzed using the LI-COR Odyssey Fc Imager (LI-COR Biosciences, Lincoln, NE, USA). The primary antibodies employed in this study included anti-Flag M2 antibody (Sigma Aldrich, St. Louis, MO, USA; Cat.#A8592), used for detecting Flag-tagged proteins, and anti-Actin antibody (Santacruz Biotechnology, SantaCruz, CA, USA, Cat.#SC-47778), serving as a loading control to ensure equal protein loading across samples.
2.5. Quantitative PCR
Total RNA was extracted from cells following two to three washes with Dulbecco’s phosphate-buffered saline (DPBS) to remove residual medium and debris. Cells were lysed in 1 mL of TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. From the isolated RNA, 1–5 μg of total RNA was reverse-transcribed into complementary DNA (cDNA) using random hexamer primers and SuperScript™ III reverse transcriptase (Invitrogen, Carlsbad, CA, USA). The resulting cDNA, corresponding to 10 ng of input total RNA, was subjected to quantitative PCR (qPCR) analysis using the QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA) and the following specific TaqMan probes: Pydc3 (Mm04206759_mH), Ifnb (Mm00439552_s1), and 18S gene (Hs99999901_s1) was used as the internal control, and the relative expression level were quantified using the comparative CT (cycle threshold) method.
2.6. ELISA
To determine IFN-β concentrations, culture supernatants were harvested from treated cells and passed through a 0.2 μm syringe filter to eliminate cellular debris. The clarified samples were subsequently analyzed using a mouse IFN-β ELISA kit (Abcam, Cambridge, MA, USA. Cat. #ab252363), following the protocol provided by the manufacturer. Each standard and sample was assayed in duplicate to ensure measurement accuracy. Absorbance values were recorded at 405 nm using an EMax Plus microplate reader (Molecular Devices, Sunnyvale, CA, USA), and cytokine concentrations were calculated from the corresponding standard curve.
2.7. RNA Extraction from Bone Tissue
Bone samples were collected aseptically into sterile containers containing phosphate-buffered saline (PBS). Approximately 160 mg of bone tissue was transferred to a sterile 10 cm Petri dish using sterile forceps. TRIzol® reagent (1 mL) was added to the tissue, which was subsequently minced into a coarse slurry using sterile scissors. Homogenization was completed using a Dounce tissue grinder until no visible fragments remained. The homogenate was transferred to a 1.5 mL microcentrifuge tube and placed on ice for 5 min. Samples were centrifuged at 12,000× g for 10 min at 4 °C, and the supernatant was collected in a fresh 1.5 mL microcentrifuge tube. To shear genomic DNA and reduce lysate viscosity, the supernatant was passed slowly through a 21-gauge needle at least 6 times. Chloroform (0.15 volumes) was added, and the tube was shaken vigorously for 15 s. Samples were centrifuged at 10,000× g for 15 min at 4 °C, and the upper aqueous phase was carefully transferred to a new 1.5 mL tube. An equal volume of isopropanol was added to the aqueous phase, followed by gentle inversion (5–6 times) to mix. Samples were incubated at room temperature for 10 min to precipitate RNA, then centrifuged at 10,000× g for 10 min at 4 °C. The supernatant was discarded, and the RNA pellet was washed with 1 mL of ice-cold 75% ethanol. Following centrifugation at 10,000× g for 5 min at 4 °C, the supernatant was removed, and tubes were briefly centrifuged again for ~15 s to remove residual ethanol. The RNA pellet was air-dried for 3 min, ensuring it was not over-dried, and subsequently dissolved in 30–80 μL of nuclease-free distilled water. RNA concentration and purity were measured with a NanoDrop spectrophotometer. The RNA was then aliquoted and stored at −70 °C or lower until further use.
2.8. Statistics
One-way ANOVA, two-way ANOVA, or 2-tailed paired Student’s t test were used for comparisons involving three or more independent groups. A p value of less than 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism software (version 10).
