Human-Induced Pluripotent Stem Cells (iPSCs) for Disease Modeling and Insulin Target Cell Regeneration in the Treatment of Insulin Resistance: A Review
Abstract
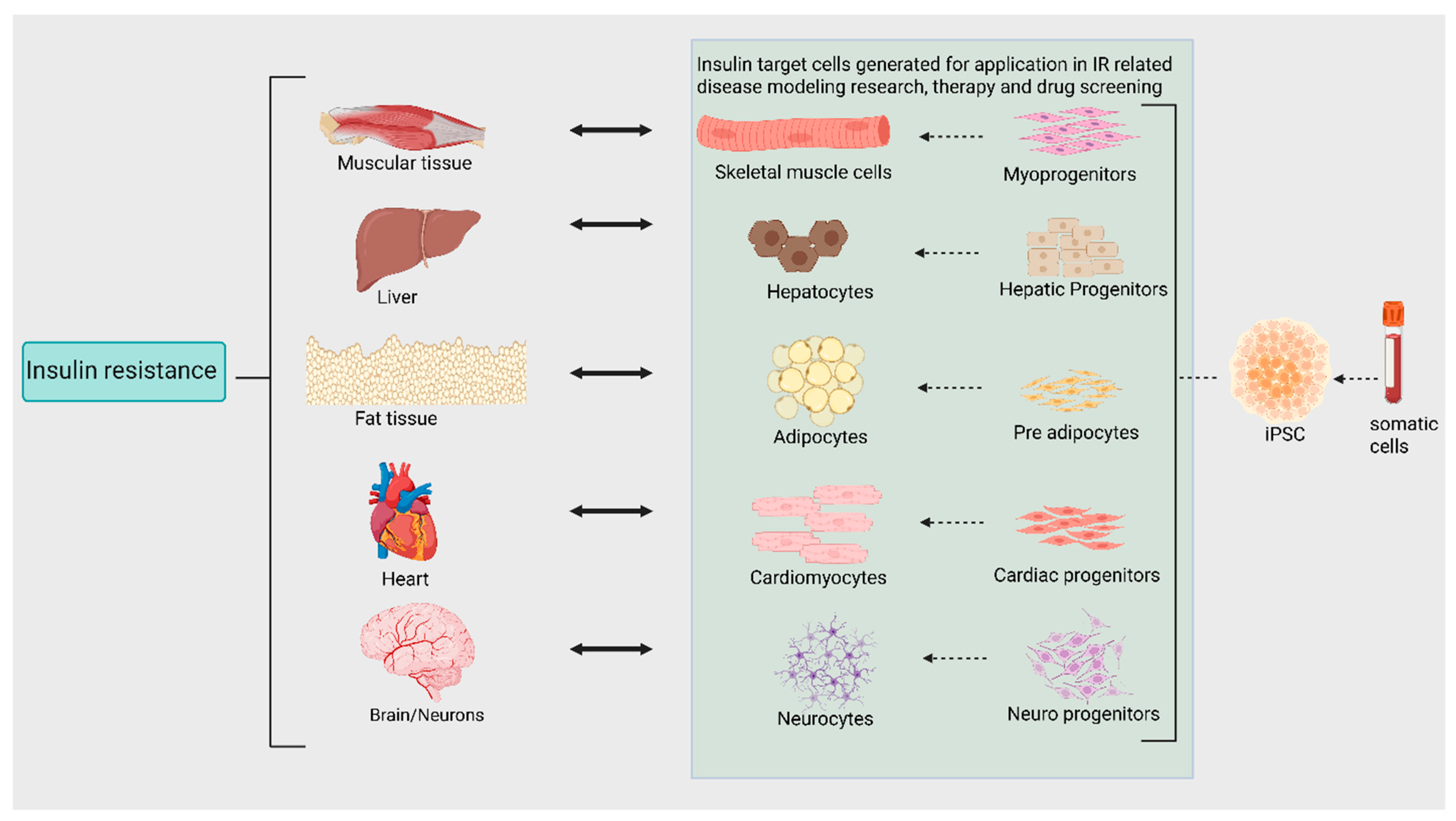
1. Introduction
2. Reprogramming Somatic Cells into iPSCs
3. iPSC-Derived Hepatocytes
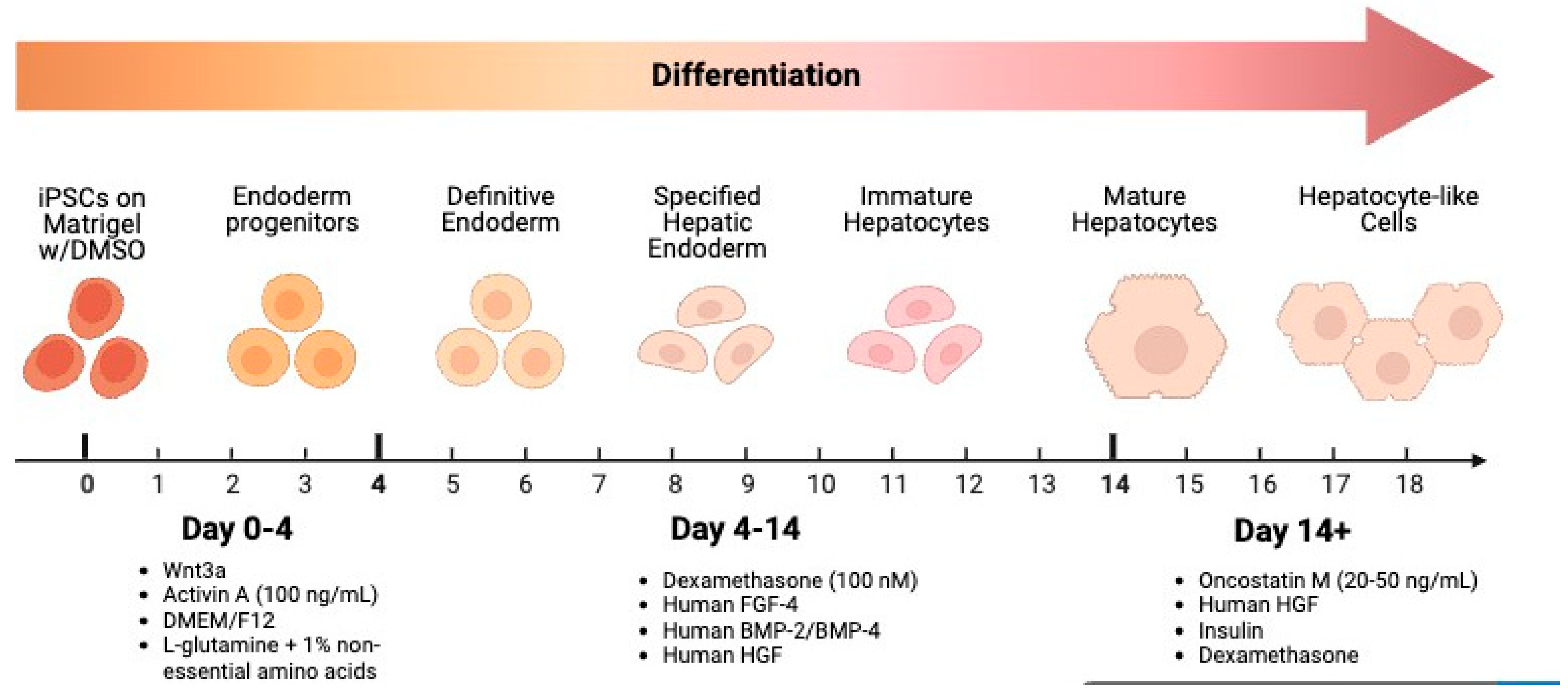
3.1. Stages, Signaling Molecules, and Growth Factor
3.1.1. Definitive Endoderm Induction
3.1.2. Hepatic Specification & Maturation
3.2. Evaluation
3.3. Application
4. iPSC-Derived Skeletal Muscle
4.1. Stages, Signaling Molecules, and Growth Factors
4.1.1. iPSC Generation
4.1.2. Mesoderm Induction and Paraxial Mesoderm Formation
4.1.3. Myoblast Specification & Proliferation
4.1.4. Myotube Formation & Maturation
4.2. Evaluation
4.3. Application
5. iPSC-Derived Adipocytes
5.1. Stages, Signaling Molecules, and Growth Factors
5.1.1. Derivation of Mesenchymal Stem Cells (MSCs)
5.1.2. Adipocyte Differentiation from MSCs
5.1.3. Protocol
5.2. Evaluation
5.3. Application
6. iPSC-Derived Cardiomyocytes
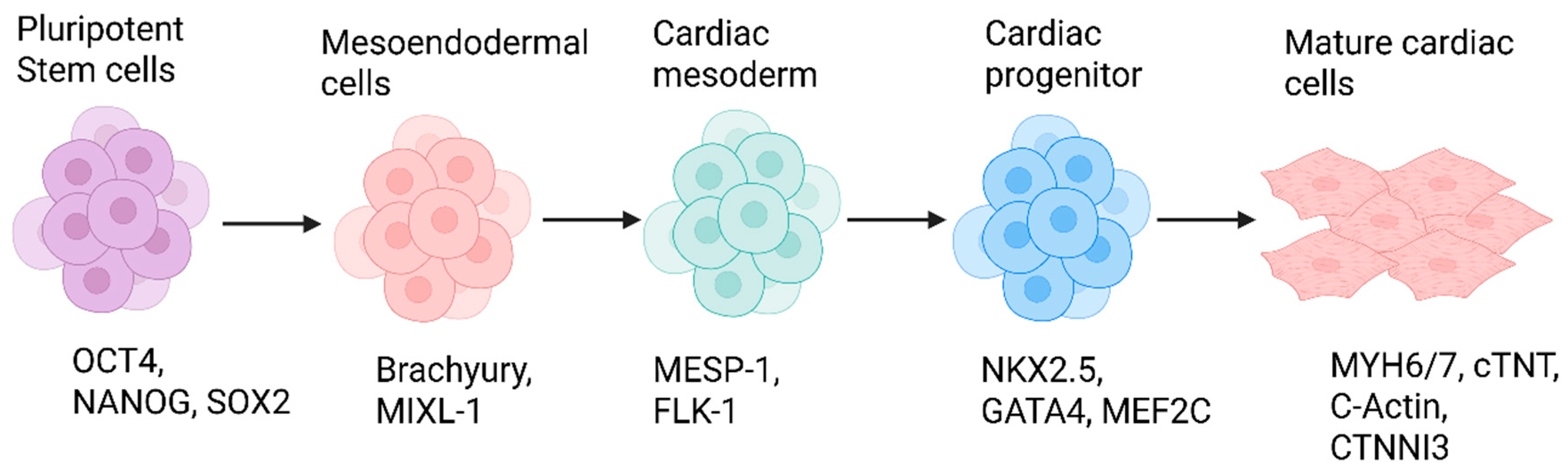
6.1. Stages and Signaling Pathways
6.1.1. Mesendoderm Induction
6.1.2. Cardiac Mesoderm Specification
6.1.3. Cardiac Progenitor Commitment
6.1.4. Cardiomyocyte Maturation
6.1.5. Protocol
6.2. Evaluation
6.3. Application
7. iPSC-Derived Neuronal Cells
7.1. Stages and Signaling Pathways
7.1.1. Neuroectoderm Specification
7.1.2. Neural Progenitor Specification
7.1.3. Neuronal Commitment
7.1.4. Neuronal Maturation
7.1.5. Protocols
7.2. Evaluation
7.3. Applications
8. Limitations
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baron, V.; Van Obberghen, E. Mechanism of insulin action. C R. Seances Soc. Biol. Fil. 1995, 189, 25–41. [Google Scholar]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.G. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef] [PubMed]
- Nakrani, M.N.; Wineland, R.H.; Anjum, F. Physiology, Glucose Metabolism. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Thakur, G.; Lee, H.J.; Jeon, R.H.; Lee, S.L.; Rho, G.J. Small Molecule-Induced Pancreatic β-Like Cell Development: Mechanistic Approaches and Available Strategies. Int. J. Mol. Sci. 2020, 21, 2388. [Google Scholar] [CrossRef]
- Xu, G.; Liu, B.; Sun, Y.; Du, Y.; Snetselaar, L.G.; Hu, F.B.; Bao, W. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: Population based study. BMJ 2018, 362, k1497. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuniga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef]
- Gheibi, S.; Singh, T.; da Cunha, J.; Fex, M.; Mulder, H. Insulin/Glucose-Responsive Cells Derived from Induced Pluripotent Stem Cells: Disease Modeling and Treatment of Diabetes. Cells 2020, 9, 2465. [Google Scholar] [CrossRef]
- Raab, S.; Klingenstein, M.; Liebau, S.; Linta, L. A Comparative View on Human Somatic Cell Sources for iPSC Generation. Stem Cells Int. 2014, 2014, 768391. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Sommer, C.A.; Mostoslavsky, G. Experimental approaches for the generation of induced pluripotent stem cells. Stem Cell Res. Ther. 2010, 1, 26. [Google Scholar] [CrossRef][Green Version]
- Steichen, C.; Luce, E.; Maluenda, J.; Tosca, L.; Moreno-Gimeno, I.; Desterke, C.; Dianat, N.; Goulinet-Mainot, S.; Awan-Toor, S.; Burks, D.; et al. Messenger RNA- versus retrovirus-based induced pluripotent stem cell reprogramming strategies: Analysis of genomic integrity. Stem Cells Transl. Med. 2014, 3, 686–691. [Google Scholar] [CrossRef]
- Shin, D.; Monga, S.P. Cellular and molecular basis of liver development. Compr. Physiol. 2013, 3, 799–815. [Google Scholar] [CrossRef]
- Andreasson, L.; Evenbratt, H.; Mobini, R.; Simonsson, S. Differentiation of induced pluripotent stem cells into definitive endoderm on Activin A-functionalized gradient surfaces. J. Biotechnol. 2021, 325, 173–178. [Google Scholar] [CrossRef]
- Horbelt, D.; Denkis, A.; Knaus, P. A portrait of Transforming Growth Factor β superfamily signalling: Background matters. Int. J. Biochem. Cell Biol. 2012, 44, 469–474. [Google Scholar] [CrossRef]
- Tsukamoto, M.; Kimura, K.; Yoshida, T.; Sugiura, K.; Hatoya, S. Canine induced pluripotent stem cells efficiently differentiate into definitive endoderm in 3D cell culture conditions using high-dose activin A. Regen. Ther. 2022, 21, 502–510. [Google Scholar] [CrossRef]
- Qiu, S.; Li, Y.; Imakura, Y.; Mima, S.; Hashita, T.; Iwao, T.; Matsunaga, T. An Efficient Method for the Differentiation of Human iPSC-Derived Endoderm toward Enterocytes and Hepatocytes. Cells 2021, 10, 812. [Google Scholar] [CrossRef]
- Kubo, A.; Shinozaki, K.; Shannon, J.M.; Kouskoff, V.; Kennedy, M.; Woo, S.; Fehling, H.J.; Keller, G. Development of definitive endoderm from embryonic stem cells in culture. Development 2004, 131, 1651–1662. [Google Scholar] [CrossRef]
- Matsuno, K.; Mae, S.I.; Okada, C.; Nakamura, M.; Watanabe, A.; Toyoda, T.; Uchida, E.; Osafune, K. Redefining definitive endoderm subtypes by robust induction of human induced pluripotent stem cells. Differentiation 2016, 92, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Luce, E.; Hussein, M.; Dubart-Kupperschmitt, A. Pluripotent-Stem-Cell-Derived Hepatic Cells: Hepatocytes and Organoids for Liver Therapy and Regeneration. Cells 2020, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Wang, N.; Zhang, J.; Yang, F.; Chen, Y.; Zhuang, Y.; Xu, Y.; Fang, J.; You, K.; Lin, X.; et al. Efficiently generate functional hepatic cells from human pluripotent stem cells by complete small-molecule strategy. Stem Cell Res. Ther. 2022, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Feng, Y.; Qiu, D.; Xu, Y.; Pang, M.; Cai, N.; Xiang, A.P.; Zhang, Q. Highly efficient and expedited hepatic differentiation from human pluripotent stem cells by pure small-molecule cocktails. Stem Cell Res. Ther. 2018, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhao, Y.; Yourick, J.J.; Sprando, R.L.; Gao, X. Phenotypical, functional and transcriptomic comparison of two modified methods of hepatocyte differentiation from human induced pluripotent stem cells. Biomed. Rep. 2022, 16, 43. [Google Scholar] [CrossRef]
- Campbell, S.A.; Stephan, T.L.; Lotto, J.; Cullum, R.; Drissler, S.; Hoodless, P.A. Signalling pathways and transcriptional regulators orchestrating liver development and cancer. Development 2021, 148, dev199814. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.D.; Yoon, S.; Kang, K.; Kim, Y.; Lee, S.B.; Seo, D.; Ryu, K.; Jeong, J.; Choi, D. Simple Maturation of Direct-Converted Hepatocytes Derived from Fibroblasts. Tissue Eng. Regen. Med. 2017, 14, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Tseng, C.Y.; Wang, H.W.; Kuo, H.C.; Yang, V.W.; Lee, O.K. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology 2012, 55, 1193–1203. [Google Scholar] [CrossRef]
- Antarianto, R.D.; Pragiwaksana, A.; Septiana, W.L.; Mazfufah, N.F.; Mahmood, A. Hepatocyte Differentiation from iPSCs or MSCs in Decellularized Liver Scaffold: Cell-ECM Adhesion, Spatial Distribution, and Hepatocyte Maturation Profile. Organogenesis 2022, 18, 2061263. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Z.N.; Rong, Z.; Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 2011, 474, 212–215. [Google Scholar] [CrossRef]
- Blackford, S.J.I.; Ng, S.S.; Segal, J.M.; King, A.J.F.; Austin, A.L.; Kent, D.; Moore, J.; Sheldon, M.; Ilic, D.; Dhawan, A.; et al. Validation of Current Good Manufacturing Practice Compliant Human Pluripotent Stem Cell-Derived Hepatocytes for Cell-Based Therapy. Stem Cells Transl. Med. 2019, 8, 124–137. [Google Scholar] [CrossRef]
- Michalopoulos, G.K.; Bowen, W.C.; Mulè, K.; Luo, J. HGF-, EGF-, and dexamethasone-induced gene expression patterns during formation of tissue in hepatic organoid cultures. Gene Expr. 2003, 11, 55–75. [Google Scholar] [CrossRef]
- Pan, T.; Chen, Y.; Zhuang, Y.; Yang, F.; Xu, Y.; Tao, J.; You, K.; Wang, N.; Wu, Y.; Lin, X.; et al. Synergistic modulation of signaling pathways to expand and maintain the bipotency of human hepatoblasts. Stem Cell Res. Ther. 2019, 10, 364. [Google Scholar] [CrossRef]
- Xie, Y.; Yao, J.; Jin, W.; Ren, L.; Li, X. Induction and Maturation of Hepatocyte-Like Cells In Vitro: Focus on Technological Advances and Challenges. Front. Cell Dev. Biol. 2021, 9, 765980. [Google Scholar] [CrossRef]
- Medine, C.N.; Lucendo-Villarin, B.; Zhou, W.; West, C.C.; Hay, D.C. Robust generation of hepatocyte-like cells from human embryonic stem cell populations. J. Vis. Exp. 2011, 56, e2969. [Google Scholar] [CrossRef]
- Kumar, S.; Curran, J.E.; Williams-Blangero, S.; Blangero, J. Efficient Generation of Functional Hepatocytes from Human Induced Pluripotent Stem Cells for Disease Modeling and Disease Gene Discovery. Methods Mol. Biol. 2022, 2549, 85–101. [Google Scholar] [CrossRef]
- Monga, S.P.; Hout, M.S.; Baun, M.J.; Micsenyi, A.; Muller, P.; Tummalapalli, L.; Ranade, A.R.; Luo, J.H.; Strom, S.C.; Gerlach, J.C. Mouse fetal liver cells in artificial capillary beds in three-dimensional four-compartment bioreactors. Am. J. Pathol. 2005, 167, 1279–1292. [Google Scholar] [CrossRef]
