Natural Killer (NK) Cell Alloreactivity in Haploidentical Stem Cell Transplantation
Abstract
1. Main Text
2. NK Cells
3. NK Cell Biology
4. Types of NK Cell Receptors
5. KIR Genes
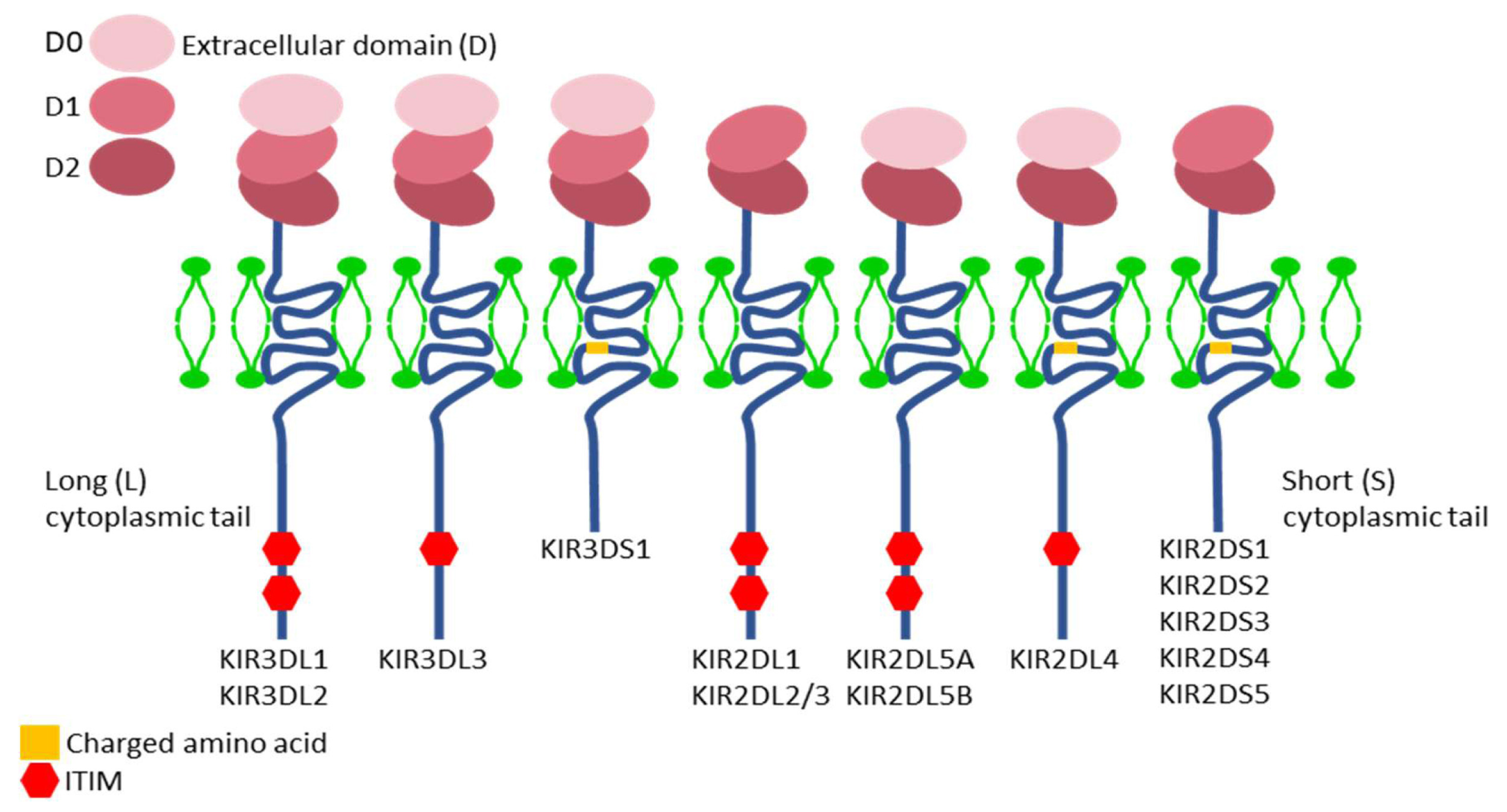
6. Nomenclature and Structure of KIR Genes
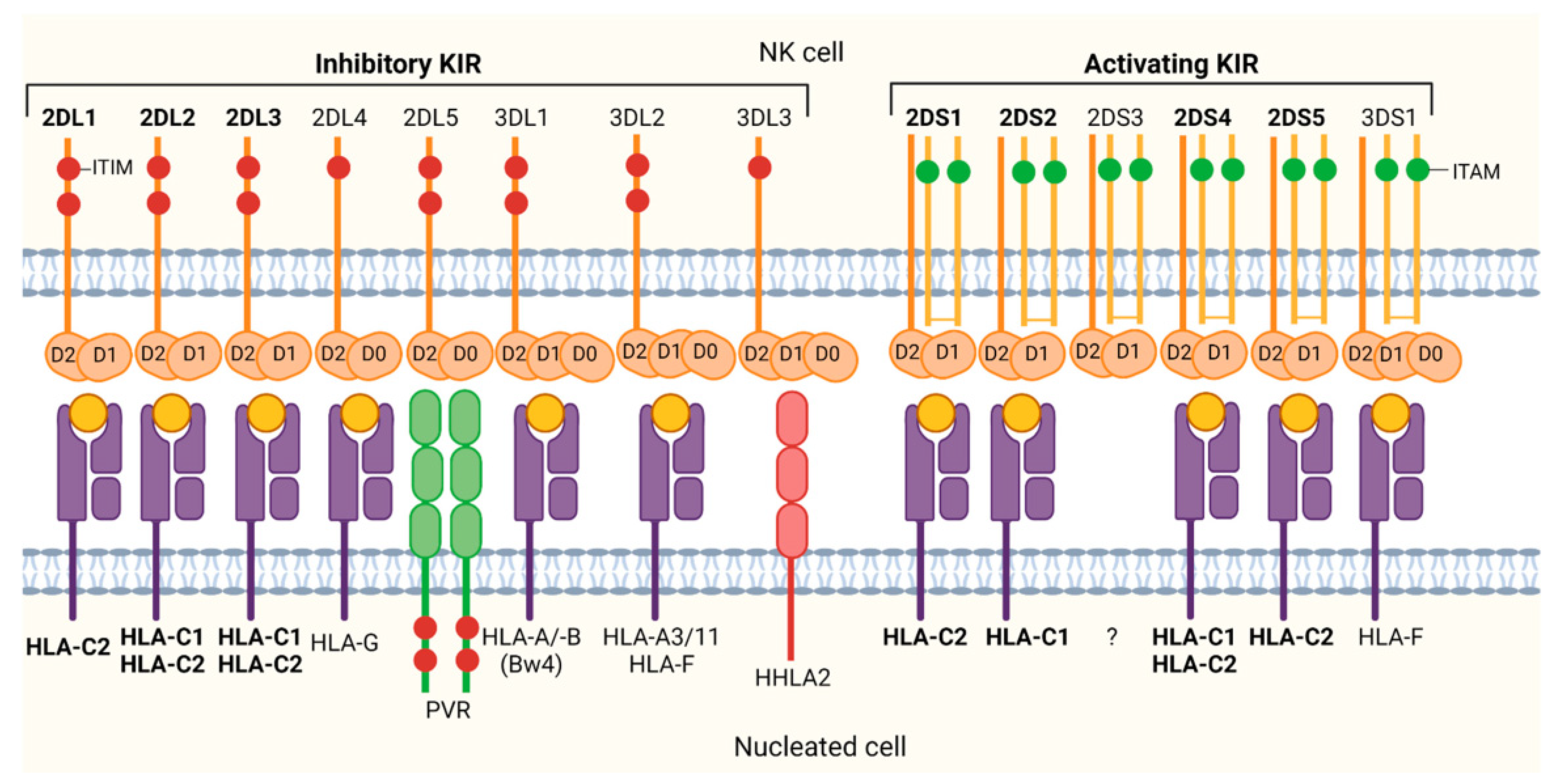
7. KIR Proteins
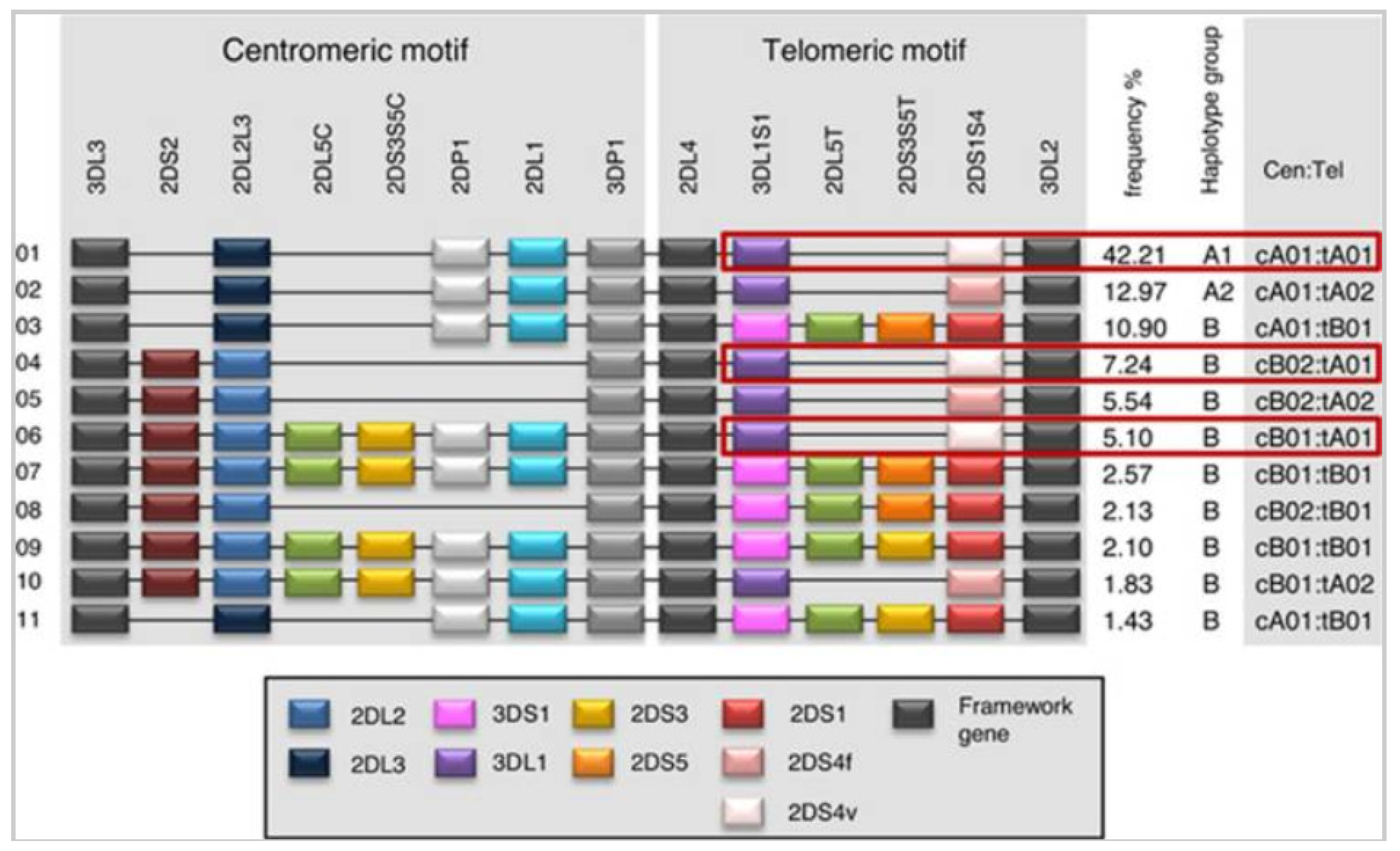
8. Genetic Organization of KIR
9. Polymorphism of KIR Genes
10. Combined Inheritance
11. HLA Ligands for KIR Receptors
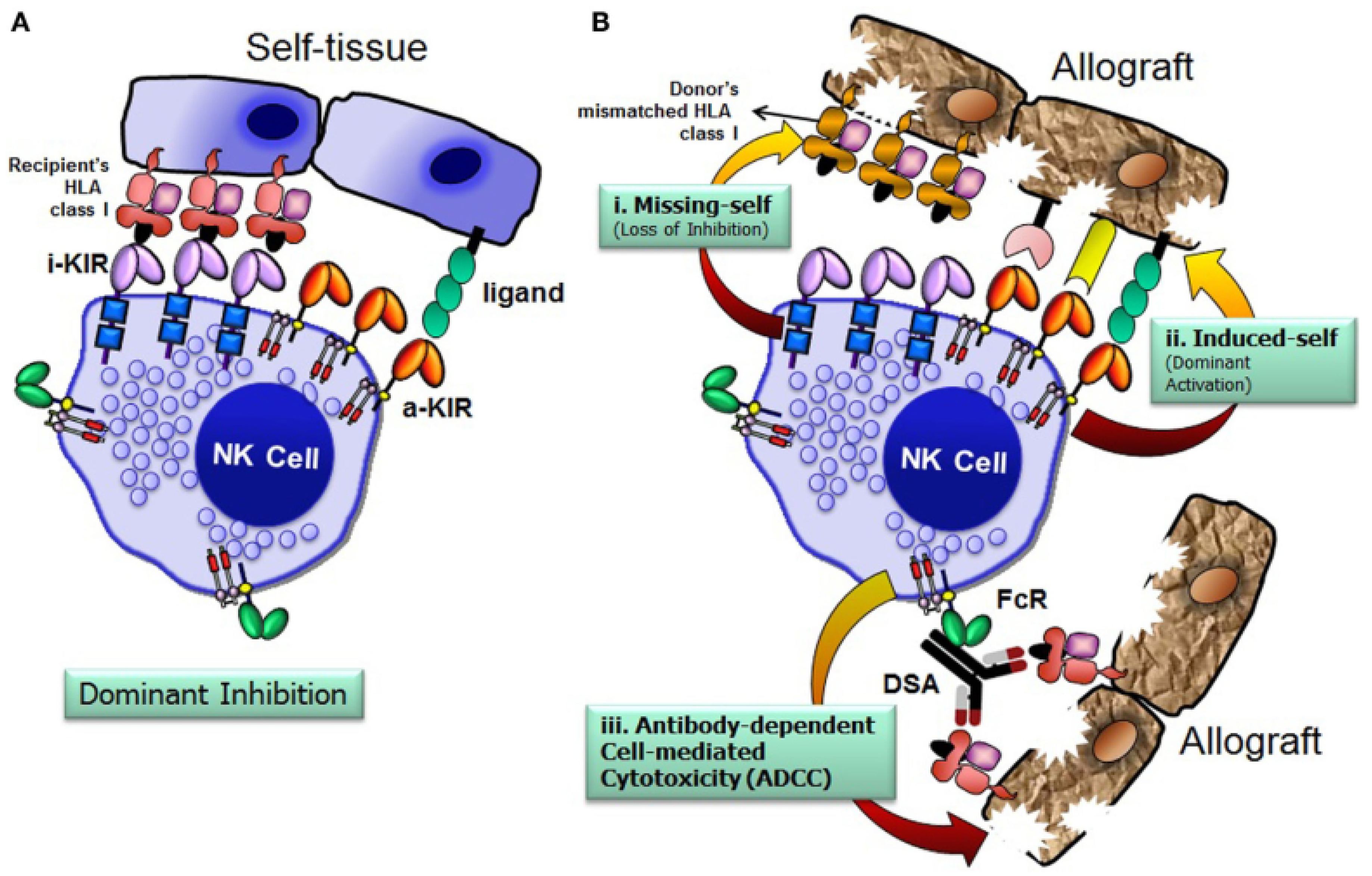
12. Role of NK Cells in HSCT
12.1. Myeloablative Conditioning and T Cell Depletion with Megadoses of CD34+ Cells
12.2. In Vivo Modulation of T Cell-Replete Grafts Using GIAC Protocol
12.3. Post-Transplant High-Dose Cyclophosphamide
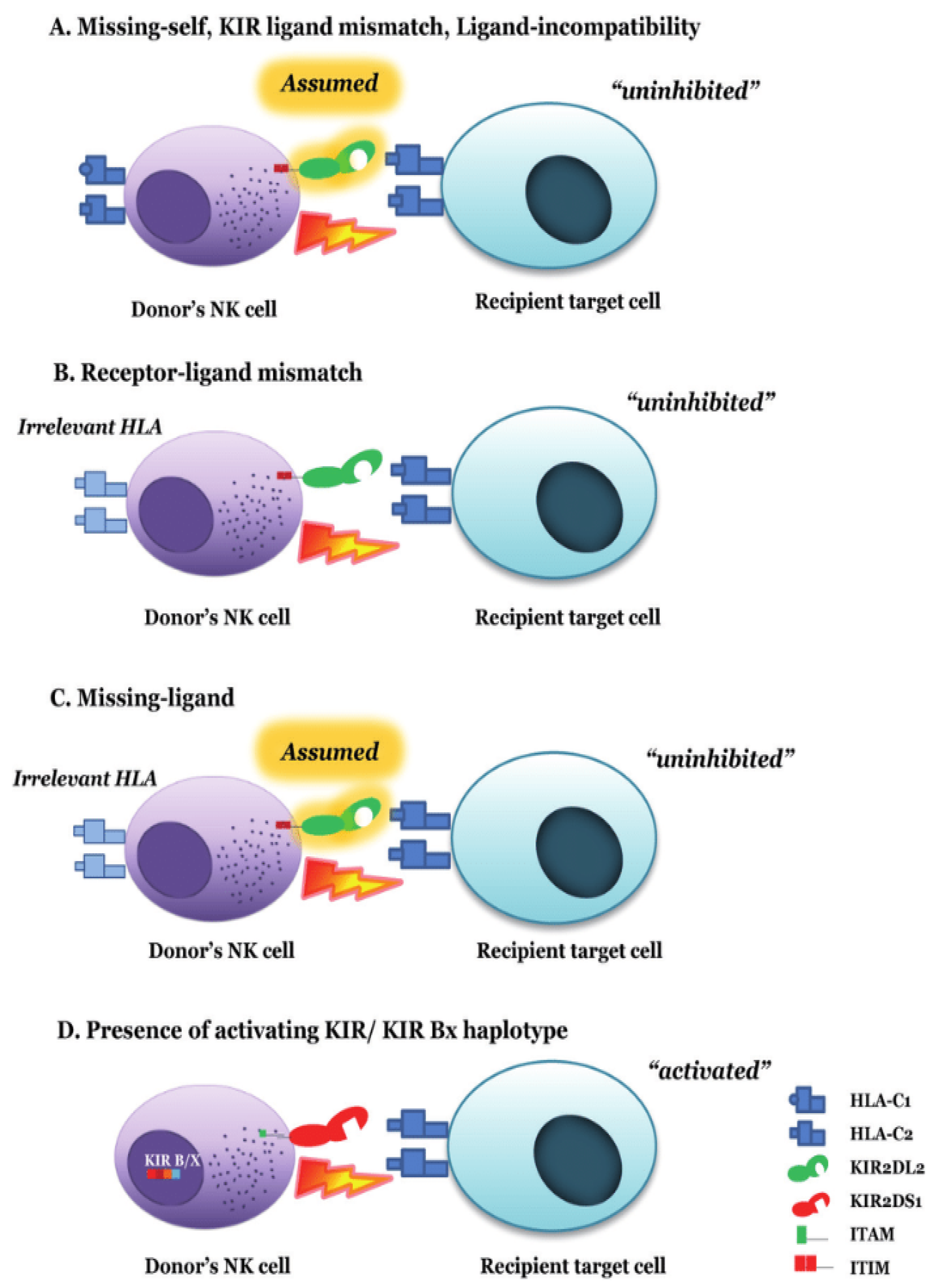
13. Prediction Models of Alloreactivity
14. NK Cell Alloreactivity in T-Cell-Replete Haploidentical Transplantation
15. Mismatched KIR Transplantation and GvHD
16. Clinical and Therapeutic Use of NK Cells and Research
17. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cooley, S.; Weisdorf, D.J.; Guethlein, L.A.; Klein, J.P.; Wang, T.; Le, C.T.; Marsh, S.G.E.; Geraghty, D.; Spellman, S.; Haagenson, M.D.; et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 2010, 116, 2411–2419. [Google Scholar] [CrossRef]
- Leung, W. Use of NK cell activity in cure by transplant. Br. J. Haematol. 2011, 155, 14–29. [Google Scholar] [CrossRef]
- Palmer, J.M.; Rajasekaran, K.; Thakar, M.S.; Malarkannan, S. Clinical relevance of natural killer cells following hematopoietic stem cell transplantation. J. Cancer 2013, 4, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Huntington, N.D.; Belz, G.T.; Seillet, C. Type 1 Innate Lymphoid Cell Biology: Lessons Learnt from Natural Killer Cells. Front. Immunol. 2016, 7, 426. [Google Scholar] [CrossRef] [PubMed]
