Role of Cellular Senescence in IUGR: Impact on Fetal Morbidity and Development
Abstract
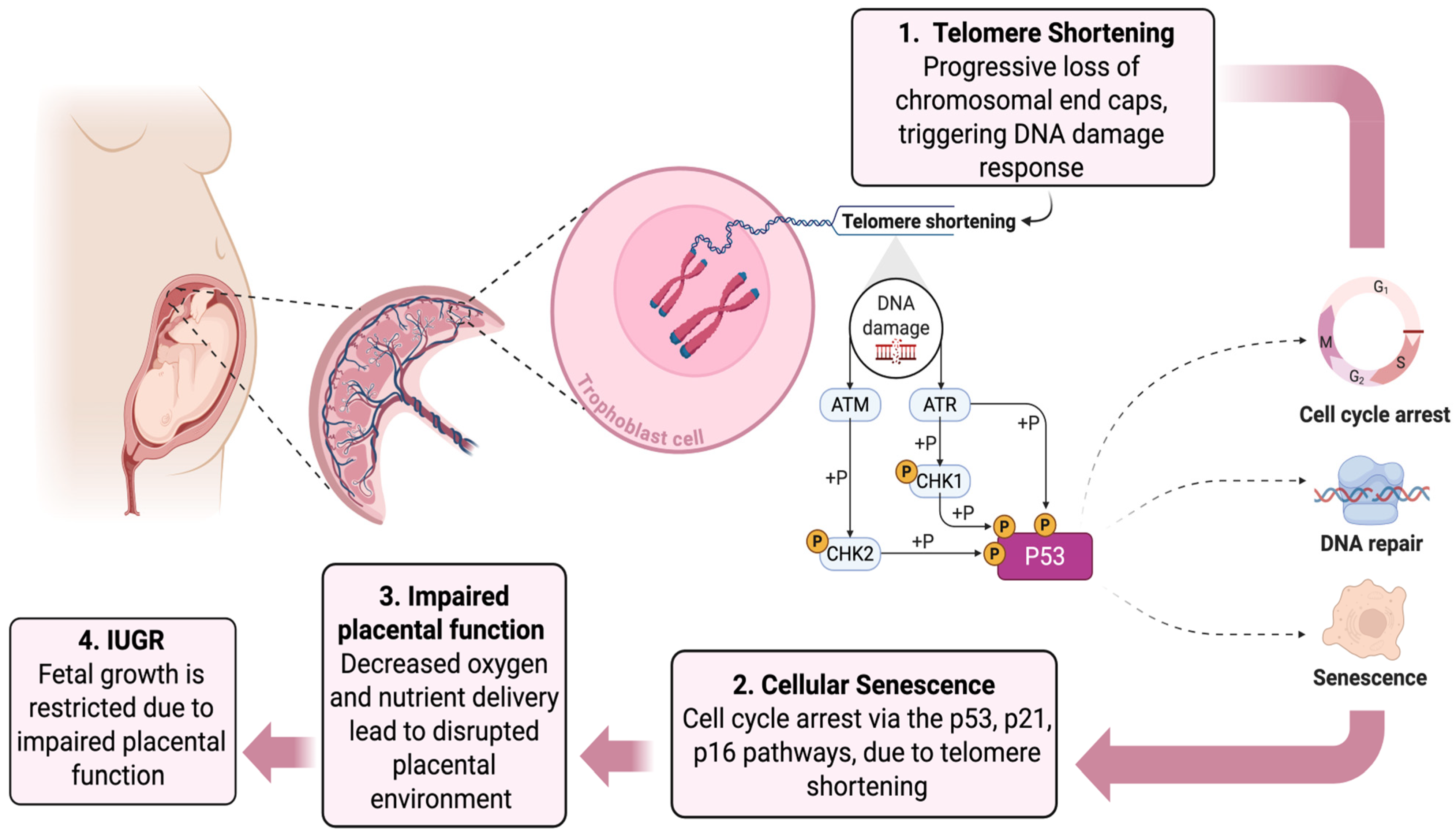
1. Introduction
2. Telomere Shortening and IUGR
2.1. Telomere Shortening in IUGR-Associated Preeclampsia (PE)
2.2. Insulin-like Growth Factor (IGF) and Telomere Length
3. Oxidative Stress, Senescence, and IUGR
3.1. Mitochondrial Dysfunction and IUGR
3.2. Oxidative Stress and Senescence in the Placenta
3.3. Oxidative Stress-Triggered ER Stress, Unfolded Proteins, and IUGR
4. Detection of Senescence
4.1. Biomarkers as Novel Translational Directions
4.2. Artificial Intelligence (AI) and Predictive Models in Precision Obstetric Medicine
5. Therapeutic Interventions and Translational Prospects
5.1. Senotherapeutics in Pregnancy
5.2. Rytvela as a Potent Senotherapeutic in IUGR and Preterm Birth
5.3. Antioxidant and Mitochondrial Therapies
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin. Med. Insights Pediatr. 2016, 10, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Damhuis, S.E.; Ganzevoort, W.; Gordijn, S.J. Abnormal Fetal Growth. Obstet. Gynecol. Clin. N. Am. 2021, 48, 267–279. [Google Scholar] [CrossRef]
- Suhag, A.; Berghella, V. Intrauterine Growth Restriction (IUGR): Etiology and Diagnosis. Curr. Obstet. Gynecol. Rep. 2013, 2, 102–111. [Google Scholar] [CrossRef]
- Barker, D.; Eriksson, J.; Forsén, T.; Osmond, C. Fetal origins of adult disease: Strength of effects and biological basis. Int. J. Epidemiol. 2002, 31, 1235–1239. [Google Scholar] [CrossRef]
- Hoffman, D.J.; Reynolds, R.M.; Hardy, D.B. Developmental origins of health and disease: Current knowledge and potential mechanisms. Nutr. Rev. 2017, 75, 951–970. [Google Scholar] [CrossRef]
- Arima, Y.; Fukuoka, H. Developmental origins of health and disease theory in cardiology. J. Cardiol. 2020, 76, 14–17. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef]
- Davenport, B.N.; Wilson, R.L.; Jones, H.N. Interventions for placental insufficiency and fetal growth restriction. Placenta 2022, 125, 4–9. [Google Scholar] [CrossRef]
- de Onis, M.; Blössner, M.; Villar, J. Levels and patterns of intrauterine growth retardation in developing countries. Eur. J. Clin. Nutr. 1998, 52 (Suppl. 1), S5–S15. [Google Scholar]
- Imdad, A.; Bhutta, Z.A. Nutritional Management of the Low Birth Weight/Preterm Infant in Community Settings: A Perspective from the Developing World. J. Pediatr. 2013, 162, S107–S114. [Google Scholar] [CrossRef] [PubMed]
- Saleem, T.; Sajjad, N.; Fatima, S.; Habib, N.; Ali, S.R.; Qadir, M. Intrauterine growth retardation—Small events, big consequences. Ital. J. Pediatr. 2011, 37, 41. [Google Scholar] [CrossRef] [PubMed]
- Pallotto, E.K.; Kilbride, H.W. Perinatal outcome and later implications of intrauterine growth restriction. Clin. Obstet. Gynecol. 2006, 49, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C. Intrauterine growth restriction--diagnosis and management. Aust. Fam. Physician 2005, 34, 717–723. [Google Scholar] [PubMed]
- Shah, D.K.; Pereira, S.; Lodygensky, G.A. Long-Term Neurologic Consequences following Fetal Growth Restriction: The Impact on Brain Reserve. Dev. Neurosci. 2025, 47, 139–146. [Google Scholar] [CrossRef]
- Vinall, J.; Grunau, R.E.; Brant, R.; Chau, V.; Poskitt, K.J.; Synnes, A.R.; Miller, S.P. Slower Postnatal Growth Is Associated with Delayed Cerebral Cortical Maturation in Preterm Newborns. Sci. Transl. Med. 2013, 5, 168ra8. [Google Scholar] [CrossRef]
- Durousseau, S.; Chavez, G.F. Associations of intrauterine growth restriction among term infants and maternal pregnancy intendedness, initial happiness about being pregnant, and sense of control. Pediatrics 2003, 111, 1171–1175. [Google Scholar] [CrossRef]
- Joss-Moore, L.A.; Lane, R.H. The developmental origins of adult disease. Curr. Opin. Pediatr. 2009, 21, 230–234. [Google Scholar] [CrossRef]
- Chen, J.-H.; Hales, C.N.; Ozanne, S.E. DNA damage, cellular senescence and organismal ageing: Causal or correlative? Nucleic Acids Res. 2007, 35, 7417–7428. [Google Scholar] [CrossRef]
