Going with the Flow: Sensorimotor Integration Along the Zebrafish GI Tract
Abstract
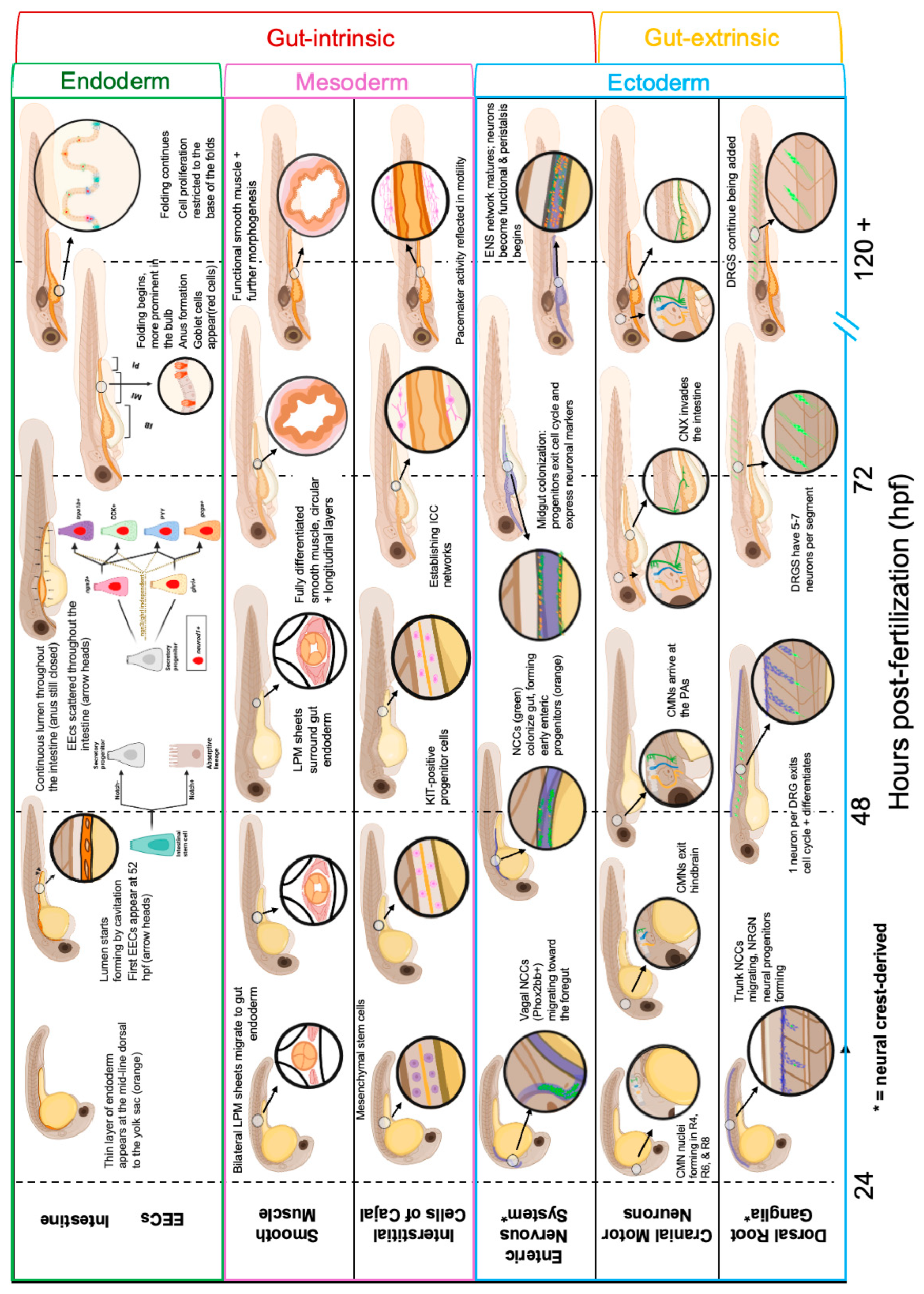
1. Introduction
2. Regulatory Elements of Gut Sensorimotor Reflexes
2.1. Gut-Extrinsic Regulators
2.1.1. Cranial Motor Neurons
2.1.2. Dorsal Root Ganglia
2.2. Gut-Intrinsic Regulators
2.2.1. Intestinal Epithelium/Lumen
2.2.2. Enteroendocrine Cells
2.2.3. Enteric Nervous System
2.2.4. Smooth Muscle
2.2.5. Interstitial Cells of Cajal
3. Integration of Sensorimotor Reflex Circuits Along the GI Tract

3.1. Swallowing Reflex
3.2. Peristalsis and Luminal pH
3.3. Churning and Enzymatic Reflexes of the Intestinal Bulb
3.4. Nutrient Sensing and Absorptive Reflexes
3.5. Evacuation Reflexes
4. Altered Sensorimotor Integration in ASD: Insights from Zebrafish and Other Animal Models
4.1. CHD7
4.2. CHD8
4.3. CNTNAP2
4.4. DYRK1A
4.5. FOXP1
4.6. MECP2
4.7. Neuroligin-3
4.8. SHANK3
4.9. TCF4
4.10. CHD2, SYNGAP1, SCN2A
5. The Search for Therapeutic Treatments
6. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | Serotonin |
| ACh | Acetylcholine |
| ASD | Autism spectrum disorder |
| CCK | Cholecystokinin |
| CHARGE | Coloboma, heart malformation, choanal atresia, retardation, genital, ear anomalies |
| CHD | Chromodomain helicase DNA-binding protein |
| CNIX | Cranial neuron 9 |
| CNS | Central nervous system |
| CNVII | Cranial neuron 7 |
| CNX | Cranial neuron 10 |
| CNTNAP2 | Contactin-associated protein 2 |
| DPF | Days post fertilization |
| DRG | Dorsal root ganglia |
| DYRK1A | Dual-specificity tyrosine phosphorylation-regulated kinase 1A |
| EEC | Enteroendocrine cell |
| EC | Enterochromaffin cell |
| ENCC | Enteric neural crest cell |
| ENS | Enteric nervous system |
| FOXP1 | Forkhead-box protein P1 |
| GI | Gastrointestinal |
| GTPase | Guanine Triphosphatase |
| HCN4 | Hyperpolarization-activated cyclic nucleotide-gated channel 4 |
| HPF | Hours post fertilization |
| ICCs | Interstitial cells of Cajal |
| IPAN | Intrinsic primary afferent neurons |
| LPM | Lateral-plate mesoderm |
| MECP2 | Methyl CpG binding protein 2 |
| NDDs | Neurodevelopment disorders |
| Neurod1 | Neurogenic differentiation 1 |
| Ngn3 | Neurogenin 3 |
| NO | Nitric oxide |
| nNOS | Neuronal nitric oxide synthase |
| PA | Pharyngeal Arches |
| PMS | Phelan–McDermid syndrome |
| PTHS | Pitt–Hopkins syndrome |
| PYY | Peptide YY |
| R | Rhombomere |
| RAS | Rat sarcoma virus |
| RNA | Ribonucleic Acid |
| RTT | Rett syndrome |
| SHANK3 | SH3 and multiple ankyrin repeat domains 3 |
| TCF4 | Transcription factor 4 |
| NTS | Nucleus tractus solitarius |
| VIP | Vasoactive intestinal peptide |
References
- Woodward, O.R.M.; Gribble, F.M.; Reimann, F.; Lewis, J.E. Gut peptide regulation of food intake—Evidence for the modulation of hedonic feeding. J. Physiol. 2022, 600, 1053–1078. [Google Scholar] [CrossRef]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef]
- Leader, G.; Forde, J.; Naughton, K.; Maher, L.; Arndt, S.; Mannion, A. Relationships among gastrointestinal symptoms, sleep problems, challenging behaviour, comorbid psychopathology and autism spectrum disorder symptoms in children and adolescents with 15q duplication syndrome. J. Intellect. Disabil. Res. 2021, 65, 32–46. [Google Scholar] [CrossRef]
- Christensen, T.J.; Ringdahl, J.E.; Bosch, J.J.; Falcomata, T.S.; Luke, J.R.; Andelman, M.S. Constipation Associated with Self-Injurious and Aggressive Behavior Exhibited by a Child Diagnosed with Autism. Educ. Treat. Child. 2009, 32, 14. [Google Scholar] [CrossRef]
- Yang, X.L.; Liang, S.; Zou, M.Y.; Sun, C.H.; Han, P.P.; Jiang, X.T.; Xia, W.; Wu, L.J. Are gastrointestinal and sleep problems associated with behavioral symptoms of autism spectrum disorder? Psychiatry Res. 2018, 259, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.A.; Holingue, C.; Jimenez-Gomez, A.; Dallman, J.E.; Moshiree, B. Gastrointestinal Dysfunction in Genetically Defined Neurodevelopmental Disorders. Semin. Neurol. 2023, 43, 645–660. [Google Scholar] [CrossRef]
- Halladay, A.; Croffie, J.; Dallman, J.; Grabenstatter, H.; Holingue, C.; Madgett, K.; Margolis, K.G.; Motil, K.J.; Jimenez-Gomez, A.; Ferguson, B.J.; et al. Conference proceedings: Inaugural meeting of the consortium for autism, genetic neurodevelopmental disorders, and digestive diseases. J. Pediatr. Gastroenterol. Nutr. 2024, 79, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.K.; Bockmann, J.; Steinestel, K.; Boeckers, T.M.; Grabrucker, A.M. Altered Intestinal Morphology and Microbiota Composition in the Autism Spectrum Disorders Associated SHANK3 Mouse Model. Int. J. Mol. Sci. 2019, 20, 2134. [Google Scholar] [CrossRef]
- Drokhlyansky, E.; Smillie, C.S.; Van Wittenberghe, N.; Ericsson, M.; Griffin, G.K.; Eraslan, G.; Dionne, D.; Cuoco, M.S.; Goder-Reiser, M.N.; Sharova, T.; et al. The Human and Mouse Enteric Nervous System at Single-Cell Resolution. Cell 2020, 182, 1606–1622.e23. [Google Scholar] [CrossRef]
