The Roles of Non-Coding RNAs in the Pathogenesis of Uterine Fibroids
Abstract
1. Introduction
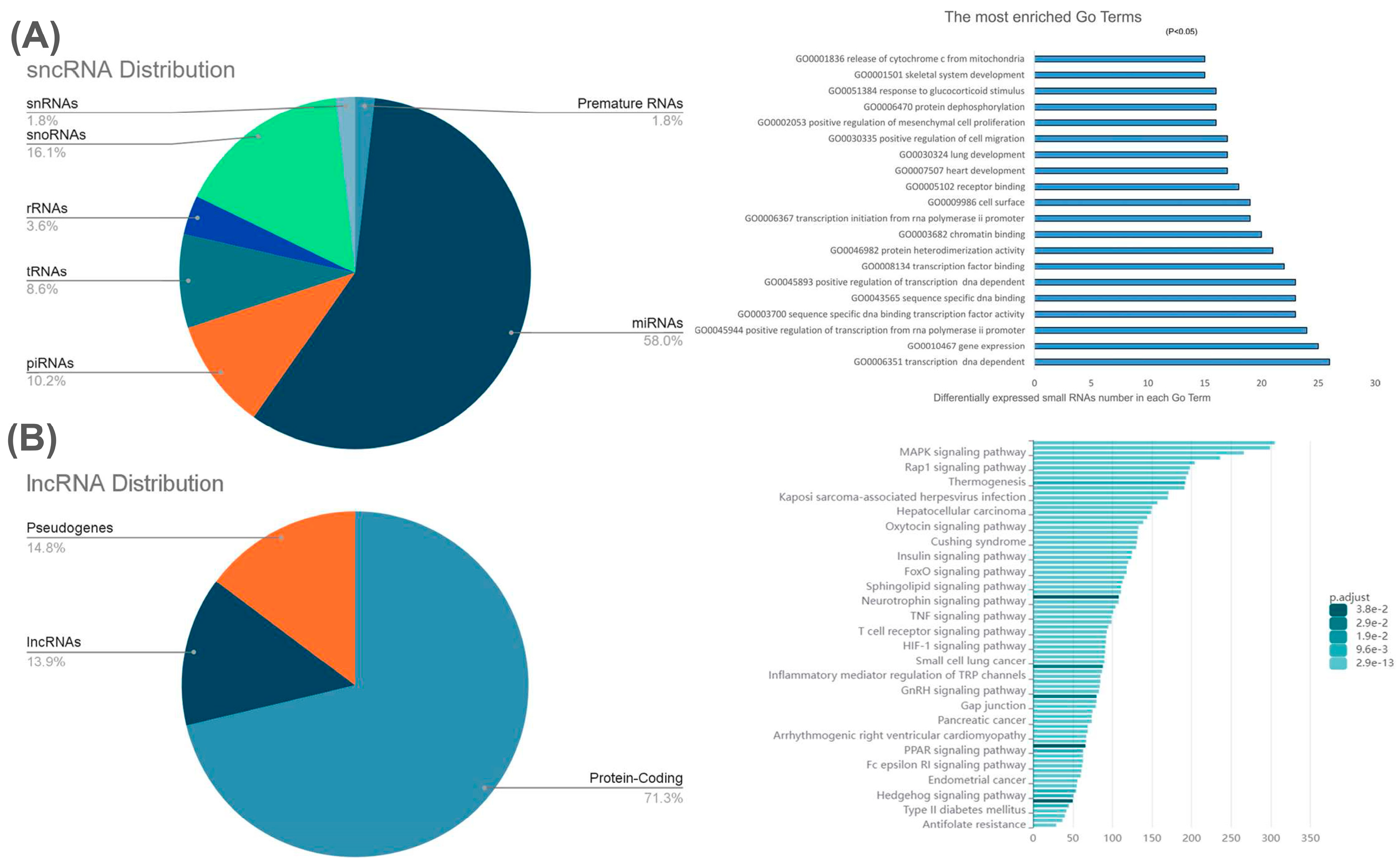
2. Non-Coding RNAs (ncRNAs)
- Long non-coding RNAs (lncRNAs): These molecules are greater than 200 nucleotides in length and share many structural features with messenger RNAs (mRNAs), including a 5′ cap and poly(A) tail [38,39,40]. LncRNAs can be classified as ‘intergenic’, ‘intronic’, ‘sense’, or ‘antisense’, depending upon which region of the genome they are transcribed from [38,39,40], and are involved in various regulatory functions, such as chromatin remodeling, gene silencing, and cellular differentiation [38,39,40].
- ○
- ○
- Enhancer RNAs (eRNAs): Enhancer RNAs (eRNAs) are typically considered to be relatively short, ranging from around 50 to 2000 nucleotides in length, with the majority falling within the range of a few hundred nucleotides (around 350 nucleotides) [25,44,45,46]. eRNAs are produced by the bidirectional transcription of enhancer regions in the genome and are identified using the epigenetic modifications H3K27ac, H3K4me1, and H3K27me3 [44,47]; however, there also exists a class of long non-coding eRNAs (elncRNAs) that are unidirectionally transcribed, polyadenylated, and more stable when compared to eRNAs [44].
- ○
- Super enhancer (SE-lncRNAs): A class of lncRNAs that are transcribed from “super-enhancer” (SE) regions of the genome. When compared to typical enhancers, SEs have a higher density of transcription factors, mediator coactivators, and epigenetic modifications [48,49]. Dysregulation in SE-lncRNAs has also been implicated in oncogenesis, specifically through their regulation of the oncogene MYC [49] and pathways such as TGF-β, MEK/ERK, and Wnt [50,51,52,53].
- ○
- Circular RNAs (circRNAs): A class of single-stranded, covalently closed ncRNAs that are master regulators of gene expression [54,55,56,57]. Regulation of gene expression is accomplished through a variety of mechanisms, such as sponging miRNAs and serving as a protein scaffold [54,55,56,57]. Recent studies implicate circRNAs in tumor development, making them a possible therapeutic target [54,55,56,57].
