Cell Culture in a Hyperbaric Chamber: A Research Model to Study the Effects of Hyperbarism (Hyperbaric Pressure) on Bone Cell Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Hyperbaric Chamber
2.2. Cell Culture
2.3. Treatment
2.4. RNA Extraction and Retrotranscription
2.5. RT-PCR
2.6. Immunofluorescence
2.7. ELISA
2.8. Superoxide and RNS Measurement
2.9. Statistical Analysis
3. Results
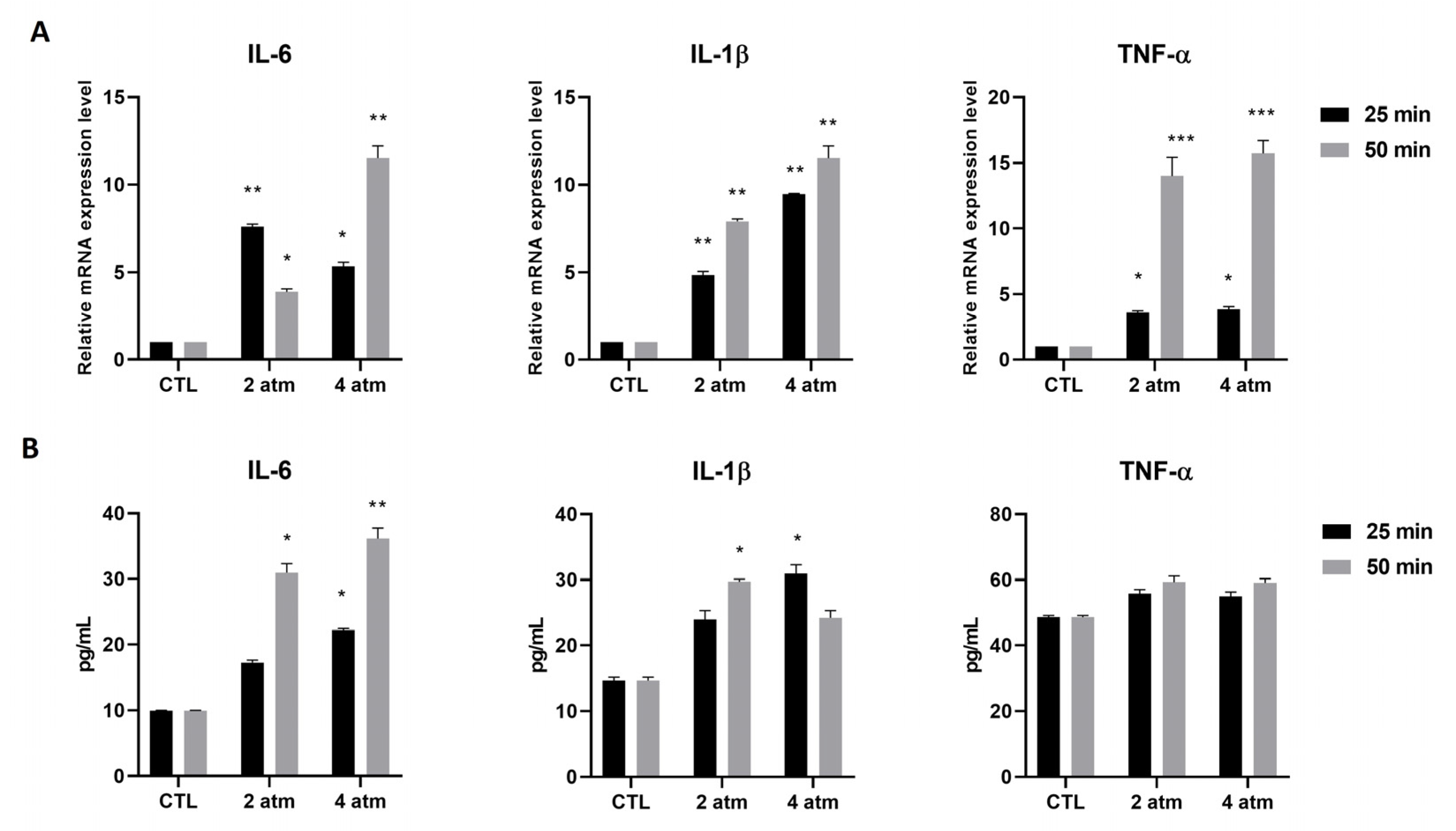
3.1. Inflammatory Pathway
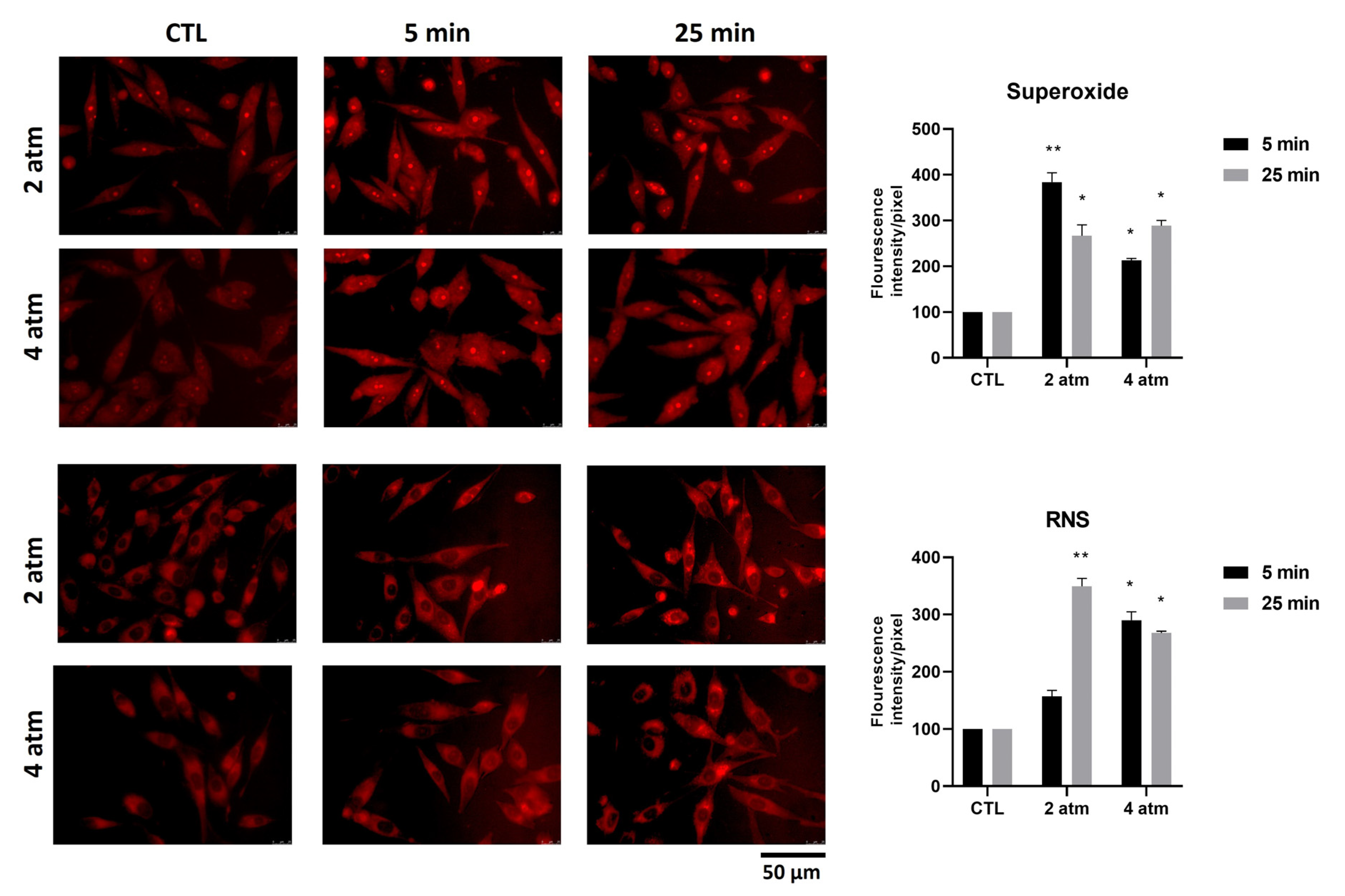
3.2. Oxygen and Nitrogen Reactive Species
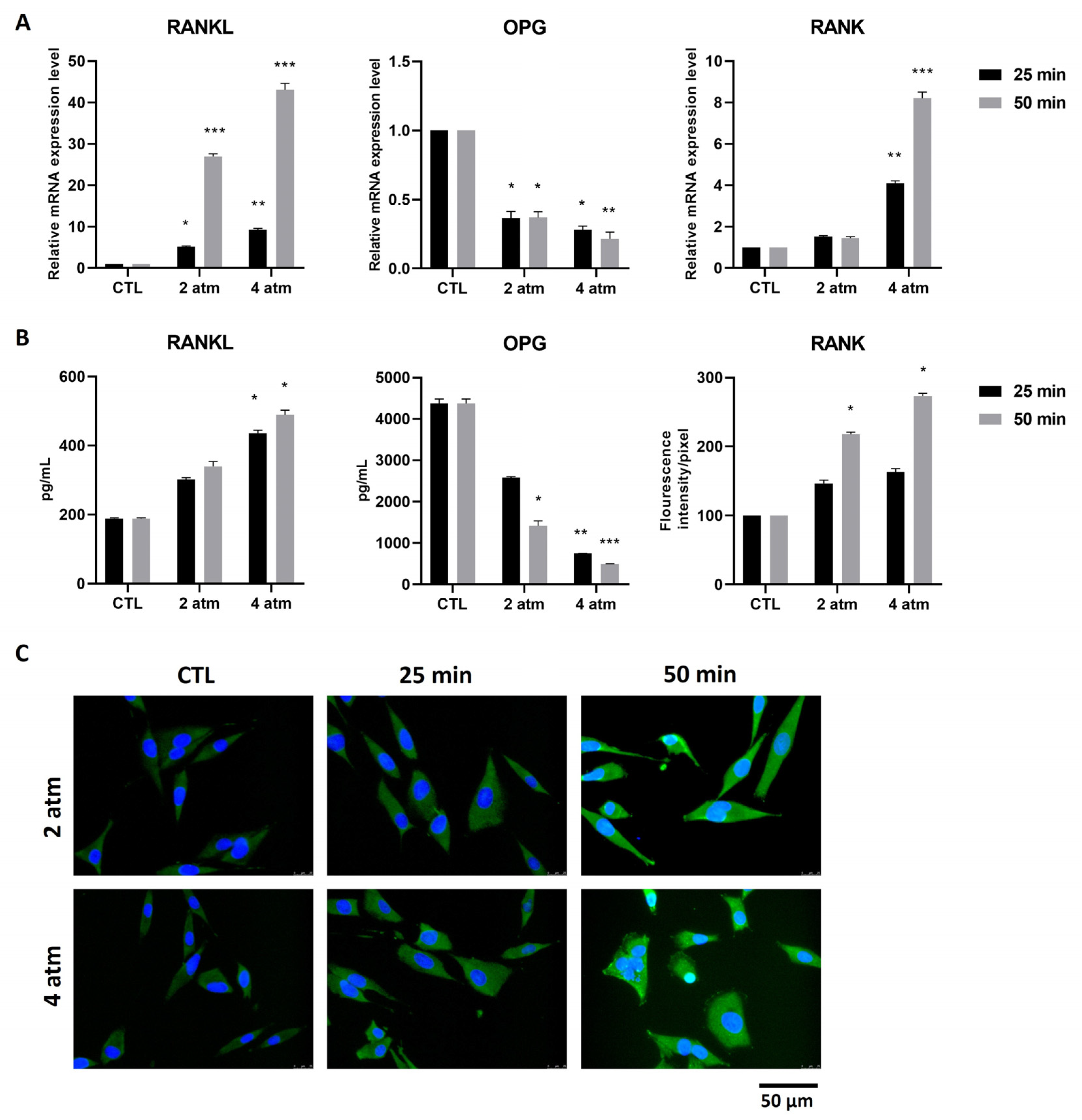
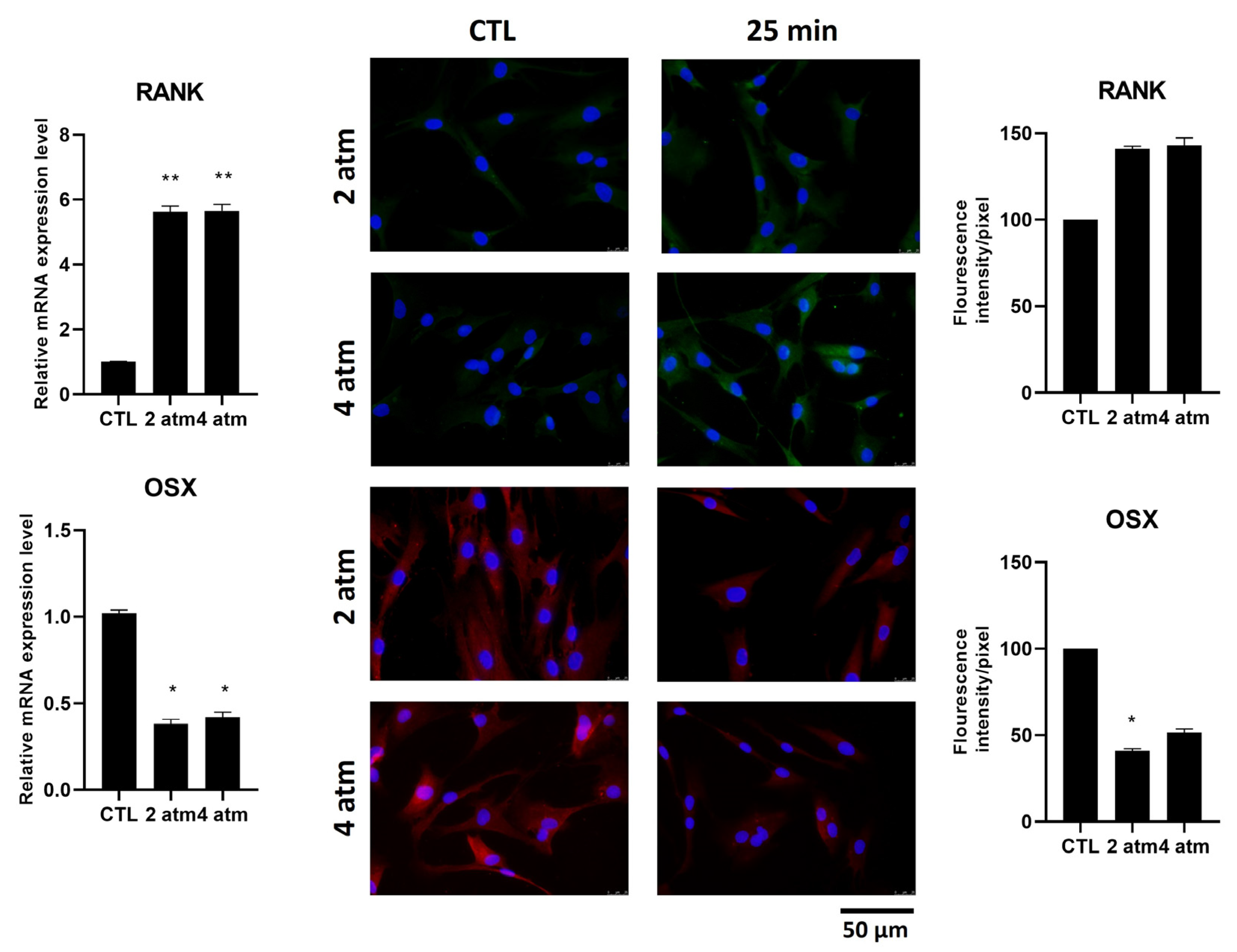
3.3. Bone Remodeling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romsbotn, S.; Eftedal, I.; Vaag, J.R. A Work Environment Under Pressure: Psychosocial Job Demands and Resources Among Saturation Divers. Front. Public Health 2022, 10, 765197. [Google Scholar] [CrossRef]
- Uguen, M.; Pougnet, R.; Uguen, A.; Loddé, B.; Dewitte, J.D. Dysbaric osteonecrosis among professional divers: A literature review. Undersea Hyperb. Med. 2014, 41, 579–587. [Google Scholar]
- Arya, A.K.; Balestra, C.; Bhopale, V.M.; Tuominen, L.J.; Räisänen-Sokolowski, A.; Dugrenot, E.; L’Her, E.; Bhat, A.R.; Thom, S.R. Elevations of Extracellular Vesicles and Inflammatory Biomarkers in Closed Circuit SCUBA Divers. Int. J. Mol. Sci. 2023, 24, 5969. [Google Scholar] [CrossRef]
- Sharareh, B.; Schwarzkopf, R. Dysbaric Osteonecrosis. Clin. J. Sport Med. 2015, 25, 153–161. [Google Scholar] [CrossRef]
