Centriole Duplication at the Crossroads of Cell Cycle Control and Oncogenesis
Abstract
1. Introduction to Centrioles and Centrosomes
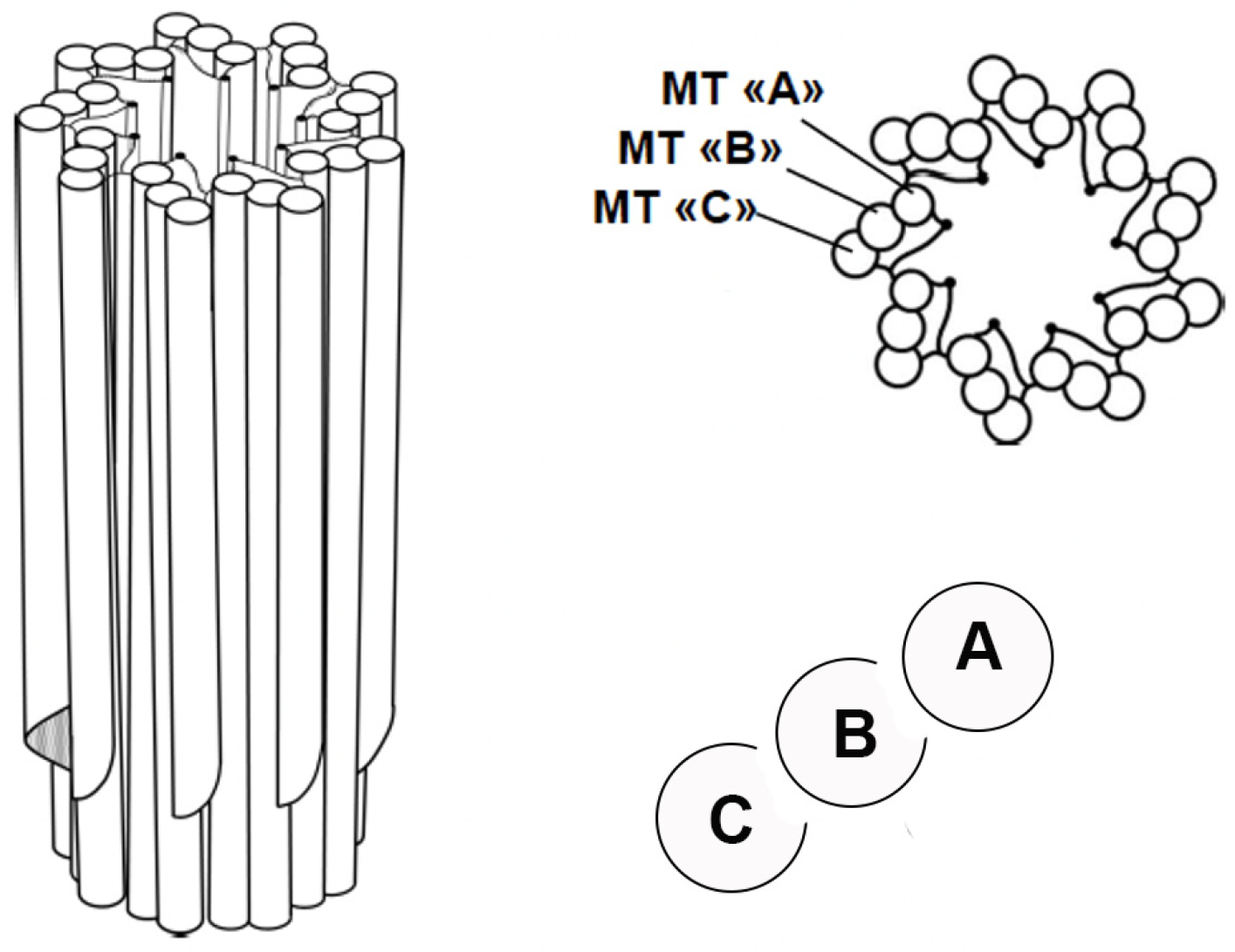
1.1. Structure of Centrioles
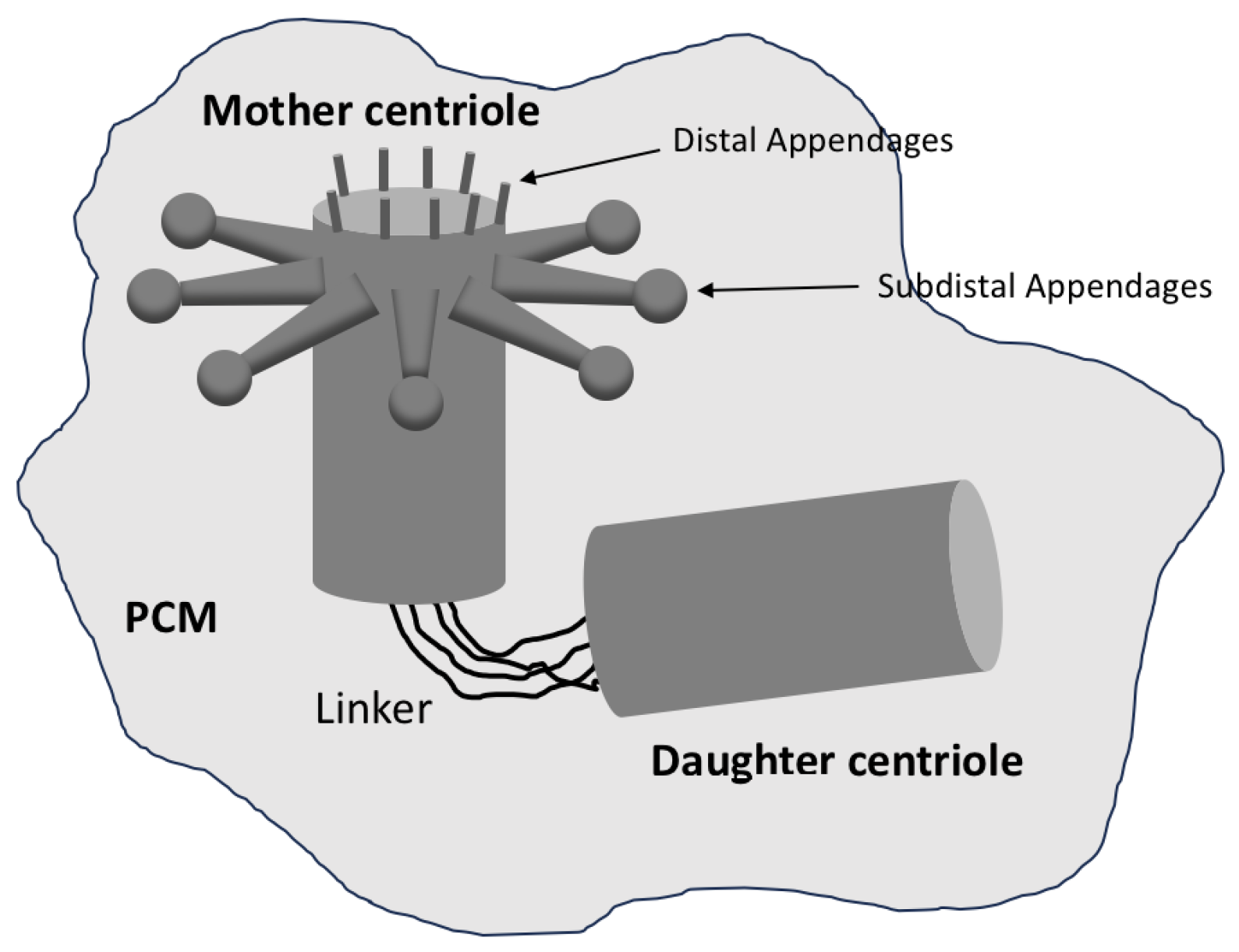
1.2. The Centrosome and Its Function
1.3. Centriole Duplication: A Brief Overview
1.4. The Importance of Centriole Number and Integrity
1.5. Historical Perspective and Discovery of Centrioles
1.6. Relevance of Centriole Research in Disease and Therapeutics
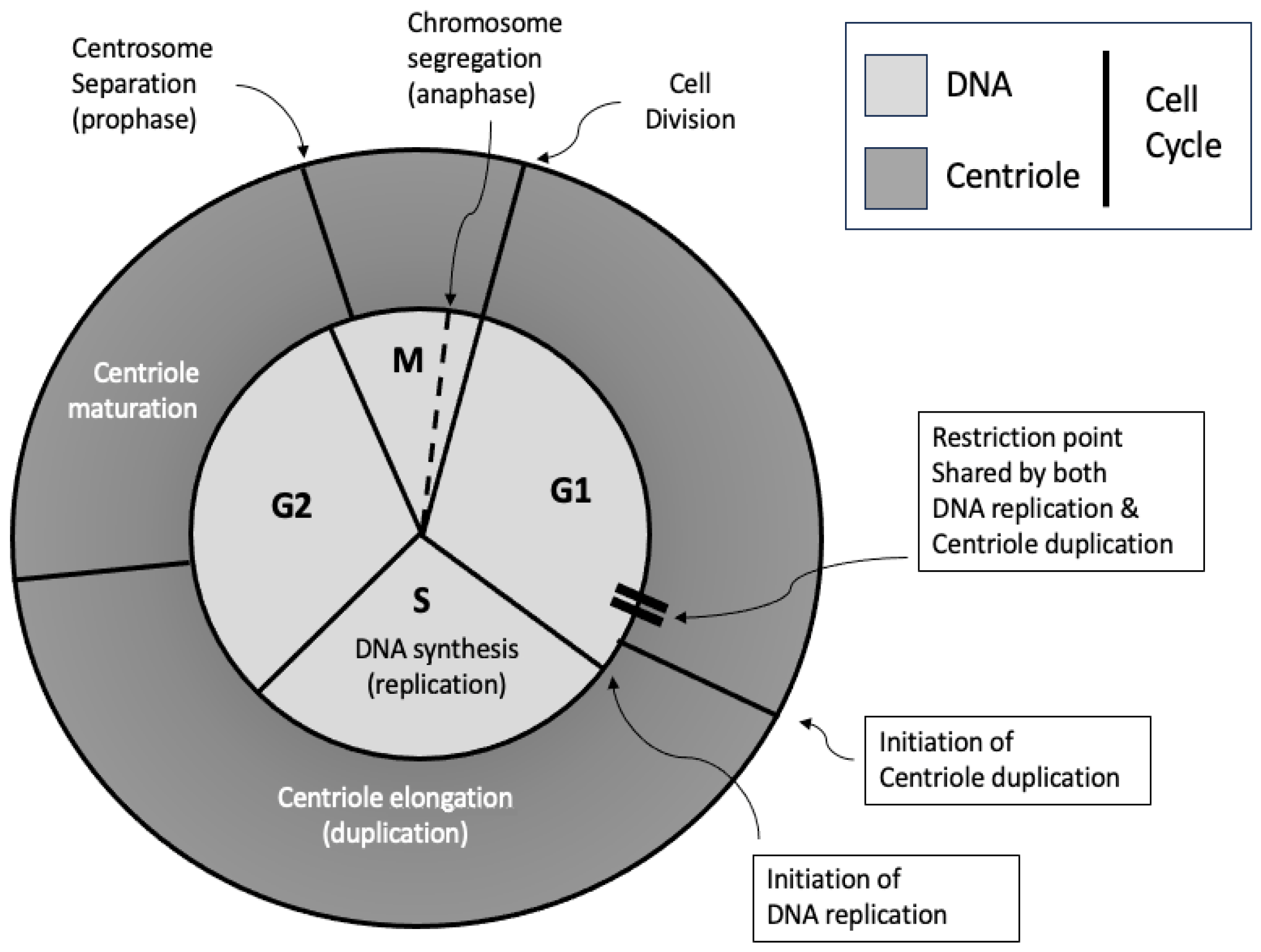
2. An Overview of the Centriole Duplication Process
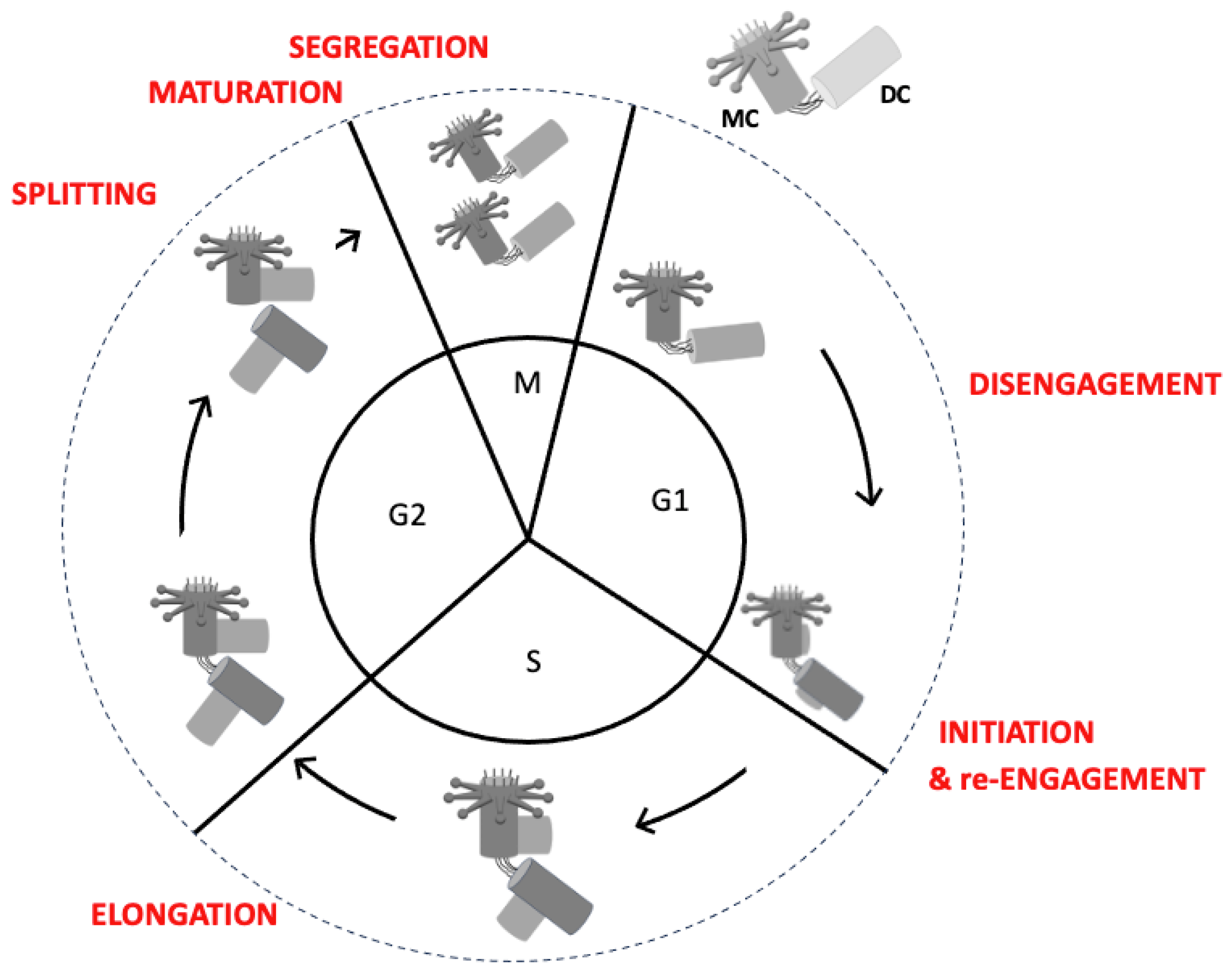
2.1. The Centriole Duplication Cycle
2.2. Key Stages of Centriole Duplication
2.2.1. Initiation: The Formation of the Procentriole
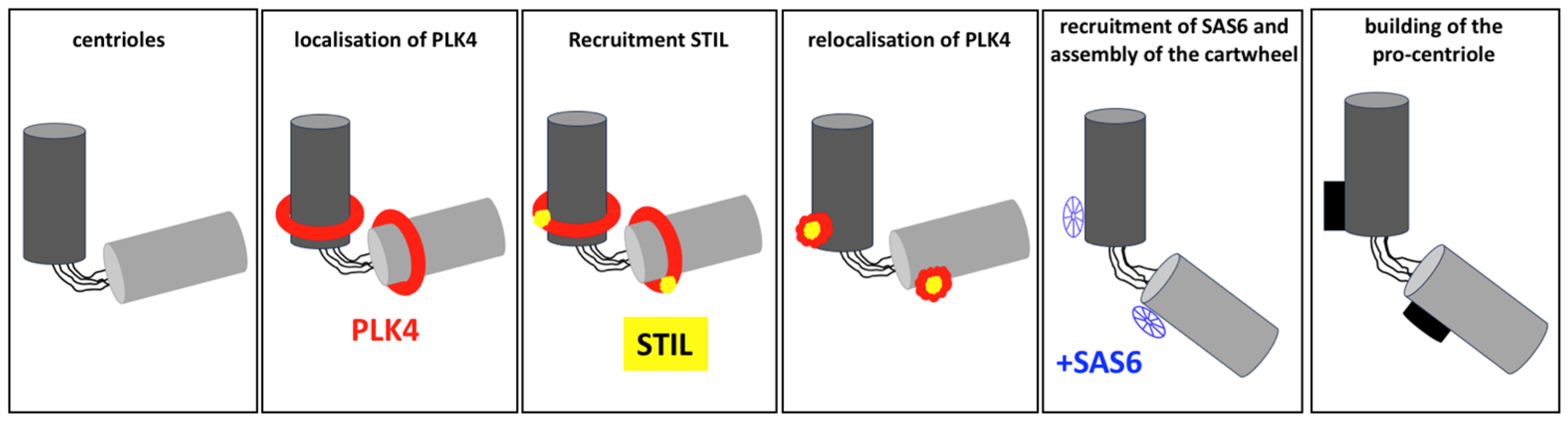
2.2.2. Elongation: The Growth of the Procentriole
2.2.3. Splitting
2.2.4. Maturation: Preparation for Function
2.2.5. Segregation: Distribution to Daughter Cells
2.2.6. Engagement–Disengagement
2.3. Mechanisms Preventing Overduplication
3. Key Molecular Players in Centriole Duplication
3.1. PLK4
3.2. STIL
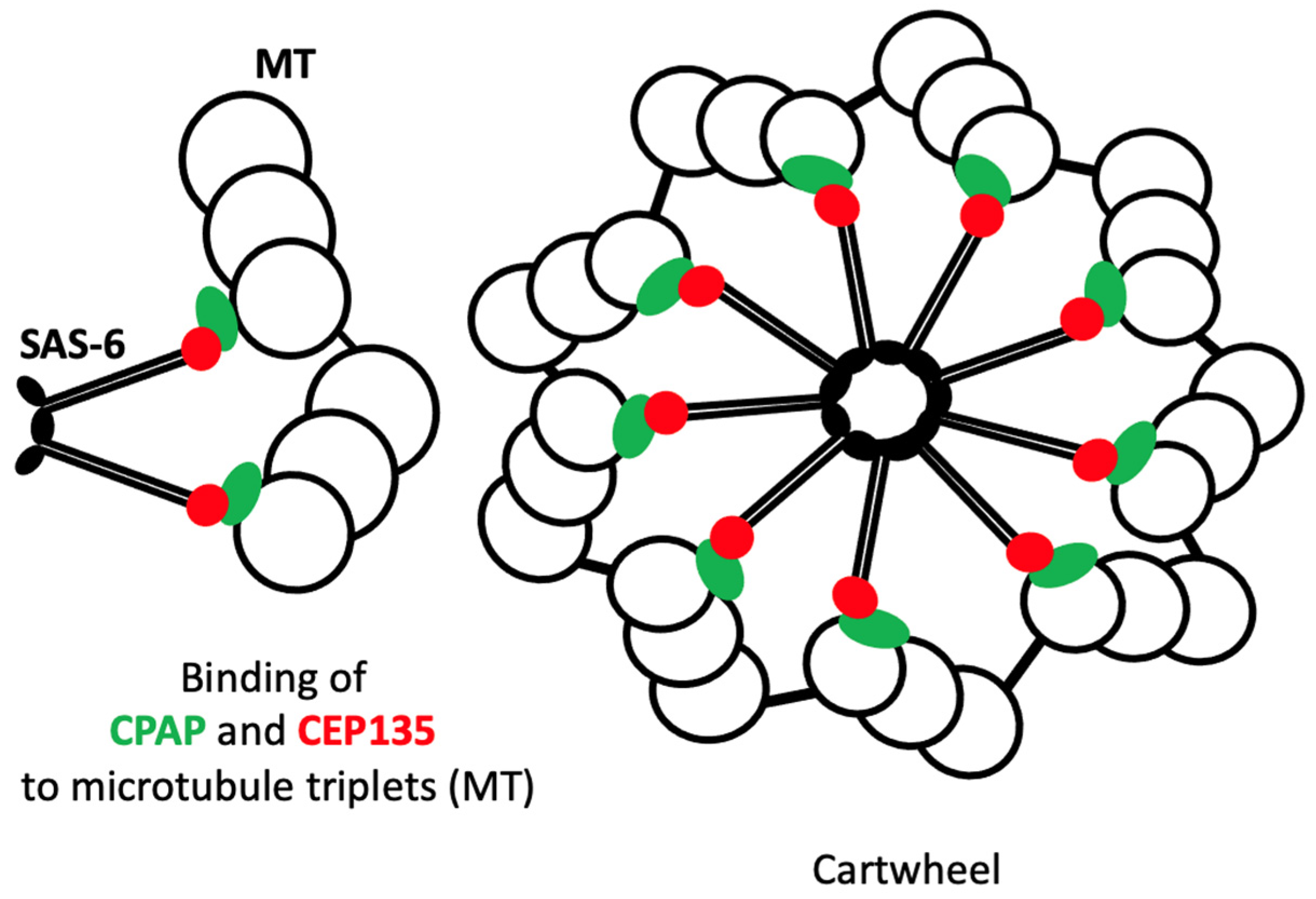
3.3. SAS-6
3.4. CPAP
3.5. CEP135
3.6. Other Proteins Involved in Centriole Biogenesis and Maintenance
4. Regulation of Centriole Duplication
4.1. Licensing and Temporal Control
4.1.1. Licensing Mechanisms
4.1.2. Preventing Overduplication
4.1.3. Checkpoints in Centriole Duplication
4.2. Ubiquitination and Proteasomal Degradation
4.3. Negative Regulation by p53 and p21
4.4. Centriole Reduplication Block
5. Aberrant Centriole Duplication and Its Consequences
5.1. Centrosome Amplification
5.1.1. Dysregulation of Centriole Duplication Proteins
5.1.2. Causes of Amplification
5.2. Cellular Consequences
5.2.1. Formation of Multipolar Spindles
5.2.2. Cancer and Chromosomal Instability
5.3. Microcephaly and Developmental Defects
6. Centriole Duplication and Disease
6.1. Cancer
Therapeutic Targeting
6.2. Neurological Disorders
6.3. Ciliopathies
7. Recent Advances in Visualising Centriole
8. Future Directions in Centriole Duplication Research
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APC | adenomatous polyposis Coli |
| APC/C | anaphase-promoting complex |
| ASD | autism spectrum disorders |
| AURKA | Aurora A kinase |
| BBS | Bardet–Biedl syndrome |
| βTrCP | beta-transducin repeat-containing protein |
| CCDC102B | Coiled-coil domain-containing protein 102B |
| CDK(s) | cyclin-dependent kinase(s) |
| CDK2 | cyclin-dependent kinase 2 |
| CEAs | centriole elongation activators |
| CEIs | centriole elongation inhibitors |
| CENP-J | centromeric protein J |
| CEP63 | Centrosomal Protein 63 |
| CEP135 | Centrosomal Protein 135 |
| CEP152 | Centrosomal Protein 152 |
| CEP192 | Centrosomal Protein 192 |
| CEP250 | Centrosomal Protein 250 |
| CEP295 | Centrosomal Protein 295 |
| C-Nap1 | Centrosomal Nek2-associated protein 1 |
| CPAP | Centrosomal P4.1-associated protein |
| CRL4 | cullin4A-RING E3 ubiquitin ligase |
| Cryo-EM | cryo-electron microscopy |
| DCAF1 | DDB1-CUL4-associated factor 1 |
| DC | daughter centriole |
| DNA | deoxyribonucleic acid |
| exM | expansion microscopy |
| γ-TuRC | γ-Tubulin Ring Complex |
| IDRs | intrinsically disordered regions |
| KIFC1 | kinesin family C1 |
| LRRC45 | Leucine-rich repeat-containing protein 45 |
| MC | mother centriole |
| Mib1 | Mindbomb Homologue-1 |
| MTOC | microtubule organising centre |
| Myc | myelocytomatosis oncogene |
| Nek1A | NimA-related protein kinase 2 |
| NimA | never in mitosis A |
| OFD1 | orofaciodigital syndrome I |
| p53 | protein 53 (or TP53 for tumour protein 53) |
| p21 | protein 21 (or CIP1 for cyclin-dependent kinase inhibitor 1) |
| PCM | pericentriolar material |
| PKD | polycystic kidney disease |
| PLK1 | polo-like kinase 1 |
| PLK4 | polo-like kinase 4 |
| PLK5 | polo-like kinase 5 |
| pRb | retinoblastoma protein |
| RNA | ribonucleic acid |
| SAS-6 | spindle assembly abnormal protein 6 |
| SCF | Skp1–Cullin1–F–box protein |
| sSgo1 | Shugoshin 1 |
| SIM | structured illumination microscopy |
| SSNA-1 | Sjoegren syndrome nuclear autoantigen 1 |
| STED | stimulated emission depletion |
| STIL | SCL/TAL1 interrupting locus |
| TRIM37 | Tripartite motif-containing protein 37 |
References
- Bornens, M. The centrosome in cells and organisms. Science 2012, 335, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Tassin, A.M.; Bornens, M. Centrosome structure and microtubule nucleation in animal cells. Biol. Cell 1999, 91, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Boveri, T. Zur Frage der Entstehung Maligner Tumoren; Gustav Fischer: Jena, Germany, 1914; Volume 40, pp. 857–859. [Google Scholar]
- Qi, F.; Zhou, J. Multifaceted roles of centrosomes in development, health, and disease. J. Mol. Cell Biol. 2021, 13, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Kiermaier, E.; Stötzel, I.; Schapfl, M.A.; Villunger, A. Amplified centrosomes-more than just a threat. EMBO Rep. 2024, 25, 4153–4167. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, D.; Vakonakis, I.; Olieric, N.; Hilbert, M.; Keller, D.; Olieric, V.; Bortfeld, M.; Erat, M.C.; Flückiger, I.; Gönczy, P.; et al. Structural basis of the 9-fold symmetry of centrioles. Cell 2011, 144, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Le Guennec, M.; Klena, N.; Aeschlimann, G.; Hamel, V.; Guichard, P. Overview of the centriole architecture. Curr. Opin. Struct. Biol. 2021, 66, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Pudlowski, R.; Xu, L.; Milenkovic, L.; Kumar, C.; Hemsworth, K.; Aqrabawi, Z.; Stearns, T.; Wang, J.T. A delta-tubulin/epsilon-tubulin/Ted protein complex is required for centriole architecture. Elife 2025, 13, RP98704. [Google Scholar] [CrossRef] [PubMed]
