Notch2 Deletion Compromises Epithelial Integrity and Enamel Formation in Rodent Incisors
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice Handling and Ethics Statement
2.2. Tissue Collection and Processing
2.3. Incisor Dissection and Length Measurement
2.4. Histological Evaluation
2.5. Immunostaining on Sections
2.6. Scanning Electron Microscopy (SEM)
2.7. Micro-Computed Tomography (μCT)
2.8. Tissue Collection for Gene Expression Analysis
2.9. LS8 Dental Epithelium-like Cell Line and In Vitro Experimentation
2.10. Quantification and Statistical Analysis
3. Results
3.1. Distribution of the Notch1 and Notch2 Proteins in Prenatal and Early Postnatal Incisors
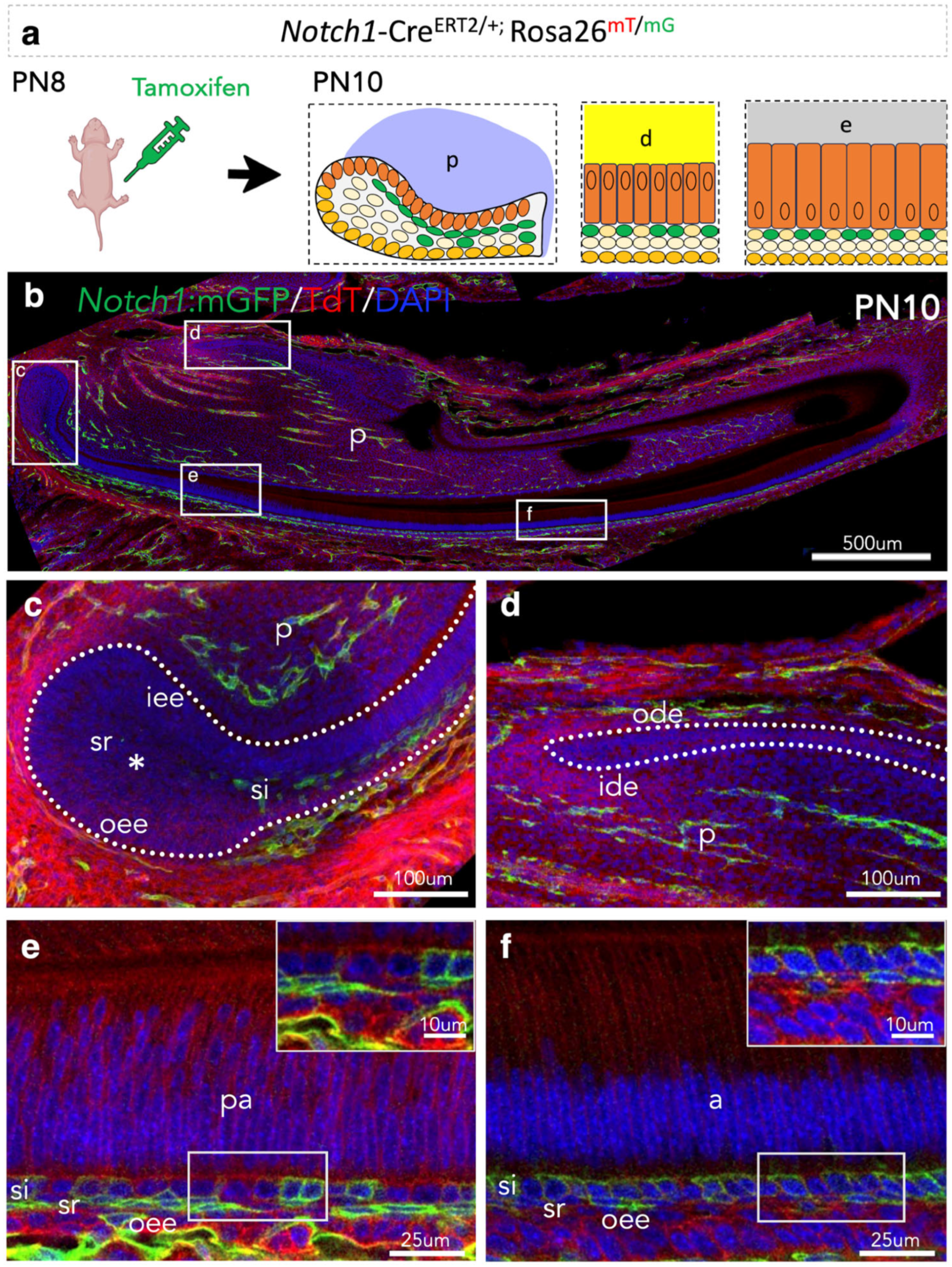
3.2. Lineage Tracing of Notch1-Expressing Cells and Their Progeny in the Epithelium of Early Postnatal Incisors
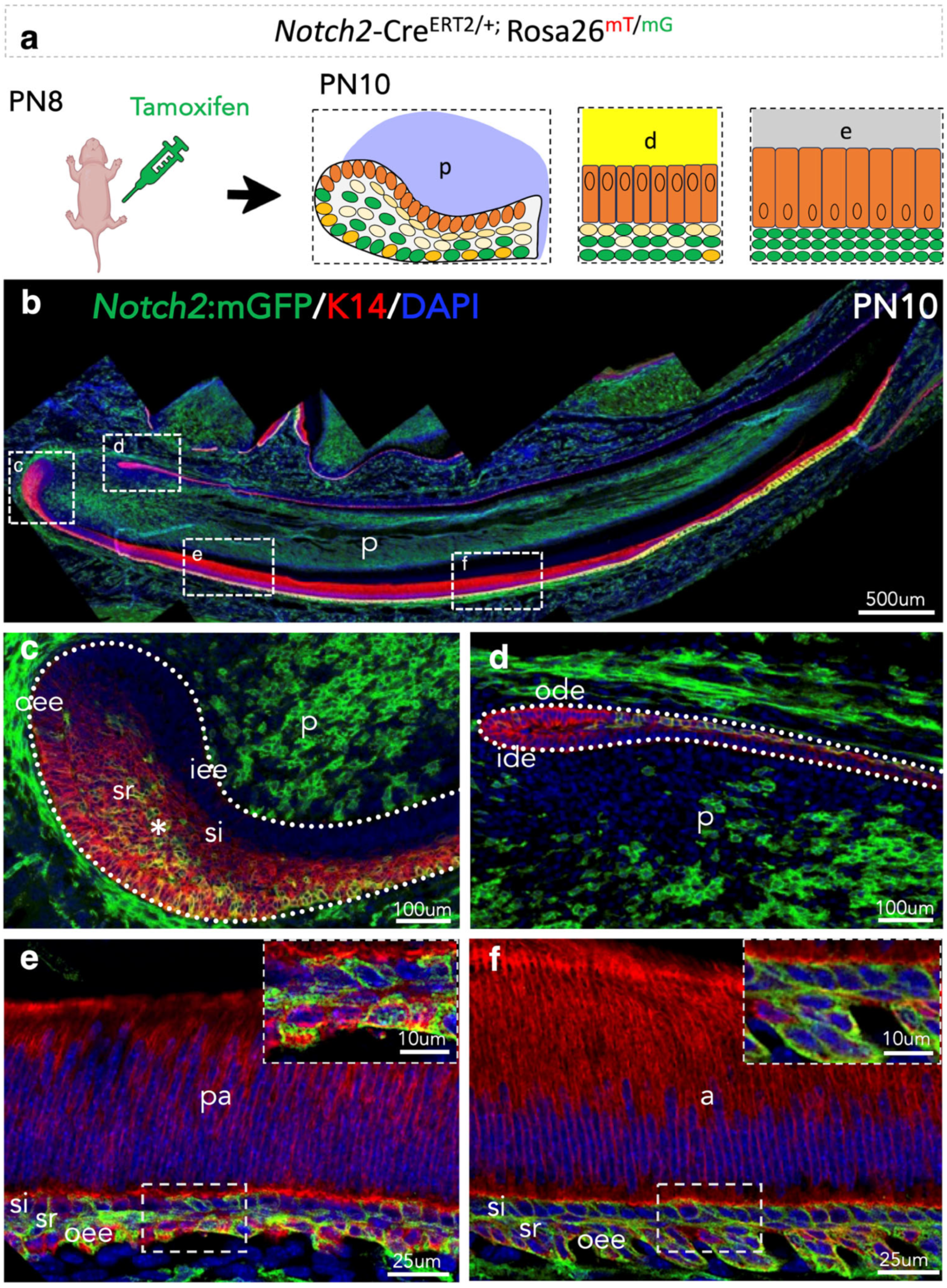
3.3. Lineage Tracing of Notch2-Expressing Cells and Their Progeny in the Epithelium of Early Postnatal Incisors
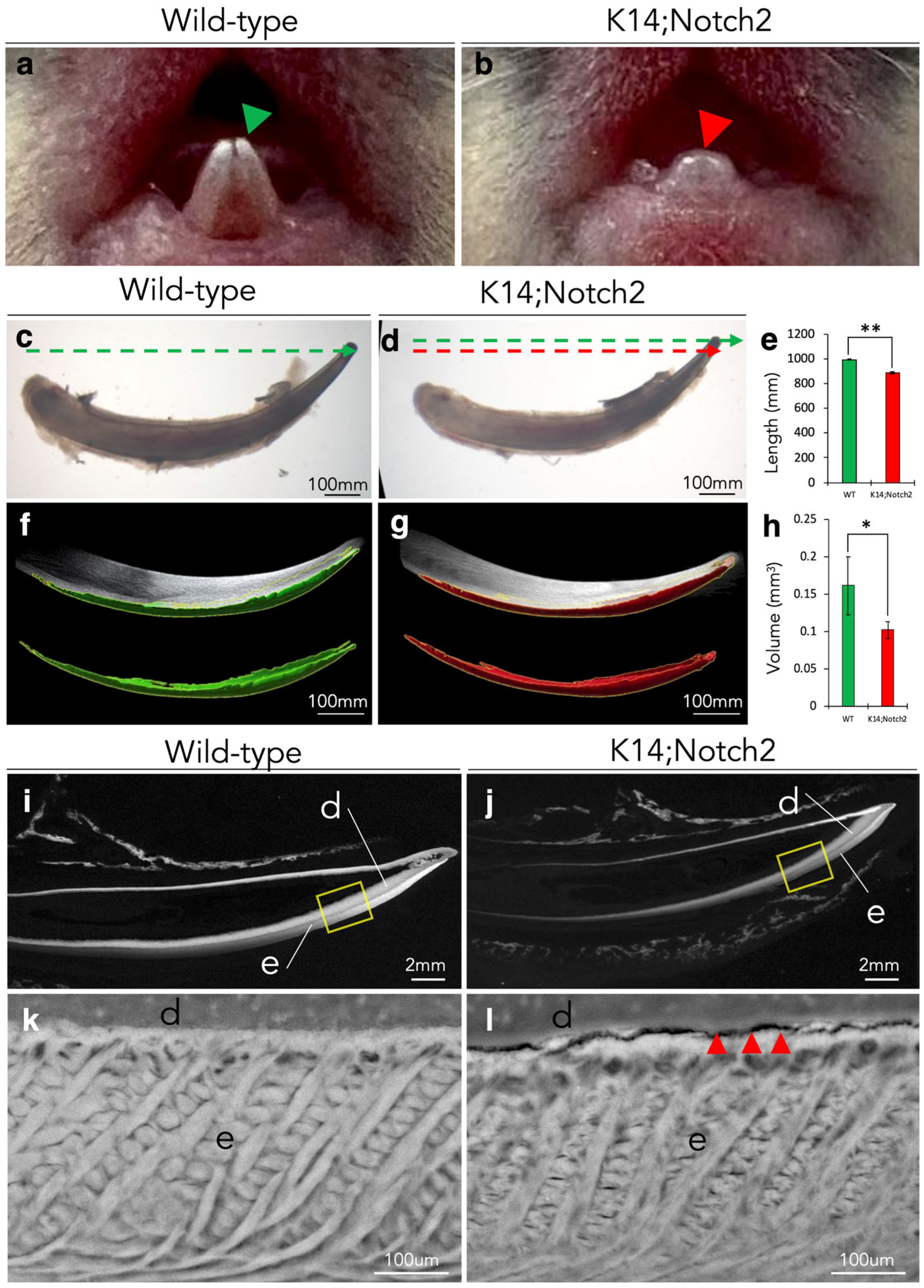
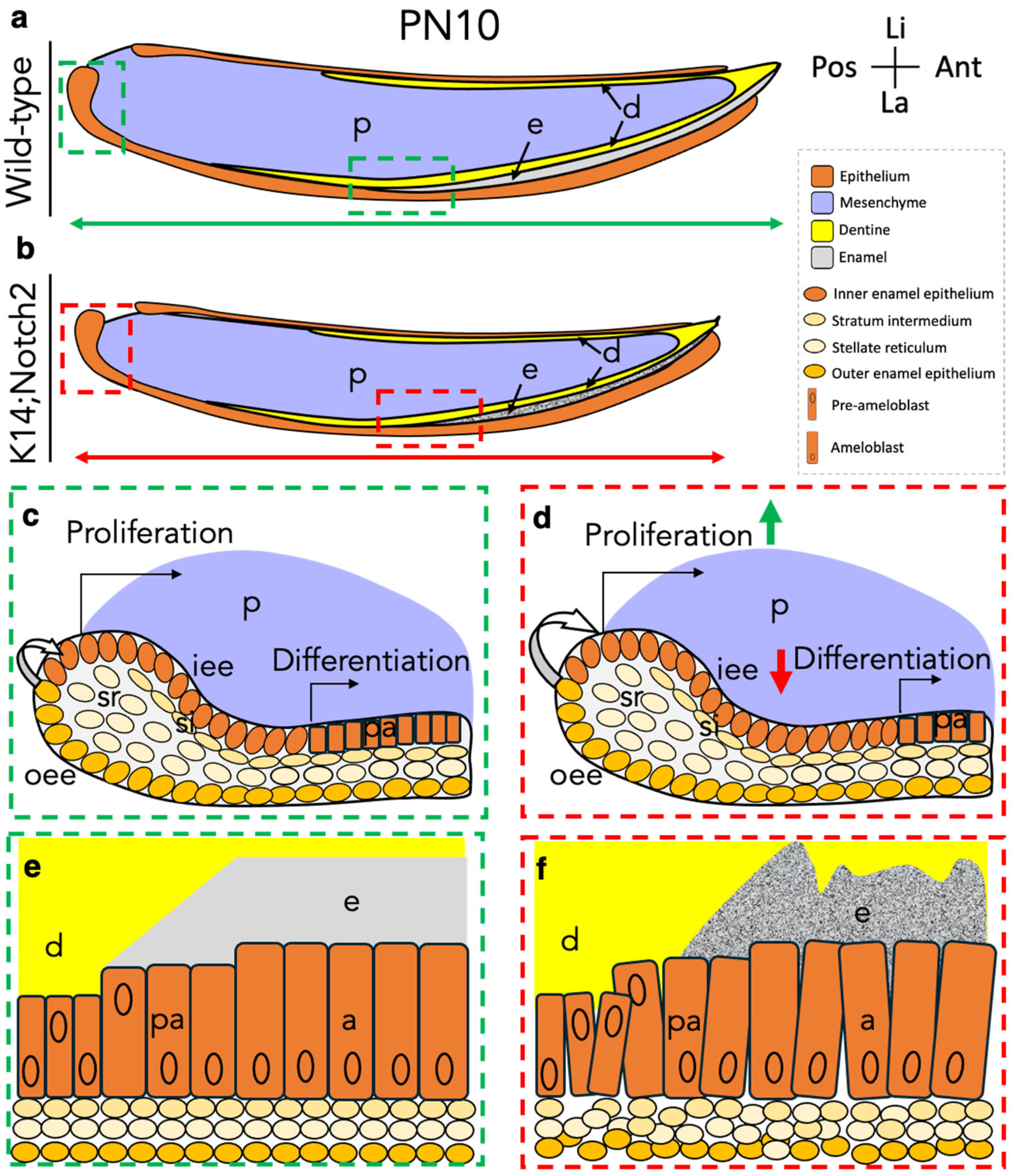
3.4. Epithelial Notch2 Deletion in Incisors Leads to Shorter Incisors and Enamel Malformation
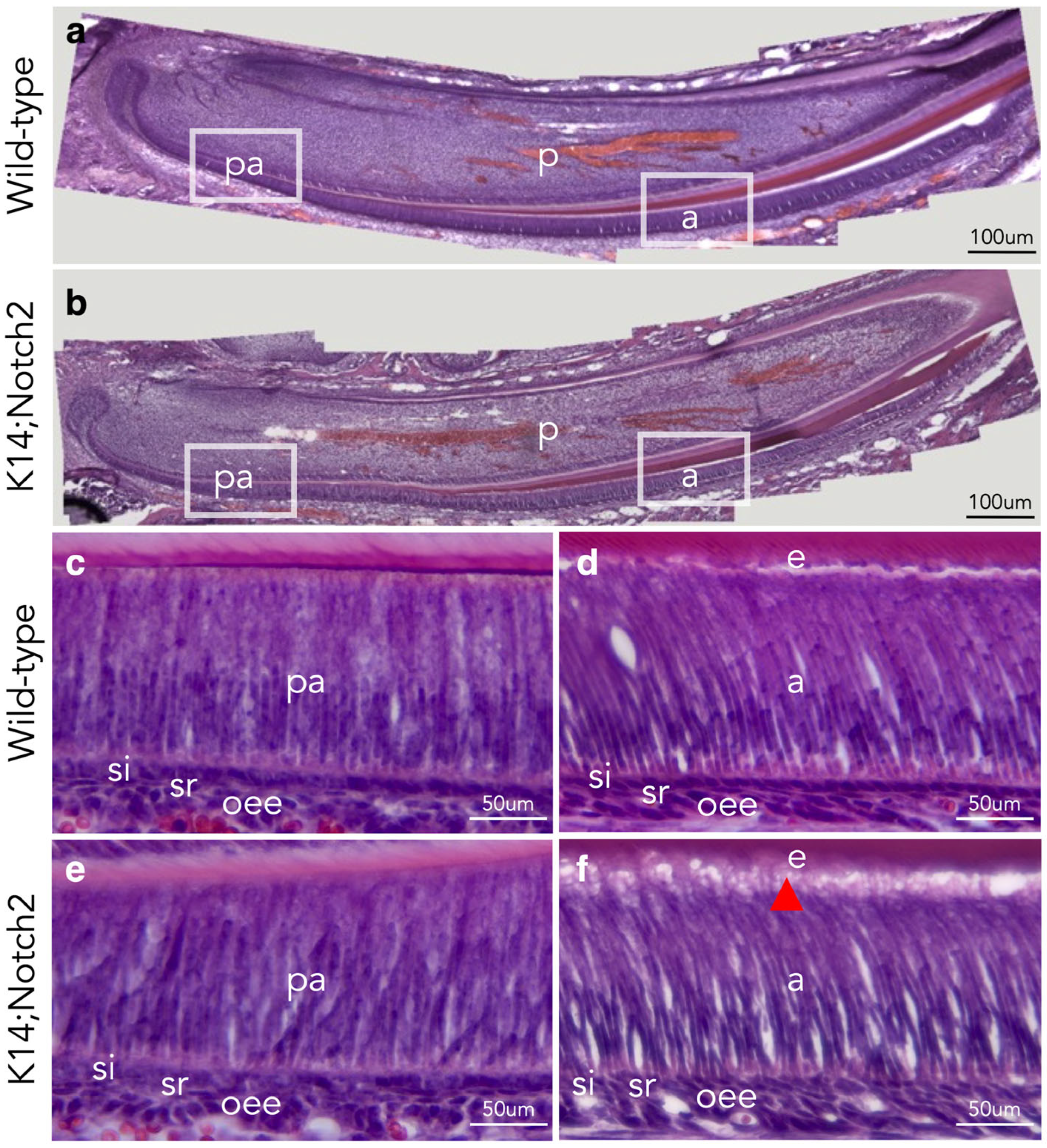
3.5. Loss of Notch2 Results in Defective Dental Epithelium
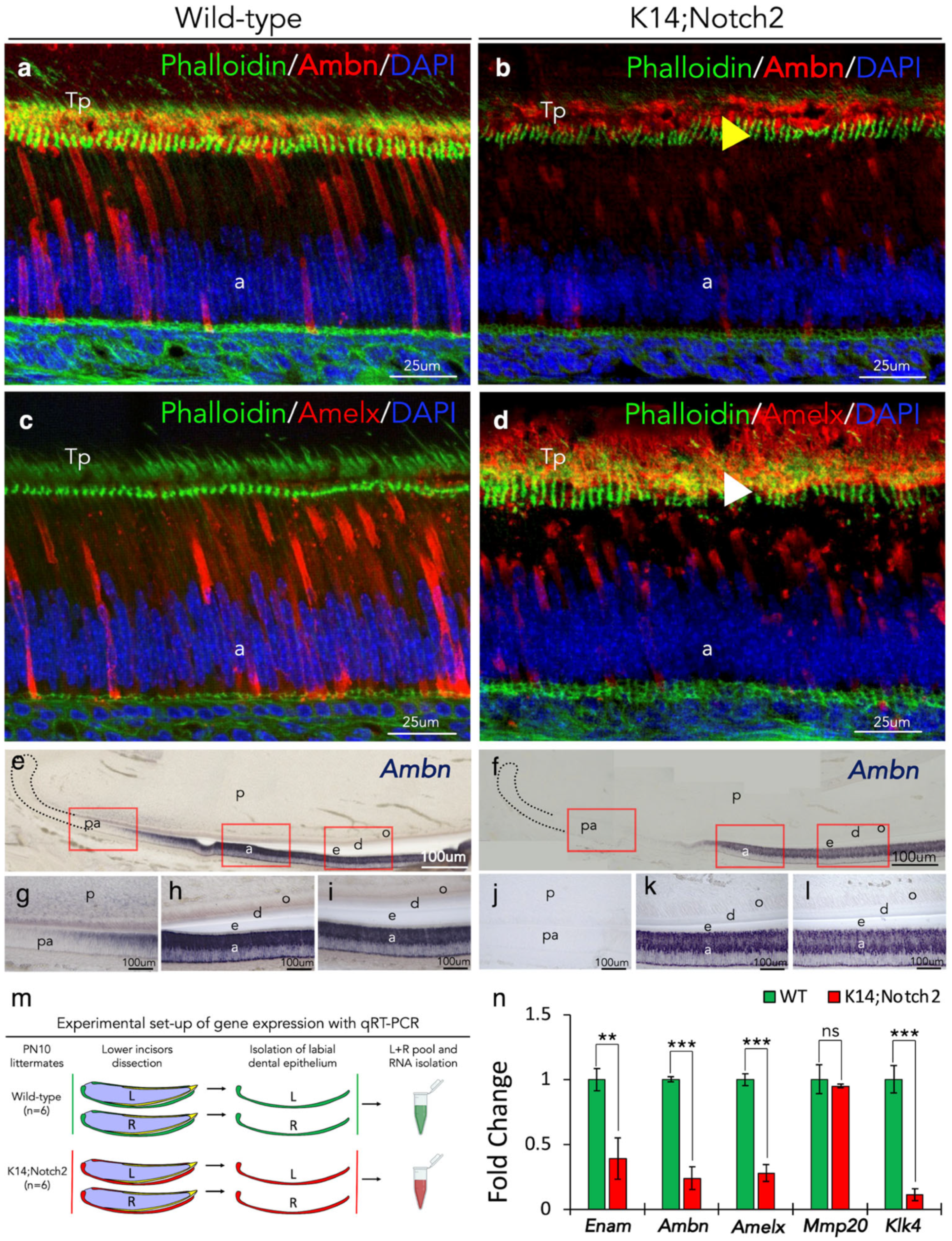
3.6. Aberrant Enamel Protein Distribution and Enamel Gene Expression in Dental Epithelium upon Notch2 Deletion
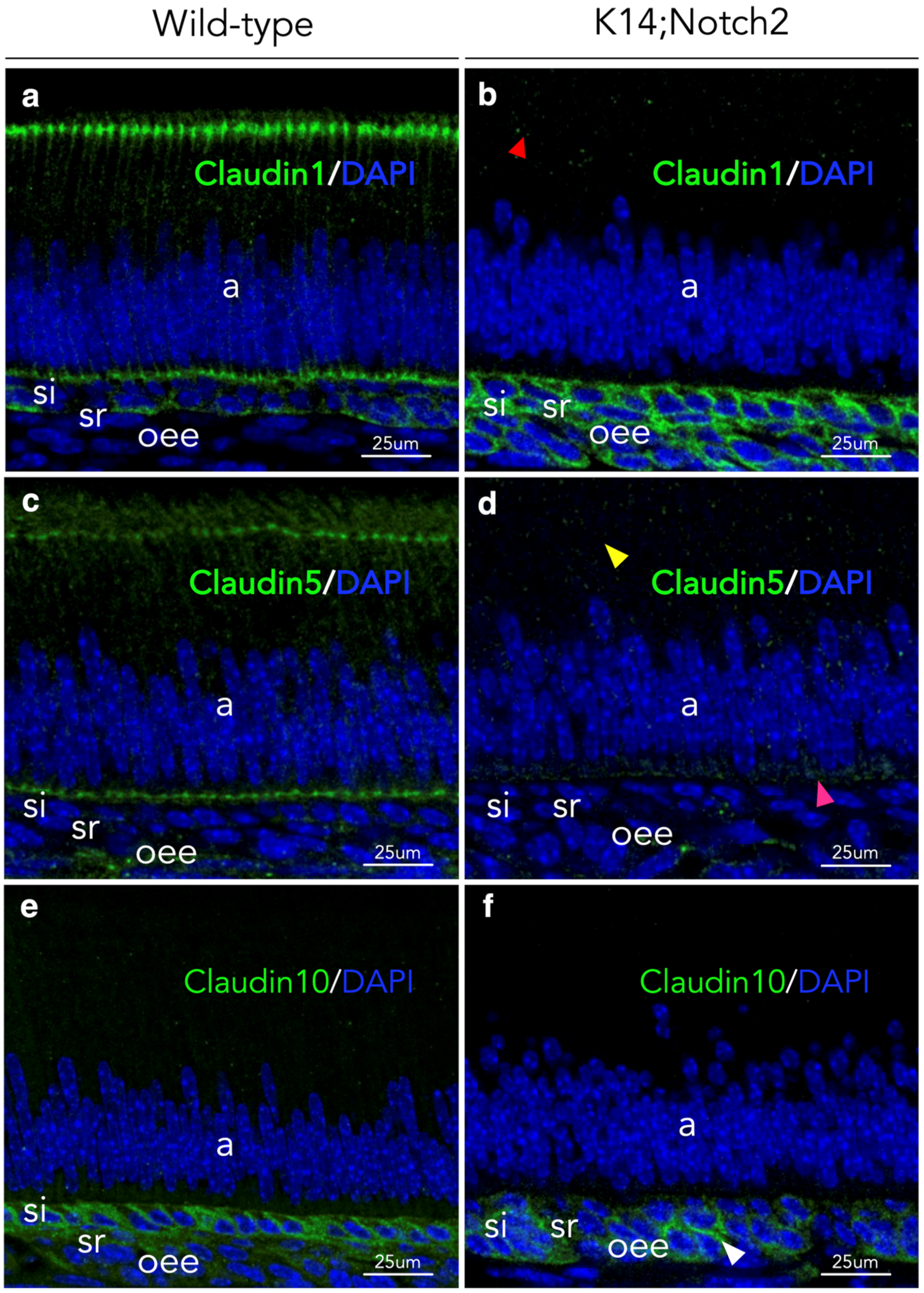
3.7. Cell-to-Cell Adhesion Disruption in Incisors’ Epithelium upon Notch2 Deletion
3.8. Modifications in the Epithelial Stem Cell Niche of Incisors upon Notch2 Deletion
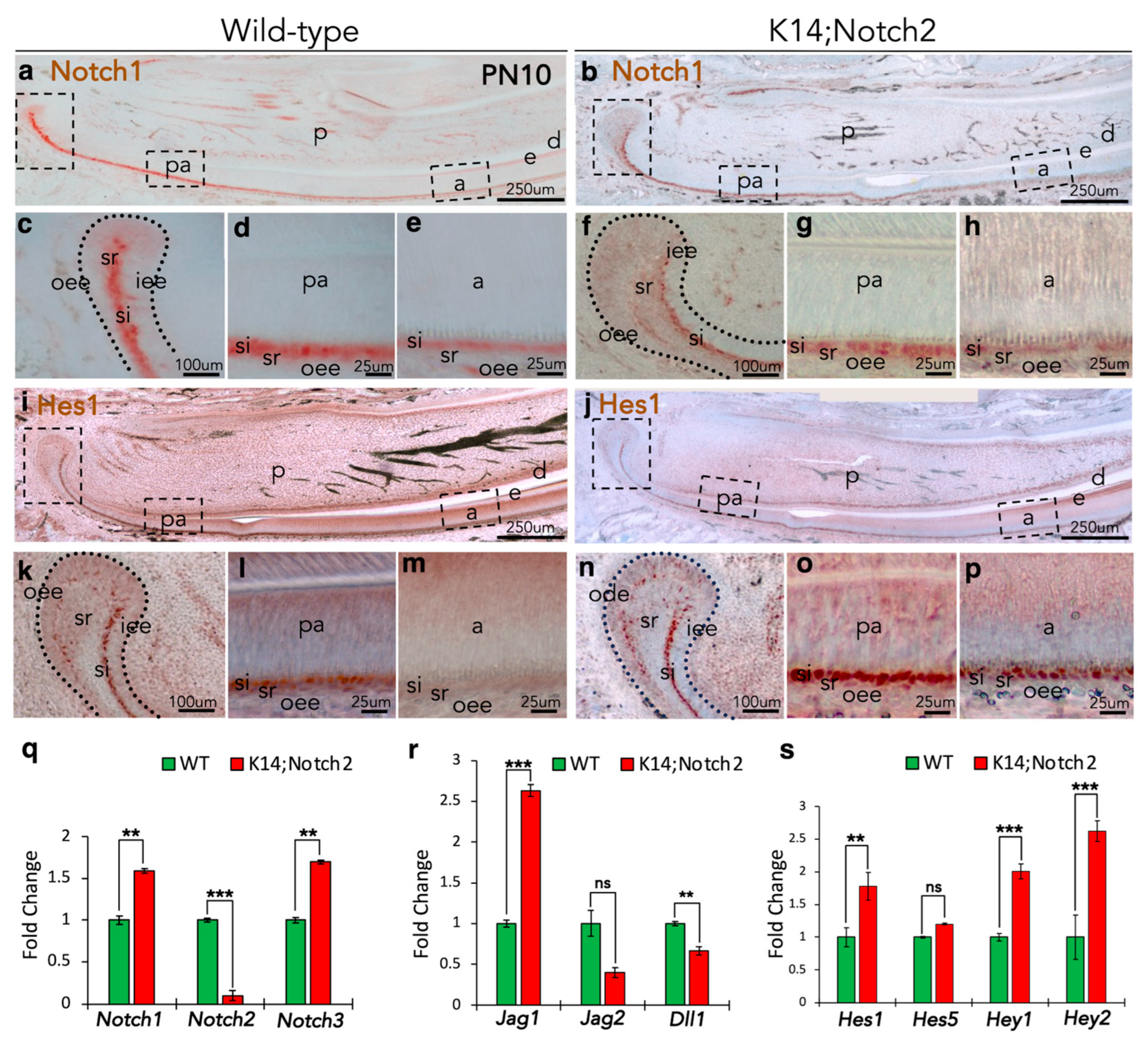
3.9. Notch2 Deletion Affects the Notch Signalling Pathway in the Incisors’ Epithelium
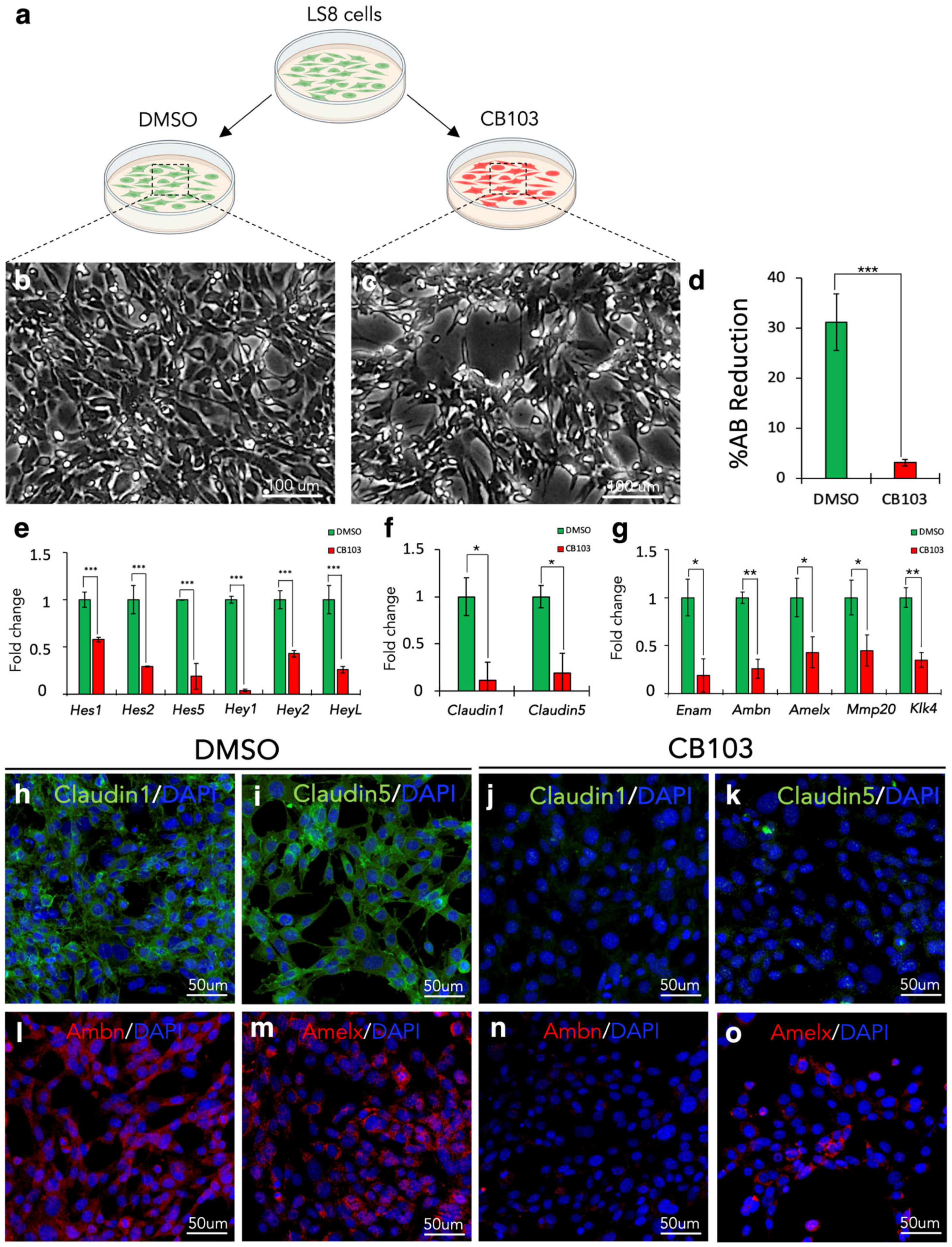
3.10. Notch Signalling Pharmacological Inhibition In Vitro Recapitulates the In Vivo Keratin14Cre/+;Notch2fl/fl Incisors’ Phenotype
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMBN | Ameloblastin |
| AMELX | Amelogenin |