- Small non-coding RNAs (sncRNAs):
- ○
- ○
- ○
- Housekeeping sncRNAs:
3. ncRNA Mechanisms of Action
3.1. miRNAs
3.2. lncRNAs
4. ncRNAs in Uterine Fibroids
4.1. miRNAs
4.1.1. miR-21a-5p
4.1.2. miR-29 Family
4.1.3. miR-200c
4.1.4. miR-93
4.1.5. hsa-let-7 Family
4.1.6. miR-197
4.1.7. miR-199a-5p
4.1.8. miR-139-5p
4.1.9. miR-150-5p
4.2. lncRNAs
4.2.1. H19
4.2.2. MIAT
4.2.3. XIST
4.2.4. LINCMD1
4.2.5. lnc-AL445665.1-4
| miRNA | Function | Expression | Location | Reference(s) |
|---|---|---|---|---|
| miR-181a-5p | * Positively associated with cellular proliferation, ECM turnover, angiogenesis, and TGFBR2/IGF2BP1 expression * Upregulation induces cellular senescence and represses AKT3 in spheroid cultures. | Upregulated | chr1:198,859,044-198,859,153 | [83] |
| miR-127-3p miR-28-3p miR-30b-5p | * Positively associated with cellular proliferation, ECM turnover, angiogenesis, and TGFBR2/IGF2BP1 expression | Upregulated | chr14:100,882,979-100,883,075 chr3:188,688,781-188,688,866 chr8:134,800,520-134,800,607 | [83] |
| let-7c | * Positively associated with cellular proliferation, ECM turnover, angiogenesis, and TGFBR2/IGF2BP1 expression * Luciferase assay shows HMGA2 as a target * Overexpression associated with reduced expression of HMGA2 * Fibroids with higher proportions of truncated binding sites for let7 in HMGA2 had higher HMGA2 expression * Inversely correlated with Ki67 | Upregulated | chr21:16,539,828-16,539,911 | [17,35,120,121,122] |
| let-7a | * Luciferase assay shows HMGA2 as a target * Overexpression associated with reduced expression of HMGA2 * Fibroids with higher proportions of truncated binding sites for let7 in HMGA2 had higher HMGA2 expression | Downregulated | chr9:94,175,957-94,176,036 | [17,35,120,121,122] |
| miR-29a | * Overexpression results in downregulation of fibrillar collagens * Inhibition promotes expression of ECM remodeling genes | Downregulated | chr7:130,876,747-130,876,810 | [67,92,99,100,101] |
| miR-29b | * Overexpression nduces cellular senescence in spheroid cultures. * Overexpression results in downregulation of fibrillar collagens (COL1A1, COL2A1, COL3A1) * Inhibition promotes expression of ECM remodeling genes | Downregulated | chr7:130,877,459-130,877,539 | [67,92,99,100,101] |
| miR-29c | * Targets cell cycle regulatory protein CDK2. * Inverse relationship with TGF-B3. * Luciferase assay confirms TGF-B3 as a target * Overexpression results in downregulation of fibrillar collagens (COL1A1, COL2A1, COL3A1) * Inhibition promotes expression of ECM remodeling genes * Overexpression inhibits protein/mRNA expression of COL3A1 and DNMT3A, secreted COL3A1, and rate of cell proliferation. * Knockdown of p65 induced expression. * Treatment with Tranilast decreased expression | Downregulated | chr1:207,801,852-207,801,939 | [67,92,99,100,101] |
| miR-200c | * Race-dependent, with lower expression in fibroids of Black patients when compared to White * Induced cellular senescence in spheroid cultures when overexpressed * Luciferase assay shows CDK2, CCND1, and E2F1 as targets * Overexpression downregulated mRNA and protein expression of CDK2 * Upregulation repressed ZEB1/2, Vimentin, and fibroid cell proliferation and increased E-cadherin. * Microarray assay confirmed TIMP2, FBLN5, and VEGFA as targets for miR-200c. * Overexpression resulted in decreased IKBKB phosphorylation and p65 transcriptional activity at the IL8 promoter while increasing caspase-3/7 activity. | Downregulated | chr12:6,963,699-6,963,766 | [33,36,94,111,112,113] |
| miR-93 | * Luciferase assay shows CDK2, CCND1, and E2F1 as targets * Overexpression downregulated mRNA and protein expression of CCND1 and E2F1 * Fibroids express significantly higher levels of its host gene MCM7. * Upregulation F3, CTGF, PAI-1, and IL8 expression. | Downregulated | hr7:100,093,768-100,093,847 | [93] |
| miR-21 | * Increased expression results in upregulation of fibronectin, COL1A1, CTGF, Versican and DPT. * Knockdown results in increased expression of apoptotic markers PDCD-4 and Caspase-3. * Knockdown results in increased expression of EEF2, a marker of cell proliferation * Induction through lentiviral infection induced expression of TGF-β and MMP-2/11 | Upregulated | chr17:59,841,266-59,841,337 | [96,97] |
| miR-199a-5p | * Regulate cell proliferation and apoptosis in-vitro. * Bioinformatics showed MED12 as a potential target for miR-199a-5p. * MED12 dependent, with MED12 mutants having lower expression | Downregulated | chr19:10,817,426-10,817,496 | [130] |
| miR-139-5p | * Restored expression results in decreased ECM contractility, cell migration * Restored expression reduced protein expression of COL1A1 and phosphorylated p38 MAPK. | Downregulated | chr11:72,615,063-72,615,130 | [133] |
| miR-542-3p | * Luciferase assay shows survivin as a predicted target. * Overexpression inhibits cell proliferation through induction of G1 and G2/M cell cycle arrest. | Downregulated | chrX:134,541,341-134,541,437 | [124] |
| miR-150-5p | * After transfection of cultured cells with miR-150 mimic, expression levels of its predicted target AKT decreased while p27Kip1 levels increased. | Downregulated | chr19:49,500,785-49,500,868 | [134] |
| miR-135b | * Confirmed to be target for LINCMD1 by Luciferase Assay * After LINCMD1 knockdown, expression was induced | Downregulated | chr1:205,448,302-205,448,398 | [153] |
| miR-146b-5p | * Targeted by Lnc-AL445665.1–4 by Luciferase Assay * Silencing Lnc-AL445665.1–4 negatively regulated miR-146b | Downregulated in MUL Upregulated in SUL | chr10:102,436,512-102,436,584 | [154] |
| miR-197 | * Decreased expression associated with increased fibroid cell proliferation and induction of cell cycle arrest * Luciferase assay shows IGFBP5 as a target | Downregulated | chr1:109,598,893-109,598,967 | [35,74,127,128,129] |
4.2.6. CAR10
4.2.7. APTR
5. Super Enhancer lncRNAs (SElncRNAs)
6. circRNA
| lncRNA | Function | Expression | Aliases | Location | Reference(s) |
|---|---|---|---|---|---|
| H19 | * Promotes expression of MED12, HMGA2, and many key ECM remodeling genes * Inverse relationship with TET1. * Uses TET3-mediated epigenetic mechanism to alter gene expression * Strong predictive marker for preoperative recurrence of fibroids * Upregulation induces TGFBR2 and TSP1 expression | Upregulated | ASM BWS WT2 ASM1 D11S813E MIR675HG LINC00008 NCRNA00008 | chr11:1995130-2001710 | [142,143] |
