The Role of Lactate in Immune Regulation: A Metabolic Rheostat via Transporters, Receptors, and Epigenetic Modifiers
Abstract
1. Introduction
2. Results
2.1. Lactate Accumulation Across Diverse Pathophysiological Contexts
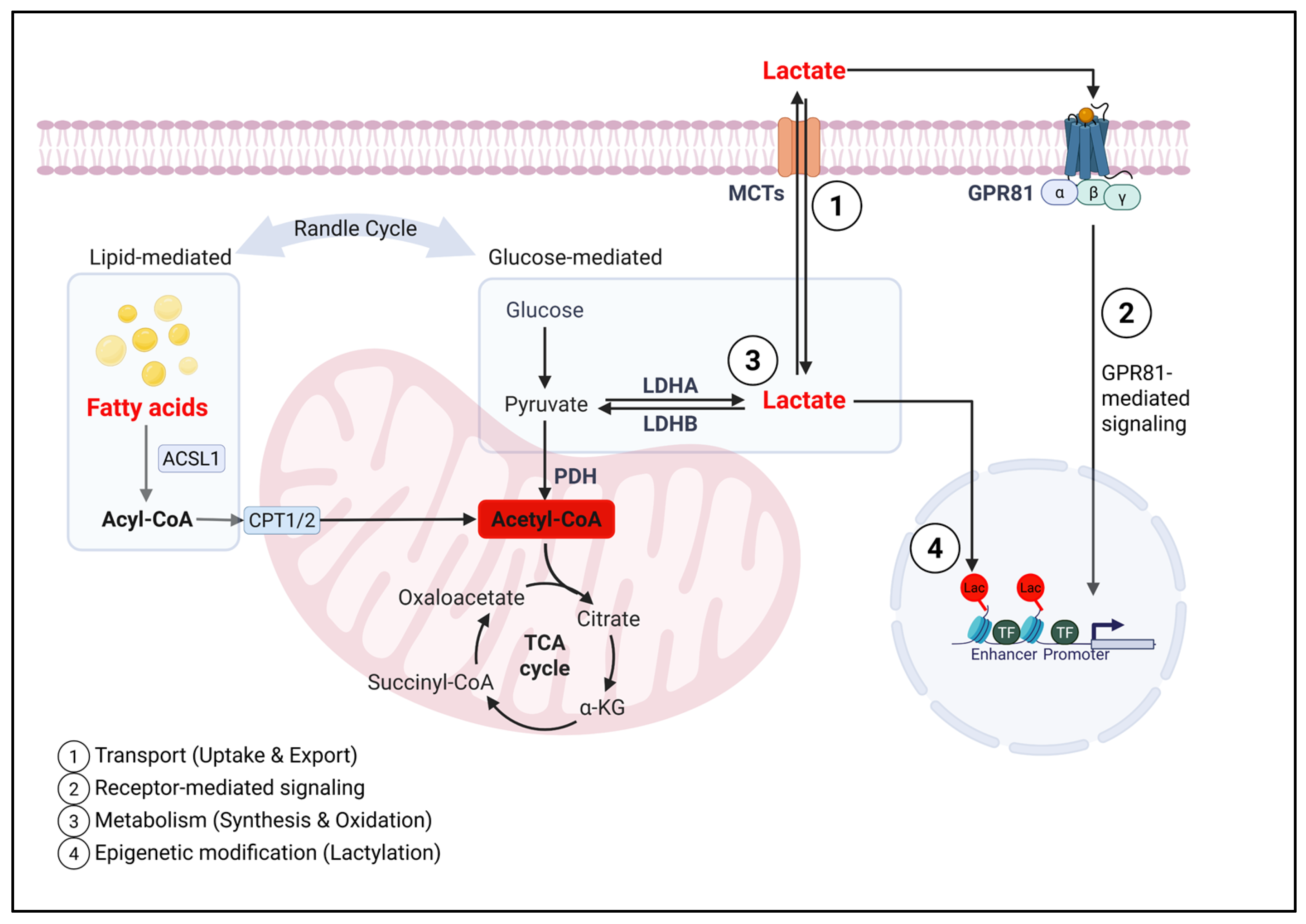
2.2. Lactate Influences Immunity Through Distinct but Integrated Mechanistic Layer
2.2.1. Transporters and Lactate Flux: Role of MCTs in Immunity
- MCT1
- MCT4
- MCT11
2.2.2. Lactate-Responsive Receptors in Immune Modulation
- GPR81
- Proton-sensing receptors (GPR65/GPR4/GPR132)
- NDRG3
2.2.3. Lactate Synthesis and Oxidation, and Metabolic Consequences in the Immune System
- LDH
- PDH
- Impact of lactate-driven NAD+/NADH balance on immune cell function
- Impact of lactate on amino acid metabolism in immune cells
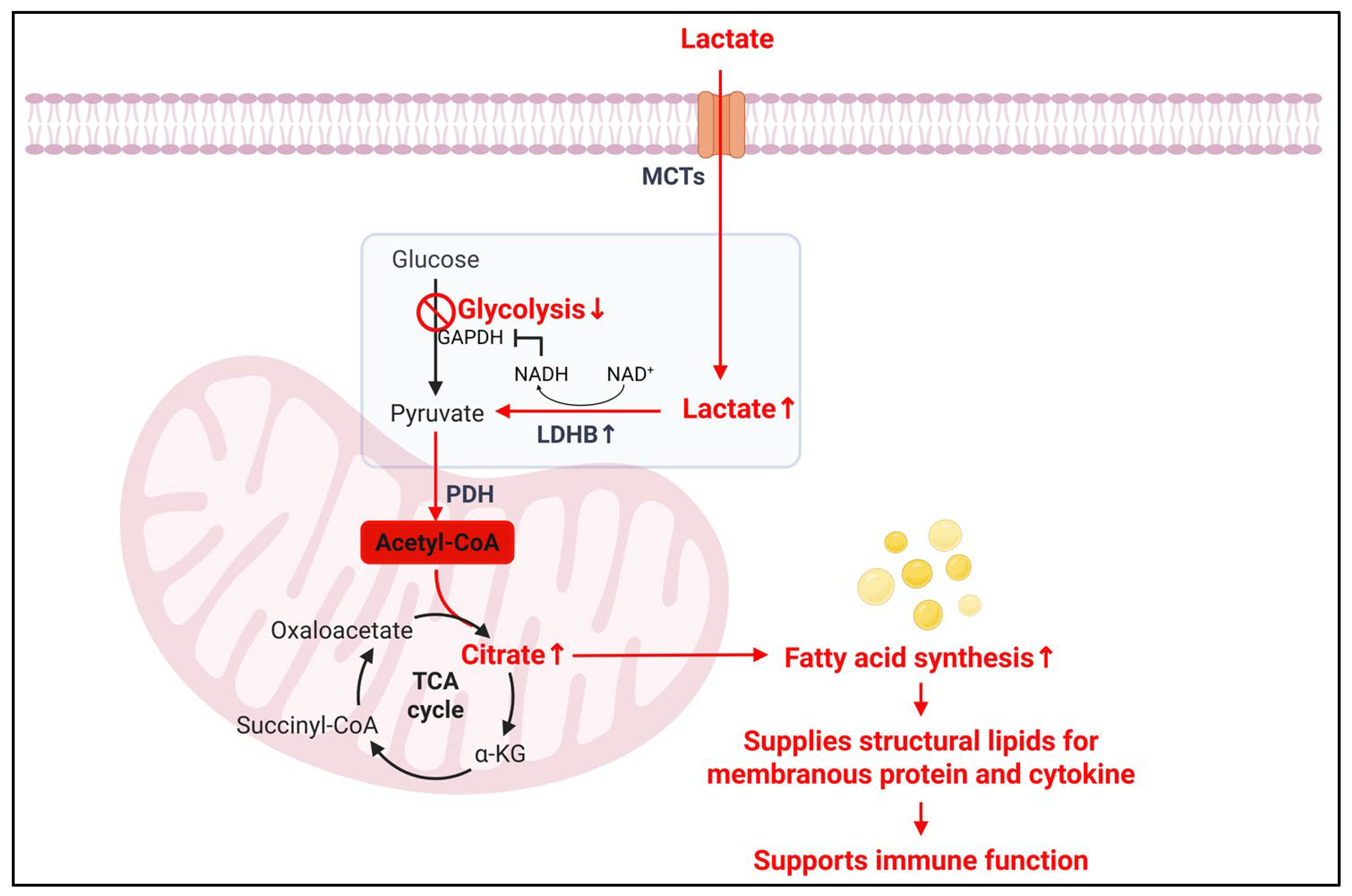
- Impact of lactate on lipid synthesis and consequences in immune cells
- Impact of lactate on reactive oxygen species (ROS) signaling and redox balance in immune cells
2.2.4. Epigenetic Effects of Lactate: Histone and Protein Lactylation
- Histone lactylation
- Non-histone protein lactylation
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Llibre, A.; Kucuk, S.; Gope, A.; Certo, M.; Mauro, C. Lactate: A key regulator of the immune response. Immunity 2025, 58, 535–554. [Google Scholar] [CrossRef]
- Buck, M.D.; Sowell, R.T.; Kaech, S.M.; Pearce, E.L. Metabolic Instruction of Immunity. Cell 2017, 169, 570–586. [Google Scholar] [CrossRef]
- Apostolova, P.; Pearce, E.L. Lactic acid and lactate: Revisiting the physiological roles in the tumor microenvironment. Trends Immunol. 2022, 43, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Pucino, V.; Certo, M.; Bulusu, V.; Cucchi, D.; Goldmann, K.; Pontarini, E.; Haas, R.; Smith, J.; Headland, S.E.; Blighe, K.; et al. Lactate Buildup at the Site of Chronic Inflammation Promotes Disease by Inducing CD4+ T Cell Metabolic Rewiring. Cell Metab. 2019, 30, 1055–1074.e8. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.B.; Rivers, E.P.; Knoblich, B.P.; Jacobsen, G.; Muzzin, A.; Ressler, J.A.; Tomlanovich, M.C. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit. Care Med. 2004, 32, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Hirschhaeuser, F.; Sattler, U.G.A.; Mueller-Klieser, W. Lactate: A metabolic key player in cancer. Cancer Res. 2011, 71, 6921–6925. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Bartoloni, B.; Mannelli, M.; Gamberi, T.; Fiaschi, T. The Multiple Roles of Lactate in the Skeletal Muscle. Cells 2024, 13, 1177. [Google Scholar] [CrossRef]
- Gu, X.Y.; Yang, J.L.; Lai, R.; Zhou, Z.J.; Tang, D.; Hu, L.; Zhao, L.J. Impact of lactate on immune cell function in the tumor microenvironment: Mechanisms and therapeutic perspectives. Front. Immunol. 2025, 16, 1563303. [Google Scholar] [CrossRef]
- Baltazar, F.; Afonso, J.; Costa, M.; Granja, S. Lactate Beyond a Waste Metabolite: Metabolic Affairs and Signaling in Malignancy. Front. Oncol. 2020, 10, 231. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, D.; Huang, M.; Ji, J.; Xu, X.; Wang, F.; Zhou, L.; Bao, B.; Jiang, F.; Xu, W.; et al. Glycolysis in the tumor microenvironment: A driver of cancer progression and a promising therapeutic target. Front. Cell Dev. Biol. 2024, 12, 1416472. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, X.; Wang, L.; Hong, X.; Yang, J. Metabolic reprogramming and crosstalk of cancer-related fibroblasts and immune cells in the tumor microenvironment. Front. Endocrinol. 2022, 13, 988295. [Google Scholar] [CrossRef] [PubMed]
