Photosynthetic Electron Transport in Winter Wheat: Responses to Low-Temperature and Weak-Light Condition
Abstract
1. Introduction
2. Materials and Methods
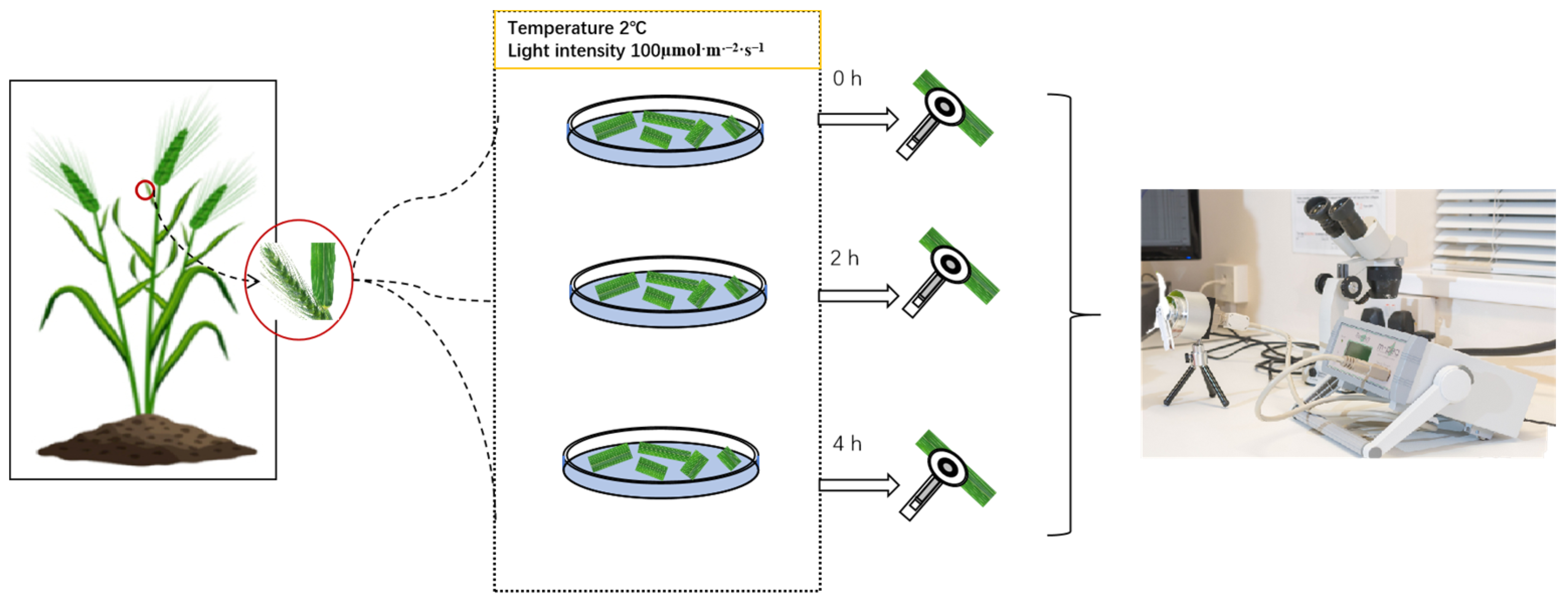
2.1. Materials and Experimental Design
2.2. Measurement of Rapid Chlorophyll Fluorescence Induction Kinetics, 820 nm Light Reflection, and Delayed Fluorescence
2.3. Statistical Analysis
3. Results
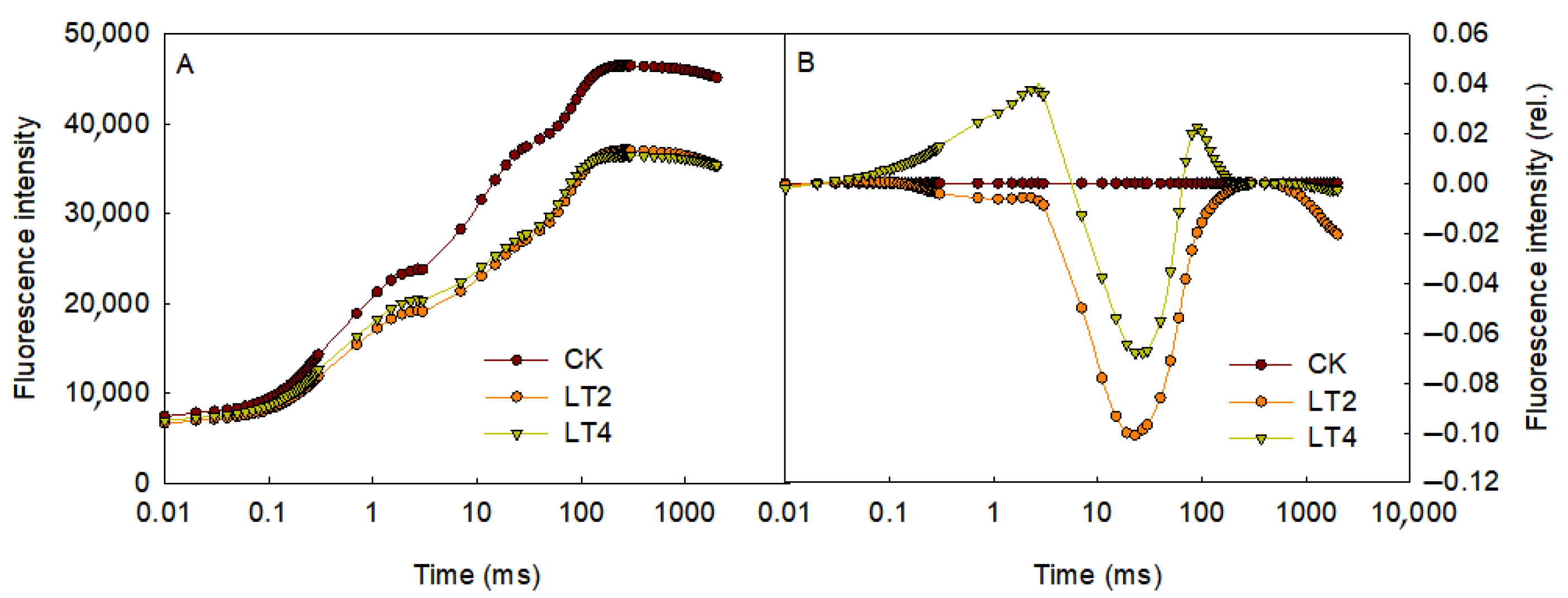
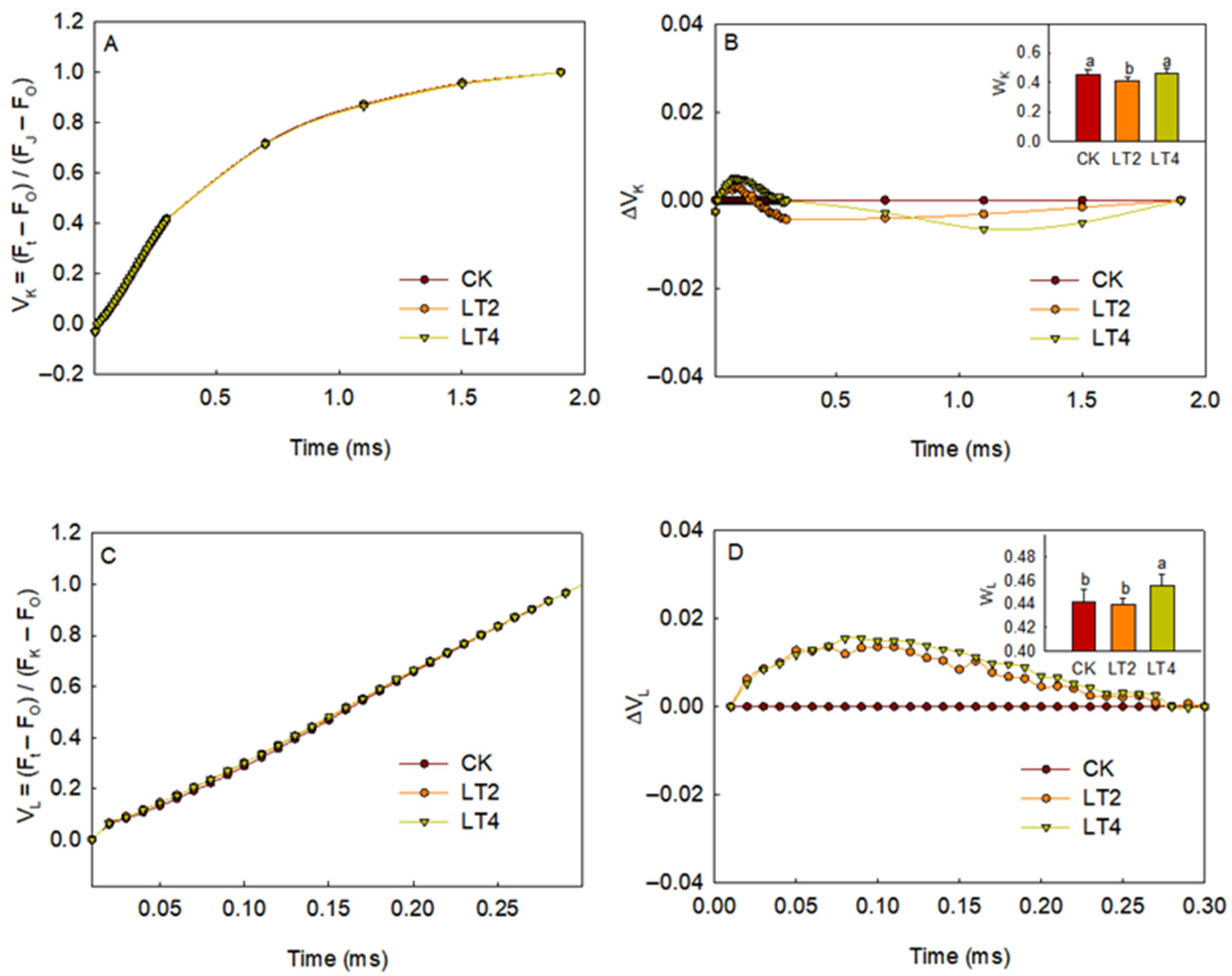
3.1. Effects of Low Temperature on Chlorophyll Fluorescence Induction Kinetics Curves
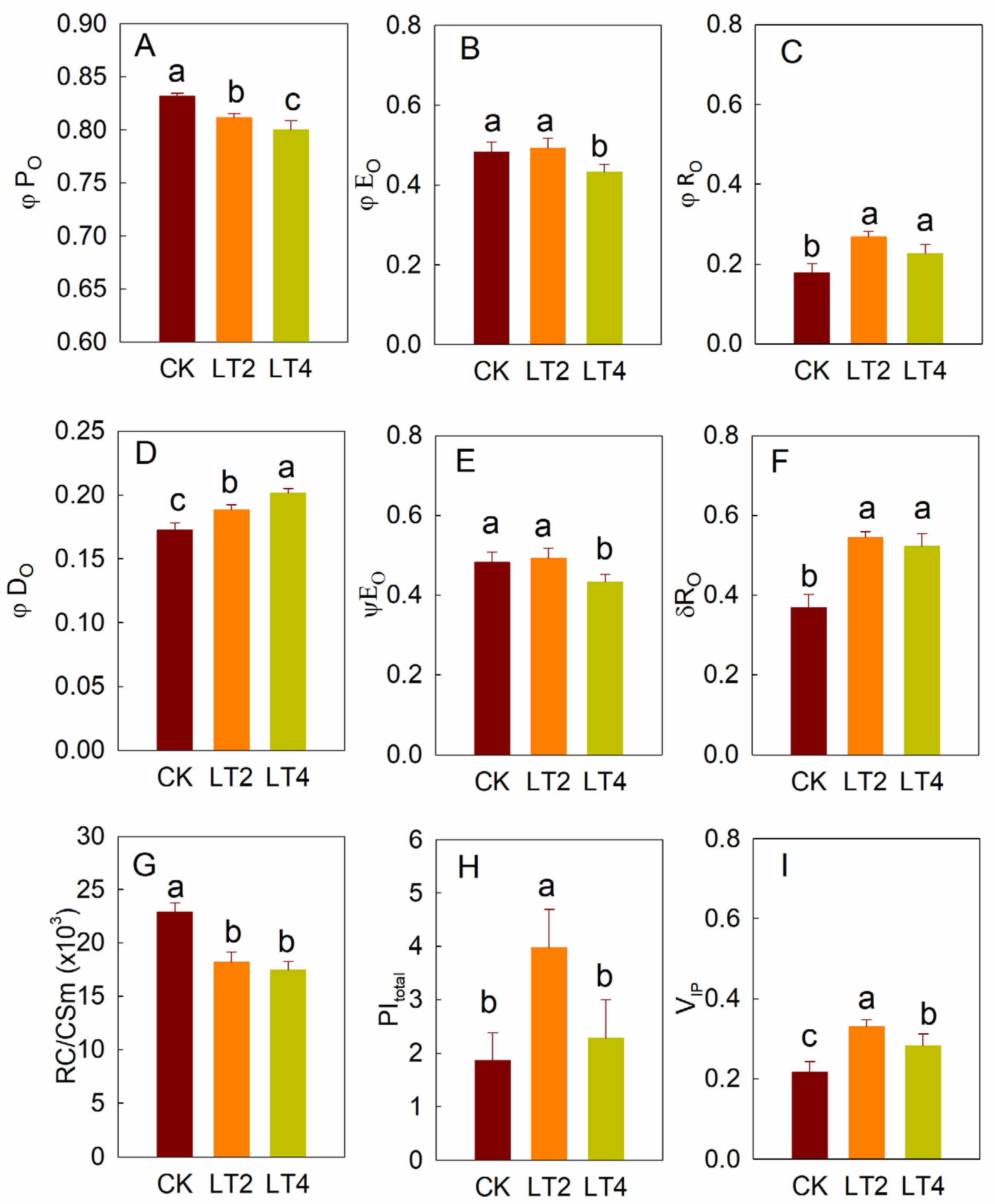
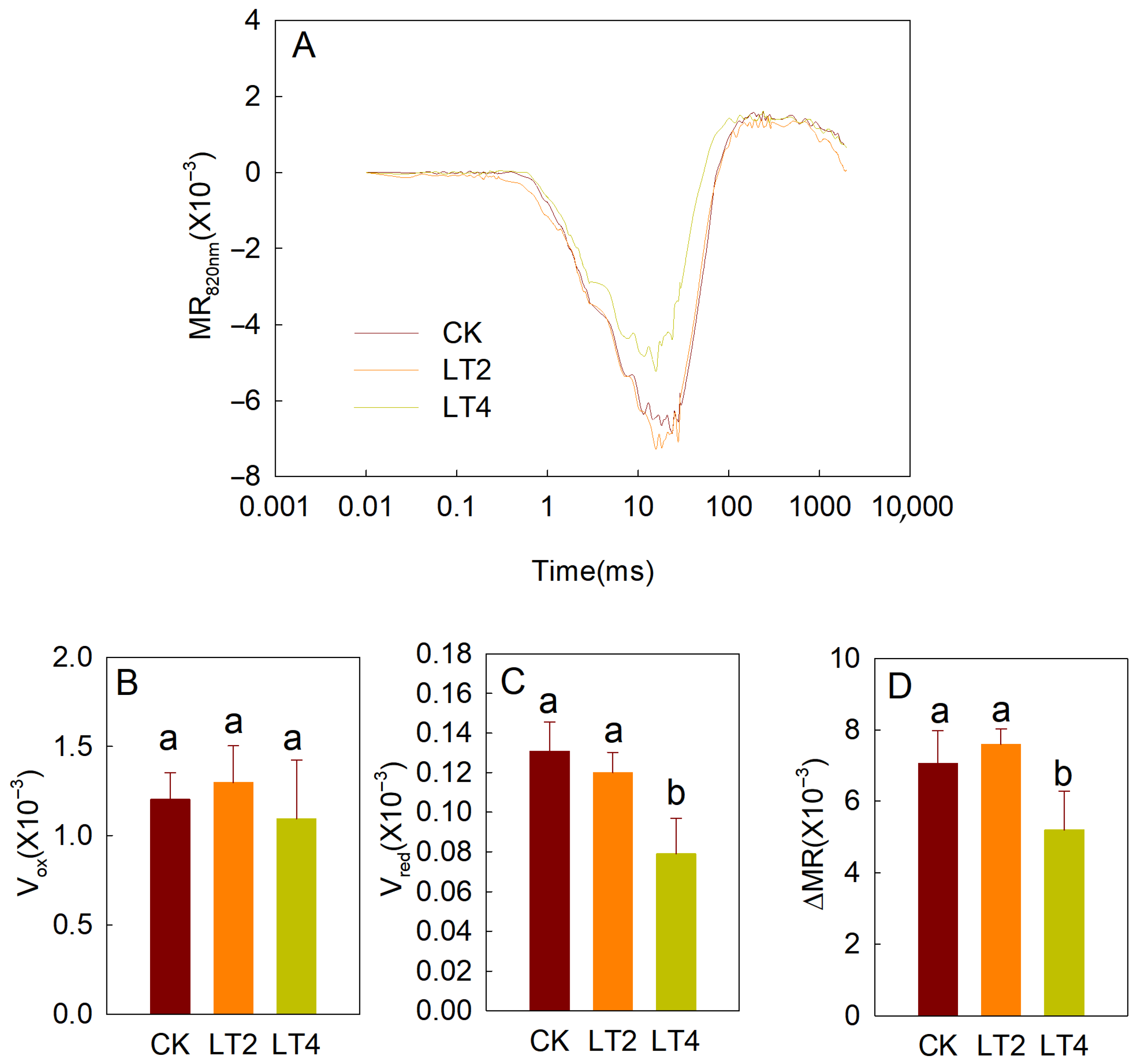
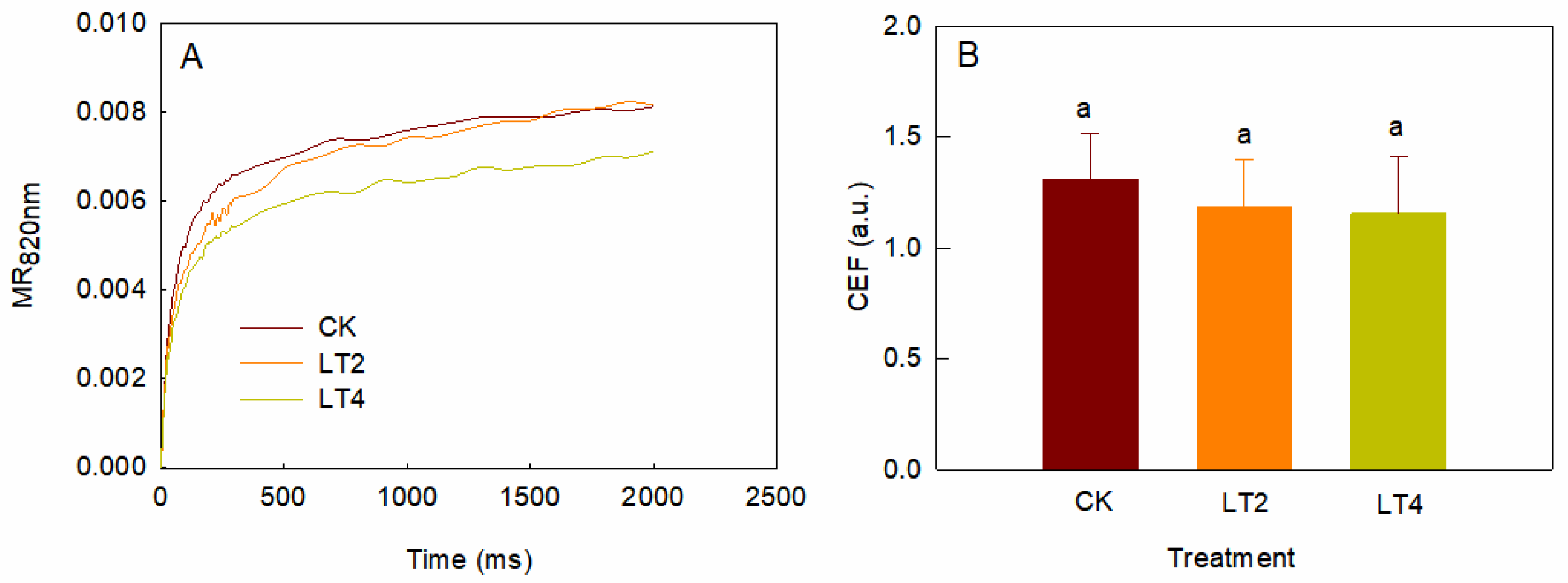
3.2. Effects of Low Temperature on MR820nm Light Reflection
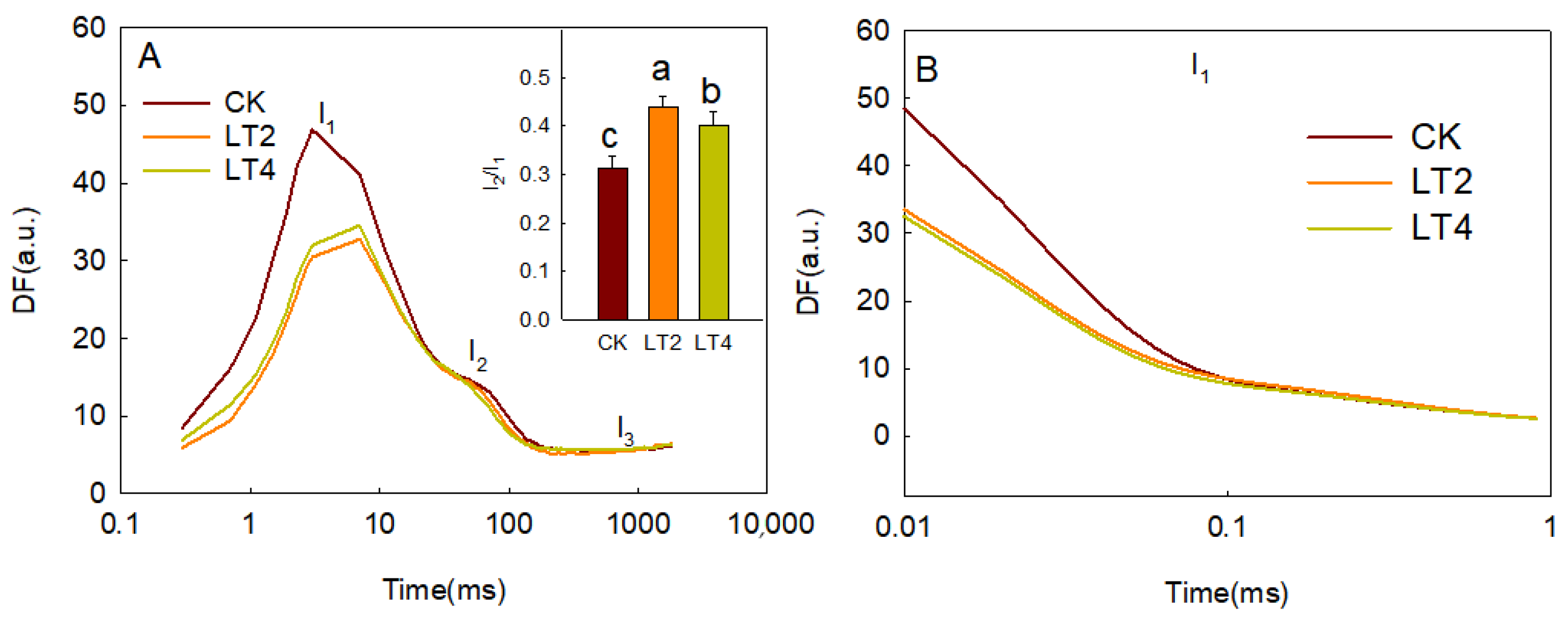
3.3. Effects of Low Temperatures on Delayed Fluorescence in Wheat Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, W.D.; Liu, J.G.; Chang, J.; Wang, J.J. Changes in Extreme Temperature and Precipitation in Henan Province During 1957–2005. Adv. Clim. Change Res. 2008, 2, 78–83. [Google Scholar]
- Gao, J.B.; Liu, L.L.; Wu, S.H. Hazards of extreme events in China under different global warming targets. Big Earth Data 2020, 4, 153–174. [Google Scholar] [CrossRef]
- Dey, R.; Lewis, S.C. Natural disasters linked to climate change. In The Impacts of Climate Change; Elsevier: Amsterdam, The Netherlands, 2021; pp. 177–193. [Google Scholar]
- Lindsey, L.E.; Alt, D.S.; Lindsey, A.J. Freeze symptoms and associated yield loss in soft red winter wheat. Crop Forage Turfgrass Manag. 2021, 7, e20078. [Google Scholar] [CrossRef]
- Subedi, K.; Floyd, C.N.; Budhathoki, C. Cool temperature-induced sterility in spring wheat at high altitudes in Nepal. Field Crops Res. 1998, 55, 141–151. [Google Scholar] [CrossRef]