- Campisi, J. Senescent Cells, Tumor Suppression, and Organismal Aging: Good Citizens, Bad Neighbors. Cell 2005, 120, 513–522. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Cañamero, M.; Maraver, A.; Gómez-López, G.; Contreras, J.; Murillo-Cuesta, S.; Rodríguez-Baeza, A.; Varela-Nieto, I.; Ruberte, J.; Collado, M.; et al. Programmed Cell Senescence during Mammalian Embryonic Development. Cell 2013, 155, 1104–1118. [Google Scholar] [CrossRef]
- Sacco, A.; Belloni, L.; Latella, L. From Development to Aging: The Path to Cellular Senescence. Antioxid. Redox Signal. 2021, 34, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Storer, M.; Mas, A.; Robert-Moreno, A.; Pecoraro, M.; Ortells, M.C.; Di Giacomo, V.; Yosef, R.; Pilpel, N.; Krizhanovsky, V.; Sharpe, J.; et al. Senescence Is a Developmental Mechanism that Contributes to Embryonic Growth and Patterning. Cell 2013, 155, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Storer, M.; Keyes, W.M. Developing senescence to remodel the embryo. Commun. Integr. Biol. 2014, 7, e970969. [Google Scholar] [CrossRef]
- Wanner, E.; Thoppil, H.; Riabowol, K. Senescence and Apoptosis: Architects of Mammalian Development. Front. Cell Dev. Biol. 2021, 8, 620089. [Google Scholar] [CrossRef]
- Rhinn, M.; Ritschka, B.; Keyes, W.M. Cellular senescence in development, regeneration and disease. Development 2019, 146, dev151837. [Google Scholar] [CrossRef]
- Menon, R.; Behnia, F.; Polettini, J.; Saade, G.R.; Campisi, J.; Velarde, M. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging 2016, 8, 216–230. [Google Scholar] [CrossRef]
- Cox, L.S.; Redman, C. The role of cellular senescence in ageing of the placenta. Placenta 2017, 52, 139–145. [Google Scholar] [CrossRef]
- Saroyo, Y.B.; Wibowo, N.; Irwinda, R.; Prijanti, A.R.; Yunihastuti, E.; Bardosono, S.; Krisnadi, S.R.; Permata, P.I.; Wijaya, S.; Santawi, V.P.A. Oxidative Stress Induced Damage and Early Senescence in Preterm Placenta. J. Pregnancy 2021, 2021, 9923761. [Google Scholar] [CrossRef]
- Vidal, M.S.; Lintao, R.C.V.; Severino, M.E.L.; Tantengco, O.A.G.; Menon, R. Spontaneous preterm birth: Involvement of multiple feto-maternal tissues and organ systems, differing mechanisms, and pathways. Front. Endocrinol. 2022, 13, 1015622. [Google Scholar] [CrossRef]
- Biron-Shental, T.; Sadeh-Mestechkin, D.; Amiel, A. Telomere homeostasis in IUGR placentas—A review. Placenta 2016, 39, 21–23. [Google Scholar] [CrossRef]
- O’Sullivan, R.J.; Karlseder, J. Telomeres: Protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 2010, 11, 171–181. [Google Scholar] [CrossRef]
- Biron-Shental, T.; Sukenik-Halevy, R.; Sharon, Y.; Laish, I.; Fejgin, M.D.; Amiel, A. Telomere shortening in intra uterine growth restriction placentas. Early Hum. Dev. 2014, 90, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.E.; Armando, R.G.; Farina, H.G.; Menna, P.L.; Cerrudo, C.S.; Ghiringhelli, P.D.; Alonso, D.F. Telomere structure and telomerase in health and disease. Int. J. Oncol. 2012, 41, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.K.; Mitchell, J.R.; Collins, K. RNA Binding Domain of Telomerase Reverse Transcriptase. Mol. Cell Biol. 2001, 21, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, P.S.; Guan, X.-Y.; Trent, J.M. Telomere capture stabilizes chromosome breakage. Nat. Genet. 1993, 4, 252–255. [Google Scholar] [CrossRef]
- Entringer, S.; de Punder, K.; Buss, C.; Wadhwa, P.D. The fetal programming of telomere biology hypothesis: An update. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170151. [Google Scholar] [CrossRef]
- Entringer, S.; Buss, C.; Wadhwa, P.D. Prenatal Stress, Telomere Biology, and Fetal Programming of Health and Disease RiskA Presentation from the European Society for Paediatric Endocrinology (ESPE) New Inroads to Child Health (NICHe) Conference on Stress Response and Child Health in Heraklion, Crete, Greece, 18 to 20 May 2012. Sci. Signal. 2012, 5, pt12. [Google Scholar] [CrossRef]
- Polettini, J.; da Silva, M.G. Telomere-Related Disorders in Fetal Membranes Associated with Birth and Adverse Pregnancy Outcomes. Front. Physiol. 2020, 11, 561771. [Google Scholar] [CrossRef]