- Kocianova, E.; Piatrikova, V.; Golias, T. Revisiting the Warburg Effect with Focus on Lactate. Cancers 2022, 14, 6028. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Jiang, C.H.; Li, N. Altered metabolism in cancer: Insights into energy pathways and therapeutic targets. Mol. Cancer 2024, 23, 203. [Google Scholar] [CrossRef]
- Li, C.; Liu, C.; Zhang, J.; Lu, Y.; Jiang, B.; Xiong, H.; Li, C. Pyruvate dehydrogenase kinase regulates macrophage polarization in metabolic and inflammatory diseases. Front. Immunol. 2023, 14, 1296687. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Kelly, B.; O’Neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- He, L. Metformin and Systemic Metabolism. Trends Pharmacol. Sci. 2020, 41, 868–881. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, L. Lactate metabolism and lactylation in kidney diseases: Insights into mechanisms and therapeutic opportunities. Ren. Fail. 2025, 47, 2469746. [Google Scholar] [CrossRef]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef]
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascon, G.A.; Hernandez, G.; Murray, P.; De Backer, D.; Workgroup, A.X. The Endothelium in Sepsis. Shock 2016, 45, 259–270. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Donadello, K.; Sakr, Y.; Ospina-Tascon, G.; Salgado, D.; Scolletta, S.; Vincent, J.L. Microcirculatory alterations in patients with severe sepsis: Impact of time of assessment and relationship with outcome. Crit. Care Med. 2013, 41, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Fu, Q.; Long, Q.; Liu, S.; Zou, Y.; Fu, D.; Xu, Q.; Jiang, Z.; Ren, X.; Zhang, G.; et al. PDK4-dependent hypercatabolism and lactate production of senescent cells promotes cancer malignancy. Nat. Metab. 2023, 5, 1887–1910. [Google Scholar] [CrossRef] [PubMed]
- Bosshart, P.D.; Kalbermatter, D.; Bonetti, S.; Fotiadis, D. Mechanistic basis of L-lactate transport in the SLC16 solute carrier family. Nat. Commun. 2019, 10, 2649. [Google Scholar] [CrossRef]
- Halestrap, A.P. The SLC16 gene family—Structure, role and regulation in health and disease. Mol. Asp. Med. 2013, 34, 337–349. [Google Scholar] [CrossRef]
- D’Aria, S.; Maquet, C.; Li, S.; Dhup, S.; Lepez, A.; Kohler, A.; Van Hee, V.F.; Dadhich, R.K.; Freniere, M.; Andris, F.; et al. Expression of the monocarboxylate transporter MCT1 is required for virus-specific mouse CD8+ T cell memory development. Proc. Natl. Acad. Sci. USA 2024, 121, e2306763121. [Google Scholar] [CrossRef]
- Renner, K.; Bruss, C.; Schnell, A.; Koehl, G.; Becker, H.M.; Fante, M.; Menevse, A.N.; Kauer, N.; Blazquez, R.; Hacker, L.; et al. Restricting Glycolysis Preserves T Cell Effector Functions and Augments Checkpoint Therapy. Cell Rep. 2019, 29, 135–150.e139. [Google Scholar] [CrossRef]
- Watson, M.J.; Vignali, P.D.A.; Mullett, S.J.; Overacre-Delgoffe, A.E.; Peralta, R.M.; Grebinoski, S.; Menk, A.V.; Rittenhouse, N.L.; DePeaux, K.; Whetstone, R.D.; et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 2021, 591, 645–651. [Google Scholar] [CrossRef]
- Peralta, R.M.; Xie, B.; Lontos, K.; Nieves-Rosado, H.; Spahr, K.; Joshi, S.; Ford, B.R.; Quann, K.; Frisch, A.T.; Dean, V.; et al. Dysfunction of exhausted T cells is enforced by MCT11-mediated lactate metabolism. Nat. Immunol. 2024, 25, 2297–2307. [Google Scholar] [CrossRef]
