Comparison of Primary Human Osteoblast-like Cells and hFOB 1.19 Cells: Contrasting Effects of Proinflammatory Cytokines
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bone Material, Ethics Approval, and Patient Information
2.3. Isolation and Culture of Primary Human Osteoblasts
2.4. Cell Culture with hFOB 1.19 Cells
2.5. Toluidine Blue, Oil Red O, and Phalloidin Staining
2.6. Alizarin Red S Staining
2.7. MTT Assay
2.8. Extracellular ALP Activity
2.9. Cell Proliferation Rate by BrdU Assay
2.10. Real-Time qPCR
2.11. Western Blot
2.12. Statistics
3. Results
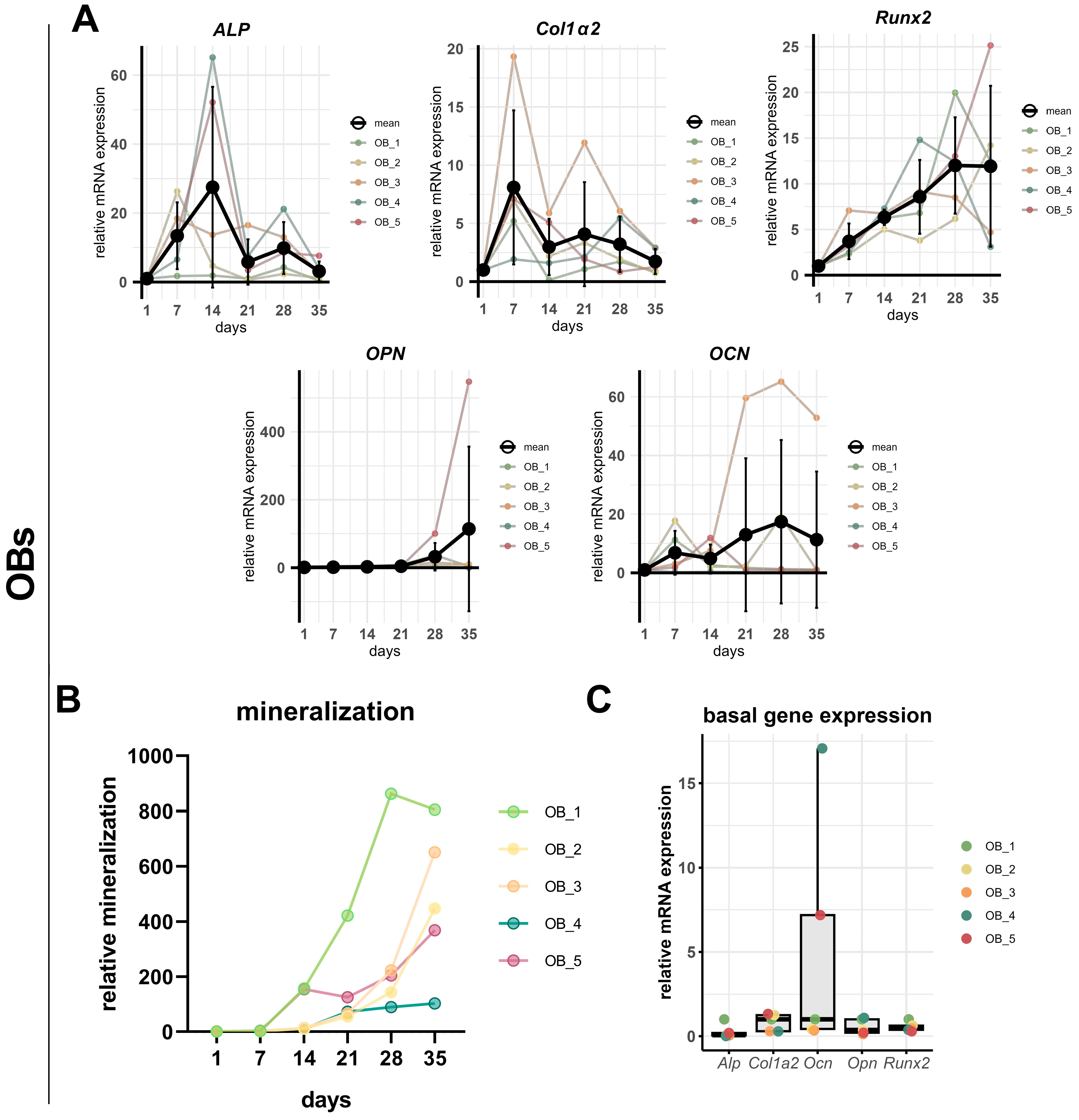
3.1. Morphological Comparison of hFOB 1.19 Cells and Primary Human Osteoblasts
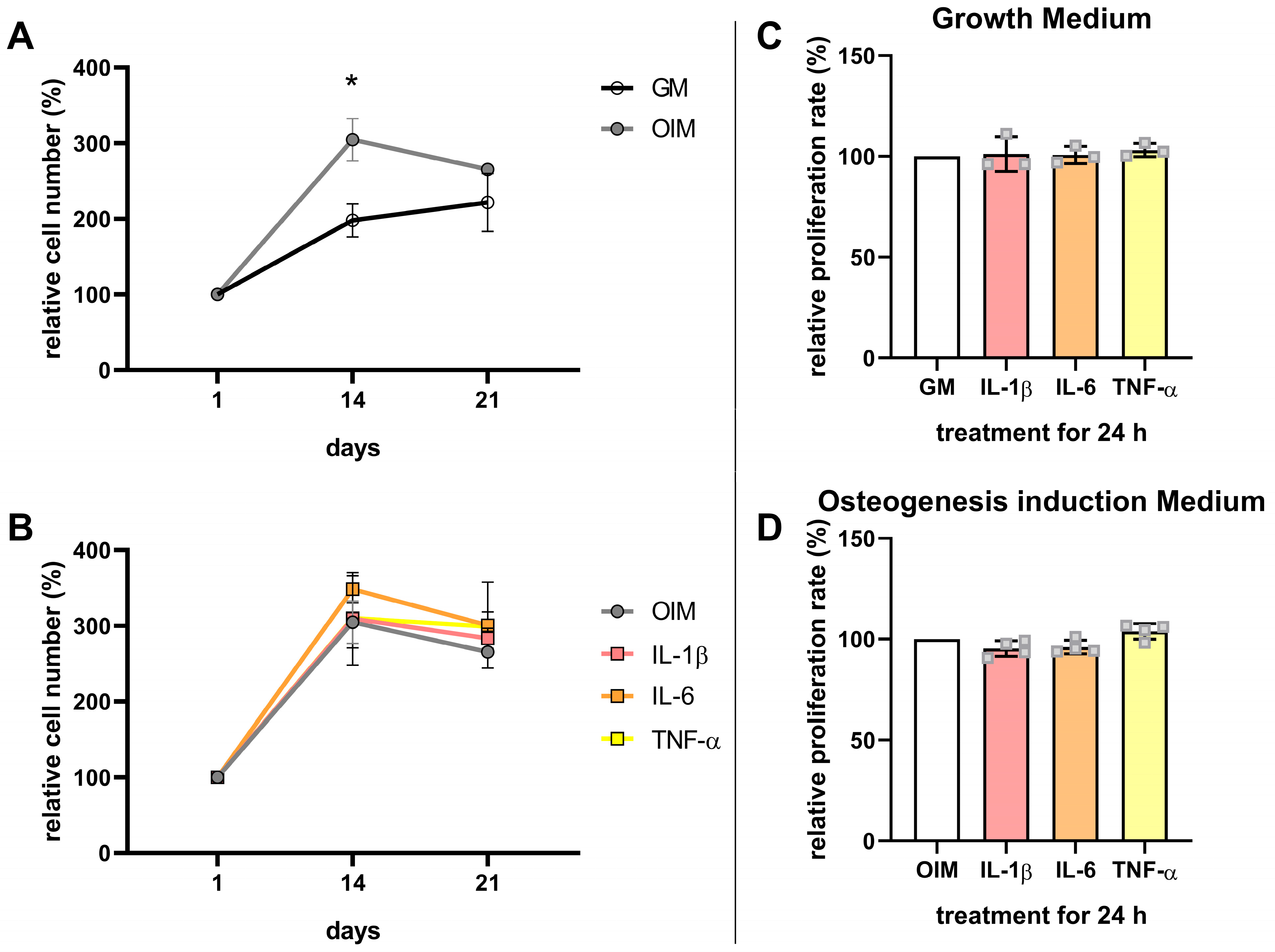
3.2. A Comparison of the Osteogenesis of hFOB 1.19 Cells and Primary Human Osteoblasts
3.3. Effects of Proinflammatory Cytokines on the Osteogenesis and Proliferation of hFOB 1.19 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| TNF-α | Tumor necrosis factor alpha |
| OBs | Primary human osteoblast-like cells |
| ALP | Alkaline phosphatase |
| MSCs | Human mesenchymal stem cells |
| Col1 | Collagen type I |
| Runx2 | Runt-related transcription factor 2 |
| Ocn | Osteocalcin |
| Opn | Osteopontin |
| DXA | Dual-energy X-ray absorptiometry |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| SD | Standard deviation |
| Pen/Strep | Penicillin/streptomycin |
| FBS | Fetal bovine serum |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid solution |
| G418 | Geneticin |
| GM | Growth medium |
| OIM | Osteogenic induction medium |
| PFA | Paraformaldehyde |
| RT | Room temperature |
| PBS | Phosphate-buffered saline |
| DAPI | 4′,6-diamidino-2-phenylindole |
| ddH2O | Double-distilled water |
| CPC | (1 hexadecyl) pyridinium chloride monohydrate |
| MTT | Dimethylthiazolyl Blue Tetrazolium Bromide |
| DMSO | Dimethyl sulfoxide |
| BrdU | Bromodeoxyuridine |
| bp | Base pairs |
| Rplp0 | Ribosomal protein lateral stalk subunit P0 |
| Tfrc | Transferrin receptor protein 1 |
| mRNA | Messenger ribonucleic acid |
| U | Units |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells |
| GSK3β | Glycogen synthase kinase-3 beta |
References
- Cai, L.; Lv, Y.; Yan, Q.; Guo, W. Cytokines: The links between bone and the immune system. Injury 2024, 55, 111203. [Google Scholar] [CrossRef]
- Umur, E.; Bulut, S.B.; Yiğit, P.; Bayrak, E.; Arkan, Y.; Arslan, F.; Baysoy, E.; Kaleli-Can, G.; Ayan, B. Exploring the Role of Hormones and Cytokines in Osteoporosis Development. Biomedicines 2024, 12, 1830. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.C.; Simmons, P.J.; Graves, S.E.; Hamilton, J.A. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: Implications for bone repair during inflammation. Osteoarthr. Cartil. 2009, 17, 735–742. [Google Scholar] [CrossRef]
