Therapeutic Effects of Neuro-Cells on Amyloid Pathology, BDNF Levels, and Insulin Signalling in APPswe/PSd1E9 Mice
Abstract
1. Introduction
2. Materials and Methods
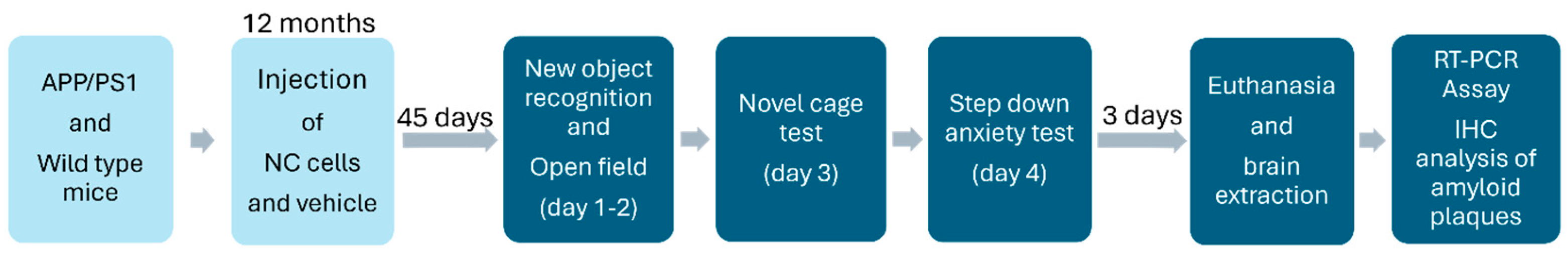
2.1. Animals and Study Design
Study Flow
2.2. Neuro-Cells Preparation
2.3. Central Administration of Neuro-Cells
2.4. Behavioural Assays for Memory, Locomotion and Anxiety
2.4.1. Open Field and Object Exploration/Recognition Paradigm
2.4.2. Novel Cage Test
2.4.3. Step-Down Anxiety Test
2.5. Culling and Brain Tissue Collection
2.6. Brain Sectioning and Histological Assays
2.7. Immunohistochemical Staining of Amyloid Plaques with 6E10
2.8. QuPath 0.4.3 Pixel Classifier
2.9. Real-Time Polymerase Chain Reaction (RT-PCR)
2.10. Statistical Analysis
3. Results
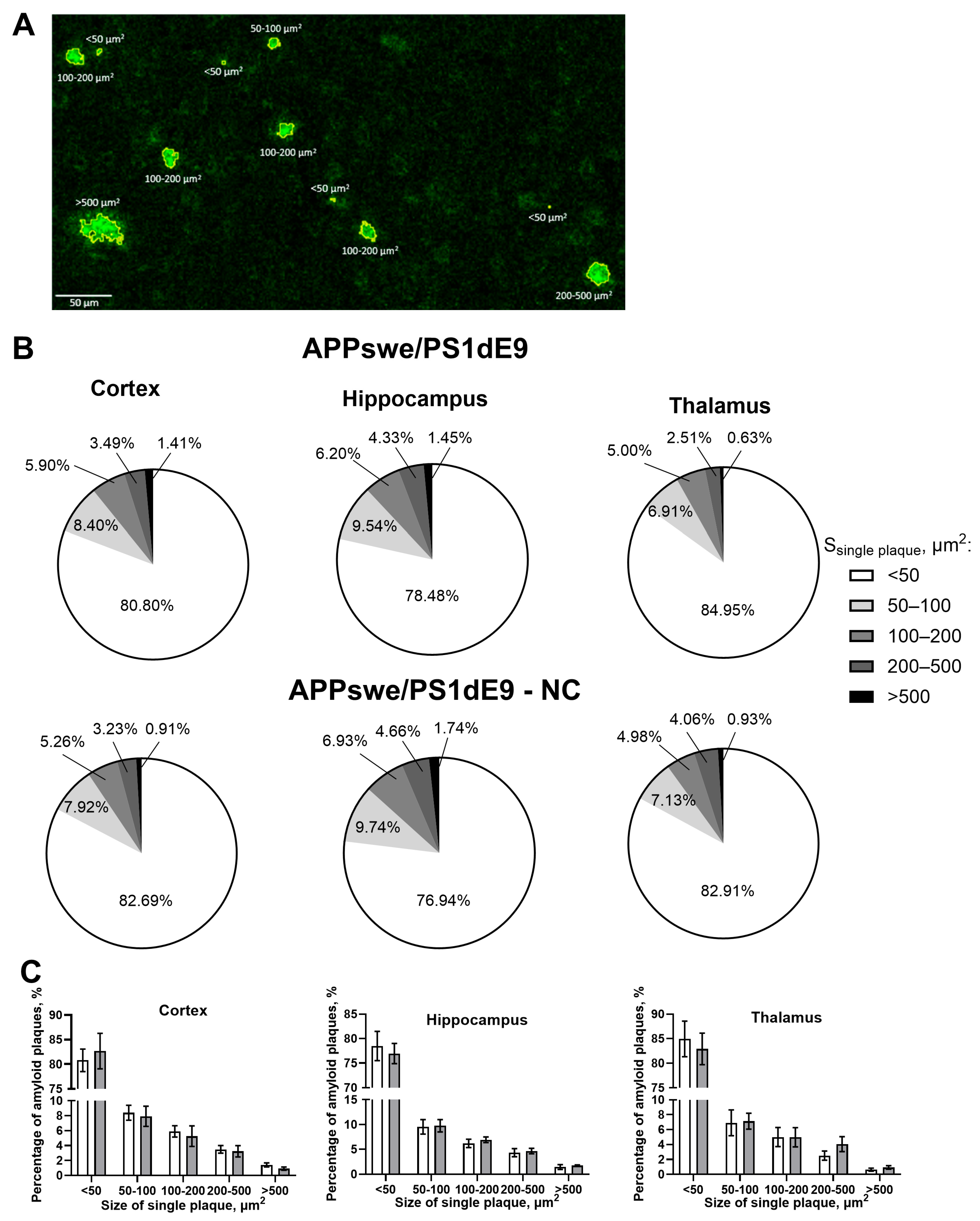
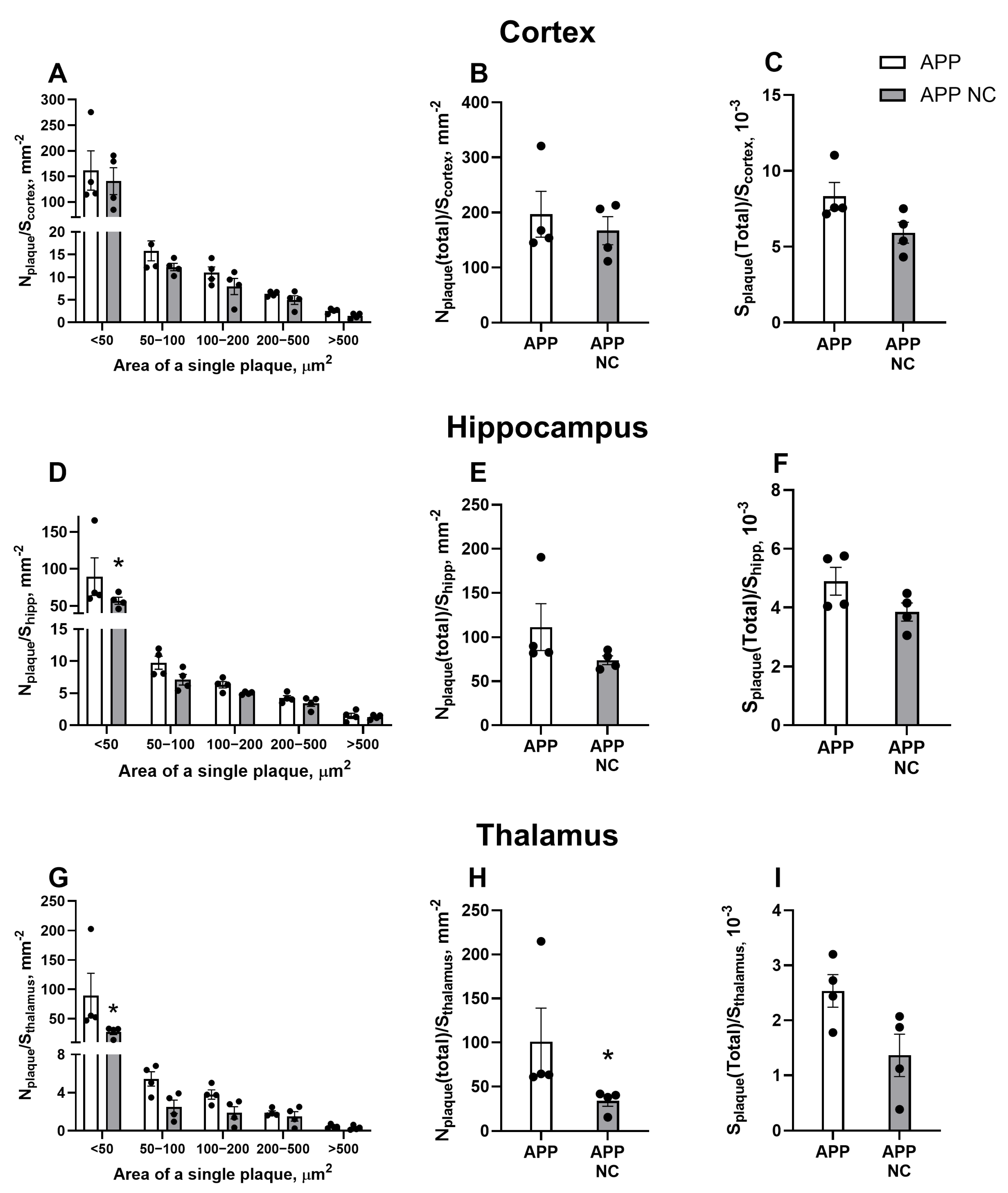
3.1. Injection of NC to APPswe/PS1dE9 Mice Reduces Amyloid Plaque Formation of the Smallest Size
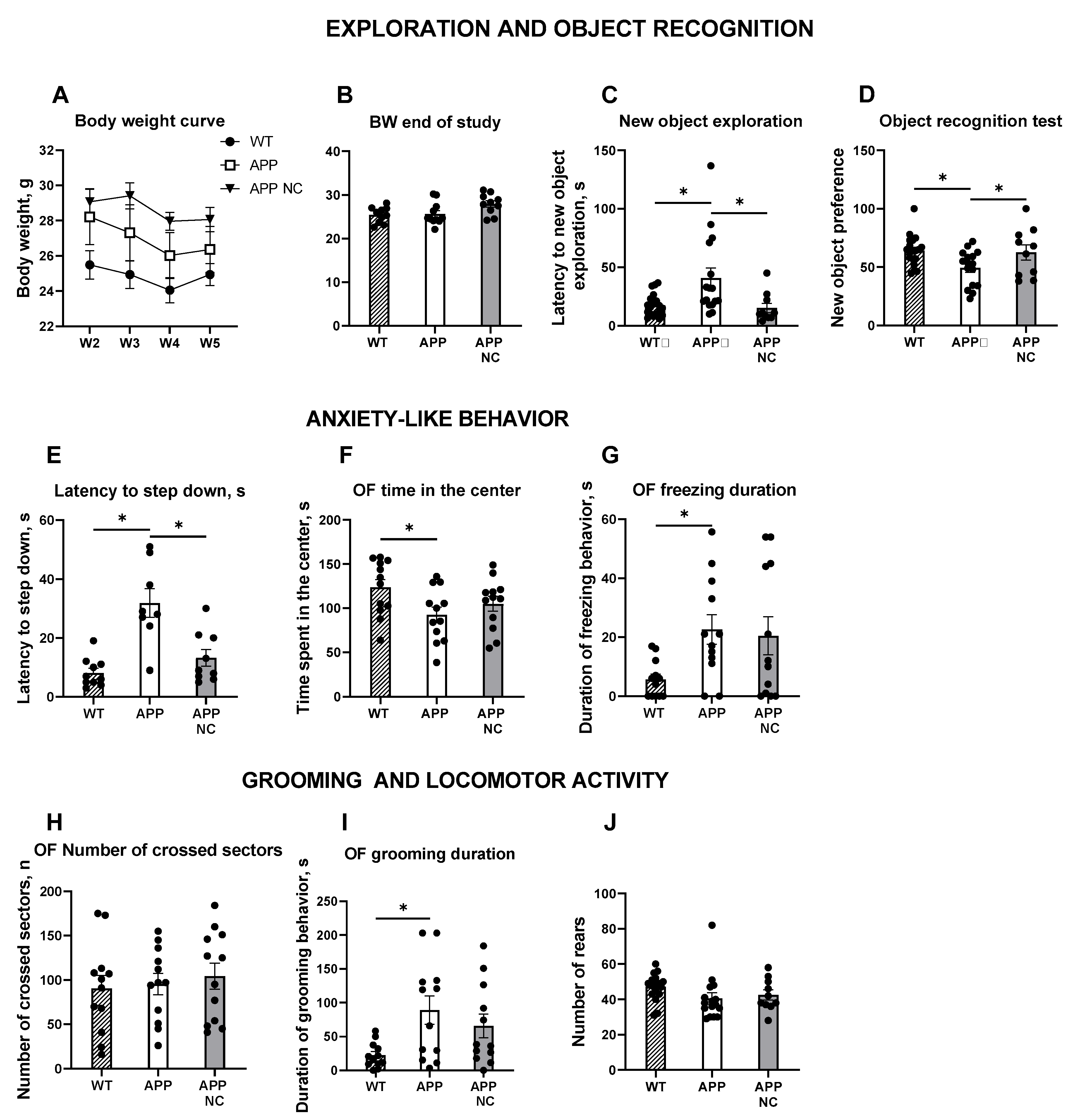
3.2. Administration of NC Ameliorates Hippocampus-Dependent Learning and Anxiety-like Behaviour in APPswe/PS1вE9-NC Mice
3.3. Gene Expression of Bdnf, Elements of IR-Mediated Signalling, and Markers of Ageing and AD in APPswe/PS1вE9-NC Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| MSCs | Mesenchymal stem cells |
| HSCs | Haematopoietic stem cells |
| Aβ | Amyloid-β |
| TNF | Tumor necrosis factor |
| IL-10 | Interleukin-10 |
| VEGF | Vascular endothelial growth factor |
| Ki-67 | Marker of proliferation Kiel 67 |
| Wnt | Wingless/Int-1 |
| BDNF | Brain-derived neurotrophic factor |
| IGF-1 | Insulin-like growth factor-1 |
| HGF | Hepatocyte growth factor |
| NGF | Neuronal growth factor |
| IR | Insulin receptor |
| TrkB | Tyrosine receptor kinase B |
| IGF-1R | Insulin-like growth factor 1 receptor |
| IGF-2 | Insulin-like growth factor 2 |
| IRS-1 | Insulin receptor substrate 1 |
| IRS-2 | Insulin receptor substrate 2 |
| GABA | gamma-aminobutyric acid |
| NC | Neuro-Cells |
| CD105 | Cluster of differentiation 105 |
| CD90 | Cluster of differentiation 90 |
| CD271 | Cluster of differentiation 271 |
| CD73 | Cluster of differentiation 73 |
| CD34 | Cluster of differentiation 34 |
| ALS | Amyotrophic lateral sclerosis |
| FDTL | Frontotemporal dementia |
| FUS | Fused in sarcoma protein |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| APP | Amyloid precursor protein |
| WT | Wild-type |
| APD | Amyloid plaque density |
| Egr1 | Early growth response 1 |
| Gdf15 | Growth differentiation factor 15 |
| Sirt1 | Sirtuin 1 |
| Irs2 | Insulin receptor substrate 2 gene |
| Igf | Insulin-like growth factor gene |
| Bdnf | Brain-derived neurotrophic factor gene |
| Syp | Synaptophysin gene |
| Pgc1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha gene |
| Sqstm | Sequestosome 1 gene |
| Arc | Activity-regulated cytoskeleton-associated protein gene |
| Cldn5 | Claudin-5 gene |
| BBB | Blood–brain barrier |
| BACE-1 | β-secretase 1 |
| P62 | P62 protein (alternate name for SQSTM1) |
| C57BL/6 | C57 Black 6 strain of laboratory mouse |
| RNA | Ribonucleic acid |
| qRT-PCR | Real-time quantitative reverse transcription polymerase chain reaction |
| cDNA | Complementary DNA |
| i.c. | Intracerebral |
| DAPI | 4′,6-diamidino-2-phenylindole |
| SEM | Standard error of the mean |
References
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Orobets, K.S.; Karamyshev, A.L. Amyloid Precursor Protein and Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 14794. [Google Scholar] [CrossRef]