| BSA | Bovine Serum Albumin |
| DEJ | Confocal Laser Scanning Microscope |
| DMSO | Dimethyl sulfoxide |
| E | Embryonic Day |
| EDU | 5-Ethynyl-2′-Deoxyuridine |
| ENAM | Enamelin |
| GFP | Green Fluorescent Protein |
| IEE | Inner Enamel Epithelium |
| IF | Immunofluorescence |
| IHC | Immunohistochemistry |
| KLK4 | Kallikrein 4 |
| KO | Knockout |
| laCL | Labial Cervical Loop |
| liCL | Lingual Cervical Loop |
| μm | Micron |
| MMP20 | Matrix Metalloprotease 20 |
| NICD | Notch Intracellular Domain |
| OEE | Outer Enamel Epithelium |
| PN | Postnatal Day |
| PCR | Polymerase Chain Reaction |
| qPCR | Quantitative Polymerase Chain Reaction |
| SI | Stratum Intermedium |
| SOX2 | SRY-box transcription factor 2 |
| SR | Stellate Reticulum |
References
- Sjöqvist, M.; Andersson, E.R. Do as I say, Not(ch) as I do: Lateral control of cell fate. Dev. Biol. 2019, 447, 58–70. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, S.; Matsuno, K.; Fortini, M.E. Notch signaling. Science 1995, 268, 225–232. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch Signaling: Cell Fate Control and Signal Integration in Development. Science 1999, 284, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Kopan, R. Notch signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, 10. [Google Scholar] [CrossRef]
- Nye, J.S.; Kopan, R. Vertebrate ligands for Notch. Curr. Biol. 1995, 5, 966–969. [Google Scholar] [CrossRef]
- Kovall, R.A.; Gebelein, B.; Sprinzak, D.; Kopan, R. The Canonical Notch Signaling Pathway: Structural and Biochemical Insights into Shape, Sugar, and Force. Dev. Cell 2017, 41, 228–241. [Google Scholar] [CrossRef]
- Hori, K.; Sen, A.; Artavanis-Tsakonas, S. Notch signaling at a glance. J. Cell Sci. 2013, 126, 2135–2140. [Google Scholar] [CrossRef]
- Siebel, C.; Lendahl, U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol. Rev. 2017, 97, 1235–1294. [Google Scholar] [CrossRef]