- Osorio-Yáñez, C.; Clemente, D.B.P.; Maitre, L.; Vives-Usano, M.; Bustamante, M.; Martinez, D.; Casas, M.; Alexander, J.; Thomsen, C.; Chatzi, L.; et al. Early life tobacco exposure and children’s telomere length: The HELIX project. Sci. Total Environ. 2020, 711, 135028. [Google Scholar] [CrossRef]
- Laganović, M.; Bendix, L.; Rubelj, I.; Kirhmajer, M.V.; Slade, N.; Lela, I.V.; Premužic, V.; Nilsson, P.M.; Jelakovic, B. Reduced telomere length is not associated with early signs of vascular aging in young men born after intrauterine growth restriction. J. Hypertens. 2014, 32, 1613–1620. [Google Scholar] [CrossRef]
- Biron-Shental, T.; Sukenik Halevy, R.; Goldberg-Bittman, L.; Kidron, D.; Fejgin, M.D.; Amiel, A. Telomeres are shorter in placental trophoblasts of pregnancies complicated with intrauterine growth restriction (IUGR). Early Hum. Dev. 2010, 86, 451–456. [Google Scholar] [CrossRef]
- Walker, A.R.; Walker, B.F. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 1421. [Google Scholar] [CrossRef] [PubMed]
- Biron-Shental, T.; Kidron, D.; Sukenik-Halevy, R.; Goldberg-Bittman, L.; Sharony, R.; Fejgin, M.D.; Amiel, A. TERC telomerase subunit gene copy number in placentas from pregnancies complicated with intrauterine growth restriction. Early Hum. Dev. 2011, 87, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Vornic, I.; Buciu, V.; Furau, C.G.; Gaje, P.N.; Ceausu, R.A.; Dumitru, C.-S.; Barb, A.C.; Novacescu, D.; Cumpanas, A.A.; Latcu, S.C.; et al. Oxidative Stress and Placental Pathogenesis: A Contemporary Overview of Potential Biomarkers and Emerging Therapeutics. Int. J. Mol. Sci. 2024, 25, 12195. [Google Scholar] [CrossRef]
- Kwong, W.Y.; Miller, D.J.; Ursell, E.; Wild, A.E.; Wilkins, A.P.; Osmond, C.; Anthony, F.W.; Fleming, T.P. Imprinted gene expression in the rat embryo–fetal axis is altered in response to periconceptional maternal low protein diet. Reproduction 2006, 132, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Tarry-Adkins, J.L.; Chen, J.H.; Smith, N.S.; Jones, R.H.; Cherif, H.; Ozanne, S.E. Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J. 2009, 23, 1521–1528. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Martin-Gronert, M.S.; Chen, J.-H.; Cripps, R.L.; Ozanne, S.E. Maternal diet influences DNA damage, aortic telomere length, oxidative stress, and antioxidant defense capacity in rats. FASEB J. 2008, 22, 2037–2044. [Google Scholar] [CrossRef]
- Pericuesta, E.; Gutiérrez-Arroyo, J.L.; Sánchez-Calabuig, M.J.; Gutiérrez-Adán, A. Postnatal Catch-Up Growth Programs Telomere Dynamics and Glucose Intolerance in Low Birth Weight Mice. Int. J. Mol. Sci. 2021, 22, 3657. [Google Scholar] [CrossRef]
- He, G.; Dai, Y.; Huang, Z.; Ling, F.; Li, P. TERT translocation as a Novel condition in Intrauterine Growth Restriction rats with early catch-up growth. PLoS ONE 2025, 20, e0312221. [Google Scholar] [CrossRef]
- Akkad, A.; Hastings, R.; Konje, J.; Bell, S.; Thurston, H.; Williams, B. Telomere length in small-for-gestational-age babies. BJOG 2006, 113, 318–323. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Joglekar, M.V.; Hardikar, A.A.; Satoor, S.N.; Thompson, J.M.D.; Jenkins, A.; Mitchell, E.A.; Murphy, R. Maternal stress during pregnancy and small for gestational age birthweight are not associated with telomere length at 11 years of age. Gene 2019, 694, 97–101. [Google Scholar] [CrossRef]
- Entringer, S.; Epel, E.S.; Kumsta, R.; Lin, J.; Hellhammer, D.H.; Blackburn, E.H.; Wüst, S.; Wadhwa, P.D. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc. Natl. Acad. Sci. USA 2011, 108, E513–E518. [Google Scholar] [CrossRef]
- Ravlić, S.; Škrobot Vidaček, N.; Nanić, L.; Laganović, M.; Slade, N.; Jelaković, B.; Rubelj, I. Mechanisms of fetal epigenetics that determine telomere dynamics and health span in adulthood. Mech. Ageing Dev. 2018, 174, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Mol, B.W.J.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; de Groot, C.J.M.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2016, 387, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Biron-Shental, T.; Sukenik-Halevy, R.; Sharon, Y.; Goldberg-Bittman, L.; Kidron, D.; Fejgin, M.D.; Amiel, A. Short telomeres may play a role in placental dysfunction in preeclampsia and intrauterine growth restriction. Am. J. Obstet. Gynecol. 2010, 202, 381.e1–381.e7. [Google Scholar] [CrossRef] [PubMed]
