Mechanistic Insights into Autophagy-Dependent Cell Death (ADCD): A Novel Avenue for Cancer Therapy
Abstract
1. Introduction
2. Molecular Mechanisms of Autophagy and Autophagy-Dependent Cell Death
2.1. Autophagy and Its Regulatory Pathways
2.1.1. Initiation of Autophagy
2.1.2. Nucleation: Formation of the Phagophore
2.1.3. Elongation and Maturation of Autophagosomes
2.1.4. Fusion with Lysosomes and Cargo Degradation
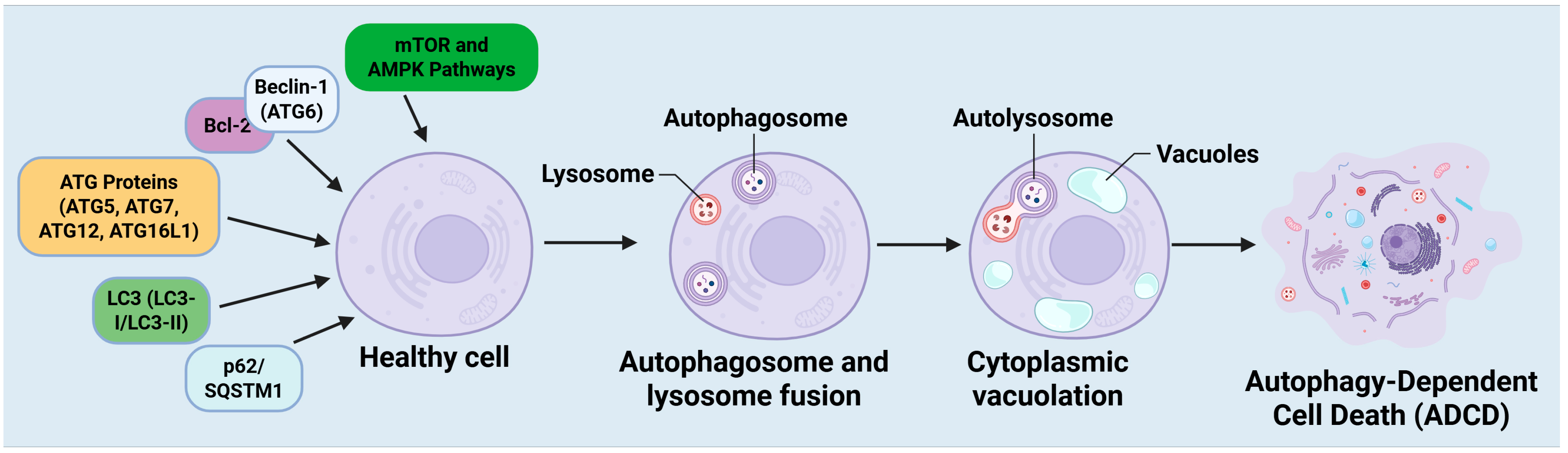
2.2. Molecular Mechanisms of Autophagy-Dependent Cell Death
2.2.1. Beclin-1 (ATG6): A Principal Regulator of Autophagy
2.2.2. ATG Proteins: Crucial for Autophagosome Formation
2.2.3. LC3: An Indicator of Autophagosome Formation
2.2.4. p62/SQSTM1: A Mediator of Autophagy and Apoptosis Interference
2.2.5. mTOR and AMPK Signaling: Principal Regulators of Autophagy
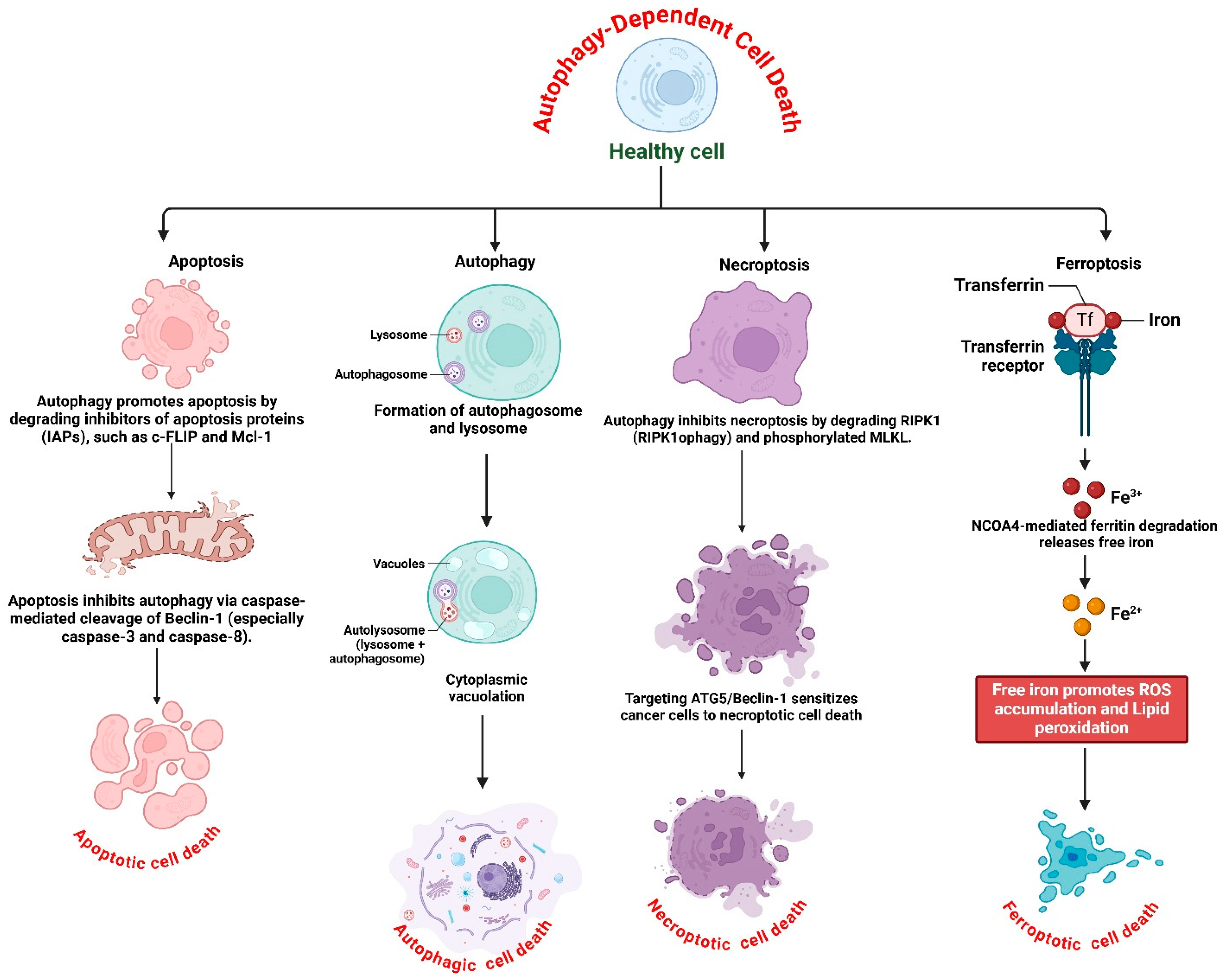
2.3. Crosstalk Between Autophagy-Dependent Cell Death and Other Cell Death Pathways
2.3.1. Autophagy and Apoptosis: A Dual Regulatory Mechanism
2.3.2. Autophagy and Necroptosis: A Complex Interplay
2.3.3. Autophagy and Ferroptosis: The Role of Ferritinophagy
3. Role of Autophagy-Dependent Cell Death in Cancer Therapy
3.1. Dual Role of Autophagy-Dependent Cell Death in Cancer
3.1.1. Autophagy-Dependent Cell Death Functions as a Tumor Suppressor
Preservation of Cellular Homeostasis and Genomic Integrity
Regulation of Oncogenes and Tumor Suppressor Genes
Mitigation of Chronic Inflammation
3.1.2. Autophagy-Dependent Cell Death as a Tumor Enhancer
Metabolic Adaptation and Resistance Under Stress
Role in Therapy Resistance
Role in Tumor Dormancy and Recurrence
3.2. Therapeutic Targeting of Autophagy in Cancer
3.2.1. Autophagy Inhibition in Cancer Therapy
Chloroquine (CQ) and Hydroxychloroquine (HCQ): Inhibiting Autophagosome-Lysosome Fusion
mTOR Inhibitors: Inhibiting Autophagy Activation in Cancers
3.2.2. Activation of Autophagy in Cancer Treatment
Resveratrol and Curcumin: Facilitating ADCD to Augment Apoptosis in Cancer Cells
AMPK Activators: Augmenting Autophagic Flux to Induce Cytotoxicity in Cancer
3.3. Combination Strategies for Enhancing Cancer Therapy
3.3.1. Inhibition of Autophagy Combined with Chemotherapy: Disrupting Mechanisms of Cancer Cell Survival
3.3.2. Activation of Autophagy and Immunotherapy: Augmenting Immune-Mediated Tumor Elimination
3.3.3. Modulation of Autophagy and Radiation Therapy: Enhancing Therapeutic Efficacy in Resistant Tumors
4. Therapeutic and Clinical Application of Autophagy-Dependent Cell Death in Cancer Therapy
5. Limitations and Future Directions of Autophagy-Dependent Cell Death in Cancer Therapy