- Chi, W.; Kang, N.; Sheng, L.; Liu, S.; Tao, L.; Cao, X.; Liu, Y.; Zhu, C.; Zhang, Y.; Wu, B.; et al. MCT1-governed pyruvate metabolism is essential for antibody class-switch recombination through H3K27 acetylation. Nat. Commun. 2024, 15, 163. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Dong, M.; Min, J.; He, X.; Tan, Y.; Liu, F.; Chen, M.; Chen, X.; Yin, Q.; et al. Macrophage MCT4 inhibition activates reparative genes and protects from atherosclerosis by histone H3 lysine 18 lactylation. Cell Rep. 2024, 43, 114180. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Xi, X. MCT1 gene silencing enhances the immune effect of dendritic cells on cervical cancer cells. Adv. Clin. Exp. Med. 2024, 33, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Felmlee, M.A.; Jones, R.S.; Rodriguez-Cruz, V.; Follman, K.E.; Morris, M.E. Monocarboxylate Transporters (SLC16): Function, Regulation, and Role in Health and Disease. Pharmacol. Rev. 2020, 72, 466–485. [Google Scholar] [CrossRef] [PubMed]
- Hertz, L.; Dienel, G.A. Lactate transport and transporters: General principles and functional roles in brain cells. J. Neurosci. Res. 2005, 79, 11–18. [Google Scholar] [CrossRef]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Dimmer, K.S.; Friedrich, B.; Lang, F.; Deitmer, J.W.; Broer, S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem. J. 2000, 350 Pt 1, 219–227. [Google Scholar] [CrossRef]
- Kaushik, D.K.; Bhattacharya, A.; Mirzaei, R.; Rawji, K.S.; Ahn, Y.; Rho, J.M.; Yong, V.W. Enhanced glycolytic metabolism supports transmigration of brain-infiltrating macrophages in multiple sclerosis. J. Clin. Investig. 2019, 129, 3277–3292. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef]
- Certo, M.; Marone, G.; de Paulis, A.; Mauro, C.; Pucino, V. Lactate: Fueling the fire starter. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1474. [Google Scholar] [CrossRef]
- Mohammad Nezhady, M.A.; Modaresinejad, M.; Zia, A.; Chemtob, S. Versatile lactate signaling via HCAR1: A multifaceted GPCR involved in many biological processes. Am. J. Physiol. Physiol. 2023, 325, C1502–C1515. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.Q.; Ren, N.; Jin, L.; Cheng, K.; Kash, S.; Chen, R.; Wright, S.D.; Taggart, A.K.; Waters, M.G. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem. Biophys. Res. Commun. 2008, 377, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, H.W.; Xing, Z.Q.; Tang, B.; Zhou, X. Lactate induces alternative polarization (M2) of macrophages under lipopolysaccharide stimulation in vitro through G-protein coupled receptor 81. Chin. Med. J. 2020, 133, 1761–1763. [Google Scholar] [CrossRef]
- Hoque, R.; Farooq, A.; Ghani, A.; Gorelick, F.; Mehal, W.Z. Lactate reduces liver and pancreatic injury in Toll-like receptor– and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 2014, 146, 1763–1774. [Google Scholar] [CrossRef]
- Ranganathan, P.; Shanmugam, A.; Swafford, D.; Suryawanshi, A.; Bhattacharjee, P.; Hussein, M.S.; Koni, P.A.; Prasad, P.D.; Kurago, Z.B.; Thangaraju, M.; et al. GPR81, a Cell-Surface Receptor for Lactate, Regulates Intestinal Homeostasis and Protects Mice from Experimental Colitis. J. Immunol. 2018, 200, 1781–1789. [Google Scholar] [CrossRef]
- Yang, K.; Xu, J.; Fan, M.; Tu, F.; Wang, X.; Ha, T.; Williams, D.L.; Li, C. Lactate Suppresses Macrophage Pro-Inflammatory Response to LPS Stimulation by Inhibition of YAP and NF-κB Activation via GPR81-Mediated Signaling. Front. Immunol. 2020, 11, 587913. [Google Scholar] [CrossRef]
- Kanemaru, H.; Mizukami, Y.; Kaneko, A.; Tagawa, H.; Kimura, T.; Kuriyama, H.; Sawamura, S.; Kajihara, I.; Makino, K.; Miyashita, A.; et al. A mechanism of cooling hot tumors: Lactate attenuates inflammation in dendritic cells. iScience 2021, 24, 103067. [Google Scholar] [CrossRef]
- Manoharan, I.; Prasad, P.D.; Thangaraju, M.; Manicassamy, S. Lactate-Dependent Regulation of Immune Responses by Dendritic Cells and Macrophages. Front. Immunol. 2021, 12, 691134. [Google Scholar] [CrossRef]