- Flicker, L. Antiamyloid treatment for dementia: Concerns outweigh hopes. Curr. Opin. Psychiatry 2025, 38, 355–360. [Google Scholar] [CrossRef]
- Miles, L.A.; Masters, C.L. The structural foundations of anti-amyloid-β immunotherapies: Unravelling antibody-antigen interactions in Alzheimer’s disease treatment. J. Alzheimers Dis. 2025, 30, 13872877251361049. [Google Scholar] [CrossRef] [PubMed]
- Bobbins, A.; Davies, M.; Lynn, E.; Roy, D.; Yeomans, A.; Shakir, S.A.W. Safety and effectiveness of the anti-amyloid monoclonal antibody (mAb) drug lecanemab for early Alzheimer’s disease: The pharmacovigilance perspective. Br. J. Clin. Pharmacol. 2025, 91, 1352–1360. [Google Scholar] [CrossRef]
- Jeremic, D.; Jiménez-Díaz, L.; Navarro-López, J.D. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef]
- de Munter, J.; Mey, J.; Strekalova, T.; Kramer, B.W.; Wolters, E.C. Why do anti-inflammatory signals of bone marrow-derived stromal cells improve neurodegenerative conditions where anti-inflammatory drugs fail? J. Neural Transm. 2020, 127, 715–727. [Google Scholar] [CrossRef]
- Wang, S.M.; Lee, C.U.; Lim, H.K. Stem cell therapies for Alzheimer’s disease: Is it time? Curr. Opin. Psychiatry 2019, 32, 105–116. [Google Scholar] [CrossRef]
- Mishra, P.; Silva, A.; Sharma, J.; Nguyen, J.; Pizzo, D.P.; Hinz, D.; Sahoo, D.; Cherqui, S. Rescue of Alzheimer’s disease phenotype in a mouse model by transplantation of wild-type hematopoietic stem and progenitor cells. Cell Rep. 2023, 42, 112956. [Google Scholar] [CrossRef] [PubMed]
- Baumheter, S.; Singer, M.S.; Henzel, W.; Hemmerich, S.; Renz, M.; Rosen, S.D.; Lasky, L.A. Binding of L-selectin to the vascular sialomucin CD34. Science 1993, 262, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Krizanac-Bengez, L.; Mayberg, M.R.; Janigro, D. The cerebral vasculature as a therapeutic target for neurological disorders and the role of shear stress in vascular homeostatis and pathophysiology. Neurol. Res. 2004, 26, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.K.; Lee, H.; Carter, J.E.; Chang, J.W.; Oh, W.; Yang, Y.S.; Suh, J.G.; Lee, B.H.; Jin, H.K.; et al. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol. Aging 2012, 33, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Godoy, M.; Saraiva, L.; de vasconcelos, A.; Carvalho, L.; Beiral, H.; Rocha, L.; Sinis, L.; Monteiro, V.; Leal, R.; Braga, C.; et al. Mesenchymal stem cells protect neurons against oxidative stress and synaptic failure induced by oligomers of the amyloid-b peptide (abos) through catalase release and abos clearance. Alzheimer’s Dement. 2016, 12, P225. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, H.N.; Park, H.J.; Shin, J.Y.; Lee, P.H. Mesenchymal Stem Cells Increase Hippocampal Neurogenesis and Neuronal Differentiation by Enhancing the Wnt Signaling Pathway in an Alzheimer’s Disease Model. Cell Transplant. 2015, 24, 1097–1109. [Google Scholar] [CrossRef]
- Yan, Y.; Ma, T.; Gong, K.; Ao, Q.; Zhang, X.; Gong, Y. Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer’s disease mice. Neural Regen. Res. 2014, 9, 798–805. [Google Scholar] [CrossRef]
- Cui, Y.; Ma, S.; Zhang, C.; Cao, W.; Liu, M.; Li, D.; Lv, P.; Xing, Q.; Qu, R.; Yao, N.; et al. Human umbilical cord mesenchymal stem cells transplantation improves cognitive function in Alzheimer’s disease mice by decreasing oxidative stress and promoting hippocampal neurogenesis. Behav. Brain Res. 2017, 320, 291–301. [Google Scholar] [CrossRef]