- Kuil, L.E.; Chauhan, R.K.; Cheng, W.W.; Hofstra, R.M.W.; Alves, M.M. Zebrafish: A Model Organism for Studying Enteric Nervous System Development and Disease. Front. Cell Dev. Biol. 2020, 8, 629073. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.N.; de Jong-Curtain, T.A.; Mawdsley, D.J.; White, S.J.; Shin, J.; Appel, B.; Dong, P.D.; Stainier, D.Y.; Heath, J.K. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev. Biol. 2005, 286, 114–135. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.N.; Akhter, S.; Smith, E.M.; Lorent, K.; Pack, M. Intestinal growth and differentiation in zebrafish. Mech. Dev. 2005, 122, 157–173. [Google Scholar] [CrossRef]
- James, D.M.; Kozol, R.A.; Kajiwara, Y.; Wahl, A.L.; Storrs, E.C.; Buxbaum, J.D.; Klein, M.; Moshiree, B.; Dallman, J.E. Intestinal dysmotility in a zebrafish (Danio rerio) shank3a;shank3b mutant model of autism. Mol. Autism 2019, 10, 3. [Google Scholar] [CrossRef]
- Hayot, G.; Massonot, M.; Keime, C.; Faure, E.; Golzio, C. Loss of autism-candidate CHD8 perturbs neural crest development and intestinal homeostatic balance. Life Sci. Alliance 2023, 6, e202201456. [Google Scholar] [CrossRef]
- McCluskey, K.E.; Stovell, K.M.; Law, K.; Kostyanovskaya, E.; Schmidt, J.D.; Exner, C.R.T.; Dea, J.; Brimble, E.; State, M.W.; Willsey, A.J.; et al. Autism gene variants disrupt enteric neuron migration and cause gastrointestinal dysmotility. Nat. Commun. 2025, 16, 2238. [Google Scholar] [CrossRef]
- Chandrasekhar, A. Turning heads: Development of vertebrate branchiomotor neurons. Dev. Dyn. 2004, 229, 143–161. [Google Scholar] [CrossRef]
- Higashijima, S.; Hotta, Y.; Okamoto, H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J. Neurosci. 2000, 20, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Wanner, S.J.; Saeger, I.; Guthrie, S.; Prince, V.E. Facial motor neuron migration advances. Curr. Opin. Neurobiol. 2013, 23, 943–950. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barsh, G.R.; Isabella, A.J.; Moens, C.B. Vagus Motor Neuron Topographic Map Determined by Parallel Mechanisms of hox5 Expression and Time of Axon Initiation. Curr. Biol. 2017, 27, 3812–3825.e3. [Google Scholar] [CrossRef]
- Isabella, A.J.; Barsh, G.R.; Stonick, J.A.; Dubrulle, J.; Moens, C.B. Retinoic Acid Organizes the Zebrafish Vagus Motor Topographic Map via Spatiotemporal Coordination of Hgf/Met Signaling. Dev. Cell 2020, 53, 344–357.e345. [Google Scholar] [CrossRef]
- Isabella, A.J.; Stonick, J.A.; Dubrulle, J.; Moens, C.B. Intrinsic positional memory guides target-specific axon regeneration in the zebrafish vagus nerve. Development 2021, 148, dev199706. [Google Scholar] [CrossRef]
- Pavan, W.J.; Raible, D.W. Specification of neural crest into sensory neuron and melanocyte lineages. Dev. Biol. 2012, 366, 55–63. [Google Scholar] [CrossRef]
- Wolfson, R.L. Spinal sensory innervation of the intestine. Curr. Opin. Neurobiol. 2025, 90, 102973. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Singh, N.; Ahsan, K.; Beiriger, A.; Prince, V.E. Neural crest development: Insights from the zebrafish. Dev. Dyn. 2020, 249, 88–111. [Google Scholar] [CrossRef]
- An, M.; Luo, R.; Henion, P.D. Differentiation and maturation of zebrafish dorsal root and sympathetic ganglion neurons. J. Comp. Neurol. 2002, 446, 267–275. [Google Scholar] [CrossRef]
- McGraw, H.F.; Nechiporuk, A.; Raible, D.W. Zebrafish dorsal root ganglia neural precursor cells adopt a glial fate in the absence of neurogenin1. J. Neurosci. 2008, 28, 12558–12569. [Google Scholar] [CrossRef]
- Ernsberger, U. Role of neurotrophin signalling in the differentiation of neurons from dorsal root ganglia and sympathetic ganglia. Cell Tissue Res. 2009, 336, 349–384. [Google Scholar] [CrossRef]
- McGraw, H.F.; Snelson, C.D.; Prendergast, A.; Suli, A.; Raible, D.W. Post-embryonic neuronal addition in zebrafish dorsal root ganglion is regulated by Notch signaling. Discov. Neurosci. 2012, 7, 23. [Google Scholar] [CrossRef]
- Wolfson, R.L.; Abdelaziz, A.; Rankin, G.; Kushner, S.; Qi, L.; Mazor, O.; Choi, S.; Sharma, N.; Ginty, D.D. DRG afferents that mediate physiologic and pathologic mechanosensation from the distal colon. Cell 2023, 186, 3368–3385.e3318. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.N.; Pack, M. Unique and conserved aspects of gut development in zebrafish. Dev. Biol. 2003, 255, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Abud, H.E.; Watson, N.; Heath, J.K. Growth of intestinal epithelium in organ culture is dependent on EGF signalling. Exp. Cell Res. 2005, 303, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Rombout, J.H.; Lamers, C.H.; Helfrich, M.H.; Dekker, A.; Taverne-Thiele, J.J. Uptake and transport of intact macromolecules in the intestinal epithelium of carp (Cyprinus carpio L.) and the possible immunological implications. Cell Tissue Res. 1985, 239, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Beasley, A.; Hu, Y.; Chen, X. A Zebrafish Model for Studies on Esophageal Epithelial Biology. PLoS ONE 2015, 10, e0143878. [Google Scholar] [CrossRef]
- Rawls, J.F.; Samuel, B.S.; Gordon, J.I. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc. Natl. Acad. Sci. USA 2004, 101, 4596–4601. [Google Scholar] [CrossRef]
- Morash, M.; Kay, R.; Wen, J.; Padilla-Mercado, G.; Ye, L.; Reimann, F.; Gribble, F.; Rawls, J. Tools for studying enteroendocrine cell subtype development and physiology in zebrafish. Physiology 2025, 38 (Suppl. S1), 5733038. [Google Scholar] [CrossRef]
- Sternini, C.; Anselmi, L.; Rozengurt, E. Enteroendocrine cells: A site of ‘taste’ in gastrointestinal chemosensing. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 73–78. [Google Scholar] [CrossRef]
