Fibroblasts Attenuate Anti-Tumor Drug Efficacy in Tumor Cells via Paracrine Interactions with Tumor Cells in 3D Plexiform Neurofibroma Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Cells and Cell Maintenance
2.3. Three-Dimensional (3D)/4D (3D in Real-Time) Culture Models
2.4. Image Acquisition for Quantitative Analysis in 3D
2.5. Drug Treatments
2.6. Live-Cell LIVE/DEADTM Assay
2.7. Generation of Fibroblast-Conditioned Media (CM)
2.8. Immunofluorescence Staining
2.9. 3D MTT Assay
2.10. Statistical Analysis
3. Results
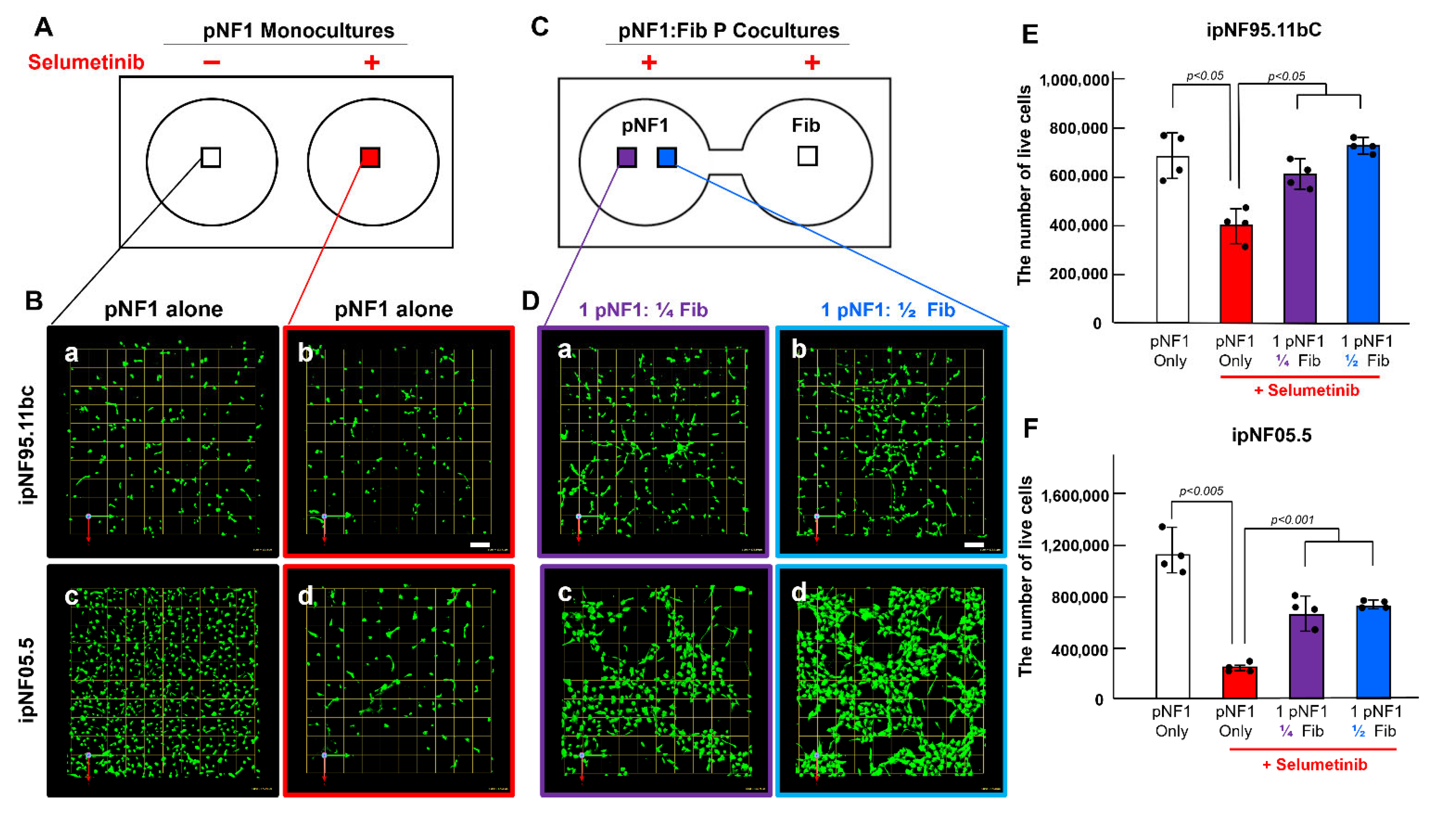
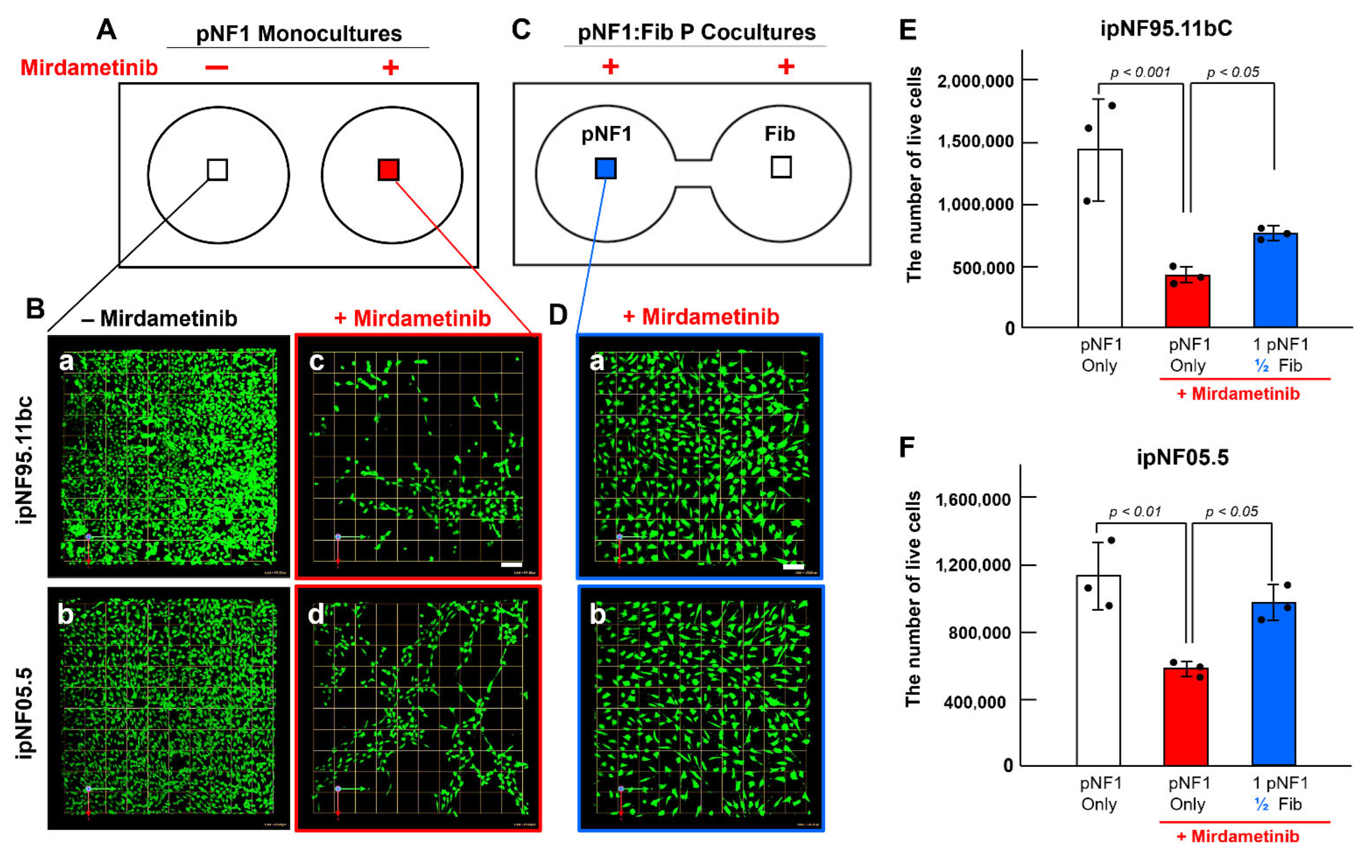
