Gene Expression Pattern Associated with Cytoskeletal Remodeling in Lipid-Loaded Human Vascular Smooth Muscle Cells: Crosstalk Between C3 Complement and the Focal Adhesion Protein Paxillin
Abstract
1. Introduction
2. Materials and Methods
2.1. hVSMC Culture and LDL Preparation
2.2. Scratch-Wound Assay
2.3. Cell Adhesion Assay
2.4. RNA Extraction and Real-Time PCR Analysis
2.5. In Silico Analysis
2.6. Western Blot Analysis
2.7. Confocal Microscopy
2.8. Statistical Analysis
3. Results
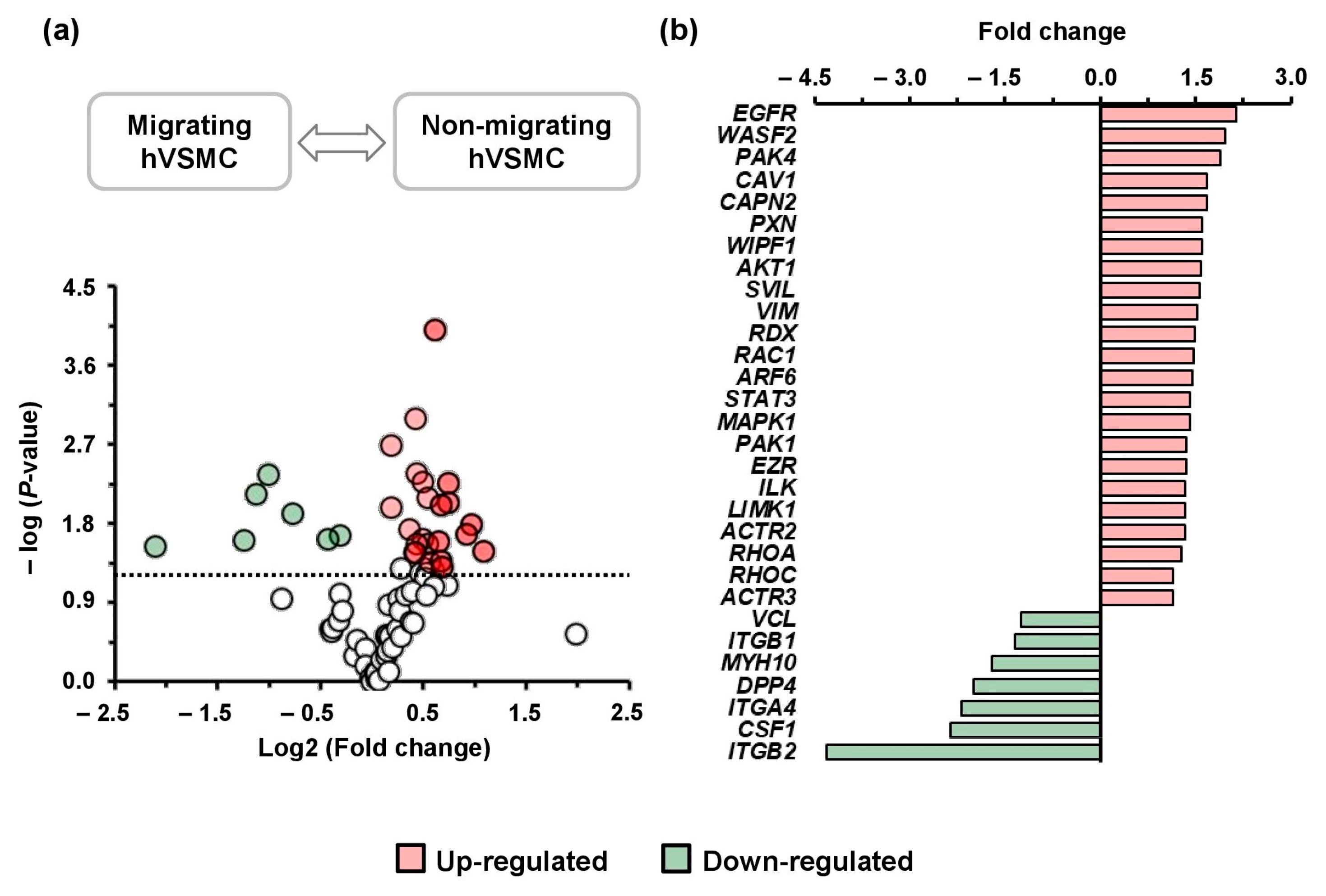
3.1. Gene Profiling Related to Cell Migration of hVSMCs
3.2. Gene Ontology (GO) Terms for the Differential Gene Pattern in Migrating hVSMCs
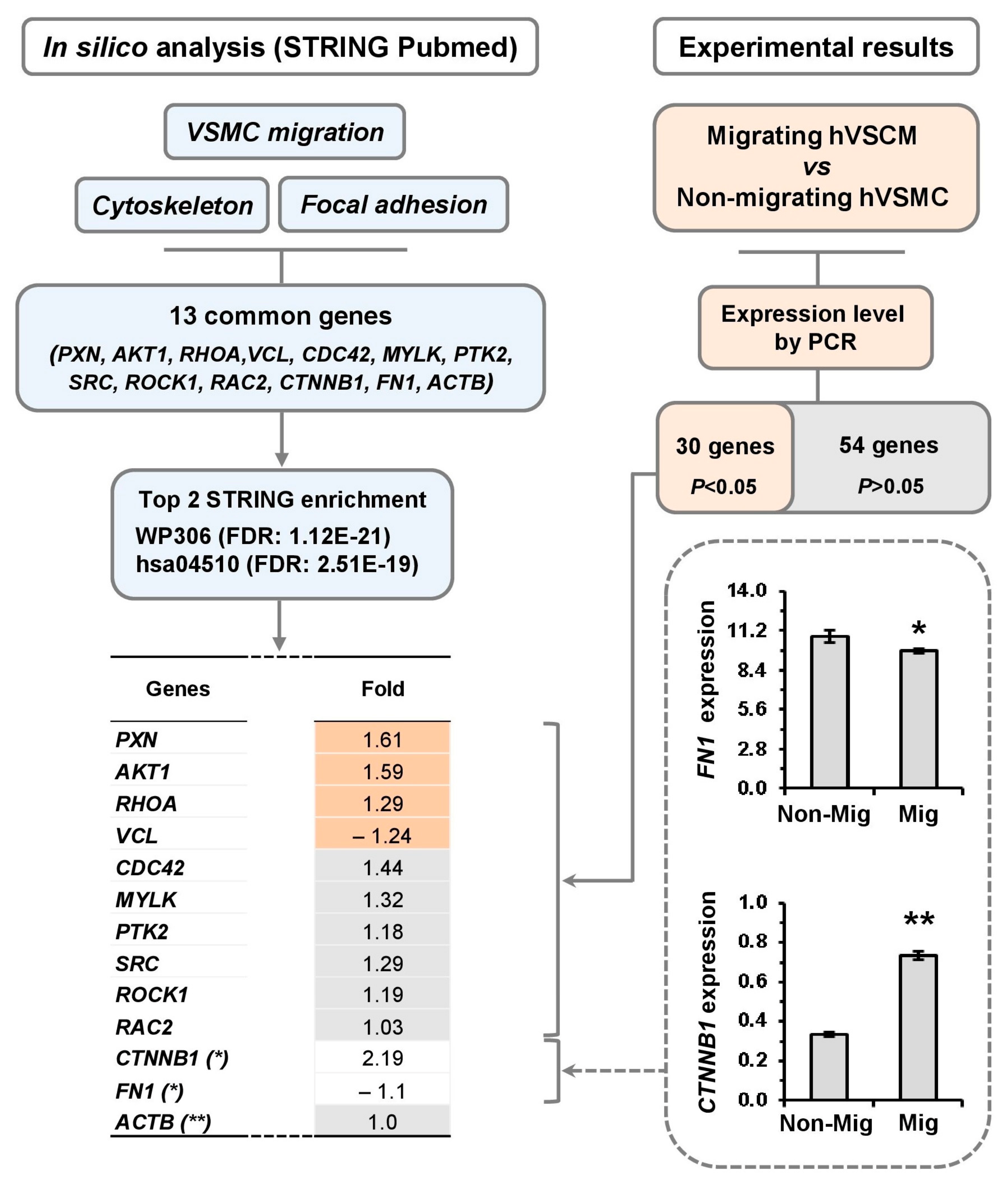
3.3. Differential Pattern of Genes Related to Actin Cytoskeleton in Migrating hVSMCs
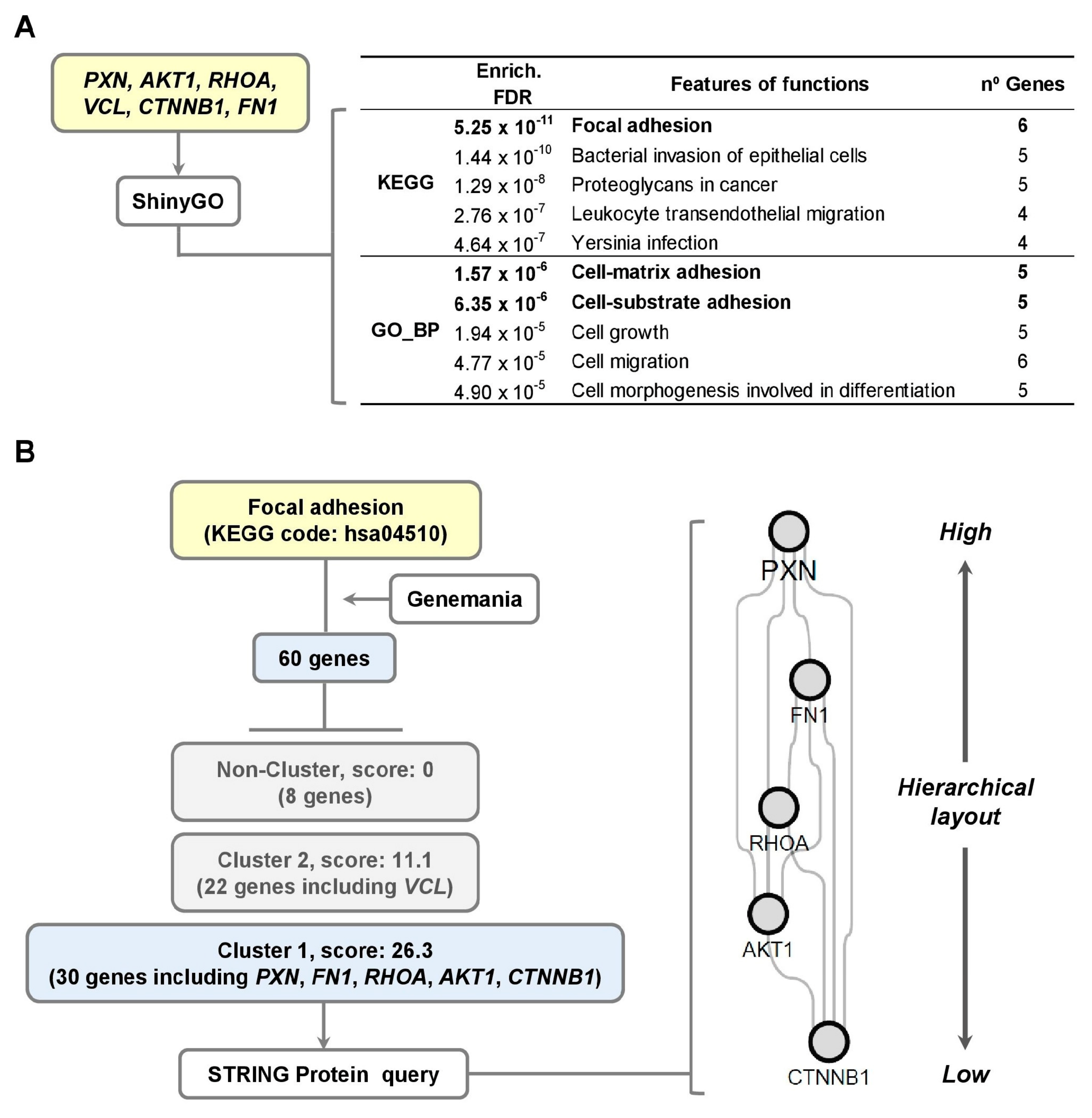
3.4. Signaling Pathway Linked to Cell Migration of hVSMCs
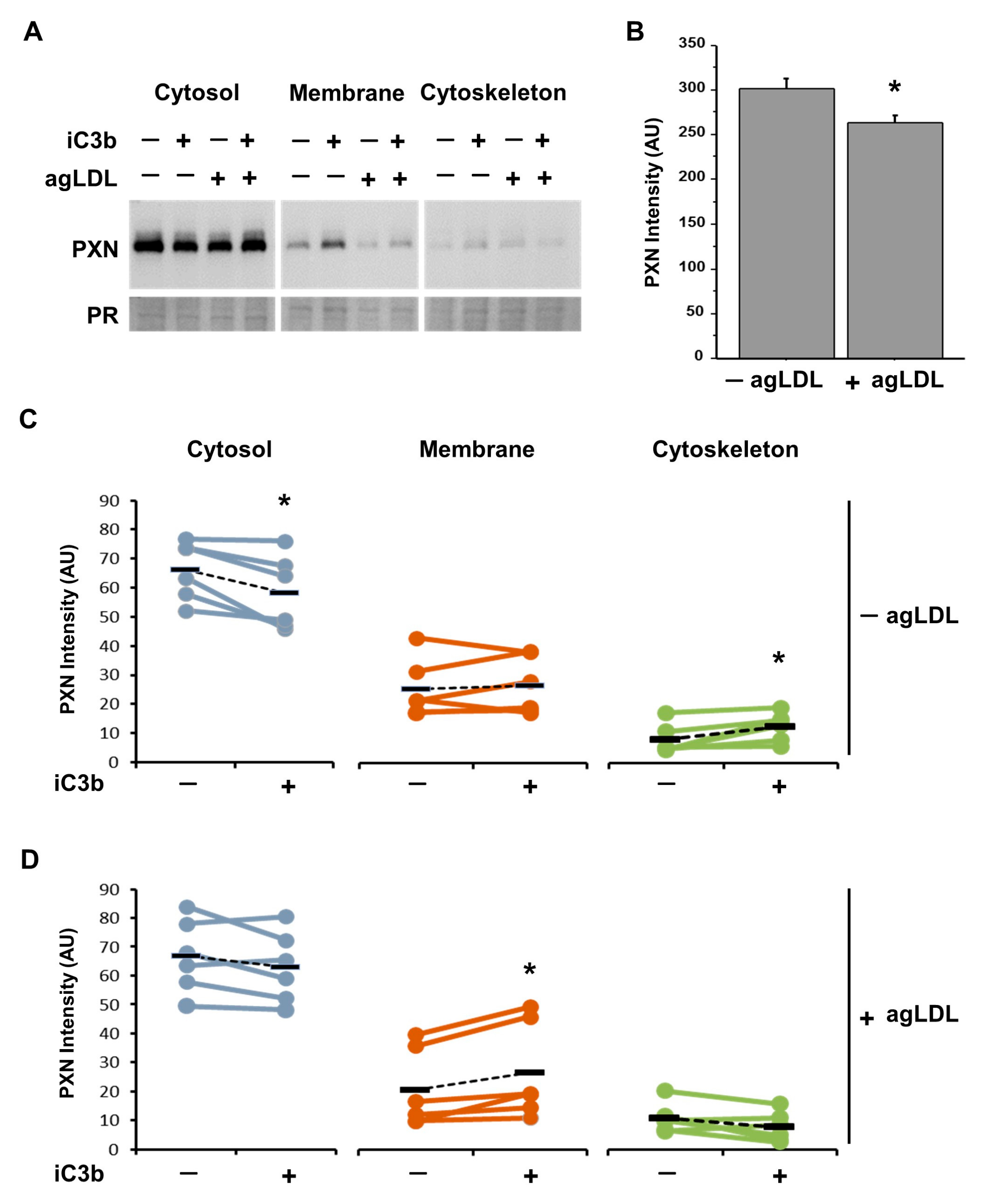
3.5. Effect of Atherogenic LDL on PXN Expression and Intracellular Localization
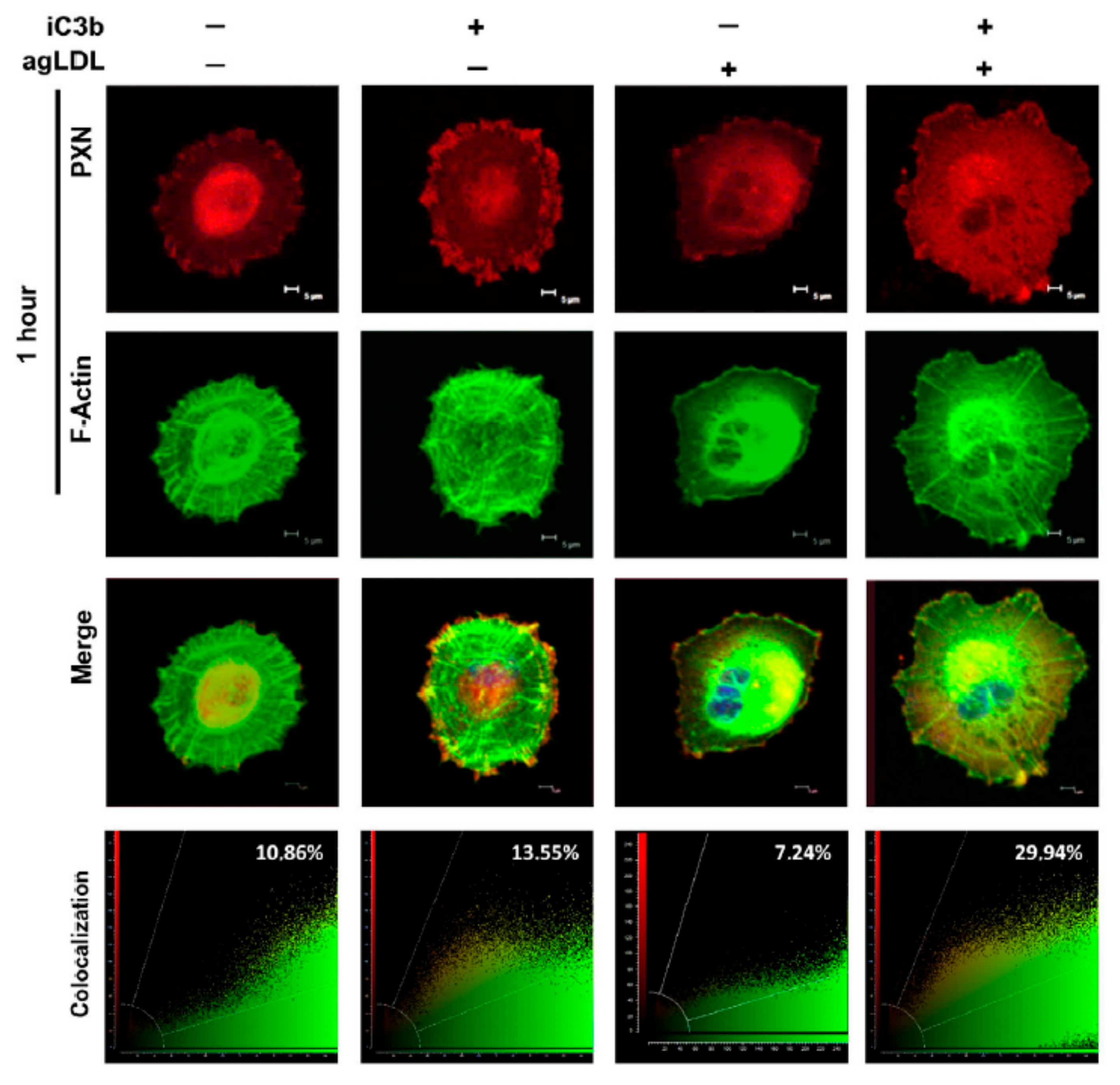
3.6. PXN as a Target of iC3b-Integrin Signaling: In Silico Predictions and Confocal Analysis