- Narni-Mancinelli, E.; Vivier, E.; Kerdiles, Y.M. The “T-cell-ness” of NK cells: Unexpected similarities between NK cells and T cells. Int. Immunol. 2011, 23, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Rajalingam, R. Human diversity of killer cell immunoglobulin-like receptors and disease. Korean J. Hematol. 2011, 46, 216–228. [Google Scholar] [CrossRef]
- Lanier, L.L. NK cell recognition. Annu. Rev. Immunol. 2005, 23, 225–274. [Google Scholar] [CrossRef]
- Ljunggren, H.G.; Kärre, K. In search of the “missing self”: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- Dębska-Zielkowska, J.; Moszkowska, G.; Zieliński, M.; Zielińska, H.; Dukat-Mazurek, A.; Trzonkowski, P.; Stefańska, K. KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease. Cells 2021, 10, 1777. [Google Scholar] [CrossRef]
- Dhuyser, A.; Aarnink, A.; Pérès, M.; Jayaraman, J.; Nemat-Gorgani, N.; Rubio, M.T.; Trowsdale, J.; Traherne, J. KIR in Allogeneic Hematopoietic Stem Cell Transplantation: Need for a Unified Paradigm for Donor Selection. Front. Immunol. 2022, 13, 821533. [Google Scholar] [CrossRef]
- Rajalingam, R. The Impact of HLA Class I-Specific Killer Cell Immunoglobulin-Like Receptors on Antibody-Dependent Natural Killer Cell-Mediated Cytotoxicity and Organ Allograft Rejection. Front. Immunol. 2016, 7, 585. [Google Scholar] [CrossRef]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef]
- Long, E.O.; Kim, H.S.; Liu, D.; Peterson, M.E.; Rajagopalan, S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013, 31, 227–258. [Google Scholar] [CrossRef]
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.-G.; Long, E.O. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 2006, 107, 159–166. [Google Scholar] [CrossRef]
- Orange, J.S. Formation and function of the lytic NK-cell immunological synapse. Nat. Rev. Immunol. 2008, 8, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Cretney, E.; Kelly, J.M.; Westwood, J.A.; Street, S.E.A.; Yagita, H.; Takeda, K.; van Dommelen, S.L.H.; Degli-Esposti, M.A.; Hayakawa, Y. Activation of NK cell cytotoxicity. Mol. Immunol. 