3.1. Paracrine Interactions Between 3D pNF1 Tumor Structures and Fibroblasts Increase Drug Resistance in 3D pNF1 Culture Model
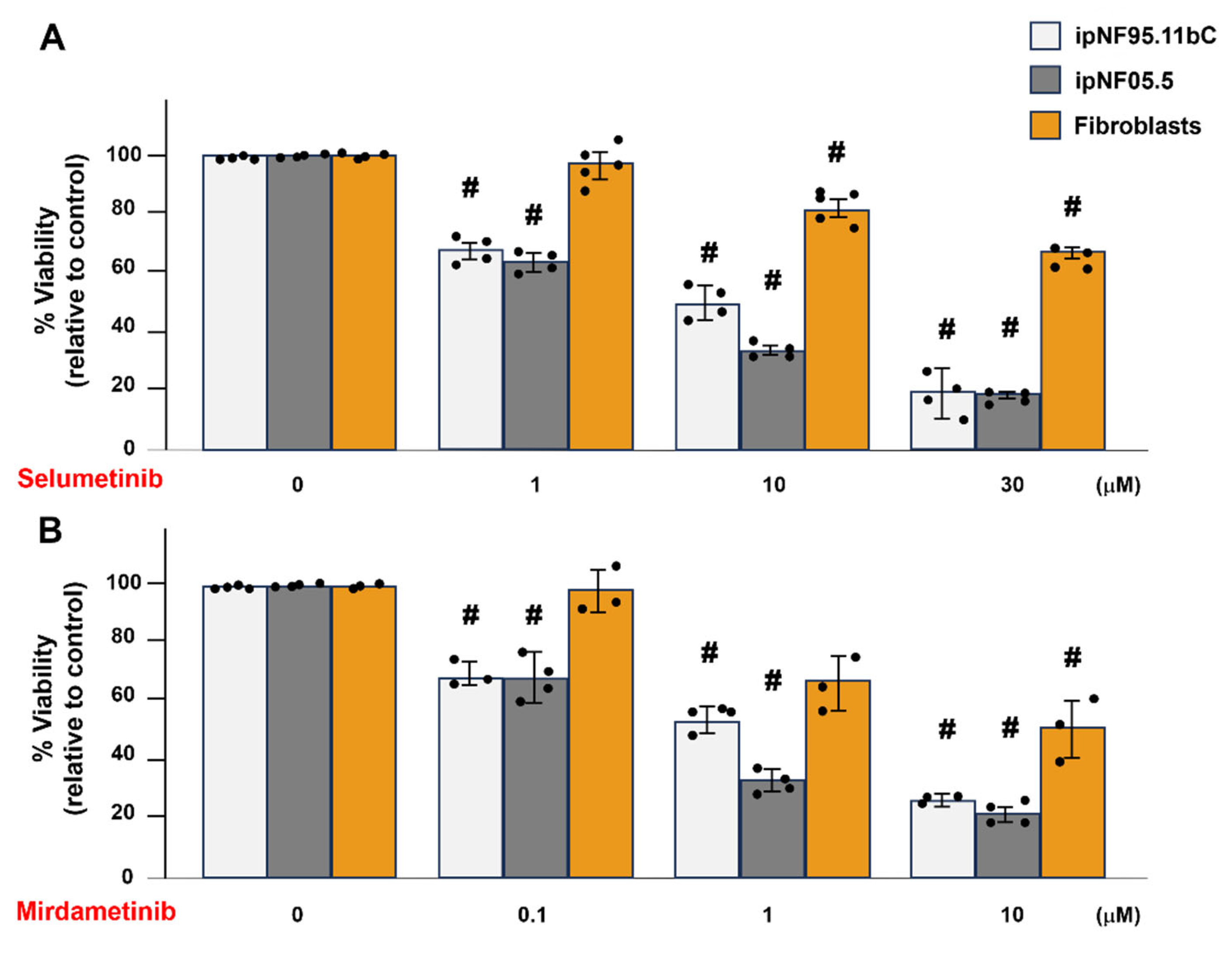
3.2. Fibroblasts Show Greater Resistance than pNF1 Tumor Structures to the Treatment of Selumetinib or Mirdametinib in 3D Cultures
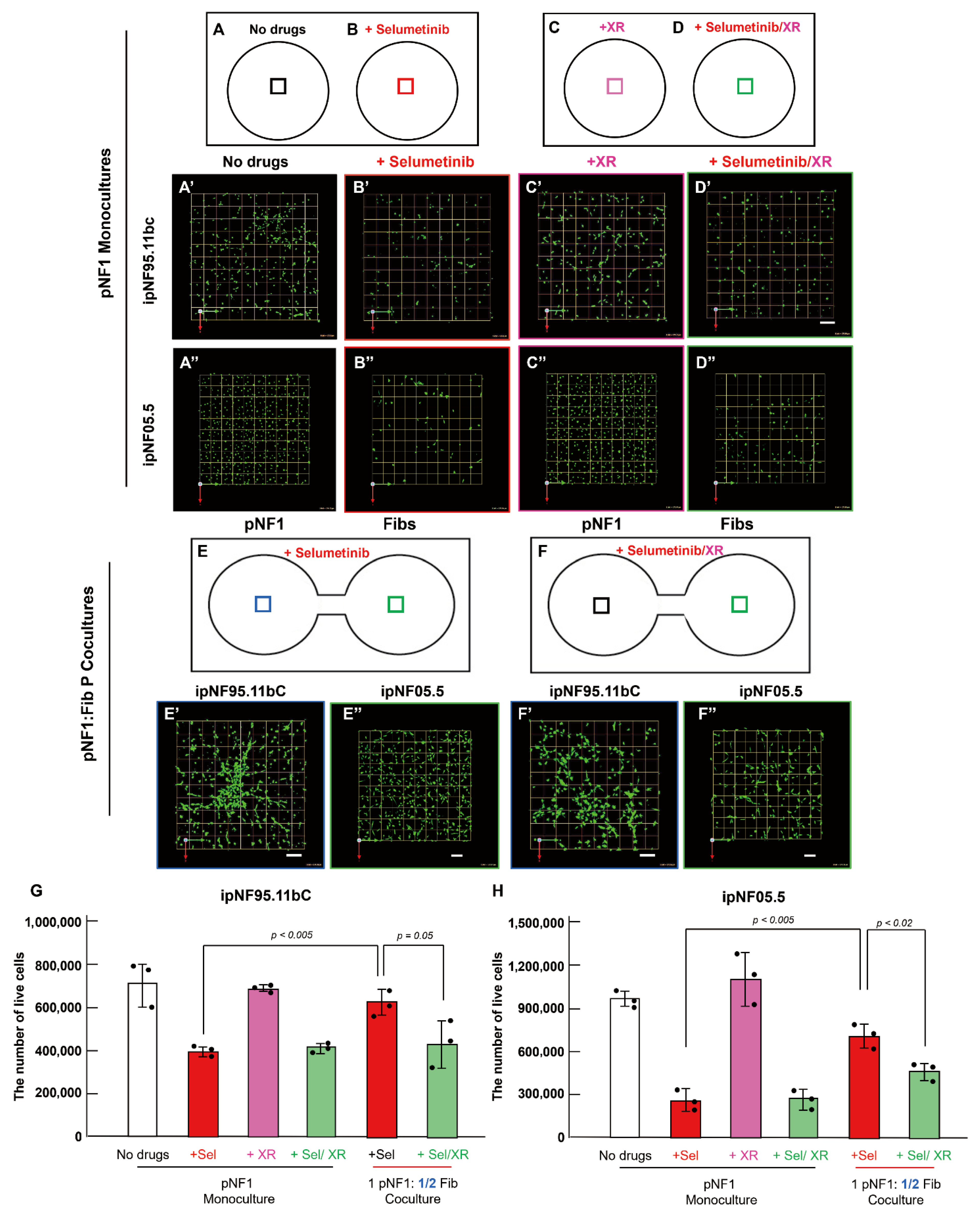
3.3. P-Glycoprotein Contributes to Fibroblast-Derived Secretome-Mediated Drug Resistance in pNF1 Tumor Structures
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cimino, P.J.; Gutmann, D.H. Neurofibromatosis type 1. Handb. Clin. Neurol. 2018, 148, 799–811. [Google Scholar] [CrossRef]