| MIAT | * MED12-dependent, with higher expression in MED12 mutant fibroids when compared to wild-type * Luciferase assays shows miR-29 as a target * Inhibition resulted in downregulation of COL1A1, COL3A1, TGFB3 * Knockdown in fibroid xenografts resulted in reduction of tumor weight, cell proliferation, expression of cell cycle regulatory genes (CCND1, CDK2, E2F1) and increased expression of the miR-29 family * Knockdown reduced mRNA/protein expression of COL3A1, FN1, TGFB3 and total collagen protein. | Upregulated | RNCR2 GOMAFU C22orf35 LINC00066 NCRNA00066 lncRNA-MIAT | chr22:26646428-26851957 | [148,149] |
| XIST | * Expression induced by 17β-Estradiol, progesterone and their combination. * Knockdown in-vitro resulted in decreased cell proliferation and increased expression of miR-29c, miR-200c. The downstream targets of miR-29c and miR-200c were also downregulated. * Immunoprecipitation analysis shows miR-29c and miR-200c as targets * Fibroid xenografts treated with siRNA for XIST in-vivo resulted in a significant reduction of tumor weight and increased expression of miR-29c and miR-200c * Knockdown significantly reduced total collagen protein, COL3A1, FN1, CDK2, SPARC, EZH2, apoptotic marker caspase-3, and the cell proliferation marker Ki67. * Associated with hypomethylation on X chromosome | Upregulated | SXI1 swd66 DXS1089 DXS399E LINC00001 NCRNA00001 | chrX:73817775-73852753 | [150,151,152] |
| lnc-AL445665.1-4 | * Luciferase assay shows miR-146b-5p as a target * Inhibition reduced fibroid cell proliferation and miR-146b-5p expression. | Downregulated in SUL Upregulated in MUL | lnc-CBWD5-4:7 NONHSAT131696 | chr9:65218523-65219575 | [154] |
| APTR | * Overexpression induced tumor cell proliferation and colony formation both in vitro and in vivo. * Functional assays showed ERα as a target of APTR * Overexpression induced expression of Wnt pathway proteins. | Upregulated | RSBN1L-AS1 | chr7:77477984-77697345 | [155] |
| LINCMD1 | * Luciferase assay shows miR-135b as target * Knockdown increased levels of miR-135b, accumulation of β-catenine, increased expression of COL1A1, and reduced expression of APC. | Downregulated | LINC-MD1 MIR133BHG | chr6:52146814-52151425 | [153] |
| CAR10 | * Knockdown inhibited proliferation of fibroid cells in vitro and downregulated its neighboring gene ADAM12 | Upregulated | ADAM12 MCMP MLTN MLTNA MCMPMltna ADAM12-OT1 | chr22:37469068-37472724 | [138] |
| TCONS_l2_00000923 | * Upregulation associated with downregulation of PLD5 and increased tumor size * Race-dependent, with Blacks having increased expression when compared to Whites | Upregulated | - | - | [37] |
| HOTAIR | * Differential effect depending on gene polymorphisms * CTGA haplotype downregulated in fibroids, but CCGA, TCGA, TTTA, and TTGA haplotypes were upregulated | Depends on Haplotype | HOXAS HOXC-AS4 HOXC11-AS1 NCRNA00072 | chr12:53962308-53974956 | [147] |
| Hsa_circ_0056686 | * Upregulation correlates with fibroid size * Upregulated in tumor associated fibroblasts (TAFs) * TAFs transfected with Hsa_circ_0056686 shRNA were unable to proliferate and induce expression of ECM proteins * Luciferase assay confirms that it is a target of miR-515-5p * miR-515-5p overexpression in TAF media containing Hsa_circ_0056686 shRNA restored Hsa_circ_0056686's maligant behaviors | Upregulated | - | - | [159,160] |
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Q.; Ciebiera, M.; Victoria Bariani, M.; Ali, M.; Elkafas, H.; Boyer, T.G.; Al-Hendy, A. Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment. Endocr. Rev. 2021, 43, 678–719. [Google Scholar] [CrossRef] [PubMed]
- Centini, G.; Cannoni, A.; Ginetti, A.; Colombi, I.; Giorgi, M.; Schettini, G.; Martire, F.G.; Lazzeri, L.; Zupi, E. Tailoring the Diagnostic Pathway for Medical and Surgical Treatment of Uterine Fibroids: A Narrative Review. Diagnostics 2024, 14, 2046. [Google Scholar] [CrossRef] [PubMed]
- Micić, J.; Macura, M.; Andjić, M.; Ivanović, K.; Dotlić, J.; Micić, D.D.; Arsenijević, V.; Stojnić, J.; Bila, J.; Babić, S.; et al. Currently Available Treatment Modalities for Uterine Fibroids. Medicina 2024, 60, 868. [Google Scholar] [CrossRef]
- Krzyżanowski, J.; Paszkowski, T.; Szkodziak, P.; Woźniak, S. Advancements and Emerging Therapies in the Medical Management of Uterine Fibroids: A Comprehensive Scoping Review. Med. Sci. Monit. 2024, 30, e943614. [Google Scholar] [CrossRef]
- Ramaiyer, M.S.; Saad, E.; Kurt, I.; Borahay, M.A. Genetic Mechanisms Driving Uterine Leiomyoma Pathobiology, Epidemiology, and Treatment. Genes 2024, 15, 558. [Google Scholar] [CrossRef]
- Ishikawa, H.; Goto, Y.; Hirooka, C.; Katayama, E.; Baba, N.; Kaneko, M.; Saito, Y.; Kobayashi, T.; Koga, K. Role of inflammation and immune response in the pathogenesis of uterine fibroids: Including their negative impact on reproductive outcomes. J. Reprod. Immunol. 2024, 165, 104317. [Google Scholar] [CrossRef]
- Wong, J.Y.; Gold, E.B.; Johnson, W.O.; Lee, J.S. Circulating Sex Hormones and Risk of Uterine Fibroids: Study of Women’s Health Across the Nation (SWAN). J. Clin. Endocrinol. Metab. 2016, 101, 123–130. [Google Scholar] [CrossRef]
- Ali, M.; Ciebiera, M.; Vafaei, S.; Alkhrait, S.; Chen, H.Y.; Chiang, Y.F.; Huang, K.C.; Feduniw, S.; Hsia, S.M.; Al-Hendy, A. Progesterone Signaling and Uterine Fibroid Pathogenesis; Molecular Mechanisms and Potential Therapeutics. Cells 2023, 12, 1117. [Google Scholar] [CrossRef]
- Yang, Q.; Al-Hendy, A. Update on the Role and Regulatory Mechanism of Extracellular Matrix in the Pathogenesis of Uterine Fibroids. Int. J. Mol. Sci. 2023, 24, 5778. [Google Scholar] [CrossRef]
- Koltsova, A.S.; Efimova, O.A.; Pendina, A.A. A View on Uterine Leiomyoma Genesis through the Prism of Genetic, Epigenetic and Cellular Heterogeneity. Int. J. Mol. Sci. 2023, 24, 5752. [Google Scholar] [CrossRef]
- Wang, H.; Shen, Q.; Ye, L.H.; Ye, J. MED12 mutations in human diseases. Protein Cell 2013, 4, 643–646. [Google Scholar] [CrossRef]
- Amendola, I.L.S.; Spann, M.; Segars, J.; Singh, B. The Mediator Complex Subunit 12 (MED-12) Gene and Uterine Fibroids: A Systematic Review. Reprod. Sci. 2024, 31, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Jain, P.; Chatterjee, S.; Rani, A.; Tripathi, A.; Dubey, P.K. Variants in exon 2 of MED12 gene causes uterine leiomyoma’s through over-expression of MMP-9 of ECM pathway. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2024, 828, 111839. [Google Scholar] [CrossRef] [PubMed]
- Al-Hendy, A.; Laknaur, A.; Diamond, M.P.; Ismail, N.; Boyer, T.G.; Halder, S.K. Silencing Med12 Gene Reduces Proliferation of Human Leiomyoma Cells Mediated via Wnt/β-Catenin Signaling Pathway. Endocrinology 2017, 158, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Galindo, L.J.; Hernández-Beeftink, T.; Salas, A.; Jung, Y.; Reyes, R.; de Oca, F.M.; Hernández, M.; Almeida, T.A. HMGA2 and MED12 alterations frequently co-occur in uterine leiomyomas. Gynecol. Oncol. 2018, 150, 562–568. [Google Scholar] [CrossRef]