2005, 42, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Zamai, L.; Del Zotto, G.; Buccella, F.; Gabrielli, S.; Canonico, B.; Artico, M.; Ortolani, C.; Papa, S. Understanding the Synergy of NKp46 and Co-Activating Signals in Various NK Cell Subpopulations: Paving the Way for More Successful NK-Cell-Based Immunotherapy. Cells 2020, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Orr, M.T.; Lanier, L.L. Natural killer cell education and tolerance. Cell 2010, 142, 847–856. [Google Scholar] [CrossRef]
- Elliott, J.M.; Yokoyama, W.M. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 2011, 32, 364–372. [Google Scholar] [CrossRef]
- Kim, S.; Poursine-Laurent, J.; Truscott, S.M.; Lybarger, L.; Song, Y.-J.; Yang, L.; French, A.R.; Sunwoo, J.B.; Lemieux, S.; Hansen, T.H.; et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 2005, 436, 709–713. [Google Scholar] [CrossRef]
- Trowsdale, J. Genetic and functional relationships between MHC and NK receptor genes. Immunity 2001, 15, 363–374. [Google Scholar] [CrossRef]
- Kuroki, K.; Furukawa, A.; Maenaka, K. Molecular recognition of paired receptors in the immune system. Front. Microbiol. 2012, 3, 429. [Google Scholar] [CrossRef]
- Barquera, D.T.-G.R. Receptores de células NK (KIR): Estructura, función y relevancia en la susceptibilidad de enfermedades. Rev. Inst. Nal. Enf. Resp. Mex. 2008, 21, 57–65. [Google Scholar]
- Dupont, B.; Selvakumar, A.; Steffens, U. The killer cell inhibitory receptor genomic region on human chromosome 19q13.4. Tissue Antigens 1997, 49, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, A.; Steffens, U.; Dupont, B. NK cell receptor gene of the KIR family with two IG domains but highest homology to KIR receptors with three IG domains. Tissue Antigens 1996, 48, 285–294. [Google Scholar] [CrossRef]