- Cialoni, D.; Brizzolari, A.; Barassi, A.; Bosco, G.; Pieri, M.; Lancellotti, V.; Marroni, A. White Blood Cells, Platelets, Red Blood Cells and Gas Bubbles in SCUBA Diving: Is There a Relationship? Healthcare 2022, 10, 182. [Google Scholar] [CrossRef]
- de Jong, F.J.M.; Wingelaar, T.T.; Brinkman, P.; van Ooij, P.-J.A.M.; Maitland-van der Zee, A.-H.; Hollmann, M.W.; van Hulst, R.A. Pulmonary Oxygen Toxicity Through Exhaled Breath Markers After Hyperbaric Oxygen Treatment Table 6. Front. Physiol. 2022, 13, 899568. [Google Scholar] [CrossRef]
- de Wolde, S.D.; Hulskes, R.H.; de Jonge, S.W.; Hollmann, M.W.; van Hulst, R.A.; Weenink, R.P.; Kox, M. The Effect of Hyperbaric Oxygen Therapy on Markers of Oxidative Stress and the Immune Response in Healthy Volunteers. Front. Physiol. 2022, 13, 826163. [Google Scholar] [CrossRef]
- Vezzoli, A.; Mrakic-Sposta, S.; Brizzolari, A.; Balestra, C.; Camporesi, E.M.; Bosco, G. Oxy-Inflammation in Humans during Underwater Activities. Int. J. Mol. Sci. 2024, 25, 3060. [Google Scholar] [CrossRef] [PubMed]
- Rocco, M.; Maggi, L.; Loffredo, C.; Pelli, M.; Benedetto, P.D.; Fiorelli, S.; Simmaco, M.; De Blasi, R.A. The impact of different gas mixtures on inflammatory responses in advanced recreational divers. Diving Hyperb. Med. J. 2021, 51, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Batle, J.M.; Capó, X.; Martorell, M.; Córdova, A.; Tur, J.A.; Pons, A. Scuba diving induces nitric oxide synthesis and the expression of inflammatory and regulatory genes of the immune response in neutrophils. Physiol. Genom. 