- Lee, K.D.; Kuo, T.K.; Whang-Peng, J.; Chung, Y.F.; Lin, C.T.; Chou, S.H.; Chen, J.R.; Chen, Y.P.; Lee, O.K. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 2004, 40, 1275–1284. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Deng, P.; Chen, W.; Guo, Y.; Tao, T.; Qin, J. In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab A Chip 2018, 18, 3606–3616. [Google Scholar] [CrossRef] [PubMed]
- Olgasi, C.; Cucci, A.; Follenzi, A. iPSC-Derived Liver Organoids: A Journey from Drug Screening, to Disease Modeling, Arriving to Regenerative Medicine. Int. J. Mol. Sci. 2020, 21, 6215. [Google Scholar] [CrossRef] [PubMed]
- Loerch, C.; Szepanowski, L.P.; Reiss, J.; Adjaye, J.; Graffmann, N. Forskolin induces FXR expression and enhances maturation of iPSC-derived hepatocyte-like cells. Front. Cell Dev. Biol. 2024, 12, 1383928. [Google Scholar] [CrossRef] [PubMed]
- Altmaier, S.; Meiser, I.; Lemesre, E.; Chanrion, B.; Steeg, R.; Leonte, L.E.; Holst, B.; Nielsen, B.S.; Clausen, C.; Schmidt, K.; et al. Human iPSC-derived hepatocytes in 2D and 3D suspension culture for cryopreservation and in vitro toxicity studies. Reprod. Toxicol. 2022, 111, 68–80. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Qi, R.; Li, K.; Wang, X.; Li, X.; Shi, B. Role of connexin 32 in the directional differentiation of induced pluripotent stem cells into hepatocytes. Int. J. Med. Sci. 2024, 21, 508–518. [Google Scholar] [CrossRef]
- Lv, Y.; Rao, Z.; Liu, L.; Jia, J.; Wu, C.; Xu, J.; Du, Y.; Liu, Y.; Liu, B.; Shi, J.; et al. The efficient generation of functional human hepatocytes from chemically induced pluripotent stem cells. Cell Prolif. 2024, 57, e13540. [Google Scholar] [CrossRef]
- Gao, X.; Liu, Y. A transcriptomic study suggesting human iPSC-derived hepatocytes potentially offer a better in vitro model of hepatotoxicity than most hepatoma cell lines. Cell Biol. Toxicol. 2017, 33, 407–421. [Google Scholar] [CrossRef]
- Takeishi, K.; Collin de l’Hortet, A.; Wang, Y.; Handa, K.; Guzman-Lepe, J.; Matsubara, K.; Morita, K.; Jang, S.; Haep, N.; Florentino, R.M.; et al. Assembly and Function of a Bioengineered Human Liver for Transplantation Generated Solely from Induced Pluripotent Stem Cells. Cell Rep. 2020, 31, 107711. [Google Scholar] [CrossRef]
- Hui, H.; Ma, W.; Cui, J.; Gong, M.; Wang, Y.; Zhang, Y.; He, T.; Bi, Y.; He, Y. Periodic acid-Schiff staining method for function detection of liver cells is affected by 2% horse serum in induction medium. Mol. Med. Rep. 2017, 16, 8062–8068. [Google Scholar] [CrossRef]
- Török, G.; Erdei, Z.; Lilienberg, J.; Apáti, Á.; Homolya, L. The importance of transporters and cell polarization for the evaluation of human stem cell-derived hepatic cells. PLoS ONE 2020, 15, e0227751. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, Y.; Toh, Y.C.; Xing, J.; Qu, Y.; Poh, J.; Li, H.; Tan, H.S.; Kanesvaran, R.; Yu, H.; Tan, M.H. Patient-specific hepatocyte-like cells derived from induced pluripotent stem cells model pazopanib-mediated hepatotoxicity. Sci. Rep. 2017, 7, 41238. [Google Scholar] [CrossRef]
- Bulutoglu, B.; Rey-Bedón, C.; Mert, S.; Tian, L.; Jang, Y.Y.; Yarmush, M.L.; Usta, O.B. A comparison of hepato-cellular in vitro platforms to study CYP3A4 induction. PLoS ONE 2020, 15, e0229106. [Google Scholar] [CrossRef] [PubMed]
- Varghese, D.S.; Alawathugoda, T.T.; Sheikh, M.A.; Challagandla, A.K.; Emerald, B.S.; Ansari, S.A. Developmental modeling of hepatogenesis using obese iPSCs-hepatocyte differentiation uncovers pathological features. Cell Death Dis. 2022, 13, 670. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.T.; Doueiry, C.; Jiang, Y.L.; Blaszkiewicz, J.; Lamprecht, M.P.; Heslop, J.A.; Peterson, Y.K.; Carten, J.D.; Traktman, P.; Yuan, Y.; et al. A human iPSC-derived hepatocyte screen identifies compounds that inhibit production of Apolipoprotein B. Commun. Biol. 2023, 6, 452. [Google Scholar] [CrossRef]
- Cayo, M.A.; Cai, J.; DeLaForest, A.; Noto, F.K.; Nagaoka, M.; Clark, B.S.; Collery, R.F.; Si-Tayeb, K.; Duncan, S.A. JD induced pluripotent stem cell-derived hepatocytes faithfully recapitulate the pathophysiology of familial hypercholesterolemia. Hepatology 2012, 56, 2163–2171. [Google Scholar] [CrossRef]
- Aghadi, M.; Elgendy, R.; Abdelalim, E.M. Loss of FOXA2 induces ER stress and hepatic steatosis and alters developmental gene expression in human iPSC-derived hepatocytes. Cell Death Dis. 2022, 13, 713. [Google Scholar] [CrossRef]
- Groeger, M.; Matsuo, K.; Heidary Arash, E.; Pereira, A.; Le Guillou, D.; Pino, C.; Telles-Silva, K.A.; Maher, J.J.; Hsiao, E.C.; Willenbring, H. Modeling and therapeutic targeting of inflammation-induced hepatic insulin resistance using human iPSC-derived hepatocytes and macrophages. Nat. Commun. 2023, 14, 3902. [Google Scholar] [CrossRef]
- Rashid, S.T.; Corbineau, S.; Hannan, N.; Marciniak, S.J.; Miranda, E.; Alexander, G.; Huang-Doran, I.; Griffin, J.; Ahrlund-Richter, L.; Skepper, J.; et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J. Clin. Investig. 2010, 120, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Yu, Y.; Nyberg, S.L. Induced Pluripotent Stem Cells for the Treatment of Liver Diseases: Novel Concepts. Cells Tissues Organs 2022, 211, 368–384. [Google Scholar] [CrossRef]
- Asgari, S.; Moslem, M.; Bagheri-Lankarani, K.; Pournasr, B.; Miryounesi, M.; Baharvand, H. Differentiation and transplantation of human induced pluripotent stem cell-derived hepatocyte-like cells. Stem Cell Rev. Rep. 2013, 9, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Hirai, H.; Asakura, Y.; Tastad, C.; Verma, M.; Keller, C.; Dutton, J.R.; Asakura, A. MyoD gene suppression by Oct4 is required for reprogramming in myoblasts to produce induced pluripotent stem cells. Stem Cells 2011, 29, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef]
- Kodaka, Y.; Rabu, G.; Asakura, A. Skeletal Muscle Cell Induction from Pluripotent Stem Cells. Stem Cells Int. 2017, 2017, 1376151. [Google Scholar] [CrossRef]
- Iberite, F.; Gruppioni, E.; Ricotti, L. Skeletal muscle differentiation of human iPSCs meets bioengineering strategies: Perspectives and challenges. NPJ Regen. Med. 2022, 7, 23. [Google Scholar] [CrossRef]
- Martin, B.L. Mesoderm induction and patterning: Insights from neuromesodermal progenitors. Semin. Cell Dev. Biol. 2022, 127, 37–45. [Google Scholar] [CrossRef]
- Schulte-Merker, S.; Smith, J.C. Mesoderm formation in response to Brachyury requires FGF signalling. Curr. Biol. 1995, 5, 62–67. [Google Scholar] [CrossRef]
- McFann, S.E.; Shvartsman, S.Y.; Toettcher, J.E. Putting in the Erk: Growth factor signaling and mesoderm morphogenesis. Curr. Top. Dev. Biol. 2022, 149, 263–310. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Selvaraj, S.; Mondragon-Gonzalez, R.; Xu, B.; Magli, A.; Kim, H.; Lainé, J.; Kiley, J.; McKee, H.; Rinaldi, F.; Aho, J.; et al. Screening identifies small molecules that enhance the maturation of human pluripotent stem cell-derived myotubes. Elife 2019, 8, e47970. [Google Scholar] [CrossRef]
- Rao, L.; Qian, Y.; Khodabukus, A.; Ribar, T.; Bursac, N. Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat. Commun. 2018, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Inami, Y.; Tamamura, Y.; Yoshikai, T.; Sehara-Fujisawa, A.; Isobe, K. Bidirectional induction toward paraxial mesodermal derivatives from mouse ES cells in chemically defined medium. Stem Cell Res. 2009, 3, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Chal, J.; Oginuma, M.; Al Tanoury, Z.; Gobert, B.; Sumara, O.; Hick, A.; Bousson, F.; Zidouni, Y.; Mursch, C.; Moncuquet, P.; et al. Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat. Biotechnol. 2015, 33, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Caron, L.; Kher, D.; Lee, K.L.; McKernan, R.; Dumevska, B.; Hidalgo, A.; Li, J.; Yang, H.; Main, H.; Ferri, G.; et al. A Human Pluripotent Stem Cell Model of Facioscapulohumeral Muscular Dystrophy-Affected Skeletal Muscles. Stem Cells Transl. Med. 2016, 5, 1145–1161. [Google Scholar] [CrossRef]
- Miyagoe-Suzuki, Y.; Takeda, S. Skeletal muscle generated from induced pluripotent stem cells—induction and application. World J. Stem Cells 2017, 9, 89–97. [Google Scholar] [CrossRef]
- Relaix, F.; Rocancourt, D.; Mansouri, A.; Buckingham, M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 2005, 435, 948–953. [Google Scholar] [CrossRef]
- Pownall, M.E.; Gustafsson, M.K.; Emerson, C.P., Jr. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 2002, 18, 747–783. [Google Scholar] [CrossRef]
- Vishal, K.; Lovato, T.L.; Bragg, C.; Chechenova, M.B.; Cripps, R.M. FGF signaling promotes myoblast proliferation through activation of wingless signaling. Dev. Biol. 2020, 464, 1–10. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Xi, Y.; Yang, C.; Sun, C.; Li, D. Sirtuin 1 promotes the proliferation of C2C12 myoblast cells via the myostatin signaling pathway. Mol. Med. Rep. 2016, 14, 1309–1315. [Google Scholar] [CrossRef]