- Moran, G.W.; Leslie, F.C.; Levison, S.E.; Worthington, J.; McLaughlin, J.T. Enteroendocrine cells: Neglected players in gastrointestinal disorders? Therap Adv. Gastroenterol. 2008, 1, 51–60. [Google Scholar] [CrossRef]
- Flasse, L.C.; Stern, D.G.; Pirson, J.L.; Manfroid, I.; Peers, B.; Voz, M.L. The bHLH transcription factor Ascl1a is essential for the specification of the intestinal secretory cells and mediates Notch signaling in the zebrafish intestine. Dev. Biol. 2013, 376, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Bermingham, N.A.; Finegold, M.J.; Zoghbi, H.Y. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 2001, 294, 2155–2158. [Google Scholar] [CrossRef]
- Crosnier, C.; Vargesson, N.; Gschmeissner, S.; Ariza-McNaughton, L.; Morrison, A.; Lewis, J. Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development 2005, 132, 1093–1104. [Google Scholar] [CrossRef]
- Jenny, M.; Uhl, C.; Roche, C.; Duluc, I.; Guillermin, V.; Guillemot, F.; Jensen, J.; Kedinger, M.; Gradwohl, G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002, 21, 6338–6347. [Google Scholar] [CrossRef]
- Barton, J.R.; Londregan, A.K.; Alexander, T.D.; Entezari, A.A.; Covarrubias, M.; Waldman, S.A. Enteroendocrine cell regulation of the gut-brain axis. Front. Neurosci. 2023, 17, 1272955. [Google Scholar] [CrossRef]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef]
- Gierl, M.S.; Karoulias, N.; Wende, H.; Strehle, M.; Birchmeier, C. The zinc-finger factor Insm1 (IA-1) is essential for the development of pancreatic beta cells and intestinal endocrine cells. Genes. Dev. 2006, 20, 2465–2478. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, A.; Tarifeno-Saldivia, E.; Pirson, J.; Reuter, A.S.; Flasse, L.; Manfroid, I.; Voz, M.L.; Peers, B. Pancreatic and intestinal endocrine cells in zebrafish share common transcriptomic signatures and regulatory programmes. BMC Biol. 2020, 18, 109. [Google Scholar] [CrossRef]
- Ye, L.; Mueller, O.; Bagwell, J.; Bagnat, M.; Liddle, R.A.; Rawls, J.F. High fat diet induces microbiota-dependent silencing of enteroendocrine cells. eLife 2019, 8, e48479. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D.; Drakontides, A.B.; Ross, L.L. Serotonin: Synthesis and Release from the Myenteric Plexus of the Mouse Intestine. Science 1965, 149, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Sikander, A.; Rana, S.V.; Prasad, K.K. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin. Chim. Acta 2009, 403, 47–55. [Google Scholar] [CrossRef]
- Ye, L.; Bae, M.; Cassilly, C.D.; Jabba, S.V.; Thorpe, D.W.; Martin, A.M.; Lu, H.Y.; Wang, J.; Thompson, J.D.; Lickwar, C.R.; et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe 2021, 29, 179–196.e9. [Google Scholar] [CrossRef]
- Alsudayri, A.; Perelman, S.; Brewer, M.; Chura, A.; McDevitt, M.; Drerup, C.; Ye, L. Gut microbiota regulate maturation and mitochondrial function of the nutrient-sensing enteroendocrine cell. Development 2024, 151, dev202544. [Google Scholar] [CrossRef]
- Bohorquez, D.V.; Shahid, R.A.; Erdmann, A.; Kreger, A.M.; Wang, Y.; Calakos, N.; Wang, F.; Liddle, R.A. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Investig. 2015, 125, 782–786. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system: Normal functions and enteric neuropathies. Neurogastroenterol. Motil. 2008, 20 (Suppl. S1), 32–38. [Google Scholar] [CrossRef]
- Li, C.; Gehring, J.; Bronner, M.E. Spatiotemporal dynamics of the developing zebrafish enteric nervous system at the whole-organ level. Dev. Cell 2025, 60, 613–629.e6. [Google Scholar] [CrossRef]
- Shepherd, I.; Eisen, J. Development of the zebrafish enteric nervous system. Methods Cell Biol. 2011, 101, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, I.T.; Pietsch, J.; Elworthy, S.; Kelsh, R.N.; Raible, D.W. Roles for GFRalpha1 receptors in zebrafish enteric nervous system development. Development 2004, 131, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, T.; Nagashimada, M.; Yonemura, S.; Enomoto, H. Diminished Ret expression compromises neuronal survival in the colon and causes intestinal aganglionosis in mice. J. Clin. Investig. 2008, 118, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Roy-Carson, S.; Natukunda, K.; Chou, H.-c.; Pal, N.; Farris, C.; Schneider, S.Q.; Kuhlman, J.A. Defining the transcriptomic landscape of the developing enteric nervous system and its cellular environment. BMC Genomics 2017, 18, 290. [Google Scholar] [CrossRef]
- Reichenbach, B.; Delalande, J.M.; Kolmogorova, E.; Prier, A.; Nguyen, T.; Smith, C.M.; Holzschuh, J.; Shepherd, I.T. Endoderm-derived Sonic hedgehog and mesoderm Hand2 expression are required for enteric nervous system development in zebrafish. Dev. Biol. 2008, 318, 52–64. [Google Scholar] [CrossRef]
- Yu, H.H.; Moens, C.B. Semaphorin signaling guides cranial neural crest cell migration in zebrafish. Dev. Biol. 2005, 280, 373–385. [Google Scholar] [CrossRef]
- Uribe, R.A.; Bronner, M.E. Meis3 is required for neural crest invasion of the gut during zebrafish enteric nervous system development. Mol. Biol. Cell 2015, 26, 3728–3740. [Google Scholar] [CrossRef]
