The Sex Difference in the Pathophysiology of Preterm Birth
Abstract
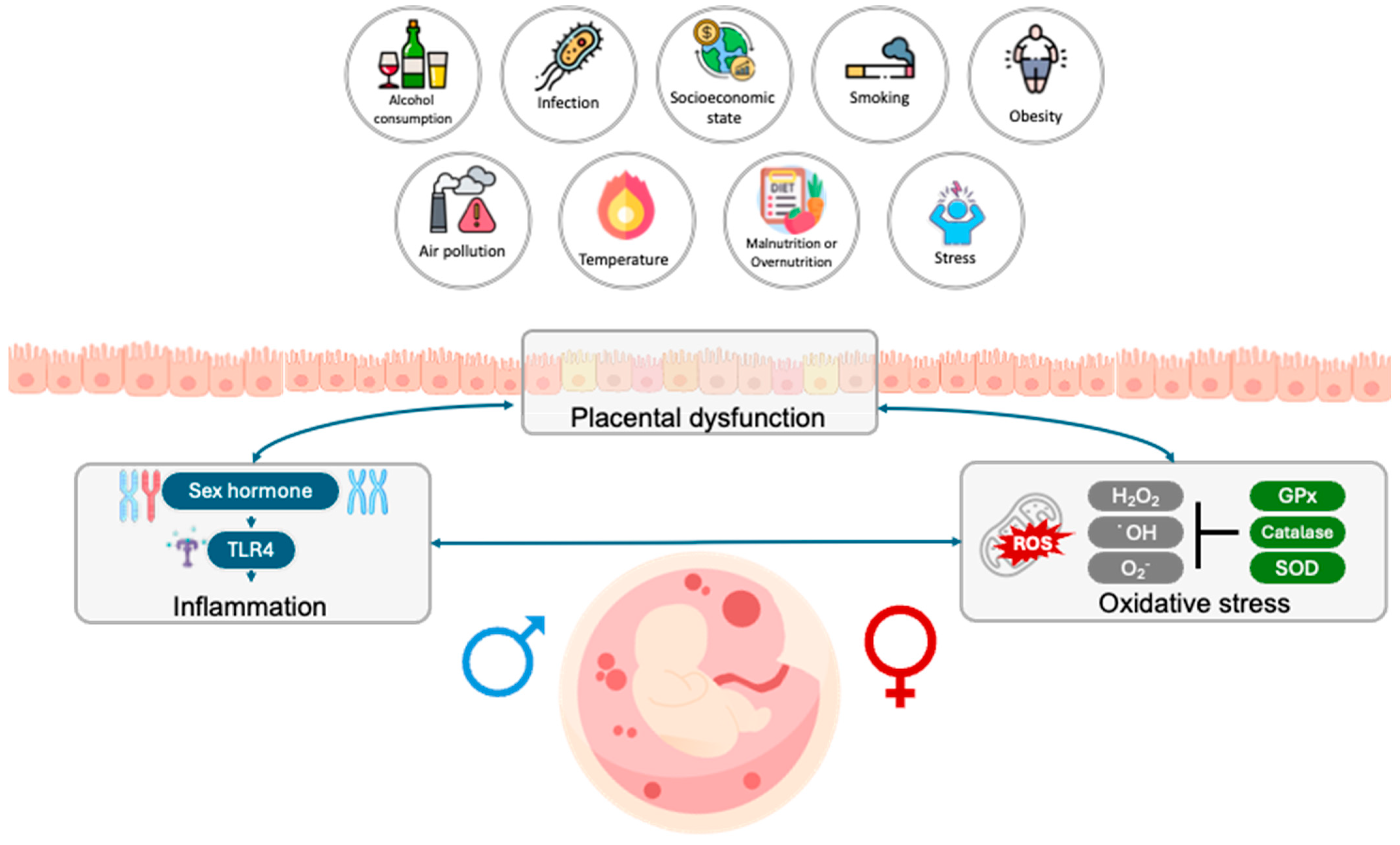
1. Introduction
2. Environmental Factors Influence Preterm Birth Prevalence According to Fetal Sex
3. Sex-Specific Modulation of Inflammation in Preterm Birth
3.1. Inflammatory Mechanism in Pregnancy
3.2. Inflammatory Dysregulation and Preterm Birth
3.3. Fetal Sex-Based Modulation of Inflammatory Responses
3.3.1. Sex Differences in Immune Regulation
3.3.2. Sex Chromosomal Contribution to Immune Expression
3.3.3. PTB-Related Immune Modulation by Fetal Sex Difference Manner
4. Placental Dysfunction in Preterm Brith
4.1. Placental Formation and Function in Pregnancy
4.2. Placenta Dysfunction and Role in Preterm Brith
4.3. Fetal Sex Hormone Contributing to Placental Dysfunction
4.3.1. Sex Difference Effects on Placental Structure
4.3.2. Growth Strategy According to Fetal Sex
4.3.3. Transcriptional and Epigenetic Differences
5. Oxidative Stress as a Trigger for Preterm Birth
5.1. Balanced Oxidative Stress in Normal Pregnancy
5.2. Imbalanced Oxidative Stress and Adverse Pregnancy Outcomes
5.3. Fetal Sex Differences in Oxidative Stress Responses
6. Additional Fetal Sex-Specific Mechanisms in PTB
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 8-OHdG | 8-oxodeoxyguanosine |
| AG | androgen receptor |
| BPD | bronchopulmonary dysplasia |
| CCL | C-C motif chemokine ligand |
| COX-2 | cyclooxygenase-2 |
| DAMPs | damage-associated molecular patterns |
| DOHaD | developmental origins of health and disease |
| E | embryonic day |
| EVs | extracellular vesicles |
| Fe2+ | ferrous iron |
| GWAS | genome-wide associated studies |
| GM-CSF | granulocyte–macrophage colony stimulating factor |
| GnRH | gonadotropin-releasing hormone |
| GPX | glutathione peroxidase |
| GR | glucocorticoid receptor |
| GRER1 | G-protein-coupled estrogen receptor 1 |
| GSH | glutathione |
| H2O2 | hydrogen peroxide |
| hCG | human chorionic gonadotropin |
| HIF | hypoxia-inducible factor |
| HO | heme oxygenase |
| HO˙ | hydroxyl radical |
| IFN | interferon |
| Ig | immunoglobulin |
| IGF | insulin-like growth factor |
| IL | interleukin |
| IUGR | intrauterine growth restriction |
| MAPK | p38-mitogen-activated protein kinase |
| MDA | malondialdehyde |
| MIAC | microbial invasion of amniotic cavity |
| MMP | matrix metalloproteinase-9 |
| NF-κb | nuclear factor kappa-light-chain-enhancer of activated B cell |
| NK | natural killer |
| nRBCs | nucleated red blood cells |
| O2 | superoxide radical |
| P4 | progesterone |
| PAMPs | pathogen-associated molecular patterns |
| PAPP | pregnancy-associated plasma protein |
| PE | preeclampsia |
| PG | prostaglandin |
| PlGF | placental growth factor |
| PIVH | periventricular–intraventricular hemorrhage |
| pPROM | preterm premature rupture of the membrane |
| PRA | progesterone receptor A |
| PTB | preterm birth |
| RDS | respiratory distress syndrome |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| SpAs | spiral arteries |
| sPTB | spontaneous preterm birth |
| SRY | sex-determining region |
| T1 | trimester 1 |
| T2 | trimester 2 |
| T3 | trimester 3 |
| TLR | toll-like receptor |
| TNF | tumor necrosis factor |
| Vegf | vascular endothelial growth factor |
| XCI | X chromosome inhibition |
References
- Beck, S.; Wojdyla, D.; Say, L.; Betran, A.P.; Merialdi, M.; Requejo, J.H.; Rubens, C.; Menon, R.; Van Look, P.F. The worldwide incidence of preterm birth: A systematic review of maternal mortality and morbidity. Bull. World Health Organ. 2010, 88, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ohuma, E.O.; Moller, A.-B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Lodefalk, M.; Chelslín, F.; Patriksson Karlsson, J.; Hansson, S.R. Placental Changes and Neuropsychological Development in Children-A Systematic Review. Cells 2023, 12, 435. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Behnia, F.; Polettini, J.; Richardson, L.S. Novel pathways of inflammation in human fetal membranes associated with preterm birth and preterm pre-labor rupture of the membranes. Semin. Immunopathol. 2020, 42, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Hirsch, E. Intrauterine infection and preterm labor. Semin. Fetal Neonatal Med. 2012, 17, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Adams Waldorf, K.M.; Singh, N.; Mohan, A.R.; Young, R.C.; Ngo, L.; Das, A.; Tsai, J.; Bansal, A.; Paolella, L.; Herbert, B.R.; et al. Uterine overdistention induces preterm labor mediated by inflammation: Observations in pregnant women and nonhuman primates. Am. J. Obs. Gynecol. 2015, 213, 830.e1–830.e19. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.F. Advances in the prevention of infection-related preterm birth. Front. Immunol. 2015, 6, 566. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, M.; Della Bella, S.; Ferrazzi, E.; Mavilio, D.; Divanovic, S. Inflammation and preterm birth. J. Leucoc. Biol. 2016, 99, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.A.; Ahmad, I.M.; Zimmerman, M.C. Oxidative stress and preterm birth: An integrative review. Biol. Res. Nurs. 2018, 20, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Dutta, E.H.; Behnia, F.; Boldogh, I.; Saade, G.R.; Taylor, B.D.; Kacerovský, M.; Menon, R. Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. MHR Basic Sci. Reprod. Med. 2016, 22, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Koullali, B.; Oudijk, M.; Nijman, T.; Mol, B.; Pajkrt, E. Risk assessment and management to prevent preterm birth. Semin. Fetal Neonatal Med. 2016, 21, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.-B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The global epidemiology of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Porpora, M.G.; Piacenti, I.; Scaramuzzino, S.; Masciullo, L.; Rech, F.; Benedetti Panici, P. Environmental contaminants exposure and preterm birth: A systematic review. Toxics 2019, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Khandre, V.; Potdar, J.; Keerti, A.; Khandre, V., Jr. Preterm birth: An overview. Cureus 2022, 14, e33006. [Google Scholar] [CrossRef] [PubMed]
- Broere-Brown, Z.A.; Adank, M.C.; Benschop, L.; Tielemans, M.; Muka, T.; Gonçalves, R.; Bramer, W.M.; Schoufour, J.D.; Voortman, T.; Steegers, E.A.P.; et al. Fetal sex and maternal pregnancy outcomes: A systematic review and meta-analysis. Biol. Sex. Differ. 2020, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Lacagnina, S. The Developmental Origins of Health and Disease (DOHaD). Am. J. Lifestyle Med. 2020, 14, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Navara, K.J.; Nelson, R.J. Prenatal environmental influences on the production of sex-specific traits in mammals. Semin. Cell Dev. Biol. 2009, 20, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Terrell, M.L.; Hartnett, K.P.; Marcus, M. Can environmental or occupational hazards alter the sex ratio at birth? A systematic review. Emerg. Health Threat. J. 2011, 4, 7109. [Google Scholar] [CrossRef] [PubMed]
- Peelen, M.J.; Kazemier, B.M.; Ravelli, A.C.; De Groot, C.J.; Van Der Post, J.A.; Mol, B.W.; Hajenius, P.J.; Kok, M. Impact of fetal gender on the risk of preterm birth, a national cohort study. Acta Obstet. Et Gynecol. Scand. 2016, 95, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Galjaard, S.; Ameye, L.; Lees, C.C.; Pexsters, A.; Bourne, T.; Timmerman, D.; Devlieger, R. Sex differences in fetal growth and immediate birth outcomes in a low-risk Caucasian population. Biol. Sex Differ. 2019, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.D.; Dickinson, C.; Kandasamy, Y. Sex difference in mortality for premature and low birth weight neonates: A systematic review. Am. J. Perinatol. 2018, 35, 707–715. [Google Scholar] [PubMed]
- Fang, K.; Yue, S.; Wang, S.; Wang, M.; Yu, X.; Ding, Y.; Lv, M.; Liu, Y.; Cao, C.; Liao, Z. The association between sex and neonatal respiratory distress syndrome. BMC Pediatr. 2024, 24, 129. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Ji, X.; Ku, T.; Li, G.; Sang, N. Sex difference in bronchopulmonary dysplasia of offspring in response to maternal PM2. 5 exposure. J. Hazard. Mater. 2020, 389, 122033. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Aly, H. Male gender is associated with intraventricular hemorrhage. Pediatrics 2010, 125, e333–e339. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.T.; Tsompanidis, A.; Radecki, M.A.; Dorfschmidt, L.; Adhya, D.; Ayeung, B.; Bamford, R.; Biron-Shental, T.; Burton, G.; Cowell, W.; et al. Sex Differences in Human Brain Structure at Birth. Biol. Sex Differ. 2024, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Winckelmans, E.; Vrijens, K.; Tsamou, M.; Janssen, B.G.; Saenen, N.D.; Roels, H.A.; Kleinjans, J.; Lefebvre, W.; Vanpoucke, C.; de Kok, T.M.; et al. Newborn sex-specific transcriptome signatures and gestational exposure to fine particles: Findings from the ENVIRONAGE birth cohort. Environ. Health 2017, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Cossi, M.; Zuta, S.; Padula, A.M.; Gould, J.B.; Stevenson, D.K.; Shaw, G.M. Role of infant sex in the association between air pollution and preterm birth. Ann. Epidemiol. 2015, 25, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kwon, E.; Lee, G.; You, Y.A.; Kim, S.M.; Hur, Y.M.; Jung, S.; Jee, Y.; Park, M.H.; Na, S.H.; et al. Effect of Particulate Matter 2.5 on Fetal Growth in Male and Preterm Infants through Oxidative Stress. Antioxidants 2023, 12, 1916. [Google Scholar] [CrossRef] [PubMed]
- Günther, V.; Alkatout, I.; Stein, A.; Maass, N.; Strauss, A.; Voigt, M. Impact of smoking and fetal gender on preterm delivery. J. Dev. Orig. Health Dis. 2021, 12, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Voigt, M.; Hermanussen, M.; Wittwer-Backofen, U.; Fusch, C.; Hesse, V. Sex-specific differences in birth weight due to maternal smoking during pregnancy. Eur. J. Pediatr. 2006, 165, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Flannigan, K.; Poole, N.; Cook, J.; Unsworth, K. Sex-related differences among individuals assessed for fetal alcohol spectrum disorder in Canada. Alcohol. Clin. Exp. Res. 2023, 47, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Darrow, L.A.; Huang, M.; Warren, J.L.; Strickland, M.J.; Holmes, H.A.; Newman, A.J.; Chang, H.H. Preterm and early-term delivery after heat waves in 50 US metropolitan areas. JAMA Netw. Open 2024, 7, e2412055. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yang, L.; Liu, M.; Wang, C.; Shen, X.; Fan, L.; Zhang, J. Extreme Temperature Exposure and Risks of Preterm Birth Subtypes Based on a Nationwide Survey in China. Environ. Health Perspect. 2023, 131, 87009. [Google Scholar] [CrossRef] [PubMed]