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tobore, T.O. On the need for the development of a cancer early detection, diagnostic, prognosis, and treatment response system. Future Sci. OA 2020, 6, FSO439. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Bhardwaj, A.; Gupta, S. Cancer treatment therapies: Traditional to modern approaches to combat cancers. Mol. Biol. Rep. 2023, 50, 9663–9676. [Google Scholar] [CrossRef]
- Gu, Y.; Yang, R.; Zhang, Y.; Guo, M.; Takehiro, K.; Zhan, M.; Yang, L.; Wang, H. Molecular mechanisms and therapeutic strategies in overcoming chemotherapy resistance in cancer. Mol. Biomed. 2025, 6, 2. [Google Scholar] [CrossRef]
- Bialik, S.; Dasari, S.K.; Kimchi, A. Autophagy-dependent cell death–where, how and why a cell eats itself to death. J. Cell Sci. 2018, 131, jcs215152. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Saikat, A.S.M.; Rahman, M.S.; Islam, M.; Parvez, M.A.K.; Kim, B. Recent update and drug target in molecular and pharmacological insights into autophagy modulation in cancer treatment and future progress. Cells 2023, 12, 458. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rhim, H. Therapeutic implication of autophagy in neurodegenerative diseases. BMB Rep. 2017, 50, 345–354. [Google Scholar] [CrossRef]
- Jalali, P.; Shahmoradi, A.; Samii, A.; Mazloomnejad, R.; Hatamnejad, M.R.; Saeed, A.; Namdar, A.; Salehi, Z. The role of autophagy in cancer: From molecular mechanism to therapeutic window. Front. Immunol. 2025, 16, 1528230. [Google Scholar] [CrossRef]
- Tang, Q.; Tang, K.; Markby, G.R.; Parys, M.; Phadwal, K.; MacRae, V.E.; Corcoran, B.M. Autophagy regulates cellular senescence by mediating the degradation of CDKN1A/p21 and CDKN2A/p16 through SQSTM1/p62-mediated selective autophagy in myxomatous mitral valve degeneration. Autophagy 2025, 21, 1433–1455. [Google Scholar] [CrossRef]
- Coutts, A.S.; La Thangue, N.B. Regulation of actin nucleation and autophagosome formation. Cell. Mol. Life Sci. 2016, 73, 3249–3263. [Google Scholar] [CrossRef]
- Chan, W.W.R.; Chow, J.; Chau, D.D.-L.; Zhai, Y.; Lau, K.-F. Beclin 1-Mediated Autophagy Is Potentiated by an Interaction with the Neuronal Adaptor FE65. Biology 2025, 14, 97. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Xu, H. Autophagy-lysosome pathway in insulin & glucagon homeostasis. Front. Endocrinol. 2025, 16, 1541794. [Google Scholar]
- Qian, Z.; Li, Z.; Peng, X.; Mao, Y.; Mao, X.; Li, J. Annexin A: Cell Death, Inflammation, and Translational Medicine. J. Inflamm. Res. 2025, 18, 5655–5672. [Google Scholar] [CrossRef]