- Chargé, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Badu-Mensah, A.; Valinski, P.; Parsaud, H.; Hickman, J.J.; Guo, X. Hyperglycemia Negatively Affects IPSC-Derived Myoblast Proliferation and Skeletal Muscle Regeneration and Function. Cells 2022, 11, 3674. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Khodabukus, A.; Rao, L.; Vandusen, K.; Abutaleb, N.; Bursac, N. Engineered skeletal muscles for disease modeling and drug discovery. Biomaterials 2019, 221, 119416. [Google Scholar] [CrossRef] [PubMed]
- Giacomazzi, G.; Holvoet, B.; Trenson, S.; Caluwé, E.; Kravic, B.; Grosemans, H.; Cortés-Calabuig, Á.; Deroose, C.M.; Huylebroeck, D.; Hashemolhosseini, S.; et al. MicroRNAs promote skeletal muscle differentiation of mesodermal iPSC-derived progenitors. Nat. Commun. 2017, 8, 1249. [Google Scholar] [CrossRef]
- Sakai-Takemura, F.; Narita, A.; Masuda, S.; Wakamatsu, T.; Watanabe, N.; Nishiyama, T.; Nogami, K.; Blanc, M.; Takeda, S.; Miyagoe-Suzuki, Y. Premyogenic progenitors derived from human pluripotent stem cells expand in floating culture and differentiate into transplantable myogenic progenitors. Sci. Rep. 2018, 8, 6555. [Google Scholar] [CrossRef]
- Truskey, G.A. Development and application of human skeletal muscle microphysiological systems. Lab. Chip 2018, 18, 3061–3073. [Google Scholar] [CrossRef]
- Ozasa, S.; Kimura, S.; Ito, K.; Ueno, H.; Ikezawa, M.; Matsukura, M.; Yoshioka, K.; Araki, K.; Yamamura, K.-I.; Abe, K.; et al. Efficient conversion of ES cells into myogenic lineage using the gene-inducible system. Biochem. Biophys. Res. Commun. 2007, 357, 957–963. [Google Scholar] [CrossRef]
- Ribeiro, S.; Gomes, A.C.; Etxebarria, I.; Lanceros-Méndez, S.; Ribeiro, C. Electroactive biomaterial surface engineering effects on muscle cells differentiation. Mater. Sci. Eng. C 2018, 92, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Omer, S.A.; McKnight, K.H.; Young, L.I.; Song, S. Stimulation strategies for electrical and magnetic modulation of cells and tissues. Cell Regen. 2023, 12, 21. [Google Scholar] [CrossRef]
- Qazi, T.H.; Mooney, D.J.; Pumberger, M.; Geissler, S.; Duda, G.N. Biomaterials based strategies for skeletal muscle tissue engineering: Existing technologies and future trends. Biomaterials 2015, 53, 502–521. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; Chen, B.; Gao, J.; Li, T.; Ye, Y.; Tian, H.; Wang, S.; Wang, F.; Jiang, J.; et al. Magnetically Actuated Biohybrid Microswimmers for Precise Photothermal Muscle Contraction. ACS Nano 2022, 16, 6515–6526. [Google Scholar] [CrossRef]
- Bou Akar, R.; Lama, C.; Aubin, D.; Maruotti, J.; Onteniente, B.; Esteves de Lima, J.; Relaix, F. Generation of highly pure pluripotent stem cell-derived myogenic progenitor cells and myotubes. Stem Cell Rep. 2024, 19, 84–99. [Google Scholar] [CrossRef]
- Rashid, M.I.; Ito, T.; Miya, F.; Shimojo, D.; Arimoto, K.; Onodera, K.; Okada, R.; Nagashima, T.; Yamamoto, K.; Khatun, Z.; et al. Simple and efficient differentiation of human iPSCs into contractible skeletal muscles for muscular disease modeling. Sci. Rep. 2023, 13, 8146. [Google Scholar] [CrossRef]
- Metzler, E.; Escobar, H.; Sunaga-Franze, D.Y.; Sauer, S.; Diecke, S.; Spuler, S. Generation of hiPSC-Derived Skeletal Muscle Cells: Exploiting the Potential of Skeletal Muscle-Derived hiPSCs. Biomedicines 2022, 10, 1204. [Google Scholar] [CrossRef]
- Xu, N.; Wu, J.; Ortiz-Vitali, J.L.; Li, Y.; Darabi, R. Directed Differentiation of Human Pluripotent Stem Cells toward Skeletal Myogenic Progenitors and Their Purification Using Surface Markers. Cells 2021, 10, 2746. [Google Scholar] [CrossRef] [PubMed]
- Al Tanoury, Z.; Zimmerman, J.F.; Rao, J.; Sieiro, D.; McNamara, H.M.; Cherrier, T.; Rodriguez-delaRosa, A.; Hick-Colin, A.; Bousson, F.; Fugier-Schmucker, C.; et al. Prednisolone rescues Duchenne muscular dystrophy phenotypes in human pluripotent stem cell-derived skeletal muscle in vitro. Proc. Natl. Acad. Sci. USA 2021, 118, e2022960118. [Google Scholar] [CrossRef]
- Cerrada, V.; Garcia-Consuegra, I.; Arenas, J.; Gallardo, M.E. Creation of an iPSC-Based Skeletal Muscle Model of McArdle Disease Harbouring the Mutation c.2392T>C (p.Trp798Arg) in the PYGM Gene. Biomedicines 2023, 11, 2434. [Google Scholar] [CrossRef] [PubMed]
- Darabi, R.; Arpke, R.W.; Irion, S.; Dimos, J.T.; Grskovic, M.; Kyba, M.; Perlingeiro, R.C. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 2012, 10, 610–619. [Google Scholar] [CrossRef]
- Kibschull, M.; Nguyen, T.T.N.; Chow, T.; Alarab, M.; Lye, S.J.; Rogers, I.; Shynlova, O. Differentiation of patient-specific void urine-derived human induced pluripotent stem cells to fibroblasts and skeletal muscle myocytes. Sci. Rep. 2023, 13, 4746. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.C.; Asada, H.H. Extracellular matrix remodelling induced by alternating electrical and mechanical stimulations increases the contraction of engineered skeletal muscle tissues. Sci. Rep. 2019, 9, 2732. [Google Scholar] [CrossRef] [PubMed]
- Noë, S.; Corvelyn, M.; Willems, S.; Costamagna, D.; Aerts, J.M.; Van Campenhout, A.; Desloovere, K. The Myotube Analyzer: How to assess myogenic features in muscle stem cells. Skelet. Muscle 2022, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Iovino, S.; Burkart, A.M.; Warren, L.; Patti, M.E.; Kahn, C.R. Myotubes derived from human-induced pluripotent stem cells mirror in vivo insulin resistance. Proc. Natl. Acad. Sci. USA 2016, 113, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.W.; Kondo, T.; Imamura, K.; Suga, M.; Enami, T.; Nagahashi, A.; Tsukita, K.; Inoue, I.; Kawaguchi, J.; Shu, T.; et al. Simple derivation of skeletal muscle from human pluripotent stem cells using temperature-sensitive Sendai virus vector. J. Cell Mol. Med. 2021, 25, 9586–9596. [Google Scholar] [CrossRef]
- Somers, S.M.; Spector, A.A.; DiGirolamo, D.J.; Grayson, W.L. Biophysical Stimulation for Engineering Functional Skeletal Muscle. Tissue Eng. Part. B Rev. 2017, 23, 362–372. [Google Scholar] [CrossRef]
- Duffy, R.M.; Feinberg, A.W. Engineered skeletal muscle tissue for soft robotics: Fabrication strategies, current applications, and future challenges. Wiley Interdiscip. Rev. Nanomed. Nanobiotech. 2014, 6, 178–195. [Google Scholar] [CrossRef]
- Swartz, E.W.; Baek, J.; Pribadi, M.; Wojta, K.J.; Almeida, S.; Karydas, A.; Gao, F.B.; Miller, B.L.; Coppola, G. A Novel Protocol for Directed Differentiation of C9orf72-Associated Human Induced Pluripotent Stem Cells Into Contractile Skeletal Myotubes. Stem Cells Transl. Med. 2016, 5, 1461–1472. [Google Scholar] [CrossRef]
- Uchimura, T.; Asano, T.; Nakata, T.; Hotta, A.; Sakurai, H. A muscle fatigue-like contractile decline was recapitulated using skeletal myotubes from Duchenne muscular dystrophy patient-derived iPSCs. Cell Rep. Med. 2021, 2, 100298. [Google Scholar] [CrossRef]
- Sun, C.; Choi, I.Y.; Rovira Gonzalez, Y.I.; Andersen, P.; Talbot, C.C., Jr.; Iyer, S.R.; Lovering, R.M.; Wagner, K.R.; Lee, G. Duchenne muscular dystrophy hiPSC-derived myoblast drug screen identifies compounds that ameliorate disease in mdx mice. JCI Insight 2020, 5, e134287. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.L.; Bloch, R.J. Skeletal muscle cell transplantation: Models and methods. J. Muscle Res. Cell Motil. 2020, 41, 297–311. [Google Scholar] [CrossRef]
- Piga, D.; Salani, S.; Magri, F.; Brusa, R.; Mauri, E.; Comi, G.P.; Bresolin, N.; Corti, S. Human induced pluripotent stem cell models for the study and treatment of Duchenne and Becker muscular dystrophies. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419833478. [Google Scholar] [CrossRef]
- Riederer, I.; Negroni, E.; Bencze, M.; Wolff, A.; Aamiri, A.; Di Santo, J.P.; Silva-Barbosa, S.D.; Butler-Browne, G.; Savino, W.; Mouly, V. Slowing down differentiation of engrafted human myoblasts into immunodeficient mice correlates with increased proliferation and migration. Mol. Ther. 2012, 20, 146–154. [Google Scholar] [CrossRef]
- Sacco, F.; Seelig, A.; Humphrey, S.J.; Krahmer, N.; Volta, F.; Reggio, A.; Marchetti, P.; Gerdes, J.; Mann, M. Phosphoproteomics Reveals the GSK3-PDX1 Axis as a Key Pathogenic Signaling Node in Diabetic Islets. Cell Metab. 2019, 29, 1422–1432.e1423. [Google Scholar] [CrossRef]
- Batista, T.M.; Jayavelu, A.K.; Wewer Albrechtsen, N.J.; Iovino, S.; Lebastchi, J.; Pan, H.; Dreyfuss, J.M.; Krook, A.; Zierath, J.R.; Mann, M.; et al. A Cell-Autonomous Signature of Dysregulated Protein Phosphorylation Underlies Muscle Insulin Resistance in Type 2 Diabetes. Cell Metab. 2020, 32, 844–859.e845. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, V.L.; Delgado-Olguín, P.; Klip, A. Deprogram and reprogram to solve the riddle of insulin resistance. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Carrageta, D.F.; Oliveira, P.F.; Monteiro, M.P.; Alves, M.G. Adipocyte Specific Signaling. In Tissue-Specific Cell Signaling; Silva, J.V., Freitas, M.J., Fardilha, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 409–436. [Google Scholar] [CrossRef]
- Wibmer, A.G.; Becher, T.; Eljalby, M.; Crane, A.; Andrieu, P.C.; Jiang, C.S.; Vaughan, R.; Schöder, H.; Cohen, P. Brown adipose tissue is associated with healthier body fat distribution and metabolic benefits independent of regional adiposity. Cell Rep. Med. 2021, 2, 100332. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef]