- Gutmann, D.H.; Ferner, R.E.; Listernick, R.H.; Korf, B.R.; Wolters, P.L.; Johnson, K.J. Neurofibromatosis type 1. Nat. Rev. Dis. Primers 2017, 3, 17004. [Google Scholar] [CrossRef] [PubMed]
- Tucker, T.; Friedman, J.M.; Friedrich, R.E.; Wenzel, R.; Funsterer, C.; Mautner, V.F. Longitudinal study of neurofibromatosis 1 associated plexiform neurofibromas. J. Med. Genet. 2009, 46, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Lakkis, M.M.; Tennekoon, G.I. Neurofibromatosis type 1. I. General overview. J. Neurosci. Res. 2000, 62, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Ferner, R.E.; Huson, S.M.; Thomas, N.; Moss, C.; Willshaw, H.; Evans, D.G.; Upadhyaya, M.; Towers, R.; Gleeson, M.; Steiger, C.; et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J. Med. Genet. 2007, 44, 81–88. [Google Scholar] [CrossRef]
- Katz, D.; Lazar, A.; Lev, D. Malignant peripheral nerve sheath tumour (MPNST): The clinical implications of cellular signalling pathways. Expert. Rev. Mol. Med. 2009, 11, e30. [Google Scholar] [CrossRef]
- FDA. FDA Approves Selumetinib for Neurofibromatosis Type 1 with Symptomatic, Inoperable Plexiform Neurofibromas. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selumetinib-neurofibromatosis-type-1-symptomatic-inoperable-plexiform-neurofibromas (accessed on 13 April 2020).
- FDA. Drugs@FDA: FDA-Approved Drugs_KOSELUGO (Selumetinib). Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process (accessed on 13 July 2025).
- FDA. FDA Approves Mirdametinib for Adult and Pediatric Patients with Neurofibromatosis Type 1 Who Have Symptomatic Plexiform Neurofibromas not Amenable to Complete Resection. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-mirdametinib-adult-and-pediatric-patients-neurofibromatosis-type-1-who-have-symptomatic#:~:text=On%20February%2011%2C%202025%2C%20the,not%20amenable%20to%20complete%20resection. (accessed on 22 April 2025).
- FDA. Drugs@FDA: FDA-Approved Drugs_GOMEKLI (Mirdametinib). Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=219379 (accessed on 13 July 2025).