- Huang, X.; Yan, H.; Xu, Z.; Yang, B.; Luo, P.; He, Q. The inducible role of autophagy in cell death: Emerging evidence and future perspectives. Cell Commun. Signal. 2025, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Palabiyik, A.A. The role of Bcl-2 in controlling the transition between autophagy and apoptosis. Mol. Med. Rep. 2025, 32, 172. [Google Scholar] [CrossRef]
- Cabrera-Serrano, A.J.; Sánchez-Maldonado, J.M.; González-Olmedo, C.; Carretero-Fernández, M.; Díaz-Beltrán, L.; Gutiérrez-Bautista, J.F.; García-Verdejo, F.J.; Gálvez-Montosa, F.; López-López, J.A.; García-Martín, P. Crosstalk between autophagy and oxidative stress in hematological malignancies: Mechanisms, implications, and therapeutic potential. Antioxidants 2025, 14, 264. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, J.; Lu, Y.; Gao, H.; Ning, E.; Yang, X.; Hao, Y.; Hu, D. The interplay between autophagy and programmed cell death in osteoarthritis: Insights into mechanisms and therapeutic targets. Mol. Cell. Biochem. 2025, 1–20. [Google Scholar] [CrossRef]
- Yun, C.W.; Jeon, J.; Go, G.; Lee, J.H.; Lee, S.H. The dual role of autophagy in cancer development and a therapeutic strategy for cancer by targeting autophagy. Int. J. Mol. Sci. 2020, 22, 179. [Google Scholar] [CrossRef]
- Kumar, P.; Raj, A. Degradation of misfolded proteins by ubiquitin-proteasome system and autophagy lysosomal proteolytic pathways. In Protein Misfolding in Neurodegenerative Diseases; Elsevier: Amsterdam, The Netherlands, 2025; pp. 253–289. [Google Scholar]
- Chen, R.; Yang, C.; Yang, F.; Yang, A.; Xiao, H.; Peng, B.; Chen, C.; Geng, B.; Xia, Y. Targeting the mTOR-Autophagy Axis: Unveiling Therapeutic Potentials in Osteoporosis. Biomolecules 2024, 14, 1452. [Google Scholar] [CrossRef]
- Lyu, J.; Kim, J.-S.; Shin, J. Autophagy flux via AMPK/mTOR/ULK1 signaling pathway. In Herbal Medical Products for Metabolic Diseases-New Integrated Pharmacological Approaches; Frontiers in Pharmacology: Lausanne, Switzerland, 2024; p. 37. [Google Scholar]
- Wang, B.; Pareek, G.; Kundu, M. ULK/Atg1: Phasing in and out of autophagy. Trends Biochem. Sci. 2024, 49, 494–505. [Google Scholar] [CrossRef]
- Sousa, C.; Videira, M. Dual Approaches in Oncology: The Promise of siRNA and Chemotherapy Combinations in Cancer Therapies. Onco 2025, 5, 2. [Google Scholar] [CrossRef]
- Ahmed, K.R.; Rahman, M.M.; Islam, M.N.; Fahim, M.M.H.; Rahman, M.A.; Kim, B. Antioxidants activities of phytochemicals perspective modulation of autophagy and apoptosis to treating cancer. Biomed. Pharmacother. 2024, 174, 116497. [Google Scholar] [CrossRef]