- Klemke, M.; Meyer, A.; Nezhad, M.H.; Bartnitzke, S.; Drieschner, N.; Frantzen, C.; Schmidt, E.H.; Belge, G.; Bullerdiek, J. Overexpression of HMGA2 in uterine leiomyomas points to its general role for the pathogenesis of the disease. Genes Chromosomes Cancer 2009, 48, 171–178. [Google Scholar] [CrossRef]
- Mello, J.B.H.; Barros-Filho, M.C.; Abreu, F.B.; Cirilo, P.D.R.; Domingues, M.A.C.; Pontes, A.; Rogatto, S.R. MicroRNAs involved in the HMGA2 deregulation and its co-occurrence with MED12 mutation in uterine leiomyoma. Mol. Hum. Reprod. 2018, 24, 556–563. [Google Scholar] [CrossRef]
- Harrison, W.J.; Andrici, J.; Maclean, F.; Madadi-Ghahan, R.; Farzin, M.; Sioson, L.; Toon, C.W.; Clarkson, A.; Watson, N.; Pickett, J.; et al. Fumarate Hydratase–deficient Uterine Leiomyomas Occur in Both the Syndromic and Sporadic Settings. Am. J. Surg. Pathol. 2016, 40, 599–607. [Google Scholar] [CrossRef]
- Yin, X.; Wei, X.; Al Shamsi, R.; Ali, F.S.; Al Kindi, F.; Zhang, X.; Liang, J.; Pan, X.; Al Masqari, M.; Zheng, L.; et al. Benign metastasizing fumarate hydratase (FH)-deficient uterine leiomyomas: Clinicopathological and molecular study with first documentation of multi-organ metastases. Virchows Arch. 2024, 485, 223–231. [Google Scholar] [CrossRef]
- Zhu, J.; Li, S.; Zhuang, Z.; Chen, H.; Chen, C.; Zhu, J. Fumarate hydratase mutation associated uterine leiomyomas: A case report and literature review. Clin. Case Rep. 2024, 12, e8526. [Google Scholar] [CrossRef]
- Ishikawa, H.; Kobayashi, T.; Kaneko, M.; Saito, Y.; Shozu, M.; Koga, K. RISING STARS: Role of MED12 mutation in the pathogenesis of uterine fibroids. J. Mol. Endocrinol. 2023, 71, e230039. [Google Scholar] [CrossRef] [PubMed]
- Orciani, M.; Caffarini, M.; Biagini, A.; Lucarini, G.; Delli Carpini, G.; Berretta, A.; Di Primio, R.; Ciavattini, A. Chronic Inflammation May Enhance Leiomyoma Development by the Involvement of Progenitor Cells. Stem Cells Int. 2018, 2018, 1716246. [Google Scholar] [CrossRef] [PubMed]
- Sevostyanova, O.; Lisovskaya, T.; Chistyakova, G.; Kiseleva, M.; Sevostyanova, N.; Remizova, I.; Buev, Y. Proinflammatory mediators and reproductive failure in women with uterine fibroids. Gynecol. Endocrinol. 2020, 36 (Suppl. S1), 33–35. [Google Scholar] [CrossRef]
- Agostini, M.; Ganini, C.; Candi, E.; Melino, G. The role of noncoding RNAs in epithelial cancer. Cell Death Discov. 2020, 6, 13. [Google Scholar] [CrossRef]
- Lekka, E.; Hall, J. Noncoding RNAs in disease. FEBS Lett. 2018, 592, 2884–2900. [Google Scholar] [CrossRef]
- Kaikkonen, M.U.; Lam, M.T.; Glass, C.K. Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc. Res. 2011, 90, 430–440. [Google Scholar] [CrossRef]
- Nothnick, W.B. Non-coding RNAs in Uterine Development, Function and Disease. Adv. Exp. Med. Biol. 2016, 886, 171–189. [Google Scholar]
- Sheng, Q.J.; Tan, Y.; Zhang, L.; Wu, Z.P.; Wang, B.; He, X.Y. Heterogeneous graph framework for predicting the association between lncRNA and disease and case on uterine fibroid. Comput. Biol. Med. 2023, 165, 107331. [Google Scholar] [CrossRef]
- Morgan, R.; da Silveira, W.A.; Kelly, R.C.; Overton, I.; Allott, E.H.; Hardiman, G. Long non-coding RNAs and their potential impact on diagnosis, prognosis, and therapy in prostate cancer: Racial, ethnic, and geographical considerations. Expert. Rev. Mol. Diagn. 2021, 21, 1257–1271. [Google Scholar] [CrossRef]
- Xue, C.; Gu, X.; Bao, Z.; Su, Y.; Lu, J.; Li, L. The Mechanism Underlying the ncRNA Dysregulation Pattern in Hepatocellular Carcinoma and Its Tumor Microenvironment. Front. Immunol. 2022, 13, 847728. [Google Scholar] [CrossRef]
- Jiao, J.; Zhao, Y.; Li, Q.; Jin, S.; Liu, Z. LncRNAs in tumor metabolic reprogramming and tumor microenvironment remodeling. Front. Immunol. 2024, 15, 1467151. [Google Scholar] [CrossRef]
- Chuang, T.D.; Ton, N.; Rysling, S.; Boos, D.; Khorram, O. The Effect of Race/Ethnicity and MED12 Mutation on the Expression of Long Non-Coding RNAs in Uterine Leiomyoma and Myometrium. Int. J. Mol. Sci. 2024, 25, 1307. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.D.; Khorram, O. Regulation of Cell Cycle Regulatory Proteins by MicroRNAs in Uterine Leiomyoma. Reprod. Sci. 2019, 26, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.D.; Quintanilla, D.; Boos, D.; Khorram, O. Differential Expression of Super-Enhancer-Associated Long Non-coding RNAs in Uterine Leiomyomas. Reprod. Sci. 2022, 29, 2960–2976. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Obijuru, L.; Laser, J.; Aris, V.; Lee, P.; Mittal, K.; Soteropoulos, P.; Wei, J.J. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer 2007, 46, 336–347. [Google Scholar] [CrossRef]
- Chuang, T.D.; Panda, H.; Luo, X.; Chegini, N. miR-200c is aberrantly expressed in leiomyomas in an ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5. Endocr.-Relat. Cancer 2012, 19, 541–556. [Google Scholar] [CrossRef]
- Aissani, B.; Zhang, K.; Mensenkamp, A.R.; Menko, F.H.; Wiener, H.W. Fine mapping of the uterine leiomyoma locus on 1q43 close to a lncRNA in the RGS7-FH interval. Endocr.-Relat. Cancer 2015, 22, 633–643. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Ma, L.; Bajic, V.B.; Zhang, Z. On the classification of long non-coding RNAs. RNA Biol. 2013, 10, 925–933. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Kritika, C. Transforming ‘Junk’ DNA into Cancer Warriors: The Role of Pseudogenes in Hepatocellular Carcinoma. Cancer Diagn. Progn. 2024, 4, 214–222. [Google Scholar] [CrossRef]
- Nakamura-García, A.K.; Espinal-Enríquez, J. Pseudogenes in Cancer: State of the Art. Cancers 2023, 15, 4024. [Google Scholar] [CrossRef] [PubMed]
- Pink, R.C.; Wicks, K.; Caley, D.P.; Punch, E.K.; Jacobs, L.; Carter, D.R. Pseudogenes: Pseudo-functional or key regulators in health and disease? RNA 2011, 17, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Chen, J.; Li, L.Y.; Wu, M. Epigenetic plasticity of enhancers in cancer. Transcription 2020, 11, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Shlyueva, D.; Stampfel, G.; Stark, A. Transcriptional enhancers: From properties to genome-wide predictions. Nat. Rev. Genet. 2014, 15, 272–286. [Google Scholar] [CrossRef]
- Qi, S.H.; Wang, Q.L.; Zhang, J.Y.; Liu, Q.; Li, C.Y. The regulatory mechanisms by enhancers during cancer initiation and progression. Yi Chuan 2022, 44, 275–288. [Google Scholar]
- Young, R.S.; Kumar, Y.; Bickmore, W.A.; Taylor, M.S. Bidirectional transcription initiation marks accessible chromatin and is not specific to enhancers. Genome Biol. 2017, 18, 242. [Google Scholar] [CrossRef]