3.7. iC3b Modulates the Cytoskeleton Remodeling of PXN in Lipid-Loaded Adherent hVSMCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ip, J.H.; Fuster, V.; Badimon, L.; Badimon, J.; Taubman, M.B.; Chesebro, J.H. Syndromes of accelerated atherosclerosis: Role of vascular injury and smooth muscle cell proliferation. J. Am. Coll. Cardiol. 1990, 15, 1667–1687. [Google Scholar] [CrossRef] [PubMed]
- Berk, B.C. Vascular smooth muscle growth: Autocrine growth mechanisms. Physiol. Rev. 2001, 81, 999–1030. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Zhuge, Y.; Zhang, J.; Qian, F.; Wen, Z.; Niu, C.; Xu, K.; Ji, H.; Rong, X.; Chu, M.; Jia, C. Role of smooth muscle cells in cardiovascular disease. Int. J. Biol. Sci. 2020, 16, 2741–2751. [Google Scholar] [CrossRef]
- Lauffenburger, D.A.; Horwitz, A.F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Fan, S.; Xu, L.; Jiang, X.; Wang, K.; Cai, B. Macrophage migration inhibitory factor activates the inflammatory response in joint capsule fibroblasts following post-traumatic joint contracture. Aging 2021, 13, 5804–5823. [Google Scholar] [CrossRef]
- Llorente-Cortés, V.; Badimon, L. LDL receptor-related protein and the vascular: Wall implications for atherothrombosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 497–504. [Google Scholar] [CrossRef]