- Rao, S.; Skulsuppaisarn, M.; Strong, L.M.; Ren, X.; Lazarou, M.; Hurley, J.H.; Hummer, G. Three-step docking by WIPI2, ATG16L1, and ATG3 delivers LC3 to the phagophore. Sci. Adv. 2024, 10, eadj8027. [Google Scholar] [CrossRef]
- Noda, N.N. Structural biology of the Atg8 and Atg12 conjugation systems. Autophagy Rep. 2023, 2, 2277582. [Google Scholar] [CrossRef] [PubMed]
- Gammoh, N. The multifaceted functions of ATG16L1 in autophagy and related processes. J. Cell Sci. 2020, 133, jcs249227. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. In Autophagosome and Phagosome; Humana Press: Totowa, NJ, USA, 2008; pp. 77–88. [Google Scholar]
- Zhou, Y.; Wang, Z.; Huang, Y.; Bai, C.; Zhang, X.; Fang, M.; Ju, Z.; Liu, B. Membrane dynamics of ATG4B and LC3 in autophagosome formation. J. Mol. Cell Biol. 2021, 13, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Lőrincz, P.; Juhász, G. Autophagosome-lysosome fusion. J. Mol. Biol. 2020, 432, 2462–2482. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Liyanage, P.; Zhong, Q.; Naren, A.P. A SNARE protein Syntaxin 17 captures CFTR to potentiate autophagosomal clearance under stress. FASEB J. 2021, 35, e21185. [Google Scholar] [CrossRef]
- Mijanovic, O.; Petushkova, A.I.; Brankovic, A.; Turk, B.; Solovieva, A.B.; Nikitkina, A.I.; Bolevich, S.; Timashev, P.S.; Parodi, A.; Zamyatnin, A.A., Jr. Cathepsin D—Managing the delicate balance. Pharmaceutics 2021, 13, 837. [Google Scholar] [CrossRef]
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar] [CrossRef]
- Xu, H.-D.; Qin, Z.-H. Beclin 1, Bcl-2 and autophagy. In Autophagy: Biology and Diseases: Basic Science; Springer: Singapore, 2019; pp. 109–126. [Google Scholar]
- Kaur, S.; Changotra, H. The beclin 1 interactome: Modification and roles in the pathology of autophagy-related disorders. Biochimie 2020, 175, 34–49. [Google Scholar] [CrossRef]
- Tran, S.; Fairlie, W.D.; Lee, E.F. BECLIN1: Protein structure, function and regulation. Cells 2021, 10, 1522. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Tian, K.; Ran, Y.; Zhou, H.; Zhou, L.; Ding, Y.; Tang, X. Beclin-1: A therapeutic target at the intersection of autophagy, immunotherapy, and cancer treatment. Front. Immunol. 2024, 15, 1506426. [Google Scholar] [CrossRef] [PubMed]
- Nakatogawa, H. Mechanisms governing autophagosome biogenesis. Nat. Rev. Mol. Cell Biol. 2020, 21, 439–458. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L. Regulation of ATG and autophagy initiation. In Autophagy: Biology and Diseases: Basic Science; Springer: Singapore, 2019; pp. 41–65. [Google Scholar]
