Fundus Autofluorescence in Inherited Retinal Disease: A Review
Abstract
1. Introduction
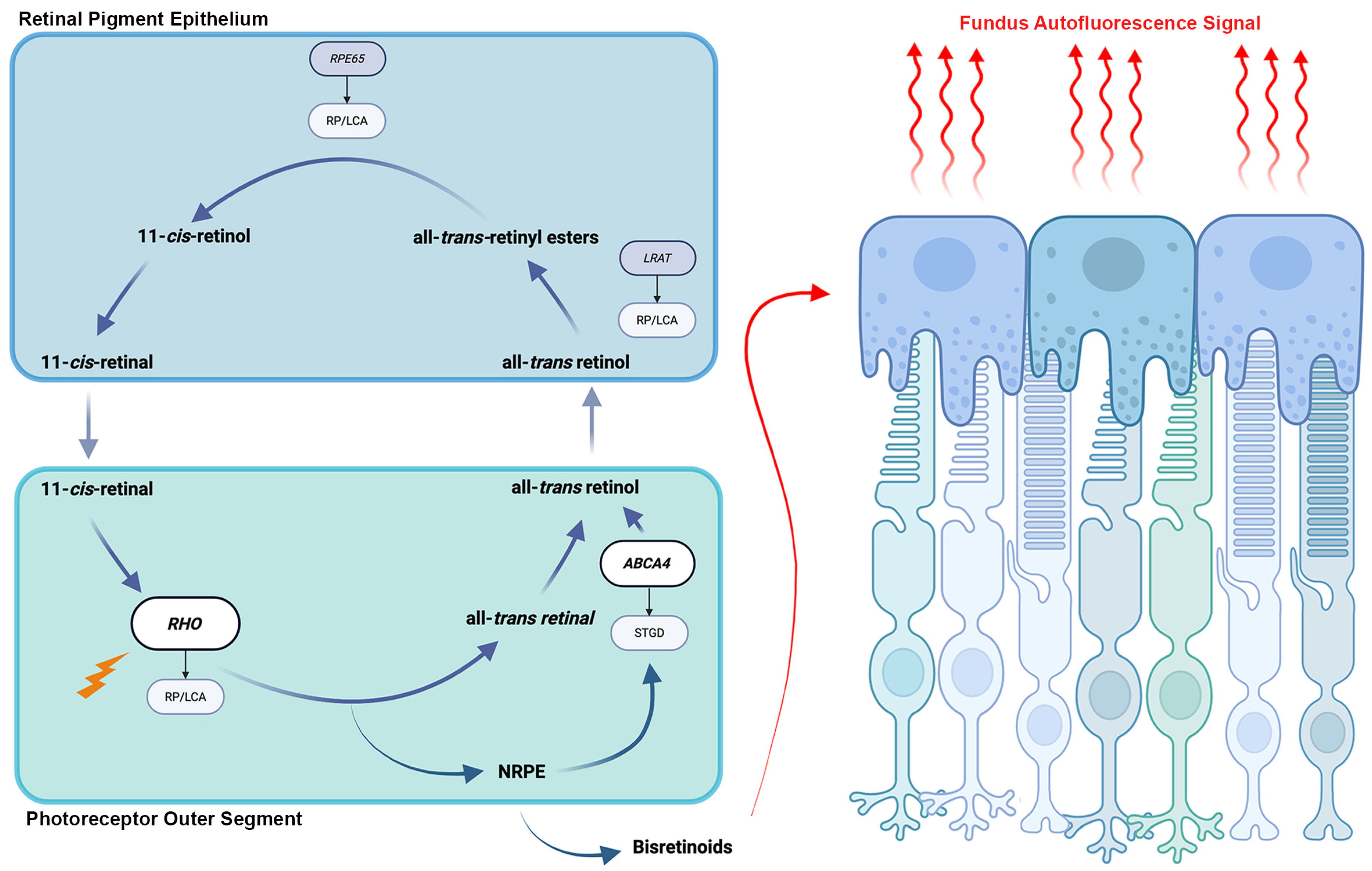
2. Physiologic Basis and Clinical Relevance
3. Fundus Autofluorescence Features Across Inherited Retinal Disease
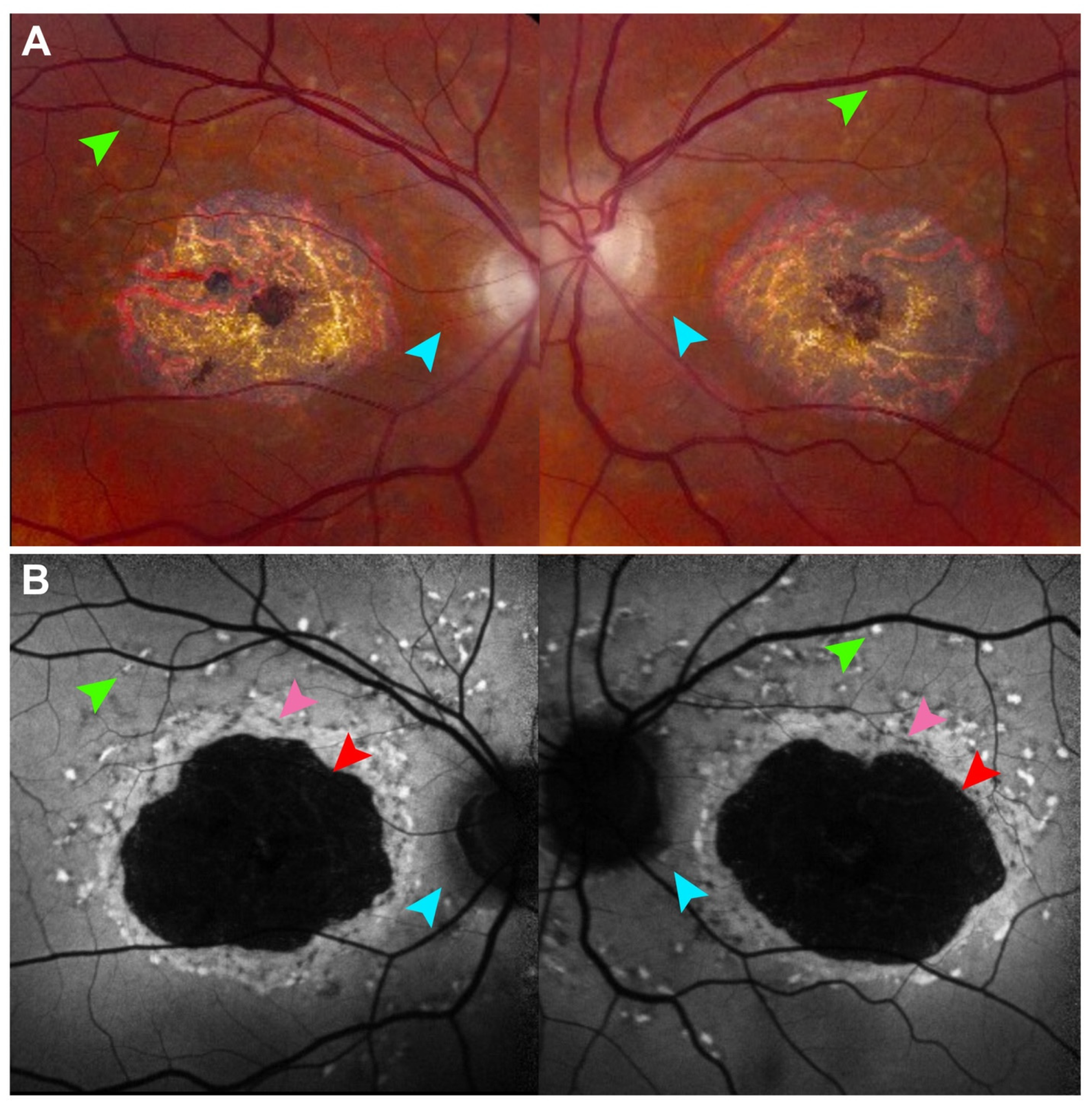
4. Retinitis Pigmentosa and Other Rod–Cone Dystrophies
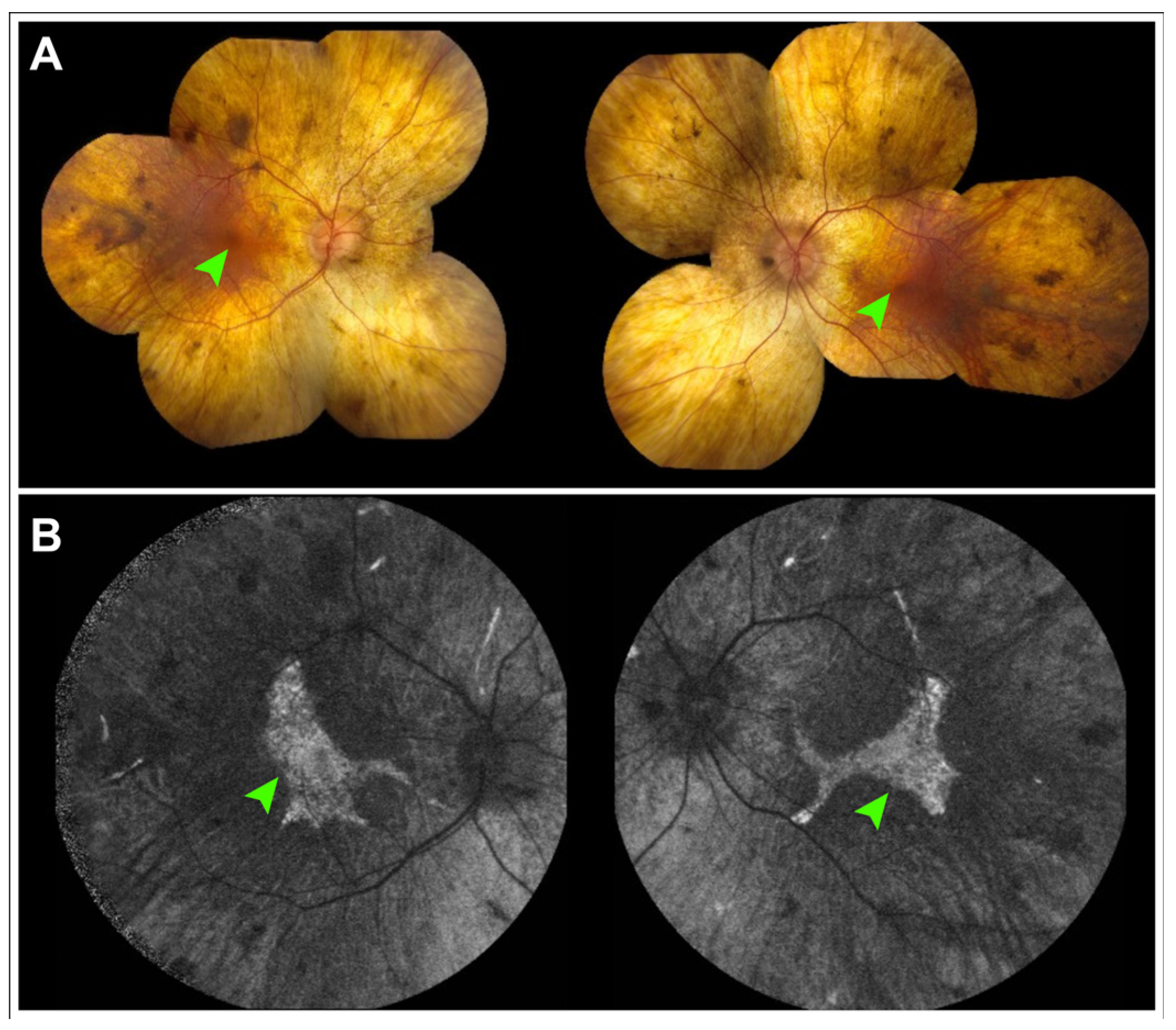
5. Choroideremia
6. Mitochondrial Disease
7. Fundus Autofluorescence as a Quantitative Outcome Measure in Inherited Retinal Disease Natural History Studies and Clinical Trials
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Delori, F.C.; Dorey, C.K.; Staurenghi, G.; Arend, O.; Goger, D.G.; Weiter, J.J. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Investig. Ophthalmol. Vis. Sci. 1995, 36, 718–729. [Google Scholar]
- von Rückmann, A.; Fitzke, F.W.; Bird, A.C. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br. J. Ophthalmol. 1995, 79, 407–412. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Gregory-Roberts, E.; Yamamoto, K.; Blonska, A.; Ghosh, S.K.; Ueda, K.; Zhou, J. The bisretinoids of retinal pigment epithelium. Prog. Retin. Eye Res. 2012, 31, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Boulton, M. RPE lipofuscin and its role in retinal pathobiology. Exp. Eye Res. 2005, 80, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Yin, D. Biochemical basis of lipofuscin, ceroid, and age pigment-like fluorophores. Free Radic. Biol. Med. 1996, 21, 871–888. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Bellmann, C.; Margaritidis, M.; Schütt, F.; Otto, T.P.; Völcker, H.E. Patterns of increased in vivo fundus autofluorescence in the junctional zone of geographic atrophy of the retinal pigment epithelium associated with age-related macular degeneration. Graefe’ s Arch. Clin. Exp. Ophthalmol. 1999, 237, 145–152. [Google Scholar] [CrossRef]
- Holz, F.G.; Bellman, C.; Staudt, S.; Schütt, F.; Völcker, H.E. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1051–1056. [Google Scholar] [CrossRef]
- Spaide, R.F. Autofluorescence imaging of acute posterior multifocal placoid pigment epitheliopathy. Retina 2006, 26, 479–482. [Google Scholar] [CrossRef]
- Haen, S.P.; Spaide, R.F. Fundus autofluorescence in multifocal choroiditis and panuveitis. Am. J. Ophthalmol. 2008, 145, 847–853. [Google Scholar] [CrossRef]
- Katz, M.L.; Eldred, G.E.; Robison, W.G., Jr. Lipofuscin autofluorescence: Evidence for vitamin A involvement in the retina. Mech. Ageing Dev. 1987, 39, 81–90. [Google Scholar] [CrossRef]
- Lenis, T.L.; Botsford, B.W.; Sarraf, D.; Papakostas, T.D. Didanosine-Associated Retinal Toxicity in a Patient with a Mutation in the CRB1 Gene. J. Vitreoretin. Dis. 2021, 6, 329–331. [Google Scholar] [CrossRef]
- Gelman, R.; Kiss, S.; Tsang, S.H. Multimodal imaging in a case of deferoxamine-induced maculopathy. Retin. Cases Brief Rep. 2014, 8, 306–309. [Google Scholar] [CrossRef]