- Jia, Y.; Chng, W.J.; Zhou, J. Super-enhancers: Critical roles and therapeutic targets in hematologic malignancies. J. Hematol. Oncol. 2019, 12, 77. [Google Scholar] [CrossRef]
- See, Y.X.; Chen, K.; Fullwood, M.J. MYC overexpression leads to increased chromatin interactions at super-enhancers and MYC binding sites. Genome Res. 2022, 32, 629–642. [Google Scholar] [CrossRef]
- Song, P.; Han, R.; Yang, F. Super enhancer lncRNAs: A novel hallmark in cancer. Cell Commun. Signal. 2024, 22, 207. [Google Scholar] [CrossRef]
- Bao, Y.; Teng, S.; Zhai, H.; Zhang, Y.; Xu, Y.; Li, C.; Chen, Z.; Ren, F.; Wang, Y. SE-lncRNAs in Cancer: Classification, Subcellular Localisation, Function and Corresponding TFs. J. Cell Mol. Med. 2024, 28, e70296. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, C.; Long, L.; Ge, L.; Guo, J.; Fan, Z.; Yu, G. A Comprehensive Analysis of SE-lncRNA/mRNA Differential Expression Profiles During Chondrogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Front. Cell Dev. Biol. 2021, 9, 721205. [Google Scholar] [CrossRef] [PubMed]
- Ropri, A.S.; DeVaux, R.S.; Eng, J.; Chittur, S.V.; Herschkowitz, J.I. Cis-acting super-enhancer lncRNAs as biomarkers to early-stage breast cancer. Breast Cancer Res. 2021, 23, 101. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, C.; Sun, H.; Wang, J.; Liang, Y.; Wang, Y.; Wong, G. The bioinformatics toolbox for circRNA discovery and analysis. Brief. Bioinform. 2021, 22, 1706–1728. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, D. The role of circRNA in breast cancer drug resistance. PeerJ 2024, 12, e18733. [Google Scholar] [CrossRef]
- Bachmayr-Heyda, A.; Reiner, A.T.; Auer, K.; Sukhbaatar, N.; Aust, S.; Bachleitner-Hofmann, T.; Mesteri, I.; Grunt, T.W.; Zeillinger, R.; Pils, D. Correlation of circular RNA abundance with proliferation--exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis, and normal human tissues. Sci. Rep. 2015, 5, 8057. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Diao, L.; Han, L. Small non-coding RNAs in human cancer: Function, clinical utility, and characterization. Oncogene 2021, 40, 1570–1577. [Google Scholar] [CrossRef]
- Hill, M.; Tran, N. miRNA interplay: Mechanisms and consequences in cancer. Dis. Model. Mech. 2021, 14, dmm047662. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Chalbatani, G.M.; Dana, H.; Memari, F.; Gharagozlou, E.; Ashjaei, S.; Kheirandish, P.; Marmari, V.; Mahmoudzadeh, H.; Mozayani, F.; Maleki, A.R.; et al. Biological function and molecular mechanism of piRNA in cancer. Pr. Lab. Med. 2019, 13, e00113. [Google Scholar] [CrossRef]
- Bagheri, M.; Khansarinejad, B.; Mondanizadeh, M.; Azimi, M.; Alavi, S. MiRNAs related in signaling pathways of women’s reproductive diseases: An overview. Mol. Biol. Rep. 2024, 51, 414. [Google Scholar] [CrossRef]
- Ciebiera, M.; Włodarczyk, M.; Zgliczyński, S.; Łoziński, T.; Walczak, K.; Czekierdowski, A. The Role of miRNA and Related Pathways in Pathophysiology of Uterine Fibroids—From Bench to Bedside. Int. J. Mol. Sci. 2020, 21, 3016. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nangia-Makker, P.; Farhana, L.; Rajendra, S.G.; Levi, E.; Majumdar, A.P. miR-21 and miR-145 cooperation in regulation of colon cancer stem cells. Mol. Cancer 2015, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.R.; Cittelly, D.M.; Howe, E.N.; Spoelstra, N.S.; McKinsey, E.L.; LaPara, K.; Elias, A.; Yee, D.; Richer, J.K. MicroRNAs link estrogen receptor alpha status and Dicer levels in breast cancer. Horm. Cancer 2010, 1, 306–319. [Google Scholar] [CrossRef]
- Chuang, T.D.; Khorram, O. Mechanisms underlying aberrant expression of miR-29c in uterine leiomyoma. Fertil. Steril. 2016, 105, 236–245.e1. [Google Scholar] [CrossRef]
- Qiang, W.; Liu, Z.; Serna, V.A.; Druschitz, S.A.; Liu, Y.; Espona-Fiedler, M.; Wei, J.J.; Kurita, T. Down-regulation of miR-29b is essential for pathogenesis of uterine leiomyoma. Endocrinology 2014, 155, 663–669. [Google Scholar] [CrossRef]
- Schmitz, S.U.; Grote, P.; Herrmann, B.G. Mechanisms of long noncoding RNA function in development and disease. Cell. Mol. Life Sci. 2016, 73, 2491–2509. [Google Scholar] [CrossRef]
- Muralimanoharan, S.; Shamby, R.; Stansbury, N.; Schenken, R.; de la Pena Avalos, B.; Javanmardi, S.; Dray, E.; Sung, P.; Boyer, T.G. Aberrant R-loop-induced replication stress in MED12-mutant uterine fibroids. Sci. Rep. 2022, 12, 6169. [Google Scholar] [CrossRef]
- Vaid, R.; Thombare, K.; Mendez, A.; Burgos-Panadero, R.; Djos, A.; Jachimowicz, D.; Lundberg, K.I.; Bartenhagen, C.; Kumar, N.; Tümmler, C.; et al. METTL3 drives telomere targeting of TERRA lncRNA through m6A-dependent R-loop formation: A therapeutic target for ALT-positive neuroblastoma. Nucleic Acids Res. 2024, 52, 2648–2671. [Google Scholar] [CrossRef]
- Luo, H.; Zhu, G.; Eshelman, M.A.; Fung, T.K.; Lai, Q.; Wang, F.; Zeisig, B.B.; Lesperance, J.; Ma, X.; Chen, S.; et al. HOTTIP-dependent R-loop formation regulates CTCF boundary activity and TAD integrity in leukemia. Mol. Cell 2022, 82, 833–851.e11. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.M.; Iijima, K.; Ogami, K.; Shinjo, K.; Murofushi, Y.; Xie, J.; Wang, X.; Kitano, Y.; Mamiya, A.; Kibe, Y.; et al. TUG1-mediated R-loop resolution at microsatellite loci as a prerequisite for cancer cell proliferation. Nat. Commun. 2023, 14, 4521. [Google Scholar] [CrossRef] [PubMed]
- Zavadil, J.; Ye, H.; Liu, Z.; Wu, J.; Lee, P.; Hernando, E.; Soteropoulos, P.; Toruner, G.A.; Wei, J.J. Profiling and functional analyses of microRNAs and their target gene products in human uterine leiomyomas. PLoS ONE 2010, 5, e12362. [Google Scholar] [CrossRef]
- Ciebiera, M.; Wlodarczyk, M.; Wrzosek, M.; Meczekalski, B.; Nowicka, G.; Lukaszuk, K.; Ciebiera, M.; Slabuszewska-Jozwiak, A.; Jakiel, G. Role of Transforming Growth Factor β in Uterine Fibroid Biology. Int. J. Mol. Sci. 2017, 18, 2435. [Google Scholar] [CrossRef]
- Malik, M.; Britten, J.; DeAngelis, A.; Catherino, W.H. Cross-talk between Janus kinase-signal transducer and activator of transcription pathway and transforming growth factor beta pathways and increased collagen1A1 production in uterine leiomyoma cells. F S Sci. 2020, 1, 206–220. [Google Scholar] [CrossRef]
- Carbajo-García, M.C.; Juarez-Barber, E.; Segura-Benítez, M.; Faus, A.; Trelis, A.; Monleón, J.; Carmona-Antoñanzas, G.; Pellicer, A.; Flanagan, J.M.; Ferrero, H. H3K4me3 mediates uterine leiomyoma pathogenesis via neuronal processes, synapsis components, proliferation, and Wnt/β-catenin and TGF-β pathways. Reprod. Biol. Endocrinol. 2023, 21, 9. [Google Scholar] [CrossRef]