- Changotra, H.; Kaur, S.; Yadav, S.S.; Gupta, G.L.; Parkash, J.; Duseja, A. ATG5: A central autophagy regulator implicated in various human diseases. Cell Biochem. Funct. 2022, 40, 650–667. [Google Scholar] [CrossRef] [PubMed]
- Goncu, E.; Tinartas, E.P.; Gunay, B.; Ordu, T.; Turgay Izzetoglu, G. Role of Atg3, Atg5 and Atg12 in the crosstalk between apoptosis and autophagy in the posterior silk gland of Bombyx mori. Insect Mol. Biol. 2025, 34, 470–485. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, C.; Wang, Q.; Song, L.; He, Z.; Liu, W.; Wan, W. Deacetylation of ATG7 drives the induction of macroautophagy and LC3-associated microautophagy. Autophagy 2024, 20, 1134–1146. [Google Scholar] [CrossRef]
- Collier, J.J.; Suomi, F.; Oláhová, M.; McWilliams, T.G.; Taylor, R.W. Emerging roles of ATG7 in human health and disease. EMBO Mol. Med. 2021, 13, e14824. [Google Scholar] [CrossRef]
- Bussi, C.; Iribarren, P.; Rodriguez, C.M. Microtubule-associated protein 1A/1B-light chain 3 (LC3)’decorates’ intracytoplasmic inclusions in a patient with chronic lymphocytic leukaemia. Br. J. Haematol. 2017, 179, 529. [Google Scholar] [CrossRef]
- Runwal, G.; Stamatakou, E.; Siddiqi, F.H.; Puri, C.; Zhu, Y.; Rubinsztein, D.C. LC3-positive structures are prominent in autophagy-deficient cells. Sci. Rep. 2019, 9, 10147. [Google Scholar] [CrossRef]
- Huang, R.; Xu, Y.; Wan, W.; Shou, X.; Qian, J.; You, Z.; Liu, B.; Chang, C.; Zhou, T.; Lippincott-Schwartz, J. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol. Cell 2015, 57, 456–466. [Google Scholar] [CrossRef]
- Rahman, M.A.; Bishayee, K.; Sadra, A.; Huh, S.-O. Oxyresveratrol activates parallel apoptotic and autophagic cell death pathways in neuroblastoma cells. Biochim. Biophys. Acta BBA-Gen. Subj. 2017, 1861, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.V.; Mills, J.; Lapierre, L.R. Selective autophagy receptor p62/SQSTM1, a pivotal player in stress and aging. Front. Cell Dev. Biol. 2022, 10, 793328. [Google Scholar] [CrossRef]
- Carroll, B.; Otten, E.G.; Manni, D.; Stefanatos, R.; Menzies, F.M.; Smith, G.R.; Jurk, D.; Kenneth, N.; Wilkinson, S.; Passos, J.F. Oxidation of SQSTM1/p62 mediates the link between redox state and protein homeostasis. Nat. Commun. 2018, 9, 256. [Google Scholar] [CrossRef]
- Orlandi, G.; Roncucci, L.; Carnevale, G.; Sena, P. Different roles of apoptosis and autophagy in the development of human colorectal cancer. Int. J. Mol. Sci. 2023, 24, 10201. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Xia, S.; Li, J.; Yang, Q.; Ding, K.; Zhang, H. Sequestosome 1/p62: A multitasker in the regulation of malignant tumor aggression. Int. J. Oncol. 2021, 59, 77. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H. Regulation of autophagy by mTOR signaling pathway. In Autophagy: Biology and Diseases: Basic Science; Springer: Singapore, 2019; pp. 67–83. [Google Scholar]