- Sukenik-Halevy, R.; Amiel, A.; Kidron, D.; Liberman, M.; Ganor-Paz, Y.; Biron-Shental, T. Telomere homeostasis in trophoblasts and in cord blood cells from pregnancies complicated with preeclampsia. Am. J. Obstet. Gynecol. 2016, 214, 283.e1–283.e7. [Google Scholar] [CrossRef]
- Harville, E.W.; Williams, M.A.; Qiu, C.; Mejia, J.; Risques, R.A. Telomere length, pre-eclampsia, and gestational diabetes. BMC Res. Notes 2010, 3, 113. [Google Scholar] [CrossRef]
- van Heemst, D. Insulin, IGF-1 and longevity. Aging Dis. 2010, 1, 147–157. [Google Scholar]
- Zia, A.; Farkhondeh, T.; Sahebdel, F.; Pourbagher-Shahri, A.M.; Samarghandian, S. Key miRNAs in Modulating Aging and Longevity: A Focus on Signaling Pathways and Cellular Targets. Curr. Mol. Pharmacol. 2022, 15, 736–762. [Google Scholar] [CrossRef]
- Hiden, U.; Glitzner, E.; Hartmann, M.; Desoye, G. Insulin and the IGF system in the human placenta of normal and diabetic pregnancies. J. Anat. 2009, 215, 60–68. [Google Scholar] [CrossRef]
- Barbieri, M.; Paolisso, G.; Kimura, M.; Gardner, J.P.; Boccardi, V.; Papa, M.; Hjelmborg, J.V.; Christensen, K.; Brimacombe, M.; Nawrot, T.S.; et al. Higher circulating levels of IGF-1 are associated with longer leukocyte telomere length in healthy subjects. Mech. Ageing Dev. 2009, 130, 771–776. [Google Scholar] [CrossRef]
- Niu, B.; Wu, J.-X.; Huang, X.-L.; Lei, S.-F.; Deng, F.-Y. Telomere Length Is a Driving Hallmark for Aging-Related Biochemical Hallmarks: Evidence from the Shared Genetic Effect and Causal Inference. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad275. [Google Scholar] [CrossRef]
- Monaghan, J.M.; Godber, I.M.; Lawson, N.; Kaur, M.; Wark, G.; Teale, D.; Hosking, D.J. Longitudinal changes of insulin-like growth factors and their binding proteins throughout normal pregnancy. Ann. Clin. Biochem. 2004, 41, 220–226. [Google Scholar] [CrossRef]
- Olausson, H.; Lof, M.; Brismar, K.; Lewitt, M.; Forsum, E.; Sohlstrom, A. Longitudinal Study of the Maternal Insulin-Like Growth Factor System before, during and after Pregnancy in Relation to Fetal and Infant Weight. Horm. Res. Paediatr. 2008, 69, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chin, E.; Bondy, C. Cellular Pattern of Insulin-Like Growth Factor-I (IGF-I) and IGF-I Receptor Gene Expression in the Developing and Mature Ovarian Follicle. Endocrinology 1991, 129, 3281–3288. [Google Scholar] [CrossRef] [PubMed]
- Duhig, K.; Chappell, L.C.; Shennan, A.H. Oxidative stress in pregnancy and reproduction. Obstet. Med. 2016, 9, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, Ö.; Durak, İ. Role of Oxidative Stress in Intrauterine Growth Restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Chong, W.; Shastri, M.; Eri, R. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Nexus Implicated in Bowel Disease Pathophysiology. Int. J. Mol. Sci. 2017, 18, 771. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The Roles of Mitochondrial Dysfunction and Reactive Oxygen Species in Aging and Senescence. Curr. Mol. Med. 2022, 22, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Bhandary, B.; Marahatta, A.; Kim, H.-R.; Chae, H.-J. An Involvement of Oxidative Stress in Endoplasmic Reticulum Stress and Its Associated Diseases. Int. J. Mol. Sci. 2012, 14, 434–456. [Google Scholar] [CrossRef]
- Vasileiou, P.; Evangelou, K.; Vlasis, K.; Fildisis, G.; Panayiotidis, M.; Chronopoulos, E.; Passias, P.G.; Kouloukoussa, M.; Gorgoulis, V.G.; Havaki, S. Mitochondrial Homeostasis and Cellular Senescence. Cells 2019, 8, 686. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell. 2016, 61, 654–666. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Li, D.; Yin, Y.; Wang, X.; Li, P.; Dangott, L.J.; Hu, W.; Wu, G. Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J. Nutr. 2008, 138, 60–66. [Google Scholar] [CrossRef]
- Devarajan, A.; Rajasekaran, N.S.; Valburg, C.; Ganapathy, E.; Bindra, S.; Freije, W.A. Maternal perinatal calorie restriction temporally regulates the hepatic autophagy and redox status in male rat. Free Radic. Biol. Med. 2019, 130, 592–600. [Google Scholar] [CrossRef]