- Nigg, E.A.; Raff, J.W. Centrioles, centrosomes, and cilia in health and disease. Cell 2009, 139, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Ameijeiras, J.; Lozano-Fernández, P.; Martí, E. Centrosome maturation—In tune with the cell cycle. J. Cell Sci. 2022, 135, jcs259395. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Dynlacht, B.D. Regulating the transition from centriole to basal body. J. Cell Biol. 2011, 193, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Godinho, S.A.; Pellman, D. Causes and consequences of centrosome abnormalities in cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130467. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.J.; Naylor, R.M.; van Deursen, J.M. Centrosome dynamics as a source of chromosomal instability. Trends Cell Biol. 2015, 25, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, I.; Dynlacht, B.D. Cilium assembly and disassembly. Nat. Cell Biol. 2016, 18, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Basto, R.; Lau, J.; Vinogradova, T.; Gardiol, A.; Woods, C.G.; Khodjakov, A.; Raff, J.W. Flies without centrioles. Cell 2006, 125, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Vorobjev, I.A.; Chentsov, Y.S. Centrioles in the cell cycle. I. Epithelial cells. J. Cell Biol. 1982, 93, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Tsou, M.F.; Stearns, T. Mechanism limiting centrosome duplication to once per cell cycle. Nature 2006, 442, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Lingle, W.L.; Barrett, S.L.; Negron, V.C.; D’Assoro, A.B.; Boeneman, K.; Liu, W.; Whitehead, C.M.; Reynolds, C.; Salisbury, J.L. Centrosome amplification drives chromosomal instability in breast tumor development. Proc. Natl. Acad. Sci. USA 2002, 99, 1978–1983. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Limeta, A.; Lukasik, K.; Kong, D.; Sullenberger, C.; Luvsanjav, D.; Sahabandu, N.; Chari, R.; Loncarek, J. CPAP insufficiency leads to incomplete centrioles that duplicate but fragment. J. Cell Biol. 2022, 221, e202108018. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.A.; Ahmad, I.; Klingseisen, A.; Hussain, M.S.; Bicknell, L.S.; Leitch, A.; Nürnberg, G.; Toliat, M.R.; Murray, J.E.; Hunt, D.; et al. Mutations in PLK4, encoding a master regulator of centriole biogenesis, cause microcephaly, growth failure and retinopathy. Nat. Genet. 2014, 46, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Sladky, V.C.; Akbari, H.; Tapias-Gomez, D.; Evans, L.T.; Drown, C.G.; Strong, M.A.; LoMastro, G.M.; Larman, T.; Holland, A.J. Centriole signaling restricts hepatocyte ploidy to maintain liver integrity. Genes Dev. 2022, 36, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Boveri, T. Zellen-Studien Heft 2, Die Befruchtung und Teilung des Eles von Ascaris megalocephala II; Verlag von Gustav Fischer: Jena, Germany, 1888. [Google Scholar]
- Boveri, T. Ueber die Natur de Centrosomen Zellen-Studien 4; G Fisher: Jena, Germany, 1900. [Google Scholar]
- Basto, R.; Brunk, K.; Vinadogrova, T.; Peel, N.; Franz, A.; Khodjakov, A.; Raff, J.W. Centrosome amplification can initiate tumorigenesis in flies. Cell 2008, 133, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Dzafic, E.; Strzyz, P.J.; Wilsch-Bräuninger, M.; Norden, C. Centriole Amplification in Zebrafish Affects Proliferation and Survival but Not Differentiation of Neural Progenitor Cells. Cell Rep. 2015, 13, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.S.; Bakker, B.; Boeckx, B.; Moyett, J.; Lu, J.; Vitre, B.; Spierings, D.C.; Lansdorp, P.M.; Cleveland, D.W.; Lambrechts, D.; et al. Centrosome Amplification Is Sufficient to Promote Spontaneous Tumorigenesis in Mammals. Dev. Cell 2017, 40, 313–322.e5. [Google Scholar] [CrossRef] [PubMed]
- D’Assoro, A.B.; Lingle, W.L.; Salisbury, J.L. Centrosome amplification and the development of cancer. Oncogene 2002, 21, 6146–6153. [Google Scholar] [CrossRef] [PubMed]
- Turan, M.G.; Orhan, M.E.; Cevik, S.; Kaplan, O.I. CiliaMiner: An integrated database for ciliopathy genes and ciliopathies. Database 2023, 2023, baad047. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.J.; Cleveland, D.W. Polo-like kinase 4 inhibition: A strategy for cancer therapy? Cancer Cell 2014, 26, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Yu, Q.; Yang, N.; Xiao, Z.; Song, C.; Zhang, R.; Yang, S.; Liu, Z.; Deng, H. Therapeutic potential of targeting polo-like kinase 4. Eur. J. Med. Chem. 2024, 265, 116115. [Google Scholar] [CrossRef] [PubMed]