- Schneider, N.; Sundaresan, Y.; Gopalakrishnan, P.; Beryozkin, A.; Hanany, M.; Levanon, E.Y.; Banin, E.; Ben-Aroya, S.; Sharon, D. Inherited retinal diseases: Linking genes, disease-causing variants, and relevant therapeutic modalities. Prog. Retin. Eye Res. 2022, 89, 101029. [Google Scholar] [CrossRef]
- Pichi, F.; Abboud, E.B.; Ghazi, N.G.; Khan, A.O. Fundus autofluorescence imaging in hereditary retinal diseases. Acta Ophthalmol. 2018, 96, e549–e561. [Google Scholar] [CrossRef]
- Eldred, G.E.; Lasky, M.R. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature 1993, 361, 724–726. [Google Scholar] [CrossRef]
- Li, J.; Yao, K.; Yu, X.; Dong, X.; Gan, L.; Luo, C.; Wu, Y. Identification of a novel lipofuscin pigment (iisoA2E) in retina and its effects in the retinal pigment epithelial cells. J. Biol. Chem. 2013, 288, 35671–35682. [Google Scholar] [CrossRef]
- Boulton, M.; Docchio, F.; Dayhaw-Barker, P.; Ramponi, R.; Cubeddu, R. Age-related changes in the morphology, absorption and fluorescence of melanosomes and lipofuscin granules of the retinal pigment epithelium. Vis. Res. 1990, 30, 1291–1303. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Nakanishi, K.; Parish, C.A. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1981–1989. [Google Scholar]
- Schultz, R.; Hasan, S.; Curcio, C.A.; Smith, R.T.; Meller, D.; Hammer, M. Spectral and lifetime resolution of fundus autofluorescence in advanced age-related macular degeneration revealing different signal sources. Acta Ophthalmol. 2022, 100, e841–e846. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.; Sauer, L.; Klemm, M.; Peters, S.; Schultz, R.; Haueisen, J. Fundus autofluorescence beyond lipofuscin: Lesson learned from ex vivo fluorescence lifetime imaging in porcine eyes. Biomed. Opt. Express 2018, 9, 3078–3091. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Valckenberg, S.; Pfau, M.; Fleckenstein, M.; Staurenghi, G.; Sparrow, J.R.; Bindewald-Wittich, A.; Spaide, R.F.; Wolf, S.; Sadda, S.R.; Holz, F.G.; et al. Fundus autofluorescence imaging. Prog. Retin. Eye Res. 2021, 81, 100893. [Google Scholar] [CrossRef]
- Duisdieker, V.; Fleckenstein, M.; Zilkens, K.M.; Steinberg, J.S.; Holz, F.G.; Schmitz-Valckenberg, S. Long-Term Follow-Up of Fundus Autofluorescence Imaging Using Wide-Field Scanning Laser Ophthalmoscopy. Ophthalmologica 2015, 234, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Keilhauer, C.N.; Delori, F.C. Near-infrared autofluorescence imaging of the fundus: Visualization of ocular melanin. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3556–3564. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, A.W.; Lappas, A.; Kirschkamp, T.; Mazinani, B.A.E.; Huth, J.K.; Mohammadi, B.; Walter, P. Fundus near infrared fluorescence correlates with fundus near infrared reflectance. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3098–3108. [Google Scholar] [CrossRef]
- Kellner, S.; Weinitz, S.; Farmand, G.; Kellner, U. Near-Infrared Autofluorescence: Early Detection of Retinal Pigment Epithelial Alterations in Inherited Retinal Dystrophies. J. Clin. Med. 2024, 13, 6886. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Duncker, T.; Schuerch, K.; Paavo, M.; de Carvalho, J.R.L., Jr. Lessons learned from quantitative fundus autofluorescence. Prog. Retin. Eye Res. 2020, 74, 100774. [Google Scholar] [CrossRef]