- Green, E.S.; Arck, P.C. Pathogenesis of preterm birth: Bidirectional inflammation in mother and fetus. Semin. Immunopathol. 2020, 42, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W.; Chavan, A.R.; Protopapas, S.; Maziarz, J.; Romero, R.; Wagner, G.P. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc. Natl. Acad. Sci. USA 2017, 114, e6566–e6575. [Google Scholar] [CrossRef] [PubMed]
- Bromfield, J.J. Seminal fluid and reproduction: Much more than previously thought. J. Assist. Reprod. Genet. 2014, 31, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Kapovic, M.; Rukavina, D. Kinetics of lymphoproliferative responses of lymphocytes harvested from the uterine draining lymph nodes during pregnancy in rats. J. Reprod. Immunol. 1991, 20, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Sivarajasingam, S.P.; Imami, N.; Johnson, M.R. Myometrial cytokines and their role in the onset of labour. J. Endocrinol. 2016, 231, R101–R119. [Google Scholar] [CrossRef] [PubMed]
- Kircheis, R.; Planz, O. The Role of Toll-like Receptors (TLRs) and Their Related Signaling Pathways in Viral Infection and Inflammation. Int. J. Mol. Sci. 2023, 24, 6701. [Google Scholar] [CrossRef] [PubMed]
- Faro, J.; Romero, R.; Schwenkel, G.; Garcia-Flores, V.; Arenas-Hernandez, M.; Leng, Y.; Xu, Y.; Miller, D.; Hassan, S.S.; Gomez-Lopez, N. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasome. Biol. Reprod. 2019, 100, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Presicce, P.; Park, C.W.; Senthamaraikannan, P.; Bhattacharyya, S.; Jackson, C.; Kong, F.; Rueda, C.M.; DeFranco, E.; Miller, L.A.; Hildeman, D.A.; et al. IL-1 signaling mediates intrauterine inflammation and chorio-decidua neutrophil recruitment and activation. JCI Insight 2018, 3, e98306. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tian, Y.; Zheng, L.; Luu, T.; Kwak-Kim, J. The Update Immune-Regulatory Role of Pro- and Anti-Inflammatory Cytokines in Recurrent Pregnancy Losses. Int. J. Mol. Sci. 2023, 24, 132. [Google Scholar] [CrossRef] [PubMed]
- Lash, G.E.; Schiessl, B.; Kirkley, M.; Innes, B.A.; Cooper, A.; Searle, R.F.; Robson, S.C.; Bulmer, J.N. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J. Leukoc. Biol. 2006, 80, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Kasahara, T.; Kato, Y.; Ishihara, Y.; Ichijo, M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine 1993, 5, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Sirtori, M.; Oyarzun, E.; Avila, C.; Mazor, M.; Callahan, R.; Sabo, V.; Athanassiadis, A.P.; Hobbins, J.C. Infection and labor V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 1989, 161, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Romero, R.; Gervasi, M.T.; Kim, J.-S.; Yoo, W.; Lee, D.-C.; Mittal, P.; Erez, O.; Kusanovic, J.P.; Hassan, S.S.; et al. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab. Investig. 2009, 89, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.G.; Marchesi, J.R.; Lee, Y.S.; Smith, A.; Lehne, B.; Kindinger, L.M.; Terzidou, V.; Holmes, E.; Nicholson, J.K.; Bennett, P.R.; et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Yuan, J.; Cha, J.; Sun, X.; Bartos, A.; Yagita, H.; Hirota, Y.; Dey, S.K. Endothelial cells in the decidual bed are potential therapeutic targets for preterm birth prevention. Cell Rep. 2019, 27, 1755–1768.e4. [Google Scholar] [CrossRef] [PubMed]
- Firmal, P.; Shah, V.K.; Chattopadhyay, S. Insight Into TLR4-Mediated Immunomodulation in Normal Pregnancy and Related Disorders. Front. Immunol. 2020, 11, 807. [Google Scholar] [CrossRef] [PubMed]
- Flis, W.; Socha, M.W. The Role of the NLRP3 Inflammasome in the Molecular and Biochemical Mechanisms of Cervical Ripening: A Comprehensive Review. Cells 2024, 13, 600. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, C.R.; Gao, L.; Montalbano, A.P. Multifactorial Regulation of Myometrial Contractility During Pregnancy and Parturition. Front. Endocrinol. 2019, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.E.; Perry, W.A.; Klein, S.L. Mechanisms and consequences of sex differences in immune responses. Nat. Rev. Nephrol. 2024, 20, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bouvier, D.L.; Divekar, A.A.; Sasidhar, M.; Du, S.; Tiwari-Woodruff, S.K.; King, J.K.; Arnold, A.P.; Singh, R.R.; Voskuhl, R.R. A role for sex chromosome complement in the female bias in autoimmune disease. J. Exp. Med. 2008, 205, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Golden, L.C.; Itoh, N.; Matsukawa, M.A.; Ren, E.; Tse, V.; Arnold, A.P.; Voskuhl, R.R. The X-linked histone demethylase Kdm6a in CD4+ T lymphocytes modulates autoimmunity. J. Clin. Investig. 2019, 129, 3852–3863. [Google Scholar] [CrossRef] [PubMed]