- Xiang, C.; Min, Y.; Wang, P.N.; Dai, W.C.; Weng, Y.; Cai, H.M.; Wu, Y.; Xu, H.; Zheng, B.Q.; Li, J.C. Effects of late spring coldness during the anther differentiation period on grain number and weight of wheat main stem at different spikelet and grain position. Chin. J. Agrometeorol. 2023, 44, 575–587. [Google Scholar]
- Liu, L.Z.; Zhang, Y.; Zhang, L.; Cai, H.M.; Yu, M.; Wei, F.Z.; Li, J.C. Effects of low temperature stress during the anther differentiation period on leaf anatomical structure and photosynthetic characteristics of wheat. Chin. J. Agrometeorol. 2023, 44, 58–70. [Google Scholar]
- Zhang, Y.; Liu, L.Z.; Chen, X.; Li, J.C. Effects of Low-Temperature Stress during the Anther Differentiation Period on Winter Wheat Photosynthetic Performance and Spike-Setting Characteristics. Plants 2022, 11, 389. [Google Scholar] [CrossRef]
- Didaran, F.; Kordrostami, M.; Ghasemi-Soloklui, A.A. The mechanisms of photoinhibition and repair in plants under high light conditions and interplay with abiotic stressors. J. Photochem. Photobiol. B 2024, 259, 113004. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Harimoto, S.; Maekawa, S.; Miyake, C.; Ifuku, K. PSII Photoinhibition as a Protective Strategy: Maintaining an Oxidative State of PSI by Suppressing PSII Activity Under Environmental Stress. Physiol. Plant 2025, 177, e70392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.S.; Jia, Y.J.; Gao, H.Y.; Zhang, L.T.; Li, H.D.; Meng, Q.W. Characterization of PSI recovery after chilling-induced photoinhibition in cucumber (Cucumis sativus L.) leaves. Planta 2011, 234, 883–889. [Google Scholar] [CrossRef] [PubMed]
- David, R.; Mark, A.S.; Omar, S.I.; Ralph, B. Structure, function, and assembly of PSI in thylakoid membranes of vascular plants. Plant Cell 2024, 36, 4080–4108. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Zhang, L.T.; Gao, H.Y. Research of the photoinhibition of PSI and PSII in leaves of cucumber under chilling stress combined with different light intensities. Sci. Agric. Sin. 2009, 42, 4288–4293. [Google Scholar]