2014, 46, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Kiboub, F.Z.; Møllerløkken, A.; Hjelde, A.; Flatberg, A.; Loennechen, Ø.; Eftedal, I. Blood Gene Expression and Vascular Function Biomarkers in Professional Saturation Diving. Front. Physiol. 2018, 9, 00937. [Google Scholar] [CrossRef]
- Marchetti, E.; Pigini, D.; Spagnoli, M.; Tranfo, G.; Buonaurio, F.; Sciubba, F.; Giampaoli, O.; Miccheli, A.; Pinto, A.; De Angelis, N.; et al. Hyperbaric Exposure of Scuba Divers Affects the Urinary Excretion of Nucleic Acid Oxidation Products and Hypoxanthine. Int. J. Environ. Res. Public Health 2022, 19, 3005. [Google Scholar] [CrossRef]
- Cooper, J.S.; Hanson, K.C. Decompression Sickness (DCS, Bends, Caisson Disease); StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Coleman, B.; Davis, F.M. Dysbaric osteonecrosis in technical divers: The new ‘at-risk’ group? Diving Hyperb. Med. J. 2020, 50, 295–299. [Google Scholar] [CrossRef]
- Uguen, M.; Pougnet, R.; Uguen, A.; Cornec, D.; Quintin-Roué, I.; Dewitte, J.D.; Loddé, B. Dysbaric osteonecrosis in professional divers: Two case reports. Undersea Hyperb. Med. 2015, 42, 363–367. [Google Scholar] [PubMed]
- Xin, X.; Fan, B.; Flammer, J.; Miller, N.R.; Jaggi, G.P.; Killer, H.E.; Meyer, P.; Neutzner, A. Meningothelial Cells React to Elevated Pressure and Oxidative Stress. PLoS ONE 2011, 6, e20142. [Google Scholar] [CrossRef] [PubMed]