- Delori, F.; Greenberg, J.P.; Woods, R.L.; Fischer, J.; Duncker, T.; Sparrow, J.; Smith, R.T. Quantitative measurements of autofluorescence with the scanning laser ophthalmoscope. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9379–9390. [Google Scholar] [CrossRef]
- Allikmets, R.; Singh, N.; Sun, H.; Shroyer, N.F.; Hutchinson, A.; Chidambaram, A.; Gerrard, B.; Baird, L.; Stauffer, D.; Peiffer, A.; et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat. Genet. 1997, 15, 236–246, Erratum in Nat. Genet. 1997, 17, 122. [Google Scholar] [CrossRef]
- Radu, R.A.; Han, Y.; Bui, T.V.; Nusinowitz, S.; Bok, D.; Lichter, J.; Widder, K.; Travis, G.H.; Mata, N.L. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: A potential therapy for treatment of lipofuscin-based retinal diseases. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4393–4401, Erratum in Investig. Ophthalmol. Vis. Sci. 2006, 47, 3735. [Google Scholar] [CrossRef]
- von Rückmann, A.; Fitzke, F.W.; Bird, A.C. In vivo fundus autofluorescence in macular dystrophies. Arch. Ophthalmol. 1997, 115, 609–615. [Google Scholar] [CrossRef]
- Burke, T.R.; Rhee, D.W.; Smith, R.T.; Tsang, S.H.; Allikmets, R.; Chang, S.; Lazow, M.A.; Hood, D.C.; Greenstein, V.C. Quantification of peripapillary sparing and macular involvement in Stargardt disease (STGD1). Investig. Ophthalmol. Vis. Sci. 2011, 52, 8006–8015. [Google Scholar] [CrossRef]
- Kong, X.; West, S.K.; Strauss, R.W.; Munoz, B.; Cideciyan, A.V.; Michaelides, M.; Ho, A.; Ahmed, M.; Schönbach, E.M.; Cheetham, J.K.; et al. Progression of Visual Acuity and Fundus Autofluorescence in Recent-Onset Stargardt Disease: ProgStar Study Report #4. Ophthalmol. Retin. 2017, 1, 514–523. [Google Scholar]
- Strauss, R.W.; Muñoz, B.; Ho, A.; Jha, A.; Michaelides, M.; Cideciyan, A.V.; Audo, I.; Birch, D.G.; Hariri, A.H.; Nittala, M.G.; et al. Progression of Stargardt Disease as Determined by Fundus Autofluorescence in the Retrospective Progression of Stargardt Disease Study (ProgStar Report No. 9). JAMA Ophthalmol. 2017, 135, 1232–1241. [Google Scholar] [CrossRef]
- Strauss, R.W.; Ho, A.; Jha, A.; Fujinami, K.; Michaelides, M.; Cideciyan, A.V.; Audo, I.; Birch, D.G.; Sadda, S.; Ip, M.; et al. Progression of Stargardt Disease as Determined by Fundus Autofluorescence over a 24-Month Period (ProgStar Report No. 17). Am. J. Ophthalmol. 2023, 250, 157–170. [Google Scholar] [CrossRef]
- Celia, W.; Greenstein, V.C.; Zernant-Rajang, J.; Smith, T.R.; Barile, G.; Allikmets, R.; Tsang, S.H. G1961E mutant allele in the Stargardt disease gene ABCA4 causes bull’s eye maculopathy. Exp. Eye Res. 2009, 89, 16–24. [Google Scholar] [CrossRef]
- Cremers, F.P.M.; Lee, W.; Collin, R.W.J.; Allikmets, R. Clinical spectrum, genetic complexity and therapeutic approaches for retinal disease caused by ABCA4 mutations. Prog. Retin. Eye Res. 2020, 79, 100861. [Google Scholar] [CrossRef]