- Whitacre, C.C.; Reingold, S.C.; O’Looney, P.A.; Blankenhorn, E.; Brinley, F.; Collier, E.; Duquette, P.; Fox, H.; Giesser, B.; Gilmore, W. A gender gap in autoimmunity: Task force on gender, multiple sclerosis and autoimmunity. Science 1999, 283, 1277–1278. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, L.A.; Rasi, C.; Malmqvist, N.; Davies, H.; Pasupulati, S.; Pakalapati, G.; Sandgren, J.; de Ståhl, T.D.; Zaghlool, A.; Giedraitis, V. Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat. Genet. 2014, 46, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.A.; Huttmann, T.D.; Liong, S.; Liong, F.; O’Leary, J.J.; Brooks, D.A.; Selemidis, S. Exploring the Contribution of TLR7 to Sex-Based Disparities in Respiratory Syncytial Virus (RSV)-Induced Inflammation and Immunity. Viruses 2025, 17, 428. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Olsson, P.-E. Sex differences in severity and mortality from COVID-19: Are males more vulnerable? Biol. Sex Differ. 2020, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hafiz, H.A.; Schafer, J.M.; Chen, X.; Xiao, T.; Gauntner, T.D.; Li, Z.; Theodorescu, D. Y chromosome loss in cancer drives growth by evasion of adaptive immunity. Nature 2023, 619, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Merz, N.B.; Barnes, P.J.; Brinton, R.D.; Carrero, J.-J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Libert, C.; Dejager, L.; Pinheiro, I. The X chromosome in immune functions: When a chromosome makes the difference. Nat. Rev. Immunol. 2010, 10, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Migeon, B. Females Are Mosaics: X Inactivation and Sex Differences in Disease; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Migeon, B.R. Why females are mosaics, X-chromosome inactivation, and sex differences in disease. Gend. Med. 2007, 4, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Wainer Katsir, K.; Linial, M. Human genes escaping X-inactivation revealed by single cell expression data. BMC Genom. 2019, 20, 201. [Google Scholar] [CrossRef] [PubMed]
- Souyris, M.; Cenac, C.; Azar, P.; Daviaud, D.; Canivet, A.; Grunenwald, S.; Pienkowski, C.; Chaumeil, J.; Mejía, J.E.; Guéry, J.-C. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 2018, 3, eaap8855. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-A.; Wang, C.-L.; Chuang, H.; Ou, C.-Y.; Hsu, T.-Y.; Yang, K.D. Prediction of elevated cord blood IgE levels by maternal IgE levels, and the neonate’s gender and gestational age. Chang. Gung Med. J. 2003, 26, 561–569. [Google Scholar] [PubMed]
- Page, S.T.; Plymate, S.R.; Bremner, W.J.; Matsumoto, A.M.; Hess, D.L.; Lin, D.W.; Amory, J.K.; Nelson, P.S.; Wu, J.D. Effect of medical castration on CD4+CD25+ T cells, CD8+ T cell IFN-γ expression, and NK cells: A physiological role for testosterone and/or its metabolites. Am. J. Physiol. -Endocrinol. Metab. 2006, 290, E856–E863. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Gonzalez, T.L.; Deng, N.; DiPentino, R.; Clark, E.L.; Lee, B.; Tang, J.; Wang, Y.; Stripp, B.R.; Yao, C. Sexually dimorphic crosstalk at the maternal-fetal interface. J. Clin. Endocrinol. Metab. 2020, 105, e4831–e4847. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.E.; Muench, K.L.; Robinson, B.G.; Wang, A.; Palmer, T.D.; Winn, V.D. Examining sex differences in the human placental transcriptome during the first fetal androgen peak. Reprod. Sci. 2021, 28, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Malik, Q.; Nadeem, M.T.; Ullah, N.; Ikram, F.; Hussain, M. Frequency Of Meningitis In Neonatal Sepsis. J. Rawalpindi Med. Coll. (JRMC) 2023, 27, 114–118. [Google Scholar]
- Haahr, T.; Clausen, T.D.; Thorsen, J.; Rasmussen, M.A.; Mortensen, M.S.; Lehtimäki, J.; Shah, S.A.; Hjelmsø, M.H.; Bønnelykke, K.; Chawes, B.L.; et al. Vaginal dysbiosis in pregnancy associates with risk of emergency caesarean section: A prospective cohort study. Clin. Microbiol. Infect. 2022, 28, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Oh, D.; Heo, H.; Lee, G.; Kim, S.M.; Ansari, A.; You, Y.A.; Jung, Y.J.; Kim, Y.H.; Lee, M.; et al. Prediction of preterm birth based on machine learning using bacterial risk score in cervicovaginal fluid. Am. J. Reprod. Immunol. 2021, 86, e13435. [Google Scholar] [CrossRef] [PubMed]
- You, Y.-A.; Park, S.; Kim, K.; Kwon, E.J.; Hur, Y.M.; Kim, S.M.; Lee, G.; Ansari, A.; Park, J.; Kim, Y.J. Transition in vaginal Lactobacillus species during pregnancy and prediction of preterm birth in Korean women. Sci. Rep. 2022, 12, 22303. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Grassi, P.; MacIntyre, D.A.; Molina, B.G.; Sykes, L.; Kundu, S.; Hsiao, C.-T.; Khoo, K.-H.; Bennett, P.R.; Dell, A. N-glycosylation of cervicovaginal fluid reflects microbial community, immune activity, and pregnancy status. Sci. Rep. 2022, 12, 16948. [Google Scholar] [CrossRef] [PubMed]