- Gross, A.M.; Wolters, P.L.; Dombi, E.; Baldwin, A.; Whitcomb, P.; Fisher, M.J.; Weiss, B.; Kim, A.; Bornhorst, M.; Shah, A.C.; et al. Selumetinib in Children with Inoperable Plexiform Neurofibromas. N. Engl. J. Med. 2020, 382, 1430–1442. [Google Scholar] [CrossRef]
- Moertel, C.L.; Hirbe, A.C.; Shuhaiber, H.H.; Bielamowicz, K.; Sidhu, A.; Viskochil, D.; Weber, M.D.; Lokku, A.; Smith, L.M.; Foreman, N.K.; et al. ReNeu: A Pivotal, Phase IIb Trial of Mirdametinib in Adults and Children with Symptomatic Neurofibromatosis Type 1-Associated Plexiform Neurofibroma. J. Clin. Oncol. 2025, 43, 716–729. [Google Scholar] [CrossRef]
- Staser, K.; Yang, F.C.; Clapp, D.W. Pathogenesis of plexiform neurofibroma: Tumor-stromal/hematopoietic interactions in tumor progression. Annu. Rev. Pathol. 2012, 7, 469–495. [Google Scholar] [CrossRef]
- Le, L.Q.; Parada, L.F. Tumor microenvironment and neurofibromatosis type I: Connecting the GAPs. Oncogene 2007, 26, 4609–4616. [Google Scholar] [CrossRef]
- Le, L.Q.; Shipman, T.; Burns, D.K.; Parada, L.F. Cell of origin and microenvironment contribution for NF1-associated dermal neurofibromas. Cell Stem Cell 2009, 4, 453–463. [Google Scholar] [CrossRef]
- Brosseau, J.P.; Sathe, A.A.; Wang, Y.; Nguyen, T.; Glass, D.A., II; Xing, C.; Le, L.Q. Human cutaneous neurofibroma matrisome revealed by single-cell RNA sequencing. Acta Neuropathol. Commun. 2021, 9, 11. [Google Scholar] [CrossRef]
- Wang, W.N.; Koguchi-Yoshioka, H.; Nimura, K.; Watanabe, R.; Tanemura, A.; Fujimoto, M.; Wataya-Kaneda, M. Distinct Transcriptional Profiles in the Different Phenotypes of Neurofibroma from the Same Subject with Neurofibromatosis 1. J. Investig. Dermatol. 2024, 144, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, J.; Penttinen, R.; Larjava, H.; Aho, H.J. Collagens in neurofibromas and neurofibroma cell cultures. Ann. N. Y. Acad. Sci. 1986, 486, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Atit, R.P.; Crowe, M.J.; Greenhalgh, D.G.; Wenstrup, R.J.; Ratner, N. The Nf1 tumor suppressor regulates mouse skin wound healing, fibroblast proliferation, and collagen deposited by fibroblasts. J. Investig. Dermatol. 1999, 112, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Mazuelas, H.; Magallon-Lorenz, M.; Fernandez-Rodriguez, J.; Uriarte-Arrazola, I.; Richaud-Patin, Y.; Terribas, E.; Villanueva, A.; Castellanos, E.; Blanco, I.; Raya, A.; et al. Modeling iPSC-derived human neurofibroma-like tumors in mice uncovers the heterogeneity of Schwann cells within plexiform neurofibromas. Cell Rep. 2022, 38, 110385. [Google Scholar] [CrossRef]
- Yang, F.C.; Chen, S.; Clegg, T.; Li, X.; Morgan, T.; Estwick, S.A.; Yuan, J.; Khalaf, W.; Burgin, S.; Travers, J.; et al. Nf1+/− mast cells induce neurofibroma like phenotypes through secreted TGF-beta signaling. Hum. Mol. Genet. 2006, 15, 2421–2437. [Google Scholar] [CrossRef]
- Ji, K.; Schwenkel, G.J.; Mattingly, R.R.; Sundararaghavan, H.G.; Zhang, Z.G.; Chopp, M. A Fibroblast-Derived Secretome Stimulates the Growth and Invasiveness of 3D Plexiform Neurofibroma Spheroids. Cancers 2024, 16, 2498. [Google Scholar] [CrossRef]
- Kraniak, J.M.; Chalasani, A.; Wallace, M.R.; Mattingly, R.R. Development of 3D culture models of plexiform neurofibroma and initial application for phenotypic characterization and drug screening. Exp. Neurol. 2018, 299, 289–298. [Google Scholar] [CrossRef]