- Lacey, K.R.; Jackson, P.K.; Stearns, T. Cyclin-dependent kinase control of centrosome duplication. Proc. Natl. Acad. Sci. USA 1999, 96, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Nigg, E.A.; Holland, A.J. Once and only once: Mechanisms of centriole duplication and their deregulation in disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, R.; Dasgupta, S.; Borisy, G.G. Independence of centriole formation and initiation of DNA synthesis in Chinese hamster ovary cells. Cell Motil. Cytoskelet. 1986, 6, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Neben, K.; Ho, A.D. Centrosome replication, genomic instability and cancer. Leukemia 2002, 16, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.J.; Murata, K.; Hwang, D.S.; Parvin, J.D. Inhibition of BRCA1 in breast cell lines causes the centrosome duplication cycle to be disconnected from the cell cycle. Oncogene 2006, 25, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Durcan, T.M.; Halpin, E.S.; Casaletti, L.; Vaughan, K.T.; Pierson, M.R.; Woods, S.; Hinchcliffe, E.H. Centrosome duplication proceeds during mimosine-induced G1 cell cycle arrest. J. Cell Physiol. 2008, 215, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Gönczy, P.; Hatzopoulos, G.N. Centriole assembly at a glance. J. Cell Sci. 2019, 132, jcs228833. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.I.; Kleylein-Sohn, J.; Westendorf, J.; Le Clech, M.; Lavoie, S.B.; Stierhof, Y.D.; Nigg, E.A. Control of centriole length by CPAP and CP110. Curr. Biol. 2009, 19, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Olieric, N.; Steinmetz, M.O. Centriole length control. Curr. Opin. Struct. Biol. 2021, 66, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sullenberger, C.; Vasquez-Limeta, A.; Kong, D.; Loncarek, J. With Age Comes Maturity: Biochemical and Structural Transformation of a Human Centriole in the Making. Cells 2020, 9, 1429. [Google Scholar] [CrossRef] [PubMed]
- Faruki, S.; Cole, R.W.; Rieder, C.L. Separating centrosomes interact in the absence of associated chromosomes during mitosis in cultured vertebrate cells. Cell Motil. Cytoskelet. 2002, 52, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Agircan, F.G.; Schiebel, E.; Mardin, B.R. Separate to operate: Control of centrosome positioning and separation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130461. [Google Scholar] [CrossRef] [PubMed]
- Habedanck, R.; Stierhof, Y.D.; Wilkinson, C.J.; Nigg, E.A. The Polo kinase Plk4 functions in centriole duplication. Nat. Cell Biol. 2005, 7, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.J.; Fachinetti, D.; Da Cruz, S.Q.; Vitre, B.; Lince-Faria, M.; Chen, D.; Parish, N.; Verma, I.M.; Bettencourt-Dias, M.; Cleveland, D.W. Polo-like kinase 4 controls centriole duplication but does not directly regulate cytokinesis. Mol. Biol. Cell 2012, 23, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.J.; Lin, S.Y.; Hsu, W.B.; Lin, Y.N.; Wu, C.T.; Lin, Y.C.; Chang, C.W.; Wu, K.S.; Tang, T.K. The human microcephaly protein STIL interacts with CPAP and is required for procentriole formation. EMBO J. 2011, 30, 4790–4804. [Google Scholar] [CrossRef] [PubMed]
- Yoshiba, S.; Tsuchiya, Y.; Ohta, M.; Gupta, A.; Shiratsuchi, G.; Nozaki, Y.; Ashikawa, T.; Fujiwara, T.; Natsume, T.; Kanemaki, M.T.; et al. HsSAS-6-dependent cartwheel assembly ensures stabilization of centriole intermediates. J. Cell Sci. 2019, 132, jcs217521. [Google Scholar] [CrossRef] [PubMed]
- Hatch, E.M.; Kulukian, A.; Holland, A.J.; Cleveland, D.W.; Stearns, T. Cep152 interacts with Plk4 and is required for centriole duplication. J. Cell Biol. 2010, 191, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Sonnen, K.F.; Gabryjonczyk, A.M.; Anselm, E.; Stierhof, Y.D.; Nigg, E.A. Human Cep192 and Cep152 cooperate in Plk4 recruitment and centriole duplication. J. Cell Sci. 2013, 126 Pt 14, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.J.; Fu, R.H.; Wu, K.S.; Hsu, W.B.; Tang, T.K. CPAP is a cell-cycle regulated protein that controls centriole length. Nat. Cell Biol. 2009, 11, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Inanç, B.; Pütz, M.; Lalor, P.; Dockery, P.; Kuriyama, R.; Gergely, F.; Morrison, C.G. Abnormal centrosomal structure and duplication in Cep135-deficient vertebrate cells. Mol. Biol. Cell 2013, 24, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Bahe, S.; Stierhof, Y.D.; Wilkinson, C.J.; Leiss, F.; Nigg, E.A. Rootletin forms centriole-associated filaments and functions in centrosome cohesion. J. Cell Biol. 2005, 171, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.M.; Mayor, T.; Meraldi, P.; Stierhof, Y.D.; Tanaka, K.; Nigg, E.A. C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J. Cell Biol. 1998, 141, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Mayor, T.; Stierhof, Y.D.; Tanaka, K.; Fry, A.M.; Nigg, E.A. The centrosomal protein C-Nap1 is required for cell cycle-regulated centrosome cohesion. J. Cell Biol. 2000, 151, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.M.; Meraldi, P.; Nigg, E.A. A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. EMBO J. 1998, 17, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Faragher, A.J.; Fry, A.M. Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles. Mol. Biol. Cell 2003, 14, 2876–2889. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Huang, N.; Bao, Y.; Zhou, H.; Teng, J.; Chen, J. LRRC45 is a centrosome linker component required for centrosome cohesion. Cell Rep. 2013, 4, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Huang, N.; Chen, Z.; Li, F.; Fan, G.; Ma, D.; Chen, J.; Teng, J. CCDC102B functions in centrosome linker assembly and centrosome cohesion. J. Cell Sci. 2018, 131, jcs222901. [Google Scholar] [CrossRef] [PubMed]
- Gould, R.R.; Borisy, G.G. The pericentriolar material in Chinese hamster ovary cells nucleates microtubule formation. J. Cell Biol. 1977, 73, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.F. Basal bodies platforms for building cilia. Curr. Top. Dev. Biol. 2008, 85, 1–22. [Google Scholar] [PubMed]