- Parichy, D.M.; Mellgren, E.M.; Rawls, J.F.; Lopes, S.S.; Kelsh, R.N.; Johnson, S.L. Mutational analysis of endothelin receptor b1 (rose) during neural crest and pigment pattern development in the zebrafish Danio rerio. Dev. Biol. 2000, 227, 294–306. [Google Scholar] [CrossRef]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Furness, J.B.; Jones, C.; Nurgali, K.; Clerc, N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog. Neurobiol. 2004, 72, 143–164. [Google Scholar] [CrossRef]
- Kuil, L.E.; Kakiailatu, N.J.M.; Windster, J.D.; Bindels, E.; Zink, J.T.M.; van der Zee, G.; Hofstra, R.M.W.; Shepherd, I.T.; Melotte, V.; Alves, M.M. Unbiased characterization of the larval zebrafish enteric nervous system at a single cell transcriptomic level. iScience 2023, 26, 107070. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.; Holmberg, A.; Holmgren, S. Development of enteric and vagal innervation of the zebrafish (Danio rerio) gut. J. Comp. Neurol. 2008, 508, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Uyttebroek, L.; Shepherd, I.T.; Harrisson, F.; Hubens, G.; Blust, R.; Timmermans, J.P.; Van Nassauw, L. Neurochemical coding of enteric neurons in adult and embryonic zebrafish (Danio rerio). J. Comp. Neurol. 2010, 518, 4419–4438. [Google Scholar] [CrossRef]
- Popowycz, N.; Uyttebroek, L.; Hubens, G.; Van Nassauw, L. Differentiation and Subtype Specification of Enteric Neurons: Current Knowledge of Transcription Factors, Signaling Molecules and Signaling Pathways Involved. J. Cell. Signal. 2022, 3, 14–27. [Google Scholar]
- Olden, T.; Akhtar, T.; Beckman, S.A.; Wallace, K.N. Differentiation of the zebrafish enteric nervous system and intestinal smooth muscle. Genesis 2008, 46, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.; Wabbersen, T.; Shepherd, I.T. In vivo visualization of the development of the enteric nervous system using a Tg(-8.3bphox2b:Kaede) transgenic zebrafish. Genesis 2014, 52, 985–990. [Google Scholar] [CrossRef]
- Howard, A.G.A.I.V.; Baker, P.A.; Ibarra-García-Padilla, R.; Moore, J.A.; Rivas, L.J.; Tallman, J.J.; Singleton, E.W.; Westheimer, J.L.; Corteguera, J.A.; Uribe, R.A. An atlas of neural crest lineages along the posterior developing zebrafish at single-cell resolution. eLife 2021, 10, e60005. [Google Scholar] [CrossRef]
- Seiler, C.; Abrams, J.; Pack, M. Characterization of zebrafish intestinal smooth muscle development using a novel sm22alpha-b promoter. Dev. Dyn. 2010, 239, 2806–2812. [Google Scholar] [CrossRef]
- Ticho, A.L.; Malhotra, P.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Bile acid receptors and gastrointestinal functions. Liver Res. 2019, 3, 31–39. [Google Scholar] [CrossRef]
- Okamoto, S.I.; Hatta, K. Ca2+-imaging and photo-manipulation of the simple gut of zebrafish larvae in vivo. Sci. Rep. 2022, 12, 2018. [Google Scholar] [CrossRef]
- Hirst, G.D. An additional role for ICC in the control of gastrointestinal motility? J. Physiol. 2001, 537, 1. [Google Scholar] [CrossRef]
- Rich, A.; Leddon, S.A.; Hess, S.L.; Gibbons, S.J.; Miller, S.; Xu, X.; Farrugia, G. Kit-like immunoreactivity in the zebrafish gastrointestinal tract reveals putative ICC. Dev. Dyn. 2007, 236, 903–911. [Google Scholar] [CrossRef]
- Ball, E.R.; Matsuda, M.M.; Dye, L.; Hoffmann, V.; Zerfas, P.M.; Szarek, E.; Rich, A.; Chitnis, A.B.; Stratakis, C.A. Ultra-structural identification of interstitial cells of Cajal in the zebrafish Danio rerio. Cell Tissue Res. 2012, 349, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Ganz, J.; Baker, R.P.; Hamilton, M.K.; Melancon, E.; Diba, P.; Eisen, J.S.; Parthasarathy, R. Image velocimetry and spectral analysis enable quantitative characterization of larval zebrafish gut motility. Neurogastroenterol. Motil. 2018, 30, e13351. [Google Scholar] [CrossRef]
- Rich, A.; Gordon, S.; Brown, C.; Gibbons, S.J.; Schaefer, K.; Hennig, G.; Farrugia, G. Kit signaling is required for development of coordinated motility patterns in zebrafish gastrointestinal tract. Zebrafish 2013, 10, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Wee, C.L.; Song, E.Y.; Johnson, R.E.; Ailani, D.; Randlett, O.; Kim, J.Y.; Nikitchenko, M.; Bahl, A.; Yang, C.T.; Ahrens, M.B.; et al. A bidirectional network for appetite control in larval zebrafish. eLife 2019, 8, e43775. [Google Scholar] [CrossRef] [PubMed]
- Wilbrink, J.; Masclee, G.; Klaassen, T.; van Avesaat, M.; Keszthelyi, D.; Masclee, A. Review on the Regional Effects of Gastrointestinal Luminal Stimulation on Appetite and Energy Intake: (Pre)clinical Observations. Nutrients 2021, 13, 1601. [Google Scholar] [CrossRef]
- Gershon, M.D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 14–21. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohorquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361, eaat5236. [Google Scholar] [CrossRef] [PubMed]
- Florie, M.; Pilz, W.; Dijkman, R.H.; Kremer, B.; Wiersma, A.; Winkens, B.; Baijens, L.W.J. The Effect of Cranial Nerve Stimulation on Swallowing: A Systematic Review. Dysphagia 2021, 36, 216–230. [Google Scholar] [CrossRef]
- Browning, K.N.; Verheijden, S.; Boeckxstaens, G.E. The Vagus Nerve in Appetite Regulation, Mood, and Intestinal Inflammation. Gastroenterology 2017, 152, 730–744. [Google Scholar] [CrossRef]
- Crucke, J.; Van de Kelft, A.; Huysseune, A. The innervation of the zebrafish pharyngeal jaws and teeth. J. Anat. 2015, 227, 62–71. [Google Scholar] [CrossRef]