- Gao, J.; Li, P.M.; Ma, F.W.; Goltsev, V. Photosynthetic performance during leaf expansion in Malus micromalus probed by chlorophyll a fluorescence and modulated 820 nm reflection. J. Photochem. Photobiol. B Biol. 2014, 137, 144–150. [Google Scholar] [CrossRef]
- Yang, C.; Du, S.; Shi, Y.; Zhang, D.; Yue, J.; Li, X.; Jin, H.; Fang, B.; Wei, F.; Zhang, Z.; et al. Differential Sensitivity of Photosynthetic Electron Transport to Dark-Induced Senescence in Wheat Flag Leaves. BMC Plant Biol. 2025, 25, 650. [Google Scholar] [CrossRef]
- Khatri, K.; Rathore, M.S. Photosystem photochemistry, prompt and delayed fluorescence, photosynthetic responses and electron flow in tobacco under drought and salt stress. Photosynthetica 2019, 57, 61–74. [Google Scholar] [CrossRef]
- Goussi, R.; Manaa, A.; Derbali, W.; Cantamessa, S.; Abdelly, C.; Barbato, R. Comparative analysis of salt stress, duration and intensity, on the chloroplast ultrastructure and photosynthetic apparatus in Thellungiella salsuginea. J. Photochem. Photobiol. B Biol. 2018, 183, 275–287. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, C.; Xia, J.; Zhang, Y.; Yu, Q.; Eamus, D. Recognition of key regions for restoration of phytoplankton communities in the Huai River basin, China. J. Hydrol. 2012, 420, 292–300. [Google Scholar] [CrossRef]
- Liu, C.; Ji, W.; Jiang, H.; Shi, Y.; He, L.; Gu, Z.; Zhu, S. Comparison of Biochemical Composition and Non-Volatile Taste Active Compounds in Raw, High Hydrostatic Pressure-Treated and Steamed Oysters Crassostrea Hongkongensis. Food Chem. 2021, 344, 128632. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, F.; Chen, Z.; Teng, Z.; Sun, K.; Li, X.; Yu, J.; Zhang, G.; Liang, Y.; Huang, X.; et al. Synergistic interplay of ABA and BR signal in regulating plant growth and adaptation. Nat. Plants 2021, 7, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, Z.S.; Yuan, Y.; Zhang, D.Q.; Jin, H.Y.; Li, Y.; Du, S.M.; Li, X.D.; Fang, B.T.; Wei, F.; et al. Natural variation in photosynthetic electron transport of wheat flag leaves in response to dark-induced senescence. J. Photochem. Photobiol. B Biol. 2024, 259, 113018. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Guo, Y.; Chang, Y.; Zhang, J.; Wang, H.; Liu, Q.; Ji, Y.; Zhang, Z.; Liu, Y.; et al. Fast chlorophyll fluorescence rise kinetics as a high-throughput diagnostic tool for evaluating the capacity of 2-amino-3-methylhexanoic acid at inducing plant resistance against high temperature. Environ. Exp. Bot. 2025, 229, 106040. [Google Scholar] [CrossRef]
- Kan, X.; Ren, J.J.; Chen, T.T.; Cui, M.C.; Li, C.; Zhou, R.H.; Zhang, Y.; Liu, H.H.; Deng, D.X.; Yin, Z.T. Effects of salinity on photosynthesis in maize probed by prompt fluorescence, delayed fluorescence and P700 signals. Environ. Exp. Bot. 2017, 140, 56–64. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. Analysis of the Fluorescence Transiet as a tool to characterize and screen photosynthetic samples. Chlorophyll A Fluoresc. Signat. Photosynth. 2004, 25, 445–483. [Google Scholar]
- Gao, D.P.; Ran, C.; Zhang, Y.H.; Wang, X.L.; Lu, S.F.; Geng, Y.Q.; Guo, L.Y.; Shao, X.W. Effect of different concentrations of foliar iron fertilizer on chlorophyll fluorescence characteristics of iron-deficient rice seedlings under saline sodic conditions. Plant Physiol. Biochem. 2022, 185, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, Z.S.; Gao, H.Y.; Liu, M.J.; Fan, X.L. Mechanisms by Which the Infection of Sclerotinia sclerotiorum (Lib.) de Bary Affects the Photosynthetic Performance in Tobacco Leaves. BMC Plant Biol. 2014, 14, 240. [Google Scholar] [CrossRef]
