Impact of the Use of 2-Phospho-L Ascorbic Acid in the Production of Engineered Stromal Tissue for Regenerative Medicine
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
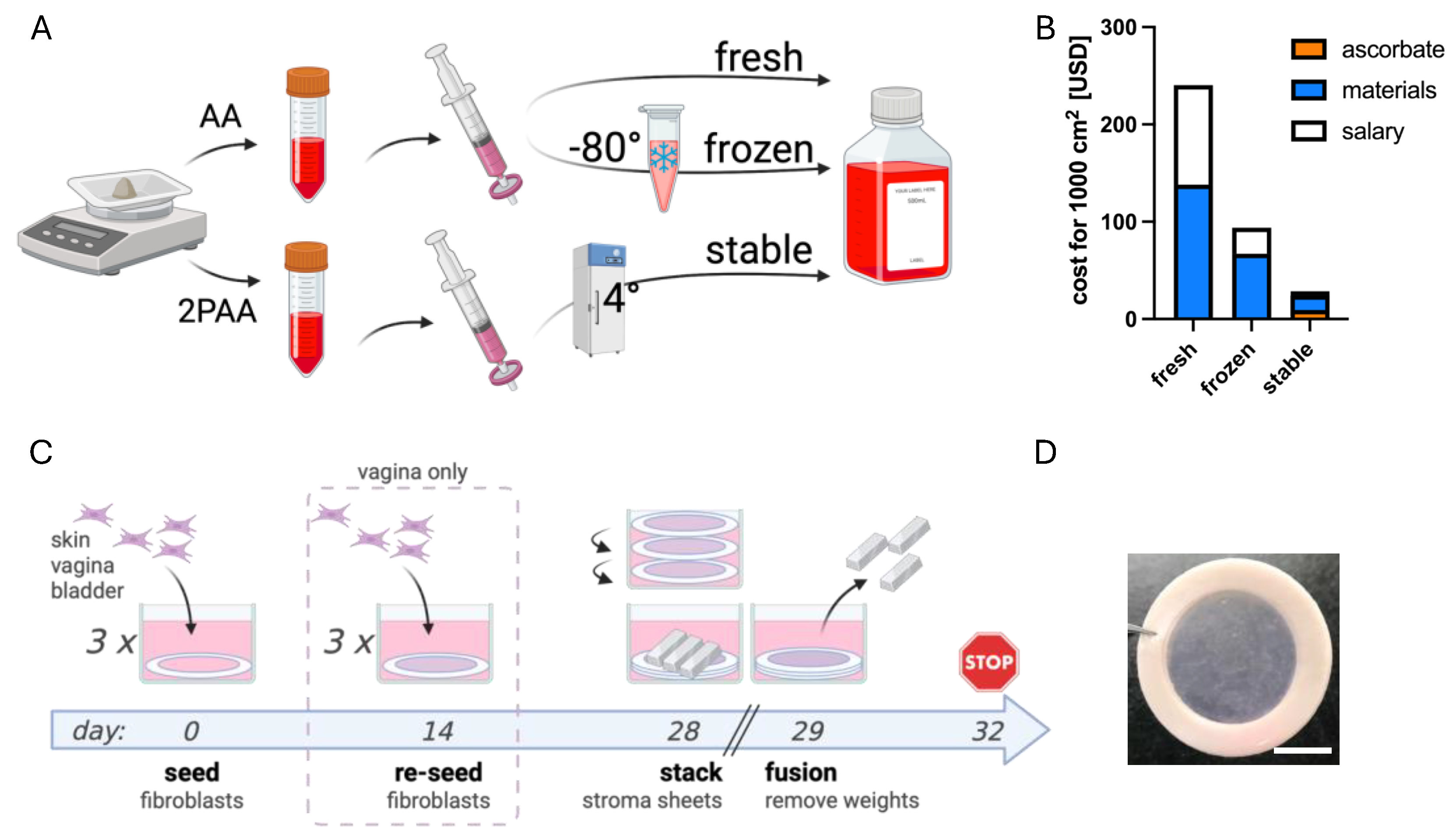
2.2. Preparation of AA and 2PAA Stock Solutions
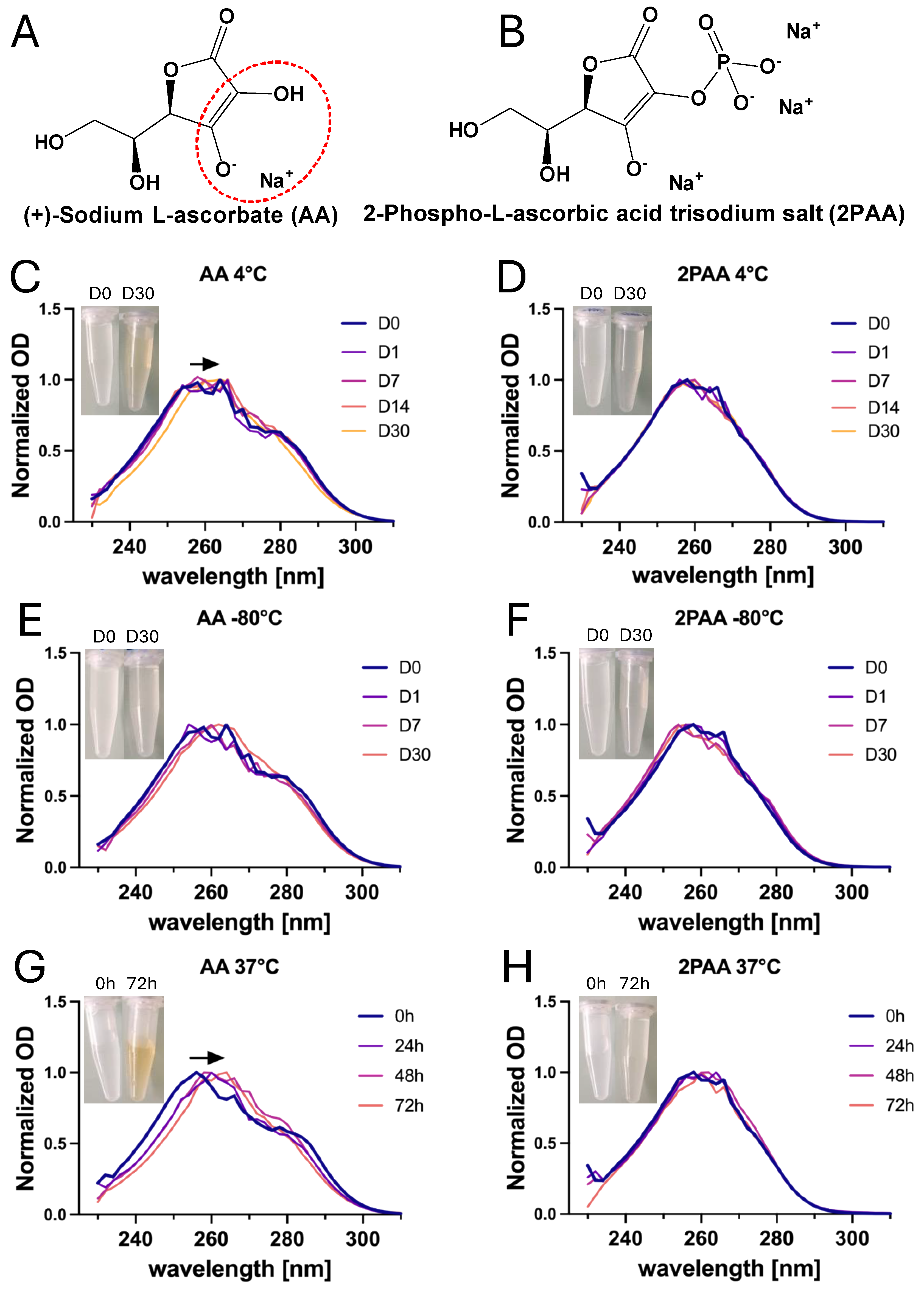
2.3. Direct Spectrophotometric Absorbance Measurement of AA and 2PAA
2.4. Cell Culture
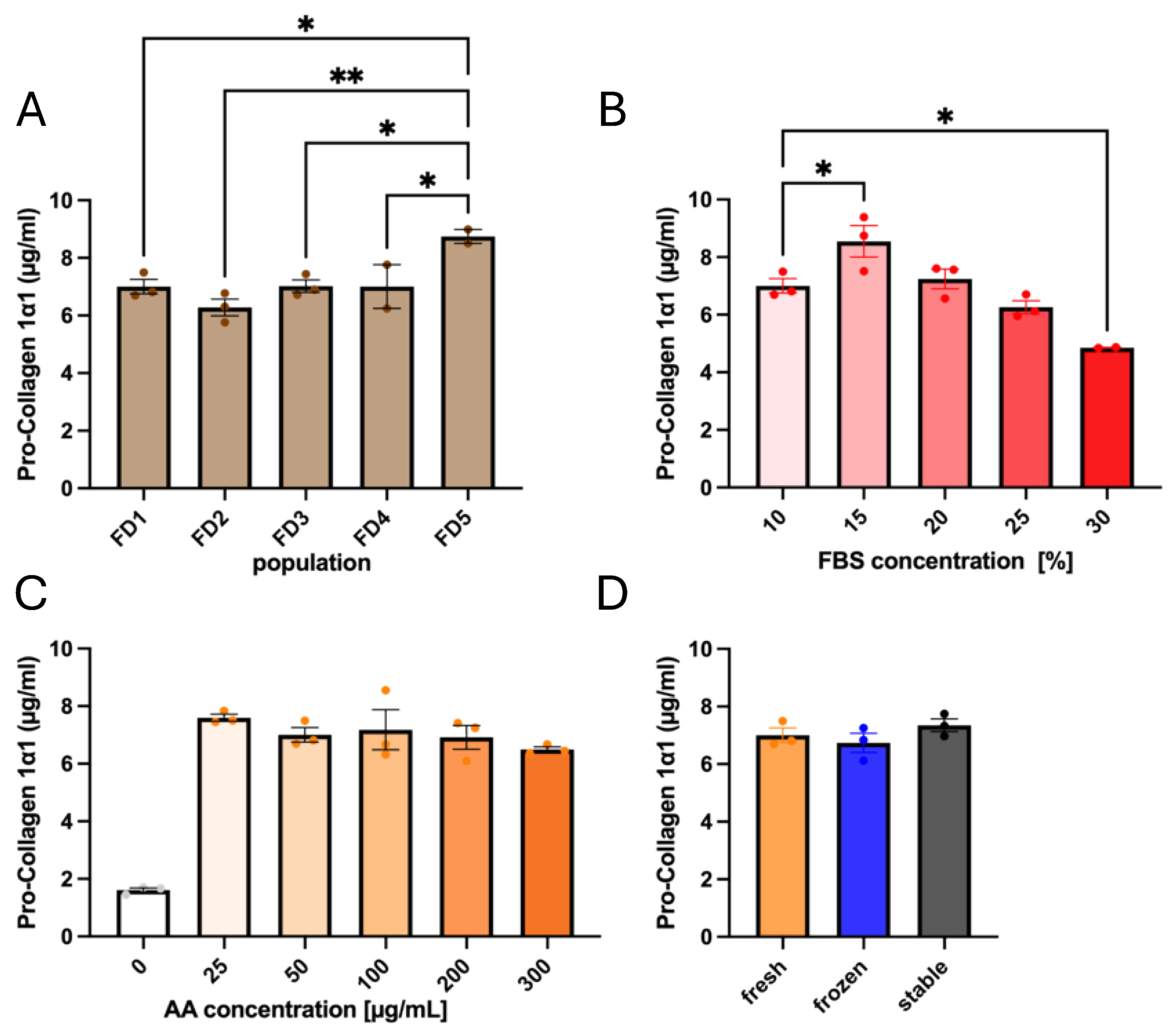
2.5. Quantification of Pro-Collagen 1α1 Secretion
2.6. Reconstruction of Stroma by Self-Assembly
2.7. Quantification of Collagen Secretion
2.8. Matrix Metalloproteinase (MMP) Activity Quantification
2.9. Quantification of Total Protein and Collagen Content
2.10. Total DNA Quantification
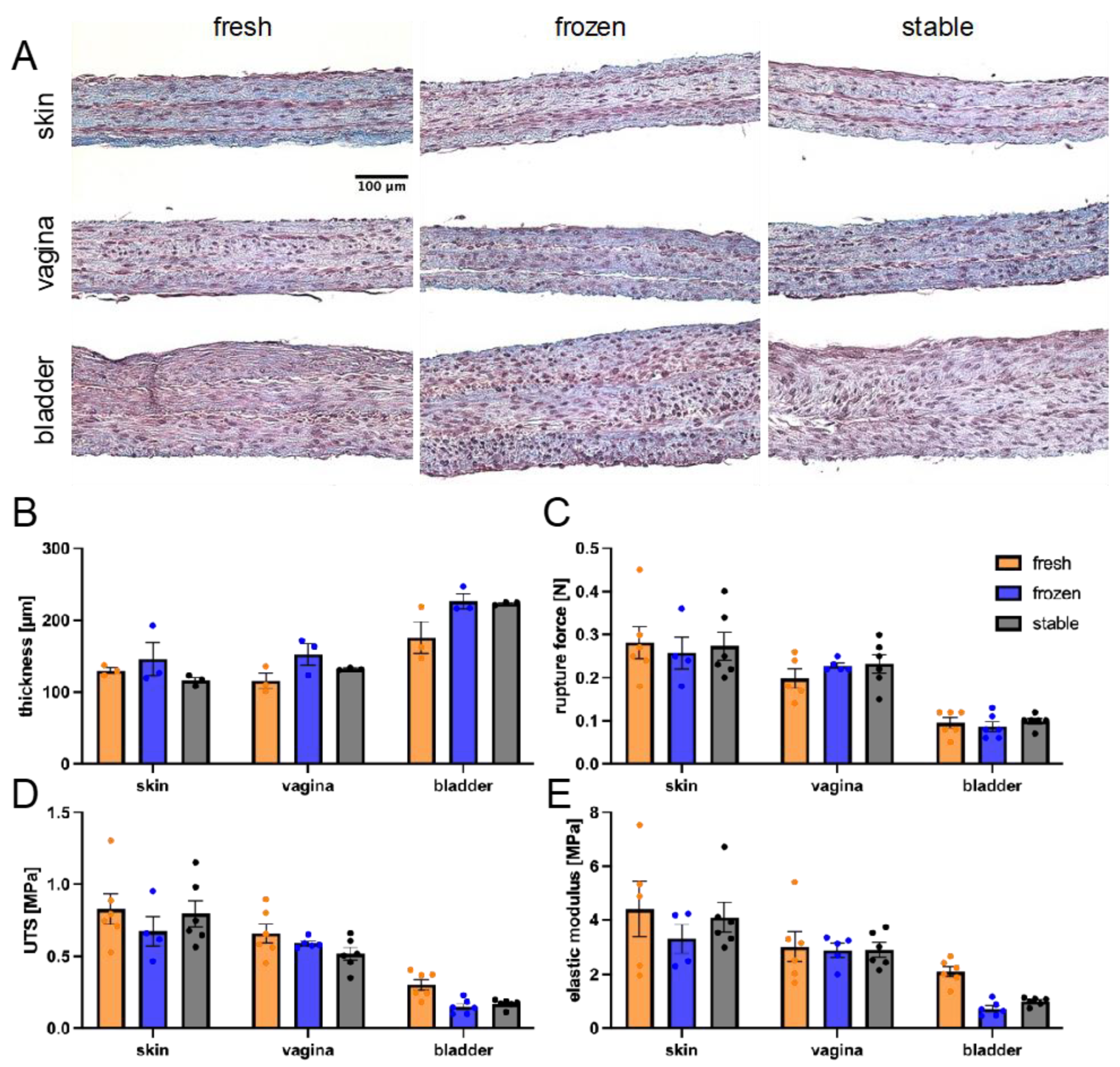
2.11. Histological Analysis
2.12. Tissue-Scale Mechanical Properties
2.13. Cell-Scale Mechanical Properties
2.14. Quantitative Polarized Light Microscopy
2.15. Statistical Analyses
3. Results