- García-Arguinzonis, M.; Padró, T.; Lugano, R.; Llorente-Cortes, V.; Badimon, L. Low-density lipoproteins induce heat shock protein 27 dephosphorylation, oligomerization, and subcellular relocalization in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1212–1219. [Google Scholar] [CrossRef]
- Padró, T.; Peña, E.; García-Arguinzonis, M.; Llorente-Cortes, V.; Badimon, L. Low-density lipoproteins impair migration of human coronary vascular smooth muscle cells and induce changes in the proteomic profile of myosin light chain. Cardiovasc. Res. 2008, 77, 211–220. [Google Scholar] [CrossRef][Green Version]
- Lugano, R.; Peña, E.; Badimon, L.; Padró, T. Aggregated low-density lipoprotein induce impairment of the cytoskeleton dynamics through urokinase-type plasminogen activator/urokinase-type plasminogen activator receptor in human vascular smooth muscle cell. J. Thromb. Haemost. 2012, 10, 2158–2167. [Google Scholar] [CrossRef]
- Garcia-Arguinzonis, M.; Diaz-Riera, E.; Peña, E.; Escate, R.; Juan-Babot, O.; Mata, P.; Badimon, L.; Padro, T. Alternative c3 complement system: Lipids and atherosclerosis. Int. J. Mol. Sci. 2021, 22, 5122. [Google Scholar] [CrossRef]
- Buono, C.; Come, C.E.; Witztum, J.L.; Maguire, G.F.; Connelly, P.W.; Carroll, M.; Lichtman, A.H. Influence of C3 deficiency on atherosclerosis. Circulation 2002, 105, 3025–3031. [Google Scholar] [CrossRef] [PubMed]
