p38α MAPK Regulation of Energy Metabolism in Skeletal Muscle Offers a Therapeutic Path for Type 2 Diabetes
Abstract
1. Introduction
1.1. Glucose Homeostasis and Type 2 Diabetes
1.2. Lipid-Induced Insulin Resistance in Peripheral Tissues: Two Prevailing Models
2. P38 MAPK in the Regulation of Glucose Homeostasis and Insulin Sensitivity
2.1. General
2.2. Whole Body Involvement of p38α MAPK in Energy Metabolism Affecting Insulin Sensitivity
2.2.1. Adipocytes
2.2.2. Liver
2.2.3. Pancreatic β Cells
2.2.4. Systemic Involvement of p38α in Energy Metabolism
3. p38α: A Key Regulator of Mitochondrial Biogenesis, Lipid Metabolism and Energy Turnover in Skeletal Muscle (Figure 2)
3.1. Metabolic Adjustment of Skeletal Muscle to Energy Requirements
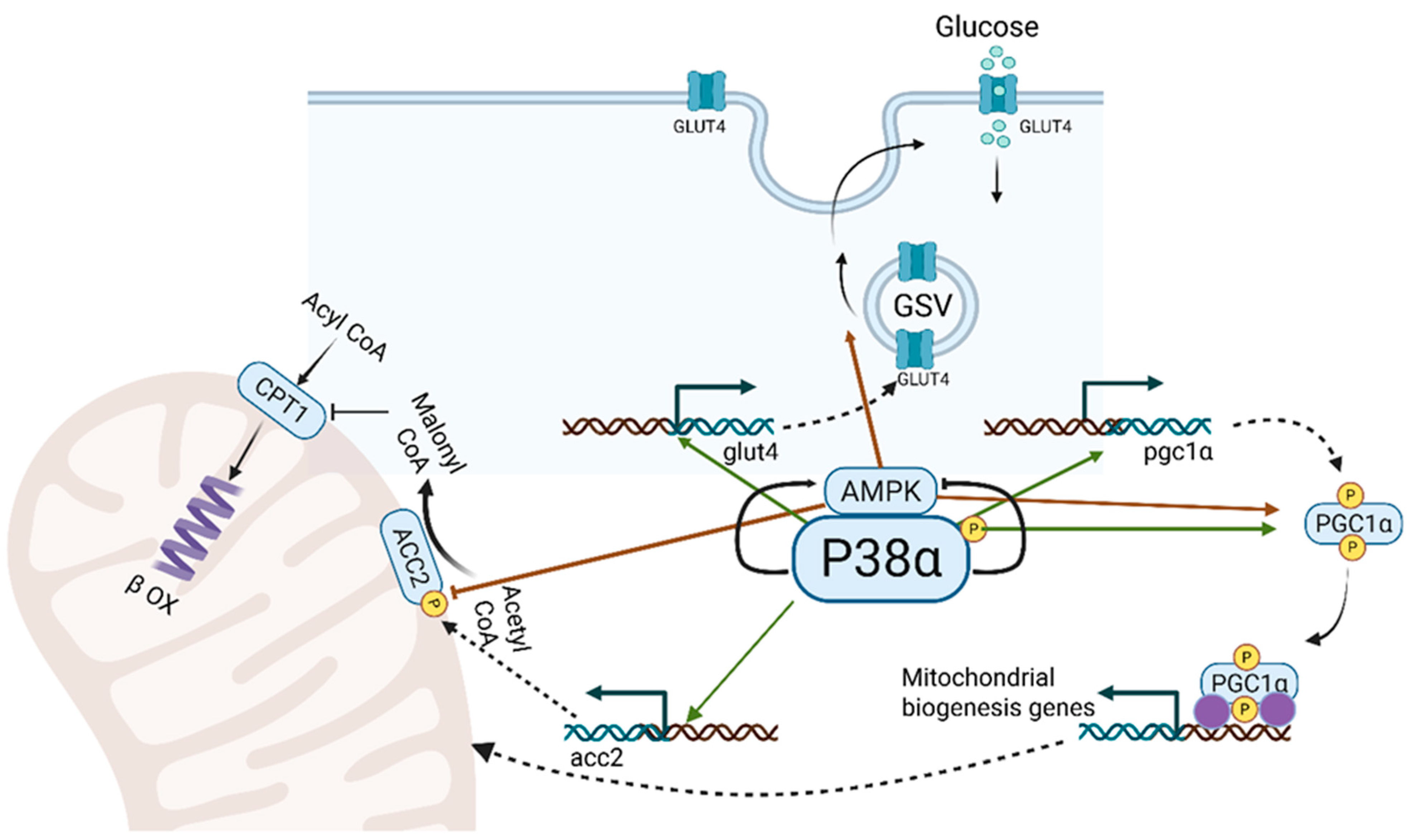
3.2. Metabolic Adaptations of Skeletal Muscle to Nutrient Excess and Energy Demand: Insights from Obesity and Endurance Exercise
3.3. p38α and γ in Mitochondrial Biogenesis
3.4. p38α and Lipid Oxidation
3.5. AMPK Cooperates with P38 MAPK in the Metabolic Adaptation to Muscle (Figure 2)
4. Thoughts About Targeting p38α for the Prevention and Treatment of Type 2 Diabetes
5. Strategies to Augment p38α Pathway Activity (Figure 3)
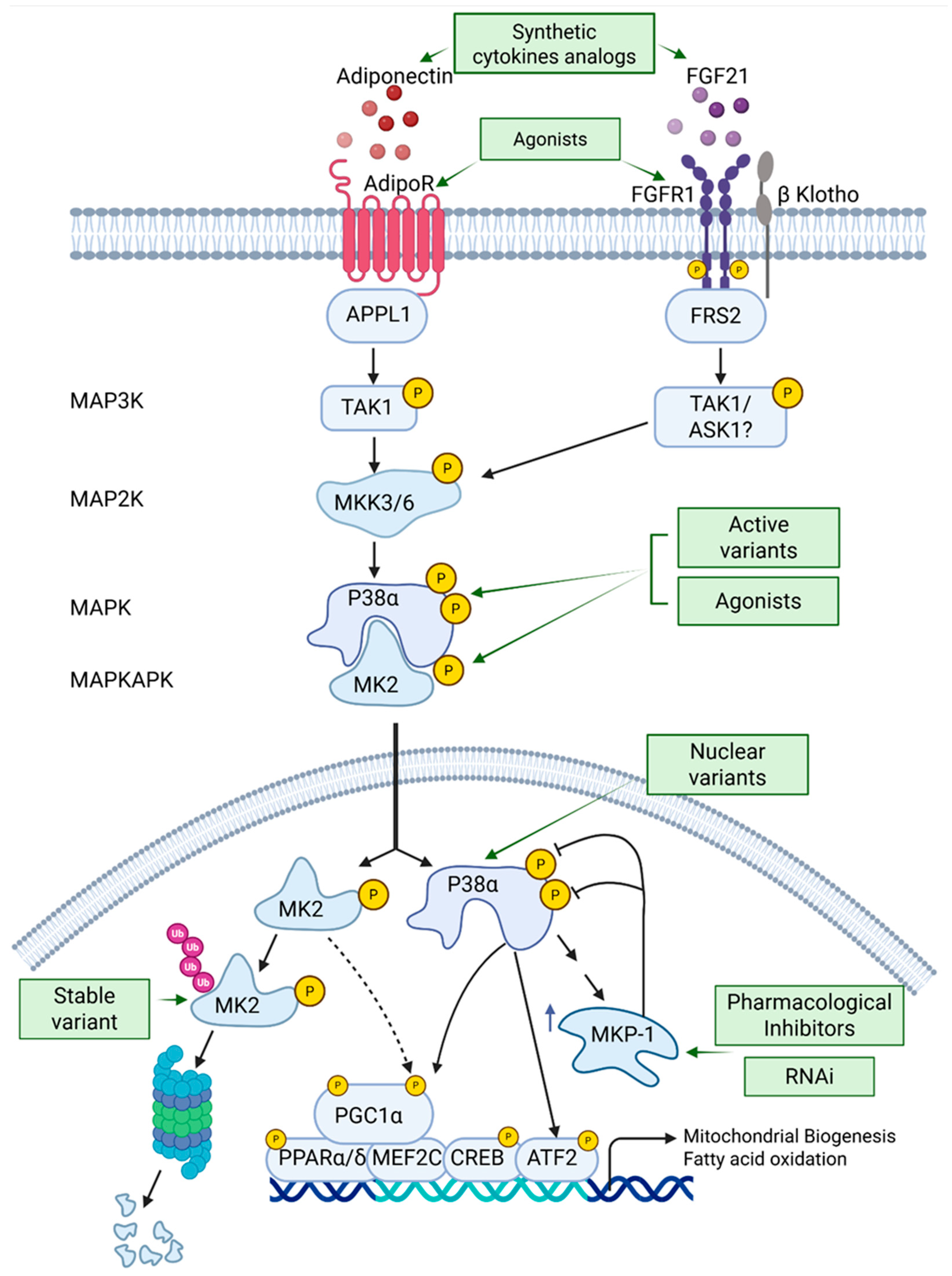
5.1. Expression of p38α Variants in Skeletal Muscles
5.1.1. Intrinsically Active Variants
5.1.2. Expression of Nuclear Localized Variants
5.2. Activation of p38α Targets Such as MK2
5.3. Recombinant Adeno-Associated Virus (rAAV) as a Tool for Therapeutics Expression of Active Variants
5.4. Inhibition of MAPK Phosphatase 1 (MKP1)
5.4.1. Pharmacological Inhibitors
5.4.2. Muscle-Specific Targeting of MKP-1 by Genetic Means
5.5. Treatment with Metabolic Hormones Analogs or Hormone Receptor Agonists That Activate p38α in Skeletal Muscle
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Haslam, D.; Rigby, N. A long look at obesity. Lancet 2010, 376, 85–86. [Google Scholar] [CrossRef]
- da Silva Rosa, S.C.; Nayak, N.; Caymo, A.M.; Gordon, J.W. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol. Rep. 2020, 8, e14607. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Holman, G.D. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E29–E37. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Laakso, M. Lipids and glucose in type 2 diabetes: What is the cause and effect? Diabetes Care 2004, 27, 2253–2259. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- Adams, S.H.; Hoppel, C.L.; Lok, K.H.; Zhao, L.; Wong, S.W.; Minkler, P.E.; Hwang, D.H.; Newman, J.W.; Garvey, W.T. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J. Nutr. 2009, 139, 1073–1081. [Google Scholar] [CrossRef]
- Aguer, C.; McCoin, C.S.; Knotts, T.A.; Thrush, A.B.; Ono-Moore, K.; McPherson, R.; Dent, R.; Hwang, D.H.; Adams, S.H.; Harper, M.E. Acylcarnitines: Potential implications for skeletal muscle insulin resistance. FASEB J. 2015, 29, 336–345. [Google Scholar] [CrossRef]
- Keung, W.; Ussher, J.R.; Jaswal, J.S.; Raubenheimer, M.; Lam, V.H.; Wagg, C.S.; Lopaschuk, G.D. Inhibition of carnitine palmitoyltransferase-1 activity alleviates insulin resistance in diet-induced obese mice. Diabetes 2013, 62, 711–720. [Google Scholar] [CrossRef]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Hwang, D.H.; Newman, J.W.; Garvey, W.T. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef]
- Pereyra, A.S.; Rajan, A.; Ferreira, C.R.; Ellis, J.M. Loss of Muscle Carnitine Palmitoyltransferase 2 Prevents Diet-Induced Obesity and Insulin Resistance despite Long-Chain Acylcarnitine Accumulation. Cell Rep. 2020, 33, 108374. [Google Scholar] [CrossRef] [PubMed]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Sabio, G.; Davis, R.J. cJun NH2-terminal kinase 1 (JNK1): Roles in metabolic regulation of insulin resistance. Trends Biochem. Sci. 2010, 35, 490–496. [Google Scholar] [CrossRef]
- Sabio, G.; Kennedy, N.J.; Cavanagh-Kyros, J.; Jung, D.Y.; Ko, H.J.; Ong, H.; Barrett, T.; Kim, J.K.; Davis, R.J. Role of muscle c-Jun NH2-terminal kinase 1 in obesity-induced insulin resistance. Mol. Cell. Biol. 2010, 30, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Rhee, J.; Lin, J.; Wu, Z.; Yoon, J.; Zhang, C.-Y.; Krauss, S.; Mootha, V.K.; Lowell, B.B.; Spiegelman, B.M. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol. Cell 2001, 8, 971–982. [Google Scholar] [CrossRef]
- Cuenda, A.; Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 2007, 1773, 1358–1375. [Google Scholar] [CrossRef]