- Ma, D.; Wang, F.; Teng, J.; Huang, N.; Chen, J. Structure and function of distal and subdistal appendages of the mother centriole. J. Cell Sci. 2023, 136, jcs260560. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, J.B.; Wueseke, O.; Hyman, A.A. Pericentriolar material structure and dynamics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130459. [Google Scholar] [CrossRef] [PubMed]
- Uzbekov, R.; Kireyev, I.; Prigent, C. Centrosome separation: Respective role of microtubules and actin filaments. Biol. Cell 2002, 94, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Giet, R.; Petretti, C.; Prigent, C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005, 15, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Schöckel, L.; Möckel, M.; Mayer, B.; Boos, D.; Stemmann, O. Cleavage of cohesin rings coordinates the separation of centrioles and chromatids. Nat. Cell Biol. 2011, 13, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; Duan, Q.; Jiang, N.; Huang, Y.; Darzynkiewicz, Z.; Dai, W. sSgo1, a major splice variant of Sgo1, functions in centriole cohesion where it is regulated by Plk1. Dev. Cell 2008, 14, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.K.; Takumi, K.; Matsuhashi, K.; Sakamoto, H.; Nagai, K.; Fukuyama, M.; Yamamoto, S.; Chinen, T.; Hata, S.; Kitagawa, D. Multimodal mechanisms of human centriole engagement and disengagement. EMBO J. 2025, 44, 1294–1321. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewski, N.; Zheng, L.; Cuevas, R.; Parry, J.; Chatterjee, P.; Anderton, B.; Duensing, A.; Münger, K.; Duensing, S. Cullin 1 functions as a centrosomal suppressor of centriole multiplication by regulating polo-like kinase 4 protein levels. Cancer Res. 2009, 69, 6668–6675. [Google Scholar] [CrossRef] [PubMed]
- Arquint, C.; Nigg, E.A. The PLK4-STIL-SAS-6 module at the core of centriole duplication. Biochem. Soc. Trans. 2016, 44, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Blow, J.J.; Laskey, R.A. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature 1988, 332, 546–548. [Google Scholar] [CrossRef] [PubMed]
- Zitouni, S.; Nabais, C.; Jana, S.C.; Guerrero, A.; Bettencourt-Dias, M. Polo-like kinases: Structural variations lead to multiple functions. Nat. Rev. Mol. Cell Biol. 2014, 15, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Vakonakis, I. The centriolar cartwheel structure: Symmetric, stacked, and polarized. Curr. Opin. Struct. Biol. 2021, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Ashikawa, T.; Nozaki, Y.; Kozuka-Hata, H.; Goto, H.; Inagaki, M.; Oyama, M.; Kitagawa, D. Direct interaction of Plk4 with STIL ensures formation of a single procentriole per parental centriole. Nat. Commun. 2014, 5, 5267. [Google Scholar] [CrossRef] [PubMed]
- Arquint, C.; Gabryjonczyk, A.M.; Imseng, S.; Böhm, R.; Sauer, E.; Hiller, S.; Nigg, E.A.; Maier, T. STIL binding to Polo-box 3 of PLK4 regulates centriole duplication. Elife 2015, 4, e07888. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Ferreira, I.; Rodrigues-Martins, A.; Bento, I.; Riparbelli, M.; Zhang, W.; Laue, E.; Callaini, G.; Glover, D.M.; Bettencourt-Dias, M. The SCF/Slimb ubiquitin ligase limits centrosome amplification through degradation of SAK/PLK4. Curr. Biol. 2009, 19, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Čajánek, L.; Glatter, T.; Nigg, E.A. The E3 ubiquitin ligase Mib1 regulates Plk4 and centriole biogenesis. J. Cell Sci. 2015, 128, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, J.; Kratz, A.S.; Kordonsky, A.; Prag, G.; Hoffmann, I. CRL4DCAF1 ubiquitin ligase regulates PLK4 protein levels to prevent premature centriole duplication. Life Sci. Alliance 2024, 7, e202402668. [Google Scholar] [CrossRef] [PubMed]
- Guderian, G.; Westendorf, J.; Uldschmid, A.; Nigg, E.A. Plk4 trans-autophosphorylation regulates centriole number by controlling betaTrCP-mediated degradation. J. Cell Sci. 2010, 123 Pt 13, 2163–2169. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Ferreira, I.; Bento, I.; Pimenta-Marques, A.; Jana, S.C.; Lince-Faria, M.; Duarte, P.; Borrego-Pinto, J.; Gilberto, S.; Amado, T.; Brito, D.; et al. Regulation of autophosphorylation controls PLK4 self-destruction and centriole number. Curr. Biol. 2013, 23, 2245–2254. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Martins, A.; Riparbelli, M.; Callaini, G.; Glover, D.M.; Bettencourt-Dias, M. Revisiting the role of the mother centriole in centriole biogenesis. Science 2007, 316, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Shamir, M.; Martin, F.J.O.; Woolfson, D.N.; Friedler, A. Molecular Mechanism of STIL Coiled-Coil Domain Oligomerization. Int. J. Mol. Sci. 2023, 24, 14616. [Google Scholar] [CrossRef] [PubMed]
- Kratz, A.S.; Bärenz, F.; Richter, K.T.; Hoffmann, I. Plk4-dependent phosphorylation of STIL is required for centriole duplication. Biol. Open. 2015, 4, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Dzhindzhev, N.S.; Tzolovsky, G.; Lipinszki, Z.; Schneider, S.; Lattao, R.; Fu, J.; Debski, J.; Dadlez, M.; Glover, D.M. Plk4 phosphorylates Ana2 to trigger Sas6 recruitment and procentriole formation. Curr. Biol. 2014, 24, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, J.; Guichard, P.; Smith, A.H.; Schwarz, H.; Agard, D.A.; Marco, S.; Avidor-Reiss, T. Self-assembling SAS-6 multimer is a core centriole building block. J. Biol. Chem. 2010, 285, 8759–8770. [Google Scholar] [CrossRef] [PubMed]