- Duncker, T.; Tsang, S.H.; Lee, W.; Zernant, J.; Allikmets, R.; Delori, F.C.; Sparrow, J.R. Quantitative fundus autofluorescence distinguishes ABCA4-associated and non-ABCA4-associated bull’s-eye maculopathy. Ophthalmology 2015, 122, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Ayyagari, R.; Borooah, S.; Durham, T.; Gelfman, C.; Bowman, A. Current and Future Directions in Developing Effective Treatments for PRPH2-Associated Retinal Diseases: A Workshop Report. Transl. Vis. Sci. Technol. 2024, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, G.; Parravano, M.; Barbano, L.; Costanzo, E.; Bertelli, M.; Medori, M.C.; Parisi, V.; Ziccardi, L. Multimodal Study of PRPH2 Gene-Related Retinal Phenotypes. Diagnostics 2022, 12, 1851. [Google Scholar] [CrossRef] [PubMed]
- Duncker, T.; Tsang, S.H.; Woods, R.L.; Lee, W.; Zernant, J.; Allikmets, R.; Delori, F.C.; Sparrow, J.R. Quantitative Fundus Autofluorescence and Optical Coherence Tomography in PRPH2/RDS- and ABCA4-Associated Disease Exhibiting Phenotypic Overlap. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3159–3170. [Google Scholar] [CrossRef]
- Duncker, T.; Greenberg, J.P.; Ramachandran, R.; Hood, D.C.; Smith, R.T.; Hirose, T.; Woods, R.L.; Tsang, S.H.; Delori, F.C.; Sparrow, J.R. Quantitative fundus autofluorescence and optical coherence tomography in best vitelliform macular dystrophy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1471–1482. [Google Scholar] [CrossRef]
- Mohler, C.W.; Fine, S.L. Long-term evaluation of patients with Best’ s vitelliform dystrophy. Ophthalmology 1981, 88, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Jarc-Vidmar, M.; Kraut, A.; Hawlina, M. Fundus autofluorescence imaging in Best’ s vitelliform dystrophy. Klin. Monatsblätter Augenheilkd. 2003, 220, 861–867. [Google Scholar]
- Parodi, M.B.; Iacono, P.; Campa, C.; Del Turco, C.; Bandello, F. Fundus autofluorescence patterns in Best vitelliform macular dystrophy. Am. J. Ophthalmol. 2014, 158, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Lima de Carvalho, J.R.; Oh, J.K.; Ragi, S.; Kim, J.; Manzi, R.S.; Sun, E.; Cabral, T.; Belfort, R.; Greenstein, V.C.; Sparrow, J.R.; et al. Multimodal Imaging and Dark-Adapted Chromatic Perimetry in BEST1 Vitelliform Macular Dystrophy: Identification of Outcome Measurements. Ophthalmol. Sci. 2025, 5, 100823. [Google Scholar] [CrossRef]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Lois, N.; Forrester, J.V. Fundus Autofluorescence; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Tojo, N.; Nakamura, T.; Fuchizawa, C.; Oiwake, T.; Hayashi, A. Adaptive optics fundus images of cone photoreceptors in the macula of patients with retinitis pigmentosa. Clin. Ophthalmol. 2013, 7, 203–210. [Google Scholar] [CrossRef][Green Version]
- Nassisi, M.; Lavia, C.; Mohand-Said, S.; Smirnov, V.; Antonio, A.; Condroyer, C.; Sancho, S.; Varin, J.; Gaudric, A.; Zeitz, C.; et al. Near-infrared fundus autofluorescence alterations correlate with swept-source optical coherence tomography angiography findings in patients with retinitis pigmentosa. Sci. Rep. 2021, 11, 3180. [Google Scholar] [CrossRef]