- Kshitiz; Ellison, D.D.; Suhail, Y.; Afzal, J.; Woo, L.; Kilic, O.; Spees, J.; Levchenko, A. Dynamic secretome of bone marrow-derived stromal cells reveals a cardioprotective biochemical cocktail. Proc. Natl. Acad. Sci. USA 2019, 116, 14374–14383. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Castro, E.; Cunningham, C.; Miller, J.; Martuscelli, L.; Aoulad-Ali, S.; Rothwell, N.J.; Kielty, C.M.; Allan, S.M.; Pinteaux, E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res. Ther. 2017, 8, 79. [Google Scholar] [CrossRef]
- Mufson, E.J.; Kroin, J.S.; Sendera, T.J.; Sobreviela, T. Distribution and retrograde transport of trophic factors in the central nervous system: Functional implications for the treatment of neurodegenerative diseases. Progress. Neurobiol. 1999, 57, 451–484. [Google Scholar] [CrossRef]
- Mackay, K.B.; Loddick, S.A.; Naeve, G.S.; Vana, A.M.; Verge, G.M.; Foster, A.C. Neuroprotective effects of insulin-like growth factor-binding protein ligand inhibitors in vitro and in vivo. J. Cereb. Blood Flow Metab. 2003, 23, 1160–1167. [Google Scholar] [CrossRef]
- Tornqvist, H.E.; Pierce, M.W.; Frackelton, A.R.; Nemenoff, R.A.; Avruch, J. Identification of insulin receptor tyrosine residues autophosphorylated in vitro. J. Biol. Chem. 1987, 262, 10212–10219. [Google Scholar] [CrossRef] [PubMed]
- Pomytkin, I.; Costa-Nunes, J.P.; Kasatkin, V.; Veniaminova, E.; Demchenko, A.; Lyundup, A.; Lesch, K.P.; Ponomarev, E.D.; Strekalova, T. Insulin receptor in the brain: Mechanisms of activation and the role in the CNS pathology and treatment. CNS Neurosci. Ther. 2018, 24, 763–774. [Google Scholar] [CrossRef]
- van der Heide, L.P.; Ramakers, G.M.; Smidt, M.P. Insulin signaling in the central nervous system: Learning to survive. Progress. Neurobiol. 2006, 79, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.; Terry, B.M.; Rivera, E.J.; Cannon, J.L.; Neely, T.R.; Tavares, R.; Xu, X.J.; Wands, J.R.; de la Monte, S.M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—Is this type 3 diabetes? J. Alzheimer’s Dis. 2005, 7, 63–80. [Google Scholar] [CrossRef]
- Neth, B.J.; Craft, S. Insulin Resistance and Alzheimer’s Disease: Bioenergetic Linkages. Front. Aging Neurosci. 2017, 9, 345. [Google Scholar] [CrossRef]
- Bessman, S.P.; Mohan, C.; Zaidise, I. Intracellular site of insulin action: Mitochondrial Krebs cycle. Proc. Natl. Acad. Sci. USA 1986, 83, 5067–5070. [Google Scholar] [CrossRef]
- Dorn, A.; Rinne, A.; Bernstein, H.G.; Hahn, H.J.; Ziegler, M. Insulin and C-peptide in human brain neurons (insulin/C-peptide/brain peptides/immunohistochemistry/radioimmunoassay). J. Hirnforsch. 1983, 24, 495–499. [Google Scholar]
- Takano, K.; Koarashi, K.; Kawabe, K.; Itakura, M.; Nakajima, H.; Moriyama, M.; Nakamura, Y. Insulin expression in cultured astrocytes and the decrease by amyloid β. Neurochem. Int. 2018, 119, 171–177. [Google Scholar] [CrossRef]
- Clarke, D.W.; Mudd, L.; Boyd, F.T., Jr.; Fields, M.; Raizada, M.K. Insulin is released from rat brain neuronal cells in culture. J. Neurochem. 1986, 47, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, T.; Kagalwala, M.N.; Onuma, Y.; Ito, Y.; Warashina, M.; Terashima, K.; Sanosaka, T.; Nakashima, K.; Gage, F.H.; Asashima, M. Insulin biosynthesis in neuronal progenitors derived from adult hippocampus and the olfactory bulb. EMBO Mol. Med. 2011, 3, 742–754. [Google Scholar] [CrossRef]
- Molnár, G.; Faragó, N.; Kocsis, Á.K.; Rózsa, M.; Lovas, S.; Boldog, E.; Báldi, R.; Csajbók, É.; Gardi, J.; Puskás, L.G.; et al. GABAergic neurogliaform cells represent local sources of insulin in the cerebral cortex. J. Neurosci. 2014, 34, 1133–1137. [Google Scholar] [CrossRef]