3.1. Influences of Donor Origin and Supplements on Pro-Collagen 1α1 Secretion
3.2. Ascorbate Stability in Both Storage and Culture Conditions
3.3. Impact of AA and 2PAA on Engineered Stroma Composition
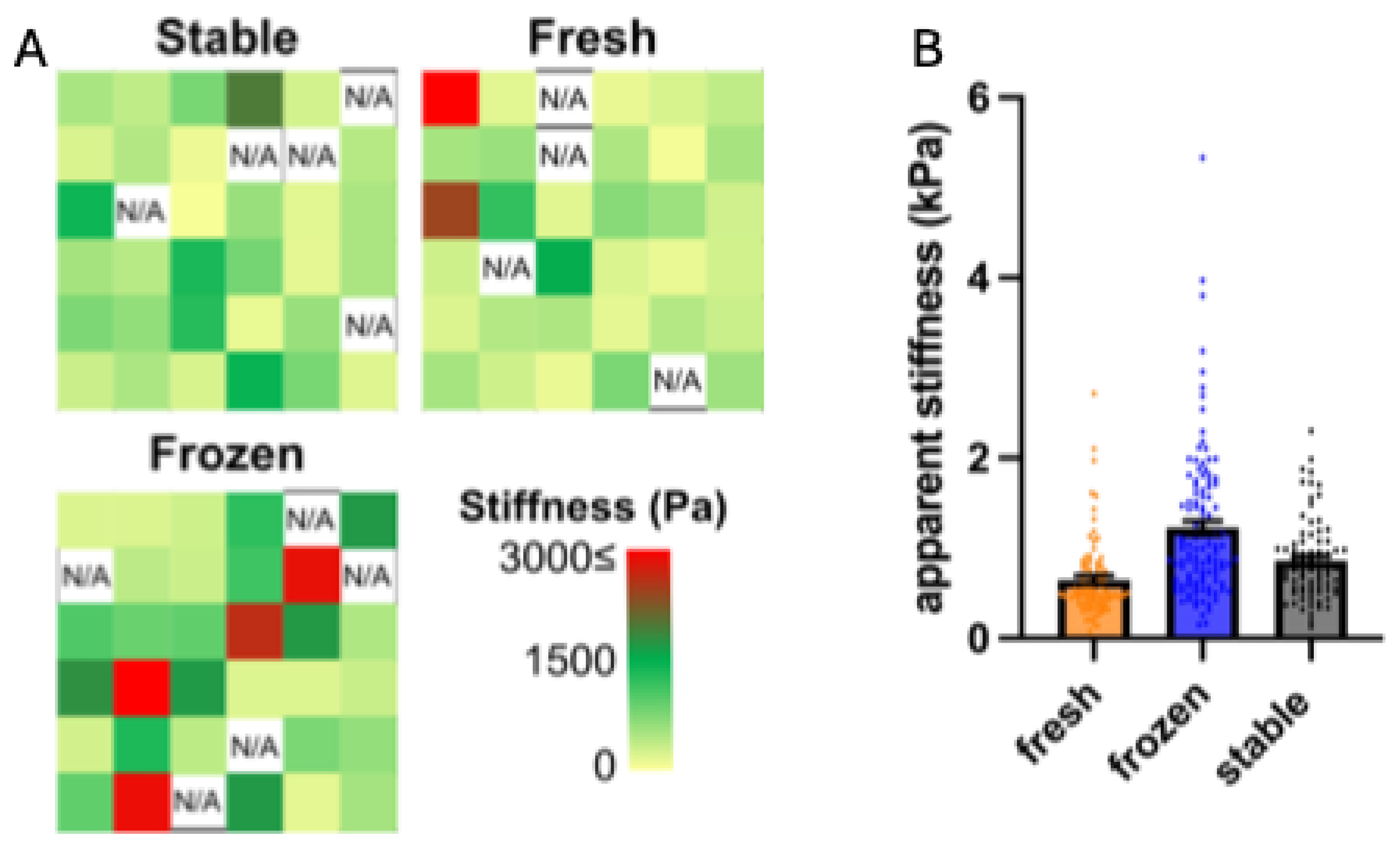
3.4. Impact of AA and 2PAA on the Physical Properties of Engineered Stroma
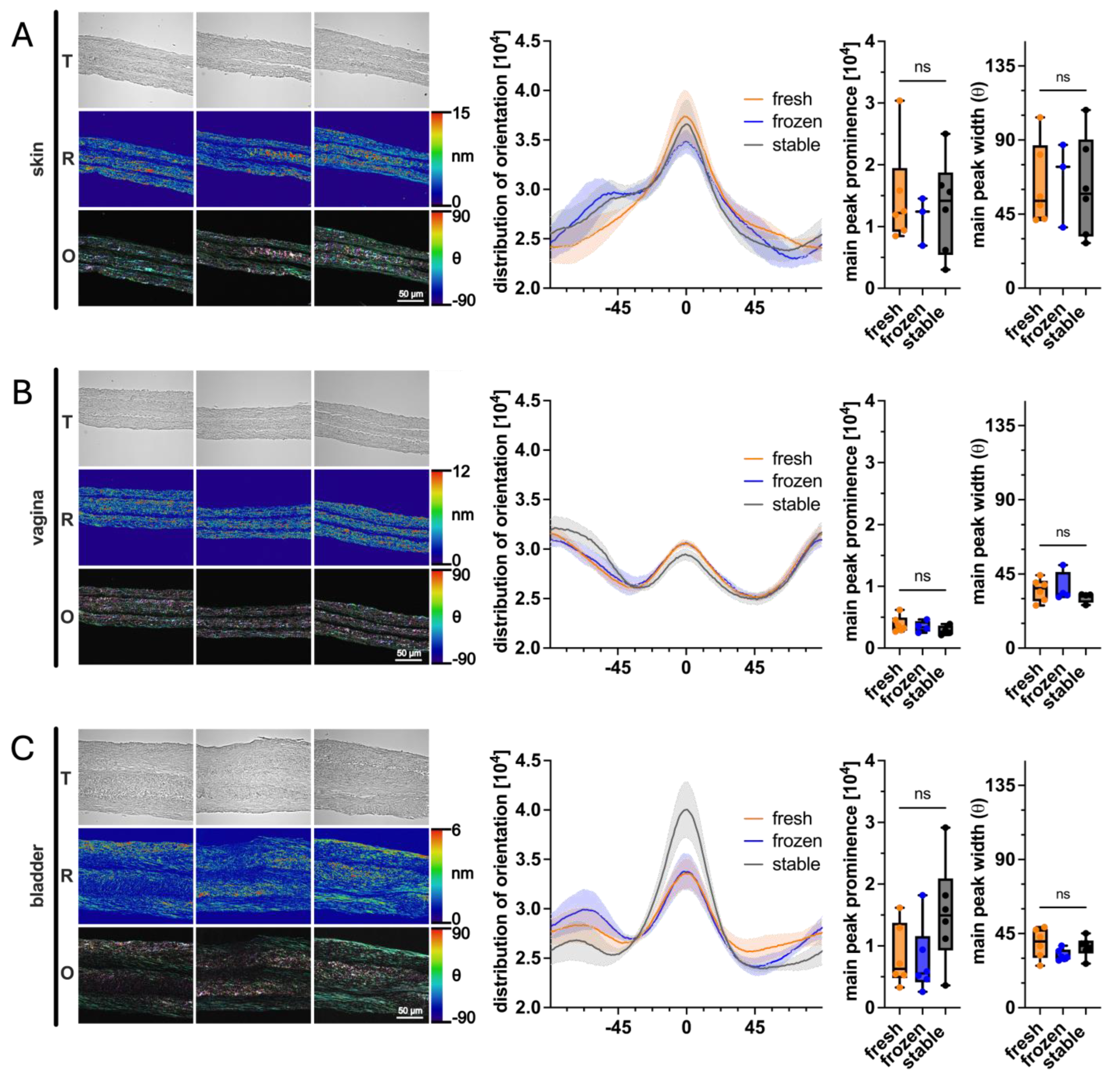
3.5. Impact of AA and 2PAA on Collagen Architecture of Engineered Stroma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galvani-Townsend, S.; Martinez, I.; Pandey, A. Is life expectancy higher in countries and territories with publicly funded health care? Global analysis of health care access and the social determinants of health. J. Glob. Health 2022, 12, 04091. [Google Scholar] [CrossRef]
- Park, J.H.; Moon, J.H.; Kim, H.J.; Kong, M.H.; Oh, Y.H. Sedentary Lifestyle: Overview of Updated Evidence of Potential Health Risks. Korean J. Fam. Med. 2020, 41, 365–373. [Google Scholar] [CrossRef]