- Canovas, B.; Nebreda, A.R. Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 346–366. [Google Scholar] [CrossRef]
- Manieri, E.; Sabio, G. Stress kinases in the modulation of metabolism and energy balance. J. Mol. Endocrinol. 2015, 55, R11–R22. [Google Scholar] [CrossRef]
- Bengal, E.; Aviram, S.; Hayek, T. p38 MAPK in Glucose Metabolism of Skeletal Muscle: Beneficial or Harmful? Int. J. Mol. Sci. 2020, 21, 6480. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, T.; Pohnert, S.C.; Li, P.; Zhang, M.; Gumbs, C.; Rosenberg, P.B.; Williams, R.S.; Yan, Z. Exercise stimulates Pgc-1α transcription in skeletal muscle through activation of the p38 MAPK pathway. J. Biol. Chem. 2005, 280, 19587–19593. [Google Scholar] [CrossRef]
- Fan, M.; Rhee, J.; St-Pierre, J.; Handschin, C.; Puigserver, P.; Lin, J.; Jäeger, S.; Erdjument-Bromage, H.; Tempst, P.; Spiegelman, B.M. Suppression of mitochondrial respiration through recruitment of p160 myb binding protein to PGC-1α: Modulation by p38 MAPK. Genes Dev. 2004, 18, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Aouadi, M.; Caron, L.; Binetruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef]
- Cao, W.; Daniel, K.W.; Robidoux, J.; Puigserver, P.; Medvedev, A.V.; Bai, X.; Floering, L.M.; Spiegelman, B.M.; Collins, S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell. Biol. 2004, 24, 3057–3067. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef]
- Wang, Q.; Li, D.; Cao, G.; Shi, Q.; Zhu, J.; Zhang, M.; Cheng, H.; Wen, Q.; Xu, H.; Zhu, L.; et al. IL-27 signalling promotes adipocyte thermogenesis and energy expenditure. Nature 2021, 600, 314–318. [Google Scholar] [CrossRef]
- Yesian, A.R.; Chalom, M.M.; Knudsen, N.H.; Hyde, A.L.; Personnaz, J.; Cho, H.; Liou, Y.H.; Starost, K.A.; Lee, C.W.; Tsai, D.Y.; et al. Preadipocyte IL-13/IL-13Rα1 signaling regulates beige adipogenesis through modulation of PPARgamma activity. J. Clin. Investig. 2025, 135, e169152. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, N.; Nikolic, I.; Leiva, M.; Pulgarin-Alfaro, M.; Santamans, A.M.; Bernardo, E.; Mora, A.; Herrera-Melle, L.; Rodríguez, E.; Beiroa, D.; et al. p38α blocks brown adipose tissue thermogenesis through p38delta inhibition. PLoS Biol. 2018, 16, e2004455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cao, H.; Li, Y.; Jing, Y.; Liu, S.; Ye, C.; Wang, H.; Yu, S.; Peng, C.; Hui, L.; et al. Metabolic benefits of inhibition of p38α in white adipose tissue in obesity. PLoS Biol. 2018, 16, e2004225. [Google Scholar] [CrossRef]
- Leiva, M.; Matesanz, N.; Pulgarin-Alfaro, M.; Nikolic, I.; Sabio, G. Uncovering the Role of p38 Family Members in Adipose Tissue Physiology. Front. Endocrinol. 2020, 11, 572089. [Google Scholar] [CrossRef]
- Cao, W.; Collins, Q.F.; Becker, T.C.; Robidoux, J.; Lupo, E.G.; Xiong, Y.; Daniel, K.W.; Floering, L.; Collins, S. p38 Mitogen-activated protein kinase plays a stimulatory role in hepatic gluconeogenesis. J. Biol. Chem. 2005, 280, 42731–42737. [Google Scholar] [CrossRef]
- Collins, Q.F.; Xiong, Y.; Lupo, E.G., Jr.; Liu, H.Y.; Cao, W. p38 Mitogen-activated protein kinase mediates free fatty acid-induced gluconeogenesis in hepatocytes. J. Biol. Chem. 2006, 281, 24336–24344. [Google Scholar] [CrossRef]
- Herzig, S.; Long, F.; Jhala, U.S.; Hedrick, S.; Quinn, R.; Bauer, A.; Rudolph, D.; Schutz, G.; Yoon, C.; Puigserver, P.; et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 2001, 413, 179–183. [Google Scholar] [CrossRef]
- Jing, Y.; Liu, W.; Cao, H.; Zhang, D.; Yao, X.; Zhang, S.; Xia, H.; Li, D.; Wang, Y.-C.; Yan, J.; et al. Hepatic p38α regulates gluconeogenesis by suppressing AMPK. J. Hepatol. 2015, 62, 1319–1327. [Google Scholar] [CrossRef]
- Yang, W.; Liao, W.; Li, X.; Ai, W.; Pan, Q.; Shen, Z.; Jiang, W.; Guo, S. Hepatic p38α MAPK controls gluconeogenesis via FOXO1 phosphorylation at S273 during glucagon signalling in mice. Diabetologia 2023, 66, 1322–1339. [Google Scholar] [CrossRef]
- Xiong, Y.; Collins, Q.F.; An, J.; Lupo, E.; Liu, H.-Y.; Liu, D.; Robidoux, J.; Liu, Z.; Cao, W. p38 mitogen-activated protein kinase plays an inhibitory role in hepatic lipogenesis. J. Biol. Chem. 2007, 282, 4975–4982. [Google Scholar] [CrossRef]
- Wu, J.J.; Roth, R.J.; Anderson, E.J.; Hong, E.G.; Lee, M.K.; Choi, C.S.; Neufer, P.D.; Shulman, G.I.; Kim, J.K.; Bennett, A.M. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab. 2006, 4, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.; Sellers, J.; Smith, S.; Bhogoju, S.; Junkins, S.; Welch, M.; Willoughby, O.; Ghimire, N.; Secunda, C.; Barmanova, M.; et al. Metabolic Impact of MKP-2 Upregulation in Obesity Promotes Insulin Resistance and Fatty Liver Disease. Nutrients 2022, 14, 2475. [Google Scholar] [PubMed]