- Timmons, J.A.; Wennmalm, K.; Larsson, O.; Walden, T.B.; Lassmann, T.; Petrovic, N.; Hamilton, D.L.; Gimeno, R.E.; Wahlestedt, C.; Baar, K.; et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl. Acad. Sci. USA 2007, 104, 4401–4406. [Google Scholar] [CrossRef]
- Avram, M.M.; Avram, A.S.; James, W.D. Subcutaneous fat in normal and diseased states: 1. Introduction. J. Am. Acad. Dermatol. 2005, 53, 663–670. [Google Scholar] [CrossRef]
- Taura, D.; Noguchi, M.; Sone, M.; Hosoda, K.; Mori, E.; Okada, Y.; Takahashi, K.; Homma, K.; Oyamada, N.; Inuzuka, M.; et al. Adipogenic differentiation of human induced pluripotent stem cells: Comparison with that of human embryonic stem cells. FEBS Lett. 2009, 583, 1029–1033. [Google Scholar] [CrossRef]
- Ng, F.; Boucher, S.; Koh, S.; Sastry, K.S.; Chase, L.; Lakshmipathy, U.; Choong, C.; Yang, Z.; Vemuri, M.C.; Rao, M.S.; et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): Transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 2008, 112, 295–307. [Google Scholar] [CrossRef]
- Mohsen-Kanson, T.; Hafner, A.L.; Wdziekonski, B.; Takashima, Y.; Villageois, P.; Carriere, A.; Svensson, M.; Bagnis, C.; Chignon-Sicard, B.; Svensson, P.A.; et al. Differentiation of human induced pluripotent stem cells into brown and white adipocytes: Role of Pax3. Stem Cells 2014, 32, 1459–1467. [Google Scholar] [CrossRef]
- Brown, S.E.; Tong, W.; Krebsbach, P.H. The derivation of mesenchymal stem cells from human embryonic stem cells. Cells Tissues Organs 2009, 189, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.K.; Madsen, L.; Pedersen, L.M.; Hallenborg, P.; Hagland, H.; Viste, K.; Døskeland, S.O.; Kristiansen, K. Cyclic AMP (cAMP)-mediated stimulation of adipocyte differentiation requires the synergistic action of Epac- and cAMP-dependent protein kinase-dependent processes. Mol. Cell Biol. 2008, 28, 3804–3816. [Google Scholar] [CrossRef] [PubMed]
- Zilberfarb, V.; Siquier, K.; Strosberg, A.D.; Issad, T. Effect of dexamethasone on adipocyte differentiation markers and tumour necrosis factor-alpha expression in human PAZ6 cells. Diabetologia 2001, 44, 377–386. [Google Scholar] [CrossRef]
- Entezari, B.; Akbaba, H.; Gurer-Orhan, H. Modulation of adipogenesis and lipogenesis by indomethacin and pantoprazole. Toxicol. Vitr. 2024, 100, 105895. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.A.; Nguyen, V.T.; Levi, B.; James, A.W. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2011, 20, 1793–1804. [Google Scholar] [CrossRef]
- Bowers, R.R.; Kim, J.W.; Otto, T.C.; Lane, M.D. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: Role of the BMP-4 gene. Proc. Natl. Acad. Sci. USA 2006, 103, 13022–13027. [Google Scholar] [CrossRef]
- Elsen, M.; Raschke, S.; Tennagels, N.; Schwahn, U.; Jelenik, T.; Roden, M.; Romacho, T.; Eckel, J. BMP4 and BMP7 induce the white-to-brown transition of primary human adipose stem cells. Am. J. Physiol. Cell Physiol. 2014, 306, C431–C440. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef]
- Choy, L.; Skillington, J.; Derynck, R. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J. Cell Biol. 2000, 149, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Zamani, N.; Brown, C.W. Emerging roles for the transforming growth factor-beta superfamily in regulating adiposity and energy expenditure. Endocr. Rev. 2011, 32, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.; Yamanaka, M.; Kawachi, H.; Matsui, T.; Yano, H. Activin A inhibits differentiation of 3T3-L1 preadipocyte. Mol. Cell Endocrinol. 2005, 232, 21–26. [Google Scholar] [CrossRef]
- Townsend, K.; Tseng, Y.H. Brown adipose tissue: Recent insights into development, metabolic function and therapeutic potential. Adipocyte 2012, 1, 13–24. [Google Scholar] [CrossRef]
- Hafner, A.L.; Dani, C. Human induced pluripotent stem cells: A new source for brown and white adipocytes. World J. Stem Cells 2014, 6, 467–472. [Google Scholar] [CrossRef]
- Li, J.; Jin, C.; Gustafsson, S.; Rao, A.; Wabitsch, M.; Park, C.Y.; Quertermous, T.; Bielczyk-Maczynska, E.; Knowles, J.W. Single-cell transcriptome dataset of human and mouse in vitro adipogenesis models. Sci Data. 2023, 10, 387–397. [Google Scholar] [CrossRef]
- Tan, X.; Zhu, T.; Zhang, L.; Fu, L.; Hu, Y.; Li, H.; Li, C.; Zhang, J.; Liang, B.; Liu, J. miR-669a-5p promotes adipogenic differentiation and induces browning in preadipocytes. Adipocyte 2022, 11, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Hernandez, M.E.; Khan, N.M.; Drissi, H. Efficient Differentiation of Human Induced Pluripotent Stem Cell (hiPSC)-Derived Mesenchymal Progenitors Into Adipocytes and Osteoblasts. Bio Protoc. 2023, 13, e4885. [Google Scholar] [CrossRef]
- Zhang, L.; Avery, J.; Yin, A.; Singh, A.M.; Cliff, T.S.; Yin, H.; Dalton, S. Generation of Functional Brown Adipocytes from Human Pluripotent Stem Cells via Progression through a Paraxial Mesoderm State. Cell Stem Cell 2020, 27, 784–797.e711. [Google Scholar] [CrossRef] [PubMed]
- Karam, M.; Younis, I.; Elareer, N.R.; Nasser, S.; Abdelalim, E.M. Scalable Generation of Mesenchymal Stem Cells and Adipocytes from Human Pluripotent Stem Cells. Cells 2020, 9, 710. [Google Scholar] [CrossRef]
- Guenantin, A.C.; Briand, N.; Capel, E.; Dumont, F.; Morichon, R.; Provost, C.; Stillitano, F.; Jeziorowska, D.; Siffroi, J.P.; Hajjar, R.J.; et al. Functional Human Beige Adipocytes From Induced Pluripotent Stem Cells. Diabetes 2017, 66, 1470–1478. [Google Scholar] [CrossRef]
- Su, S.; Guntur, A.R.; Nguyen, D.C.; Fakory, S.S.; Doucette, C.C.; Leech, C.; Lotana, H.; Kelley, M.; Kohli, J.; Martino, J.; et al. A Renewable Source of Human Beige Adipocytes for Development of Therapies to Treat Metabolic Syndrome. Cell Rep. 2018, 25, 3215–3228.e3219. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Gaggini, M.; DeFronzo, R.A. Role of Adipose Tissue Insulin Resistance in the Natural History of Type 2 Diabetes: Results from the San Antonio Metabolism Study. Diabetes 2017, 66, 815–822. [Google Scholar] [CrossRef]
- Macrae, J.W.; Tholpady, S.S.; Katz, A.J.; Gampper, T.G.; Drake, D.B.; Ogle, R.C.; Morgan, R.F. Human adipocyte viability testing: A new assay. Aesthet. Surg. J. 2003, 23, 265–269. [Google Scholar] [CrossRef]
- Mårin, P.; Rebuffé-Scrive, M.; Smith, U.; Björntorp, P. Glucose uptake in human adipose tissue. Metabolism 1987, 36, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Stöckli, J.; Fazakerley, D.J.; James, D.E. GLUT4 exocytosis. J. Cell Sci. 2011, 124 Pt 24, 4147–4159. [Google Scholar] [CrossRef]
- Shi, J.; Kandror, K.V. Study of glucose uptake in adipose cells. Methods Mol. Biol. 2008, 456, 307–315. [Google Scholar] [CrossRef]
- Urbonas, T.; Kievišas, M.; Petrikaitė, V.; Gibieža, P.; Baranauskas, G.; Mištautas, T.; Suslavičius, K.; Rutkauskas, I.; Stukas, D.; Jasukaitiene, A. Assessing Adipocyte Viability and Surgeons’ Work Efficiency by Comparing Different Liposuction Methods. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5190. [Google Scholar] [CrossRef]
- Lee, J.H.; Kirkham, J.C.; McCormack, M.C.; Medina, M.A.; Nicholls, A.M.; Randolph, M.A.; Austen, W.G., Jr. A novel approach to adipocyte analysis. Plast. Reconstr. Surg. 2012, 129, 380–387. [Google Scholar] [CrossRef]
- Park, Y.J.; Choe, S.S.; Sohn, J.H.; Kim, J.B. The role of glucose-6-phosphate dehydrogenase in adipose tissue inflammation in obesity. Adipocyte 2017, 6, 147–153. [Google Scholar] [CrossRef]
- Ham, M.; Choe, S.S.; Shin, K.C.; Choi, G.; Kim, J.W.; Noh, J.R.; Kim, Y.H.; Ryu, J.W.; Yoon, K.H.; Lee, C.H.; et al. Glucose-6-Phosphate Dehydrogenase Deficiency Improves Insulin Resistance With Reduced Adipose Tissue Inflammation in Obesity. Diabetes 2016, 65, 2624–2638. [Google Scholar] [CrossRef]
- Pilkington, A.C.; Paz, H.A.; Wankhade, U.D. Beige Adipose Tissue Identification and Marker Specificity-Overview. Front. Endocrinol. 2021, 12, 599134. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef]
- Qiu, Y.; Nguyen, K.D.; Odegaard, J.I.; Cui, X.; Tian, X.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014, 157, 1292–1308. [Google Scholar] [CrossRef] [PubMed]
- Carobbio, S.; Guenantin, A.C.; Bahri, M.; Rodriguez-Fdez, S.; Honig, F.; Kamzolas, I.; Samuelson, I.; Long, K.; Awad, S.; Lukovic, D.; et al. Unraveling the Developmental Roadmap toward Human Brown Adipose Tissue. Stem Cell Rep. 2021, 16, 641–655. [Google Scholar] [CrossRef]
- Gavrilova, O.; Marcus-Samuels, B.; Graham, D.; Kim, J.K.; Shulman, G.I.; Castle, A.L.; Vinson, C.; Eckhaus, M.; Reitman, M.L. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J. Clin. Investig. 2000, 105, 271–278. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Li, Y.; Wuang, L.; Liu, Y.; Pang, N.; Luo, Y.; He, J.; Zhang, L.; Chen, N.; et al. Transplantation of Normal Adipose Tissue Improves Blood Flow and Reduces Inflammation in High Fat Fed Mice with Hindlimb Ischemia. Front. Physiol. 2018, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef]
- Coleman, S.R. Facial recontouring with lipostructure. Clin. Plast. Surg. 1997, 24, 347–367. [Google Scholar] [CrossRef]