- Cuende, N.; Cuende, J.I.; Fajardo, J.; Huet, J.; Alonso, M. Effect of Population Aging on the International Organ Donation Rates and the Effectiveness of the Donation Process. Am. J. Transplant. 2007, 7, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Koukoura, A.; Tsianos, G.-I.; Gargavanis, A.A.; Nielsen, A.A.; Vassiliadis, E. Organ donation in the US and Europe: The supply vs demand imbalance. Transplant. Rev. 2021, 35, 100585. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, C.A. The history of tissue engineering. J. Cell. Mol. Med. 2006, 10, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, T.; Khademhosseini, A.; Langer, R. Chasing the Paradigm: Clinical Translation of 25 Years of Tissue Engineering. Tissue Eng. Part A 2019, 25, 679–687. [Google Scholar] [CrossRef]
- Capella-Monsonís, H.; Crum, R.J.; Hussey, G.S.; Badylak, S.F. Advances, challenges, and future directions in the clinical translation of ECM biomaterials for regenerative medicine applications. Adv. Drug Deliv. Rev. 2024, 211, 115347. [Google Scholar] [CrossRef]
- O’Donnell, B.T.; Ives, C.J.; Mohiuddin, O.A.; Bunnell, B.A. Beyond the Present Constraints That Prevent a Wide Spread of Tissue Engineering and Regenerative Medicine Approaches. Front. Bioeng. Biotechnol. 2019, 7, 95. [Google Scholar] [CrossRef]
- Food and Drug Administration. STRATAGRAFT, Vaccines, Blood & Biologics. 2024. Available online: https://www.fda.gov/vaccines-blood-biologics/stratagraft (accessed on 3 September 2024).
- Food and Drug Administration. MACI (Autologous Cultured Chondrocytes on Porcine Collagen Membrane), Cellular & Gene Therapy Products. 2024. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/maci-autologous-cultured-chondrocytes-porcine-collagen-membrane (accessed on 3 September 2024).
- Food and Drug Administration. GINTUIT (Allogeneic Cultured Keratinocytes and Fibroblasts in Bovine Collagen), Cellular & Gene Therapy Products. 2018. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/gintuit-allogeneic-cultured-keratinocytes-and-fibroblasts-bovine-collagen (accessed on 3 September 2024).
- Food and Drug Administration. APLIGRAF (GRAFTSKIN), Medical Devices, Databases, Premarket Approval. 2024. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P950032S016 (accessed on 3 September 2024).
- Food and Drug Administration. TRANCYTE HUMAN FIBROBLAST-DERIVED TEMPORARY SKIN SUBSTITUTE, Medical Devices, Databases, Premarket Approval. 2024. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P960007 (accessed on 3 September 2024).
- Food and Drug Administration. RETHYMIC, Vaccines, Blood & Biologics. 2021. Available online: https://www.fda.gov/vaccines-blood-biologics/rethymic (accessed on 3 September 2024).
- Food and Drug Administration. EPICEL (Cultured Epidermal Autografts), Approved Blood Products. 2018. Available online: https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/epicel-cultured-epidermal-autografts (accessed on 3 September 2024).