- Pitts, T.; Iceman, K.E. Deglutition and the Regulation of the Swallow Motor Pattern. Physiology 2023, 38, 10–24. [Google Scholar] [CrossRef]
- Hernandez, L.P.; Patterson, S.E.; Devoto, S.H. The development of muscle fiber type identity in zebrafish cranial muscles. Anat. Embryol. 2005, 209, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Day, S.W.; Higham, T.E.; Holzman, R.; Van Wassenbergh, S. Morphology, Kinematics, and Dynamics: The Mechanics of Suction Feeding in Fishes. Integr. Comp. Biol. 2015, 55, 21–35. [Google Scholar] [CrossRef]
- Schilling, T.F.; Kimmel, C.B. Musculoskeletal patterning in the pharyngeal segments of the zebrafish embryo. Development 1997, 124, 2945–2960. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.M.; Miller, A.J. Sensory input pathways and mechanisms in swallowing: A review. Dysphagia 2010, 25, 323–333. [Google Scholar] [CrossRef]
- Goyal, R.K.; Chaudhury, A. Physiology of normal esophageal motility. J. Clin. Gastroenterol. 2008, 42, 610–619. [Google Scholar] [CrossRef]
- Kuo, B.; Urma, D. Esophagus—Anatomy and development. GI Motil. Online 2006. [Google Scholar] [CrossRef]
- Burnstock, G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J. Physiol. 1981, 313, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Rich, A. Improved Imaging of Zebrafish Motility. Neurogastroenterol. Motil. 2018, 30, e13435. [Google Scholar] [CrossRef] [PubMed]
- Wiles, T.J.; Jemielita, M.; Baker, R.P.; Schlomann, B.H.; Logan, S.L.; Ganz, J.; Melancon, E.; Eisen, J.S.; Guillemin, K.; Parthasarathy, R. Host Gut Motility Promotes Competitive Exclusion within a Model Intestinal Microbiota. PLoS Biol. 2016, 14, e1002517. [Google Scholar] [CrossRef]
- Hamilton, M.K.; Wall, E.S.; Robinson, C.D.; Guillemin, K.; Eisen, J.S. Enteric nervous system modulation of luminal pH modifies the microbial environment to promote intestinal health. PLoS Pathog. 2022, 18, e1009989. [Google Scholar] [CrossRef]
- Lickwar, C.R.; Camp, J.G.; Weiser, M.; Cocchiaro, J.L.; Kingsley, D.M.; Furey, T.S.; Sheikh, S.Z.; Rawls, J.F. Genomic dissection of conserved transcriptional regulation in intestinal epithelial cells. PLoS Biol. 2017, 15, e2002054. [Google Scholar] [CrossRef]
- Hui, J.C.M.; Du, P.; Webb, S.E.; Liu, J.Y.H.; Ngan, M.P.; Lu, Z.; Ng, H.S.H.; Yang, L.; Khalid, A.; Liu, L.; et al. Imaging analytical technique to assess gastrointestinal motility in vivo using zebrafish larvae with diabetes mellitus-like traits. PLoS ONE 2024, 19, e0314515. [Google Scholar] [CrossRef]
- Ward, S.M.; Beckett, A.H.; Wang, X.; Baker, F.; Khoyi, M.; Sanders, K.M. Interstitial Cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J. Neurosci. 2000, 20, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Nakajo, K.; Egashira, Y.; Yamamoto, Y.; Kitada, K.; Taniguchi, K.; Kawai, M.; Tomiyama, H.; Kawakami, K.; Uchiyama, K.; et al. Gastrointestinal Neurons Expressing HCN4 Regulate Retrograde Peristalsis. Cell Rep. 2020, 33, 108314. [Google Scholar] [CrossRef]
- Wen, J.; Mercado, G.P.; Volland, A.; Doden, H.L.; Lickwar, C.R.; Crooks, T.; Kakiyama, G.; Kelly, C.; Cocchiaro, J.L.; Ridlon, J.M.; et al. Fxr signaling and microbial metabolism of bile salts in the zebrafish intestine. Sci. Adv. 2021, 7, eabg1371. [Google Scholar] [CrossRef] [PubMed]
- Ganz, J. Gut feelings: Studying enteric nervous system development, function, and disease in the zebrafish model system. Dev. Dyn. 2018, 247, 268–278. [Google Scholar] [CrossRef]
- Park, J.; Levic, D.S.; Sumigray, K.D.; Bagwell, J.; Eroglu, O.; Block, C.L.; Eroglu, C.; Barry, R.; Lickwar, C.R.; Rawls, J.F.; et al. Lysosome-Rich Enterocytes Mediate Protein Absorption in the Vertebrate Gut. Dev. Cell 2019, 51, 7–20.e6. [Google Scholar] [CrossRef] [PubMed]
- Childers, L.; Park, J.; Wang, S.; Liu, R.; Barry, R.; Watts, S.A.; Rawls, J.F.; Bagnat, M. Protein absorption in the zebrafish gut is regulated by interactions between lysosome rich enterocytes and the microbiome. eLife 2025, 13, RP100611. [Google Scholar] [CrossRef]
- Wachsmuth, H.R.; Weninger, S.N.; Duca, F.A. Role of the gut-brain axis in energy and glucose metabolism. Exp. Mol. Med. 2022, 54, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.; Holmgren, S. The control of gut motility. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 128, 481–503. [Google Scholar] [CrossRef]
- Field, H.A.; Kelley, K.A.; Martell, L.; Goldstein, A.M.; Serluca, F.C. Analysis of gastrointestinal physiology using a novel intestinal transit assay in zebrafish. Neurogastroenterol. Motil. 2009, 21, 304–312. [Google Scholar] [CrossRef]
- Cassar, S.; Huang, X.; Cole, T. High-throughput Measurement of Gut Transit Time Using Larval Zebrafish. J. Vis. Exp. 2018, 140, 58497. [Google Scholar] [CrossRef]
- Wei, Y.; Martin, S.C.; Heinrich, G.; Mojsov, S. Cloning and functional characterization of PACAP-specific receptors in zebrafish. Ann. N. Y. Acad. Sci. 1998, 865, 45–48. [Google Scholar] [CrossRef]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 2017, 170, 185–198.e16. [Google Scholar] [CrossRef]