- Baitule, S.; Patel, A.H.; Murthy, N.; Sankar, S.; Kyrou, I.; Ali, A.; Randeva, H.S.; Robbins, T. A Systematic Review to Assess the Impact of Hyperbaric Oxygen Therapy on Glycaemia in People with Diabetes Mellitus. Medicina 2021, 57, 1134. [Google Scholar] [CrossRef]
- Brouwer, R.J.; Lalieu, R.C.; Hoencamp, R.; van Hulst, R.A.; Ubbink, D.T. A systematic review and meta-analysis of hyperbaric oxygen therapy for diabetic foot ulcers with arterial insufficiency. J. Vasc. Surg. 2020, 71, 682–692.e1. [Google Scholar] [CrossRef]
- Roeckl-Wiedmann, I.; Bennett, M.; Kranke, P. Systematic review of hyperbaric oxygen in the management of chronic wounds. Br. J. Surg. 2005, 92, 24–32. [Google Scholar] [CrossRef]
- Pérez-Vielma, N.M.; Valencia Gutiérrez, M.M.; Sánchez Camacho, J.V.; González Hernández, J.E.; García, Á.M.; Ochoa, C.; Labovitz, J.; López, M.G. The effect of hyperbaric oxygen therapy on oxidative stress and inflammation in patients with diabetic foot ulcers: A preliminary study. Heliyon 2024, 10, e40586. [Google Scholar] [CrossRef]
- Ristic, P.; Savic, M.; Bolevich, S.; Bolevich, S.; Orlova, A.; Mikhaleva, A.; Kartashova, A.; Yavlieva, K.; Nikolic Turnic, T.; Pindovic, B.; et al. Examining the Effects of Hyperbaric Oxygen Therapy on the Cardiovascular System and Oxidative Stress in Insulin-Treated and Non-Treated Diabetic Rats. Animals 2023, 13, 2847. [Google Scholar] [CrossRef]