- Mitsiadis, T.A.; Graf, D.; Luder, H.; Gridley, T.; Bluteau, G. BMPs and FGFs target Notch signalling via jagged 2 to regulate tooth morphogenesis and cytodifferentiation. Development 2010, 137, 3025–3035. [Google Scholar] [CrossRef]
- Mitsiadis, T.A.; Jimenez-Rojo, L.; Balic, A.; Weber, S.; Saftig, P.; Pagella, P. Adam10-dependent Notch signaling establishes dental epithelial cell boundaries required for enamel formation. iScience 2022, 25, 10. [Google Scholar] [CrossRef]
- Mitsiadis, T.A.; Pagella, P.; Gomes-Rodrigues, H.; Tsouknidas, A.; Ramenzoni, L.L.; Radtke, F.; Mehl, A.; Viriot, L. Notch Signaling Pathway in Tooth Shape Variations throughout Evolution. Cells 2023, 12, 761. [Google Scholar] [CrossRef] [PubMed]
- Mitsiadis, T.A.; Pagella, P.; Capellini, T.D.; Smith, M.M. The Notch-mediated circuitry in the evolution and generation of new cell lineages: The tooth model. Cell. Mol. Life Sci. 2023, 80, 182. [Google Scholar] [CrossRef] [PubMed]
- Jernvall, J.; Thesleff, I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech. Dev. 2000, 92, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Mitsiadis, T.A.; Henrique, D.; Thesleff, I.; Lendahl, U. Mouse Serrate-1 (Jagged-1): Expression in the developing tooth is regulated by epithelial-mesenchymal interactions and fibroblast growth factor-4. Development 1997, 124, 1473–1483. [Google Scholar] [CrossRef]
- Li, J.; Feng, J.; Liu, Y.; Ho, T.V.; Grimes, W.; Ho, H.A.; Park, S.; Wang, S.; Chai, Y. BMP-SHH signaling network controls epithelial stem cell fate via regulation of its niche in the developing tooth. Dev. Cell 2015, 33, 125–135. [Google Scholar] [CrossRef]
- Liu, H.; Yan, X.; Pandya, M.; Luan, X.; Diekwisch, T.G.H. Daughters of the enamel organ: Development, fate, and function of the stratum intermedium, stellate reticulum, and outer enamel epithelium. Stem Cells Dev. 2016, 25, 1580–1590. [Google Scholar] [CrossRef]
- Rodrigues, H.G.; Renaud, S.; Charles, C.; Le Poul, Y.; Solé, F.; Aguilar, J.P.; Michaux, J.; Tafforeau, P.; Headon, D.; Jernvall, J.; et al. Roles of dental development and adaptation in rodent evolution. Nat. Commun. 2013, 4, 2504. [Google Scholar] [CrossRef]