- Datkhayeva, Z.; Iskakova, A.; Mireeva, A.; Seitaliyeva, A.; Skakova, R.; Kulniyazova, G.; Shayakhmetova, A.; Koshkimbayeva, G.; Sarmuldayeva, C.; Nurseitova, L. The Multifactorial Pathogenesis of Endometriosis: A Narrative Review Integrating Hormonal, Immune, and Microbiome Aspects. Medicina 2025, 61, 811. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.; Zhang, X.; Zhang, L.; Han, Z.; Jie, R.; Duan, P.; Cao, M.; Yang, A. Electroacupuncture as a promising therapeutic strategy for doxorubicin-induced heart failure: Insights into the PI3K/AKT/mTOR/ULK1 and AMPK/mTOR/ULK1 pathways. Colloids Surf. B Biointerfaces 2025, 251, 114590. [Google Scholar] [CrossRef]
- Zhang, C.; Shu, X.; Yin, C.; Hu, S.; Liu, P. The role of the mTOR pathway in breast cancer stem cells (BCSCs): Mechanisms and therapeutic potentials. Stem Cell Res. Ther. 2025, 16, 156. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.-Y.; Lei, Y.-X.; Zhou, J.-T.; Liu, L.-J.; Yang, T.; Zhou, Y.; Ge, J.-W.; Xu, C.; Mei, Z.-G. Insight into interplay between PANoptosis and autophagy: Novel therapeutics in ischemic stroke. Front. Mol. Neurosci. 2025, 17, 1482015. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, W.; Liao, Y.; Wang, W.; Deng, X.; Wang, C.; Shi, W. Autophagy: A double-edged sword in ischemia–reperfusion injury. Cell. Mol. Biol. Lett. 2025, 30, 1–47. [Google Scholar] [CrossRef]
- Liao, Y.X.; Yu, H.Y.; Lv, J.Y.; Cai, Y.R.; Liu, F.; He, Z.M.; He, S.S. Targeting autophagy is a promising therapeutic strategy to overcome chemoresistance and reduce metastasis in osteosarcoma. Int. J. Oncol. 2019, 55, 1213–1222. [Google Scholar] [CrossRef]
- Wei, S.; Han, C.; Mo, S.; Huang, H.; Luo, X. Advancements in programmed cell death research in antitumor therapy: A comprehensive overview. Apoptosis 2025, 30, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Jalouli, M. Emerging Role of Hypoxia-Inducible Factors (HIFs) in Modulating Autophagy: Perspectives on Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 1752. [Google Scholar] [CrossRef] [PubMed]
- Mosadegh, M.; Noori Goodarzi, N.; Erfani, Y. A Comprehensive Insight into Apoptosis: Molecular Mechanisms, Signaling Pathways, and Modulating Therapeutics. Cancer Investig. 2025, 43, 33–58. [Google Scholar] [CrossRef]
- Selvarani, R. Using Novel RIPK3 and Mlkl Knock-In Mouse Models to Study the Role of Necroptosis in Chronic Liver Disease and Liver Cancer. Ph.D. Thesis, The University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA, 2024. [Google Scholar]