- Rohrich, R.J.; Abraham, J.T. Hand Rejuvenation with Fat Grafting. Plast. Reconstr. Surg. 2023, 151, 614e–617e. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, S.C.; Piston, D.W. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes 2012, 61, 674–682. [Google Scholar] [CrossRef]
- Zhu, Z.; Spicer, E.G.; Gavini, C.K.; Goudjo-Ako, A.J.; Novak, C.M.; Shi, H. Enhanced sympathetic activity in mice with brown adipose tissue transplantation (transBATation). Physiol. Behav. 2014, 125, 21–29. [Google Scholar] [CrossRef]
- Sharma, A.; Burridge, P.W.; McKeithan, W.L.; Serrano, R.; Shukla, P.; Sayed, N.; Churko, J.M.; Kitani, T.; Wu, H.; Holmstrom, A.; et al. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci. Transl. Med. 2017, 9, eaaf2584. [Google Scholar] [CrossRef]
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy revisited. Circulation 2007, 115, 3213–3223. [Google Scholar] [CrossRef]
- Kattman, S.J.; Witty, A.D.; Gagliardi, M.; Dubois, N.C.; Niapour, M.; Hotta, A.; Ellis, J.; Keller, G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 2011, 8, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, D.W.; van Berlo, J.H. The Role of TGF-β Signaling in Cardiomyocyte Proliferation. Curr. Heart Fail. Rep. 2020, 17, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Umbarkar, P.; Guo, Y.; Force, T.; Gupte, M.; Lal, H. Inhibition of GSK-3 to induce cardiomyocyte proliferation: A recipe for in situ cardiac regeneration. Cardiovasc. Res. 2019, 115, 20–30. [Google Scholar] [CrossRef]
- Lewandowski, J.; Kolanowski, T.J.; Kurpisz, M. Techniques for the induction of human pluripotent stem cell differentiation towards cardiomyocytes. J. Tissue Eng. Regen. Med. 2017, 11, 1658–1674. [Google Scholar] [CrossRef]
- Kempf, H.; Zweigerdt, R. Scalable Cardiac Differentiation of Pluripotent Stem Cells Using Specific Growth Factors and Small Molecules. Adv. Biochem. Eng. Biotechnol. 2018, 163, 39–69. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef] [PubMed]
- Protze, S.I.; Liu, J.; Nussinovitch, U.; Ohana, L.; Backx, P.H.; Gepstein, L.; Keller, G.M. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat. Biotechnol. 2017, 35, 56–68. [Google Scholar] [CrossRef]
- Jara-Avaca, M.; Kempf, H.; Rückert, M.; Robles-Diaz, D.; Franke, A.; de la Roche, J.; Fischer, M.; Malan, D.; Sasse, P.; Solodenko, W.; et al. EBIO Does Not Induce Cardiomyogenesis in Human Pluripotent Stem Cells but Modulates Cardiac Subtype Enrichment by Lineage-Selective Survival. Stem Cell Rep. 2017, 8, 305–317. [Google Scholar] [CrossRef]
- Uosaki, H.; Fukushima, H.; Takeuchi, A.; Matsuoka, S.; Nakatsuji, N.; Yamanaka, S.; Yamashita, J.K. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS ONE 2011, 6, e23657. [Google Scholar] [CrossRef]
- Sala, L.; Gnecchi, M.; Schwartz, P.J. Long QT Syndrome Modelling with Cardiomyocytes Derived from Human-induced Pluripotent Stem Cells. Arrhythm. Electrophysiol. Rev. 2019, 8, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Birket, M.J.; Ribeiro, M.C.; Kosmidis, G.; Ward, D.; Leitoguinho, A.R.; van de Pol, V.; Dambrot, C.; Devalla, H.D.; Davis, R.P.; Mastroberardino, P.G.; et al. Contractile Defect Caused by Mutation in MYBPC3 Revealed under Conditions Optimized for Human PSC-Cardiomyocyte Function. Cell Rep. 2015, 13, 733–745. [Google Scholar] [CrossRef]
- Lee, Y.K.; Ng, K.M.; Chan, Y.C.; Lai, W.H.; Au, K.W.; Ho, C.Y.; Wong, L.Y.; Lau, C.P.; Tse, H.F.; Siu, C.W. Triiodothyronine promotes cardiac differentiation and maturation of embryonic stem cells via the classical genomic pathway. Mol. Endocrinol. 2010, 24, 1728–1736. [Google Scholar] [CrossRef]
- Yang, X.; Rodriguez, M.L.; Leonard, A.; Sun, L.; Fischer, K.A.; Wang, Y.; Ritterhoff, J.; Zhao, L.; Kolwicz, S.C., Jr.; Pabon, L.; et al. Fatty Acids Enhance the Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Stem Cell Rep. 2019, 13, 657–668. [Google Scholar] [CrossRef]
- Hsueh, Y.C.; Pratt, R.E.; Dzau, V.J.; Hodgkinson, C.P. Novel method of differentiating human induced pluripotent stem cells to mature cardiomyocytes via Sfrp2. Sci. Rep. 2023, 13, 3920. [Google Scholar] [CrossRef]
- Mummery, C.; Oostwaard, D.; Doevendans, P.; Spijker, R.; Brink, S.; Hassink, R.; van der Heyden, M.; Opthof, T.; Pera, M.; Riviere, A.; et al. Differentiation of human embryonic stem cells to cardiomyocytes: Role of coculture with visceral endoderm-like cells. Circulation 2003, 107, 2733–2740. [Google Scholar] [CrossRef]
- Guo, N.N.; Liu, L.P.; Zheng, Y.W.; Li, Y.M. Inducing human induced pluripotent stem cell differentiation through embryoid bodies: A practical and stable approach. World J. Stem Cells 2020, 12, 25–34. [Google Scholar] [CrossRef]
- Batalov, I.; Feinberg, A.W. Differentiation of Cardiomyocytes from Human Pluripotent Stem Cells Using Monolayer Culture. Biomark. Insights 2015, 10 (Suppl. S1), 71–76. [Google Scholar] [CrossRef]
- Yuan, Q.; Verbueken, D.; Dinani, R.; Kim, R.; Schoger, E.; Morsink, C.D.; Simkooei, S.A.; Kemna, L.J.M.; Hjortnaes, J.; Kuster, D.W.D.; et al. Glycogen synthase kinase-3 inhibition and insulin enhance proliferation and inhibit maturation of human iPSC-derived cardiomyocytes via TCF and FOXO signaling. Stem Cell Rep. 2025, 20, 102371. [Google Scholar] [CrossRef]
- Prondzynski, M.; Berkson, P.; Trembley, M.A.; Tharani, Y.; Shani, K.; Bortolin, R.H.; Sweat, M.E.; Mayourian, J.; Yucel, D.; Cordoves, A.M.; et al. Efficient and reproducible generation of human iPSC-derived cardiomyocytes and cardiac organoids in stirred suspension systems. Nat. Commun. 2024, 15, 5929. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zou, J. Differentiation of Cardiomyocytes from Human Pluripotent Stem Cells in Fully Chemically Defined Conditions. STAR Protoc. 2020, 1, 100015. [Google Scholar] [CrossRef]
- Balafkan, N.; Mostafavi, S.; Schubert, M.; Siller, R.; Liang, K.X.; Sullivan, G.; Bindoff, L.A. A method for differentiating human induced pluripotent stem cells toward functional cardiomyocytes in 96-well microplates. Sci. Rep. 2020, 10, 18498. [Google Scholar] [CrossRef] [PubMed]
- Karakikes, I.; Ameen, M.; Termglinchan, V.; Wu, J.C. Human induced pluripotent stem cell-derived cardiomyocytes: Insights into molecular, cellular, and functional phenotypes. Circ. Res. 2015, 117, 80–88. [Google Scholar] [CrossRef]
- Pasqualini, F.S.; Sheehy, S.P.; Agarwal, A.; Aratyn-Schaus, Y.; Parker, K.K. Structural phenotyping of stem cell-derived cardiomyocytes. Stem Cell Rep. 2015, 4, 340–347. [Google Scholar] [CrossRef]
- Ma, J.; Guo, L.; Fiene, S.J.; Anson, B.D.; Thomson, J.A.; Kamp, T.J.; Kolaja, K.L.; Swanson, B.J.; January, C.T. High purity human-induced pluripotent stem cell-derived cardiomyocytes: Electrophysiological properties of action potentials and ionic currents. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2006–H2017. [Google Scholar] [CrossRef] [PubMed]
- Geraets, I.M.E.; Chanda, D.; van Tienen, F.H.J.; van den Wijngaard, A.; Kamps, R.; Neumann, D.; Liu, Y.; Glatz, J.F.C.; Luiken, J.; Nabben, M. Human embryonic stem cell-derived cardiomyocytes as an in vitro model to study cardiac insulin resistance. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 (5 Pt. B), 1960–1967. [Google Scholar] [CrossRef]
- Granéli, C.; Hicks, R.; Brolén, G.; Synnergren, J.; Sartipy, P. Diabetic Cardiomyopathy Modelling Using Induced Pluripotent Stem Cell Derived Cardiomyocytes: Recent Advances and Emerging Models. Stem Cell Rev. Rep. 2019, 15, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K.; Sheikh, F.; Gupta, R.M.; Houser, S.R.; Maher, K.O.; Milan, D.J.; Terzic, A.; Wu, J.C.; On behalf of the American Heart Association Council on Functional Genomics and Translational Biology; Council on Cardiovascular Disease in the Young; et al. Induced Pluripotent Stem Cells for Cardiovascular Disease Modeling and Precision Medicine: A Scientific Statement from the American Heart Association. Circ. Genom. Precis. Med. 2018, 11, e000043. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.G.; Kwon, Y.W.; Lee, T.W.; Park, G.T.; Kim, J.H. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater. Res. 2018, 22, 36. [Google Scholar] [CrossRef]
- Dakhore, S.; Nayer, B.; Hasegawa, K. Human Pluripotent Stem Cell Culture: Current Status, Challenges, and Advancement. Stem Cells Int. 2018, 2018, 7396905. [Google Scholar] [CrossRef]
- Hamledari, H.; Asghari, P.; Jayousi, F.; Aguirre, A.; Maaref, Y.; Barszczewski, T.; Ser, T.; Moore, E.; Wasserman, W.; Klein Geltink, R.; et al. Using human induced pluripotent stem cell-derived cardiomyocytes to understand the mechanisms driving cardiomyocyte maturation. Front. Cardiovasc. Med. 2022, 9, 967659. [Google Scholar] [CrossRef] [PubMed]
- Braam, S.R.; Tertoolen, L.; van de Stolpe, A.; Meyer, T.; Passier, R.; Mummery, C.L. Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 2010, 4, 107–116. [Google Scholar] [CrossRef]
- Burridge, P.W.; Li, Y.F.; Matsa, E.; Wu, H.; Ong, S.G.; Sharma, A.; Holmstrom, A.; Chang, A.C.; Coronado, M.J.; Ebert, A.D.; et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat. Med. 2016, 22, 547–556. [Google Scholar] [CrossRef]
- Drawnel, F.M.; Boccardo, S.; Prummer, M.; Delobel, F.; Graff, A.; Weber, M.; Gerard, R.; Badi, L.; Kam-Thong, T.; Bu, L.; et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014, 9, 810–821. [Google Scholar] [CrossRef]