- Yu, Y.H.; Zhang, H.J.; Yang, F.; Xu, L.; Liu, H. Curcumol, a major terpenoid from Curcumae Rhizoma, attenuates human uterine leiomyoma cell development via the p38MAPK/NF-κB pathway. J. Ethnopharmacol. 2023, 310, 116311. [Google Scholar] [CrossRef]
- Bao, H.; Sin, T.K.; Zhang, G. Activin A induces leiomyoma cell proliferation, extracellular matrix (ECM) accumulation and myofibroblastic transformation of myometrial cells via p38 MAPK. Biochem. Biophys. Res. Commun. 2018, 504, 447–453. [Google Scholar] [CrossRef]
- Yu, L.; Moore, A.B.; Castro, L.; Gao, X.; Huynh, H.L.; Klippel, M.; Flagler, N.D.; Lu, Y.; Kissling, G.E.; Dixon, D. Estrogen Regulates MAPK-Related Genes through Genomic and Nongenomic Interactions between IGF-I Receptor Tyrosine Kinase and Estrogen Receptor-Alpha Signaling Pathways in Human Uterine Leiomyoma Cells. J. Signal Transduct. 2012, 2012, 204236. [Google Scholar] [CrossRef]
- Ono, M.; Yin, P.; Navarro, A.; Moravek, M.B.; Coon, J.S., V.; Druschitz, S.A.; Serna, V.A.; Qiang, W.; Brooks, D.C.; Malpani, S.S.; et al. Paracrine activation of WNT/beta-catenin pathway in uterine leiomyoma stem cells promotes tumor growth. Proc. Natl. Acad. Sci. USA 2013, 110, 17053–17058. [Google Scholar] [CrossRef]
- Neblett, M.F., II.; Ducharme, M.T.; Meridew, J.A.; Haak, A.J.; Girard, S.; Tschumperlin, D.J.; Stewart, E.A. Evaluation of the In Vivo Efficacy of the JAK Inhibitor AZD1480 in Uterine Leiomyomas Using a Patient-derived Xenograft Murine Model. Reprod. Sci. 2025, 32, 417–427. [Google Scholar] [CrossRef]
- Kim, M.; Kang, D.; Kwon, M.Y.; Lee, H.J.; Kim, M.J. MicroRNAs as potential indicators of the development and progression of uterine leiomyoma. PLoS ONE 2022, 17, e0268793. [Google Scholar] [CrossRef]
- Lazzarini, R.; Caffarini, M.; Delli Carpini, G.; Ciavattini, A.; Di Primio, R.; Orciani, M. From 2646 to 15: Differentially regulated microRNAs between progenitors from normal myometrium and leiomyoma. Am. J. Obstet. Gynecol. 2020, 222, 596.e1–596.e9. [Google Scholar] [CrossRef] [PubMed]
- Marsh, E.E.; Lin, Z.; Yin, P.; Milad, M.; Chakravarti, D.; Bulun, S.E. Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertil. Steril. 2008, 89, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.W.; Abell, G.C.; Kim, K.H.; Nam, Y.D.; Bae, J.W. Comparing microarrays and next-generation sequencing technologies for microbial ecology research. Trends Biotechnol. 2010, 28, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Qin, D. Next-generation sequencing and its clinical application. Cancer Biol. Med. 2019, 16, 4–10. [Google Scholar] [CrossRef]
- Hurd, P.J.; Nelson, C.J. Advantages of next-generation sequencing versus the microarray in epigenetic research. Brief. Funct. Genom. Proteom. 2009, 8, 174–183. [Google Scholar] [CrossRef]
- Chuang, T.D.; Khorram, O. Expression Profiling of lncRNAs, miRNAs, and mRNAs and Their Differential Expression in Leiomyoma Using Next-Generation RNA Sequencing. Reprod. Sci. 2018, 25, 246–255. [Google Scholar] [CrossRef]
- Chuang, T.D.; Xie, Y.; Yan, W.; Khorram, O. Next-generation sequencing reveals differentially expressed small noncoding RNAs in uterine leiomyoma. Fertil. Steril. 2018, 109, 919–929. [Google Scholar] [CrossRef]
- Georgieva, B.; Milev, I.; Minkov, I.; Dimitrova, I.; Bradford, A.P.; Baev, V. Characterization of the uterine leiomyoma microRNAome by deep sequencing. Genomics 2012, 99, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.-D.; Khorram, O. Cross-talk between miR-29c and transforming growth factor-β3 is mediated by an epigenetic mechanism in leiomyoma. Fertil. Steril. 2019, 112, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.D.; Luo, X.; Panda, H.; Chegini, N. miR-93/106b and their host gene, MCM7, are differentially expressed in leiomyomas and functionally target F3 and IL-8. Mol. Endocrinol. 2012, 26, 1028–1042. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.D.; Khorram, O. miR-200c Regulates IL8 Expression by Targeting IKBKB: A Potential Mediator of Inflammation in Leiomyoma Pathogenesis. PLoS ONE 2014, 9, e95370. [Google Scholar] [CrossRef]
- Chuang, T.D.; Ton, N.; Rysling, S.; Baghdasarian, D.; Khorram, O. Differential Expression of Small Non-Coding RNAs in Uterine Leiomyomas. Int. J. Mol. Sci. 2025, 26, 1688. [Google Scholar] [CrossRef]
- Fitzgerald, J.B.; Chennathukuzhi, V.; Koohestani, F.; Nowak, R.A.; Christenson, L.K. Role of microRNA-21 and programmed cell death 4 in the pathogenesis of human uterine leiomyomas. Fertil. Steril. 2012, 98, 726–734.e2. [Google Scholar] [CrossRef]
- Cardozo, E.R.; Foster, R.; Karmon, A.E.; Lee, A.E.; Gatune, L.W.; Rueda, B.R.; Styer, A.K. MicroRNA 21a-5p overexpression impacts mediators of extracellular matrix formation in uterine leiomyoma. Reprod. Biol. Endocrinol. 2018, 16, 46. [Google Scholar] [CrossRef]
- Bormann, T.; Maus, R.; Stolper, J.; Tort Tarrés, M.; Brandenberger, C.; Wedekind, D.; Jonigk, D.; Welte, T.; Gauldie, J.; Kolb, M.; et al. Role of matrix metalloprotease-2 and MMP-9 in experimental lung fibrosis in mice. Respir. Res. 2022, 23, 180. [Google Scholar] [CrossRef]
- Nguyen, T.T.P.; Suman, K.H.; Nguyen, T.B.; Nguyen, H.T.; Do, D.N. The Role of miR-29s in Human Cancers—An Update. Biomedicines 2022, 10, 2121. [Google Scholar] [CrossRef]
- Marsh, E.E.; Steinberg, M.L.; Parker, J.B.; Wu, J.; Chakravarti, D.; Bulun, S.E. Decreased expression of microRNA-29 family in leiomyoma contributes to increased major fibrillar collagen production. Fertil. Steril. 2016, 106, 766–772. [Google Scholar] [CrossRef]
- Huang, D.; Xue, H.; Shao, W.; Wang, X.; Liao, H.; Ye, Y. Inhibiting effect of miR-29 on proliferation and migration of uterine leiomyoma via the STAT3 signaling pathway. Aging 2022, 14, 1307–1320. [Google Scholar] [CrossRef]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef]
- Casacuberta-Serra, S.; González-Larreategui, Í.; Capitán-Leo, D.; Soucek, L. MYC and KRAS cooperation: From historical challenges to therapeutic opportunities in cancer. Signal Transduct. Target. Ther. 2024, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Mahdadi, S.; Vidal, M.; Desbène-Finck, S. Non-nucleoside inhibitors of DNMT1 and DNMT3 for targeted cancer therapy. Pharmacol. Res. 2024, 207, 107328. [Google Scholar] [CrossRef] [PubMed]
- Davletgildeeva, A.T.; Kuznetsov, N.A. The Role of DNMT Methyltransferases and TET Dioxygenases in the Maintenance of the DNA Methylation Level. Biomolecules 2024, 14, 1117. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. Febs J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular matrix composition of connective tissues: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 10542. [Google Scholar] [CrossRef]