- Goltsev, V.; Zaharieva, I.; Chernev, P. Delayed fluorescence in photosynthesis. Photosynth Res. 2009, 101, 217–232. [Google Scholar] [CrossRef]
- Goltsev, V.; Zaharieva, I.; Chernev, P.; Strasser, R. Delayed Chlorophyll Fluorescence as a Monitor for Physiological State of Photosynthetic Apparatus. Biotechnol. Biotechnol. Equip. 2009, 23, 452–457. [Google Scholar] [CrossRef]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Chen, S.Y.; Zheng, Q.X.; Qi, Z.Y.; Ding, J.; Song, X.W.; Xia, X.J. Stress-induced delay of the I-P rise of the fast chlorophyll a fluorescence transient in tomato. Sci. Hortic. 2024, 326, 112741. [Google Scholar] [CrossRef]
- Cheng, D.D.; Zhang, Z.S.; Sun, X.B.; Zhao, M.; Sun, G.Y. Photoinhibition and photoinhibition-like damage to the photosynthetic apparatus in tobacco leaves induced by Pseudomonas syringae pv. Tabaci under light and dark conditions. BMC Plant Biol. 2016, 16, 29. [Google Scholar]
- Kalisz, A.; Kornaś, A.; Skoczowski, A.; Oliwa, J.; Jurkow, R.; Gil, J.; Sękara, A.; Sałata, A.; Caruso, G. Leaf chlorophyll fluorescence and reflectance of oakleaf lettuce exposed to metal and metal(oid) oxide nanoparticles. BMC Plant Biol. 2023, 23, 329. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, M.H.; Goltsev, V.N.; Żuk-Gołaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll fluorescence: Understanding Crop Performance—Basics and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Tikkanen, M.; Mekala, N.R.; Aro, E.M. Photosystem II photoinhibition-repair cycle protects Photosystem I from irreversible damage. Biochim. Biophys. Acta (BBA) Bioenerg. 2014, 1837, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Kudoh, H.; Sonoike, K. Irreversible damage to photosystem I by chilling in the light: Cause of the degradation of chlorophyll after returning to normal growth temperature. Planta 2002, 215, 541–548. [Google Scholar] [CrossRef]