- Oberweis, C.V.; Marchal, J.A.; López-Ruiz, E.; Gálvez-Martín, P. A Worldwide Overview of Regulatory Frameworks for Tissue-Based Products. Tissue Eng. Part B Rev. 2020, 26, 181–196. [Google Scholar] [CrossRef]
- Robertson, W.v.B.; Schwartz, B. ASCORBIC ACID AND THE FORMATION OF COLLAGEN. J. Biol. Chem. 1953, 201, 689–696. [Google Scholar] [CrossRef]
- Barnes, M.J. FUNCTION OF ASCORBIC ACID IN COLLAGEN METABOLISM. Ann. N. Y. Acad. Sci. 1975, 258, 264–277. [Google Scholar] [CrossRef]
- Switzer, B.R.; Summer, G.K. Collagen Synthesis in Human Skin Fibroblasts: Effects of Ascorbate, α-Ketoglutarate and Ferrous Ion on Proline Hydroxylation. J. Nutr. 1972, 102, 721–728. [Google Scholar] [CrossRef]
- Germain, L.; Larouche, D.; Nedelec, B.; Perreault, I.; Duranceau, L.; Bortoluzzi, P.; Beaudoin Cloutier, C.; Genest, H.; Caouette-Laberge, L.; Dumas, A.; et al. Autologous bilayered self-assembled skin substitutes (SASSs) as permanent grafts: A case series of 14 severely burned patients indicating clinical effectiveness. Eur. Cell Mater. 2018, 36, 128–141. [Google Scholar] [CrossRef]
- Caneparo, C.; Chabaud, S.; Fradette, J.; Bolduc, S. Engineered human organ-specific urethra as a functional substitute. Sci. Rep. 2022, 12, 21346. [Google Scholar] [CrossRef]
- Orabi, H.; Saba, I.; Rousseau, A.; Bolduc, S. Novel three-dimensional autologous tissue-engineered vaginal tissues using the self-assembly technique. Transl. Res. 2017, 180, 22–36. [Google Scholar] [CrossRef]
- Hoben, G.M.; Hu, J.C.; James, R.A.; Athanasiou, K.A. Self-Assembly of Fibrochondrocytes and Chondrocytes for Tissue Engineering of the Knee Meniscus. Tissue Eng. 2007, 13, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Ofek, G.; Revell, C.M.; Hu, J.C.; Allison, D.D.; Grande-Allen, K.J.; Athanasiou, K.A. Matrix Development in Self-Assembly of Articular Cartilage. PLoS ONE 2008, 3, e2795. [Google Scholar] [CrossRef]
- Labbé, B.; Marceau-Fortier, G.; Fradette, J. Cell Sheet Technology for Tissue Engineering: The Self-Assembly Approach Using Adipose-Derived Stromal Cells. In Adipose-Derived Stem Cells: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2011; pp. 429–441. [Google Scholar] [CrossRef]
- Proulx, S.; d’Arc Uwamaliya, J.; Carrier, P.; Deschambeault, A.; Audet, C.; Giasson, C.J.; Guérin, S.L.; Auger, F.A.; Germain, L. Reconstruction of a human cornea by the self-assembly approach of tissue engineering using the three native cell types. Mol. Vis. 2010, 16, 2192–2201. [Google Scholar] [PubMed]
- Alanazi, H.; Rouabhia, M. Effect of e-cigarette aerosol on gingival mucosa structure and proinflammatory cytokine response. Toxicol. Rep. 2022, 9, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Paquette, J.-S.; Moulin, V.; Tremblay, P.; Bernier, V.; Boutet, M.; Laviolette, M.; Auger, F.A.; Boulet, L.-P.; Goulet, F. Tissue-engineered human asthmatic bronchial equivalents. Eur. Cell Mater. 2004, 7, 11; discussion 1–11. [Google Scholar] [CrossRef]
- Robayo, L.M.; Moulin, V.J.; Tremblay, P.; Cloutier, R.; Lamontagne, J.; Larkin, A.-M.; Chabaud, S.; Simon, F.; Islam, N.; Goulet, F. New ligament healing model based on tissue-engineered collagen scaffolds. Wound Repair Regen. 2011, 19, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Saba, I.; Barat, C.; Chabaud, S.; Reyjon, N.; Leclerc, M.; Jakubowska, W.; Orabi, H.; Lachhab, A.; Pelletier, M.; Tremblay, M.J.; et al. Immunocompetent Human 3D Organ-Specific Hormone-Responding Vaginal Mucosa Model of HIV-1 Infection. Tissue Eng. Part C Methods 2021, 27, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Caneparo, C.; Brownell, D.; Chabaud, S.; Bolduc, S. Genitourinary Tissue Engineering: Reconstruction and Research Models. Bioengineering 2021, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Bouhout, S.; Chabaud, S.; Bolduc, S. Organ-specific matrix self-assembled by mesenchymal cells improves the normal urothelial differentiation in vitro. World J. Urol. 2016, 34, 121–130. [Google Scholar] [CrossRef]
- Saba, I.; Jakubowska, W.; Bolduc, S.; Chabaud, S. Engineering Tissues without the Use of a Synthetic Scaffold: A Twenty-Year History of the Self-Assembly Method. BioMed Res. Int. 2018, 2018, 5684679. [Google Scholar] [CrossRef]
- Attiogbe, E.; Larochelle, S.; Chaib, Y.; Mainzer, C.; Mauroux, A.; Bordes, S.; Closs, B.; Gilbert, C.; Moulin, V.J. An in vitro autologous, vascularized, and immunocompetent Tissue Engineered Skin model obtained by the self-assembled approach. Acta Biomater. 2023, 168, 361–371. [Google Scholar] [CrossRef]
- Magnan, L.; Labrunie, G.; Fénelon, M.; Dusserre, N.; Foulc, M.-P.; Lafourcade, M.; Svahn, I.; Gontier, E.; Vélez V., J.H.; McAllister, T.N.; et al. Human textiles: A cell-synthesized yarn as a truly “bio” material for tissue engineering applications. Acta Biomater. 2020, 105, 111–120. [Google Scholar] [CrossRef]