- Wang, G.; Jiang, X.; Torabian, P.; Yang, Z. Investigating autophagy and intricate cellular mechanisms in hepatocellular carcinoma: Emphasis on cell death mechanism crosstalk. Cancer Lett. 2024, 588, 216744. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Jadhav, K.; Rawat, N.; Verma, R.K.; Kumar, J. Current strategies for targeting autophagy and ros for cancer treatment. In Role of Autophagy and Reactive Oxygen Species in Cancer Treatment: Principles and Current Strategies; Springer: Berlin/Heidelberg, Germany, 2024; pp. 287–307. [Google Scholar]
- Karlowitz, R.; Van Wijk, S.J. Surviving death: Emerging concepts of RIPK3 and MLKL ubiquitination in the regulation of necroptosis. FEBS J. 2023, 290, 37–54. [Google Scholar] [CrossRef]
- Snyder, A.G.; Oberst, A. The antisocial network: Cross talk between cell death programs in host defense. Annu. Rev. Immunol. 2021, 39, 77–101. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, Y.; Yin, X. Autophagy, ferroptosis, apoptosis and pyroptosis in metabolic dysfunction-associated steatotic liver disease. Front. Biosci.-Landmark 2024, 29, 30. [Google Scholar] [CrossRef]
- Yuan, J.; Amin, P.; Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 2019, 20, 19–33. [Google Scholar] [CrossRef]
- Martinez-Osorio, V.; Abdelwahab, Y.; Ros, U. The many faces of MLKL, the executor of necroptosis. Int. J. Mol. Sci. 2023, 24, 10108. [Google Scholar] [CrossRef]
- Samson, A.L.; Zhang, Y.; Geoghegan, N.D.; Gavin, X.J.; Davies, K.A.; Mlodzianoski, M.J.; Whitehead, L.W.; Frank, D.; Garnish, S.E.; Fitzgibbon, C. MLKL trafficking and accumulation at the plasma membrane control the kinetics and threshold for necroptosis. Nat. Commun. 2020, 11, 3151. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xiong, A.; Liu, J.; Li, X.; Wang, J.; Zhang, L.; Liu, Y.; Xiong, Y.; Li, G.; He, X. PANoptosis: Bridging apoptosis, pyroptosis, and necroptosis in cancer progression and treatment. Cancer Gene Ther. 2024, 31, 970–983. [Google Scholar] [CrossRef]
- Zheng, J.; Conrad, M. Ferroptosis: When metabolism meets cell death. Physiol. Rev. 2025, 105, 651–706. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, Y.; Chen, D.; Sun, Y.; Li, D.; Meng, Y.; Zhou, Q.; Zeng, F.; Deng, G.; Chen, X. Targeting regulated cell death: Apoptosis, necroptosis, pyroptosis, ferroptosis, and cuproptosis in anticancer immunity. J. Transl. Intern. Med. 2025, 13, 10–32. [Google Scholar] [CrossRef]