- Milovanovic, I.; Njuieyon, F.; Deghmoun, S.; Chevenne, D.; Levy-Marchal, C.; Beltrand, J. SGA children with moderate catch-up growth are showing the impaired insulin secretion at the age of 4. PLoS ONE 2014, 9, e100337. [Google Scholar] [CrossRef]
- Li, C.; Johnson, M.S.; Goran, M.I. Effects of low birth weight on insulin resistance syndrome in caucasian and African-American children. Diabetes Care 2001, 24, 2035–2042. [Google Scholar] [CrossRef][Green Version]
- Jensen, C.B.; Storgaard, H.; Dela, F.; Holst, J.J.; Madsbad, S.; Vaag, A.A. Early differential defects of insulin secretion and action in 19-year-old caucasian men who had low birth weight. Diabetes 2002, 51, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Rashid, C.S.; Bansal, A.; Simmons, R.A. Oxidative Stress, Intrauterine Growth Restriction, and Developmental Programming of Type 2 Diabetes. Physiology 2018, 33, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.A.; Suponitsky-Kroyter, I.; Selak, M.A. Progressive accumulation of mitochondrial DNA mutations and decline in mitochondrial function lead to beta-cell failure. J. Biol. Chem. 2005, 280, 28785–28791. [Google Scholar] [CrossRef] [PubMed]
- Theys, N.; Clippe, A.; Bouckenooghe, T.; Reusens, B.; Remacle, C. Early low protein diet aggravates unbalance between antioxidant enzymes leading to islet dysfunction. PLoS ONE 2009, 4, e6110. [Google Scholar] [CrossRef]
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front. Endocrinol. 2020, 11, 611929. [Google Scholar] [CrossRef]
- Oke, S.L.; Hardy, D.B. The Role of Cellular Stress in Intrauterine Growth Restriction and Postnatal Dysmetabolism. Int. J. Mol. Sci. 2021, 22, 6986. [Google Scholar] [CrossRef]
- Ben-Porath, I.; Weinberg, R.A. When cells get stressed: An integrative view of cellular senescence. J. Clin. Investig. 2004, 113, 8–13. [Google Scholar] [CrossRef]
- Londero, A.P.; Orsaria, M.; Marzinotto, S.; Grassi, T.; Fruscalzo, A.; Calcagno, A.; Bertozzi, S.; Nardini, N.; Stella, E.; Lellé, R.J.; et al. Placental aging and oxidation damage in a tissue micro-array model: An immunohistochemistry study. Histochem. Cell Biol. 2016, 146, 191–204. [Google Scholar] [CrossRef]
- Romero, R.; Chaiworapongsa, T.; Alpay Savasan, Z.; Xu, Y.; Hussein, Y.; Dong, Z.; Kusanovic, J.P.; Kim, C.J.; Hassan, S.S. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: A study of the alarmin HMGB1. J. Matern. Fetal Neonatal Med. 2011, 24, 1444–1455. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Romero, R.; Plazyo, O.; Panaitescu, B.; Furcron, A.E.; Miller, D.; Roumayah, T.; Flom, E.; Hassan, S.S. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am. J. Reprod. Immunol. 2016, 75, 3–7. [Google Scholar] [CrossRef]
- Menon, R. Initiation of human parturition: Signaling from senescent fetal tissues via extracellular vesicle mediated paracrine mechanism. Obstet. Gynecol. Sci. 2019, 62, 199–211. [Google Scholar] [CrossRef]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Arrigo, T.; Barberi, I.; Reiter, R.J.; Gitto, E. Oxidative stress-mediated aging during the fetal and perinatal periods. Oxid. Med. Cell. Longev. 2014, 2014, 358375. [Google Scholar] [CrossRef] [PubMed]
- Nye, G.A.; Ingram, E.; Johnstone, E.D.; Jensen, O.E.; Schneider, H.; Lewis, R.M.; Chernyavsky, I.L.; Brownbill, P. Human placental oxygenation in late gestation: Experimental and theoretical approaches. J. Physiol. 2018, 596, 5523–5534. [Google Scholar] [CrossRef] [PubMed]
- Hart, B.; Morgan, E.; Alejandro, E.U. Nutrient sensor signaling pathways and cellular stress in fetal growth restriction. J. Mol. Endocrinol. 2019, 62, R155–R165. [Google Scholar] [CrossRef]
- Rosenstein, M.G.; Cheng, Y.W.; Snowden, J.M.; Nicholson, J.M.; Caughey, A.B. Risk of stillbirth and infant death stratified by gestational age. Obstet. Gynecol. 2012, 120, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Maslovich, M.M.B.L. Intrauterine Fetal Demise; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ferrari, F.; Facchinetti, F.; Saade, G.; Menon, R. Placental telomere shortening in stillbirth: A sign of premature senescence? J. Matern. Fetal Neonatal Med. 2016, 29, 1283–1288. [Google Scholar] [CrossRef]