- Yu, T.; Li, Z.; Jia, Z.; Clapcote, S.J.; Liu, C.; Li, S.; Asrar, S.; Pao, A.; Chen, R.; Fan, N.; et al. A mouse model of Down syndrome trisomic for all human chromosome 21 syntenic regions. Hum. Mol. Genet. 2010, 19, 2780–2791. [Google Scholar] [CrossRef]
- Takahashi, F.; Patel, P.; Kitsuka, T.; Arai, K. The Exciting Realities and Possibilities of iPS-Derived Cardiomyocytes. Bioengineering 2023, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Amenov, A.; Ignatyeva, N.; Koschinski, A.; Xu, H.; Soong, P.L.; Tiburcy, M.; Linke, W.A.; Zaccolo, M.; Hasenfuss, G.; et al. Troponin destabilization impairs sarcomere-cytoskeleton interactions in iPSC-derived cardiomyocytes from dilated cardiomyopathy patients. Sci. Rep. 2020, 10, 209. [Google Scholar] [CrossRef]
- Chuang, J.H.; Tung, L.C.; Lin, Y. Neural differentiation from embryonic stem cells in vitro: An overview of the signaling pathways. World J. Stem Cells 2015, 7, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhang, S.C. Neural Subtype Specification from Human Pluripotent Stem Cells. Cell Stem Cell 2016, 19, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef]
- Pasca, A.M.; Sloan, S.A.; Clarke, L.E.; Tian, Y.; Makinson, C.D.; Huber, N.; Kim, C.H.; Park, J.Y.; O’Rourke, N.A.; Nguyen, K.D.; et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 2015, 12, 671–678. [Google Scholar] [CrossRef]
- Cohen, M.A.; Itsykson, P.; Reubinoff, B.E. The role of FGF-signaling in early neural specification of human embryonic stem cells. Dev. Biol. 2010, 340, 450–458. [Google Scholar] [CrossRef]
- Borghese, L.; Dolezalova, D.; Opitz, T.; Haupt, S.; Leinhaas, A.; Steinfarz, B.; Koch, P.; Edenhofer, F.; Hampl, A.; Brustle, O. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells 2010, 28, 955–964. [Google Scholar] [CrossRef]
- Le Dreau, G.; Marti, E. Dorsal-ventral patterning of the neural tube: A tale of three signals. Dev. Neurobiol. 2012, 72, 1471–1481. [Google Scholar] [CrossRef]
- Bejoy, J.; Bijonowski, B.; Marzano, M.; Jeske, R.; Ma, T.; Li, Y. Wnt-Notch Signaling Interactions During Neural and Astroglial Patterning of Human Stem Cells. Tissue Eng. Part. A 2020, 26, 419–431. [Google Scholar] [CrossRef]
- Ho, S.M.; Hartley, B.J.; Tcw, J.; Beaumont, M.; Stafford, K.; Slesinger, P.A.; Brennand, K.J. Rapid Ngn2-induction of excitatory neurons from hiPSC-derived neural progenitor cells. Methods 2016, 101, 113–124. [Google Scholar] [CrossRef]
- Denham, M.; Dottori, M. Neural differentiation of induced pluripotent stem cells. Methods Mol. Biol. 2011, 793, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Abu-Bonsrah, K.D.; Viventi, S.; Newgreen, D.F.; Dottori, M. Generation of Neural Crest Progenitors from Human Pluripotent Stem Cells. Methods Mol. Biol. 2019, 1976, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Sloan, S.A.; Darmanis, S.; Huber, N.; Khan, T.A.; Birey, F.; Caneda, C.; Reimer, R.; Quake, S.R.; Barres, B.A.; Pasca, S.P. Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron 2017, 95, 779–790.e776. [Google Scholar] [CrossRef] [PubMed]
- Autar, K.; Guo, X.; Rumsey, J.W.; Long, C.J.; Akanda, N.; Jackson, M.; Narasimhan, N.S.; Caneus, J.; Morgan, D.; Hickman, J.J. A functional hiPSC-cortical neuron differentiation and maturation model and its application to neurological disorders. Stem Cell Rep. 2022, 17, 96–109. [Google Scholar] [CrossRef]
- Yuan, S.H.; Martin, J.; Elia, J.; Flippin, J.; Paramban, R.I.; Hefferan, M.P.; Vidal, J.G.; Mu, Y.; Killian, R.L.; Israel, M.A.; et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS ONE 2011, 6, e17540. [Google Scholar] [CrossRef]
- Kriks, S.; Shim, J.W.; Piao, J.; Ganat, Y.M.; Wakeman, D.R.; Xie, Z.; Carrillo-Reid, L.; Auyeung, G.; Antonacci, C.; Buch, A.; et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011, 480, 547–551. [Google Scholar] [CrossRef]
- Kurus, M.; Akbari, S.; Eskier, D.; Bursali, A.; Ergin, K.; Erdal, E.; Karakulah, G. Transcriptome Dynamics of Human Neuronal Differentiation From iPSC. Front. Cell Dev. Biol. 2021, 9, 727747. [Google Scholar] [CrossRef]
- Valiulahi, P.; Vidyawan, V.; Puspita, L.; Oh, Y.; Juwono, V.B.; Sittipo, P.; Friedlander, G.; Yahalomi, D.; Sohn, J.W.; Lee, Y.K.; et al. Generation of caudal-type serotonin neurons and hindbrain-fate organoids from hPSCs. Stem Cell Rep. 2021, 16, 1938–1952. [Google Scholar] [CrossRef]
- Solomon, E.; Davis-Anderson, K.; Hovde, B.; Micheva-Viteva, S.; Harris, J.F.; Twary, S.; Iyer, R. Global transcriptome profile of the developmental principles of in vitro iPSC-to-motor neuron differentiation. BMC Mol. Cell Biol. 2021, 22, 13. [Google Scholar] [CrossRef]
- Kim, T.W.; Piao, J.; Koo, S.Y.; Kriks, S.; Chung, S.Y.; Betel, D.; Socci, N.D.; Choi, S.J.; Zabierowski, S.; Dubose, B.N.; et al. Biphasic Activation of WNT Signaling Facilitates the Derivation of Midbrain Dopamine Neurons from hESCs for Translational Use. Cell Stem Cell 2021, 28, 343–355.e345. [Google Scholar] [CrossRef]
- Shimojo, D.; Onodera, K.; Doi-Torii, Y.; Ishihara, Y.; Hattori, C.; Miwa, Y.; Tanaka, S.; Okada, R.; Ohyama, M.; Shoji, M.; et al. Rapid, efficient, and simple motor neuron differentiation from human pluripotent stem cells. Mol. Brain 2015, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.J.; Dariolli, R.; Jorge, F.M.; Monteiro, M.R.; Maximino, J.R.; Martins, R.S.; Strauss, B.E.; Krieger, J.E.; Callegaro, D.; Chadi, G. Gene expression profiling for human iPS-derived motor neurons from sporadic ALS patients reveals a strong association between mitochondrial functions and neurodegeneration. Front. Cell Neurosci. 2015, 9, 289. [Google Scholar] [CrossRef]
- Madhu, V.; Dighe, A.S.; Cui, Q.; Deal, D.N. Dual Inhibition of Activin/Nodal/TGF-β and BMP Signaling Pathways by SB431542 and Dorsomorphin Induces Neuronal Differentiation of Human Adipose Derived Stem Cells. Stem Cells Int. 2016, 2016, 1035374. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Chen, X.; Gong, S.; Yu, P.; Yau, S.; Su, Z.; Zhou, L.; Yu, J.; Pan, G.; Shi, L. Characteristic analyses of a neural differentiation model from iPSC-derived neuron according to morphology, physiology, and global gene expression pattern. Sci. Rep. 2017, 7, 12233. [Google Scholar] [CrossRef]
- Tcw, J.; Wang, M.; Pimenova, A.A.; Bowles, K.R.; Hartley, B.J.; Lacin, E.; Machlovi, S.I.; Abdelaal, R.; Karch, C.M.; Phatnani, H.; et al. An Efficient Platform for Astrocyte Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Rep. 2017, 9, 600–614. [Google Scholar] [CrossRef]
- Leventoux, N.; Morimoto, S.; Imaizumi, K.; Sato, Y.; Takahashi, S.; Mashima, K.; Ishikawa, M.; Sonn, I.; Kondo, T.; Watanabe, H.; et al. Human Astrocytes Model Derived from Induced Pluripotent Stem Cells. Cells 2020, 9, 2680. [Google Scholar] [CrossRef]
- Majd, H.; Amin, S.; Ghazizadeh, Z.; Cesiulis, A.; Arroyo, E.; Lankford, K.; Majd, A.; Farahvashi, S.; Chemel, A.K.; Okoye, M.; et al. Deriving Schwann cells from hPSCs enables disease modeling and drug discovery for diabetic peripheral neuropathy. Cell Stem Cell 2023, 30, 632–647.e610. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, D.; Zarnowska, E.D.; Du, Z.; Werbel, B.; Valliere, C.; Pearce, R.A.; Thomson, J.A.; Zhang, S.C. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 2005, 23, 781–790. [Google Scholar] [CrossRef]
- Hu, B.Y.; Weick, J.P.; Yu, J.; Ma, L.X.; Zhang, X.Q.; Thomson, J.A.; Zhang, S.C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA 2010, 107, 4335–4340. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.; Pearlman, J.; Ruan, T.; Manion, J.; Waller, M.; Neely, G.G.; Caron, L. Human Pluripotent Stem Cells-Based Therapies for Neurodegenerative Diseases: Current Status and Challenges. Cells 2020, 9, 2517. [Google Scholar] [CrossRef]
- Wainger, B.J.; Kiskinis, E.; Mellin, C.; Wiskow, O.; Han, S.S.; Sandoe, J.; Perez, N.P.; Williams, L.A.; Lee, S.; Boulting, G.; et al. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 2014, 7, 1–11. [Google Scholar] [CrossRef]
- Choe, M.S.; Yeo, H.C.; Kim, J.S.; Lee, J.; Lee, H.J.; Kim, H.R.; Baek, K.M.; Jung, N.Y.; Choi, M.; Lee, M.Y. Simple modeling of familial Alzheimer’s disease using human pluripotent stem cell-derived cerebral organoid technology. Stem Cell Res. Ther. 2024, 15, 118. [Google Scholar] [CrossRef]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, S.H.; D’Avanzo, C.; Hebisch, M.; Sliwinski, C.; Bylykbashi, E.; Washicosky, K.J.; Klee, J.B.; Brüstle, O.; Tanzi, R.E.; et al. A 3D human neural cell culture system for modeling Alzheimer’s disease. Nat. Protoc. 2015, 10, 985–1006. [Google Scholar] [CrossRef]
- Zagare, A.; Kurlovics, J.; Almeida, C.; Ferrante, D.; Frangenberg, D.; Neises, L.; Vitali, A.; Gomez-Giro, G.; Jäger, C.; Antony, P.; et al. Insulin resistance compromises midbrain organoid neural activity and metabolic efficiency predisposing to Parkinson’s disease pathology. J. Tissue Eng. 2025, 16, 20417314241295928–20417314241295947. [Google Scholar] [CrossRef] [PubMed]