- van Breugel, M.; Hirono, M.; Andreeva, A.; Yanagisawa, H.A.; Yamaguchi, S.; Nakazawa, Y.; Morgner, N.; Petrovich, M.; Ebong, I.O.; Robinson, C.V.; et al. Structures of SAS-6 suggest its organization in centrioles. Science 2011, 331, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Hiraki, M.; Kamiya, R.; Hirono, M. SAS-6 is a cartwheel protein that establishes the 9-fold symmetry of the centriole. Curr. Biol. 2007, 17, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Gönczy, P. Towards a molecular architecture of centriole assembly. Nat. Rev. Mol. Cell Biol. 2012, 13, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, M.; Noga, A.; Frey, D.; Hamel, V.; Guichard, P.; Kraatz, S.H.; Pfreundschuh, M.; Hosner, S.; Flückiger, I.; Jaussi, R.; et al. SAS-6 engineering reveals interdependence between cartwheel and microtubules in determining centriole architecture. Nat. Cell Biol. 2016, 18, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Cottee, M.A.; Raff, J.W.; Lea, S.M.; Roque, H. SAS-6 oligomerization: The key to the centriole? Nat. Chem. Biol. 2011, 7, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.Y.; Tang, C.J.; Tang, T.K. Protein 4.1 R-135 interacts with a novel centrosomal protein (CPAP) which is associated with the gamma-tubulin complex. Mol. Cell Biol. 2000, 20, 7813–7825. [Google Scholar] [CrossRef] [PubMed]
- Garcez, P.P.; Diaz-Alonso, J.; Crespo-Enriquez, I.; Castro, D.; Bell, D.; Guillemot, F. Cenpj/CPAP regulates progenitor divisions and neuronal migration in the cerebral cortex downstream of Ascl1. Nat. Commun. 2015, 6, 6474. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ramani, A.; Soni, K.; Gottardo, M.; Zheng, S.; Ming Gooi, L.; Li, W.; Feng, S.; Mariappan, A.; Wason, A.; et al. Molecular basis for CPAP-tubulin interaction in controlling centriolar and ciliary length. Nat. Commun. 2016, 7, 11874. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chang, C.W.; Hsu, W.B.; Tang, C.J.; Lin, Y.N.; Chou, E.J.; Wu, C.T.; Tang, T.K. Human microcephaly protein CEP135 binds to hSAS-6 and CPAP, and is required for centriole assembly. EMBO J. 2013, 32, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.S.; Baig, S.M.; Neumann, S.; Nürnberg, G.; Farooq, M.; Ahmad, I.; Alef, T.; Hennies, H.C.; Technau, M.; Altmüller, J.; et al. A truncating mutation of CEP135 causes primary microcephaly and disturbed centrosomal function. Am. J. Hum. Genet. 2012, 90, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Bamborschke, D.; Daimagüler, H.S.; Hahn, A.; Hussain, M.S.; Nürnberg, P.; Cirak, S. Mutation in CEP135 causing primary microcephaly and subcortical heterotopia. Am. J. Med. Genet. A. 2020, 182, 2450–2453. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Park, J.E.; Shukla, A.; Choi, S.; Murugan, R.N.; Lee, J.H.; Ahn, M.; Rhee, K.; Bang, J.K.; Kim, B.Y.; et al. Hierarchical recruitment of Plk4 and regulation of centriole biogenesis by two centrosomal scaffolds, Cep192 and Cep152. Proc. Natl. Acad. Sci. USA 2013, 110, E4849–E4857. [Google Scholar] [CrossRef] [PubMed]
- Cizmecioglu, O.; Arnold, M.; Bahtz, R.; Settele, F.; Ehret, L.; Haselmann-Weiss, U.; Antony, C.; Hoffmann, I. Cep152 acts as a scaffold for recruitment of Plk4 and CPAP to the centrosome. J. Cell Biol. 2010, 191, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Hsu, W.B.; Tsai, J.J.; Tang, C.J.; Tang, T.K. CEP295 interacts with microtubules and is required for centriole elongation. J. Cell Sci. 2016, 129, 2501–2513. [Google Scholar] [CrossRef] [PubMed]
- Löffler, H.; Fechter, A.; Matuszewska, M.; Saffrich, R.; Mistrik, M.; Marhold, J.; Hornung, C.; Westermann, F.; Bartek, J.; Krämer, A. Cep63 recruits Cdk1 to the centrosome: Implications for regulation of mitotic entry, centrosome amplification, and genome maintenance. Cancer Res. 2011, 71, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, R.; Teixeira, L.K. Cyclin E/CDK2: DNA Replication, Replication Stress and Genomic Instability. Front. Cell Dev. Biol. 2021, 9, 774845. [Google Scholar] [CrossRef] [PubMed]
- Tarapore, P.; Okuda, M.; Fukasawa, K. A mammalian in vitro centriole duplication system: Evidence for involvement of CDK2/cyclin E and nucleophosmin/B23 in centrosome duplication. Cell Cycle 2002, 1, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, X.; Gurian-West, M.; Roberts, J.M. Loss of cyclin-dependent kinase 2 (CDK2) inhibitory phosphorylation in a CDK2AF knock-in mouse causes misregulation of DNA replication and centrosome duplication. Mol. Cell Biol. 2012, 32, 1421–1432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okuda, M.; Horn, H.F.; Tarapore, P.; Tokuyama, Y.; Smulian, A.G.; Chan, P.K.; Knudsen, E.S.; Hofmann, I.A.; Snyder, J.D.; Bove, K.E.; et al. Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell 2000, 103, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Fisk, H.A.; Winey, M. The mouse Mps1p-like kinase regulates centrosome duplication. Cell 2001, 106, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Leidel, S.; Delattre, M.; Cerutti, L.; Baumer, K.; Gönczy, P. SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells . Nat. Cell Biol. 2005, 7, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Peel, N.; Stevens, N.R.; Basto, R.; Raff, J.W. Overexpressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Curr. Biol. 2007, 17, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Strnad, P.; Leidel, S.; Vinogradova, T.; Euteneuer, U.; Khodjakov, A.; Gönczy, P. Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev. Cell 2007, 13, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.M. Integration of the centrosome in cell cycle control, stress response and signal transduction pathways. Curr. Opin. Cell Biol. 2002, 14, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.I. The role of p34 kinases in the G1 to S-phase transition. Annu. Rev. Cell Biol. 1992, 8, 529–561. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M.; Brill, J.A.; Fink, G.R.; Weinberg, R.A. Collaboration of G1 cyclins in the functional inactivation of the retinoblastoma protein. Genes. Dev. 1994, 8, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Lentini, L.; Iovino, F.; Amato, A.; Di Leonardo, A. Centrosome amplification induced by hydroxyurea leads to aneuploidy in pRB deficient human and mouse fibroblasts. Cancer Lett. 2006, 238, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Yuen, V.W.; Chiu, D.K.; Goh, C.C.; Thu, K.L.; Cescon, D.W.; Soria-Bretones, I.; Law, C.T.; Cheu, J.W.; Lee, D.; et al. Polo-like kinase 4 inhibitor CFI-400945 suppresses liver cancer through cell cycle perturbation and eliciting antitumor immunity. Hepatology 2023, 77, 729–744. [Google Scholar] [CrossRef] [PubMed]
- Pellizzari, S.; Bhat, V.; Athwal, H.; Cescon, D.W.; Allan, A.L.; Parsyan, A. PLK4 as a potential target to enhance radiosensitivity in triple-negative breast cancer. Radiat. Oncol. 2024, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Calvo, A.; Gönczy, P.; Holland, A.J.; Balestra, F.R. TRIM37: A critical orchestrator of centrosome function. Cell Cycle 2021, 20, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Balestra, F.R.; Strnad, P.; Flückiger, I.; Gönczy, P. Discovering regulators of centriole biogenesis through siRNA-based functional genomics in human cells. Dev. Cell 2013, 25, 555–571. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Siddik, Z.H.; Huang, Z.; Wang, R.; Koomen, J.; Kobayashi, R.; Khokhar, A.R.; Kuang, J. Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene 2005, 24, 2929–2943. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Saito, H.; Takekawa, M. SAPK pathways and p53 cooperatively regulate PLK4 activity and centrosome integrity under stress. Nat Commun. 2013, 4, 1775. [Google Scholar] [CrossRef] [PubMed]
- Tarapore, P.; Fukasawa, K. Loss of p53 and centrosome hyperamplification. Oncogene 2002, 21, 6234–6240. [Google Scholar] [CrossRef] [PubMed]
- Lambrus, B.G.; Uetake, Y.; Clutario, K.M.; Daggubati, V.; Snyder, M.; Sluder, G.; Holland, A.J. p53 protects against genome instability following centriole duplication failure. J. Cell Biol. 2015, 210, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Lipsick, J. A History of Cancer Research: The P53 Pathway. Cold Spring Harb. Perspect. Med. 2025, 15, a035931. [Google Scholar] [PubMed]
- Sabat-Pośpiech, D.; Fabian-Kolpanowicz, K.; Prior, I.A.; Coulson, J.M.; Fielding, A.B. Targeting centrosome amplification, an Achilles’ heel of cancer. Biochem. Soc. Trans. 2019, 47, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Delattre, M.; Gönczy, P. The arithmetic of centrosome biogenesis. J. Cell Sci. 2004, 117 Pt 9, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Remo, A.; Li, X.; Schiebel, E.; Pancione, M. The Centrosome Linker and Its Role in Cancer and Genetic Disorders. Trends Mol Med. 2020, 26, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Kong, D.; Sharma, M.; Magidson, V.; Loncarek, J. Plk1 relieves centriole block to reduplication by promoting daughter centriole maturation. Nat. Commun. 2015, 6, 8077. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.J.; Lan, W.; Niessen, S.; Hoover, H.; Cleveland, D.W. Polo-like kinase 4 kinase activity limits centrosome overduplication by autoregulating its own stability. J. Cell Biol. 2010, 188, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kleylein-Sohn, J.; Westendorf, J.; Le Clech, M.; Habedanck, R.; Stierhof, Y.D.; Nigg, E.A. Plk4-induced centriole biogenesis in human cells. Dev. Cell 2007, 13, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Meraldi, P.; Honda, R.; Nigg, E.A. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53-/- cells. EMBO J. 2002, 21, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Inanç, B.; Dodson, H.; Morrison, C.G. A centrosome-autonomous signal that involves centriole disengagement permits centrosome duplication in G2 phase after DNA damage. Mol. Biol. Cell 2010, 21, 3866–3877. [Google Scholar] [CrossRef] [PubMed]
- Denu, R.A.; Shabbir, M.; Nihal, M.; Singh, C.K.; Longley, B.J.; Burkard, M.E.; Ahmad, N. Centriole Overduplication is the Predominant Mechanism Leading to Centrosome Amplification in Melanoma. Mol. Cancer Res. 2018, 16, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Denu, R.A.; Burkard, M.E. Analysis of the “centrosome-ome” identifies MCPH1 deletion as a cause of centrosome amplification in human cancer. Sci. Rep. 2020, 10, 11921. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.M.; Sun, X.J.; Huang, C.C.; Chen, Q.; He, Y.M.; Zhang, S.M.; Guan, H.; Song, M.; Zhou, P.K.; Hou, J. Inhibition of c-Myc expression accounts for an increase in the number of multinucleated cells in human cervical epithelial cells. Oncol. Lett. 2017, 14, 2878–2886. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Courapied, S.; Cherier, J.; Vigneron, A.; Troadec, M.B.; Giraud, S.; Valo, I.; Prigent, C.; Gamelin, E.; Coqueret, O.; Barré, B. Regulation of the Aurora-A gene following topoisomerase I inhibition: Implication of the Myc transcription factor. Mol. Cancer. 2010, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Khodjakov, A.; Rieder, C.L. Mitosis: Too much of a good thing (can be bad). Curr. Biol. 2009, 19, R1032-4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, S.; Guerrero-Haughton, E.; Foijer, F. Chromosomal Instability-Driven Cancer Progression: Interplay with the Tumour Microenvironment and Therapeutic Strategies. Cells 2023, 12, 2712. [Google Scholar] [CrossRef] [PubMed]