- Tasnim, A.; Alkislar, I.; Hakim, R.; Turecek, J.; Abdelaziz, A.; Orefice, L.L.; Ginty, D.D. The developmental timing of spinal touch processing alterations predicts behavioral changes in genetic mouse models of autism spectrum disorders. Nat. Neurosci. 2024, 27, 484–496. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Faucherre, A.; Nargeot, J.; Mangoni, M.E.; Jopling, C. piezo2b regulates vertebrate light touch response. J. Neurosci. 2013, 33, 17089–17094. [Google Scholar] [CrossRef] [PubMed]
- Faucherre, A.; Kissa, K.; Nargeot, J.; Mangoni, M.E.; Jopling, C. Piezo1 plays a role in erythrocyte volume homeostasis. Haematologica 2014, 99, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Dobbelsteyn, C.; Peacocke, S.D.; Blake, K.; Crist, W.; Rashid, M. Feeding difficulties in children with CHARGE syndrome: Prevalence, risk factors, and prognosis. Dysphagia 2008, 23, 127–135. [Google Scholar] [CrossRef]
- Pagon, R.A.; Graham, J.M., Jr.; Zonana, J.; Yong, S.L. Coloboma, congenital heart disease, and choanal atresia with multiple anomalies: CHARGE association. J. Pediatr. 1981, 99, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Cloney, K.; Steele, S.L.; Stoyek, M.R.; Croll, R.P.; Smith, F.M.; Prykhozhij, S.V.; Brown, M.M.; Midgen, C.; Blake, K.; Berman, J.N. Etiology and functional validation of gastrointestinal motility dysfunction in a zebrafish model of CHARGE syndrome. FEBS J. 2018, 285, 2125–2140. [Google Scholar] [CrossRef]
- Patten, S.A.; Jacobs-McDaniels, N.L.; Zaouter, C.; Drapeau, P.; Albertson, R.C.; Moldovan, F. Role of Chd7 in zebrafish: A model for CHARGE syndrome. PLoS ONE 2012, 7, e31650. [Google Scholar] [CrossRef]
- Bernier, R.; Golzio, C.; Xiong, B.; Stessman, H.A.; Coe, B.P.; Penn, O.; Witherspoon, K.; Gerdts, J.; Baker, C.; Vulto-van Silfhout, A.T.; et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell 2014, 158, 263–276. [Google Scholar] [CrossRef]
- Batsukh, T.; Schulz, Y.; Wolf, S.; Rabe, T.I.; Oellerich, T.; Urlaub, H.; Schaefer, I.M.; Pauli, S. Identification and characterization of FAM124B as a novel component of a CHD7 and CHD8 containing complex. PLoS ONE 2012, 7, e52640. [Google Scholar] [CrossRef]
- Mohrle, D.; Fernandez, M.; Penagarikano, O.; Frick, A.; Allman, B.; Schmid, S. What we can learn from a genetic rodent model about autism. Neurosci. Biobehav. Rev. 2020, 109, 29–53. [Google Scholar] [CrossRef]
- Poliak, S.; Salomon, D.; Elhanany, H.; Sabanay, H.; Kiernan, B.; Pevny, L.; Stewart, C.L.; Xu, X.; Chiu, S.Y.; Shrager, P.; et al. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J. Cell Biol. 2003, 162, 1149–1160. [Google Scholar] [CrossRef]
- Belloso, J.M.; Bache, I.; Guitart, M.; Caballin, M.R.; Halgren, C.; Kirchhoff, M.; Ropers, H.H.; Tommerup, N.; Tumer, Z. Disruption of the CNTNAP2 gene in a t(7;15) translocation family without symptoms of Gilles de la Tourette syndrome. Eur. J. Hum. Genet. 2007, 15, 711–713. [Google Scholar] [CrossRef]
- The Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 2011, 43, 969–976. [Google Scholar] [CrossRef]
- Robinson, B.G.; Oster, B.A.; Robertson, K.; Kaltschmidt, J.A. Loss of ASD-related molecule Cntnap2 affects colonic motility in mice. Front. Neurosci. 2023, 17, 1287057. [Google Scholar] [CrossRef]
- Rea, V.; Van Raay, T.J. Using Zebrafish to Model Autism Spectrum Disorder: A Comparison of ASD Risk Genes Between Zebrafish and Their Mammalian Counterparts. Front. Mol. Neurosci. 2020, 13, 575575. [Google Scholar] [CrossRef]
- Hoffman, E.J.; Turner, K.J.; Fernandez, J.M.; Cifuentes, D.; Ghosh, M.; Ijaz, S.; Jain, R.A.; Kubo, F.; Bill, B.R.; Baier, H.; et al. Estrogens Suppress a Behavioral Phenotype in Zebrafish Mutants of the Autism Risk Gene, CNTNAP2. Neuron 2016, 89, 725–733. [Google Scholar] [CrossRef]
- Courraud, J.; Quartier, A.; Drouot, N.; Zapata-Bodalo, I.; Gilet, J.; Benchoua, A.; Mandel, J.L.; Piton, A. DYRK1A roles in human neural progenitors. Front. Neurosci. 2025, 19, 1533253. [Google Scholar] [CrossRef]
- Kim, O.H.; Cho, H.J.; Han, E.; Hong, T.I.; Ariyasiri, K.; Choi, J.H.; Hwang, K.S.; Jeong, Y.M.; Yang, S.Y.; Yu, K.; et al. Zebrafish knockout of Down syndrome gene, DYRK1A, shows social impairments relevant to autism. Mol. Autism 2017, 8, 50. [Google Scholar] [CrossRef]
- Palmesino, E.; Rousso, D.L.; Kao, T.J.; Klar, A.; Laufer, E.; Uemura, O.; Okamoto, H.; Novitch, B.G.; Kania, A. Foxp1 and lhx1 coordinate motor neuron migration with axon trajectory choice by gating Reelin signalling. PLoS Biol. 2010, 8, e1000446. [Google Scholar] [CrossRef]
- Braccioli, L.; Vervoort, S.J.; Adolfs, Y.; Heijnen, C.J.; Basak, O.; Pasterkamp, R.J.; Nijboer, C.H.; Coffer, P.J. FOXP1 Promotes Embryonic Neural Stem Cell Differentiation by Repressing Jagged1 Expression. Stem Cell Rep. 2017, 9, 1530–1545. [Google Scholar] [CrossRef]
- Frohlich, H.; Kollmeyer, M.L.; Linz, V.C.; Stuhlinger, M.; Groneberg, D.; Reigl, A.; Zizer, E.; Friebe, A.; Niesler, B.; Rappold, G. Gastrointestinal dysfunction in autism displayed by altered motility and achalasia in Foxp1(+/−) mice. Proc. Natl. Acad. Sci. USA 2019, 116, 22237–22245. [Google Scholar] [CrossRef]
- Amir, R.E.; Zoghbi, H.Y. Rett Syndrome: Methyl-CpG-binding protein 2 mutations and genotype-phenotype correlations. Am. J. Med. Genet. 2000, 97, 147–152. [Google Scholar] [CrossRef]
- Operto, F.F.; Mazza, R.; Pastorino, G.M.G.; Verrotti, A.; Coppola, G. Epilepsy and genetic in Rett syndrome: A review. Brain Behav. 2019, 9, e01250. [Google Scholar] [CrossRef]