- Brown, T.P.; Bhattacharjee, P.; Ramachandran, S.; Sivaprakasam, S.; Ristic, B.; Sikder, M.O.F.; Ganapathy, V. The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene 2020, 39, 3292–3304. [Google Scholar] [CrossRef]
- Luo, Y.; Li, L.; Chen, X.; Gou, H.; Yan, K.; Xu, Y. Effects of lactate in immunosuppression and inflammation: Progress and prospects. Int. Rev. Immunol. 2022, 41, 19–29. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Y.; Hang, J.; Zhang, J.; Zhang, T.; Huo, Y.; Liu, J.; Lai, S.; Luo, D.; Wang, L.; et al. Lactate-Modulated Immunosuppression of Myeloid-Derived Suppressor Cells Contributes to the Radioresistance of Pancreatic Cancer. Cancer Immunol. Res. 2020, 8, 1440–1451. [Google Scholar] [CrossRef]
- Justus, C.R.; Dong, L.; Yang, L.V. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front. Physiol. 2013, 4, 354. [Google Scholar] [CrossRef]
- Gao, F.; Shah, R.; Xin, G.; Wang, R. Metabolic Dialogue Shapes Immune Response in the Tumor Microenvironment. Eur. J. Immunol. 2025, 55, e202451102. [Google Scholar] [CrossRef]
- Mogi, C.; Tobo, M.; Tomura, H.; Murata, N.; He, X.D.; Sato, K.; Kimura, T.; Ishizuka, T.; Sasaki, T.; Sato, T.; et al. Involvement of proton-sensing TDAG8 in extracellular acidification-induced inhibition of proinflammatory cytokine production in peritoneal macrophages. J. Immunol. 2009, 182, 3243–3251. [Google Scholar] [CrossRef]
- Xie, L.; McKenzie, C.I.; Qu, X.; Mu, Y.; Wang, Q.; Bing, N.; Naidoo, K.; Alam, M.J.; Yu, D.; Gong, F.; et al. pH and Proton Sensor GPR65 Determine Susceptibility to Atopic Dermatitis. J. Immunol. 2021, 207, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zuo, H.; Xiong, H.; Kolar, M.J.; Chu, Q.; Saghatelian, A.; Siegwart, D.J.; Wan, Y. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc. Natl. Acad. Sci. USA 2017, 114, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.P.; Ganapathy, V. Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol. Ther. 2020, 206, 107451. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Sohn, H.A.; Park, Z.Y.; Oh, S.; Kang, Y.K.; Lee, K.M.; Kang, M.; Jang, Y.J.; Yang, S.J.; Hong, Y.K.; et al. A lactate-induced response to hypoxia. Cell 2015, 161, 595–609. [Google Scholar] [CrossRef]
- Park, K.C.; Lee, D.C.; Yeom, Y.I. NDRG3-mediated lactate signaling in hypoxia. BMB Rep. 2015, 48, 301–302. [Google Scholar] [CrossRef]
- Hobson-Gutierrez, S.A.; Carmona-Fontaine, C. The metabolic axis of macrophage and immune cell polarization. Dis. Model. Mech. 2018, 11, dmm034462. [Google Scholar] [CrossRef]
- Vrieling, F.; van Dierendonck, X.; Jaeger, M.; Janssen, A.W.M.; Hijmans, A.; Netea, M.G.; Tack, C.J.; Stienstra, R. Glycolytic activity in human immune cells: Inter-individual variation and functional implications during health and diabetes. Immunometabolism 2022, 4, e00008. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.L.; Poffenberger, M.C.; Chang, C.H.; Jones, R.G. Fueling immunity: Insights into metabolism and lymphocyte function. Science 2013, 342, 1242454. [Google Scholar] [CrossRef] [PubMed]
- Susser, L.I.; Nguyen, M.A.; Geoffrion, M.; Emerton, C.; Ouimet, M.; Khacho, M.; Rayner, K.J. Mitochondrial Fragmentation Promotes Inflammation Resolution Responses in Macrophages via Histone Lactylation. Mol. Cell. Biol. 2023, 43, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Osis, G.; Zmijewska, A.A.; Traylor, A.; Thukral, S.; Wilson, L.; Barnes, S.; George, J.F.; Agarwal, A. Macrophage-Specific Lactate Dehydrogenase Expression Modulates Inflammatory Function In Vitro. Kidney360 2025, 6, 197–207. [Google Scholar] [CrossRef]