- Mitsiadis, T.A.; Luder, H. Genetic basis for tooth malformations: From mice to men and back again. Clin. Genet. 2011, 80, 319–329. [Google Scholar] [CrossRef]
- Jernvall, J.; Thesleff, I. Tooth shape formation and tooth renewal: Evolving with the same signals. Development 2012, 139, 3487–3497. [Google Scholar] [CrossRef]
- Thesleff, I.; Tummers, M. Tooth organogenesis and regeneration. In StemBook; Harvard Stem Cell Institute: Cambridge, MA, USA, 2009; pp. 1–12. [Google Scholar]
- Mitsiadis, T.A.; Barrandon, O.; Rochat, A.; Barrandon, Y.; De Bari, C. Stem cell niches in mammals. Exp. Cell Res. 2007, 313, 3377–3385. [Google Scholar] [CrossRef]
- Sharir, A.; Marangoni, P.; Zilionis, R.; Wan, M.; Wald, T.; Hu, J.K.; Kawaguchi, K.; Castillo-Azofeifa, D.; Epstein, L.; Pagella, P.; et al. A large pool of actively cycling progenitors orchestrates self- renewal and injury repair of an ectodermal appendage. Nat. Cell Biol. 2019, 21, 1102–1112. [Google Scholar] [CrossRef]
- Caton, J.; Luder, H.U.; Zoupa, M.; Bradman, M.; Bluteau, G.; Tucker, A.S.; Klein, O.; Mitsiadis, T.A. Enamel-free teeth: Tbx1 deletion affects amelogenesis in rodent incisors. Dev. Biol. 2009, 328, 493–505. [Google Scholar] [CrossRef]
- Harada, H.; Kettunen, P.; Jung, H.S.; Mustonen, T.; Wang, Y.A.; Thesleff, I. Localization of putative stem cells in dental epithelium and their association with Notch and FGF Signaling. J. Cell Biol. 1999, 147, 105–120. [Google Scholar] [CrossRef]
- Bei, M. Molecular genetics of ameloblast cell lineage. J. Exp. Zool. B Mol. Dev. Evol. 2009, 312, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Jheon, A.H.; Prochazkova, M.; Meng, B.; Wen, T.; Lim, Y.J.; Naveau, A.; Espinoza, R.; Cox, T.C.; Sone, E.D.; Ganss, B.; et al. Inhibition of Notch signaling during mouse incisor renewal leads to enamel defects. J. Bone Miner. Res. 2016, 31, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Jheon, A.H.; Mostowfi, P.; Snead, M.L.; Ihrie, R.A.; Sone, E.; Pramparo, T.; Attardi, L.D.; Klein, O.D. PERP regulates enamel formation via effects on cell-cell adhesion and gene expression. J. Cell Sci. 2011, 124, 745–754. [Google Scholar] [CrossRef]