- Ercan, A.; Kohrt, W.M.; Cui, J.; Deane, K.D.; Pezer, M.; Yu, E.W.; Hausmann, J.S.; Campbell, H.; Kaiser, U.B.; Rudd, P.M.; et al. Estrogens regulate glycosylation of IgG in women and men. JCI Insight 2017, 2, e89703. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.H.; Schubert, W.-D.; Heinz, D.W. Adhesins and invasins of pathogenic bacteria: A structural view. Microbes Infect. 2004, 6, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Cindrova-Davies, T.; Sferruzzi-Perri, A.N. Human placental development and function. Semin. Cell Dev. Biol. 2022, 131, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. What is the placenta? Am. J. Obstet. Gynecol. 2015, 213, S6.e1–S6.e4. [Google Scholar] [CrossRef] [PubMed]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Whitley, G.S.J.; Cartwright, J.E. Trophoblast-mediated spiral artery remodelling: A role for apoptosis. J. Anat. 2009, 215, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Farrelly, R.; Kennedy, M.G.; Spencer, R.; Forbes, K. Extracellular vesicles as markers and mediators of pregnancy complications: Gestational diabetes, pre-eclampsia, preterm birth and fetal growth restriction. J. Physiol. 2023, 601, 4973–4988. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A. The endocrine function of human placenta: An overview. Reprod. Biomed. Online 2016, 32, 14–43. [Google Scholar] [CrossRef] [PubMed]
- Massimiani, M.; Lacconi, V.; La Civita, F.; Ticconi, C.; Rago, R.; Campagnolo, L. Molecular Signaling Regulating Endometrium-Blastocyst Crosstalk. Int. J. Mol. Sci. 2019, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Preston, M.; Hall, M.; Shennan, A.; Story, L. The role of placental insufficiency in spontaneous preterm birth: A literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 295, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Faye-Petersen, O. The placenta in preterm birth. J. Clin. Pathol. 2008, 61, 1261–1275. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.; Strauss, J.F. Premature rupture of the fetal membranes. N. Engl. J. Med. 1998, 338, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Bujold, E.; Chaiworapongsa, T.; Gomez, R.; Yoon, B.H.; Thaler, H.T.; Rotmensch, S.; Romero, R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 2003, 189, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Loftness, B.C.; Bernstein, I.; McBride, C.A.; Cheney, N.; McGinnis, E.W.; McGinnis, R.S. Preterm Preeclampsia Risk Modelling: Examining Hemodynamic, Biochemical, and Biophysical Markers Prior to Pregnancy. medRxiv 2023, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cindrova-Davies, T.; Fogarty, N.M.; Jones, C.J.; Kingdom, J.; Burton, G.J. Evidence of oxidative stress-induced senescence in mature, post-mature and pathological human placentas. Placenta 2018, 68, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.C.; Heazell, A.E.P.; Sibley, C.; Wright, R.; Bischof, H.; Beards, F.; Guevara, T.; Girard, S.; Jones, R.L. Hypoxia and oxidative stress induce sterile placental inflammation in vitro. Sci. Rep. 2021, 11, 7281. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.; Sultana, Z.; Aitken, R.J.; Morris, J.; Park, F.; Andrew, B.; Riley, S.C.; Smith, R. Evidence that fetal death is associated with placental aging. Am. J. Obstet. Gynecol. 2017, 217, 441.e1–441.e14. [Google Scholar] [CrossRef] [PubMed]
- Johns, J.; Jauniaux, E. Threatened miscarriage as a predictor of obstetric outcome. Obstet. Gynecol. 2006, 107, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.; Metsäranta, M.; Gissler, M.; Luukkaala, T.; Hiilesmaa, V.; Ylikorkala, O.; Paavonen, J.; Andersson, S.; Nuutila, M. Male fetal sex is associated with earlier onset of placental abruption. Acta Obs. Gynecol. Scand. 2010, 89, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Tchaicha, J.H.; Reyes, S.B.; Shin, J.; Hossain, M.G.; Lang, F.F.; McCarty, J.H. Glioblastoma angiogenesis and tumor cell invasiveness are differentially regulated by β8 integrin. Cancer Res. 2011, 71, 6371–6381. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, T.L.; Sun, T.; Koeppel, A.F.; Lee, B.; Wang, E.T.; Farber, C.R.; Rich, S.S.; Sundheimer, L.W.; Buttle, R.A.; Chen, Y.-D.I.; et al. Sex differences in the late first trimester human placenta transcriptome. Biol. Sex Differ. 2018, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Orzack, S.H.; Stubblefield, J.W.; Akmaev, V.R.; Colls, P.; Munné, S.; Scholl, T.; Steinsaltz, D.; Zuckerman, J.E. The human sex ratio from conception to birth. Proc. Natl. Acad. Sci. USA 2015, 112, E2102–E2111. [Google Scholar] [CrossRef] [PubMed]
- Buckberry, S.; Bianco-Miotto, T.; Bent, S.J.; Dekker, G.A.; Roberts, C.T. Integrative transcriptome meta-analysis reveals widespread sex-biased gene expression at the human fetal–maternal interface. Mol. Hum. Reprod. 2014, 20, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Saddiki, H.; Zhang, X.; Colicino, E.; Wilson, A.; Kloog, I.; Wright, R.O.; Wright, R.J.; Lesseur, C. DNA methylation profiles reveal sex-specific associations between gestational exposure to ambient air pollution and placenta cell-type composition in the PRISM cohort study. Clin. Epigenetics 2023, 15, 188. [Google Scholar] [CrossRef] [PubMed]