- Cabral, T.; Sengillo, J.D.; Duong, J.K.; Justus, S.; Boudreault, K.; Schuerch, K.; Belfort, R.; Mahajan, V.B.; Sparrow, J.R.; Tsang, S.H. Retrospective Analysis of Structural Disease Progression in Retinitis Pigmentosa Utilizing Multimodal Imaging. Sci. Rep. 2017, 7, 10347. [Google Scholar] [CrossRef]
- Jauregui, R.; Takahashi, V.K.L.; Park, K.S.; Cui, X.; Takiuti, J.T.; de Carvalho, J.R.L.; Tsang, S.H. Multimodal structural disease progression of retinitis pigmentosa according to mode of inheritance. Sci. Rep. 2019, 9, 10712. [Google Scholar] [CrossRef]
- Iga, Y.; Hasegawa, T.; Ikeda, H.O.; Hirota, Y.; Miyata, M.; Numa, S.; Otsuka, Y.; Tsujikawa, A. Progression of retinitis pigmentosa on static perimetry, optical coherence tomography, and fundus autofluorescence. Sci. Rep. 2023, 13, 22040. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, V.K.L.; Takiuti, J.T.; Carvalho-Jr, J.R.L.; Xu, C.L.; Duong, J.K.; Mahajan, V.B.; Tsang, S.H. Fundus autofluorescence and ellipsoid zone (EZ) line width can be an outcome measurement in RHO-associated autosomal dominant retinitis pigmentosa. Graefe’ s Arch. Clin. Exp. Ophthalmol. 2019, 257, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.S.; Theofylaktopoulos, V.; Mitsios, A.; Houston, S.; Hagag, A.M.; Dubis, A.M.; Moosajee, M. Investigating Biomarkers for USH2A Retinopathy Using Multimodal Retinal Imaging. Int. J. Mol. Sci. 2022, 23, 4198. [Google Scholar] [CrossRef]
- Maguire, M.G.; Birch, D.G.; Duncan, J.L.; Ayala, A.R.; Ayton, L.N.; Cheetham, J.K.; Cheng, P.; Durham, T.A.; Ferris, F.L.; Hoyng, C.B.; et al. Endpoints and Design for Clinical Trials in USH2A-Related Retinal Degeneration: Results and Recommendations from the RUSH2A Natural History Study. Transl. Vis. Sci. Technol. 2024, 13, 15. [Google Scholar] [CrossRef]
- Marques, J.P.; Porto, F.B.O.; Carvalho, A.L.; Neves, E.; Chen, R.; Sampaio, S.A.M.; Murta, J.; Saraiva, J.; Silva, R. EYS-Associated Sector Retinitis Pigmentosa. Graefe’ s Arch. Clin. Exp. Ophthalmol. 2022, 260, 1405–1413. [Google Scholar] [CrossRef]
- Jauregui, R.; Chan, L.; Oh, J.K.; Cho, A.; Sparrow, J.R.; Tsang, S.H. Disease asymmetry and hyperautofluorescent ring shape in retinitis pigmentosa patients. Sci. Rep. 2020, 10, 3364. [Google Scholar] [CrossRef]
- Dowd-Schoeman, T.J.; Rosenbloom, J.; Ameri, H. Patterns of Autofluorescence in Common Genotypes of Retinitis Pigmentosa. Ophthalmic Surg. Lasers Imaging Retin. 2021, 52, 426–431. [Google Scholar] [CrossRef]
- Oh, J.K.; Lima de Carvalho, J.R., Jr.; Ryu, J.; Tsang, S.H.; Sparrow, J.R. Short-Wavelength and Near-Infrared Autofluorescence in Patients with Deficiencies of the Visual Cycle and Phototransduction. Sci. Rep. 2020, 10, 8998. [Google Scholar] [CrossRef]
- Lorenz, B.; Wabbels, B.; Wegscheider, E.; Hamel, C.P.; Drexler, W.; Preising, M.N. Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. Ophthalmology 2004, 111, 1585–1594. [Google Scholar] [CrossRef]