- Maoldomhnaigh, C.Ó.; Cox, D.J.; Phelan, J.J.; Mitermite, M.; Murphy, D.M.; Leisching, G.; Thong, L.; O’Leary, S.M.; Gogan, K.M.; McQuaid, K.; et al. Lactate Alters Metabolism in Human Macrophages and Improves Their Ability to Kill Mycobacterium tuberculosis. Front. Immunol. 2021, 12, 663695. [Google Scholar] [CrossRef]
- Colegio, O.R.; Chu, N.Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef]
- Frank, A.C.; Raue, R.; Fuhrmann, D.C.; Sirait-Fischer, E.; Reuse, C.; Weigert, A.; Lutjohann, D.; Hiller, K.; Syed, S.N.; Brune, B. Lactate dehydrogenase B regulates macrophage metabolism in the tumor microenvironment. Theranostics 2021, 11, 7570–7588. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Kang, S.; Gnanaprakasam, J.R.; Wang, R. The lactate dehydrogenase (LDH) isoenzyme spectrum enables optimally controlling T cell glycolysis and differentiation. Sci. Adv. 2023, 9, eadd9554. [Google Scholar] [CrossRef]
- Decking, S.M.; Bruss, C.; Babl, N.; Bittner, S.; Klobuch, S.; Thomas, S.; Feuerer, M.; Hoffmann, P.; Dettmer, K.; Oefner, P.J.; et al. LDHB Overexpression Can Partially Overcome T Cell Inhibition by Lactic Acid. Int. J. Mol. Sci. 2022, 23, 5970. [Google Scholar] [CrossRef]
- Patel, M.S.; Nemeria, N.S.; Furey, W.; Jordan, F. The pyruvate dehydrogenase complexes: Structure-based function and regulation. J. Biol. Chem. 2014, 289, 16615–16623. [Google Scholar] [CrossRef]
- Jeon, J.H.; Thoudam, T.; Choi, E.J.; Kim, M.J.; Harris, R.A.; Lee, I.K. Loss of metabolic flexibility as a result of overexpression of pyruvate dehydrogenase kinases in muscle, liver and the immune system: Therapeutic targets in metabolic diseases. J. Diabetes Investig. 2021, 12, 21–31. [Google Scholar] [CrossRef]
- Min, B.K.; Park, S.; Kang, H.J.; Kim, D.W.; Ham, H.J.; Ha, C.M.; Choi, B.J.; Lee, J.Y.; Oh, C.J.; Yoo, E.K.; et al. Pyruvate Dehydrogenase Kinase Is a Metabolic Checkpoint for Polarization of Macrophages to the M1 Phenotype. Front. Immunol. 2019, 10, 944. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Long, D.; Zabalawi, M.; Ingram, B.; Yoza, B.K.; Stacpoole, P.W.; McCall, C.E. Stimulating pyruvate dehydrogenase complex reduces itaconate levels and enhances TCA cycle anabolic bioenergetics in acutely inflamed monocytes. J. Leukoc. Biol. 2020, 107, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Gerriets, V.A.; Kishton, R.J.; Nichols, A.G.; Macintyre, A.N.; Inoue, M.; Ilkayeva, O.; Winter, P.S.; Liu, X.; Priyadharshini, B.; Slawinska, M.E.; et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Investig. 2015, 125, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jeon, J.H.; Lee, Y.J.; Kim, M.J.; Kwon, W.H.; Chanda, D.; Thoudam, T.; Pagire, H.S.; Pagire, S.H.; Ahn, J.H.; et al. Inhibition of Pyruvate Dehydrogenase Kinase 4 in CD4(+) T Cells Ameliorates Intestinal Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 439–461. [Google Scholar] [CrossRef]
- Quinn, W.J., 3rd; Jiao, J.; TeSlaa, T.; Stadanlick, J.; Wang, Z.; Wang, L.; Akimova, T.; Angelin, A.; Schafer, P.M.; Cully, M.D.; et al. Lactate Limits T Cell Proliferation via the NAD(H) Redox State. Cell Rep. 2020, 33, 108500. [Google Scholar] [CrossRef]
- Tilton, W.M.; Seaman, C.; Carriero, D.; Piomelli, S. Regulation of glycolysis in the erythrocyte: Role of the lactate/pyruvate and NAD/NADH ratios. J. Lab. Clin. Med. 1991, 118, 146–152. [Google Scholar]
- Ma, G.; Zhang, Z.; Li, P.; Zhang, Z.; Zeng, M.; Liang, Z.; Li, D.; Wang, L.; Chen, Y.; Liang, Y.; et al. Reprogramming of glutamine metabolism and its impact on immune response in the tumor microenvironment. Cell Commun. Signal. 2022, 20, 114. [Google Scholar] [CrossRef]