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: A review. Front. Immunol. 2023, 14, 1222129. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Ghali, O.; Lencel, P.; Broux, O.; Chauveau, C.; Devedjian, J.C.; Hardouin, P.; Magne, D. TNF-α and IL-1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009, 84, 499–504. [Google Scholar] [CrossRef]
- Sonomoto, K.; Yamaoka, K.; Oshita, K.; Fukuyo, S.; Zhang, X.; Nakano, K.; Okada, Y.; Tanaka, Y. Interleukin-1β induces differentiation of human mesenchymal stem cells into osteoblasts via the Wnt-5a/receptor tyrosine kinase–like orphan receptor 2 pathway. Arthritis Rheum. 2012, 64, 3355–3363. [Google Scholar] [CrossRef]
- Briolay, A.; Lencel, P.; Bessueille, L.; Caverzasio, J.; Buchet, R.; Magne, D. Autocrine stimulation of osteoblast activity by Wnt5a in response to TNF-α in human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2013, 430, 1072–1077. [Google Scholar] [CrossRef]
- Bousch, J.F.; Beyersdorf, C.; Schultz, K.; Windolf, J.; Suschek, C.V.; Maus, U. Proinflammatory Cytokines Enhance the Mineralization, Proliferation, and Metabolic Activity of Primary Human Osteoblast-like Cells. Int. J. Mol. Sci. 2024, 25, 12358. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stensløkken, K.O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Gromolak, S.; Krawczenko, A.; Antończyk, A.; Buczak, K.; Kiełbowicz, Z.; Klimczak, A. Biological Characteristics and Osteogenic Differentiation of Ovine Bone Marrow Derived Mesenchymal Stem Cells Stimulated with FGF-2 and BMP-2. Int. J. Mol. Sci. 2020, 21, 9726. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.; Zhang, Y. Osteoinduction: A Review of Old Concepts with New Standards. J. Dent. Res. 2012, 91, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, W.; Masson, A.; Li, Y.P. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Voegele, T.J.; Voegele-Kadletz, M.; Esposito, V.; Macfelda, K.; Oberndorfer, U.; Vecsei, V.; Schabus, R. The effect of different isolation techniques on human osteoblast-like cell growth. Anticancer. Res. 2000, 20, 3575–3581. [Google Scholar]
- Fedarko, N.S.; Vetter, U.K.; Weinstein, S.; Robey, P.G. Age-related changes in hyaluronan, proteoglycan, collagen, and osteonectin synthesis by human bone cells. J. Cell. Physiol. 1992, 151, 215–227. [Google Scholar] [CrossRef]