- Verma, A.; Khan, M.A.; Satrusal, S.R.; Datta, D. Emerging role of EZH2 in solid tumor metastasis. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189253. [Google Scholar] [CrossRef]
- Chuang, T.D.; Khorram, O. Tranilast Inhibits Genes Functionally Involved in Cell Proliferation, Fibrosis, and Epigenetic Regulation and Epigenetically Induces miR-29c Expression in Leiomyoma Cells. Reprod. Sci. 2017, 24, 1253–1263. [Google Scholar] [CrossRef]
- Chuang, T.D.; Rehan, A.; Khorram, O. Tranilast induces MiR-200c expression through blockade of RelA/p65 activity in leiomyoma smooth muscle cells. Fertil. Steril. 2020, 113, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Howe, E.N.; Cochrane, D.R.; Cittelly, D.M.; Richer, J.K. miR-200c targets a NF-κB up-regulated TrkB/NTF3 autocrine signaling loop to enhance anoikis sensitivity in triple negative breast cancer. PLoS ONE 2012, 7, e49987. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yan, Y.; Gui, A.; Zhu, S.; Qiu, S.; Chen, F.; Liu, W.; Zuo, J.; Yang, L. A Regulatory Loop Involving miR-200c and NF-κB Modulates Mortalin Expression and Increases Cisplatin Sensitivity in an Ovarian Cancer Cell Line Model. Int. J. Mol. Sci. 2022, 23, 15300. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Islam, M.S.; Protic, O.; Ciavattini, A.; Giannubilo, S.R.; Tranquilli, A.L.; Catherino, W.H.; Castellucci, M.; Ciarmela, P. Tranilast, an orally active antiallergic compound, inhibits extracellular matrix production in human uterine leiomyoma and myometrial cells. Fertil. Steril. 2014, 102, 597–606. [Google Scholar] [CrossRef]
- Shime, H.; Kariya, M.; Orii, A.; Momma, C.; Kanamori, T.; Fukuhara, K.; Kusakari, T.; Tsuruta, Y.; Takakura, K.; Nikaido, T.; et al. Tranilast inhibits the proliferation of uterine leiomyoma cells in vitro through G1 arrest associated with the induction of p21waf1 and p53. J. Clin. Endocrinol. Metab. 2002, 87, 5610–5617. [Google Scholar] [CrossRef]
- Waugh, D.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Teijeira, A.; Garasa, S.; Ochoa, M.C.; Villalba, M.; Olivera, I.; Cirella, A.; Eguren-Santamaria, I.; Berraondo, P.; Schalper, K.A.; de Andrea, C.E.; et al. IL8, Neutrophils, and NETs in a Collusion against Cancer Immunity and Immunotherapy. Clin. Cancer Res. 2021, 27, 2383–2393. [Google Scholar] [CrossRef]
- Ahmadi, S.E.; Shabannezhad, A.; Kahrizi, A.; Akbar, A.; Safdari, S.M.; Hoseinnezhad, T.; Zahedi, M.; Sadeghi, S.; Mojarrad, M.G.; Safa, M. Tissue factor (coagulation factor III): A potential double-edge molecule to be targeted and re-targeted toward cancer. Biomark. Res. 2023, 11, 60. [Google Scholar] [CrossRef]
- Laser, J.; Lee, P.; Wei, J.J. Cellular senescence in usual type uterine leiomyoma. Fertil. Steril. 2010, 93, 2020–2026. [Google Scholar] [CrossRef]
- Peng, Y.; Laser, J.; Shi, G.; Mittal, K.; Melamed, J.; Lee, P.; Wei, J.J. Antiproliferative effects by Let-7 repression of high-mobility group A2 in uterine leiomyoma. Mol. Cancer Res. 2008, 6, 663–673. [Google Scholar] [CrossRef]
- Klemke, M.; Meyer, A.; Hashemi Nezhad, M.; Belge, G.; Bartnitzke, S.; Bullerdiek, J. Loss of let-7 binding sites resulting from truncations of the 3′ untranslated region of HMGA2 mRNA in uterine leiomyomas. Cancer Genet. Cytogenet. 2010, 196, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ye, S.; Liu, H.; Zhao, Y.; Mao, Y.; Zhang, W. HMGA2 promotes cancer metastasis by regulating epithelial–mesenchymal transition. Front. Oncol. 2024, 14, 1320887. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Choi, Y.C.; Lee, S.; Jeong, Y.; Yoon, J.; Baek, K. Induction of growth arrest by miR-542-3p that targets survivin. FEBS Lett. 2010, 584, 4048–4052. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, G.; Tomasello, L.; Giordano, C.; Pizzolanti, G. Survivin (BIRC5): Implications in cancer therapy. Life Sci. 2024, 350, 122788. [Google Scholar] [CrossRef]
- Chuwa, A.H.; Mvunta, D.H. Prognostic and clinicopathological significance of survivin in gynecological cancer. Oncol. Rev. 2024, 18, 1444008. [Google Scholar] [CrossRef]
- Ling, J.; Jiang, L.; Zhang, C.; Dai, J.; Wu, Q.; Tan, J. Upregulation of miR-197 inhibits cell proliferation by directly targeting IGFBP5 in human uterine leiomyoma cells. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 835–842. [Google Scholar] [CrossRef]
- Ling, J.; Wu, X.; Fu, Z.; Tan, J.; Xu, Q. Systematic analysis of gene expression pattern in has-miR-197 over-expressed human uterine leiomyoma cells. Biomed. Pharmacother. 2015, 75, 226–233. [Google Scholar] [CrossRef]
- Wu, X.; Ling, J.; Fu, Z.; Ji, C.; Wu, J.; Xu, Q. Effects of miRNA-197 overexpression on proliferation, apoptosis and migration in levonorgestrel treated uterine leiomyoma cells. Biomed. Pharmacother. 2015, 71, 1–6. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, Y.; Chen, L.; Sun, Y.; Fan, S. Effects of miRNA-199a-5p on cell proliferation and apoptosis of uterine leiomyoma by targeting MED12. Open Med. 2022, 17, 151–159. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Chai, B.; Wu, Z.; Gu, Z.; Zou, H.; Zhang, H.; Li, Y.; Sun, Q.; Fang, W.; et al. miR-199a-3p/5p regulate tumorgenesis via targeting Rheb in non-small cell lung cancer. Int. J. Biol. Sci. 2022, 18, 4187–4202. [Google Scholar] [CrossRef]
- Meng, W.; Li, Y.; Chai, B.; Liu, X.; Ma, Z. miR-199a: A Tumor Suppressor with Noncoding RNA Network and Therapeutic Candidate in Lung Cancer. Int. J. Mol. Sci. 2022, 23, 8518. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Kim, H.; Lee, I.; Lee, J.H.; Cho, S.; Choi, Y.S. MicroRNA-139-5p Regulates Fibrotic Potentials via Modulation of Collagen Type 1 and Phosphorylated p38 MAPK in Uterine Leiomyoma. Yonsei Med. J. 2021, 62, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, Y.S.; Park, J.H.; Kim, H.; Lee, I.; Won, Y.B.; Yun, B.H.; Park, J.H.; Seo, S.K.; Lee, B.S.; et al. MiR-150-5p May Contribute to Pathogenesis of Human Leiomyoma via Regulation of the Akt/p27Kip1 Pathway In Vitro. Int. J. Mol. Sci. 2019, 20, 2684. [Google Scholar] [CrossRef] [PubMed]
- Falahati, Z.; Mohseni-Dargah, M.; Mirfakhraie, R. Emerging Roles of Long Non-coding RNAs in Uterine Leiomyoma Pathogenesis: A Review. Reprod. Sci. 2022, 29, 1086–1101. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, X.; Chen, S.; Zhang, S. Long noncoding RNAs: Fine-tuners hidden in the cancer signaling network. Cell Death Discov. 2021, 7, 283. [Google Scholar] [CrossRef]
- Paraskevopoulou, M.D.; Hatzigeorgiou, A.G. Analyzing MiRNA-LncRNA Interactions. Methods Mol. Biol. 2016, 1402, 271–286. [Google Scholar]