- Chen, S.-J.; Yu, C.-T.; Cheng, Y.-L.; Yu, S.-Y.; Lo, H.-C. Effects of hyperbaric oxygen therapy on circulating interleukin-8, nitric oxide, and insulin-like growth factors in patients with type 2 diabetes mellitus. Clin. Biochem. 2007, 40, 30–36. [Google Scholar] [CrossRef]
- Lopreiato, M.; Mariano, A.; Cocchiola, R.; Longo, G.; Dalla Vedova, P.; Scandurra, R.; Scotto d’Abusco, A. Nanostructured TiC Layer is Highly Suitable Surface for Adhesion, Proliferation and Spreading of Cells. Condens. Matter 2020, 5, 29. [Google Scholar] [CrossRef]
- Chen, X.; Zhi, X.; Wang, J.; Su, J. RANKL signaling in bone marrow mesenchymal stem cells negatively regulates osteoblastic bone formation. Bone Res. 2018, 6, 34. [Google Scholar] [CrossRef]
- Ersson, A.; Walles, M.; Ohlsson, K.; Ekholm, A. Chronic hyperbaric exposure activates proinflammatory mediators in humans. J. Appl. Physiol. 2002, 92, 2375–2380. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, W.; Masson, A.; Li, Y.-P. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024, 10, 71. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise—Induced Bone Remodeling. BioMed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef] [PubMed]
- Sigl, V.; Jones, L.P.; Penninger, J.M. RANKL/RANK: From bone loss to the prevention of breast cancer. Open Biol. 2016, 6, 160230. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef] [PubMed]