- Symons, H.J.; Leffell, M.S.; Rossiter, N.D.; Zahurak, M.; Jones, R.J.; Fuchs, E.J. Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol. Blood Marrow Transpl. 2010, 16, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Muntasell, A.; López-Botet, M. Natural Killer Cell-Based Immunotherapy in Acute Myeloid Leukemia: Lessons for the Future. Clin. Cancer Res. 2016, 22, 1831–1833. [Google Scholar] [CrossRef]
- Vilches, C.; Parham, P. KIR: Diverse, rapidly evolving receptors of innate and adaptive immunity. Annu. Rev. Immunol. 2002, 20, 217–251. [Google Scholar] [CrossRef] [PubMed]
- Espeli, M.; Niederer, H.A.; Traherne, J.A.; Trowsdale, J.; Smith, K.G. Genetic variation, Fcγ receptors, KIRs and infection: The evolution of autoimmunity. Curr. Opin. Immunol. 2010, 22, 715–722. [Google Scholar] [CrossRef]
- Pollock, N.R.; Harrison, G.F.; Norman, P.J. Immunogenomics of Killer Cell Immunoglobulin-Like Receptor (KIR) and HLA Class I: Coevolution and Consequences for Human Health. J. Allergy Clin. Immunol. Pract. 2022, 10, 1763–1775. [Google Scholar] [CrossRef]
- Gómez-Lozano, N.; Gardiner, C.M.; Parham, P.; Vilches, C. Some human KIR haplotypes contain two KIR2DL5 genes: KIR2DL5A and KIR2DL5B. Immunogenetics 2002, 54, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Freitas, E.M.; Witt, C.S.; Christiansen, F.T. The genomic organization and evolution of the natural killer immunoglobulin-like receptor (KIR) gene cluster. Immunogenetics 2000, 51, 268–280. [Google Scholar] [CrossRef]
- Martin, A.M.; Kulski, J.K.; Gaudieri, S.; Witt, C.S.; Freitas, E.M.; Trowsdale, J.; Christiansen, F.T. Comparative genomic analysis, diversity and evolution of two KIR haplotypes A and B. Gene 2004, 335, 121–131. [Google Scholar] [CrossRef]
- Traherne, J.A.; Jiang, W.; Valdes, A.M.; Hollenbach, J.A.; Jayaraman, J.; Lane, J.A.; Johnson, C.; Trowsdale, J.; Noble, J.A. KIR haplotypes are associated with late-onset type 1 diabetes in European-American families. Genes Immun. 2016, 17, 8–12. [Google Scholar] [CrossRef]
- IPD-KIR Database. Available online: https://www.ebi.ac.uk/ipd/kir/ (accessed on 2 March 2022).