- Kalisch-Smith, J.I.; Simmons, D.G.; Pantaleon, M.; Moritz, K.M. Sex differences in rat placental development: From pre-implantation to late gestation. Biol. Sex Differ. 2017, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Clifton, V.L. Review: Sex and the Human Placenta: Mediating Differential Strategies of Fetal Growth and Survival. Placenta 2010, 31, S33–S39. [Google Scholar] [CrossRef] [PubMed]
- Sandman, C.A.; Glynn, L.M.; Davis, E.P. Is there a viability–vulnerability tradeoff? Sex differences in fetal programming. J. Psychosom. Res. 2013, 75, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Ao, A.; Erickson, R.P.; Winston, R.M.; Handysude, A.H. Transcription of paternal Y-linked genes in the human zygote as early as the pronucleate stage. Zygote 1994, 2, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, P.S. A Y-chromosomal effect on blastocyst cell number in mice. Development 1993, 117, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Schalekamp-Timmermans, S.; Arends, L.R.; Alsaker, E.; Chappell, L.; Hansson, S.; Harsem, N.K.; Jälmby, M.; Jeyabalan, A.; Laivuori, H.; Lawlor, D.A.; et al. Fetal sex-specific differences in gestational age at delivery in pre-eclampsia: A meta-analysis. Int. J. Epidemiol. 2017, 46, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Helle, S.; Lummaa, V. A trade-off between having many sons and shorter maternal post-reproductive survival in pre-industrial Finland. Biol. Lett. 2013, 9, 20130034. [Google Scholar] [CrossRef] [PubMed]
- Helle, S.; Lummaa, V.; Jokela, J. Sons reduced maternal longevity in preindustrial humans. Science 2002, 296, 1085. [Google Scholar] [CrossRef] [PubMed]
- Sheiner, E.; Shoham-Vardi, I.; Hadar, A.; Hallak, M.; Hackmon, R.; Mazor, M. Incidence, obstetric risk factors and pregnancy outcome of preterm placental abruption: A retrospective analysis. J. Matern.-Fetal Neonatal Med. 2002, 11, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.S.; Usher, R.H.; Pollack, R.; Boyd, M.; Usher, S. Etilogic Determinants of Abruptio Placentae. Obstet. Gynecol. 1997, 89, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Hassold, T.; Quillen, S.; Yamane, J. Sex ratio in spontaneous abortions. Ann. Hum. Genet. 1983, 47, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.; Warburton, D.; Opitz, J.M.; Reynolds, J.F. Male excess among anatomically normal fetuses in spontaneous abortions. Am. J. Med. Genet. 1987, 26, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Ghidini, A.; Salafia, C.M. Gender differences of placental dysfunction in severe prematurity. BJOG 2005, 112, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Okashita, N.; Maeda, R.; Kuroki, S.; Sasaki, K.; Uno, Y.; Koopman, P.; Tachibana, M. Maternal iron deficiency causes male-to-female sex reversal in mouse embryos. Nature 2025, 643, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Dohle, G.R.; Smit, M.; Weber, R.F.A. Androgens and male fertility. World J. Urol. 2003, 21, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Makieva, S.; Saunders, P.T.K.; Norman, J.E. Androgens in pregnancy: Roles in parturition. Hum. Reprod. Update 2014, 20, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Cvitic, S.; Longtine, M.S.; Hackl, H.; Wagner, K.; Nelson, M.D.; Desoye, G.; Hiden, U. The human placental sexome differs between trophoblast epithelium and villous vessel endothelium. PLoS ONE 2013, 8, e79233. [Google Scholar] [CrossRef] [PubMed]
- Yaron, Y.; Wolman, I.; Kupferminc, M.J.; Ochshorn, Y.; Many, A.; Orr-Urtreger, A. Effect of fetal gender on first trimester markers and on Down syndrome screening. Prenat. Diagn. 2001, 21, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.O.; Wøjdemann, K.R.; Shalmi, A.C.; Sundberg, K.; Christiansen, M.; Tabor, A. Gender impact on first trimester markers in Down syndrome screening. Prenat. Diagn. 2002, 22, 1207–1208. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.; Ong, C.Y.; Liao, A.W.; Papademetriou, D.; Nicolaides, K.H. The influence of fetal sex in screening for trisomy 21 by fetal nuchal translucency, maternal serum free beta-hCG and PAPP-A at 10-14 weeks of gestation. Prenat. Diagn. 2000, 20, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Menon, R. Oxidative Stress Damage as a Detrimental Factor in Preterm Birth Pathology. Front. Immunol. 2014, 5, 567. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.P.; Skepper, J.N.; Burton, G.J. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.L.; Créton, R.; Wessel, G.M. The Oxidative Burst at Fertilization Is Dependent upon Activation of the Dual Oxidase Udx1. Dev. Cell 2004, 7, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sekizawa, A.; Purwosunu, Y.; Okazaki, S.; Farina, A.; Wibowo, N.; Shimizu, H.; Okai, T. Cellular mRNA expressions of anti-oxidant factors in the blood of preeclamptic women. Prenat. Diagn. Publ. Affil. Int. Soc. Prenat. Diagn. 2009, 29, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agarwal, A.; Banerjee, J.; Alvarez, J.G. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: A systematic review. Obs. Gynecol. Surv. 2007, 62, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, Oxidants, and Aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Subash, P.; Gurumurthy, P.; Sarasabharathi, A.; Cherian, K. Urinary 8-OHdG: A marker of oxidative stress to DNA and total antioxidant status in essential hypertension with South Indian population. Indian J. Clin. Biochem. 2010, 25, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Negi, R.; Pande, D.; Kumar, A.; Khanna, R.S.; Khanna, H.D. In vivo oxidative DNA damage and lipid peroxidation as a biomarker of oxidative stress in preterm low-birthweight infants. J. Trop. Pediatr. 2012, 58, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Abdel Ghany, E.A.G.; Alsharany, W.; Ali, A.A.; Youness, E.R.; Hussein, J.S. Anti-oxidant profiles and markers of oxidative stress in preterm neonates. Paediatr. Int. Child Health 2016, 36, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; Pathak, R.; Ahmed, T.; Ahmed, R.S.; Tripathi, A.; Guleria, K.; Banerjee, B. Association of glutathione S-transferase M1 and T1 gene polymorphisms and oxidative stress markers in preterm labor. Clin. Biochem. 2010, 43, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Castro, J.; Pulido-Moran, M.; Moreno-Fernandez, J.; Kajarabille, N.; de Paco, C.; Garrido-Sanchez, M.; Prados, S.; Ochoa, J.J. Gender specific differences in oxidative stress and inflammatory signaling in healthy term neonates and their mothers. Pediatr. Res. 2016, 80, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Borrás, C.; Sastre, J.; García-Sala, D.; Lloret, A.; Pallardó, F.V.; Viña, J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic. Biol. Med. 2003, 34, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Marazziti, D.; Baroni, S.; Mucci, F.; Piccinni, A.; Moroni, I.; Giannaccini, G.; Carmassi, C.; Massimetti, E.; Dell’Osso, L. Sex-Related Differences in Plasma Oxytocin Levels in Humans. Clin. Pr. Epidemiol. Ment. Health 2019, 15, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.R.; Nielsen, J.B.; Nielsen, F.; Grandjean, P. Antioxidative enzyme activities in human erythrocytes. Clin. Chem. 1997, 43, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Sugioka, K.; Shimosegawa, Y.; Nakano, M. Estrogens as natural antioxidants of membrane phospholipid peroxidation. FEBS Lett. 1987, 210, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.S.; Rajagopaul, A.; de Vrijer, B.; Eastabrook, G.; Regnault, T.R.H. Fetal sex impacts birth to placental weight ratio and umbilical cord oxygen values with implications for regulatory mechanisms. Biol. Sex Differ. 2022, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, B.; Men, J.; Pang, Y.; Gao, J.; Bai, Y.; Wang, H.; Zhang, J.; Zhao, L.; Xu, X.; et al. Oxidative stress and energy metabolism in male reproductive damage from single and combined high-power microwave exposure at 1.5 and 4.3 GHz. Reprod. Toxicol. 2025, 132, 108759. [Google Scholar] [CrossRef] [PubMed]
- Lien, Y.-C.; Zhang, Z.; Cheng, Y.; Polyak, E.; Sillers, L.; Falk, M.J.; Ischiropoulos, H.; Parry, S.; Simmons, R.A. Human placental transcriptome reveals critical alterations in inflammation and energy metabolism with fetal sex differences in spontaneous preterm birth. Int. J. Mol. Sci. 2021, 22, 7899. [Google Scholar] [CrossRef] [PubMed]
- Akram, K.M.; Kulkarni, N.S.; Brook, A.; Wyles, M.D.; Anumba, D.O.C. Transcriptomic analysis of the human placenta reveals trophoblast dysfunction and augmented Wnt signalling associated with spontaneous preterm birth. Front. Cell Dev. Biol. 2022, 10, 987740. [Google Scholar] [CrossRef] [PubMed]
- Greenough, A.; Lagercrantz, H.; Pool, J.; Dahlin, I. Plasma catecholamine levels in preterm infants: Effect of birth asphyxia and Apgar score. Acta Pædiatrica 1987, 76, 54–59. [Google Scholar] [CrossRef] [PubMed]
- El-Khodor, B.F.; Boksa, P. Differential vulnerability of male versus female rats to long-term effects of birth insult on brain catecholamine levels. Exp. Neurol. 2003, 182, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Saif, Z.; Hodyl, N.; Hobbs, E.; Tuck, A.; Butler, M.; Osei-Kumah, A.; Clifton, V. The human placenta expresses multiple glucocorticoid receptor isoforms that are altered by fetal sex, growth restriction and maternal asthma. Placenta 2014, 35, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Hodyl, N.; Stark, M.; Butler, M.; Clifton, V. Placental P-glycoprotein is unaffected by timing of antenatal glucocorticoid therapy but reduced in SGA preterm infants. Placenta 2013, 34, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Clifton, V.L.; McDonald, M.; Morrison, J.L.; Holman, S.L.; Lock, M.C.; Saif, Z.; Meakin, A.; Wooldridge, A.L.; Gatford, K.L.; Wallace, M.J. Placental glucocorticoid receptor isoforms in a sheep model of maternal allergic asthma. Placenta 2019, 83, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Clifton, V.; Cuffe, J.; Moritz, K.; Cole, T.; Fuller, P.; Lu, N.; Kumar, S.; Chong, S.; Saif, Z. The role of multiple placental glucocorticoid receptor isoforms in adapting to the maternal environment and regulating fetal growth. Placenta 2017, 54, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.; Perez-Garcia, V.; Hemberger, M. Regulation of placental development and its impact on fetal growth—New insights from mouse models. Front. Endocrinol. 2018, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Meakin, A.S.; Cuffe, J.S.M.; Darby, J.R.T.; Morrison, J.L.; Clifton, V.L. Let’s Talk about Placental Sex, Baby: Understanding Mechanisms That Drive Female- and Male-Specific Fetal Growth and Developmental Outcomes. Int. J. Mol. Sci. 2021, 22, 6386. [Google Scholar] [CrossRef] [PubMed]