- Croes, M.; Oner, F.C.; Kruyt, M.C.; Blokhuis, T.J.; Bastian, O.; Dhert, W.J.A.; Alblas, J. Proinflammatory Mediators Enhance the Osteogenesis of Human Mesenchymal Stem Cells after Lineage Commitment. PLoS ONE 2015, 10, e0132781. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Zhou, Z.; Zhu, L.; Hu, X.; Lu, J.; Shi, C.; Chen, F.; Chen, A. TNF-α suppresses osteogenic differentiation of MSCs by accelerating P2Y2 receptor in estrogen-deficiency induced osteoporosis. Bone 2018, 117, 161–170. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, J.; Lei, L.; Sun, W.; Wu, Y.; Ding, P.; Chen, L. The positive effects of secreting cytokines IL-17 and IFN-γ on the early-stage differentiation and negative effects on the calcification of primary osteoblasts in vitro. Int. Immunopharmacol. 2018, 57, 1–10. [Google Scholar] [CrossRef]
- Gilbert, L.; He, X.; Farmer, P.; Boden, S.; Kozlowski, M.; Rubin, J.; Nanes, M.S. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology 2000, 141, 3956–3964. [Google Scholar] [CrossRef]
- Kumar, S.; Votta, B.J.; Rieman, D.J.; Badger, A.M.; Gowen, M.; Lee, J.C. IL-1- and TNF-induced bone resorption is mediated by p38 mitogen activated protein kinase. J. Cell. Physiol. 2001, 187, 294–303. [Google Scholar] [CrossRef]
- Kartsogiannis, V.; Ng, K.W. Cell lines and primary cell cultures in the study of bone cell biology. Mol. Cell. Endocrinol. 2004, 228, 79–102. [Google Scholar] [CrossRef]
- Harris, S.A.; Enger, R.J.; Riggs, B.L.; Spelsberg, T.C. Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J. Bone Miner. Res. Off J. Am. Soc. Bone Miner. Res. 1995, 10, 178–186. [Google Scholar] [CrossRef]
- Subramaniam, M.; Jalal, S.M.; Rickard, D.J.; Harris, S.A.; Bolander, M.E.; Spelsberg, T.C. Further characterization of human fetal osteoblastic hFOB 1.19 and hFOB/ERα cells: Bone formation in vivo and karyotype analysis using multicolor fluorescent in situ hybridization. J. Cell. Biochem. 2002, 87, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002, 359, 1929–1936. [Google Scholar] [CrossRef]
- Jaiswal, N.; Haynesworth, S.E.; Caplan, A.I.; Bruder, S.P. Osteogenic Differentiation of Purified, Culture-Expanded Human Mesenchymal Stem Cells In Vitro. J. Cell. Biochem. 1998, 64, 295–312. [Google Scholar] [CrossRef]
- Ji, M.J.; Ryu, H.J.; Jeong, H.H. Synovial Fluid of Patient with Rheumatoid Arthritis Enhanced Osmotic Sensitivity Through the Cytotoxic Edema Module in Synoviocytes. Front. Cell Dev. Biol. 2021, 9, 700879. [Google Scholar] [CrossRef] [PubMed]