- Munter, J.P.; Beugels, J.; Munter, S.; Jansen, L.; Cillero-Pastor, B.; Moskvin, O.; Brook, G.; Pavlov, D.; Strekalova, T.; Kramer, B.W.; et al. Standardized human bone marrow-derived stem cells infusion improves survival and recovery in a rat model of spinal cord injury. J. Neurol. Sci. 2019, 402, 16–29. [Google Scholar] [CrossRef]
- de Munter, J.; Shafarevich, I.; Liundup, A.; Pavlov, D.; Wolters, E.C.; Gorlova, A.; Veniaminova, E.; Umriukhin, A.; Kalueff, A.; Svistunov, A.; et al. Neuro-Cells therapy improves motor outcomes and suppresses inflammation during experimental syndrome of amyotrophic lateral sclerosis in mice. CNS Neurosci. Ther. 2020, 26, 504–517. [Google Scholar] [CrossRef]
- Sweeney, G.; Song, J. The association between PGC-1α and Alzheimer’s disease. Anat. Cell Biol. 2016, 49, 1–6. [Google Scholar] [CrossRef]
- de Munter, J.; Babaevskaya, D.; Wolters, E.C.; Pavlov, D.; Lysikova, E.; Kalueff, A.V.; Gorlova, A.; Oplatchikova, M.; Pomytkin, I.A.; Proshin, A.; et al. Molecular and behavioural abnormalities in the FUS-tg mice mimic frontotemporal lobar degeneration: Effects of old and new anti-inflammatory therapies. J. Cell. Mol. Med. 2020, 17, 10251–10257. [Google Scholar] [CrossRef]
- Jęśko, H.; Wencel, P.; Strosznajder, R.P.; Strosznajder, J.B. Sirtuins and Their Roles in Brain Aging and Neurodegenerative Disorders. Neurochem. Res. 2017, 42, 876–890. [Google Scholar] [CrossRef]
- Chiariello, A.; Valente, S.; Pasquinelli, G.; Baracca, A.; Sgarbi, G.; Solaini, G.; Medici, V.; Fantini, V.; Poloni, T.E.; Tognocchi, M.; et al. The expression pattern of GDF15 in human brain changes during aging and in Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1058665. [Google Scholar] [CrossRef] [PubMed]
- Sze, C.I.; Troncoso, J.C.; Kawas, C.; Mouton, P.; Price, D.L.; Martin, L.J. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1997, 56, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Bozon, B.; Davis, S.; Laroche, S. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron 2003, 40, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wang, Y.; Paudel, H.K. Early Growth Response 1 (Egr-1) Is a Transcriptional Activator of β-Secretase 1 (BACE-1) in the Brain. J. Biol. Chem. 2016, 291, 22276–22287. [Google Scholar] [CrossRef]
- Greene, C.; Hanley, N.; Campbell, M. Claudin-5: Gatekeeper of neurological function. Fluids Barriers CNS 2019, 16, 3. [Google Scholar] [CrossRef]
- Jackson, K.; Barisone, G.A.; Diaz, E.; Jin, L.W.; DeCarli, C.; Despa, F. Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann. Neurol. 2013, 74, 517–526. [Google Scholar] [CrossRef]
- Kilgore, M.; Miller, C.A.; Fass, D.M.; Hennig, K.M.; Haggarty, S.J.; Sweatt, J.D.; Rumbaugh, G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2010, 35, 870–880. [Google Scholar] [CrossRef]
- Onos, K.D.; Uyar, A.; Keezer, K.J.; Jackson, H.M.; Preuss, C.; Acklin, C.J.; O’Rourke, R.; Buchanan, R.; Cossette, T.L.; Sukoff Rizzo, S.J.; et al. Enhancing face validity of mouse models of Alzheimer’s disease with natural genetic variation. PLoS Genet. 2019, 15, e1008155. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.; Kirby, A.; Niu, J.; VanderHorst, V. Stereotaxic Surgical Approach to Microinject the Caudal Brainstem and Upper Cervical Spinal Cord via the Cisterna Magna in Mice. J. Vis. Exp. 2022. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B.J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, 4th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Schroeter, C.A.; Gorlova, A.; Sicker, M.; Umriukhin, A.; Burova, A.; Shulgin, B.; Morozov, S.; Costa-Nunes, J.P.; Strekalova, T. Unveiling the Mechanisms of a Remission in Major Depressive Disorder (MDD)-like Syndrome: The Role of Hippocampal Palmitoyltransferase Expression and Stress Susceptibility. Biomolecules 2025, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Anthony, D.C.; Dolgov, O.; Anokhin, K.; Kubatiev, A.; Steinbusch, H.M.; Schroeter, C. The differential effects of chronic imipramine or citalopram administration on physiological and behavioral outcomes in naïve mice. Behav. Brain Res. 2013, 245, 101–106. [Google Scholar] [CrossRef]
- Vignisse, J.; Steinbusch, H.W.; Grigoriev, V.; Bolkunov, A.; Proshin, A.; Bettendorff, L.; Bachurin, S.; Strekalova, T. Concomitant manipulation of murine NMDA- and AMPA-receptors to produce pro-cognitive drug effects in mice. Eur. Neuropsychopharmacol. 2014, 24, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Bahzenova, N.; Trofimov, A.; Schmitt-Böhrer, A.G.; Markova, N.; Grigoriev, V.; Zamoyski, V.; Serkova, T.; Redkozubova, O.; Vinogradova, D.; et al. Pro-neurogenic, Memory-Enhancing and Anti-stress Effects of DF302, a Novel Fluorine Gamma-Carboline Derivative with Multi-target Mechanism of Action. Mol. Neurobiol. 2018, 55, 335–349. [Google Scholar] [CrossRef]
- Vignisse, J.; Sambon, M.; Gorlova, A.; Pavlov, D.; Caron, N.; Malgrange, B.; Shevtsova, E.; Svistunov, A.; Anthony, D.C.; Markova, N.; et al. Thiamine and benfotiamine prevent stress-induced suppression of hippocampal neurogenesis in mice exposed to predation without affecting brain thiamine diphosphate levels. Mol. Cell. Neurosci. 2017, 82, 126–136. [Google Scholar] [CrossRef]
- Couch, Y.; Anthony, D.C.; Dolgov, O.; Revischin, A.; Festoff, B.; Santos, A.I.; Steinbusch, H.W.; Strekalova, T. Microglial activation, increased TNF and SERT expression in the prefrontal cortex define stress-altered behaviour in mice susceptible to anhedonia. Brain Behav. Immun. 2013, 29, 136–146. [Google Scholar] [CrossRef]
- Strekalova, T.; Pavlov, D.; Trofimov, A.; Anthony, D.C.; Svistunov, A.; Proshin, A.; Umriukhin, A.; Lyundup, A.; Lesch, K.P.; Cespuglio, R. Hippocampal Over-Expression of Cyclooxygenase-2 (COX-2) Is Associated with Susceptibility to Stress-Induced Anhedonia in Mice. Int. J. Mol. Sci. 2022, 23, 2061. [Google Scholar] [CrossRef]
- Aleksandrova, Y.; Semakov, A.; Tsypyshev, D.; Chaprov, K.; Klochkov, S.; Neganova, M. Neuroprotective Effects and Cognitive Enhancement of Allomargaritarine in 5xFAD Alzheimer’s Disease Mice Model. OBM Neurobiol. 2024, 08, 1–33. [Google Scholar] [CrossRef]
- Lysikova, E.A.; Kuzubova, E.V.; Radchenko, A.I.; Patrakhanov, E.A.; Chaprov, K.D.; Korokin, M.V.; Deykin, A.V.; Gudyrev, O.S.; Pokrovskii, M.V. [APPswe/PS1dE9/Blg Transgenic Mouse Line for Modeling Cerebral Amyloid Angiopathy Associated with Alzheimer’s Disease]. Mol. Biol. 2023, 57, 85–94. [Google Scholar] [CrossRef]
- Humphries, M.P.; Maxwell, P.; Salto-Tellez, M. QuPath: The global impact of an open source digital pathology system. Comput. Struct. Biotechnol. J. 2021, 19, 852–859. [Google Scholar] [CrossRef]
- Lee, A.; Jiang, Z.; Lee, L.; Ladiges, W. QuPath. A new digital imaging tool for geropathology. Aging Pathobiol. Ther. 2020, 2, 114–116. [Google Scholar] [CrossRef]