- Levi, S.R.; Oh, J.K.; de Carvalho, J.R.L., Jr.; Mahajan, V.B.; Tsang, S.H.; Sparrow, J.R. Quantitative Autofluorescence Following Gene Therapy with Voretigene Neparvovec. JAMA Ophthalmol. 2020, 138, 919–921. [Google Scholar] [CrossRef]
- Kolesnikova, M.; Lima de Carvalho, J.R., Jr.; Parmann, R.; Kim, A.H.; Mahajan, V.B.; Tsang, S.H.; Sparrow, J.R. Chorioretinal atrophy following voretigene neparvovec despite the presence of fundus autofluorescence. Mol. Genet. Genom. Med. 2022, 10, e2038. [Google Scholar] [CrossRef]
- Lorenz, B.; Künzel, S.H.; Preising, M.N.; Scholz, J.P.; Chang, P.; Holz, F.G.; Herrmann, P. Single Center Experience with Voretigene Neparvovec Gene Augmentation Therapy in RPE65 Mutation-Associated Inherited Retinal Degeneration in a Clinical Setting. Ophthalmology 2024, 131, 161–178. [Google Scholar] [CrossRef]
- van den Hurk, J.A.; Schwartz, M.; van Bokhoven, H.; Van de Pol, T.J.R.; Bogerd, L.; Pinckers, A.J.L.G.; Bleeker-Wagemakers, E.M.; Pawlowitzki, I.H.; Rüther, K.; Ropers, H.-H.; et al. Molecular basis of choroideremia (CHM): Mutations involving the rab escort protein-1 (REP-1) gene. Hum. Mutat. 1997, 9, 110–117. [Google Scholar] [CrossRef]
- Poli, F.E.; MacLaren, R.E.; Cehajic-Kapetanovic, J. Retinal Patterns and the Role of Autofluorescence in Choroideremia. Genes 2024, 15, 1471. [Google Scholar] [CrossRef] [PubMed]
- Poli, F.E.; Yusuf, I.H.; Jolly, J.K.; Taylor, L.J.; Adeyoju, D.; Josan, A.S.; Birtel, J.; Issa, P.C.; Cehajic-Kapetanovic, J.; Da Cruz, L.; et al. Correlation Between Fundus Autofluorescence Pattern and Retinal Function on Microperimetry in Choroideremia. Transl. Vis. Sci. Technol. 2023, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Oldani, M.; Jolly, J.K.; Edwards, T.; Groppe, M.; Downes, S.M.; MacLaren, R. Correlation of Optical Coherence Tomography and Autofluorescence in the Outer Retina and Choroid of Patients With Choroideremia. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3674–3684. [Google Scholar] [CrossRef]
- Maclaren, R.E.; Lam, B.L.; Fischer, M.D.; Holz, F.G.; Pennesi, M.E.; Birch, D.G.; Sankila, E.-M.; Meunier, I.A.; Stepien, K.E.; Sallum, J.M.F.; et al. A Prospective, Observational, Non-interventional Clinical Study of Participants With Choroideremia: The NIGHT Study. Am. J. Ophthalmol. 2024, 263, 35–49. [Google Scholar] [CrossRef]
- Jauregui, R.; Park, K.S.; Tanaka, A.J.; Cho, A.; Paavo, M.; Zernant, J.; Francis, J.H.; Allikmets, R.; Sparrow, J.R.; Tsang, S.H. Spectrum of Disease Severity and Phenotype in Choroideremia Carriers. Am. J. Ophthalmol. 2019, 207, 77–86. [Google Scholar] [CrossRef]
- Paavo, M.; Carvalho, J.R.L., Jr.; Lee, W.; Sengillo, J.D.; Tsang, S.H.; Sparrow, J.R. Patterns and Intensities of Near-Infrared and Short-Wavelength Fundus Autofluorescence in Choroideremia Probands and Carriers. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3752–3761. [Google Scholar] [CrossRef]
- Goto, Y.; Nonaka, I.; Horai, S. A mutation in the tRNALeu(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 1990, 348, 651–653. [Google Scholar] [CrossRef]