- Henckel, E.; Svenson, U.; Nordlund, B.; Berggren Broström, E.; Hedlin, G.; Degerman, S.; Bohlin, K. Telomere length was similar in school-age children with bronchopulmonary dysplasia and allergic asthma. Acta Paediatr. 2018, 107, 1395–1401. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Chen, D.; Yu, B.; He, J.; Mao, X.; Huang, Z.; Luo, J.; Yu, J.; Luo, Y. Low Birth Weight Disturbs the Intestinal Redox Status and Mitochondrial Morphology and Functions in Newborn Piglets. Animals 2021, 11, 2561. [Google Scholar] [CrossRef]
- Modaresinejad, M.; Yang, X.; Mohammad Nezhady, M.A.; Zhu, T.; Bajon, E.; Hou, X.; Tahiri, H.; Hardy, P.; Rivera, J.C.; Lachapelle, P.; et al. Endoplasmic Reticulum Stress Delays Choroid Development in the HCAR1 Knockout Mouse. Am. J. Pathol. 2024, 194, 2382–2397. [Google Scholar] [CrossRef]
- Giorgi, C.; Missiroli, S.; Patergnani, S.; Duszynski, J.; Wieckowski, M.R.; Pinton, P. Mitochondria-associated membranes: Composition, molecular mechanisms, and physiopathological implications. Antioxid. Redox Signal. 2015, 22, 995–1019. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J. Endoplasmic Reticulum (ER) Stress and Its Role in Pancreatic β-Cell Dysfunction and Senescence in Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 4843. [Google Scholar] [CrossRef]
- Pluquet, O.; Pourtier, A.; Abbadie, C. The unfolded protein response and cellular senescence. A Review in the Theme: Cellular Mechanisms of Endoplasmic Reticulum Stress Signaling in Health and Disease. Am. J. Physiol. Cell Physiol. 2015, 308, C415–C425. [Google Scholar]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.; Talwar, P.; Parimisetty, A.; Lefebvre d’Hellencourt, C.; Ravanan, P. A Molecular Web: Endoplasmic Reticulum Stress, Inflammation, and Oxidative Stress. Front. Cell Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Gye, M.C. Endoplasmic reticulum stress in periimplantation embryos. Clin. Exp. Reprod. Med. 2015, 42, 1–7. [Google Scholar] [CrossRef]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.K.; Nicholson, C.J.; Holloway, A.C.; Hardy, D.B. Maternal Nicotine Exposure Leads to Impaired Disulfide Bond Formation and Augmented Endoplasmic Reticulum Stress in the Rat Placenta. PLoS ONE 2015, 10, e0122295. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Y.; Wang, J.; Zhu, L.; Gao, L. Dysregulation in the Unfolded Protein Response in the FGR Rat Pancreas. Int. J. Endocrinol. 2020, 2020, 5759182. [Google Scholar] [CrossRef]
- Sohi, G.; Revesz, A.; Hardy, D.B. Nutritional mismatch in postnatal life of low birth weight rat offspring leads to increased phosphorylation of hepatic eukaryotic initiation factor 2 α in adulthood. Metabolism 2013, 62, 1367–1374. [Google Scholar] [CrossRef]
- Vo, T.X.; Revesz, A.; Sohi, G.; Ma, N.; Hardy, D.B. Maternal protein restriction leads to enhanced hepatic gluconeogenic gene expression in adult male rat offspring due to impaired expression of the liver X receptor. J. Endocrinol. 2013, 218, 85–97. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Gao, L.; Jiao, Y.; Liu, C. Maternal Protein Restriction Induces Alterations in Hepatic Unfolded Protein Response-Related Molecules in Adult Rat Offspring. Front. Endocrinol. 2018, 9, 676. [Google Scholar] [CrossRef]
- Deodati, A.; Argemí, J.; Germani, D.; Puglianiello, A.; Alisi, A.; De Stefanis, C.; Ferrero, R.; Nobili, V.; Aragón, T.; Cianfarani, S. The exposure to uteroplacental insufficiency is associated with activation of unfolded protein response in postnatal life. PLoS ONE 2018, 13, e0198490. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Luo, S.; Baumeister, P.; Huang, J.-M.; Gogia, R.K.; Li, M.; Lee, A.S. Underglycosylation of ATF6 as a Novel Sensing Mechanism for Activation of the Unfolded Protein Response. J. Biol. Chem. 2004, 279, 11354–11363. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, D.; Li, Y.; Xin, Y. Maternal Protein Restriction Increases Autophagy in the Pancreas of Newborn Rats. J. Nutr. Sci. Vitaminol. 2020, 66, 168–175. [Google Scholar] [CrossRef]