- Gopal Ramaswamy, V.; Ahirwar, M.; Ryan, G.; Dugger, B.N.; Al Dalahmah, O.; Signaevsky, M.; Purohit, D.P.; Haroutunian, V.; Finkbeiner, S. Generalizable Prediction of Alzheimer Disease Pathologies with a Scalable Annotation Tool and an High-Accuracy Model. medRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Mondal, R.; Sandhu, Y.K.; Kamalia, V.M.; Delaney, B.A.; Syed, A.U.; Nguyen, G.A.H.; Moran, T.R.; Limpengco, R.R.; Liang, C.; Mukherjee, J. Measurement of Aβ Amyloid Plaques and Tau Protein in Postmortem Human Alzheimer’s Disease Brain by Autoradiography Using [18F]Flotaza, [125I]IBETA, [124/125I]IPPI and Immunohistochemistry Analysis Using QuPath. Biomedicines 2023, 11, 1033. [Google Scholar] [CrossRef]
- Qin, C.; Lu, Y.; Wang, K.; Bai, L.; Shi, G.; Huang, Y.; Li, Y. Transplantation of bone marrow mesenchymal stem cells improves cognitive deficits and alleviates neuropathology in animal models of Alzheimer’s disease: A meta-analytic review on potential mechanisms. Transl. Neurodegener. 2020, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Brody, M.; Agronin, M.; Herskowitz, B.J.; Bookheimer, S.Y.; Small, G.W.; Hitchinson, B.; Ramdas, K.; Wishard, T.; McInerney, K.F.; Vellas, B.; et al. Results and insights from a phase I clinical trial of Lomecel-B for Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, 261–273. [Google Scholar] [CrossRef]
- Skok, M. Mesenchymal stem cells as a potential therapeutic tool to cure cognitive impairment caused by neuroinflammation. World J. Stem Cells 2021, 13, 1072–1083. [Google Scholar] [CrossRef]
- Park, B.N.; Kim, J.H.; Lim, T.S.; Park, S.H.; Kim, T.G.; Yoon, B.S.; Son, K.S.; Yoon, J.K.; An, Y.S. Therapeutic effect of mesenchymal stem cells in an animal model of Alzheimer’s disease evaluated by β-amyloid positron emission tomography imaging. Aust. N. Z. J. Psychiatry 2020, 54, 883–891. [Google Scholar] [CrossRef]
- de Godoy, M.A.; Saraiva, L.M.; de Carvalho, L.R.P.; Vasconcelos-Dos-Santos, A.; Beiral, H.J.V.; Ramos, A.B.; Silva, L.R.P.; Leal, R.B.; Monteiro, V.H.S.; Braga, C.V.; et al. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. J. Biol. Chem. 2018, 293, 1957–1975. [Google Scholar] [CrossRef]
- Russell, C.L.; Semerdjieva, S.; Empson, R.M.; Austen, B.M.; Beesley, P.W.; Alifragis, P. Amyloid-β acts as a regulator of neurotransmitter release disrupting the interaction between synaptophysin and VAMP2. PLoS ONE 2012, 7, e43201. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. AMS 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Frittitta, L. Type 2 Diabetes Mellitus and Alzheimer’s Disease: Role of Insulin Signalling and Therapeutic Implications. Int. J. Mol. Sci. 2018, 19, 3306. [Google Scholar] [CrossRef]
- Akhtar, A.; Sah, S.P. Insulin signaling pathway and related molecules: Role in neurodegeneration and Alzheimer’s disease. Neurochem. Int. 2020, 135, 104707. [Google Scholar] [CrossRef]
- Hu, Y.-T.; Chen, X.-L.; Huang, S.-H.; Zhu, Q.-B.; Yu, S.-Y.; Shen, Y.; Sluiter, A.; Verhaagen, J.; Zhao, J.; Swaab, D.; et al. Early growth response-1 regulates acetylcholinesterase and its relation with the course of Alzheimer’s disease. Brain Pathol. 2019, 29, 502–512. [Google Scholar] [CrossRef]
- Lu, T.T.; Wan, C.; Yang, W.; Cai, Z. Role of Cdk5 in Amyloid-beta Pathology of Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 1206–1215. [Google Scholar] [CrossRef]
- Wu, J.; Petralia, R.S.; Kurushima, H.; Patel, H.; Jung, M.Y.; Volk, L.; Chowdhury, S.; Shepherd, J.D.; Dehoff, M.; Li, Y.; et al. Arc/Arg3.1 regulates an endosomal pathway essential for activity-dependent β-amyloid generation. Cell 2011, 147, 615–628. [Google Scholar] [CrossRef]