- Hagberg, B.; Aicardi, J.; Dias, K.; Ramos, O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: Report of 35 cases. Ann. Neurol. 1983, 14, 471–479. [Google Scholar] [CrossRef] [PubMed]
- May, D.M.; Neul, J.; Pina-Garza, J.E.; Kponee-Shovein, K.; Satija, A.; Mahendran, M.; Downes, N.; Sheng, K.; Lema, N.; Boca, A.; et al. Gastrointestinal manifestations in pediatric and adult patients with Rett syndrome: An analysis of US claims and physician survey data. J. Comp. Eff. Res. 2024, 13, e230054. [Google Scholar] [CrossRef] [PubMed]
- Wahba, G.; Schock, S.C.; Cudd, S.; Grynspan, D.; Humphreys, P.; Staines, W.A. Activity and MeCP2-dependent regulation of nNOS levels in enteric neurons. Neurogastroenterol. Motil. 2016, 28, 1723–1730. [Google Scholar] [CrossRef]
- Millar-Buchner, P.; Philp, A.R.; Gutierrez, N.; Villanueva, S.; Kerr, B.; Flores, C.A. Severe changes in colon epithelium in the Mecp2-null mouse model of Rett syndrome. Mol. Cell Pediatr. 2016, 3, 37. [Google Scholar] [CrossRef]
- van der Vaart, M.; Svoboda, O.; Weijts, B.G.; Espin-Palazon, R.; Sapp, V.; Pietri, T.; Bagnat, M.; Muotri, A.R.; Traver, D. Mecp2 regulates tnfa during zebrafish embryonic development and acute inflammation. Dis. Model. Mech. 2017, 10, 1439–1451. [Google Scholar] [CrossRef]
- Hosie, S.; Ellis, M.; Swaminathan, M.; Ramalhosa, F.; Seger, G.O.; Balasuriya, G.K.; Gillberg, C.; Rastam, M.; Churilov, L.; McKeown, S.J.; et al. Gastrointestinal dysfunction in patients and mice expressing the autism-associated R451C mutation in neuroligin-3. Autism Res. 2019, 12, 1043–1056. [Google Scholar] [CrossRef]
- Herath, M.; Bornstein, J.C.; Hill-Yardin, E.L.; Franks, A.E. Mice expressing the autism-associated neuroligin-3 R451C variant exhibit increased mucus density and altered distributions of intestinal microbiota. ISME J. 2025, 19, wraf037. [Google Scholar] [CrossRef]
- Herath, M.; Cho, E.; Marklund, U.; Franks, A.E.; Bornstein, J.C.; Hill-Yardin, E.L. Quantitative Spatial Analysis of Neuroligin-3 mRNA Expression in the Enteric Nervous System Reveals a Potential Role in Neuronal-Glial Synapses and Reduced Expression in Nlgn3(R451C) Mice. Biomolecules 2023, 13, 1063. [Google Scholar] [CrossRef]
- Phelan, K.; McDermid, H.E. The 22q13.3 Deletion Syndrome (Phelan-McDermid Syndrome). Mol. Syndromol. 2012, 2, 186–201. [Google Scholar] [CrossRef]
- Betancur, C.; Buxbaum, J.D. SHANK3 haploinsufficiency: A “common” but underdiagnosed highly penetrant monogenic cause of autism spectrum disorders. Mol. Autism 2013, 4, 17. [Google Scholar] [CrossRef]
- Bockers, T.M.; Mameza, M.G.; Kreutz, M.R.; Bockmann, J.; Weise, C.; Buck, F.; Richter, D.; Gundelfinger, E.D.; Kreienkamp, H.J. Synaptic scaffolding proteins in rat brain. Ankyrin repeats of the multidomain Shank protein family interact with the cytoskeletal protein alpha-fodrin. J. Biol. Chem. 2001, 276, 40104–40112. [Google Scholar] [CrossRef]
- James, D.M.; Davidson, E.A.; Yanes, J.; Moshiree, B.; Dallman, J.E. The Gut-Brain-Microbiome Axis and Its Link to Autism: Emerging Insights and the Potential of Zebrafish Models. Front. Cell Dev. Biol. 2021, 9, 662916. [Google Scholar] [CrossRef]
- Asta, L.; Ricciardello, A.; Cucinotta, F.; Turriziani, L.; Boncoddo, M.; Bellomo, F.; Angelini, J.; Gnazzo, M.; Scandolo, G.; Pisano, G.; et al. Clinical, developmental and serotonemia phenotyping of a sample of 70 Italian patients with Phelan-McDermid Syndrome. J. Neurodev. Disord. 2024, 16, 57. [Google Scholar] [CrossRef]
- Matuleviciene, A.; Siauryte, K.; Kuiper, E.; Grabrucker, A.M.; European Phelan-McDermid syndrome guideline, c. Consensus recommendations on chewing, swallowing and gastrointestinal problems in Phelan-McDermid syndrome. Eur. J. Med. Genet. 2023, 66, 104763. [Google Scholar] [CrossRef]
- Pfaender, S.; Sauer, A.K.; Hagmeyer, S.; Mangus, K.; Linta, L.; Liebau, S.; Bockmann, J.; Huguet, G.; Bourgeron, T.; Boeckers, T.M.; et al. Zinc deficiency and low enterocyte zinc transporter expression in human patients with autism related mutations in SHANK3. Sci. Rep. 2017, 7, 45190. [Google Scholar] [CrossRef]
- Eberly, G.L.; Manthey, M.; Pang, K.K.L.; Hussein, H.; Vargas Paniagua, E.; Machen, S.; Klingensmith, S.M.; Anikeeva, P. Shank3 mutation manifests in abnormal gastrointestinal morphology and function in mice. Front. Neurosci. 2025, 19, 1552369. [Google Scholar] [CrossRef]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattoli, M. Mechanisms underlying microbial-mediated changes in social behavior in mouse models ofAutism Spectrum Disorder. Neuron 2019, 101, 246–259. [Google Scholar] [CrossRef]
- Zhang, L.; Bang, S.; He, Q.; Matsuda, M.; Luo, X.; Jiang, Y.-H.; Ji, R.-R. SHANK3 in vagal sensory neurons regulates body temperature, systemic inflammation, and sepsis. Front. Immunol. 2023, 14, 1124356. [Google Scholar] [CrossRef]
- Watkins, A.; Bissell, S.; Moss, J.; Oliver, C.; Clayton-Smith, J.; Haye, L.; Heald, M.; Welham, A. Behavioural and psychological characteristics in Pitt-Hopkins syndrome: A comparison with Angelman and Cornelia de Lange syndromes. J. Neurodev. Disord. 2019, 11, 24. [Google Scholar] [CrossRef]