- Cantù, C.; Pagella, P.; Shajiei, T.D.; Zimmerli, D.; Valenta, T.; Hausmann, G.; Basler, K.; Mitsiadis, T.A. A cytoplasmic role of Wnt/β-catenin transcriptional cofactors Bcl9, Bcl9l, and Pygopus in tooth enamel formation. Sci. Signal. 2017, 10, 465. [Google Scholar] [CrossRef]
- Pagella, P.; Lai, C.F.; Pirenne, L.; Cantù, C.; Schwab, M.E.; Mitsiadis, T.A. An unexpected role of Nogo-A as regulator of tooth enamel formation. Int. J. Oral Sci. 2024, 16, 60. [Google Scholar] [CrossRef]
- Felszeghy, S.; Suomalainen, M.; Thesleff, I. Notch signalling is required for the survival of epithelial stem cells in the continuously growing mouse incisor. Differentiation 2010, 80, 241–248. [Google Scholar] [CrossRef]
- Dassule, H.R.; Lewis, P.; Bei, M.; Maas, R.; McMahon, A.P. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 2000, 127, 4775–4785. [Google Scholar] [CrossRef]
- Fre, S.; Hannezo, E.; Sale, S.; Huyghe, M.; Lafkas, D.; Kissel, H.; Louvi, A.; Greve, J.; Louvard, D.; Artavanis-Tsakonas, S. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PLoS ONE 2011, 6, e25785. [Google Scholar] [CrossRef]
- Natsiou, D.; Granchi, Z.; Mitsiadis, T.A.; Jimenez-Rojo, L. Generation of spheres from dental epithelial stem cells. Front. Physiol. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Simanian, E.J.; Tuggy, S.Y.; Bartlett, J.D.; Snead, M.L.; Sugiyama, T.; Paine, M.L. Comparison of two mouse ameloblast-like cell lines for enamel-specific gene expression. Front. Physiol. 2014, 5, 277. [Google Scholar] [CrossRef] [PubMed]