- Shilling, H.G.; Guethlein, L.A.; Cheng, N.W.; Gardiner, C.M.; Rodriguez, R.; Tyan, D.; Parham, P. Allelic polymorphism synergizes with variable gene content to individualize human KIR genotype. J. Immunol. 2002, 168, 2307–2315. [Google Scholar] [CrossRef]
- Shilling, H.G.; Young, N.; Guethlein, L.A.; Cheng, N.W.; Gardiner, C.M.; Tyan, D.; Parham, P. Genetic control of human NK cell repertoire. J. Immunol. 2002, 169, 239–247. [Google Scholar] [CrossRef]
- Middleton, D.; Gonzelez, F. The extensive polymorphism of KIR genes. Immunology 2010, 129, 8–19. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Long, E.O. Understanding how combinations of HLA and KIR genes influence disease. J. Exp. Med. 2005, 201, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Martin, M.P.; Carrington, M. The Yin and Yang of HLA and KIR in human disease. Semin. Immunol. 2008, 20, 343–352. [Google Scholar] [CrossRef]
- Mehta, R.S.; Rezvani, K. Can we make a better match or mismatch with KIR genotyping? Hematol. Am. Soc. Hematol. Educ. Program. 2016, 2016, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Sabouri Ghannad, M.; Hajilooi, M.; Solgi, G. HLA-KIR Interactions and Immunity to Viral Infections. Res. Mol. Med. 2014, 2, 1–20. [Google Scholar] [CrossRef]
- Pende, D.; Falco, M.; Vitale, M.; Cantoni, C.; Vitale, C.; Munari, E.; Bertaina, A.; Moretta, F.; Del Zotto, G.; Pietra, G.; et al. Killer Ig-Like Receptors (KIRs): Their Role in NK Cell Modulation and Developments Leading to Their Clinical Exploitation. Front. Immunol. 2019, 10, 1179. [Google Scholar] [CrossRef] [PubMed]
- Vollmers, S.; Lobermeyer, A.; Körner, C. The New Kid on the Block: HLA-C, a Key Regulator of Natural Killer Cells in Viral Immunity. Cells 2021, 10, 3108. [Google Scholar] [CrossRef] [PubMed]
- Van Bergen, J.; Trowsdale, J. Ligand specificity of Killer cell Immunoglobulin-like Receptors: A brief history of KIR. Front. Immunol. 2012, 3, 394. [Google Scholar] [CrossRef]
- Velardi, A. Role of KIRs and KIR ligands in hematopoietic transplantation. Curr. Opin. Immunol. 2008, 20, 581–587. [Google Scholar] [CrossRef]
- Rettman, P.; Willem, C.; Volteau, C.; Legrand, N.; Chevallier, P.; Lodé, L.; Esbelin, J.; Cesbron, A.; Bonneville, M.; Moreau, P.; et al. Impact of Graft-Versus-Graft Natural Killer Cell Alloreactivity on Single Unit Dominance After Double Umbilical Cord Blood Transplantation. Transplantation 2017, 101, 2092–2101. [Google Scholar] [CrossRef]
- Lutz, C.T. Human leukocyte antigen Bw4 and Bw6 epitopes recognized by antibodies and natural killer cells. Curr. Opin. Organ. Transpl. 2014, 19, 436–441. [Google Scholar] [CrossRef]
- Luznik, L.; O’Donnell, P.V.; Fuchs, E.J. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin. Oncol. 2012, 39, 683–693. [Google Scholar] [CrossRef]
- Kanakry, C.G.; Fuchs, E.J.; Luznik, L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat. Rev. Clin. Oncol. 2016, 13, 10–24. [Google Scholar] [CrossRef]
- Kernan, N.A.; Collins, N.H.; Juliano, L.; Cartagena, T.; Dupont, B.; O’Reilly, R.J. Clonable T lymphocytes in T cell-depleted bone marrow transplants correlate with development of graft-v-host disease. Blood 1986, 68, 770–773. [Google Scholar] [CrossRef]
- Kernan, N.A.; Flomenberg, N.; Dupont, B.; O’Reilly, R.J. Graft rejection in recipients of T-cell-depleted HLA-nonidentical marrow transplants for leukemia. Identification of host-derived antidonor allocytotoxic T lymphocytes. Transplantation 1987, 43, 842–847. [Google Scholar] [CrossRef]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Delmonte, J.; Jalonen, C.K.; Ferrara, J.L. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood 1995, 86, 4422–4429. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Dejbakhsh-Jones, S.; Strober, S. Granulocyte colony-stimulating factor reduces the capacity of blood mononuclear cells to induce graft-versus-host disease: Impact on blood progenitor cell transplantation. Blood 1997, 90, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-J.; Liu, D.-H.; Liu, K.-Y.; Xu, L.-P.; Chen, H.; Han, W.; Chen, Y.-H.; Wang, J.-Z.; Gao, Z.-Y.; Zhang, Y.-C.; et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transpl. 2006, 38, 291–297. [Google Scholar] [CrossRef]
- Schwartz, R.; Dameshek, W. Drug-induced immunological tolerance. Nature 1959, 183, 1682–1683. [Google Scholar] [CrossRef]
- Berenbaum, M.C.; Brown, I.N. Prolongation of Homograft Survival in Mice with Single Doses of Cyclophosphamide. Nature 1963, 200, 84. [Google Scholar] [CrossRef]
- Oevermann, L.; Handgretinger, R. New strategies for haploidentical transplantation. Pediatr. Res. 2012, 71, 418–426. [Google Scholar] [CrossRef][Green Version]
- Kwon, M.; Bailén, R.; Díez-Martín, J.L. Evolution of the role of haploidentical stem cell transplantation: Past, present, and future. Expert. Rev. Hematol. 2020, 13, 835–850. [Google Scholar] [CrossRef]
- Al-Homsi, A.S.; Roy, T.S.; Cole, K.; Feng, Y.; Duffner, U. Post-transplant high-dose cyclophosphamide for the prevention of graft-versus-host disease. Biol. Blood Marrow Transpl. 2015, 21, 604–611. [Google Scholar] [CrossRef]
- Kasamon, Y.L.; Luznik, L.; Leffell, M.S.; Kowalski, J.; Tsai, H.-L.; Bolaños-Meade, J.; Morris, L.E.; Crilley, P.A.; O’Donnell, P.V.; Rossiter, N.; et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: Effect of HLA disparity on outcome. Biol. Blood Marrow Transpl. 2010, 16, 482–489. [Google Scholar] [CrossRef]