- Lee, J.; Sun, C.; Zhou, Y.; Lee, J.; Gokalp, D.; Herrema, H.; Park, S.W.; Davis, R.J.; Ozcan, U. p38 MAPK-mediated regulation of Xbp1s is crucial for glucose homeostasis. Nat. Med. 2011, 17, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Wang, X.; Rodrigues, R.M.; Ma, J.; He, Y.; Seo, W.; Park, S.H.; Kim, S.; Feng, D.; Gao, B. Protective and Detrimental Roles of p38α Mitogen-Activated Protein Kinase in Different Stages of Nonalcoholic Fatty Liver Disease. Hepatology 2020, 72, 873–891. [Google Scholar] [CrossRef]
- Cicuendez, B.; Ruiz-Garrido, I.; Mora, A.; Sabio, G. Stress kinases in the development of liver steatosis and hepatocellular carcinoma. Mol. Metab. 2021, 50, 101190. [Google Scholar] [CrossRef]
- Darlyuk-Saadon, I.; Bai, C.; Heng, C.K.M.; Gilad, N.; Yu, W.-P.; Lim, P.Y.; Cazenave-Gassiot, A.; Zhang, Y.; Wong, W.S.F.; Engelberg, D. Active p38α causes macrovesicular fatty liver in mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2018069118. [Google Scholar] [CrossRef]
- Gehart, H.; Kumpf, S.; Ittner, A.; Ricci, R. MAPK signalling in cellular metabolism: Stress or wellness? EMBO Rep. 2010, 11, 834–840. [Google Scholar] [CrossRef]
- Ou, Y.; Zheng, Z.; Niu, B.; Su, J.; Su, H. Different MAPK signal transduction pathways play different roles in the impairment of glucose-stimulated insulin secretion in response to IL-1β. Mol. Med. Rep. 2020, 22, 2973–2980. [Google Scholar] [CrossRef]
- Wei, X.; Gu, N.; Feng, N.; Guo, X.; Ma, X. Inhibition of p38 mitogen-activated protein kinase exerts a hypoglycemic effect by improving β cell function via inhibition of β cell apoptosis in db/db mice. J. Enzym. Inhib. Med. Chem. 2018, 33, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.S.M.; Le Guezennec, X.; Demidov, O.N.; Marshall, N.T.; Wang, S.T.; Krishnamurthy, J.; Sharpless, N.E.; Dunn, N.R.; Bulavin, D.V. p38MAPK controls expression of multiple cell cycle inhibitors and islet proliferation with advancing age. Dev. Cell 2009, 17, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Sumara, G.; Formentini, I.; Collins, S.; Sumara, I.; Windak, R.; Bodenmiller, B.; Ramracheya, R.; Caille, D.; Jiang, H.; Platt, K.A.; et al. Regulation of PKD by the MAPK p38δ in Insulin Secretion and Glucose Homeostasis. Cell 2009, 136, 235–248. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Zhao, L.; Chen, D.; Yu, B.; Yu, J. Regulation of fibroblast growth factor 15/19 and 21 on metabolism: In the fed or fasted state. J. Transl. Med. 2016, 14, 63. [Google Scholar] [CrossRef]
- Quesada-López, T.; Cereijo, R.; Turatsinze, J.-V.; Planavila, A.; Cairó, M.; Gavaldà-Navarro, A.; Peyrou, M.; Moure, R.; Iglesias, R.; Giralt, M.; et al. The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat. Commun. 2016, 7, 13479. [Google Scholar] [CrossRef]
- Geller, S.; Arribat, Y.; Netzahualcoyotzi, C.; Lagarrigue, S.; Carneiro, L.; Zhang, L.; Amati, F.; Lopez-Mejia, I.C.; Pellerin, L. Tanycytes Regulate Lipid Homeostasis by Sensing Free Fatty Acids and Signaling to Key Hypothalamic Neuronal Populations via FGF21 Secretion. Cell Metab. 2019, 30, 833–844.e837. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, H.; Cao, X.; Yang, D.; Yan, Y.; Lu, J.; Wang, X.; Wang, H. Endurance Exercise-Induced Fgf21 Promotes Skeletal Muscle Fiber Conversion through TGF-beta1 and p38 MAPK Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 11401. [Google Scholar] [CrossRef]
- Jin, L.; Diaz-Canestro, C.; Wang, Y.; Tse, M.A.; Xu, A. Exerkines and cardiometabolic benefits of exercise: From bench to clinic. EMBO Mol. Med. 2024, 16, 432–444. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Kikani, C.K.; Riojas, R.A.; Langlais, P.; Wang, L.; Ramos, F.J.; Fang, Q.; Christ-Roberts, C.Y.; Hong, J.Y.; Kim, R.Y.; et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol. 2006, 8, 516–523. [Google Scholar] [CrossRef]
- Yoon, M.J.; Lee, G.Y.; Chung, J.J.; Ahn, Y.H.; Hong, S.H.; Kim, J.B. Adiponectin Increases Fatty Acid Oxidation in Skeletal Muscle Cells by Sequential Activation of AMP-Activated Protein Kinase, p38 Mitogen-Activated Protein Kinase, and Peroxisome Proliferator–Activated Receptor α. Diabetes 2006, 55, 2562–2570. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin Stimulates Browning of White Adipocytes Through Mitogen-Activated Protein Kinase p38 MAP Kinase and ERK MAP Kinase Signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef]