- Le Norcy, E.; Lesieur, J.; Sadoine, J.; Rochefort, G.Y.; Chaussain, C.; Poliard, A. Phosphorylated and non-phosphorylated Leucine rich Amelogenin peptide differentially affect ameloblast mineralization. Front. Physiol. 2018, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Jerbaka, F.; Gribova, V.; Rey, T.; Elfaloussi, S.; Kawczynski, M.; Kharouf, N.; Herault, Y.; Arntz, Y.; Bloch-Zupan, A.; Bugueno, I.M. Organotypic 3D cellular models mimicking the epithelio-ectomesenchymal bilayer during odontogenesis. Tissue Eng. Part A 2025, 31, 471–488. [Google Scholar] [CrossRef]
- Lehal, R.; Zaric, J.; Vigolo, M.; Urech, C.; Frismantas, V.; Zangger, N.; Cao, L.; Berger, A.; Chicote, I.; Loubéry, S.; et al. Pharmacological disruption of the Notch transcription factor complex. Proc. Natl. Acad. Sci. USA 2020, 117, 16292–16301. [Google Scholar] [CrossRef]
- Li, C.Y.; Cha, W.; Luder, H.U.; Charles, R.P.; McMahon, M.; Mitsiadis, T.A.; Klein, O.D. E-cadherin regulates the behavior and fate of epithelial stem cells and their progeny in the mouse incisor. Dev. Biol. 2012, 366, 357–366. [Google Scholar] [CrossRef]
- Nishikawal, S.; Tsukita, S.; Sasa, S. Localization of adherens junction proteins along the possible sliding interface between secretory ameloblasts of the rat incisor. Cell Struct. Funct. 1990, 15, 245–249. [Google Scholar] [CrossRef]
- Bray, S.J. Notch signalling in context. Nat. Rev. Mol. Cell Biol. 2016, 17, 722–735. [Google Scholar] [CrossRef]
- Moreau, T.; Evans, A.L.; Vasquez, L.; Tijssen, M.R.; Yan, Y.; Trotter, M.W.; Howard, D.; Colzani, M.; Arumugam, M.; Wu, W.H.; et al. Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nat. Commun. 2016, 7, 11208. [Google Scholar] [CrossRef]
- Harada, H.; Ichimori, Y.; Yokohama-Tamaki, T.; Ohshima, H.; Kawano, S.; Katsube, K.; Wakisaka, S. Stratum intermedium lineage diverges from ameloblast lineage via Notch signaling. Biochem. Biophys. Res. Commun. 2006, 340, 611–616. [Google Scholar] [CrossRef]
- Hu, J.C.C.; Chun, Y.H.P.; Al-Hazzazzi, T.; Simmer, J.P. Enamel formation and Amelogenesis Imperfecta. Cells Tissues Organs 2007, 186, 78–85. [Google Scholar] [CrossRef]
- Smith, C.E.L.; Poulter, J.A.; Antanaviciute, A.; Kirkham, J.; Brookes, S.J.; Inglehearn, C.F.; Mighell, A.J. Amelogenesis Imperfecta; Genes, Proteins, and Pathways. Front. Physiol. 2017, 8, 435. [Google Scholar] [CrossRef]