- Robinson, J.; Guethlein, L.A.; Cereb, N.; Yang, S.Y.; Norman, P.J.; Marsh, S.G.E.; Parham, P. Distinguishing functional polymorphism from random variation in the sequences of >10,000 HLA-A, -B and -C alleles. PLoS Genet. 2017, 13, e1006862. [Google Scholar] [CrossRef] [PubMed]
- Farag, S.S.; Fehniger, T.A.; Ruggeri, L.; Velardi, A.; Caligiuri, M.A. Natural killer cell receptors: New biology and insights into the graft-versus-leukemia effect. Blood 2002, 100, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Barao, I.; Murphy, W.J. The immunobiology of natural killer cells and bone marrow allograft rejection. Biol. Blood Marrow Transpl. 2003, 9, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Chaisri, S.; Leelayuwat, C. Natural Killer (NK) Cell Alloreactivities against Leukemic Cells: Functions beyond Defense. In Cancer Immunotherapy and Biological Cancer Treatments; IntechOpen: London, UK, 2019. [Google Scholar]
- Heidenreich, S.; Kröger, N. Reduction of Relapse after Unrelated Donor Stem Cell Transplantation by KIR-Based Graft Selection. Front. Immunol. 2017, 8, 41. [Google Scholar] [CrossRef]
- Bessoles, S.; Grandclément, C.; Alari-Pahissa, E.; Gehrig, J.; Jeevan-Raj, B.; Held, W. Adaptations of Natural Killer Cells to Self-MHC Class, I. Front. Immunol. 2014, 5, 349. [Google Scholar] [CrossRef]
- Vivier, E.; Ugolini, S.; Blaise, D.; Chabannon, C.; Brossay, L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 2012, 12, 239–252. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Yu, X.-X.; Xu, Z.-L.; Cao, X.-H.; Huo, M.-R.; Zhao, X.-S.; Chang, Y.-J.; Wang, Y.; Zhang, X.-H.; Xu, L.-P.; et al. Donor and host coexpressing KIR ligands promote NK education after allogeneic hematopoietic stem cell transplantation. Blood Adv. 2019, 3, 4312–4325. [Google Scholar] [CrossRef]
- Shang, Q.-N.; Yu, X.-X.; Xu, Z.-L.; Cao, X.-H.; Liu, X.-F.; Zhao, X.-S.; Chang, Y.-J.; Wang, Y.; Zhang, X.-H.; Xu, L.-P.; et al. Functional Competence of NK Cells via the KIR/MHC Class I Interaction Correlates with DNAM-1 Expression. J. Immunol. 2022, 208, 492–500. [Google Scholar] [CrossRef]
- Leung, W.; Iyengar, R.; Turner, V.; Lang, P.; Bader, P.; Conn, P.; Niethammer, D.; Handgretinger, R. Determinants of antileukemia effects of allogeneic NK cells. J. Immunol. 2004, 172, 644–650. [Google Scholar] [CrossRef]
- Lotze, M.T.; Thomson, A.W. Natural Killer Cells: Basic Science and Clinical Application; Elsevier: Amsterdam, The Netherlands; Academic Press: Boston, MA, USA, 2010. [Google Scholar]
- Hsu, K.C.; Keever-Taylor, C.A.; Wilton, A.; Pinto, C.; Heller, G.; Arkun, K.; O’Reilly, R.J.; Horowitz, M.M.; Dupont, B. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood 2005, 105, 4878–4884. [Google Scholar] [CrossRef]
- Beksaç, M.; Dalva, K. Role of killer immunoglobulin-like receptor and ligand matching in donor selection. Bone Marrow Res. 2012, 2012, 271695. [Google Scholar] [CrossRef] [PubMed]
- Gagne, K.; Brizard, G.; Gueglio, B.; Milpied, N.; Herry, P.; Bonneville, F.; Chéneau, M.L.; Schleinitz, N.; Cesbron, A.; Folléa, G.; et al. Relevance of KIR gene polymorphisms in bone marrow transplantation outcome. Hum. Immunol. 2002, 63, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Oreiro, M.B. Influencia de la Alorreactividad KIR en el Trasplante Alogénico de Progenitores Hematopoyéticos Haploidéntico. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2017. [Google Scholar]
- Sahin, U.; Dalva, K.; Gungor, F.; Ustun, C.; Beksac, M. Donor-recipient killer immunoglobulin like receptor (KIR) genotype matching has a protective effect on chronic graft versus host disease and relapse incidence following HLA-identical sibling hematopoietic stem cell transplantation. Ann. Hematol. 2018, 97, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Dubreuil, L.; Maniangou, B.; Chevallier, P.; Quéméner, A.; Legrand, N.; Béné, M.C.; Willem, C.; David, G.; Alizadeh, M.; Makanga, D.R.; et al. Centromeric KIR AA Individuals Harbor Particular KIR Alleles Conferring Beneficial NK Cell Features with Implications in Haplo-Identical Hematopoietic Stem Cell Transplantation. Cancers 2020, 12, 3595. [Google Scholar] [CrossRef]
- Solomon, S.R.; Aubrey, M.T.; Zhang, X.; Piluso, A.; Freed, B.M.; Brown, S.; Jackson, K.C.; Morris, L.E.; Holland, H.K.; Solh, M.M.; et al. Selecting the Best Donor for Haploidentical Transplant: Impact of HLA, Killer Cell Immunoglobulin-Like Receptor Genotyping, and Other Clinical Variables. Biol. Blood Marrow Transpl. 2018, 24, 789–798. [Google Scholar] [CrossRef]
- Elagab, E.A.; Ibrahim, A.M.; Shediwah, A.; Alqahtani, S.M.; TalbAllah, S.M. Role of Centromeric and Telomeric Haplotypes of Killer-Cell Immunoglobulin-Like Receptors (KIRs) in Disease Susceptibility: A Research Review. Cureus 2025, 17, e83728. [Google Scholar] [CrossRef]
- Mehta, R.S.; Oran, B. The Optimal Killer Cell Immunoglobulin-Like Receptor Donor-We Can Recognize, but Can We Search? Biol. Blood Marrow Transpl. 2019, 25, e3–e4. [Google Scholar] [CrossRef]