- Papagerakis, P.; Berdal, A.; Mesbah, M.; Peuchmaur, M.; Malaval, L.; Nydegger, J.; Simmer, J.; Macdougall, M. Investigation of osteocalcin, osteonectin, and dentin sialophosphoprotein in developing human teeth. Bone 2002, 30, 377–385. [Google Scholar] [CrossRef]
- Mazzucchi, G.; Mariano, A.; Serafini, G.; Lamazza, L.; Scotto d’Abusco, A.; De Biase, A.; Lollobrigida, M. Osteoinductive Properties of Autologous Dentin: An Ex Vivo Study on Extracted Teeth. J. Funct. Biomater. 2024, 15, 162. [Google Scholar] [CrossRef]
- Moser, S.C.; van der Eerden, B.C.J. Osteocalcin—A Versatile Bone-Derived Hormone. Front. Endocrinol. 2019, 9, 00794. [Google Scholar] [CrossRef]
- Bordukalo-Nikšić, T.; Kufner, V.; Vukičević, S. The Role Of BMPs in the Regulation of Osteoclasts Resorption and Bone Remodeling: From Experimental Models to Clinical Applications. Front. Immunol. 2022, 13, 869422. [Google Scholar] [CrossRef]
- Ramazzotti, G.; Fiume, R.; Chiarini, F.; Campana, G.; Ratti, S.; Billi, A.M.; Manzoli, L.; Follo, M.Y.; Suh, P.-G.; McCubrey, J.; et al. Phospholipase C-β1 interacts with cyclin E in adipose- derived stem cells osteogenic differentiation. Adv. Biol. Regul. 2019, 71, 1–9. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Renn, J.; Winkler, C. Osterix-mCherry transgenic medaka for in vivo imaging of bone formation. Dev. Dyn. 2009, 238, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, M.; Wang, S.; Xiao, Z.; Xiong, Y.; Wang, G. Recent Advances of Osterix Transcription Factor in Osteoblast Differentiation and Bone Formation. Front. Cell Dev. Biol. 2020, 8, 601224. [Google Scholar] [CrossRef]