- Whitehead, A.; Krause, F.N.; Moran, A.; MacCannell, A.D.V.; Scragg, J.L.; McNally, B.D.; Boateng, E.; Murfitt, S.A.; Virtue, S.; Wright, J.; et al. Brown and Beige Adipose Tissue Regulate Systemic Metabolism through a Metabolite Interorgan Signaling Axis. Nat. Commun. 2021, 12, 1905. [Google Scholar] [CrossRef] [PubMed]
- Zurlo, F.; Larson, K.; Bogardus, C.; Ravussin, E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Investig. 1990, 86, 1423–1427. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J.P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Chinsomboon, J.; Ruas, J.; Gupta, R.K.; Thom, R.; Shoag, J.; Rowe, G.C.; Sawada, N.; Raghuram, S.; Arany, Z. The transcriptional coactivator PGC-1α mediates exercise-induced angiogenesis in skeletal muscle. Proc. Natl. Acad. Sci. USA 2009, 106, 21401–21406. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Katsiaras, A.; Kelley, D.E. Enhanced Fat Oxidation Through Physical Activity Is Associated With Improvements in Insulin Sensitivity in Obesity. Diabetes 2003, 52, 2191–2197. [Google Scholar] [CrossRef]
- Holloszy, J.O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 1967, 242, 2278–2282. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Li, P.; An, J.; Akimoto, T.; Slentz, D.; Ilkayeva, O.; Dohm, G.L.; Yan, Z.; Newgard, C.B.; Muoio, D.M. Peroxisome proliferator-activated receptor-gamma co-activator 1α-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J. Biol. Chem. 2005, 280, 33588–33598. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Sandri, M.; Murgia, M. Activity-Dependent Signaling Pathways Controlling Muscle Diversity and Plasticity. Physiology 2007, 22, 269–278. [Google Scholar] [CrossRef]
- Short, K.R.; Vittone, J.L.; Bigelow, M.L.; Proctor, D.N.; Rizza, R.A.; Coenen-Schimke, J.M.; Nair, K.S. Impact of Aerobic Exercise Training on Age-Related Changes in Insulin Sensitivity and Muscle Oxidative Capacity. Diabetes 2003, 52, 1888–1896. [Google Scholar] [CrossRef]
- Thyfault, J.P.; Cree, M.G.; Zheng, D.; Zwetsloot, J.J.; Tapscott, E.B.; Koves, T.R.; Ilkayeva, O.; Wolfe, R.R.; Muoio, D.M.; Dohm, G.L. Contraction of insulin-resistant muscle normalizes insulin action in association with increased mitochondrial activity and fatty acid catabolism. Am. J. Physiol. Physiol. 2007, 292, C729–C739. [Google Scholar] [CrossRef]
- Coen, P.M.; Dubé, J.J.; Amati, F.; Stefanovic-Racic, M.; Ferrell, R.E.; Toledo, F.G.; Goodpaster, B.H. Insulin Resistance Is Associated With Higher Intramyocellular Triglycerides in Type I but Not Type II Myocytes Concomitant With Higher Ceramide Content. Diabetes 2010, 59, 80–88. [Google Scholar] [CrossRef]
- Wolins, N.E.; Mittendorfer, B. The athlete’s paradOXpat. J. Physiol. 2018, 596, 755–756. [Google Scholar] [CrossRef]
- Fritzen, A.M.; Lundsgaard, A.M.; Kiens, B. Tuning fatty acid oxidation in skeletal muscle with dietary fat and exercise. Nat. Rev. Endocrinol. 2020, 16, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Karamizrak, O.; Noakes, T.D.; Dennis, S.C.; Lambert, E.V. Time Course of the Effects of a High-Fat Diet and Voluntary Exercise on Muscle Enzyme Activity in Long-Evans Rats. Physiol. Behav. 1997, 61, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Powers, S.K.; Duarte, J.; Kavazis, A.N.; Talbert, E.E. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp. Physiol. 2010, 95, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nordgaard, C.; Vind, A.C.; Stonadge, A.; Kjobsted, R.; Snieckute, G.; Antas, P.; Blasius, M.; Reinert, M.S.; Del Val, A.M.; Bekker-Jensen, D.B.; et al. ZAKbeta is activated by cellular compression and mediates contraction-induced MAP kinase signaling in skeletal muscle. EMBO J. 2022, 41, e111650. [Google Scholar] [CrossRef]
- Coffey, V.G.; Zhong, Z.; Shield, A.; Canny, B.J.; Chibalin, A.V.; Zierath, J.R.; Hawley, J.A. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J. 2006, 20, 190–192. [Google Scholar] [CrossRef]
- Yu, M.; Stepto, N.K.; Chibalin, A.V.; Fryer, L.G.; Carling, D.; Krook, A.; Hawley, J.A.; Zierath, J.R. Metabolic and mitogenic signal transduction in human skeletal muscle after intense cycling exercise. J. Physiol. 2003, 546, 327–335. [Google Scholar] [CrossRef]