- Godinho, S.A.; Picone, R.; Burute, M.; Dagher, R.; Su, Y.; Leung, C.T.; Polyak, K.; Brugge, J.S.; Théry, M.; Pellman, D. Oncogene-like induction of cellular invasion from centrosome amplification. Nature 2014, 510, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Kulukian, A.; Holland, A.J.; Vitre, B.; Naik, S.; Cleveland, D.W.; Fuchs, E. Epidermal development, growth control, and homeostasis in the face of centrosome amplification. Proc. Natl. Acad. Sci. USA 2015, 112, E6311–E6320. [Google Scholar] [CrossRef] [PubMed]
- Marthiens, V.; Rujano, M.A.; Pennetier, C.; Tessier, S.; Paul-Gilloteaux, P.; Basto, R. Centrosome amplification causes microcephaly. Nat Cell Biol. 2013, 15, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Thornton, G.K.; Woods, C.G. Primary microcephaly: Do all roads lead to Rome? Trends Genet. 2009, 25, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Chavali, P.L.; Pütz, M.; Gergely, F. Small organelle, big responsibility: The role of centrosomes in development and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130468. [Google Scholar] [CrossRef] [PubMed]
- Goundiam, O.; Basto, R. Centrosomes in disease: How the same music can sound so different? Curr. Opin. Struct. Biol. 2021, 66, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y. A clinical overview of centrosome amplification in human cancers. Int. J. Biol. Sci. 2011, 7, 1122–1144. [Google Scholar] [CrossRef] [PubMed]
- Marteil, G.; Guerrero, A.; Vieira, A.F.; de Almeida, B.P.; Machado, P.; Mendonça, S.; Mesquita, M.; Villarreal, B.; Fonseca, I.; Francia, M.E.; et al. Over-elongation of centrioles in cancer promotes centriole amplification and chromosome missegregation. Nat. Commun. 2018, 9, 1258. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, E.; Kanai, M.; Matsui, A.; Onodera, M.; Schwab, M.; Miwa, M. Enhanced expression of MYCN leads to centrosome hyperamplification after DNA damage in neuroblastoma cells. Oncogene 2004, 23, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, F.C.; Kroes, J.; Lewin, J. Targeting the centrosome and polo-like kinase 4 in osteosarcoma. Carcinogenesis 2019, 40, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Méniel, V.; Megges, M.; Young, M.A.; Cole, A.; Sansom, O.J.; Clarke, A.R. Apc and p53 interaction in DNA damage and genomic instability in hepatocytes. Oncogene 2015, 34, 4118–4129. [Google Scholar] [CrossRef] [PubMed]
- Caulin, C.; Nguyen, T.; Lang, G.A.; Goepfert, T.M.; Brinkley, B.R.; Cai, W.W.; Lozano, G.; Roop, D.R. An inducible mouse model for skin cancer reveals distinct roles for gain- and loss-of-function p53 mutations. J. Clin. Investig. 2007, 117, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, X. PLK4: A promising target for cancer therapy. J. Cancer Res. Clin. Oncol. 2019, 145, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, M.; Li, L.; Pamarthy, D.; Lawrence, T.S.; Sun, Y. SAK, a new polo-like kinase, is transcriptionally repressed by p53 and induces apoptosis upon RNAi silencing. Neoplasia 2005, 7, 312–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, Y.; Xue, Y.; Liu, H.; Mu, S.; Sun, P.; Sun, Y.; Wang, L.; Wang, H.; Wang, J.; Wu, T.; et al. Discovery of CZS-241: A Potent, Selective, and Orally Available Polo-Like Kinase 4 Inhibitor for the Treatment of Chronic Myeloid Leukemia. J. Med. Chem. 2023, 66, 2396–2421. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.; Mason, J.M.; Leber, B.; Bray, M.R.; Chan, S.M.; Gupta, V.; Khalaf, D.; Maze, D.; McNamara, C.J.; Schimmer, A.D.; et al. Preclinical characterization and clinical trial of CFI-400945, a polo-like kinase 4 inhibitor, in patients with relapsed/refractory acute myeloid leukemia and higher-risk myelodysplastic neoplasms. Leukemia 2024, 38, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xie, B. PLK4 inhibitor exhibits antitumor effect and synergizes sorafenib via arresting cell cycle and inactivating Wnt/β-catenin pathway in anaplastic thyroid cancer. Cancer Biol. Ther. 2023, 24, 2223383. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, M.; Meitinger, F.; Ohta, M. PLK4: Master Regulator of Centriole Duplication and Its Therapeutic Potential. Cytoskeleton, 2025; Epub ahead of print. [Google Scholar]