- Smith, C.E. Cellular and chemical events during enamel maturation. Crit. Rev. Oral Biol. Med. 1998, 9, 128–161. [Google Scholar] [CrossRef]
- Simmer, J.P.; Fincham, A.G. Molecular mechanisms of dental enamel formation. Crit. Rev. Oral Biol. Med. 1995, 6, 84–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Le, M.; Nakano, Y.; Chan, B.; Huang, Y.; Torbaty, P.M.; Kohwi, Y.; Marcucio, R.; Habelitz, S.; et al. SATB1 establishes ameloblast cell polarity and regulates directional amelogenin secretion for enamel formation. BMC Biol. 2019, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Kawamoto, T.; Kawai, M.; Yamamoto, T. Differential expression patterns of the tight junction-associated proteins occludin and claudins in secretory and mature ameloblasts in mouse incisor. Med. Mol. Morphol. 2010, 2, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chiba, Y.; Jia, L.; Yoshizaki, K.; Saito, K.; Yamada, A.; Qin, M.; Fukumoto, S. Expression patterns of Claudin family members during tooth development and the role of Claudin-10 (Cldn10) in cytodifferentiation of stratum intermedium. Front. Cell Dev. Biol. 2020, 8, 595593. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S. Cytoskeleton, intercellular junctions, planar cell polarity, and cell movement in amelogenesis. J. Oral Biosci. 2017, 59, 197–204. [Google Scholar] [CrossRef]
- Shi, Q.; Xue, C.; Zeng, Y.; Yuan, X.; Chu, Q.; Jiang, S.; Wang, J.; Zhang, Y.; Zhu, D.; Li, L. Notch signaling pathway in cancer: From mechanistic insights to targeted therapies. Signal Transduct. Target. Ther. 2024, 9, 128. [Google Scholar] [CrossRef]
- Watt, F.M.; Estrach, S.; Ambler, C.A. Epidermal Notch signalling: Differentiation, cancer and adhesion. Curr. Opin. Cell Biol. 2008, 2, 171–179. [Google Scholar] [CrossRef]