- Oevermann, L.; Michaelis, S.U.; Mezger, M.; Lang, P.; Toporski, J.; Bertaina, A.; Zecca, M.; Moretta, L.; Locatelli, F.; Handgretinger, R. KIR B haplotype donors confer a reduced risk for relapse after haploidentical transplantation in children with ALL. Blood 2014, 124, 2744–2747. [Google Scholar] [CrossRef]
- Escudero, A.; Martínez-Romera, I.; Fernández, L.; Valentín, J.; González-Vicent, M.; Vicario, J.L.; Madero-Jarabo, R.; Diaz, M.Á.; Pérez-Martínez, A. Donor KIR Genotype Impacts on Clinical Outcome after T Cell-Depleted HLA Matched Related Allogeneic Transplantation for High-Risk Pediatric Leukemia Patients. Biol. Blood Marrow Transpl. 2018, 24, 2493–2500. [Google Scholar] [CrossRef]
- Byrnes, C.P.; Hastings, A.; Lacej, I.; Palanicawandar, R.; Olavarria, E.; Anand, A. A retrospective analysis to evaluate if KIR B haplotype donors associate with a reduced risk of relapse in patients with haematological malignancies following haploidentical transplantation at the Blood and Bone Marrow Transplant Unit at Hammersmith Hospital ICHNHST. HLA 2024, 103, e15214. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, S.O.; Champlin, R.E. Donor selection in T cell-replete haploidentical hematopoietic stem cell transplantation: Knowns, unknowns, and controversies. Biol. Blood Marrow Transpl. 2013, 19, 180–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ciurea, S.O.; Al Malki, M.M.; Kongtim, P.; Fuchs, E.J.; Luznik, L.; Huang, X.-J.; Ciceri, F.; Locatelli, F.; Aversa, F.; Castagna, L.; et al. The European Society for Blood and Marrow Transplantation (EBMT) consensus recommendations for donor selection in haploidentical hematopoietic cell transplantation. Bone Marrow Transpl. 2020, 55, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.J. Haploidentical transplantation for hematologic malignancies: Where do we stand? Hematol. Am. Soc. Hematol. Educ. Program. 2012, 2012, 230–236. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Huang, X.-J.; Liu, K.-Y.; Xu, L.-P.; Liu, D.-H. Reconstitution of natural killer cell receptor repertoires after unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation: Analyses of CD94:NKG2A and killer immunoglobulin-like receptor expression and their associations with clinical outcome. Biol. Blood Marrow Transpl. 2007, 13, 734–744. [Google Scholar] [CrossRef][Green Version]
- Sivori, S.; Carlomagno, S.; Falco, M.; Romeo, E.; Moretta, L.; Moretta, A. Natural killer cells expressing the KIR2DS1-activating receptor efficiently kill T-cell blasts and dendritic cells: Implications in haploidentical HSCT. Blood 2011, 117, 4284–4292. [Google Scholar] [CrossRef]
- Gao, F.; Ye, Y.; Gao, Y.; Huang, H.; Zhao, Y. Influence of KIR and NK Cell Reconstitution in the Outcomes of Hematopoietic Stem Cell Transplantation. Front. Immunol. 2020, 11, 2022. [Google Scholar] [CrossRef]
- Hosokai, R.; Masuko, M.; Shibasaki, Y.; Saitoh, A.; Furukawa, T.; Imai, C. Donor Killer Immunoglobulin-Like Receptor Haplotype B/x Induces Severe Acute Graft-versus-Host Disease in the Presence of Human Leukocyte Antigen Mismatch in T Cell-Replete Hematopoietic Cell Transplantation. Biol. Blood Marrow Transpl. 2017, 23, 606–611. [Google Scholar] [CrossRef]
- Shimoni, A.; Labopin, M.; Lorentino, F.; Van Lint, M.T.; Koc, Y.; Gülbas, Z.; Tischer, J.; Bruno, B.; Blaise, D.; Pioltelli, P.; et al. Killer cell immunoglobulin-like receptor ligand mismatching and outcome after haploidentical transplantation with post-transplant cyclophosphamide. Leukemia 2019, 33, 230–239. [Google Scholar] [CrossRef]
- Russo, A.; Oliveira, G.; Berglund, S.; Greco, R.; Gambacorta, V.; Cieri, N.; Toffalori, C.; Zito, L.; Lorentino, F.; Piemontese, S.; et al. NK cell recovery after haploidentical HSCT with posttransplant cyclophosphamide: Dynamics and clinical implications. Blood 2018, 131, 247–262. [Google Scholar] [CrossRef]
- Boudreau, J.E.; Liu, X.-R.; Zhao, Z.; Zhang, A.; Shultz, L.D.; Greiner, D.L.; Dupont, B.; Hsu, K.C. Cell-Extrinsic MHC Class I Molecule Engagement Augments Human NK Cell Education Programmed by Cell-Intrinsic MHC Class, I. Immunity 2016, 45, 280–291. [Google Scholar] [CrossRef]
- Willem, C.; Makanga, D.R.; Guillaume, T.; Maniangou, B.; Legrand, N.; Gagne, K.; Peterlin, P.; Garnier, A.; Béné, M.C.; Cesbron, A.; et al. Impact of KIR/HLA Incompatibilities on NK Cell Reconstitution and Clinical Outcome after T Cell-Replete Haploidentical Hematopoietic Stem Cell Transplantation with Posttransplant Cyclophosphamide. J. Immunol. 2019, 202, 2141–2152. [Google Scholar] [CrossRef]
- Zou, J.; Kongtim, P.; Srour, S.A.; Greenbaum, U.; Schetelig, J.; Heidenreich, F.; Baldauf, H.; Moore, B.; Saengboon, S.; Carmazzi, Y.; et al. Donor selection for KIR alloreactivity is associated with superior survival in haploidentical transplant with PTCy. Front. Immunol. 2022, 13, 1033871. [Google Scholar] [CrossRef] [PubMed]
- Luis-Hidalgo, M.; Planelles, D.; Piñana, J.L.; Carbonell, J.; Amat, P.; Gómez-Seguí, I.; Guerreiro, M.; Caballero, A.; Torío, A.; Pascual-Cascón, M.J.; et al. Frequency and Distribution of KIR Genotypes of Donors-Recipient Pairs in the Haploidentical Haematopoietic Stem Cell Transplantation Setting: Collaborative Study by the Spanish Working Group in Histocompatibility and Transplant Immunology (GETHIT) and the Spanish Haematopoietic Transplantation and Cell Therapy Group (GETH-TC). HLA 2025, 105, e70248. [Google Scholar] [CrossRef] [PubMed]