- Farcy, S.; Hachour, H.; Bahi-Buisson, N.; Passemard, S. Genetic Primary Microcephalies: When Centrosome Dysfunction Dictates Brain and Body Size. Cells 2023, 12, 1807. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Girimaji, S.C.; Duvvari, M.R.; Blanton, S.H. Mutations in STIL, encoding a pericentriolar and centrosomal protein, cause primary microcephaly. Am. J. Hum. Genet. 2009, 84, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.N.; Lee, Y.S.; Li, S.K.; Tang, T.K. Loss of CPAP in developing mouse brain and its functional implication for human primary microcephaly. J. Cell Sci. 2020, 133, jcs243592. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.P.; Holland, A.J. Time is of the essence: The molecular mechanisms of primary microcephaly. Genes. Dev. 2021, 35, 1551–1578. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hu, X.; Ma, J.; Shi, S.H. Centrosome regulation and function in mammalian cortical neurogenesis. Curr. Opin. Neurobiol. 2021, 69, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Papuc, S.M.; Erbescu, A.; Glangher, A.; Streata, I.; Riza, A.L.; Budisteanu, M.; Arghir, A. Autistic Behavior as Novel Clinical Finding in OFD1 Syndrome. Genes 2023, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Chen, D.Z.; Engchuan, W.; Leal, T.P.; Thiruvahindrapuram, B.; Trost, B.; Howe, J.L.; Pellecchia, G.; Nalpathamkalam, T.; Alexandrova, R.; et al. Chromosome X-wide common variant association study in autism spectrum disorder. Am. J. Hum. Genet. 2025, 112, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, M.R.; Stearns, T. Supernumerary centrosomes nucleate extra cilia and compromise primary cilium signaling. Curr. Biol. 2012, 22, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Karalis, V.; Donovan, K.E.; Sahin, M. Primary Cilia Dysfunction in Neurodevelopmental Disorders beyond Ciliopathies. J. Dev. Biol. 2022, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Fandrey, J.; Leu, T. Primary cilia as antennas for oxygen. Am. J. Physiol. Cell Physiol. 2025, 328, C381–C386. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Roy, S.; Li, L.; Ma, M. Polycystic kidney disease: Novel insights into polycystin function. Trends Mol. Med. 2023, 29, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, Z.; Yang, F.; Zhou, T.; Xie, S. Deciphering cilia and ciliopathies using proteomic approaches. FEBS J. 2023, 290, 2590–2603. [Google Scholar] [CrossRef] [PubMed]
- Chrétien, D.; Buendia, B.; Fuller, S.D.; Karsenti, E. Reconstruction of the centrosome cycle from cryoelectron micrographs. J. Struct. Biol. 1997, 120, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Kantsadi, A.L.; Hatzopoulos, G.N.; Gönczy, P.; Vakonakis, I. Structures of SAS-6 coiled coil hold implications for the polarity of the centriolar cartwheel. Structure 2022, 30, 671–684.e5. [Google Scholar] [CrossRef] [PubMed]
- Agostini, L.; Pfister, J.; Basnet, N.; Ding, J.; Zhang, R.; Biertümpfel, C.; O’Connell, K.F.; Mizuno, N. Structural insights into SSNA1 self-assembly and its microtubule binding for centriole maintenance. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fernandez, J.J.; Marshall, W.F.; Agard, D.A. Electron cryo-tomography provides insight into procentriole architecture and assembly mechanism. Elife 2019, 8, e43434. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Hofer, F.W.; Filbeck, S.; Vermeulen, B.J.A.; Würtz, M.; Neuner, A.; Kaplan, C.; Zezlina, M.; Sala, C.; Shin, H.; et al. Structural mechanisms for centrosomal recruitment and organization of the microtubule nucleator γ-TuRC. Nat. Commun. 2025, 16, 2453. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.; Lee, Y.L.; Sahl, S.J.; Stearns, T.; Moerner, W.E. STED microscopy with optimized labeling density reveals 9-fold arrangement of a centriole protein. Biophys. J. 2012, 102, 2926–2935. [Google Scholar] [CrossRef] [PubMed]
- Sonnen, K.F.; Schermelleh, L.; Leonhardt, H.; Nigg, E.A. 3D-structured illumination microscopy provides novel insight into architecture of human centrosomes. Biol. Open. 2012, 1, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Laporte, M.H.; Gambarotto, D.; Bertiaux, É.; Bournonville, L.; Louvel, V.; Nunes, J.M.; Borgers, S.; Hamel, V.; Guichard, P. Time-series reconstruction of the molecular architecture of human centriole assembly. Cell 2024, 187, 2158–2174.e19. [Google Scholar] [CrossRef] [PubMed]
- Woglar, A.; Pierron, M.; Schneider, F.Z.; Jha, K.; Busso, C.; Gönczy, P. Molecular architecture of the C. elegans centriole. PLoS Biol. 2022, 20, e3001784. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, F.; Mahdessian, D.; Axelsson, U.; Sullivan, D.; Uhlén, M.; Andersen, J.S.; Thul, P.J.; Lundberg, E. Spatial Characterization of the Human Centrosome Proteome Opens Up New Horizons for a Small but Versatile Organelle. Proteomics 2020, 20, e1900361. [Google Scholar] [CrossRef] [PubMed]
- Cerulo, L.; Pezzella, N.; Caruso, F.P.; Parente, P.; Remo, A.; Giordano, G.; Forte, N.; Busselez, J.; Boschi, F.; Galiè, M.; et al. Single-cell proteo-genomic reveals a comprehensive map of centrosome-associated spliceosome components. iScience 2023, 26, 106602. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, A.P.; Gavard, O.; Gagné, J.P.; Rogalska, M.E.; Behera, A.K.; Mancini, E.; Bertolin, G.; Courtheoux, T.; Kumari, B.; Cailloce, J.; et al. Proteomic study identifies Aurora-A-mediated regulation of alternative splicing through multiple splicing factors. J. Biol. Chem. 2025, 301, 108000. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prigent, C. Centriole Duplication at the Crossroads of Cell Cycle Control and Oncogenesis. Cells 2025, 14, 1094. https://doi.org/10.3390/cells14141094
Prigent C. Centriole Duplication at the Crossroads of Cell Cycle Control and Oncogenesis. Cells. 2025; 14(14):1094. https://doi.org/10.3390/cells14141094
Chicago/Turabian StylePrigent, Claude. 2025. "Centriole Duplication at the Crossroads of Cell Cycle Control and Oncogenesis" Cells 14, no. 14: 1094. https://doi.org/10.3390/cells14141094
APA StylePrigent, C. (2025). Centriole Duplication at the Crossroads of Cell Cycle Control and Oncogenesis. Cells, 14(14), 1094. https://doi.org/10.3390/cells14141094