- VanDongen, A. Arc: A therapeutic hub for Alzheimer’s disease. bioRxiv 2025. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Li, Q.; Xing, S.; Liu, Y.; Liu, Y.; Chen, Y.; Liu, W.; Feng, F.; Sun, H. p62/SQSTM1, a Central but Unexploited Target: Advances in Its Physiological/Pathogenic Functions and Small Molecular Modulators. J. Med. Chem. 2020, 63, 10135–10157. [Google Scholar] [CrossRef]
- Frölich, L.; Blum-Degen, D.; Bernstein, H.G.; Engelsberger, S.; Humrich, J.; Laufer, S.; Muschner, D.; Thalheimer, A.; Türk, A.; Hoyer, S.; et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J. Neural Transm. 1998, 105, 423–438. [Google Scholar] [CrossRef]
- Craft, S.; Peskind, E.; Schwartz, M.W.; Schellenberg, G.D.; Raskind, M.; Porte, D., Jr. Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: Relationship to severity of dementia and apolipoprotein E genotype. Neurology 1998, 50, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.; Wilcox, K.C.; Tortelli, V.; Diniz, L.P.; Oliveira, M.S.; Dobbins, C.; Yu, X.-W.; Nandamuri, S.; Gomes, F.C.A.; DiNunno, N.; et al. Neuroprotective astrocyte-derived insulin/insulin-like growth factor 1 stimulates endocytic processing and extracellular release of neuron-bound Aβ oligomers. Mol. Biol. Cell 2017, 28, 2623–2636. [Google Scholar] [CrossRef]
- Ingelsson, M.; Fukumoto, H.; Newell, K.L.; Growdon, J.H.; Hedley-Whyte, E.T.; Frosch, M.P.; Albert, M.S.; Hyman, B.T.; Irizarry, M.C. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 2004, 62, 925–931. [Google Scholar] [CrossRef]
- Turgeman, G. The therapeutic potential of mesenchymal stem cells in Alzheimer’s disease: Converging mechanisms. Neural Regen. Res. 2015, 10, 698–699. [Google Scholar] [CrossRef]
- Giannakopoulos, P.; Herrmann, F.R.; Bussière, T.; Bouras, C.; Kövari, E.; Perl, D.P.; Morrison, J.H.; Gold, G.; Hof, P.R. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 2003, 60, 1495–1500. [Google Scholar] [CrossRef]
- Torres, A.; Jara, C.; Park-Kang, H.; Polanco, C.; Tapia, D.; Alarcón, F.; De La Peña, A.; Llanquinao, J.; Vargas-Mardones, G.; Indo, J.; et al. Synaptic Mitochondria: An Early Target of Amyloid-β and Tau in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 84, 1391–1414. [Google Scholar] [CrossRef]
- Dewachter, I.; Reversé, D.; Caluwaerts, N.; Ris, L.; Kuipéri, C.; Van den Haute, C.; Spittaels, K.; Umans, L.; Serneels, L.; Thiry, E.; et al. Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein [V717I] transgenic mice. J. Neurosci. 2002, 22, 3445–3453. [Google Scholar] [CrossRef]
- Ge, M.; Zhang, Y.; Hao, Q.; Zhao, Y.; Dong, B. Effects of mesenchymal stem cells transplantation on cognitive deficits in animal models of Alzheimer’s disease: A systematic review and meta-analysis. Brain Behav. 2018, 8, e00982. [Google Scholar] [CrossRef]
- Gabbouj, S.; Ryhänen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskainen, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered Insulin Signaling in Alzheimer’s Disease Brain—Special Emphasis on PI3K-Akt Pathway. Front. Neurosci. 2019, 18, 629. [Google Scholar] [CrossRef]
- Tanokashira, D.; Fukuokaya, W.; Taguchi, A. Involvement of insulin receptor substrates in cognitive impairment and Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1330–1334, Erratum in Neural Regen. Res. 2020, 15, 1165. https://doi.org/10.4103/1673-5374.270163. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, W.; Chen, K.L.; Shen, X.N.; Tan, L.; Yu, J.T. Alzheimer’s Disease Neuroimaging Initiative. Mild behavioral impairment correlates of cognitive impairments in older adults without dementia: Mediation by amyloid pathology. Transl. Psychiatry 2021, 11, 577. [Google Scholar] [CrossRef]