- Boelen, L.; Debebe, B.; Silveira, M.; Salam, A.; Makinde, J.; Roberts, C.H.; Wang, E.C.Y.; Frater, J.; Gilmour, J.; Twigger, K.; et al. Inhibitory killer cell immunoglobulin-like receptors strengthen CD8+ T cell-mediated control of HIV-1, HCV, and HTLV-1. Sci. Immunol. 2018, 3, eaao2892. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, K.; Kroeger, I.; Bauer, R.; Ganss, F.; Ovsiy, I.; Rothamer, J.; Büttner, M.; Atreya, I.; Waldner, M.; Bittrich, M.; et al. Identification and characterization of the specific murine NK cell subset supporting graft- versus -leukemia- and reducing graft- versus -host-effects. OncoImmunology 2015, 4, e981483. [Google Scholar] [CrossRef]
- Passweg, J.R.; Tichelli, A.; Meyer-Monard, S.; Heim, D.; Stern, M.; Kühne, T.; Favre, G.; Gratwohl, A. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia 2004, 18, 1835–1838. [Google Scholar] [CrossRef]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef]
- Rubnitz, J.E.; Inaba, H.; Ribeiro, R.C.; Pounds, S.; Rooney, B.; Bell, T.; Pui, C.-H.; Leung, W. NKAML: A pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J. Clin. Oncol. 2010, 28, 955–959. [Google Scholar] [CrossRef]
- Shah, N.N.; Baird, K.; Delbrook, C.P.; Fleisher, T.A.; Kohler, M.E.; Rampertaap, S.; Lemberg, K.; Hurley, C.K.; Kleiner, D.E.; Merchant, M.S.; et al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell–depleted stem cell transplantation. Blood 2015, 125, 784–792. [Google Scholar] [CrossRef]
- Simonetta, F.; Alvarez, M.; Negrin, R.S. Natural Killer Cells in Graft-versus-Host-Disease after Allogeneic Hematopoietic Cell Transplantation. Front. Immunol. 2017, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, K.; Rouce, R.; Liu, E.; Shpall, E. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol. Ther. 2017, 25, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Lanier, L.L. NK cell development, homeostasis and function: Parallels with CD8+ T cells. Nat. Rev. Immunol. 2011, 11, 645–657. [Google Scholar] [CrossRef]
- Naik, S.; Li, Y.; Talleur, A.C.; Selukar, S.; Ashcraft, E.; Cheng, C.; Madden, R.M.; Mamcarz, E.; Qudeimat, A.; Sharma, A.; et al. Memory T-cell enriched haploidentical transplantation with NK cell addback results in promising long-term outcomes: A phase II trial. J. Hematol. Oncol. 2024, 17, 50. [Google Scholar] [CrossRef]
- Lee, D.A.; Denman, C.J.; Rondon, G.; Woodworth, G.; Chen, J.; Fisher, T.; Kaur, I.; Fernandez-Vina, M.; Cao, K.; Ciurea, S.; et al. Haploidentical Natural Killer Cells Infused before Allogeneic Stem Cell Transplantation for Myeloid Malignancies: A Phase I Trial. Biol. Blood Marrow Transpl. 2016, 22, 1290–1298. [Google Scholar] [CrossRef]
- Cong, J.; Wang, X.; Zheng, X.; Wang, D.; Fu, B.; Sun, R.; Tian, Z.; Wei, H. Dysfunction of Natural Killer Cells by FBP1-Induced Inhibition of Glycolysis during Lung Cancer Progression. Cell Metab. 2018, 28, 243–255.e5. [Google Scholar] [CrossRef]
- Cooper, M.A.; Elliott, J.M.; Keyel, P.A.; Yang, L.; Carrero, J.A.; Yokoyama, W.M. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 1915–1919. [Google Scholar] [CrossRef]
- Ruggeri, L.; Vago, L.; Eikema, D.-J.; de Wreede, L.C.; Ciceri, F.; Diaz, M.A.; Locatelli, F.; Jindra, P.; Milone, G.; Diez-Martin, J.L.; et al. Natural killer cell alloreactivity in HLA-haploidentical hematopoietic transplantation: A study on behalf of the CTIWP of the EBMT. Bone Marrow Transpl. 2021, 56, 1900–1907. [Google Scholar] [CrossRef]
- Bishara, A.; De Santis, D.; Witt, C.C.; Brautbar, C.; Christiansen, F.T.; Or, R.; Nagler, A.; Slavin, S. The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD. Tissue Antigens 2004, 63, 204–211. [Google Scholar] [CrossRef]
| Centromeric (2DS2, 2DL2, 2DL3) | |
|---|---|
| Cen-A/A | 2DL3 only |
| Cen-A/B | 2DL3 with 2DS2 and/or 2DL2 |
| Cen-B/B | 2DS2 and/or 2DL2; no 2DL3 |
| Telomeric (3DL1, 3DS1, 2DS1, 2DS4) | |
| Tel-A/A | 3DL1 and 2DS4 only |
| Tel-A/B | 3DL1 and 2DS4 with 3DS1 and/or 2DS1 |
| Tel-B/B | Lacking 3DL1 and/or 2DS4 |
| KIR Genotype | B-Content Score | Cen Haplotypes | Tel Haplotypes | Category |
|---|---|---|---|---|
| A/A | 0 | A/A | A/A | Neutral |
| B/x | 1 | A/A | A/B | Neutral |
| 1 | A/B | A/A | Neutral | |
| 2 | A/A | B/B | Better | |
| 2 | A/B | A/B | Better | |
| 2 | B/B | A/A | Best | |
| 3 | A/B | B/B | Better | |
| 3 | B/B | A/B | Best | |
| 4 | B/B | B/B | Best |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luis-Hidalgo, M.; Piñana, J.L.; Solano, C.; Planelles, D. Natural Killer (NK) Cell Alloreactivity in Haploidentical Stem Cell Transplantation. Cells 2025, 14, 1091. https://doi.org/10.3390/cells14141091
Luis-Hidalgo M, Piñana JL, Solano C, Planelles D. Natural Killer (NK) Cell Alloreactivity in Haploidentical Stem Cell Transplantation. Cells. 2025; 14(14):1091. https://doi.org/10.3390/cells14141091
Chicago/Turabian StyleLuis-Hidalgo, Mar, José Luis Piñana, Carlos Solano, and Dolores Planelles. 2025. "Natural Killer (NK) Cell Alloreactivity in Haploidentical Stem Cell Transplantation" Cells 14, no. 14: 1091. https://doi.org/10.3390/cells14141091
APA StyleLuis-Hidalgo, M., Piñana, J. L., Solano, C., & Planelles, D. (2025). Natural Killer (NK) Cell Alloreactivity in Haploidentical Stem Cell Transplantation. Cells, 14(14), 1091. https://doi.org/10.3390/cells14141091