- Rath, P.P.; Jenkins, S.; Michaelides, M.; Smith, A.; Sweeney, M.G.; Davis, M.B.; Fitzke, F.W.; Bird, A.C. Characterisation of the macular dystrophy in patients with the A3243G mitochondrial DNA point mutation with fundus autofluorescence. Br. J. Ophthalmol. 2008, 92, 623–629. [Google Scholar] [CrossRef]
- Ovens, C.A.; Ahmad, K.; Fraser, C.L. Fundus Autofluorescence in Maternally Inherited Diabetes and Deafness: The Gold Standard for Monitoring Maculopathy? Neuroophthalmology 2019, 44, 168–173. [Google Scholar] [CrossRef]
- Müller, P.L.; Treis, T.; Pfau, M.; Degli Esposti, S.; Alsaedi, A.; Maloca, P.; Balaskas, K.; Webster, A.; Egan, C.; Tufail, A. Progression of Retinopathy Secondary to Maternally Inherited Diabetes and Deafness—Evaluation of Predicting Parameters. Am. J. Ophthalmol. 2020, 213, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Birtel, J.; von Landenberg, C.; Gliem, M.; Gliem, C.; Reimann, J.; Kunz, W.S.; Herrmann, P.; Betz, C.; Caswell, R.; Nesbitt, V.; et al. Mitochondrial Retinopathy. Ophthalmol. Retin. 2022, 6, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Lima de Carvalho, J.R., Jr.; Nuzbrokh, Y.; Ryu, J.; Chemudupati, T.; Mahajan, V.B.; Sparrow, J.R.; Tsang, S.H. Retinal Manifestations of Mitochondrial Oxidative Phosphorylation Disorders. Investig. Ophthalmol. Vis. Sci. 2020, 61, 12. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Bandello, F.; Boon, C.J.F.; Carelli, V.; Lenaers, G.; Reibaldi, M.; Sadda, S.R.; Sadun, A.A.; Sarraf, D.; Yu-Wai-Man, P.; et al. Mitochondrial retinopathies and optic neuropathies: The impact of retinal imaging on modern understanding of pathogenesis, diagnosis, and management. Prog. Retin. Eye Res. 2024, 101, 101264. [Google Scholar] [CrossRef]
- Meunier, I.; Bocquet, B.; Charif, M.; Dhaenens, C.-M.; Manes, G.; Amati-Bonneau, P.; Roubertie, A.; Zanlonghi, X.; Lenaers, G. A Rod-Cone Dystrophy Is Systematically Associated to the RTN4IP1 Recessive Optic Atrophy. Retina 2021, 41, 1771–1779. [Google Scholar] [CrossRef]
- Del Dotto, V.; Ullah, F.; Di Meo, I.; Magini, P.; Gusic, M.; Maresca, A.; Caporali, L.; Palombo, F.; Tagliavini, F.; Baugh, E.H.; et al. SSBP1 mutations cause mtDNA depletion underlying a complex optic atrophy disorder. J. Clin. Investig. 2020, 130, 108–125. [Google Scholar] [CrossRef]
- Tombolini, B.; Battista, M.; Borrelli, E.; Frontino, G.; Bandello, F.; Barboni, P.; Cascavilla, M.L. Wolfram Syndrome: Only a Neurodegenerative Disease or Also a Maculopathy? J. Neuroophthalmol. 2024, 44, e544–e546. [Google Scholar] [CrossRef]
- Fisher, C.R.; Ferrington, D.A. Perspective on AMD pathobiology: A bioenergetic crisis in the RPE. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD41–AMD47. [Google Scholar] [CrossRef]
- Terluk, M.R.; Kapphahn, R.J.; Soukup, L.M.; Gong, H.; Gallardo, C.; Montezuma, S.R.; Ferrington, D.A. Investigating mitochondria as a target for treating age-related macular degeneration. J. Neurosci. 2015, 35, 7304–7311. [Google Scholar] [CrossRef]