- Oaks, B.M.; Adu-Afarwuah, S.; Ashorn, P.; Lartey, A.; Laugero, K.D.; Okronipa, H.; Stewart, C.P.; Dewey, K.G. Increased risk of preterm delivery with high cortisol during pregnancy is modified by fetal sex: A cohort study. BMC Pregnancy Childbirth 2022, 22, 727. [Google Scholar] [CrossRef] [PubMed]
- Cooperstock, M.; Campbell, J. Excess males in preterm birth: Interactions with gestational age, race, and multiple birth. Obs. Gynecol. 1996, 88, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Feenstra, B.; Bacelis, J.; Liu, X.; Muglia, L.M.; Juodakis, J.; Miller, D.E.; Litterman, N.; Jiang, P.-P.; Russell, L. Genetic associations with gestational duration and spontaneous preterm birth. N. Engl. J. Med. 2017, 377, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Kumasaka, N.; Nakabayashi, K.; Kamura, H.; Maehara, K.; Kasuga, Y.; Hata, K.; Tanaka, M. Genome-wide association study of preterm birth and gestational age in a Japanese population. Hum. Genome Var. 2023, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, R.; Singh, R.; Lessner, L.; Friedman, J.M. Impact of BRCA mutations on female fertility and offspring sex ratio. Am. J. Hum. Biol. Off. J. Hum. Biol. Assoc. 2010, 22, 201–205. [Google Scholar] [CrossRef] [PubMed]



| Environmental Factors | Exposure Window | Study (Author, Year) | Cohort (Ethnicities) | Sample Size | Trend in Fetal Sex | Significance | Key Findings/Conclusion |
|---|---|---|---|---|---|---|---|
| NO2 | T2 | Cossi et al., 2015 [28] | San Joaquin Valley of California (Hispanic) | 253,704 | M > F | p < 0.01 | Exposure to NO2 during T2 was associated with a high risk of PTB (GW 20–27) in M infant. |
| PM2.5 | Entire, T1 | Park et al., 2023 [29] | Retrospective birth cohort (Korean) | 1880 | M > F | p = 0.01, p < 0.01 | The higher risk of LBW was associated with exposure to PM2.5 during T1 (OR:1.05 [95% CI: 1.01–1.10]) and T2 (OR: 1.07 [95% CI; 1.03–1.12]). |
| Smoking | Entire (Survey) | Günther et al., 2020 [30] | Database of Schleswig-Holstein (German) | 220,339 | M > F | p < 0.001 | The rate of PTB subdivided into the smoking severity. M > F for nonsmokers; M > F for: 1–7 cigarettes/day; M > F: 8–14 cigarettes/day; M > F: 15–21 cigarettes/day; M = F: ≥22 cigarettes/day. |
| Smoking | Entire (Survey) | Voigt et al., 2006 [31] | German birth statistics from Deutsche Perinatalerhebung (German) | 888,632 | M < F | p < 0.001 | Severe smokers (>21 cigarettes/day) have a higher risk for SGA in F (3.51-fold) and in M (3.15-fold) vs. non-smoker. In mild smokers (1–5/day), the risk of SGA was 1.7275-fold in F, but was 1.7143-fold in M. |
| Alcohol | Not suggested | Flannigan et al., 2023 [32] | Canada | 2574 | FASD w/wo SFF: M = F NDF: M > F EP: M < F | p < 0.001 | M = F: FASD diagnostic F: ↑EP anxiety, ↑depressive/mood disorders, ↑trauma. M: ↑NDF impairment, ↑ADHD, ↑conduct disorder, ↑oppositional, ↑defiant disorder. The differences were clearest in adolescents (13–17 years) and adults (≥25 years). |
| Heat waves | 4-day (or 7-day) # | Darrow et al., 2024 [33] | National Vital Statistics System at the National Center for Health Statistics Data (##) | 55,748,869 | M < F | RR (95% CI) F: 1.011 (1.001–1.020) M: 1.006 (0.997–1.015) | Subgroup analysis of RR per 1 °C increase. F: PTB and early PTB > 1 M: PTB RR > 1. |
| Extreme temperature | 1 to 2 weeks before delivery | Yu et al., 2023 [34] | Large-scale multicenter study (Chinese) | 82,221 | M < F | OR (95% CI) ### Fifth: 1.09 (1.04, 1.13) 10th day: 1.07 (1.04, 1.12) 10th 2D: 1.13 (1.04, 1.23) | F > M: cold spells; heat waves, ↑northern and western regions in China. Exposure to cold spells was relevant with ↑risk of PTB, especially late. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.; Andrade, G.M.; Kim, Y.J.; Anumba, D.O.C. The Sex Difference in the Pathophysiology of Preterm Birth. Cells 2025, 14, 1084. https://doi.org/10.3390/cells14141084
Lee G, Andrade GM, Kim YJ, Anumba DOC. The Sex Difference in the Pathophysiology of Preterm Birth. Cells. 2025; 14(14):1084. https://doi.org/10.3390/cells14141084
Chicago/Turabian StyleLee, Gain, Gisela Martinez Andrade, Young Ju Kim, and Dilly O. C. Anumba. 2025. "The Sex Difference in the Pathophysiology of Preterm Birth" Cells 14, no. 14: 1084. https://doi.org/10.3390/cells14141084
APA StyleLee, G., Andrade, G. M., Kim, Y. J., & Anumba, D. O. C. (2025). The Sex Difference in the Pathophysiology of Preterm Birth. Cells, 14(14), 1084. https://doi.org/10.3390/cells14141084