4. Discussion
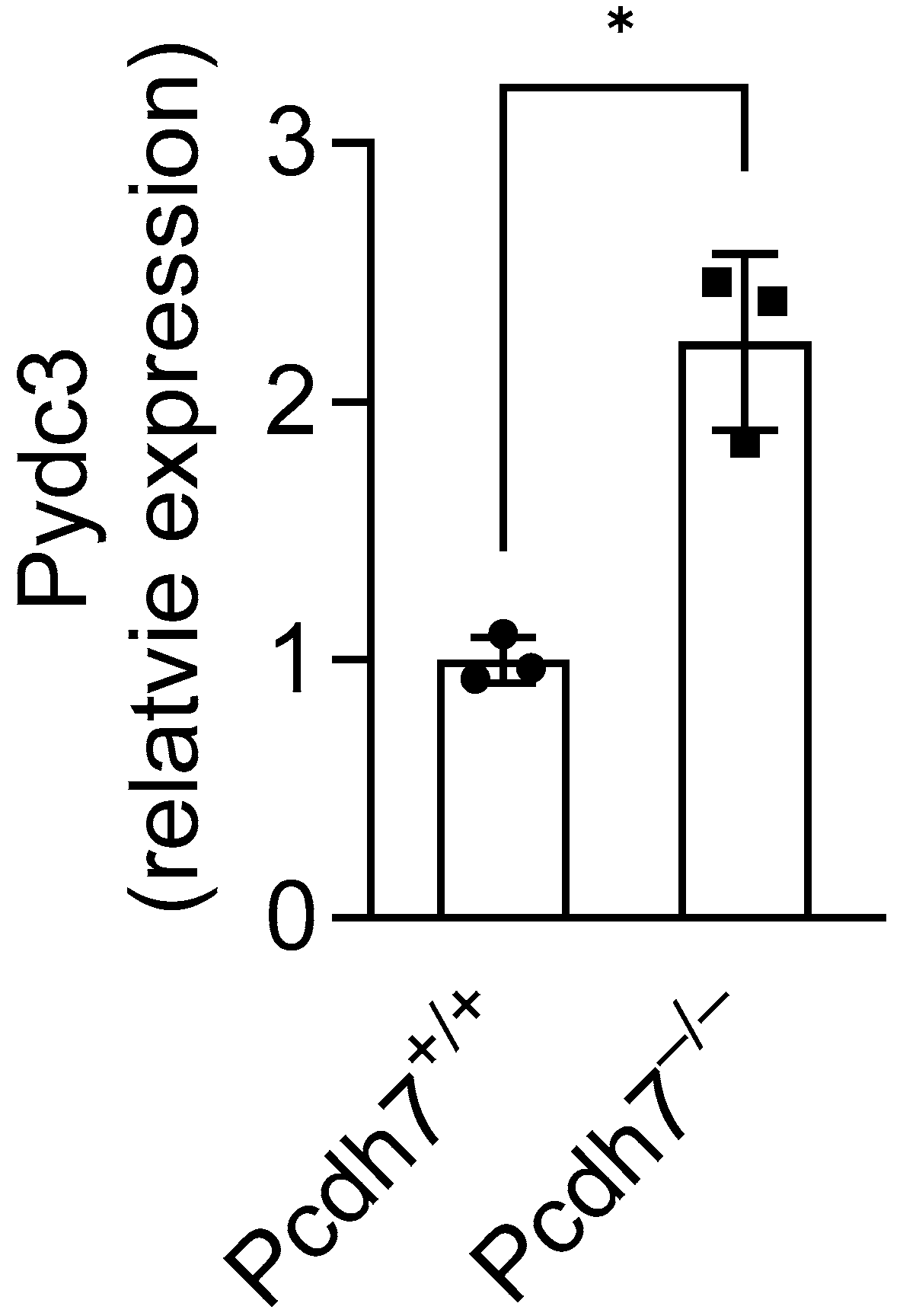
In this study, we investigated the gene regulatory role of Pcdh7 and identified Pydc3 as a novel mediator that negatively regulates osteoclast differentiation. Consistent with the results obtained from in vitro experiments, we found significant upregulation of
Pydc3 expression in Pcdh7
−/− mouse bone tissues (
Figure 5), where osteoclasts and their precursors reside, supporting the critical role of Pcdh7 in the regulation of Pydc3 expression in vivo. We also found that Pcdh7 is involved in IFN-β signaling. However, the trigger for
Pydc3 expression in Pcdh7
−/− cells remains unclear. The elevated production of IFN-β in Pcdh7
−/− cells likely contributes to the upregulation of
Pydc3, as both
Pydc3 and its human analog POP3 are known to be induced by IFN-β [
12,
14]. In turn,
Pydc3 may also enhance IFN-β production, as previously reported [
12,
14]. These observations suggest that Pydc3 functions as a positive feedback regulator of IFN-β. This feedback loop—in which IFN-β induces
Pydc3 expression, and elevated
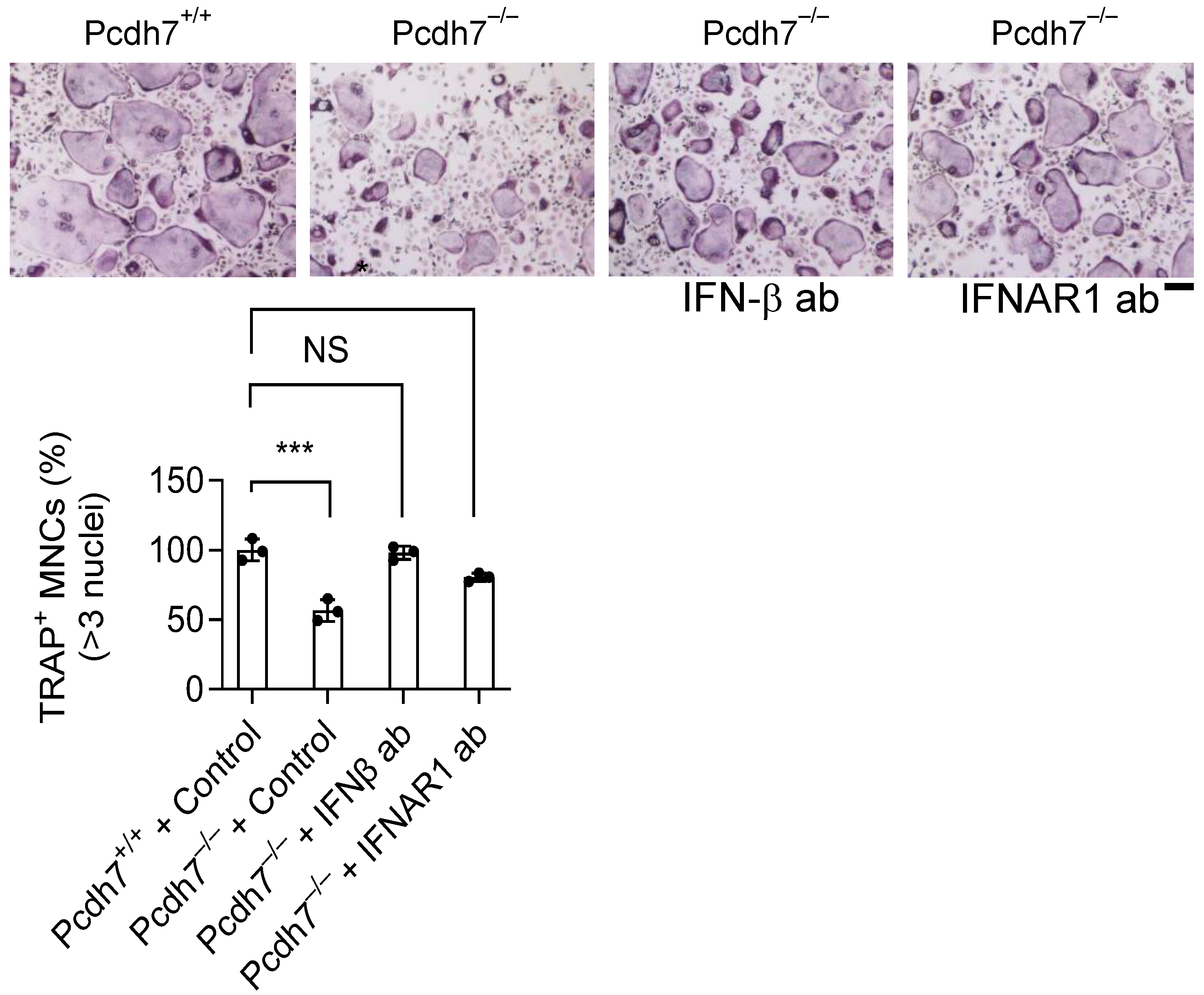
Pydc3 further amplifies IFN-β production—ultimately inhibits osteoclast differentiation. Indeed, neutralization of IFN-β signaling using anti-IFN-β or anti-IFNAR1 antibodies restored osteoclast differentiation in Pcdh7
−/− cells. Similarly, RNAi-mediated knockdown of Pydc3 fully restored the impaired osteoclast differentiation in Pcdh7-deficient cultures. Osteoclasts are positively and negatively regulated through autocrine factors such as Osteopontin, SFRP4 and IFN-β [