- Ferrington, D.A.; Fisher, C.R.; Kowluru, R.A. Mitochondrial defects drive degenerative retinal diseases. Trends Mol. Med. 2020, 26, 105–118. [Google Scholar] [CrossRef]
- MacLaren, R.E.; Duncan, J.L.; Fischer, M.D.; Lam, B.L.; Meunier, I.; Pennesi, M.E.; Sankila, E.-M.K.; Gow, J.A.; Li, J.; Tsang, S.-F.; et al. XOLARIS: A 24-Month, Prospective, Natural History Study of 201 Participants with Retinitis Pigmentosa GTPase Regulator-Associated X-Linked Retinitis Pigmentosa. Ophthalmol. Sci. 2024, 5, 100595. [Google Scholar] [CrossRef]
- Hashem, S.A.; Georgiou, M.; Wright, G.; Fujinami-Yokokawa, Y.; Laich, Y.; Varela, M.D.; de Guimaraes, T.A.; Mahroo, O.A.; Webster, A.R.; Fujinami, K.; et al. PDE6A-Associated Retinitis Pigmentosa, Clinical Characteristics, Genetics, and Natural History. Ophthalmol. Retin. 2025, 9, 278–287. [Google Scholar] [CrossRef] [PubMed]
- de Guimaraes, T.A.C.; Robson, A.G.; de Guimaraes, I.M.C.; Laich, Y.; Aychoua, N.; Wright, G.; Kalitzeos, A.; Mahroo, O.A.; Webster, A.R.; Michaelides, M. CDH23-Associated Usher Syndrome: Clinical Features, Retinal Imaging, and Natural History. Investig. Ophthalmol. Vis. Sci. 2024, 65, 27. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.D.; Bailey, J.A.; Exinor, A.; Heaps, N.; Duong, J.; Hou, B.; Piamjitchol, C.; Demirkol, A.; Brodie, S.E.; Sparrow, J.R.; et al. Ellipsoid Zone Loss as an Outcome Measure in MAK-Associated Retinitis Pigmentosa. Am. J. Ophthalmol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.L.; Greenstein, V.C.; Carlson, J.N.; Tsang, S.H.; Smith, R.T.; Carr, R.E.; Hood, D.C.; Chang, S. A comparison of fundus autofluorescence and retinal structure in patients with Stargardt disease. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3953–3959. [Google Scholar] [CrossRef]
- Kuehlewein, L.; Hariri, A.H.; Ho, A.; Dustin, L.; Wolfson, Y.; Strauss, R.W.; Scholl, H.P.N.; Sadda, S.R. Comparison of Manual and Semiautomated Fundus Autofluorescence Analysis of Macular Atrophy in Stargardt Disease Phenotype. Retina 2016, 36, 1216–1221. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Branham, K.; Schlegel, D.; Fahim, A.T.; Jayasundera, K.T. Automated Segmentation of Autofluorescence Lesions in Stargardt Disease. Ophthalmol. Retin. 2022, 6, 1098–1104. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.K.; Moussa, O.; Lam, B.L.; Sengillo, J.D. Fundus Autofluorescence in Inherited Retinal Disease: A Review. Cells 2025, 14, 1092. https://doi.org/10.3390/cells14141092
Oh JK, Moussa O, Lam BL, Sengillo JD. Fundus Autofluorescence in Inherited Retinal Disease: A Review. Cells. 2025; 14(14):1092. https://doi.org/10.3390/cells14141092
Chicago/Turabian StyleOh, Jin Kyun, Omar Moussa, Byron L. Lam, and Jesse D. Sengillo. 2025. "Fundus Autofluorescence in Inherited Retinal Disease: A Review" Cells 14, no. 14: 1092. https://doi.org/10.3390/cells14141092
APA StyleOh, J. K., Moussa, O., Lam, B. L., & Sengillo, J. D. (2025). Fundus Autofluorescence in Inherited Retinal Disease: A Review. Cells, 14(14), 1092. https://doi.org/10.3390/cells14141092