- Egan, B.; Carson, B.P.; Garcia-Roves, P.M.; Chibalin, A.V.; Sarsfield, F.M.; Barron, N.; McCaffrey, N.; Moyna, N.M.; Zierath, J.R.; O’gOrman, D.J. Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor γ coactivator-1α mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J. Physiol. 2010, 588, 1779–1790. [Google Scholar] [CrossRef]
- Wright, D.C.; Han, D.H.; Garcia-Roves, P.M.; Geiger, P.C.; Jones, T.E.; Holloszy, J.O. Exercise-induced Mitochondrial Biogenesis Begins before the Increase in Muscle PGC-1α Expression. J. Biol. Chem. 2007, 282, 194–199. [Google Scholar] [CrossRef]
- Herrera-Melle, L.; Cicuéndez, B.; López, J.A.; Dumesic, P.A.; Wilensky, S.E.; Rodríguez, E.; Leiva-Vega, L.; Caballero, A.; León, M.; Vázquez, J.; et al. p38α kinase governs muscle strength through PGC1α in mice. Acta Physiol. 2024, 240, e14234. [Google Scholar] [CrossRef]
- Yamauchi, N.; Ashida, Y.; Naito, A.; Tokuda, N.; Niibori, A.; Motohashi, N.; Aoki, Y.; Yamada, T. Fatigue Resistance and Mitochondrial Adaptations to Isometric Interval Training in Dystrophin-Deficient Muscle: Role of Contractile Load. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2025, 39, e70631. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Spiegelman, B.M. Peroxisome proliferator-activated receptor-gamma coactivator 1 α (PGC-1 α): Transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.-Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef]
- Handschin, C.; Chin, S.; Li, P.; Liu, F.; Maratos-Flier, E.; Lebrasseur, N.K.; Yan, Z.; Spiegelman, B.M. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1α muscle-specific knock-out animals. J. Biol. Chem. 2007, 282, 30014–30021. [Google Scholar] [CrossRef]
- Wende, A.R.; Schaeffer, P.J.; Parker, G.J.; Zechner, C.; Han, D.-H.; Chen, M.M.; Hancock, C.R.; Lehman, J.J.; Huss, J.M.; McClain, D.A.; et al. A role for the transcriptional coactivator PGC-1α in muscle refueling. J. Biol. Chem. 2007, 282, 36642–36651. [Google Scholar] [CrossRef]
- Arany, Z.; Foo, S.-Y.; Ma, Y.; Ruas, J.L.; Bommi-Reddy, A.; Girnun, G.; Cooper, M.; Laznik, D.; Chinsomboon, J.; Rangwala, S.M.; et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature 2008, 451, 1008–1012. [Google Scholar] [CrossRef]
- Michael, L.F.; Wu, Z.; Cheatham, R.B.; Puigserver, P.; Adelmant, G.; Lehman, J.J.; Kelly, D.P.; Spiegelman, B.M. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc. Natl. Acad. Sci. USA 2001, 98, 3820–3825. [Google Scholar] [CrossRef]
- Calvo, J.A.; Daniels, T.G.; Wang, X.; Paul, A.; Lin, J.; Spiegelman, B.M.; Stevenson, S.C.; Rangwala, S.M. Muscle-specific expression of PPARgamma coactivator-1α improves exercise performance and increases peak oxygen uptake. J. Appl. Physiol. 2008, 104, 1304–1312. [Google Scholar] [CrossRef]
- Wong, K.E.; Mikus, C.R.; Slentz, D.H.; Seiler, S.E.; DeBalsi, K.L.; Ilkayeva, O.R.; Crain, K.I.; Kinter, M.T.; Kien, C.L.; Stevens, R.D.; et al. Muscle-Specific Overexpression of PGC-1α Does Not Augment Metabolic Improvements in Response to Exercise and Caloric Restriction. Diabetes 2015, 64, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Choi, C.S.; Chin, S.; Kim, S.; Kawamori, D.; Kurpad, A.J.; Neubauer, N.; Hu, J.; Mootha, V.K.; Kim, Y.B.; et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1α knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J. Clin. Investig. 2007, 117, 3463–3474. [Google Scholar] [CrossRef]
- Puigserver, P.; Adelmant, G.; Wu, Z.; Fan, M.; Xu, J.; O’Malley, B.; Spiegelman, B.M. Activation of PPARgamma coactivator-1 through transcription factor docking. Science 1999, 286, 1368–1371. [Google Scholar] [CrossRef]
- McGee, S.L.; Hargreaves, M. Exercise and Myocyte Enhancer Factor 2 Regulation in Human Skeletal Muscle. Diabetes 2004, 53, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Pogozelski, A.R.; Geng, T.; Li, P.; Yin, X.; Lira, V.A.; Zhang, M.; Chi, J.T.; Yan, Z. p38γ mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS ONE 2009, 4, e7934. [Google Scholar] [CrossRef]
- Foster, W.H.; Tidball, J.G.; Wang, Y. p38γ activity is required for maintenance of slow skeletal muscle size. Muscle Nerve. 2012, 45, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Brien, P.; Pugazhendhi, D.; Woodhouse, S.; Oxley, D.; Pell, J.M. p38α MAPK Regulates Adult Muscle Stem Cell Fate by Restricting Progenitor Proliferation During Postnatal Growth and Repair. Stem Cells 2013, 31, 1597–1610. [Google Scholar] [CrossRef]
- Odeh, M.; Tamir-Livne, Y.; Haas, T.; Bengal, E. P38α MAPK coordinates the activities of several metabolic pathways that together induce atrophy of denervated muscles. FEBS J. 2020, 287, 73–93. [Google Scholar] [CrossRef]