- Khoramjoo, S.M.; Kazemifard, N.; Baradaran-Ghavami, S.; Farmani, M.; Shahrokh, S.; Asadzadeh, H.; Sherkat, G.; Zali, M.R. Overview of three proliferation pathways (Wnt, Notch, and Hippo) in intestine and immune system and their role in Inflammatory Bowel Diseases (IBDs). Front. Med. 2022, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- McDaniell, R.; Warthen, D.M.; Sanchez-Lara, P.A.; Pai, A.; Krantz, I.D.; Piccoli, D.A.; Spinner, N.B. Notch2 mutations cause Alagille Syndrome, a heterogeneous disorder of the Notch signaling pathway. Am. J. Hum. Genet. 2006, 79, 169–173. [Google Scholar] [CrossRef]
- Nowell, C.; Radtke, F. Cutaneous notch signaling in health and disease. Cold Spring Harb. Perspect. Med. 2013, 3, 12. [Google Scholar] [CrossRef]
- Anacleto, M.A.; Melo, C.F.R.; Oliveira, R.P.; Da Silva, L.C.P.; Taitson, P.F. Alagille syndrome: Oral manifestations—A case report. Spec. Care Dent. 2021, 41, 741–749. [Google Scholar] [CrossRef]
- Bonnet, A.L.; Greset, V.; Davit-Beal, T. Oral manifestations of Alagille syndrome. BMJ Case Rep. 2020, 13, e234689. [Google Scholar] [CrossRef]
- Banjac, I.; Maimets, M.; Jensen, K.B. Maintenance of high-turnover tissues during and beyond homeostasis. Cell Stem Cell 2023, 4, 348–361. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamprou, A.; Porcheri, C.; Mitsiadis, T.A. Notch2 Deletion Compromises Epithelial Integrity and Enamel Formation in Rodent Incisors. Cells 2025, 14, 1224. https://doi.org/10.3390/cells14151224
Lamprou A, Porcheri C, Mitsiadis TA. Notch2 Deletion Compromises Epithelial Integrity and Enamel Formation in Rodent Incisors. Cells. 2025; 14(15):1224. https://doi.org/10.3390/cells14151224
Chicago/Turabian StyleLamprou, Argyro, Cristina Porcheri, and Thimios A. Mitsiadis. 2025. "Notch2 Deletion Compromises Epithelial Integrity and Enamel Formation in Rodent Incisors" Cells 14, no. 15: 1224. https://doi.org/10.3390/cells14151224
APA StyleLamprou, A., Porcheri, C., & Mitsiadis, T. A. (2025). Notch2 Deletion Compromises Epithelial Integrity and Enamel Formation in Rodent Incisors. Cells, 14(15), 1224. https://doi.org/10.3390/cells14151224