7,
17,
18,
19]. Our study revealed a role for Pcdh7 and Pydc3 in the regulation of IFN-β, highlighting the interplay between adhesion molecules and innate immune signaling in the regulation of autocrine-mediated osteoclast differentiation. RANKL stimulation attenuated Pydc3 mRNA expression in Pcdh7
−/− BMMs, suggesting that RANKL signaling may negatively regulate Pydc3 expression. Further studies are needed to elucidate the upstream regulatory mechanisms that control
Pydc3 transcription during osteoclast differentiation.
Human POP3 has been shown to inhibit ALR inflammasome activation and regulate the production of inflammatory cytokines such as IL-1 [
12]. Pydc3 has also been implicated in the regulation of virus-mediated IL-1β production [
14]. In this study, we focused on the role of Pydc3 in regulating IFN-β, as our RNA-seq analysis pointed to type I IFN involvement in the context of Pcdh7 deficiency. However, we did not directly address the potential role of Pydc3 in inflammasome activation. Given that inflammation can promote osteoclast differentiation [
20,
21,
22], it is possible that Pydc3 may also influence this process through inflammasome-related mechanisms. Future studies will be required to determine whether Pydc3-mediated inflammasome activity contributes to the downstream effects of Pcdh7 deficiency.
We previously demonstrated that Pcdh7 functions as a signal transducer: Pcdh7 ligation activates PP2A, which subsequently activates GSK3β, leading to activation of small GTPases such as RhoA, and ultimately promoting osteoclast differentiation and multinucleation [
4,
15,
16]. Indeed, the impaired osteoclast differentiation in Pcdh7
−/− cells was fully rescued by overexpression of constitutive active forms of RhoA and Rac1 [
15]. In the present study, we similarly showed that osteoclast differentiation in Pcdh7
−/− cells was fully restored by Pydc3 knockdown and/or IFN-β signaling neutralization. This raises an important mechanistic question: Is there a link between small GTPases signaling and IFN-β? One study reported that the absence of IFN-β signaling enhanced RhoA activation [
23], while another showed that IFN-β signaling inhibited Rac1 activation via suppressor of cytokine signaling 1 (SOCS1) [
24]. These findings suggest that IFN-β may negatively regulate the activation of small GTPases. Therefore, neutralization of IFN-β signaling could relieve this inhibition and promote GTPase activity, contributing to the rescue of osteoclast differentiation in Pcdh7-deficient cells. Further investigation is required to determine whether and how Pcdh7 coordinates IFN-β signaling and small GTPase activity to regulate osteoclast differentiation.
Taken together, our findings reveal a previously unrecognized role for Pcdh7 in regulating IFN-β signaling. Specifically, we demonstrate that Pcdh7 suppresses Pydc3 expression and IFN-β production, thereby facilitating proper osteoclast differentiation.