- Zeng, F.L.; Wang, G.J.; Liang, Y.P.; Guo, N.H.; Zhu, L.; Wang, Q.; Chen, H.W.; Ma, D.; Wang, J.Y. Disentangling the photosynthesis performance in japonica rice during natural leaf senescence using OJIP fluorescence transient analysis. Funct. Plant Biol. 2021, 48, 206–217. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Shimakawa, G.; Krieger-Liszkay, A.; Roach, T. ROS derived lipid peroxidation is prevented in barley leaves during senescence. Physiol. Plant. 2022, 174, e13769. [Google Scholar] [CrossRef]
- Viljevac-Vuletić, M.; Španić, V. Characterization of photosynthetic performance during natural leaf senescence in winter wheat: Multivariate analysis as a tool for phenotypic characterization. Photosynthetica 2020, 58, 301–313. [Google Scholar] [CrossRef]
- Fan, J.B.; Chen, K.; Xu, J.L.; ABM, K.; Chen, Y.; Chen, L.; Yan, X.B. Physiological effects induced by aluminium and fluoride stress in tall fescue (Festuca arundinacea Schreb). Ecotoxicol. Environ. Saf. 2022, 231, 113192. [Google Scholar] [CrossRef]
- Paunov, M.; Koleva, L.; Vassilev, A.; Vangronsveld, J.; Goltsev, V. Effects of different metals on photosynthesis: Cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int. J. Mol. Sci. 2018, 19, 787. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.B.; Ma, L.; Xi, H.F.; Duan, W.; Li, S.H.; Loescher, W.; Wang, J.F.; Wang, L.J. Photosynthetic responses to heat treatments at different temperatures and following recovery in grapevine (Vitis amurensis L.) leaves. PLoS ONE 2011, 6, e23033. [Google Scholar] [CrossRef]
- Strasser, B.J. Donor side capacity of photosystem II probed by chlorophyll a fluorescence transients. Photosynth. Res. 1997, 52, 147–155. [Google Scholar] [CrossRef]