- Mor, M.E.; Harvey, A.; Familari, M.; St Clair-Glover, M.; Viventi, S.; de Iongh, R.U.; Cameron, F.J.; Dottori, M. Neural differentiation medium for human pluripotent stem cells to model physiological glucose levels in human brain. Brain Res. Bull. 2021, 173, 141–149. [Google Scholar] [CrossRef]
- Han, G.; Wei, P.; Han, Q. Application of IPSC and Müller glia derivatives in retinal degenerative diseases. Prog. Mol. Biol. Transl. Sci. 2023, 199, 351–362. [Google Scholar] [CrossRef]
- Okawa, T.; Kamiya, H.; Himeno, T.; Kato, J.; Seino, Y.; Fujiya, A.; Kondo, M.; Tsunekawa, S.; Naruse, K.; Hamada, Y.; et al. Transplantation of neural crest-like cells derived from induced pluripotent stem cells improves diabetic polyneuropathy in mice. Cell Transplant. 2013, 22, 1767–1783. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.H.; Liang, X.; Ren, Y.Y.; Shu, L.W.; Yan, Y.H.; Cui, X. Neurons derived from human-induced pluripotent stem cells express mu and kappa opioid receptors. Neural Regen. Res. 2021, 16, 653–658. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 2007, 448, 313–317. [Google Scholar] [CrossRef]
- Stadtfeld, M.; Brennand, K.; Hochedlinger, K. Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Curr. Biol. 2008, 18, 890–894. [Google Scholar] [CrossRef]
- Tiemann, U.; Sgodda, M.; Warlich, E.; Ballmaier, M.; Scholer, H.R.; Schambach, A.; Cantz, T. Optimal reprogramming factor stoichiometry increases colony numbers and affects molecular characteristics of murine induced pluripotent stem cells. Cytom. A 2011, 79, 426–435. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Hirano, K.; Nagata, S.; Tada, T. Sox2 expression effects on direct reprogramming efficiency as determined by alternative somatic cell fate. Stem Cell Res. 2011, 6, 177–186. [Google Scholar] [CrossRef]
- Gutierrez-Aranda, I.; Ramos-Mejia, V.; Bueno, C.; Munoz-Lopez, M.; Real, P.J.; Macia, A.; Sanchez, L.; Ligero, G.; Garcia-Parez, J.L.; Menendez, P. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells 2010, 28, 1568–1570. [Google Scholar] [CrossRef]
- Yasuda, S.; Kusakawa, S.; Kuroda, T.; Miura, T.; Tano, K.; Takada, N.; Matsuyama, S.; Matsuyama, A.; Nasu, M.; Umezawa, A.; et al. Tumorigenicity-associated characteristics of human iPS cell lines. PLoS ONE 2018, 13, e0205022. [Google Scholar] [CrossRef] [PubMed]
- Wernig, M.; Meissner, A.; Cassady, J.P.; Jaenisch, R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell 2008, 2, 10–12. [Google Scholar] [CrossRef]
- Lee, M.O.; Moon, S.H.; Jeong, H.C.; Yi, J.Y.; Lee, T.H.; Shim, S.H.; Rhee, Y.H.; Lee, S.H.; Oh, S.J.; Lee, M.Y.; et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc. Natl. Acad. Sci. USA 2013, 110, E3281–E3290. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.Y.; Bagheri, R.; Savatier, P.; Saric, T.; Moradi, S. Elimination of tumorigenic pluripotent stem cells from their differentiated cell therapy products: An important step toward ensuring safe cell therapy. Stem Cell Rep. 2025, 20, 102543. [Google Scholar] [CrossRef] [PubMed]
- Howe, S.J.; Mansour, M.R.; Schwarzwaelder, K.; Bartholomae, C.; Hubank, M.; Kempski, H.; Brugman, M.H.; Pike-Overzet, K.; Chatters, S.J.; de Ridder, D.; et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Investig. 2008, 118, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- Gorecka, J.; Kostiuk, V.; Fereydooni, A.; Gonzalez, L.; Luo, J.; Dash, B.; Isaji, T.; Ono, S.; Liu, S.; Lee, S.R.; et al. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res. Ther. 2019, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Volpato, V.; Webber, C. Addressing variability in iPSC-derived models of human disease: Guidelines to promote reproducibility. Dis. Model. Mech. 2020, 13, dmm042317. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Deng, G.; Sai, X.; Guo, H.; Huang, H.; Zhu, P. Maturation strategies and limitations of induced pluripotent stem cell-derived cardiomyocytes. Biosci. Rep. 2021, 41, BSR20200833. [Google Scholar] [CrossRef]
- George, B. Regulations and guidelines governing stem cell based products: Clinical considerations. Perspect. Clin. Res. 2011, 2, 94–99. [Google Scholar] [CrossRef]
- Moradi, S.; Mahdizadeh, H.; Saric, T.; Kim, J.; Harati, J.; Shahsavarani, H.; Greber, B.; Moore, J.B.t. Research and therapy with induced pluripotent stem cells (iPSCs): Social, legal, and ethical considerations. Stem Cell Res. Ther. 2019, 10, 341. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Behdarvand Dehkordi, F.; Chehelgerdi, M.; Kabiri, H.; Salehian-Dehkordi, H.; Abdolvand, M.; Salmanizadeh, S.; Rashidi, M.; Niazmand, A.; Ahmadi, S.; et al. Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy. Mol. Cancer 2023, 22, 189. [Google Scholar] [CrossRef]

| Cell Origin | Culture Conditions | Protocol | Functional Analysis | Key Features of the Protocol | Reference |
|---|---|---|---|---|---|
| Human iPSC (Renal cells isolated from urine) | Small molecule-induced | Stage 1: Definitive endoderm: Days 1–3 Day 1: CHIR99021, Activin A Day 2–3: Activin A Stage 2: 4 days: Hepatic endoderm induction: DMEM-F12, 1%DMSO, KOSR, Doxycycline, 2-Mercaptoethanol, Glx Stage 3: HLC: days 12–15: DMEM F12, insulin, hepatocyte growth factor (HGF), dexamethasone (Dex), recombinant-human Oncostatin M (rhOSM209a.a), and forskolin |
|
| [39] |
| Human iPSC (Windy, K,FF-2(Commercial cell lines) | Small molecule induced | Stage 1: Endoderm induction: 5 days Activin A Stage 2: Subculturing of endoderm in 1% DMSO for 7 days, followed by Cos medium with HGF, Oncostatin-M, dexamethasone, and valproic acid for 7 days. The last 3 days: celecoxib. Stage 3: 3 days: Cos medium 004, HGF, Oncostatin M, dexamethasone, celecoxib. Stage 4: 4 days: Cos medium 004, celecoxib. |
|
| [17] |
| BIONi010-C-CYP3A4–2TA-Nluc A reporter line containing a T2A-Nanoluciferase gene immediately upstream of the stop codon of the CYP3A4 gene, BIONi010 derived from Fibroblast of skin | Small molecule induced | Stage 1: Definitive endoderm: Days 1–3 Day 1: CHIR99021, Activin A Days 2–3: Activin A Stage 2: Hepatic Progenitor: 7-Day Reseeding: Progenitor Media, KOSR and DMSO Stage 3: Hepatocyte Maturation: 7 Days: Maturation Media, Dexamethasone, HGF, Oncostatin M, Hydrocortisone, Cholesterol Lipid Concentrate |
|
| [40] |
| iPSCs from urinary derived epithelial cells | Small molecule induced | Stage1: Definitive endoderm: Days 1–3 Day1: CHIR99021, Activin A Stage 2: Hepatic endoderm: 5 days: FGF, BMP-4, DMSO Stage 3: 5 days: Immature hepatocyte culture media: HGF, DMSO Stage 4: Mature hepatocyte culture media 10–12 days, HGF, DMSO, Oncostatin-M, dexamethasone |
|
| [41] |
| hCiPSCs human adult skin fibroblast and human adipose derived mesenchymal stromal cells | Small molecule induced | Stage 1 Definitive endoderm: 4 days: Activin A, BMP4, bFGF, Y27632, and CHIR99021 for 1 day in RPMI1640 medium with B27 supplement. Activin A, BMP4, Y27632, bFGF, and B27 supplement for another 3 days Foregut endoderm: 2 days: KGF, Y27632, and SB431542. hepatoblasts 1: 3 days: KGF, BMP4, BMP2, Y27632, and bFGF. Replating in hepatoblast 1 media with 10 μM Y27632 (Selleck, S1049) for 1 day. Hepatoblasts2: 3 days: William’s medium E, forskolin, EGF, and Y27632. hiHPC expansion media: DMEM/F12 mixed with William’s medium E in the ratio of 1:1 containing B27 supplement and forskolin, SB431542, EGF, CHIR99021, LP, dexamethasone and S1P, Nicotinamide, PVC, Heparin. hiHPCs maturation media: Williams’s medium E containing B27 supplement, forskolin, and SB431542. |
|
| [42] |
| iPSC Origin | Type of Differentiation | Protocol | Culture Type | Key Features of the Protocol | Reference |
|---|---|---|---|---|---|
| HiPSC-AFR1 | Small molecule induced | Stage 1: EB to myoblast (18 days): CHIR99021, the transforming growth factor-β (TGF-β) inhibitor SB431542, fibroblast growth factor-2 (FGF2), insulin growth factor-1 (IGF1), and heregulin-β-1 Stage 2: Myogenic amplification media (4–7 days): IGF1, FGF2, Heregulin-B-1 and Forskolin Stage 3: Myogenic maturation media (7 days) | EB+monolayer |
| [86] |
| MYOD1-hiPSCs | Small molecule induced | Stage 1: Myotube generation; Day 1: hESC medium without FGF-2 Day 3: Skeletal muscle induction media: αMEM supplemented with 10% KSR, 2% Ultroser G, and 2-ME. Stage 2: myotube maturation: Day 6–12 DMEM (high glucose, 1500 mg/L supplemented with 5% horse serum, recombinant human insulin-like growth factor 1 (IGF-1), and SB431542. | Monolayer |
| [87] |
| Human hiPSC (MiPS and BiPS) | Small molecule induced | Stage1: Day0–3 DICL (DMEM, ITS, CHIR, LDN) Stage 2: day 3–6 DICL+FGF Stage 3: Days 6–8 DK-LHIF medium (DMEM, KSR, LDN, HGF, IGF, FGF) Stage 4: Days 8–12 DKI (DMEM KSR, IGF) Stage 5: day12–30 DKI+ HGF Stage 6: (iMPCs): days 30–45 Skeletal muscle media(iMCs) Stage 7: days 45–60 terminal differentiation | Monolayer |
| [88] |
| hPSC | Small molecule induced | Stage 1: mesoderm induction day1–4: MDM1-CHIR, SB, EGF, Insulin, dexamethasone Stage 2: Somite or myotome induction: Days 5–14 MDM2 LDN, SB, EGF, FGF, HGF, IGF-1 Stage 3: Cell sorting and expansion of myogenic progenitor: MDM 2 media Stage 4: Terminal differentiation: MDM3,15%KSR, IMDM, IGF | Monolayer |
| [89] |