- Magnan, L.; Kawecki, F.; Labrunie, G.; Gluais, M.; Izotte, J.; Marais, S.; Foulc, M.-P.; Lafourcade, M.; L’Heureux, N. In vivo remodeling of human cell-assembled extracellular matrix yarns. Biomaterials 2021, 273, 120815. [Google Scholar] [CrossRef]
- Roudier, G.; Hourques, M.; Da Silva, N.; Gluais, M.; Binyet, E.; Olive, J.-M.; L’Heureux, N. Effects of weaving parameters on the properties of completely biological tissue-engineered vascular grafts. Biofabrication 2024, 16, 015015. [Google Scholar] [CrossRef]
- Winkler, B.S. In vitro oxidation of ascorbic acid and its prevention by GSH. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1987, 925, 258–264. [Google Scholar] [CrossRef]
- Hata, R.-I.; Senoo, H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J. Cell. Physiol. 1989, 138, 8–16. [Google Scholar] [CrossRef]
- Pellerin, F.A.; Caneparo, C.; Pellerin, E.; Chabaud, S.; Pelletier, M.; Bolduc, S. Heat-Inactivation of Fetal and Newborn Sera Did Not Impair the Expansion and Scaffold Engineering Potentials of Fi-broblasts. Bioengineering 2021, 8, 184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jakubowska, W.; Chabaud, S.; Saba, I.; Galbraith, T.; Berthod, F.; Bolduc, S. Prevascularized Tissue-Engineered Human Vaginal Mucosa: In Vitro Optimization and In Vivo Validation. Tissue Eng. Part A 2020, 26, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Corriveau, M.P.; Boufaied, I.; Lessard, J.; Chabaud, S.; Senécal, J.L.; Grodzicky, T.; Chartier, S.; Raymond, Y.; Moulin, V.J. The fibrotic phenotype of systemic sclerosis fibroblasts varies with disease duration and severity of skin involvement: Re-constitution of skin fibrosis development using a tissue engineering approach. J. Pathol. 2009, 217, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Miller, J.P.; Pannullo, S.C.; Reinhart-King, C.A.; Bordeleau, F. Quantitative assessment of cell contractility using polarized light microscopy. J. Biophotonics 2018, 11, e201800008. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Chung, L.Y.; Andrews, A.M.; Turner, T.D. Toxicity of L-ascorbic acid to L929 fibroblast cultures: Relevance to biocompatibility testing of materials for use in wound management. J. Biomed. Mater. Res. 1993, 27, 521–530. [Google Scholar] [CrossRef]
- Proulx, S.; Bourget, J.-M.; Gagnon, N.; Martel, S.; Deschambeault, A.; Carrier, P.; Giasson, C.J.; Auger, F.A.; Brunette, I.; Germain, L. Optimization of culture conditions for porcine corneal endothelial cells. Mol. Vis. 2007, 13, 524–533. [Google Scholar]
- Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef]
- Gamov, G.A.; Yarullin, D.N.; Gudyrina, M.A.; Pogodina, E.I.; Medvedeva, A.S.; Zavalishin, M.N. Protonation of l-Ascorbic Acid in an Aqueous Solution at T = 298.2 K, p = 0.1 MPa, and I = 0.10–5.0 mol L−1 (NaCl). J. Chem. Eng. Data 2022, 67, 1358–1364. [Google Scholar] [CrossRef]
- Nimni, M.E.; Harkness, R.D. Molecular Structures and Functions of Collagen. In Collagen; Nimni, M.E., Ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Chabaud, S.; Rousseau, A.; Marcoux, T.-L.; Bolduc, S. Inexpensive production of near-native engineered stromas. J. Tissue Eng. Regen. Med. 2017, 11, 1377–1389. [Google Scholar] [CrossRef]
- Rosalia, L.; Hallou, A.; Cochrane, L.; Savin, T. A magnetically actuated, optically sensed tensile testing method for mechanical characterization of soft biological tissues. Sci. Adv. 2023, 9, eade2522. [Google Scholar] [CrossRef]
- Tower, T.T.; Neidert, M.R.; Tranquillo, R.T. Fiber Alignment Imaging During Mechanical Testing of Soft Tissues. Ann. Biomed. Eng. 2002, 30, 1221–1233. [Google Scholar] [CrossRef]
- Zhai, Y.; Colmenarez, J.A.; Mendoza, V.O.; Dong, P.; Nunes, K.; Suh, D.; Gu, L. Multiscale Mechanical Characterization of Cornea With AFM, SEM, and Uniaxial Tensile Test. In Proceedings of the ASME 2023 International Mechanical Engineering Congress and Exposition, New Orleans, LA, USA, 29 October–2 November 2023; Volume 5: Biomedical and Biotechnology. American Society of Mechanical Engineers: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Assunção, M.; Dehghan-Baniani, D.; Yiu, C.H.K.; Später, T.; Beyer, S.; Blocki, A. Cell-Derived Extracellular Matrix for Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 602009. [Google Scholar] [CrossRef] [PubMed]
- Paternoster, J.L.; Vranckx, J.J. State of the Art of Clinical Applications of Tissue Engineering in 2021. Tissue Eng. Part B Rev. 2022, 28, 592–612. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Brey, D.M.; Burdick, J.A. High-Throughput and Combinatorial Technologies for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2009, 15, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef]
- Kojima, H.; Seidle, T.; Spielmann, H. (Eds) Alternatives to Animal Testing; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Ban on Animal Testing—European Commission. Available online: https://single-market-economy.ec.europa.eu/sectors/cosmetics/ban-animal-testing_en (accessed on 5 September 2024).