- Westacott, C.I.; Whicher, J.T.; Barnes, I.C.; Thompson, D.; Swan, A.J.; Dieppe, P.A. Synovial fluid concentration of five different cytokines in rheumatic diseases. Ann. Rheum. Dis. 1990, 49, 676–681. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: The MTT assay. In Cancer Cell Culture. Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 731, pp. 237–245. [Google Scholar] [CrossRef]
- Sabokbar, A.; Millett, P.J.; Myer, B.; Rushton, N. A rapid, quantitative assay for measuring alkaline phosphatase activity in osteoblastic cells in vitro. Bone Miner. 1994, 27, 57–67. [Google Scholar] [CrossRef]
- Xie, F.; Wang, J.; Zhang, B. RefFinder: A web-based tool for comprehensively analyzing and identifying reference genes. Funct. Integr. Genom. 2023, 23, 125. [Google Scholar] [CrossRef]
- Nolan, T.; Hands, R.E.; Bustin, S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gilbert, L.; He, X.; Farmer, P.; Rubin, J.; Drissi, H.; van Wijnen, A.J.; Lian, J.B.; Stein, G.S.; Nanes, M.S. Expression of the Osteoblast Differentiation Factor RUNX2 (Cbfa1/AML3/Pebp2αA) Is Inhibited by Tumor Necrosis Factor-α*. J. Biol. Chem. 2002, 277, 2695–2701. [Google Scholar] [CrossRef]
- Gavazzo, P.; Viti, F.; Donnelly, H.; Oliva, M.A.G.; Salmeron-Sanchez, M.; Dalby, M.J.; Vassalli, M. Biophysical phenotyping of mesenchymal stem cells along the osteogenic differentiation pathway. Cell Biol. Toxicol. 2021, 37, 915–933. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, Y.; Peng, P.; Huang, X.T.; Qiu, Z.H.; Liu, B.C.; Yang, T.L.; Yang, B.; Guo, Y. Isolation and culture of human primary osteoblasts: Comparing the effects of differences in method details on osteoblast characteristics. Genes Dis. 2023, 11, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Putra, V.D.L.; Kilian, K.A.; Knothe Tate, M.L. Biomechanical, biophysical and biochemical modulators of cytoskeletal remodelling and emergent stem cell lineage commitment. Commun. Biol. 2023, 6, 75. [Google Scholar] [CrossRef]
- Grewal, S.I.S.; Moazed, D. Heterochromatin and Epigenetic Control of Gene Expression. Science 2003, 301, 798–802. [Google Scholar] [CrossRef]
- Marozin, S.; Simon-Nobbe, B.; Irausek, S.; Chung, L.; Lepperdinger, G. Kinship of conditionally immortalized cells derived from fetal bone to human bone-derived mesenchymal stroma cells. Sci. Rep. 2021, 11, 10933. [Google Scholar] [CrossRef] [PubMed]
- Kaneshiro, S.; Ebina, K.; Shi, K.; Higuchi, C.; Hirao, M.; Okamoto, M.; Koizumi, K.; Morimoto, T.; Yoshikawa, H.; Hashimoto, J. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J. Bone Miner. Metab. 2014, 32, 378–392. [Google Scholar] [CrossRef]