- Persson, L.; Borén, J.; Robertson, A.-K.L.; Wallenius, V.; Hansson, G.K.; Pekna, M. Lack of complement factor C3, but not factor B, increases hyperlipidemia and atherosclerosis in apolipoprotein E-/- low-density lipoprotein receptor-/- mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E. Paxillin and focal adhesion signalling. Nat. Cell Biol. 2000, 2, E231–E236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kishi, H.; Morita, T.; Kobayashi, S. Paxillin controls actin stress fiber formation and migration of vascular smooth muscle cells by directly binding to the active Fyn. FASEB J. 2021, 35, e22012. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Song, S.; Sun, Z.; Li, Y.; Yang, L.; Xie, Z.; Cai, Y.; Zhao, Y. Mitochondria spatially and temporally modulate VSMC phenotypes via interacting with cytoskeleton in cardiovascular diseases. Redox Biol. 2023, 64, 102778. [Google Scholar] [CrossRef]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 1955, 34, 1345–1353. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Escate, R.; Padro, T.; Badimon, L. LDL accelerates monocyte to macrophage differentiation: Effects on adhesion and anoikis. Atherosclerosis 2016, 246, 177–186. [Google Scholar] [CrossRef]
- Escate, R.; Mata, P.; Cepeda, J.M.; Padró, T.; Badimon, L. MiR-505-3p controls chemokine receptor up-regulation in macrophages: Role in familial hypercholesterolemia. FASEB J. 2018, 32, 601–612. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef]
- Xu, Q.; Huff, L.P.; Fujii, M.; Griendling, K.K. Redox regulation of the actin cytoskeleton and its role in the vascular system. Free Radic. Biol. Med. 2017, 109, 84–107. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Bennett, M.R. Vascular smooth muscle cells in atherosclerosis: Time for a re-assessment. Cardiovasc. Res. 2021, 117, 2326–2339. [Google Scholar] [CrossRef] [PubMed]
- López-Colomé, A.M.; Lee-Rivera, I.; Benavides-Hidalgo, R.; López, E. Paxillin: A crossroad in pathological cell migration. J. Hematol. Oncol. 2017, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ventura, J.L.; Martinez-Lopez, D.; Roldan-Montero, R.; Gomez-Guerrero, C.; Blanco-Colio, L.M. Role of complement system in pathological remodeling of the vascular wall. Mol. Immunol. 2019, 114, 207–215. [Google Scholar] [CrossRef]
- Vlaicu, S.I.; Tatomir, A.; Rus, V.; Mekala, A.P.; Mircea, P.A.; Niculescu, F.; Rus, H. The role of complement activation in atherogenesis: The first 40 years. Immunol. Res. 2016, 64, 1–13. [Google Scholar] [CrossRef]