- Guo, H.; Zhang, X.; Dong, R.; Liu, X.; Li, Y.; Lu, S.; Xu, L.; Wang, Y.; Wang, X.; Hou, D.; et al. Integrated analysis of long noncoding RNAs and mRNAs reveals their potential roles in the pathogenesis of uterine leiomyomas. Oncotarget 2014, 5, 8625–8636. [Google Scholar] [CrossRef]
- Meng, F.; Ji, Y.; Chen, X.; Wang, Y.; Hua, M. An integrative analysis of an lncRNA–mRNA competing endogenous RNA network to identify functional lncRNAs in uterine leiomyomas with RNA sequencing. Front. Genet. 2023, 13, 1053845. [Google Scholar] [CrossRef]
- Chuang, T.D.; Rysling, S.; Ton, N.; Baghdasarian, D.; Khorram, O. Comparative Analysis of Differentially Expressed Long Non-Coding RNA in Pre- and Postmenopausal Fibroids. Int. J. Mol. Sci. 2025, 26, 6798. [Google Scholar] [CrossRef]
- Akbari, M.; Yassaee, F.; Aminbeidokhti, M.; Abedin-Do, A.; Mirfakhraie, R. LncRNA SRA1 may play a role in the uterine leiomyoma tumor growth regarding the MED12 mutation pattern. Int. J. Womens Health 2019, 11, 495–500. [Google Scholar] [CrossRef]
- Cao, T.; Jiang, Y.; Wang, Z.; Zhang, N.; Al-Hendy, A.; Mamillapalli, R.; Kallen, A.N.; Kodaman, P.; Taylor, H.S.; Li, D.; et al. H19 lncRNA identified as a master regulator of genes that drive uterine leiomyomas. Oncogene 2019, 38, 5356–5366. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Zhou, H.; Sun, Y.; Shen, B.; Chou, D. Long non-coding ribonucleic acid H19 and ten-eleven translocation enzyme 1 messenger RNA expression levels in uterine fibroids may predict their postoperative recurrence. Clinics 2021, 76, e2671. [Google Scholar] [CrossRef] [PubMed]
- Rainho, C.A.; Pontes, A.; Rogatto, S.R. Expression and imprinting of insulin-like growth factor II (IGF2) and H19 genes in uterine leiomyomas. Gynecol. Oncol. 1999, 74, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Aissani, B.; Zhang, K.; Wiener, H. Follow-up to genome-wide linkage and admixture mapping studies implicates components of the extracellular matrix in susceptibility to and size of uterine fibroids. Fertil. Steril. 2015, 103, 528–534.e13. [Google Scholar] [CrossRef]
- He, Y.F.; Li, B.Z.; Li, Z.; Liu, P.; Wang, Y.; Tang, Q.; Ding, J.; Jia, Y.; Chen, Z.; Li, L.; et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 2011, 333, 1303–1307. [Google Scholar] [CrossRef]
- Farzaneh, F.; Saravani, M.; Esmailpoor, M.; Mokhtari, M.; Teimoori, B.; Rezaei, M.; Salimi, S. Association of HOTAIR gene polymorphisms and haplotypes with uterine leiomyoma susceptibility in southeast of Iran. Mol. Biol. Rep. 2019, 46, 4271–4277. [Google Scholar] [CrossRef]
- Chuang, T.D.; Ton, N.; Manrique, N.; Rysling, S.; Khorram, O. Targeting the long non-coding RNA MIAT for the treatment of fibroids in an animal model. Clin. Sci. 2024, 138, 699–709. [Google Scholar] [CrossRef]
- Chuang, T.D.; Quintanilla, D.; Boos, D.; Khorram, O. Long Noncoding RNA MIAT Modulates the Extracellular Matrix Deposition in Leiomyomas by Sponging MiR-29 Family. Endocrinology 2021, 162, bqab186. [Google Scholar] [CrossRef]
- Chuang, T.D.; Rehan, A.; Khorram, O. Functional role of the long noncoding RNA X-inactive specific transcript in leiomyoma pathogenesis. Fertil. Steril. 2021, 115, 238–247. [Google Scholar] [CrossRef]
- Chuang, T.D.; Ton, N.; Rysling, S.; Khorram, O. The in vivo effects of knockdown of long non-coding RNA XIST on fibroid growth and gene expression. Faseb J. 2024, 38, e70140. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Maekawa, R.; Yamagata, Y.; Asada, H.; Tamura, I.; Lee, L.; Okada, M.; Tamura, H.; Sugino, N. Potential mechanisms of aberrant DNA hypomethylation on the x chromosome in uterine leiomyomas. J. Reprod. Dev. 2014, 60, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.D.; Ton, N.; Rysling, S.; Khorram, O. The Functional Role of the Long Non-Coding RNA LINCMD1 in Leiomyoma Pathogenesis. Int. J. Mol. Sci. 2024, 25, 11539. [Google Scholar] [CrossRef]
- Yang, E.; Xue, L.; Li, Z.; Yi, T. Lnc-AL445665.1–4 may be involved in the development of multiple uterine leiomyoma through interacting with miR-146b-5p. BMC Cancer 2019, 19, 709. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, G.; Li, B.; Qu, J.; Zhang, Y. LncRNA APTR Promotes Uterine Leiomyoma Cell Proliferation by Targeting ERα to Activate the Wnt/β-Catenin Pathway. Front. Oncol. 2021, 11, 536346. [Google Scholar] [CrossRef]
- Koutsi, M.A.; Pouliou, M.; Champezou, L.; Vatsellas, G.; Giannopoulou, A.I.; Piperi, C.; Agelopoulos, M. Typical Enhancers, Super-Enhancers, and Cancers. Cancers 2022, 14, 4375. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, S.; Qin, T.; Wang, W. Circular RNA (circ-0075804) promotes the proliferation of retinoblastoma via combining heterogeneous nuclear ribonucleoprotein K (HNRNPK) to improve the stability of E2F transcription factor 3 E2F3. J. Cell. Biochem. 2020, 121, 3516–3525. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Tian, Y.; Gao, Y.; Dong, X.; Chen, W.; Yuan, X.; Yin, W.; Xu, J.; Chen, K.; et al. CircRNA inhibits DNA damage repair by interacting with host gene. Mol. Cancer 2020, 19, 128. [Google Scholar] [CrossRef]
- Suo, M.; Lin, Z.; Guo, D.; Zhang, A. Hsa_circ_0056686, derived from cancer-associated fibroblasts, promotes cell proliferation and suppresses apoptosis in uterine leiomyoma through inhibiting endoplasmic reticulum stress. PLoS ONE 2022, 17, e0266374. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, L.; Wang, J.; Zhang, X.; Liu, G. Circular RNA expression profiling identifies novel biomarkers in uterine leiomyoma. Cell. Signal. 2020, 76, 109784. [Google Scholar] [CrossRef]
- Arun, G.; Diermeier, S.D.; Spector, D.L. Therapeutic Targeting of Long Non-Coding RNAs in Cancer. Trends Mol. Med. 2018, 24, 257–277. [Google Scholar] [CrossRef]
- Sangeeth, A.; Malleswarapu, M.; Mishra, A.; Gutti, R.K. Long Non-coding RNA Therapeutics: Recent Advances and Challenges. Curr. Drug Targets 2022, 23, 1457–1464. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boos, D.; Chuang, T.-D.; Khorram, O. The Roles of Non-Coding RNAs in the Pathogenesis of Uterine Fibroids. Cells 2025, 14, 1290. https://doi.org/10.3390/cells14161290
Boos D, Chuang T-D, Khorram O. The Roles of Non-Coding RNAs in the Pathogenesis of Uterine Fibroids. Cells. 2025; 14(16):1290. https://doi.org/10.3390/cells14161290
Chicago/Turabian StyleBoos, Drake, Tsai-Der Chuang, and Omid Khorram. 2025. "The Roles of Non-Coding RNAs in the Pathogenesis of Uterine Fibroids" Cells 14, no. 16: 1290. https://doi.org/10.3390/cells14161290
APA StyleBoos, D., Chuang, T.-D., & Khorram, O. (2025). The Roles of Non-Coding RNAs in the Pathogenesis of Uterine Fibroids. Cells, 14(16), 1290. https://doi.org/10.3390/cells14161290