- Ji, K.; Zhao, Z.; Sameni, M.; Moin, K.; Xu, Y.; Gillies, R.J.; Sloane, B.F.; Mattingly, R.R. Modeling Tumor: Lymphatic Interactions in Lymphatic Metastasis of Triple Negative Breast Cancer. Cancers 2021, 13, 6044. [Google Scholar] [CrossRef]
- Chalasani, A.; Ji, K.; Sameni, M.; Mazumder, S.H.; Xu, Y.; Moin, K.; Sloane, B.F. Live-Cell Imaging of Protease Activity: Assays to Screen Therapeutic Approaches. Methods Mol. Biol. 2017, 1574, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, L.; Wagner, P.; Ibrahim, N.; Rivera, E.; Theriault, R.; Booser, D.; Symmans, F.W.; Wong, F.; Blumenschein, G.; Fleming, D.R.; et al. Phase II study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer 2005, 104, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.; Bates, S.E. Tariquidar (XR9576): A P-glycoprotein drug efflux pump inhibitor. Expert. Rev. Anticancer Ther. 2007, 7, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, S.D.; Sharma, R.; White, J.K.; Aggarwal, N.; Chalasani, A.; Sameni, M.; Moin, K.; Vieira, P.C.; Turro, C.; Kodanko, J.J.; et al. Imaging Sites of Inhibition of Proteolysis in Pathomimetic Human Breast Cancer Cultures by Light-Activated Ruthenium Compound. PLoS ONE 2015, 10, e0142527. [Google Scholar] [CrossRef]
- Sameni, M.; Tovar, E.A.; Essenburg, C.J.; Chalasani, A.; Linklater, E.S.; Borgman, A.; Cherba, D.M.; Anbalagan, A.; Winn, M.E.; Graveel, C.R.; et al. Cabozantinib (XL184) Inhibits Growth and Invasion of Preclinical TNBC Models. Clin. Cancer Res. 2016, 22, 923–934. [Google Scholar] [CrossRef]
- Sameni, M.; Cavallo-Medved, D.; Franco, O.E.; Chalasani, A.; Ji, K.; Aggarwal, N.; Anbalagan, A.; Chen, X.; Mattingly, R.R.; Hayward, S.W.; et al. Pathomimetic avatars reveal divergent roles of microenvironment in invasive transition of ductal carcinoma in situ. Breast Cancer Res. 2017, 19, 56. [Google Scholar] [CrossRef]
- Callaghan, R.; Luk, F.; Bebawy, M. Inhibition of the multidrug resistance P-glycoprotein: Time for a change of strategy? Drug Metab. Dispos. 2014, 42, 623–631. [Google Scholar] [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef]
- Lai, J.I.; Tseng, Y.J.; Chen, M.H.; Huang, C.F.; Chang, P.M. Clinical Perspective of FDA Approved Drugs with P-Glycoprotein Inhibition Activities for Potential Cancer Therapeutics. Front. Oncol. 2020, 10, 561936. [Google Scholar] [CrossRef]
- Dong, J.; Yuan, L.; Hu, C.; Cheng, X.; Qin, J.J. Strategies to overcome cancer multidrug resistance (MDR) through targeting P-glycoprotein (ABCB1): An updated review. Pharmacol. Ther. 2023, 249, 108488. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hou, J.; Yu, S.; Li, W.; Wang, X.; Sun, H.; Qin, T.; Claret, F.X.; Guo, H.; Liu, Z. Role of cancer-associated fibroblasts in the resistance to antitumor therapy, and their potential therapeutic mechanisms in non-small cell lung cancer. Oncol. Lett. 2021, 21, 413. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Wu, J.; Shen, B.; Jiang, F.; Feng, J. Cancer-associated fibroblasts and resistance to anticancer therapies: Status, mechanisms, and countermeasures. Cancer Cell Int. 2022, 22, 166. [Google Scholar] [CrossRef] [PubMed]