- Floerkemeier, T.; Budde, S.; Willbold, E.; Schwarze, M.; Niehof, M.; Lichtinghagen, R.; Windhagen, H.; Weizbauer, A.; Reifenrath, J. Do biomarkers allow a differentiation between osteonecrosis of the femoral head and osteoarthritis of the hip?—A biochemical, histological and gene expression analysis. Osteoarthr. Cartil. 2021, 29, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Mazzotti, A.; Arceri, A. Osteonecrosis of the knee: A concise review of the current literature. Int. J. Bone Fragility 2022, 2, 11–15. [Google Scholar] [CrossRef]


| Pressure | Time | 1.2 atm | 0.9 atm | 0.6 atm | 0.3 atm |
|---|---|---|---|---|---|
| 2 atm | 5 min | 3 min | |||
| 25 min | 3 min | ||||
| 50 min | 7 min | ||||
| 4 atm | 5 min | 3 min | |||
| 25 min | 5 min | 15 min | |||
| 50 min | 3 min | 10 min | 25 min | 45 min |
| Gene | Primer Sequences (Fw-Rv) |
|---|---|
| IL-6 NM_000600 | 5′-GATGGATGCTTCCAATCTG-3′ 5′-CTCTAGGTATACCTCAAACTCC-3′ |
| IL-1β NM_000576 | 5′-ACGAATCTCCGACCACCACTA-3′ 5′-TCCATGGCCACAACAACTGA-3′ |
| TNF-α NM_000594 | 5′-TCAGATCATCTTCTCGAACC-3′ 5′-CAGATAGATGGGCTCATACC-3′ |
| RANK NM_003839 | 5′-CCTACGCACAAGGCGAAGATGC-3′ 5′-CGTAGACCACGATGATGTCGCC-3′ |
| RANKL NM_033012 | 5′-TCAGCCTTTTGCTCATCTCACTAT-3′ 5′-CCAAGAGGACAGACTCACTTTATGG-3′ |
| OPG NM_002546 | 5′-CGGCACATTGGACATGCTAA-3′ 5′-TCCCGGTAAGCTTTCCATCA-3′ |
| Coll I NM_000088 | 5′-AAGGGTGAGACAGGCGAACA-3′ 5′-GACCCTGGAGGCCAGAGAA-3′ |