- Ottander, C.; Hundal, T.; Andersson, B.; Huner, N.P.; Öquist, G. Photosystem II reaction centres stay intact during low temperature photoinhibition. Photosynth. Res. 1993, 35, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Sonoike, K. Photoinhibition and Protection of Photosystem I. In Photosystem I. Advances in Photosynthesis and Respiration; Golbeck, J.H., Ed.; Springer: Dordrecht, The Netherlands, 2006; Volume 24. [Google Scholar]
- Ceppi, M.G.; Oukarroum, A.; Çiçek, N.; Strasser, R.J.; Schansker, G. The IP amplitude of the fluorescence rise OJIP is sensitive to changes in the photosystem I content of leaves: A study on plants exposed to magnesium and sulfate deficiencies, drought stress and salt stress. Physiol. Plant. 2012, 144, 277–288. [Google Scholar] [CrossRef]
- Ferroni, L.; Živčak, M.; Kovar, M.; Colpo, A.; Pancaldi, S.; Allakhverdiev, S.I.; Brestič, M. Fast chlorophyll a fluorescence induction (OJIP) phenotyping of chlorophyll-deficient wheat suggests that an enlarged acceptor pool size of Photosystem I helps compensate for a deregulated photosynthetic electron flow. J. Photochem. Photobiol. B Biol. 2022, 234, 112549. [Google Scholar] [CrossRef]
- Guan, Y.N.; Huang, Z.L.; Zhang, W.J.; Shi, X.D.; Zhang, P.P. Effects of low temperature stress on photosynthetic performance of different genotypes wheat cultivars. Chin. J. Appl. Ecol. 2013, 24, 1895–1899. [Google Scholar]
| Parameter | Method of Calculation |
|---|---|
| FM | Maximum fluorescence intensity obtained under light after dark adaptation |
| FO | Fluorescence intensity at 20 μs of OJIP curve |
| Fv | Fv = FM − FO |
| Ft | Fluorescence intensity at t time |
| FK | Fluorescence intensity at the K point (0.3 ms) |
| FJ | Fluorescence intensity at the J point (3 ms) |
| FI | Fluorescence intensity at the I point (30 ms) |
| VJ | VJ = (FJ − FO)/(FM − FO) |
| VI | VI = (FI − FO)/(FM − FO) |