- Rizzolio, S.; Giordano, S.; Corso, S. The importance of being CAFs (in cancer resistance to targeted therapies). J. Exp. Clin. Cancer Res. 2022, 41, 319. [Google Scholar] [CrossRef]
- Yang, D.; Liu, J.; Qian, H.; Zhuang, Q. Cancer-associated fibroblasts: From basic science to anticancer therapy. Exp. Mol. Med. 2023, 55, 1322–1332. [Google Scholar] [CrossRef]
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef]
- Gillet, J.P.; Gottesman, M.M. Mechanisms of multidrug resistance in cancer. Methods Mol. Biol. 2010, 596, 47–76. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Bharadwaj, A.; Rastogi, A.; Pandey, S.; Gupta, S.; Sohal, J.S. Multidrug-Resistant Bacteria: Their Mechanism of Action and Prophylaxis. Biomed. Res. Int. 2022, 2022, 5419874. [Google Scholar] [CrossRef]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci. Rep. 2018, 8, 967. [Google Scholar] [CrossRef]
- Wu, C.P.; Hung, C.Y.; Murakami, M.; Wu, Y.S.; Lin, C.L.; Huang, Y.H.; Hung, T.H.; Yu, J.S.; Ambudkar, S.V. P-glycoprotein Mediates Resistance to the Anaplastic Lymphoma Kinase Inhiitor Ensartinib in Cancer Cells. Cancers 2022, 14, 2341. [Google Scholar] [CrossRef]
- Mistry, P.; Stewart, A.J.; Dangerfield, W.; Okiji, S.; Liddle, C.; Bootle, D.; Plumb, J.A.; Templeton, D.; Charlton, P. In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer Res. 2001, 61, 749–758. [Google Scholar]
- Syed, S.B.; Arya, H.; Fu, I.H.; Yeh, T.K.; Periyasamy, L.; Hsieh, H.P.; Coumar, M.S. Targeting P-glycoprotein: Investigation of piperine analogs for overcoming drug resistance in cancer. Sci. Rep. 2017, 7, 7972. [Google Scholar] [CrossRef]
- Zhang, H.; Bian, S.; Xu, Z.; Gao, M.; Wang, H.; Zhang, J.; Zhang, M.; Ke, Y.; Wang, W.; Chen, Z.S.; et al. The effect and mechanistic study of encequidar on reversing the resistance of SW620/AD300 cells to doxorubicin. Biochem. Pharmacol. 2022, 205, 115258. [Google Scholar] [CrossRef]
- Farschtschi, S.; Park, S.J.; Sawitzki, B.; Oh, S.J.; Kluwe, L.; Mautner, V.F.; Kurtz, A. Effector T cell subclasses associate with tumor burden in neurofibromatosis type 1 patients. Cancer Immunol. Immunother. 2016, 65, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.P.; Pradhan, S.; Chen, Z.; Patel, A.J.; Booker, R.C.; Le, L.Q. The role of nerve microenvironment for neurofibroma development. Oncotarget 2016, 7, 61500–61508. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, K.; Schwenkel, G.J. Fibroblasts Attenuate Anti-Tumor Drug Efficacy in Tumor Cells via Paracrine Interactions with Tumor Cells in 3D Plexiform Neurofibroma Cultures. Cells 2025, 14, 1276. https://doi.org/10.3390/cells14161276
Ji K, Schwenkel GJ. Fibroblasts Attenuate Anti-Tumor Drug Efficacy in Tumor Cells via Paracrine Interactions with Tumor Cells in 3D Plexiform Neurofibroma Cultures. Cells. 2025; 14(16):1276. https://doi.org/10.3390/cells14161276
Chicago/Turabian StyleJi, Kyungmin, and George J. Schwenkel. 2025. "Fibroblasts Attenuate Anti-Tumor Drug Efficacy in Tumor Cells via Paracrine Interactions with Tumor Cells in 3D Plexiform Neurofibroma Cultures" Cells 14, no. 16: 1276. https://doi.org/10.3390/cells14161276
APA StyleJi, K., & Schwenkel, G. J. (2025). Fibroblasts Attenuate Anti-Tumor Drug Efficacy in Tumor Cells via Paracrine Interactions with Tumor Cells in 3D Plexiform Neurofibroma Cultures. Cells, 14(16), 1276. https://doi.org/10.3390/cells14161276