- Heuser, S.K.; Li, J.; Pudewell, S.; LoBue, A.; Li, Z.; Cortese-Krott, M.M. Biochemistry, pharmacology, and in vivo function of arginases. Pharmacol. Rev. 2025, 77, 100015. [Google Scholar] [CrossRef]
- Rath, M.; Muller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef]
- Wang, B.; Pei, J.; Xu, S.; Liu, J.; Yu, J. A glutamine tug-of-war between cancer and immune cells: Recent advances in unraveling the ongoing battle. J. Exp. Clin. Cancer Res. 2024, 43, 74. [Google Scholar] [CrossRef] [PubMed]
- Paneque, A.; Fortus, H.; Zheng, J.; Werlen, G.; Jacinto, E. The Hexosamine Biosynthesis Pathway: Regulation and Function. Genes 2023, 14, 933. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Pearce, E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016, 213, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Plebanek, M.P.; Xue, Y.; Nguyen, Y.V.; DeVito, N.C.; Wang, X.; Holtzhausen, A.; Beasley, G.M.; Theivanthiran, B.; Hanks, B.A. A lactate-SREBP2 signaling axis drives tolerogenic dendritic cell maturation and promotes cancer progression. Sci. Immunol. 2024, 9, eadi4191. [Google Scholar] [CrossRef]
- Tauffenberger, A.; Fiumelli, H.; Almustafa, S.; Magistretti, P.J. Lactate and pyruvate promote oxidative stress resistance through hormetic ROS signaling. Cell Death Dis. 2019, 10, 653. [Google Scholar] [CrossRef]
- Sun, Z.; Ji, Z.; Meng, H.; He, W.; Li, B.; Pan, X.; Zhou, Y.; Yu, G. Lactate facilitated mitochondrial fission-derived ROS to promote pulmonary fibrosis via ERK/DRP-1 signaling. J. Transl. Med. 2024, 22, 479. [Google Scholar] [CrossRef]
- Manoharan, R.R.; Prasad, A.; Pospisil, P.; Kzhyshkowska, J. ROS signaling in innate immunity via oxidative protein modifications. Front. Immunol. 2024, 15, 1359600. [Google Scholar] [CrossRef]
- Zhao, K.; An, R.; Xiang, Q.; Li, G.; Wang, K.; Song, Y.; Liao, Z.; Li, S.; Hua, W.; Feng, X.; et al. Acid-sensing ion channels regulate nucleus pulposus cell inflammation and pyroptosis via the NLRP3 inflammasome in intervertebral disc degeneration. Cell Prolif. 2021, 54, e12941. [Google Scholar] [CrossRef]
- Manosalva, C.; Quiroga, J.; Hidalgo, A.I.; Alarcon, P.; Anseoleaga, N.; Hidalgo, M.A.; Burgos, R.A. Role of Lactate in Inflammatory Processes: Friend or Foe. Front. Immunol. 2021, 12, 808799. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, N.; Yan, T.; Wei, J.; Hao, L.; Sun, C.; Zhao, H.; Jiang, S. Lactate-mediated metabolic reprogramming of tumor-associated macrophages: Implications for tumor progression and therapeutic potential. Front. Immunol. 2025, 16, 1573039. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Lone, A.; Betts, D.H.; Cumming, R.C. Lactate preconditioning promotes a HIF-1α-mediated metabolic shift from OXPHOS to glycolysis in normal human diploid fibroblasts. Sci. Rep. 2020, 10, 8388. [Google Scholar] [CrossRef]
- Lee, G.; Won, H.S.; Lee, Y.M.; Choi, J.W.; Oh, T.I.; Jang, J.H.; Choi, D.K.; Lim, B.O.; Kim, Y.J.; Park, J.W.; et al. Oxidative Dimerization of PHD2 is Responsible for its Inactivation and Contributes to Metabolic Reprogramming via HIF-1α Activation. Sci. Rep. 2016, 6, 18928. [Google Scholar] [CrossRef]