- Waingerten-Kedem, L.; Aviram, S.; Blau, A.; Hayek, T.; Bengal, E. P38α MAPK Coordinates Mitochondrial Adaptation to Caloric Surplus in Skeletal Muscle. Int. J. Mol. Sci. 2024, 25, 7789. [Google Scholar] [CrossRef]
- Rynders, C.A.; Blanc, S.; DeJong, N.; Bessesen, D.H.; Bergouignan, A. Sedentary behaviour is a key determinant of metabolic inflexibility. J. Physiol. 2018, 596, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Dohlmann, T.L.; Hindso, M.; Dela, F.; Helge, J.W.; Larsen, S. High-intensity interval training changes mitochondrial respiratory capacity differently in adipose tissue and skeletal muscle. Physiol. Rep. 2018, 6, e13857. [Google Scholar] [CrossRef]
- O’Neill, H.M.; Lally, J.S.; Galic, S.; Thomas, M.; Azizi, P.D.; Fullerton, M.D.; Smith, B.K.; Pulinilkunnil, T.; Chen, Z.; Samaan, M.C.; et al. AMPK phosphorylation of ACC2 is required for skeletal muscle fatty acid oxidation and insulin sensitivity in mice. Diabetologia 2014, 57, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, H.M.; Lally, J.S.; Galic, S.; Pulinilkunnil, T.; Ford, R.J.; Dyck, J.R.; van Denderen, B.J.; Kemp, B.E.; Steinberg, G.R. Skeletal muscle ACC2 S212 phosphorylation is not required for the control of fatty acid oxidation during exercise. Physiol. Rep. 2015, 3, e12444. [Google Scholar] [CrossRef]
- Lawan, A.; Min, K.; Zhang, L.; Canfran-Duque, A.; Jurczak, M.J.; Camporez, J.P.G.; Nie, Y.; Gavin, T.P.; Shulman, G.I.; Fernandez-Hernando, C.; et al. Skeletal Muscle–Specific Deletion of MKP-1 Reveals a p38 MAPK/JNK/Akt Signaling Node That Regulates Obesity-Induced Insulin Resistance. Diabetes 2018, 67, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Winder, W.W.; Hardie, D.G. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am. J. Physiol. Metab. 1996, 270, E299–E304. [Google Scholar] [CrossRef]
- Winder, W.W.; Holmes, B.F.; Rubink, D.S.; Jensen, E.B.; Chen, M.; Holloszy, J.O. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J. Appl. Physiol. 2000, 88, 2219–2226. [Google Scholar] [CrossRef]
- Wojtaszewski, J.F.; Nielsen, P.; Hansen, B.F.; Richter, E.A.; Kiens, B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J. Physiol. 2000, 528 Pt 1, 221–226. [Google Scholar] [CrossRef]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A. PGC-1α regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Combes, A.; Dekerle, J.; Webborn, N.; Watt, P.; Bougault, V.; Daussin, F.N. Exercise-induced metabolic fluctuations influence AMPK, p38-MAPK and CaMKII phosphorylation in human skeletal muscle. Physiol. Rep. 2015, 3, e12462. [Google Scholar] [CrossRef]
- Ihsan, M.; Markworth, J.F.; Watson, G.; Choo, H.C.; Govus, A.; Pham, T.; Hickey, A.; Cameron-Smith, D.; Abbiss, C.R. Regular postexercise cooling enhances mitochondrial biogenesis through AMPK and p38 MAPK in human skeletal muscle. Am. J. Physiol. Integr. Comp. Physiol. 2015, 309, R286–R294. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; McGee, S.L.; Garnham, A.P.; Howlett, K.F.; Snow, R.J.; Hargreaves, M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1α in human skeletal muscle. J. Appl. Physiol. 2009, 106, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Tanno, M.; Bassi, R.; Gorog, D.A.; Saurin, A.T.; Jiang, J.; Heads, R.J.; Martin, J.L.; Davis, R.J.; Flavell, R.A.; Marber, M.S. Diverse mechanisms of myocardial p38 mitogen-activated protein kinase activation: Evidence for MKK-independent activation by a TAB1-associated mechanism contributing to injury during myocardial ischemia. Circ. Res. 2003, 93, 254–261. [Google Scholar] [CrossRef]
- Li, J.; Miller, E.J.; Ninomiya-Tsuji, J.; Russell, R.R., III; Young, L.H. AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MAPK to TAB1 in the ischemic heart. Circ. Res. 2005, 97, 872–879. [Google Scholar] [CrossRef]
- Ho, R.C.; Fujii, N.; Witters, L.A.; Hirshman, M.F.; Goodyear, L.J. Dissociation of AMP-activated protein kinase and p38 mitogen-activated protein kinase signaling in skeletal muscle. Biochem. Biophys. Res. Commun. 2007, 362, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Zhou, L.; Reyes, C.M.; Liu, F.; Dong, L.Q. APPL1 mediates adiponectin-stimulated p38 MAPK activation by scaffolding the TAK1-MKK3-p38 MAPK pathway. Am. J. Physiol. Metab. 2011, 300, E103–E110. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef]