- Hansson, G.K.; Holm, J.; Kral, J.G. Accumulation of IgG and complement factor C3 in human arterial endothelium and atherosclerotic lesions. Acta Pathol. Microbiol. Immunol. Scand. A 1984, 92, 429–435. [Google Scholar] [CrossRef]
- Ge, X.; Xu, C.; Liu, Y.; Zhu, K.; Zeng, H.; Su, J.; Huang, J.; Ji, Y.; Tan, Y.; Hou, Y. Complement activation in the arteries of patients with severe atherosclerosis. Int. J. Clin. Exp. Pathol. 2018, 11, 1–9. [Google Scholar]
- Graham, I.L.; Anderson, D.C.; VM, V.M.H.; Brown, E.J. Complement receptor 3 (CR3, Mac-1, integrin alpha M beta 2, CD11b/CD18) is required for tyrosine phosphorylation of paxillin in adherent and nonadherent neutrophils. J. Cell Biol. vol. 1997, 127, 1139–1147. [Google Scholar] [CrossRef]
- Zhang, Y.; Kishi, H.; Kobayashi, S. Direct active Fyn-paxillin interaction regulates vascular smooth muscle cell migration. J. Smooth Muscle Res. 2023, 59, 58–66. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Arguinzonis, M.; Escate, R.; Lugano, R.; Peña, E.; Borrell-Pages, M.; Badimon, L.; Padro, T. Gene Expression Pattern Associated with Cytoskeletal Remodeling in Lipid-Loaded Human Vascular Smooth Muscle Cells: Crosstalk Between C3 Complement and the Focal Adhesion Protein Paxillin. Cells 2025, 14, 1245. https://doi.org/10.3390/cells14161245