- Cohen, S.J.; Stackman, R.W., Jr. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 2015, 285, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Ettcheto, M.; Petrov, D.; Abad, S.; Pedrós, I.; Marin, M.; Olloquequi, J.; Camins, A. Review of the advances in treatment for Alzheimer disease: Strategies for combating β-amyloid protein. Neurologia 2018, 33, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Moskalenko, A.M.; Ikrin, A.N.; Kozlova, A.V.; Mukhamadeev, R.R.; de Abreu, M.S.; Riga, V.; Kolesnikova, T.O.; Kalueff, A.V. Decoding Molecular Bases of Rodent Social Hetero-Grooming Behavior Using in Silico Analyses and Bioinformatics Tools. Neuroscience 2024, 554, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Freude, S.; Leeser, U.; Müller, M.; Hettich, M.M.; Udelhoven, M.; Schilbach, K.; Tobe, K.; Kadowaki, T.; Köhler, C.; Schröder, H.; et al. IRS-2 branch of IGF-1 receptor signaling is essential for appropriate timing of myelination. J. Neurochem. 2008, 107, 907–917. [Google Scholar] [CrossRef]
- Mansoor, S.; Jindal, A.; Badu, N.Y.A.; Katiki, C.; Ponnapalli, V.J.S.; Desai, K.J.; Nassar, S.T. Role of Neurotrophins in the Development and Treatment of Neurodegenerative Diseases: A Systematic Review. Cureus 2024, 16, e74048. [Google Scholar] [CrossRef]


| Gene | Relative Normalised Expression | ||
|---|---|---|---|
| WT | APP | APP NC | |
| Insr | 1.02 ± 0.08 | ↑1.80 ± 0.27 * | ↑1.44 ± 0.15 |
| Igf1r | 1.78 ± 0.06 | ↑1.44 ± 0.08 * | ↓1.08 ± 0.13 |
| Igf1 | 1.17 ± 0.18 | ↑1.53 ± 0.15 | ↓0.93 ± 0.13 ● |
| Irs2 | 1.05 ± 0.15 | 1.12 ± 0.31 | ↑2.08 ± 0.33 * |
| Bdnf | 1.02 ± 0.10 | ↓0.41 ± 0.09 * | ↑1.32 ± 0.23 ● |
| Syp | 1.03 ± 0.11 | ↑1.49 ± 0.08 * | ↓1.10 ± 0.13 |
| Pcg | 1.04 ± 0.14 | ↓0.74 ± 0.08 | ↑1.20 ± 0.11 ● |
| Sirt1 | 0.80 ± 0.09 | ↑1.84 ± 0.51 * | ↓0.79 ± 0.11 ● |
| Gdf15 | 1.16 ± 0.29 | ↑3.46 ± 1.21 * | ↓1.45 ± 0.23 ● |
| Cldn5 | 1.02 ± 0.09 | ↑2.67 ± 0.84 * | ↓1.29 ± 0.16 ● |
| Egr1 | 1.11 ± 0.19 | ↑2.53 ± 0.24 * | ↓0.70 ± 0.07 ● |
| Arc | 1.04 ± 0.13 | ↑3.48 ± 0.86 * | ↓0.97 ± 0.15 ● |
| Sqstm1 | 1.04 ± 0.13 | ↑1.47 ± 0.38 | ↓0.96 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Munter, J.P.J.M.; Tsoy, A.; Sitdikova, K.; Wolters, E.C.; Chaprov, K.; Yenkoyan, K.B.; Torosyan, H.; Askarova, S.; Anthony, D.C.; Strekalova, T. Therapeutic Effects of Neuro-Cells on Amyloid Pathology, BDNF Levels, and Insulin Signalling in APPswe/PSd1E9 Mice. Cells 2025, 14, 1293. https://doi.org/10.3390/cells14161293
de Munter JPJM, Tsoy A, Sitdikova K, Wolters EC, Chaprov K, Yenkoyan KB, Torosyan H, Askarova S, Anthony DC, Strekalova T. Therapeutic Effects of Neuro-Cells on Amyloid Pathology, BDNF Levels, and Insulin Signalling in APPswe/PSd1E9 Mice. Cells. 2025; 14(16):1293. https://doi.org/10.3390/cells14161293
Chicago/Turabian Stylede Munter, Johannes P. J. M., Andrey Tsoy, Kseniia Sitdikova, Erik Ch. Wolters, Kirill Chaprov, Konstantin B. Yenkoyan, Hamlet Torosyan, Sholpan Askarova, Daniel C. Anthony, and Tatyana Strekalova. 2025. "Therapeutic Effects of Neuro-Cells on Amyloid Pathology, BDNF Levels, and Insulin Signalling in APPswe/PSd1E9 Mice" Cells 14, no. 16: 1293. https://doi.org/10.3390/cells14161293
APA Stylede Munter, J. P. J. M., Tsoy, A., Sitdikova, K., Wolters, E. C., Chaprov, K., Yenkoyan, K. B., Torosyan, H., Askarova, S., Anthony, D. C., & Strekalova, T. (2025). Therapeutic Effects of Neuro-Cells on Amyloid Pathology, BDNF Levels, and Insulin Signalling in APPswe/PSd1E9 Mice. Cells, 14(16), 1293. https://doi.org/10.3390/cells14161293