- Feng, T.; Zhao, X.; Gu, P.; Yang, W.; Wang, C.; Guo, Q.; Long, Q.; Liu, Q.; Cheng, Y.; Li, J.; et al. Adipocyte-derived lactate is a signalling metabolite that potentiates adipose macrophage inflammation via targeting PHD2. Nat. Commun. 2022, 13, 5208. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Li, W.; Zhou, X. Lactylation, an emerging hallmark of metabolic reprogramming: Current progress and open challenges. Front. Cell Dev. Biol. 2022, 10, 972020. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huang, H.; Zhao, Y. Interplay between metabolic reprogramming and post-translational modifications: From glycolysis to lactylation. Front. Immunol. 2023, 14, 1211221. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liu, Z.; Yu, X.; Huang, T.; Chen, J.; Wang, J.; Wilhelm, J.; Li, S.; Song, J.; Li, W.; et al. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat. Commun. 2022, 13, 4981. [Google Scholar] [CrossRef]
- Raychaudhuri, D.; Singh, P.; Chakraborty, B.; Hennessey, M.; Tannir, A.J.; Byregowda, S.; Natarajan, S.M.; Trujillo-Ocampo, A.; Im, J.S.; Goswami, S. Histone lactylation drives CD8+ T cell metabolism and function. Nat. Immunol. 2024, 25, 2140–2151. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, Y.; Gao, Q. Lysine lactylation in the regulation of tumor biology. Trends Endocrinol. Metab. 2024, 35, 720–731. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Wang, Q.; Li, X.; Guo, Y. Ubiquitous protein lactylation in health and diseases. Cell. Mol. Biol. Lett. 2024, 29, 23. [Google Scholar] [CrossRef]
- Feng, F.; Wu, J.; Chi, Q.; Wang, S.; Liu, W.; Yang, L.; Song, G.; Pan, L.; Xu, K.; Wang, C. Lactylome Analysis Unveils Lactylation-Dependent Mechanisms of Stemness Remodeling in the Liver Cancer Stem Cells. Adv. Sci. 2024, 11, e2405975. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, D.; Zhao, S.; Zhang, Z.; Zeng, Z.; Wang, X. Lactate and Lactylation: Clinical Applications of Routine Carbon Source and Novel Modification in Human Diseases. Mol. Cell. Proteom. 2023, 22, 100641. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Wang, D.; Qu, Y.; Li, J.; An, K.; Mao, Z.; Li, J.; Xiong, Y.; Min, Z.; Xue, Z. Enhanced glycolysis-derived lactate promotes microglial activation in Parkinson’s disease via histone lactylation. npj Park. Dis. 2025, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Fan, M.; Wang, X.; Xu, J.; Wang, Y.; Tu, F.; Gill, P.S.; Ha, T.; Liu, L.; Williams, D.L.; et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2022, 29, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhang, J.; Zhou, Q.; He, X.; Zheng, Z.; Wei, Y.; Zhou, K.; Lin, Y.; Yu, H.; Zhang, H.; et al. Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation. Cell Res. 2024, 34, 13–30. [Google Scholar] [CrossRef]
- Niveau, C.; Cettour-Cave, M.; Mouret, S.; Sosa Cuevas, E.; Pezet, M.; Roubinet, B.; Gil, H.; De Fraipont, F.; Landemarre, L.; Charles, J.; et al. MCT1 lactate transporter blockade re-invigorates anti-tumor immunity through metabolic rewiring of dendritic cells in melanoma. Nat. Commun. 2025, 16, 1083. [Google Scholar] [CrossRef]
- Ahmed, K.; Tunaru, S.; Tang, C.; Muller, M.; Gille, A.; Sassmann, A.; Hanson, J.; Offermanns, S. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 2010, 11, 311–319. [Google Scholar] [CrossRef]
- Lee, W.D.; Weilandt, D.R.; Liang, L.; MacArthur, M.R.; Jaiswal, N.; Ong, O.; Mann, C.G.; Chu, Q.; Hunter, C.J.; Ryseck, R.P.; et al. Lactate homeostasis is maintained through regulation of glycolysis and lipolysis. Cell Metab. 2025, 37, 758–771.e8. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, Y.; Zhao, X.; Yu, J. From metabolic byproduct to immune modulator: The role of lactate in tumor immune escape. Front. Immunol. 2024, 15, 1492050. [Google Scholar] [CrossRef]
- Halford, S.; Veal, G.J.; Wedge, S.R.; Payne, G.S.; Bacon, C.M.; Sloan, P.; Dragoni, I.; Heinzmann, K.; Potter, S.; Salisbury, B.M.; et al. A Phase I Dose-escalation Study of AZD3965, an Oral Monocarboxylate Transporter 1 Inhibitor, in Patients with Advanced Cancer. Clin. Cancer Res. 2023, 29, 1429–1439. [Google Scholar] [CrossRef]