| OSX NM_001173467.3 | 5′-AGAGCAACTGCTGGAGATC-3′ 5′-AAGCAGTGGTCTAGAGAGCC-3′ |
| ALP NM_000478 | 5′-TGCGGAAGAACCCCAAAG-3′ 5′-ATGGTGCCCGTGGTCAAT-3′ |
| 18S NM_003286 | 5′-CGCCGCTAGAGGTGAAATTC-3′ 5′-CATTCTTGGCAAATGCTTTCG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariano, A.; Consalvi, V.; Marchetti, E.; Rodio, A.; Scotto d’Abusco, A.; Fattorini, L. Cell Culture in a Hyperbaric Chamber: A Research Model to Study the Effects of Hyperbarism (Hyperbaric Pressure) on Bone Cell Culture. Cells 2025, 14, 1287. https://doi.org/10.3390/cells14161287
Mariano A, Consalvi V, Marchetti E, Rodio A, Scotto d’Abusco A, Fattorini L. Cell Culture in a Hyperbaric Chamber: A Research Model to Study the Effects of Hyperbarism (Hyperbaric Pressure) on Bone Cell Culture. Cells. 2025; 14(16):1287. https://doi.org/10.3390/cells14161287
Chicago/Turabian StyleMariano, Alessia, Valerio Consalvi, Enrico Marchetti, Angelo Rodio, Anna Scotto d’Abusco, and Luigi Fattorini. 2025. "Cell Culture in a Hyperbaric Chamber: A Research Model to Study the Effects of Hyperbarism (Hyperbaric Pressure) on Bone Cell Culture" Cells 14, no. 16: 1287. https://doi.org/10.3390/cells14161287
APA StyleMariano, A., Consalvi, V., Marchetti, E., Rodio, A., Scotto d’Abusco, A., & Fattorini, L. (2025). Cell Culture in a Hyperbaric Chamber: A Research Model to Study the Effects of Hyperbarism (Hyperbaric Pressure) on Bone Cell Culture. Cells, 14(16), 1287. https://doi.org/10.3390/cells14161287