| MO | MO = 4(F300μs − FO)/(FM − FO) |
| ΨO | ψO = 1 − VJ |
| φPO | φPO = Fv/FM = (FM − FO)/FM |
| φEO | φEo = ETo/ABS = [1 − (FO/FM)] × ψ |
| φDO | φDO = 1 − φPO |
| φRO | φRO = φPO × (1 − VI) |
| σRO | σRO = (1 − VI)/(1 − VJ) |
| RC/CSm | RC/CSm = φPO × (VJ/MO) × FM |
| PItotal | PItatal = (RC/ABS) × [φPO/(1 − φPO)] × [ψO/(1 − ψEO)] × [φRO/ABS(1 − φRO)] |
| VIP | VIP = 1 − VI |
| Parameters | Low-Temperature Treatment | ||
|---|---|---|---|
| CK | LT2 | LT4 | |
| L1 | 46.89 ± 2.03 a | 37.66 ± 1.28 b | 37.09 ± 1.98 b |
| L2 | 7.75 ± 0.7 a | 7.84 ± 1.19 a | 6.86 ± 0.65 a |
| L3 | 2.32 ± 0.41 a | 1.83 ± 0.37 b | 1.83 ± 0.26 b |
| τ1 | 0.02 ± 0 a | 0.02 ± 0 a | 0.02 ± 0 a |
| τ2 | 0.3 ± 0.05 a | 0.31 ± 0.04 a | 0.3 ± 0.04 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Liu, M.; Du, S.; Zhang, D.; Li, X.; Wu, L.; Shi, Y.; Fang, B.; Yan, G.; Wei, F. Photosynthetic Electron Transport in Winter Wheat: Responses to Low-Temperature and Weak-Light Condition. Cells 2025, 14, 1275. https://doi.org/10.3390/cells14161275
Yang C, Liu M, Du S, Zhang D, Li X, Wu L, Shi Y, Fang B, Yan G, Wei F. Photosynthetic Electron Transport in Winter Wheat: Responses to Low-Temperature and Weak-Light Condition. Cells. 2025; 14(16):1275. https://doi.org/10.3390/cells14161275
Chicago/Turabian StyleYang, Cheng, Minghan Liu, Simeng Du, Deqi Zhang, Xiangdong Li, Liting Wu, Yanhua Shi, Baoting Fang, Ge Yan, and Fang Wei. 2025. "Photosynthetic Electron Transport in Winter Wheat: Responses to Low-Temperature and Weak-Light Condition" Cells 14, no. 16: 1275. https://doi.org/10.3390/cells14161275
APA StyleYang, C., Liu, M., Du, S., Zhang, D., Li, X., Wu, L., Shi, Y., Fang, B., Yan, G., & Wei, F. (2025). Photosynthetic Electron Transport in Winter Wheat: Responses to Low-Temperature and Weak-Light Condition. Cells, 14(16), 1275. https://doi.org/10.3390/cells14161275