- Oh, W.; Kim, A.M.J.; Dhawan, D.; Knapp, D.W.; Lim, S.O. Lactic acid inhibits the interaction between PD-L1 protein and PD-L1 antibody in the PD-1/PD-L1 blockade therapy-resistant tumor. Mol. Ther. 2025, 33, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Cooper, C.R.; Gouw, A.M.; Dinavahi, R.; Maitra, A.; Deck, L.M.; Royer, R.E.; Vander Jagt, D.L.; Semenza, G.L.; Dang, C.V. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA 2010, 107, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- Farabegoli, F.; Vettraino, M.; Manerba, M.; Fiume, L.; Roberti, M.; Di Stefano, G. Galloflavin, a new lactate dehydrogenase inhibitor, induces the death of human breast cancer cells with different glycolytic attitude by affecting distinct signaling pathways. Eur. J. Pharm. Sci. 2012, 47, 729–738. [Google Scholar] [CrossRef]
- Berrell, N.; Sadeghirad, H.; Blick, T.; Bidgood, C.; Leggatt, G.R.; O’Byrne, K.; Kulasinghe, A. Metabolomics at the tumor microenvironment interface: Decoding cellular conversations. Med. Res. Rev. 2024, 44, 1121–1146. [Google Scholar] [CrossRef]
- He, M.J.; Pu, W.; Wang, X.; Zhang, W.; Tang, D.; Dai, Y. Comparing DESI-MSI and MALDI-MSI Mediated Spatial Metabolomics and Their Applications in Cancer Studies. Front. Oncol. 2022, 12, 891018. [Google Scholar] [CrossRef]
- Carmona-Fontaine, C.; Deforet, M.; Akkari, L.; Thompson, C.B.; Joyce, J.A.; Xavier, J.B. Metabolic origins of spatial organization in the tumor microenvironment. Proc. Natl. Acad. Sci. USA 2017, 114, 2934–2939. [Google Scholar] [CrossRef] [PubMed]


| Cell Type | MCT1 | MCT4 | Metabolic Characteristics | Immunologic Consequences | References |
|---|---|---|---|---|---|
| Effector T cells | ↑ | ↑ | Glycolysis ↑ lactate export ↑ | Supports rapid proliferation and effector cytokine production | [27,28] |
| Regulatory T cells | ↑ | ↓ | Lactate uptake ↑ Oxidative metabolism ↑ | Enhances survival and suppressive function in acidic environments | [29,30] |
| Activated B cells | ↑ | - | Glycolysis ↑ lactate export ↑ | Supports proliferation and antibody production | [31] |
| M1 Macrophages | ↓ | ↑ | Glycolysis↑ Lactate export ↑ | Promotes pro-inflammatory phenotype and cytokine secretion | [32] |
| M2 Macrophages | ↑ | ↓ | Lactate uptake ↑ Oxidative metabolism ↑ | Facilitates expression of anti-inflammatory genes | [32] |
| Dendritic cells | ↑ | - | Lactate uptake ↑ Oxidative metabolism ↑ | Facilitates expression of anti-inflammatory genes | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, E.J.; Jang, Y.Y.; Choi, E.J.; Oh, C.J. The Role of Lactate in Immune Regulation: A Metabolic Rheostat via Transporters, Receptors, and Epigenetic Modifiers. Cells 2025, 14, 1096. https://doi.org/10.3390/cells14141096
Choi EJ, Jang YY, Choi EJ, Oh CJ. The Role of Lactate in Immune Regulation: A Metabolic Rheostat via Transporters, Receptors, and Epigenetic Modifiers. Cells. 2025; 14(14):1096. https://doi.org/10.3390/cells14141096
Chicago/Turabian StyleChoi, Eun Jung, Yoon Young Jang, Eun Joo Choi, and Chang Joo Oh. 2025. "The Role of Lactate in Immune Regulation: A Metabolic Rheostat via Transporters, Receptors, and Epigenetic Modifiers" Cells 14, no. 14: 1096. https://doi.org/10.3390/cells14141096
APA StyleChoi, E. J., Jang, Y. Y., Choi, E. J., & Oh, C. J. (2025). The Role of Lactate in Immune Regulation: A Metabolic Rheostat via Transporters, Receptors, and Epigenetic Modifiers. Cells, 14(14), 1096. https://doi.org/10.3390/cells14141096