- Van Balkom, I.D.; Vuijk, P.J.; Franssens, M.; Hoek, H.W.; Hennekam, R.C. Development, cognition, and behaviour in Pitt-Hopkins syndrome. Dev. Med. Child. Neurol. 2012, 54, 925–931. [Google Scholar] [CrossRef]
- Amiel, J.; Rio, M.; de Pontual, L.; Redon, R.; Malan, V.; Boddaert, N.; Plouin, P.; Carter, N.P.; Lyonnet, S.; Munnich, A.; et al. Mutations in TCF4, encoding a class I basic helix-loop-helix transcription factor, are responsible for Pitt-Hopkins syndrome, a severe epileptic encephalopathy associated with autonomic dysfunction. Am. J. Hum. Genet. 2007, 80, 988–993. [Google Scholar] [CrossRef]
- Zollino, M.; Zweier, C.; Van Balkom, I.D.; Sweetser, D.A.; Alaimo, J.; Bijlsma, E.K.; Cody, J.; Elsea, S.H.; Giurgea, I.; Macchiaiolo, M.; et al. Diagnosis and management in Pitt-Hopkins syndrome: First international consensus statement. Clin. Genet. 2019, 95, 462–478. [Google Scholar] [CrossRef]
- de Winter, C.F.; Baas, M.; Bijlsma, E.K.; van Heukelingen, J.; Routledge, S.; Hennekam, R.C. Phenotype and natural history in 101 individuals with Pitt-Hopkins syndrome through an internet questionnaire system. Orphanet J. Rare Dis. 2016, 11, 37. [Google Scholar] [CrossRef]
- Grubisic, V.; Kennedy, A.J.; Sweatt, J.D.; Parpura, V. Pitt-Hopkins Mouse Model has Altered Particular Gastrointestinal Transits In Vivo. Autism Res. 2015, 8, 629–633. [Google Scholar] [CrossRef]
- Muncan, V.; Faro, A.; Haramis, A.P.; Hurlstone, A.F.; Wienholds, E.; van Es, J.; Korving, J.; Begthel, H.; Zivkovic, D.; Clevers, H. T-cell factor 4 (Tcf7l2) maintains proliferative compartments in zebrafish intestine. EMBO Rep. 2007, 8, 966–973. [Google Scholar] [CrossRef]
- Simons Vip, C. Simons Variation in Individuals Project (Simons VIP): A genetics-first approach to studying autism spectrum and related neurodevelopmental disorders. Neuron 2012, 73, 1063–1067. [Google Scholar] [CrossRef]
- Jordi, J.; Guggiana-Nilo, D.; Soucy, E.; Song, E.Y.; Lei Wee, C.; Engert, F. A high-throughput assay for quantifying appetite and digestive dynamics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R345–R357. [Google Scholar] [CrossRef]
- Rihel, J.; Prober, D.A.; Arvanites, A.; Lam, K.; Zimmerman, S.; Jang, S.; Haggarty, S.J.; Kokel, D.; Rubin, L.L.; Peterson, R.T.; et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science 2010, 327, 348–351. [Google Scholar] [CrossRef]
- Ijaz, S.; Hoffman, E.J. Zebrafish: A Translational Model System for Studying Neuropsychiatric Disorders. J. Am. Acad. Child. Adolesc. Psychiatry 2016, 55, 746–748. [Google Scholar] [CrossRef]
- Naumann, E.A.; Fitzgerald, J.E.; Dunn, T.W.; Rihel, J.; Sompolinsky, H.; Engert, F. From Whole-Brain Data to Functional Circuit Models: The Zebrafish Optomotor Response. Cell 2016, 167, 947–960.e920. [Google Scholar] [CrossRef]
- Irons, T.D.; Kelly, P.E.; Hunter, D.L.; Macphail, R.C.; Padilla, S. Acute administration of dopaminergic drugs has differential effects on locomotion in larval zebrafish. Pharmacol. Biochem. Behav. 2013, 103, 792–813. [Google Scholar] [CrossRef]
- Rosa, J.G.S.; Lima, C.; Lopes-Ferreira, M. Zebrafish Larvae Behavior Models as a Tool for Drug Screenings and Pre-Clinical Trials: A Review. Int. J. Mol. Sci. 2022, 23, 6647. [Google Scholar] [CrossRef]
- Filosa, A.; Barker, A.J.; Dal Maschio, M.; Baier, H. Feeding State Modulates Behavioral Choice and Processing of Prey Stimuli in the Zebrafish Tectum. Neuron 2016, 90, 596–608. [Google Scholar] [CrossRef]
- Pardo-Martin, C.; Chang, T.Y.; Koo, B.K.; Gilleland, C.L.; Wasserman, S.C.; Yanik, M.F. High-throughput in vivo vertebrate screening. Nat. Methods 2010, 7, 634–636. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, S.Y.; Zhang, Y.; Li, C.Q. Human prokinetic drugs promote gastrointestinal motility in zebrafish. Neurogastroenterol. Motil. 2014, 26, 589–595. [Google Scholar] [CrossRef]
- Barone, J.A. Domperidone: A peripherally acting dopamine2-receptor antagonist. Ann. Pharmacother. 1999, 33, 429–440. [Google Scholar] [CrossRef]
- Coomer, C.E.; Naumova, D.; Talay, M.; Zolyomi, B.; Snell, N.J.; Sorkac, A.; Chanchu, J.M.; Cheng, J.; Roman, I.; Li, J.; et al. Transsynaptic labeling and transcriptional control of zebrafish neural circuits. Nat. Neurosci. 2025, 28, 189–200. [Google Scholar] [CrossRef]
- Farnsworth, D.R.; Saunders, L.M.; Miller, A.C. A single-cell transcriptome atlaas for zebrafish development. Dev. Biol. 2020, 459, 100–108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, M.E.; Garcia-Pradas, L.; Thom, S.A.; Vazquez, R.A.; Dallman, J.E. Going with the Flow: Sensorimotor Integration Along the Zebrafish GI Tract. Cells 2025, 14, 1170. https://doi.org/10.3390/cells14151170
Rogers ME, Garcia-Pradas L, Thom SA, Vazquez RA, Dallman JE. Going with the Flow: Sensorimotor Integration Along the Zebrafish GI Tract. Cells. 2025; 14(15):1170. https://doi.org/10.3390/cells14151170
Chicago/Turabian StyleRogers, Millie E., Lidia Garcia-Pradas, Simone A. Thom, Roberto A. Vazquez, and Julia E. Dallman. 2025. "Going with the Flow: Sensorimotor Integration Along the Zebrafish GI Tract" Cells 14, no. 15: 1170. https://doi.org/10.3390/cells14151170
APA StyleRogers, M. E., Garcia-Pradas, L., Thom, S. A., Vazquez, R. A., & Dallman, J. E. (2025). Going with the Flow: Sensorimotor Integration Along the Zebrafish GI Tract. Cells, 14(15), 1170. https://doi.org/10.3390/cells14151170