- Chang, J.; Liu, F.; Lee, M.; Wu, B.; Ting, K.; Zara, J.N.; Soo, C.; Hezaimi, K.A.; Zou, W.; Chen, X.; et al. NF-κB inhibits osteogenic differentiation of mesenchymal stem cells by promoting β-catenin degradation. Proc. Natl. Acad. Sci. USA 2013, 110, 9469–9474. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Spicer, P.P.; Kasper, F.K.; Mikos, A.G. Harnessing and Modulating Inflammation in Strategies for Bone Regeneration. Tissue Eng. Part B Rev. 2011, 17, 393–402. [Google Scholar] [CrossRef]
- Komatsu, N.; Takayanagi, H. Inflammation and Bone Destruction in Arthritis: Synergistic Activity of Immune and Mesenchymal Cells in Joints. Front. Immunol. 2012, 3, 77. [Google Scholar] [CrossRef]
- Khodabandehloo, F.; Taleahmad, S.; Aflatoonian, R.; Rajaei, F.; Zandieh, Z.; Nassiri-Asl, M.; Eslaminejad, M.B. Microarray analysis identification of key pathways and interaction network of differential gene expressions during osteogenic differentiation. Hum. Genom. 2020, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Hess, K.; Ushmorov, A.; Fiedler, J.; Brenner, R.E.; Wirth, T. TNFα promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-κB signaling pathway. Bone 2009, 45, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.M.; Luo, L.; Stow, J.L. ERK1/2 in immune signalling. Biochem. Soc. Trans. 2022, 50, 1341–1352. [Google Scholar] [CrossRef]
- Kang, B.-S.; Park, Y.-G.; Cho, J.-Y.; Kim, J.-K.; Lee, T.-K.; Kim, D.-W.; Gu, Y.-H.; Suzuki, I.; Chang, Y.-C.; Kim, C.-H. Interleukin-1 and Tumor Necrosis Factor-α Induce Collagenolysis and Bone Resorption by Regulation of Matrix Metalloproteinase-2 in Mouse Calvarial Bone Cells. Immunopharmacol. Immunotoxicol. 2003, 25, 347–364. [Google Scholar] [CrossRef]
- Boskey, A.L.; Maresca, M.; Ullrich, W.; Doty, S.B.; Butler, W.T.; Prince, C.W. Osteopontin-hydroxyapatite interactions in vitro: Inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 1993, 22, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Gericke, A.; Qin, C.; Spevak, L.; Fujimoto, Y.; Butler, W.T.; Sørensen, E.S.; Boskey, A.L. Importance of Phosphorylation for Osteopontin Regulation of Biomineralization. Calcif. Tissue Int. 2005, 77, 45–54. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. J. Virtual Libr. 2007, 12, 3068–3092. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Ralphs, J.R.; Richards, R.G.; Hayes, J.S. A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing. J. Biomed. Mater. Res. Part A 2014, 102, 2636–2643. [Google Scholar] [CrossRef]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and Regulation of Runx2 in Osteogenesis. Eur. Cells Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Guillot, P.V.; De Bari, C.; Dell’Accio, F.; Kurata, H.; Polak, J.; Fisk, N.M. Comparative osteogenic transcription profiling of various fetal and adult mesenchymal stem cell sources. Differentiation 2008, 76, 946–957. [Google Scholar] [CrossRef]