- Hindi, S.M.; Sato, S.; Xiong, G.; Bohnert, K.R.; Gibb, A.A.; Gallot, Y.S.; McMillan, J.D.; Hill, B.G.; Uchida, S.; Kumar, A. TAK1 regulates skeletal muscle mass and mitochondrial function. JCI Insight 2018, 3, e98441. [Google Scholar] [CrossRef]
- Irrcher, I.; Ljubicic, V.; Kirwan, A.F.; Hood, D.A. AMP-Activated Protein Kinase-Regulated Activation of the PGC-1α Promoter in Skeletal Muscle Cells. PLoS ONE 2008, 3, e3614. [Google Scholar] [CrossRef]
- Boden, G.; Shulman, G.I. Free fatty acids in obesity and type 2 diabetes: Defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Investig. 2002, 32 (Suppl. 3), 14–23. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef]
- Sin, T.K.; Zhang, G.; Zhang, Z.; Zhu, J.Z.; Zuo, Y.; Frost, J.A.; Li, M.; Li, Y.P. Cancer-Induced Muscle Wasting Requires p38β MAPK Activation of p300. Cancer Res. 2021, 81, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Li, L.; Su, Z.; Wei, L.; Pu, W.; Zhao, C.; Ding, Y.; Wazir, J.; Cao, W.; Song, S.; et al. An integrative transcriptome study reveals Ddit4/Redd1 as a key regulator of cancer cachexia in rodent models. Cell Death Dis 2021, 12, 652. [Google Scholar] [CrossRef] [PubMed]
- Belova, S.P.; Mochalova, E.P.; Kostrominova, T.Y.; Shenkman, B.S.; Nemirovskaya, T.L. P38α-MAPK Signaling Inhibition Attenuates Soleus Atrophy during Early Stages of Muscle Unloading. Int. J. Mol. Sci. 2020, 21, 2756. [Google Scholar] [CrossRef] [PubMed]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef]
- Narkar, V.A.; Downes, M.; Yu, R.T.; Embler, E.; Wang, Y.X.; Banayo, E.; Mihaylova, M.M.; Nelson, M.C.; Zou, Y.; Juguilon, H.; et al. AMPK and PPARdelta agonists are exercise mimetics. Cell 2008, 134, 405–415. [Google Scholar] [CrossRef]
- Qiao, L.; MacDougald, O.A.; Shao, J. CCAAT/enhancer-binding protein α mediates induction of hepatic phosphoenolpyruvate carboxykinase by p38 mitogen-activated protein kinase. J. Biol. Chem. 2006, 281, 24390–24397. [Google Scholar] [CrossRef]
- Jorgensen, S.B.; Richter, E.A.; Wojtaszewski, J.F. Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. J. Physiol. 2006, 574, 17–31. [Google Scholar] [CrossRef]
- Kjobsted, R.; Roll, J.L.W.; Jorgensen, N.O.; Birk, J.B.; Foretz, M.; Viollet, B.; Chadt, A.; Al-Hasani, H.; Wojtaszewski, J.F.P. AMPK and TBC1D1 Regulate Muscle Glucose Uptake After, but Not During, Exercise and Contraction. Diabetes 2019, 68, 1427–1440. [Google Scholar] [CrossRef]
- Koistinen, H.A.; Chibalin, A.V.; Zierath, J.R. Aberrant p38 mitogen-activated protein kinase signalling in skeletal muscle from Type 2 diabetic patients. Diabetologia 2003, 46, 1324–1328. [Google Scholar] [CrossRef]
- Leng, Y.; Steiler, T.L.; Zierath, J.R. Effects of insulin, contraction, and phorbol esters on mitogen-activated protein kinase signaling in skeletal muscle from lean and ob/ob mice. Diabetes 2004, 53, 1436–1444. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, J.; Yan, W.; Zhou, K.; Cao, Y.; Cai, W. p38α MAPK antagonizing JNK to control the hepatic fat accumulation in pediatric patients onset intestinal failure. Cell Death Dis. 2017, 8, e3110. [Google Scholar] [CrossRef]
- Qiu, B.; Lawan, A.; Xirouchaki, C.E.; Yi, J.S.; Robert, M.; Zhang, L.; Brown, W.; Fernandez-Hernando, C.; Yang, X.; Tiganis, T.; et al. MKP1 promotes nonalcoholic steatohepatitis by suppressing AMPK activity through LKB1 nuclear retention. Nat. Commun. 2023, 14, 5405. [Google Scholar] [CrossRef] [PubMed]
- Heng, C.K.M.; Gilad, N.; Darlyuk-Saadon, I.; Wong, W.S.F.; Engelberg, D. Targeting the p38α pathway in chronic inflammatory diseases: Could activation, not inhibition, be the appropriate therapeutic strategy? Pharmacol. Ther. 2022, 235, 108153. [Google Scholar] [CrossRef]
- Maik-Rachline, G.; Lifshits, L.; Seger, R. Nuclear P38: Roles in Physiological and Pathological Processes and Regulation of Nuclear Translocation. Int. J. Mol. Sci. 2020, 21, 6102. [Google Scholar] [CrossRef]
- Morrison, D.K.; Davis, R.J. Regulation of MAP Kinase Signaling Modules by Scaffold Proteins in Mammals. Annu. Rev. Cell Dev. Biol. 2003, 19, 91–118. [Google Scholar] [CrossRef]
- Ben-Levy, R.; Hooper, S.; Wilson, R.; Paterson, H.F.; Marshall, C.J. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr. Biol. 1998, 8, 1049–1057. [Google Scholar] [CrossRef]
- Bulaklak, K.; Xiao, X. Therapeutic advances in musculoskeletal AAV targeting approaches. Curr. Opin. Pharmacol. 2017, 34, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bassel-Duby, R.; Olson, E.N. CRISPR-Cas9 Correction of Duchenne Muscular Dystrophy in Mice by a Self-Complementary AAV Delivery System. Methods Mol. Biol. 2023, 2587, 411–425. [Google Scholar] [PubMed]