- Yung, H.; Calabrese, S.; Hynx, D.; Hemmings, B.A.; Cetin, I.; Charnock-Jones, D.S.; Burton, G.J. Evidence of Placental Translation Inhibition and Endoplasmic Reticulum Stress in the Etiology of Human Intrauterine Growth Restriction. Am. J. Pathol. 2008, 173, 451–462. [Google Scholar] [CrossRef]
- Subramanian, A.; Weiss, D.; Nyhan, K.; Dewan, A.; Jukic, A.M.Z. Circulating miRNAs in the first trimester and pregnancy complications: A systematic review. Epigenetics 2023, 18, 2152615. [Google Scholar] [CrossRef]
- Tsochandaridis, M.; Nasca, L.; Toga, C.; Levy-Mozziconacci, A. Circulating microRNAs as clinical biomarkers in the predictions of pregnancy complications. BioMed Res. Int. 2015, 2015, 294954. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Ivankova, K.; Krofta, L. First trimester screening of circulating C19MC microRNAs and the evaluation of their potential to predict the onset of preeclampsia and IUGR. PLoS ONE 2017, 12, e0171756. [Google Scholar] [CrossRef]
- Wu, D.-M.; Yan, Y.-E.; Ma, L.-P.; Liu, H.-X.; Qu, W.; Ping, J. Intrauterine growth retardation-associated syncytin b hypermethylation in maternal rat blood revealed by DNA methylation array analysis. Pediatr. Res. 2017, 82, 704–711. [Google Scholar] [CrossRef]
- Menon, R.; Conneely, K.N.; Smith, A.K. DNA methylation: An epigenetic risk factor in preterm birth. Reprod. Sci. 2012, 19, 6–13. [Google Scholar] [CrossRef]
- Taglauer, E.S.; Wilkins-Haug, L.; Bianchi, D.W. Review: Cell-free fetal DNA in the maternal circulation as an indication of placental health and disease. Placenta 2014, 35, S64–S68. [Google Scholar] [CrossRef]
- Al Nakib, M.; Desbriere, R.; Bonello, N.; Bretelle, F.; Boubli, L.; Gabert, J.; Levy-Mozziconacci, A. Total and fetal cell-free DNA analysis in maternal blood as markers of placental insufficiency in intrauterine growth restriction. Fetal Diagn. Ther. 2009, 26, 24–28. [Google Scholar] [CrossRef]
- Marić, I.; Stevenson, D.K.; Aghaeepour, N.; Gaudillière, B.; Wong, R.J.; Angst, M.S. Predicting Preterm Birth Using Proteomics. Clin. Perinatol. 2024, 51, 391–409. [Google Scholar] [CrossRef]
- Tarca, A.L.; Pataki, B.Á.; Romero, R.; Sirota, M.; Guan, Y.; Kutum, R.; Gomez-Lopez, N.; Done, B.; Bhatti, G.; Yu, T.; et al. Crowdsourcing assessment of maternal blood multi-omics for predicting gestational age and preterm birth. Cell Rep. Med. 2021, 2, 100323. [Google Scholar] [CrossRef] [PubMed]
- Seong, D.; Espinosa, C.; Aghaeepour, N. Computational Approaches for Predicting Preterm Birth and Newborn Outcomes. Clin. Perinatol. 2024, 51, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, C.; Becker, M.; Marić, I.; Wong, R.J.; Shaw, G.M.; Gaudilliere, B.; Aghaeepour, N.; Stevenson, D.K.; Stelzer, I.A.; Peterson, L.S.; et al. Data-Driven Modeling of Pregnancy-Related Complications. Trends Mol. Med. 2021, 27, 762–776. [Google Scholar] [CrossRef]
- De Francesco, D.; Reiss, J.D.; Roger, J.; Tang, A.S.; Chang, A.L.; Becker, M.; Phongpreecha, T.; Espinosa, C.; Morin, S.; Berson, E.; et al. Data-driven longitudinal characterization of neonatal health and morbidity. Sci. Transl. Med. 2023, 15, eadc9854. [Google Scholar] [CrossRef]
- Surendiran, R.; Aarthi, R.; Thangamani, M.; Sugavanam, S.; Sarumathy, R. A Systematic Review Using Machine Learning Algorithms for Predicting Preterm Birth. Int. J. Eng. Trends Technol. 2022, 70, 46–59. [Google Scholar] [CrossRef]
- Włodarczyk, T.; Płotka, S.; Szczepański, T.; Rokita, P.; Sochacki-Wójcicka, N.; Wojcicki, J.; Lipa, M.; Trzciński, T. Machine learning methods for preterm birth prediction: A review. Electronics 2021, 10, 586. [Google Scholar] [CrossRef]
- Huang, C.; Long, X.; van der Ven, M.; Kaptein, M.; Oei, S.G.; van den Heuvel, E. Predicting preterm birth using electronic medical records from multiple prenatal visits. BMC Pregnancy Childbirth 2024, 24, 843. [Google Scholar] [CrossRef]
- Lelarge, V.; Capelle, R.; Oger, F.; Mathieu, T.; Le Calvé, B. Senolytics: From pharmacological inhibitors to immunotherapies, a promising future for patients’ treatment. npj Aging 2024, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef] [PubMed]