- Gilbert, L.C.; Rubin, J.; Nanes, M.S. The p55 TNF receptor mediates TNF inhibition of osteoblast differentiation independently of apoptosis. Am. J. Physiol.-Endocrinol. Metab. 2005, 288, E1011–E1018. [Google Scholar] [CrossRef] [PubMed]





| Target Gene | Forward Primer (5′►3′) | Reverse Primer (5′►3′) | Product Length [bp] | Primer Concentration [µM] |
|---|---|---|---|---|
| Opn | CACTCCAGTTGTCCCCACAGTAG | TCTGTAGCATCAGGGTACTGGATGT | 120 | 0.2 |
| Runx2 | AGTGGACGAGGCAAGAGTTT | TGTCTGTGCCTTCTGGGTTC | 125 | 0.2 |
| Alp | GCAGGCAGCTTGACCTCCTC | GCATGGGGGCCAGACCAAAG | 121 | 0.2 |
| Rplp0 | TTCTCGCTTCCTGGAGGGTGT | CCAGGACTCGTTTGTACCCGT | 113 | 0.4 |
| Ocn | CTCCTCGCCCTATTGGCCCT | CTGCTTGGACACAAAGGCTGC | 105 | 0.3 |
| Tfrc | TTCAGGTCAAAGACAGCGCTCA | CTATACGCCACATAACCCCCAGG | 100 | 0.4 |
| Col1α2 | CCTGGTGCTAAAGGAGAAAGAGG | ATCACCACGACTTCCAGCAGGA | 135 | 0.4 |
| Target Protein | Company | Reference Number | Dilution |
|---|---|---|---|
| RUNX2 | Santa Cruz Biotechnology (Dallas, TX, USA) | Sc-390351 | 1:100 |
| OPN | Invitrogen (Carlsbad, CA, USA) | MA5-17180 | 1:500 |
| β-catenin | Abcam (Cambridge, UK) | Ab16051 | 1:4000 |
| GSK3β | Cell Signaling Technology (Cambridge, UK) | 9315 | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bousch, J.F.; Beyersdorf, C.; Schultz, K.; Schnitker, M.; Suschek, C.V.; Maus, U. Comparison of Primary Human Osteoblast-like Cells and hFOB 1.19 Cells: Contrasting Effects of Proinflammatory Cytokines. Cells 2025, 14, 1264. https://doi.org/10.3390/cells14161264
Bousch JF, Beyersdorf C, Schultz K, Schnitker M, Suschek CV, Maus U. Comparison of Primary Human Osteoblast-like Cells and hFOB 1.19 Cells: Contrasting Effects of Proinflammatory Cytokines. Cells. 2025; 14(16):1264. https://doi.org/10.3390/cells14161264
Chicago/Turabian StyleBousch, Juliana Franziska, Christoph Beyersdorf, Katharina Schultz, Matthis Schnitker, Christoph Viktor Suschek, and Uwe Maus. 2025. "Comparison of Primary Human Osteoblast-like Cells and hFOB 1.19 Cells: Contrasting Effects of Proinflammatory Cytokines" Cells 14, no. 16: 1264. https://doi.org/10.3390/cells14161264
APA StyleBousch, J. F., Beyersdorf, C., Schultz, K., Schnitker, M., Suschek, C. V., & Maus, U. (2025). Comparison of Primary Human Osteoblast-like Cells and hFOB 1.19 Cells: Contrasting Effects of Proinflammatory Cytokines. Cells, 14(16), 1264. https://doi.org/10.3390/cells14161264