- Caunt, C.J.; Keyse, S.M. Dual-specificity MAP kinase phosphatases (MKPs): Shaping the outcome of MAP kinase signalling. FEBS J. 2013, 280, 489–504. [Google Scholar] [CrossRef]
- Dickinson, R.J.; Keyse, S.M. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J. Cell Sci. 2006, 119, 4607–4615. [Google Scholar] [CrossRef]
- Lawan, A.; Zhang, L.; Gatzke, F.; Min, K.; Jurczak, M.J.; Al-Mutairi, M.; Richter, P.; Camporez, J.P.; Couvillon, A.; Pesta, D.; et al. Hepatic Mitogen-Activated Protein Kinase Phosphatase 1 Selectively Regulates Glucose Metabolism and Energy Homeostasis. Mol. Cell. Biol. 2015, 35, 26–40. [Google Scholar] [CrossRef]
- Bennett, A.M.; Lawan, A. Improving Obesity and Insulin Resistance by Targeting Skeletal Muscle MKP-1. J. Cell. Signal. 2020, 1, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Gumpena, R.; Lountos, G.T.; Raran-Kurussi, S.; Tropea, J.E.; Cherry, S.; Waugh, D.S. Crystal structure of the human dual specificity phosphatase 1 catalytic domain. Protein Sci. 2018, 27, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Doddareddy, M.R.; Rawling, T.; Ammit, A.J. Targeting Mitogen-Activated Protein Kinase Phosphatase-1 (MKP-1): Structure-Based Design of MKP-1 Inhibitors and Upregulators. Curr. Med. Chem. 2012, 19, 163–173. [Google Scholar] [CrossRef]
- Xia, X.; Pollock, N.; Zhou, J.; Rossi, J. Tissue-Specific Delivery of Oligonucleotides. Methods Mol. Biol. 2019, 2036, 17–50. [Google Scholar] [PubMed]
- Giragossian, C.; Vage, C.; Li, J.; Pelletier, K.; Piche-Nicholas, N.; Rajadhyaksha, M.; Liras, J.; Logan, A.; Calle, R.A.; Weng, Y. Mechanistic investigation of the preclinical pharmacokinetics and interspecies scaling of PF-05231023, a fibroblast growth factor 21-antibody protein conjugate. Drug Metab. Dispos. Biol. Fate Chem. 2015, 43, 803–811. [Google Scholar] [CrossRef]
- Guo, Y.; Bao, Y.; Chen, Z.; Rao, Z.; Luo, Y.; Ye, S.; Liu, S. Novel FGF21 analogues through structure-based optimization for therapeutic development. Acta Biochim. Biophys. Sin. 2024, 57, 582–587. [Google Scholar] [CrossRef]
- Ahuja, A.; Zboinski, E.; das, S.; Zhu, X.; Ma, Q.; Xie, Y.; Tu, Q.; Chen, J. Antidiabetic features of AdipoAI, a novel AdipoR agonist. Cell Biochem. Funct. 2024, 42, e3910. [Google Scholar] [CrossRef]
- Iwabu, M.; Okada-Iwabu, M.; Tanabe, H.; Ohuchi, N.; Miyata, K.; Kobori, T.; Odawara, S.; Kadowaki, Y.; Yokoyama, S.; Yamauchi, T.; et al. AdipoR agonist increases insulin sensitivity and exercise endurance in AdipoR-humanized mice. Commun. Biol. 2021, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Joharapurkar, A.A.; Khan, M.P.; Mishra, J.S.; Singh, N.; Yadav, M.; Hossain, Z.; Khan, K.; Kumar, S.; Dhanesha, N.A.; et al. Orally Active Osteoanabolic Agent GTDF Binds to Adiponectin Receptors, With a Preference for AdipoR1, Induces Adiponectin-Associated Signaling, and Improves Metabolic Health in a Rodent Model of Diabetes. Diabetes 2014, 63, 3530–3544. [Google Scholar] [CrossRef]
- Keipert, S.; Ost, M. Stress-induced FGF21 and GDF15 in obesity and obesity resistance. Trends Endocrinol. Metab. 2021, 32, 904–915. [Google Scholar] [CrossRef]
- Fisher, F.M.; Chui, P.C.; Antonellis, P.J.; Bina, H.A.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E. Obesity Is a Fibroblast Growth Factor 21 (FGF21)-Resistant State. Diabetes 2010, 59, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.Y.; Koh, M.S.; Moon, A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin. Investig. Drugs 2009, 18, 1893–1905. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bengal, E.; Aviram, S. p38α MAPK Regulation of Energy Metabolism in Skeletal Muscle Offers a Therapeutic Path for Type 2 Diabetes. Cells 2025, 14, 1277. https://doi.org/10.3390/cells14161277
Bengal E, Aviram S. p38α MAPK Regulation of Energy Metabolism in Skeletal Muscle Offers a Therapeutic Path for Type 2 Diabetes. Cells. 2025; 14(16):1277. https://doi.org/10.3390/cells14161277
Chicago/Turabian StyleBengal, Eyal, and Sharon Aviram. 2025. "p38α MAPK Regulation of Energy Metabolism in Skeletal Muscle Offers a Therapeutic Path for Type 2 Diabetes" Cells 14, no. 16: 1277. https://doi.org/10.3390/cells14161277
APA StyleBengal, E., & Aviram, S. (2025). p38α MAPK Regulation of Energy Metabolism in Skeletal Muscle Offers a Therapeutic Path for Type 2 Diabetes. Cells, 14(16), 1277. https://doi.org/10.3390/cells14161277