- Portugal-Cohen, M.; Cohen, D.; Kohen, R.; Oron, M. Exploitation of alternative skin models from academia to industry: Proposed functional categories to answer needs and regulation demands. Front. Physiol. 2023, 14, 1215266. [Google Scholar] [CrossRef]
- Hata, R.; Sunada, H.; Arai, K.; Sat, T.; Ninomiya, Y.; Nagai, Y.; Senoo, H. Regulation of collagen metabolism and cell growth by epidermal growth factor and ascorbate in cultured human skin fibroblasts. Eur. J. Biochem. 1988, 173, 261–267. [Google Scholar] [CrossRef]
- Foroughi, F.; Aibibu, D.; Aulin, C.; Hilborn, J.; Brown, R.A. Bulk collagen incorporation rates into knitted stiff fibre polymer in tissue-engineered scaffolds: The rate-limiting step. J. Tissue Eng. Regen. Med. 2008, 2, 507–514. [Google Scholar] [CrossRef]
- Onursal, C.; Dick, E.; Angelidis, I.; Schiller, H.B.; Staab-Weijnitz, C.A.; Biosynthesis, C. Processing, and Maturation in Lung Ageing. Front. Med. 2021, 8, 593874. [Google Scholar] [CrossRef]
- Lin, A.C.; Pirrung, F.; Niestrawska, J.A.; Ondruschka, B.; Pinter, G.; Henyš, P.; Hammer, N. Shape or size matters? Towards standard reporting of tensile testing parameters for human soft tissues: Systematic review and finite element analysis. Front. Bioeng. Biotechnol. 2024, 12, 1368383. [Google Scholar] [CrossRef]
- Guilak, F.; Butler, D.L.; Goldstein, S.A.; Baaijens, F.P.T. Biomechanics and mechanobiology in functional tissue engineering. J. Biomech. 2014, 47, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Sacks, M.S.; Sun, W. Multiaxial Mechanical Behavior of Biological Materials. Annu. Rev. Biomed. Eng. 2003, 5, 251–284. [Google Scholar] [CrossRef] [PubMed]
- Pach, E.; Kümper, M.; Fromme, J.E.; Zamek, J.; Metzen, F.; Koch, M.; Mauch, C.; Zigrino, P. Extracellular Matrix Remodeling by Fibroblast-MMP14 Regulates Melanoma Growth. Int. J. Mol. Sci. 2021, 22, 12276. [Google Scholar] [CrossRef] [PubMed]
- Millet, M.; Bollmann, E.; Goulet, C.R.; Bernard, G.; Chabaud, S.; Huot, M.-É.; Pouliot, F.; Bolduc, S.; Bordeleau, F. Cancer-Associated Fibroblasts in a 3D Engineered Tissue Model Induce Tumor-like Matrix Stiffening and EMT Transition. Cancers 2022, 2022, 3810. [Google Scholar] [CrossRef]
- Deng, B.; Zhao, Z.; Kong, W.; Han, C.; Shen, X.; Zhou, C. Biological role of matrix stiffness in tumor growth and treatment. J. Transl. Med. 2022, 20, 540. [Google Scholar] [CrossRef]
- Corominas-Murtra, B.; Petridou, N.I. Viscoelastic Networks: Forming Cells and Tissues. Front. Phys. 2021, 9, 666916. [Google Scholar] [CrossRef]
- Ge, H.; Tian, M.; Pei, Q.; Tan, F.; Pei, H. Extracellular Matrix Stiffness: New Areas Affecting Cell Metabolism. Front. Oncol. 2021, 11, 631991. [Google Scholar] [CrossRef]
- Piérard, G.E.; Lapière, C.M. Microanatomy of the Dermis in Relation to Relaxed Skin Tension Lines and Langer’s Lines. Am. J. Dermatopathol. 1987, 9, 219–224. [Google Scholar] [CrossRef]
- Gauvin, R.; Parenteau-Bareil, R.; Larouche, D.; Marcoux, H.; Bisson, F.; Bonnet, A.; Auger, F.A.; Bolduc, S.; Germain, L. Dynamic mechanical stimulations induce anisotropy and improve the tensile properties of engineered tissues produced without exogenous scaffolding. Acta Biomater. 2011, 7, 3294–3301. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brownell, D.; Carignan, L.; Alavi, R.; Caneparo, C.; Labroy, M.; Galbraith, T.; Chabaud, S.; Berthod, F.; Gibot, L.; Bordeleau, F.; et al. Impact of the Use of 2-Phospho-L Ascorbic Acid in the Production of Engineered Stromal Tissue for Regenerative Medicine. Cells 2025, 14, 1123. https://doi.org/10.3390/cells14141123
Brownell D, Carignan L, Alavi R, Caneparo C, Labroy M, Galbraith T, Chabaud S, Berthod F, Gibot L, Bordeleau F, et al. Impact of the Use of 2-Phospho-L Ascorbic Acid in the Production of Engineered Stromal Tissue for Regenerative Medicine. Cells. 2025; 14(14):1123. https://doi.org/10.3390/cells14141123
Chicago/Turabian StyleBrownell, David, Laurence Carignan, Reza Alavi, Christophe Caneparo, Maxime Labroy, Todd Galbraith, Stéphane Chabaud, François Berthod, Laure Gibot, François Bordeleau, and et al. 2025. "Impact of the Use of 2-Phospho-L Ascorbic Acid in the Production of Engineered Stromal Tissue for Regenerative Medicine" Cells 14, no. 14: 1123. https://doi.org/10.3390/cells14141123
APA StyleBrownell, D., Carignan, L., Alavi, R., Caneparo, C., Labroy, M., Galbraith, T., Chabaud, S., Berthod, F., Gibot, L., Bordeleau, F., & Bolduc, S. (2025). Impact of the Use of 2-Phospho-L Ascorbic Acid in the Production of Engineered Stromal Tissue for Regenerative Medicine. Cells, 14(14), 1123. https://doi.org/10.3390/cells14141123