Garcia-Arguinzonis M, Escate R, Lugano R, Peña E, Borrell-Pages M, Badimon L, Padro T. Gene Expression Pattern Associated with Cytoskeletal Remodeling in Lipid-Loaded Human Vascular Smooth Muscle Cells: Crosstalk Between C3 Complement and the Focal Adhesion Protein Paxillin. Cells. 2025; 14(16):1245. https://doi.org/10.3390/cells14161245
Chicago/Turabian StyleGarcia-Arguinzonis, Maisa, Rafael Escate, Roberta Lugano, Esther Peña, Maria Borrell-Pages, Lina Badimon, and Teresa Padro. 2025. "Gene Expression Pattern Associated with Cytoskeletal Remodeling in Lipid-Loaded Human Vascular Smooth Muscle Cells: Crosstalk Between C3 Complement and the Focal Adhesion Protein Paxillin" Cells 14, no. 16: 1245. https://doi.org/10.3390/cells14161245
APA StyleGarcia-Arguinzonis, M., Escate, R., Lugano, R., Peña, E., Borrell-Pages, M., Badimon, L., & Padro, T. (2025). Gene Expression Pattern Associated with Cytoskeletal Remodeling in Lipid-Loaded Human Vascular Smooth Muscle Cells: Crosstalk Between C3 Complement and the Focal Adhesion Protein Paxillin. Cells, 14(16), 1245. https://doi.org/10.3390/cells14161245