- Astrike-Davis, E.M.; Coryell, P.; Loeser, R.F. Targeting cellular senescence as a novel treatment for osteoarthritis. Curr. Opin. Pharmacol. 2022, 64, 102213. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, D.L.; Kellogg, D.L.; Musi, N.; Nambiar, A.M. Cellular Senescence in Idiopathic Pulmonary Fibrosis. Curr. Mol. Biol. Rep. 2021, 7, 31–40. [Google Scholar] [CrossRef]
- Quiniou, C.; Sapieha, P.; Lahaie, I.; Hou, X.; Brault, S.; Beauchamp, M.; Leduc, M.; Rihakova, L.; Joyal, J.S.; Nadeau, S.; et al. Development of a Novel Noncompetitive Antagonist of IL-1 Receptor. J. Immunol. 2008, 180, 6977–6987. [Google Scholar] [CrossRef]
- Nadeau-Vallée, M.; Chin, P.-Y.; Belarbi, L.; Brien, M.-È.; Pundir, S.; Berryer, M.H.; Beaudry-Richard, A.; Madaan, A.; Sharkey, D.J.; Lupien-Meilleur, A.; et al. Antenatal Suppression of IL-1 Protects against Inflammation-Induced Fetal Injury and Improves Neonatal and Developmental Outcomes in Mice. J. Immunol. 2017, 198, 2047–2062. [Google Scholar] [CrossRef]
- Pierre, W.C.; Londono, I.; Quiniou, C.; Chemtob, S.; Lodygensky, G.A. Modulatory effect of IL-1 inhibition following lipopolysaccharide-induced neuroinflammation in neonatal microglia and astrocytes. Int. J. Dev. Neurosci. 2022, 82, 243–260. [Google Scholar] [CrossRef]
- Chin, P.Y.; Moldenhauer, L.M.; Lubell, W.D.; Olson, D.M.; Chemtob, S.; Keelan, J.A.; Robertson, S.A. Inhibition of interleukin-1 signaling protects against Group B streptococcus-induced preterm birth and fetal loss in mice. J. Reprod. Immunol. 2025, 169, 104520. [Google Scholar] [CrossRef]
- Takahashi, Y.; Saito, M.; Usuda, H.; Takahashi, T.; Watanabe, S.; Hanita, T.; Sato, S.; Kumagai, Y.; Koshinami, S.; Ikeda, H.; et al. Direct administration of the non-competitive interleukin-1 receptor antagonist rytvela transiently reduced intrauterine inflammation in an extremely preterm sheep model of chorioamnionitis. PLoS ONE 2021, 16, e0257847. [Google Scholar] [CrossRef]
- Polyak, E.; Ostrovsky, J.; Peng, M.; Dingley, S.D.; Tsukikawa, M.; Kwon, Y.J.; McCormack, S.E.; Bennett, M.; Xiao, R.; Seiler, C.; et al. N-acetylcysteine and vitamin E rescue animal longevity and cellular oxidative stress in pre-clinical models of mitochondrial complex I disease. Mol. Genet. Metab. 2018, 123, 449–462. [Google Scholar] [CrossRef]
- Orihuela-Campos, R.C.; Tamaki, N.; Mukai, R.; Fukui, M.; Miki, K.; Terao, J.; Ito, H.O. Biological impacts of resveratrol, quercetin, and N-acetylcysteine on oxidative stress in human gingival fibroblasts. J. Clin. Biochem. Nutr. 2015, 56, 220–227. [Google Scholar] [CrossRef]
- Lowes, D.A.; Thottakam, B.M.V.; Webster, N.R.; Murphy, M.P.; Galley, H.F. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide–peptidoglycan model of sepsis. Free Radic. Biol. Med. 2008, 45, 1559–1565. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Mater. Au. 2023, 3, 600–619. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Liaw, W.-S.; Chen, C.-A.; Zhou, Q.A. Exosomes─Nature’s Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics. ACS Nano 2022, 16, 17802–17846. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zia, A.; Sahebdel, F.; Er-Reguyeg, Y.; Desjarlais, M.; Mars, J.-C.; Lodygensky, G.A.; Chemtob, S. Role of Cellular Senescence in IUGR: Impact on Fetal Morbidity and Development. Cells 2025, 14, 1097. https://doi.org/10.3390/cells14141097
Zia A, Sahebdel F, Er-Reguyeg Y, Desjarlais M, Mars J-C, Lodygensky GA, Chemtob S. Role of Cellular Senescence in IUGR: Impact on Fetal Morbidity and Development. Cells. 2025; 14(14):1097. https://doi.org/10.3390/cells14141097
Chicago/Turabian StyleZia, Aliabbas, Faezeh Sahebdel, Yosra Er-Reguyeg, Michel Desjarlais, Jean-Clement Mars, Gregory A. Lodygensky, and Sylvain Chemtob. 2025. "Role of Cellular Senescence in IUGR: Impact on Fetal Morbidity and Development" Cells 14, no. 14: 1097. https://doi.org/10.3390/cells14141097
APA StyleZia, A., Sahebdel, F., Er-Reguyeg, Y., Desjarlais, M., Mars, J.-C., Lodygensky, G. A., & Chemtob, S. (2025). Role of Cellular Senescence in IUGR: Impact on Fetal Morbidity and Development. Cells, 14(14), 1097. https://doi.org/10.3390/cells14141097