| Human iPSC | Small molecule induced | Primary differentiation: 3–4 weeks Proliferation: 1–2 days (Skeletal muscle growth media (SKGM)) Secondary differentiation: 1–2 weeks. KC (KSR/CHIR), KCTi (KSR/CHIR, TGF-β inhibitor SB431542), KCTiP (KSR/CHIR, TGF-β inhibitor SB431542, prednisolone) | Monolayer |
| [90] |
| NSV44.1 and McA2.7 | Small molecule induced | Stage 1: Primary Differentiation Day 0–Day 3: DiCL (DMEM-ITS-CHIR-LDN) Day 3–Day 6: DiCLF (DMEM-ITS-CHIR, LDN-FGF) Day 6–Day 8: DK-HiFL (DMEM, KSR, HGF, IGF, FGF, LDN) Day 8–Day 12: DK I (DMEM, KSR, IGF) Day 12–Day 30: DK-Hi (DMEM, KSR, HGF, IGF) Stage 2: Final Differentiation; 7–14 Days KCTiP (KSR/CHIR, TGF-β inhibitor SB431542, prednisolone) | Monolayer |
| [91] |
| iPSC Origin | Method of Differentiation | Differentiation Media and Cocktail | Type of Adipocyte | Functional Analysis | Key Features of the Protocol | Reference |
|---|---|---|---|---|---|---|
| 3T3-L1 embryonic fibroblastic cell line and a C3H10T1/2 mesenchymal stem cell line | Monolayer | 3T3-L1 cell line Stage 1: 3days; DMEM, insulin, dexamethasone, isobutylmethylxanthine (IB), and Rosiglitazone. Day 4 DMEM and insulin Stage 2: Day 5–10; DMEM C3H10T1/2 Stage 1: DMEM, insulin, triiodothyronine, IBMX, dexamethasone, and indomethacin Stage 2: 4 days: DMEM, FBS, insulin, and T3 | Brown |
|
| [131] |
| HDFa-YK27-hiPSC human dermal fibroblast line and YK27-iPSC–derived iMSCs | Derivation of iMSCs from hiPSCs through embryoid bodies (EBs) formation | Stage 1: mesoderm induction: EB formation Stage 2: iMSC expansion- Stemline II, VEGF, BMP Stage 3: Preadipocyte growth: Preadipocyte basal media Stage 4: Adipocyte induction: DMEM, insulin, IBMX, dexamethasone and indomethacin | NA |
|
| [132] |
| The human embryonic stem cell (hESCs) WA09, induced pluripotent stem cell (hiPSCs) line K3 and N4, generated from human neonatal foreskin fibroblasts | Direct differentiation in rotation culture from a Paraxial mesoderm (PM) precursor | Stage 1: Paraxial mesoderm induction: Day 1: hPSC MM media Day 2: hPSC CMM media Day3–4: Mesoderm induction media: DM, BMP4, bFGF, human IGF-I and rapamycin Day5–7 Paraxial mesoderm induction: DM (defined base medium), bFGF, human IGF-I, Rapamycin, WNT3, Noggin, (2′Z,3′E)-6-Bromoindirubin-3′-oxime (BIO) and forskolin. Stage 2: Brown adipocyte priming: BA1 media; DM bFGF, BMP7, human IGF-I, Y-27632 dihydrochloride, Rosiglitazone, Dexamethasone, T3 thyroid hormone, IBMX (3-Isobutyl-1-methylxanthine) and SB 431542 Stage 3: Brown adipocyte maturation BA2: BA2 is same as BA1 excluding SB 431542 and supplemented with Chemically Defined Lipid Concentrate. | Brown |
|
| [133] |
| hESC lines (H1 and H9) | MSC through EB in a retinoic acid-based method | Stage 1: EB formation; Day 0–7. DMEM+ RA Stage 2: differentiation into MSC; Days 7–12; Differentiation media Stage 3: MSC expansion; Differentiation media and bFGF, Stage 4: Adipogenic differentiation Protocol 1 (Pr1): knockout DMEM-F12, KSR, 3-isobutyl-1-methylxanthine (IBMX), dexamethasone, insulin, indomethacin and pioglitazone. Protocol 2 (Pr2), MEM-alpha, IBMX, dexamethasone, insulin, indomethacin and Roziglitazone | NA |
|
| [134] |
| hiPSC lines reprogrammed from fibroblasts | Monolayer | Stage 1: Mesoderm differentiation; Day 0–4; STEM Pro34, Glutamax, Ascorbic acid, BMP-4, Activin A. Stage 2: Adipocyte differentiation; Days 5–10; DMEM/F12, insulin, methylxanthine (IBMX), dexamethasone, and indomethacin Stage3: Adipocyte maturation; Day 10–20: DMEM/F12, Insulin | Beige |
|
| [135] |
| Human iPSC | Monolayer, through MSC to adipocyte precursor and to adipocytes | Stage 1: Mesoderm Induction Days 0–5: MIM. Stage 2: Generation of MSC from mesoderm; Days 5–12 MesenCult-ACF Plus medium Stage 3: Beige adipogenic precursor induction; Days 0–2, MesenCult-ACF, SB 431542, IL-4. Stage 4: Beige adipocyte induction media: days 2–5; insulin, T3 Roziglitazone, isobutylmethylxanthine (IBMX), dexamethasone, indomethacin, SB 431542, EGM-2 Stage 5: Beige adipocyte maintenance; Days 5–14: EGM-2, SB 431542, insulin, T3, Roziglitazone, | Beige |
|
| [136] |
| Cell Origin | Type of Differentiation | Protocol | Functional Analysis | Key Features of the Protocol | References |
|---|---|---|---|---|---|
| HiPSC (SCVI-273, SCVI-114, SCVI-202, and SCVI-111) All are commercial cell line derived from peripheral blood | Small molecule induced | Stage 1: 3 days: Day 1; CHIR99021 RPMI/B27-insulin, Day 2–3 RPMI/B27-insulin Stage 2: Day 3 Combined media and IWP2 Day5: RPMI/B27-insulin Day7: RPMI/B27-insulin every 3 days |
|
| [164] [176] |
| WTC-11 WTC-Cas9 (generated by inserting CAG-rtTA::TetO-Cas9 in WTC-11) derived from skin fibroblasts | Small molecule induced | Stage 1: 2 days: CHIR99021 Stage 2: 2 days: IWR1 Days 7–15: Basic media with insulin |
|
| [177] |
| H1 (WA01), H9 (WA09) mND2–0 (embryonic stem cells) | Small molecule induced | Stage 1: mesoendoderm induction: Day 0: CDM-A: Cardiac Differentiation Basal Medium (CDBM) + CHIR99021 Day 1; CDM-B: CDBM + heparin Stage 2: Cardiac progenitor induction: Day 2–4: CDM-C-: CDBM + heparin + IWP2 Stage3: Cardiac differentiation: Day 5–6: CDM-B Stage 4: Cardiomyocyte maturation Day 7: CDM-D-: CDBM + Insulin |
|
| [178] |
| Human Ips Cells (derived from skin fibroblast) | Small molecule induced | Stage 1: 1 day: CHIR99021 Stage 2: days 2: WntC59, XAV939, human Sfrp2, human Wnt3a protein Stage 3: Day 5–9: differentiation media (RPMI1640+ Ascorbic acid) Day 9–14: RPMI1640+ B27 |
|
| [172] |
| H1, H9 (Embryonic stem cells), iPSC reprogrammed from fibroblast) | Small molecule induced | Stage 1: 1 day: CHIR9902 Days 2–3: RPMI1640 with B27 without insulin Stage 2: 2 days: IWP2 Stage 3: 2 days: RPMI1640 with B27 without insulin Day 7: RPMI-B27 with insulin |
|
| [179] |
| Cell Origin | Culture Conditions | Protocol | Functional Analysis | Key Features of the Protocol | Reference |
|---|---|---|---|---|---|
| WT1and WT2 (fibroblast derived cell line) and WT2 (Commercial cell line) | STEMdiff™ Neural System | Stage 1: Neural induction: 9 days: Neural induction media with SMADi and 10 µM Y-27632 Stage 2: subculturing Stage 3: generation of neuronal precursor: 7 days: Neural differentiation media Stage 4: Maturation: Neural maturation media |
|
| [210] |
| HiPSC Coriell ND41865 (commercial cell line derived from skin fibroblast) | Small molecule induced | Differentiation media 1: SB431542, LDN193189, DMH-1, and recombinant human DKK-1 protein Differentiation media 2: SB431542, LDN193189, DMH-1, and cyclopamine Differentiation media 3: Neurobasal media with 1X Glutamax,1X N-2 supplement, 1X B27 without Vitamin A, BDNF, GDNF, Ascorbic Acid, cAMP, Laminin, and 1X Antimycotic-Antibiotic. |
|
| [207] |
| H9 hESC (embryonic stem cell line), L2122mutation in PINK1 (PTEN-induced kinase) gene, and L2131 (familial control of PINK1 mutated gene hiPSC lines derived from skin fibroblast) | Small molecule induced | Stage 1: LDN193189 and SB431542 and 2μM each of purmorphamine and RA Stage 2: NB/B27 medium supplemented with BDNF. |
|
| [211] |
| hiPSC (WTC-11 (Commercial cell line derived from skin fibroblast)) | Small molecule induced | Stage1: Days 0–6: CHIR99021, DMH-1 and SB431542 Stage 2: days 6–12: Stage 1 media with RA and Pur Stage 3: RA and Pur Stage 4: RA, Pur, CpdE, IGF-1, BDNF, and CNTF |
|
| [212] |
| hiPSC (H9 and MEL1 (Commercial Embryonic stem cell lines), and J1 human induced iPSC derived from fibroblast cells) | Small molecule induced | Stage 1: L-glutamine, SHH C25II, LDN, B431542, CHIR99021 (from day4 different concentration of CHIR), and Rock inhibitor (Y-27632) Stage 2: Neurobasal/B27/L-Glu supplemented with BDNF (brain-derived neurotrophic factor, ascorbic acid, GDNF (glial cell line-derived neurotrophic factor, TGFβ3 (transforming growth factor type β3, dibutyryl cAMP, and CHIR Stage3: NB/B27/L-Glu, BDNF, ascorbic acid, GDNF, dbcAMP, and TGFβ3 until day 16, with adding DAPT |
|
| [213] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiab, S.; Azeez, J.M.; Anala, A.; Nanda, M.; Khan, S.; Butler, A.E.; Nandakumar, M. Human-Induced Pluripotent Stem Cells (iPSCs) for Disease Modeling and Insulin Target Cell Regeneration in the Treatment of Insulin Resistance: A Review. Cells 2025, 14, 1188. https://doi.org/10.3390/cells14151188
Thiab S, Azeez JM, Anala A, Nanda M, Khan S, Butler AE, Nandakumar M. Human-Induced Pluripotent Stem Cells (iPSCs) for Disease Modeling and Insulin Target Cell Regeneration in the Treatment of Insulin Resistance: A Review. Cells. 2025; 14(15):1188. https://doi.org/10.3390/cells14151188
Chicago/Turabian StyleThiab, Sama, Juberiya M. Azeez, Alekya Anala, Moksha Nanda, Somieya Khan, Alexandra E. Butler, and Manjula Nandakumar. 2025. "Human-Induced Pluripotent Stem Cells (iPSCs) for Disease Modeling and Insulin Target Cell Regeneration in the Treatment of Insulin Resistance: A Review" Cells 14, no. 15: 1188. https://doi.org/10.3390/cells14151188
APA StyleThiab, S., Azeez, J. M., Anala, A., Nanda, M., Khan, S., Butler, A. E., & Nandakumar, M. (2025). Human-Induced Pluripotent Stem Cells (iPSCs) for Disease Modeling and Insulin Target Cell Regeneration in the Treatment of Insulin Resistance: A Review. Cells, 14(15), 1188. https://doi.org/10.3390/cells14151188