- Li, S.; Huang, P.; Lai, F.; Zhang, T.; Guan, J.; Wan, H.; He, Y. Mechanisms of ferritinophagy and ferroptosis in diseases. Mol. Neurobiol. 2024, 61, 1605–1626. [Google Scholar] [CrossRef]
- Li, J.Y.; Feng, Y.H.; Li, Y.X.; He, P.Y.; Zhou, Q.Y.; Tian, Y.P.; Yao, R.Q.; Yao, Y.M. Ferritinophagy: A novel insight into the double-edged sword in ferritinophagy–ferroptosis axis and human diseases. Cell Prolif. 2024, 57, e13621. [Google Scholar] [CrossRef]
- Sui, X.; Wang, J.; Zhao, Z.; Liu, B.; Liu, M.; Liu, M.; Shi, C.; Feng, X.; Fu, Y.; Shi, D. Phenolic compounds induce ferroptosis-like death by promoting hydroxyl radical generation in the Fenton reaction. Commun. Biol. 2024, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef]
- Feng, F.; He, S.; Li, X.; He, J.; Luo, L. Mitochondria-mediated ferroptosis in diseases therapy: From molecular mechanisms to implications. Aging Dis. 2024, 15, 714. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Z.-X.; Bin, J.-L.; Chen, Y.-X.; Ma, J.; Deng, J.-H.; Huang, X.-W.; Zhou, J.; Lu, G.-D. Pharmacological approaches for targeting lysosomes to induce ferroptotic cell death in cancer. Cancer Lett. 2024, 587, 216728. [Google Scholar] [CrossRef]
- Shao, B.; Bu, H.; Li, G.; Kang, D.; Ju, Q. Autophagy-dependent apoptosis induction by oridonin are mediated by ROS-dependent AMPK-mTOR-ULK1 pathway in colon cancer. Am. J. Cancer Res. 2025, 15, 1902. [Google Scholar] [CrossRef] [PubMed]
- Van Houten, B.; Hunter, S.E.; Meyer, J.N. Mitochondrial DNA damage induced autophagy, cell death, and disease. Front. Biosci. Landmark Ed. 2016, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Mrakovcic, M.; Fröhlich, L.F. p53-mediated molecular control of autophagy in tumor cells. Biomolecules 2018, 8, 14. [Google Scholar] [CrossRef]
- Rahman, M.A.; Jalouli, M.; Bhajan, S.K.; Al-Zharani, M.; Harrath, A.H. The Role of Hypoxia-Inducible Factor-1α (HIF-1α) in the Progression of Ovarian Cancer: Perspectives on Female Infertility. Cells 2025, 14, 437. [Google Scholar] [CrossRef]
- Takahama, M.; Akira, S.; Saitoh, T. Autophagy limits activation of the inflammasomes. Immunol. Rev. 2018, 281, 62–73. [Google Scholar] [CrossRef]
- Cau, R.; Saba, L. Interlinking pathways: A narrative review on the role of IL-6 in cancer and atherosclerosis. Cardiovasc. Diagn. Ther. 2024, 14, 1186. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.R.; Neves, S.P.; Santos, L.D.S.; Dias, R.B.; Bezerra, D.P. Challenges and therapeutic opportunities of autophagy in cancer therapy. Cancers 2020, 12, 3461. [Google Scholar] [CrossRef]
- Wang, H.; Sun, P.; Yuan, X.; Xu, Z.; Jiang, X.; Xiao, M.; Yao, X.; Shi, Y. Autophagy in tumor immune escape and immunotherapy. Mol. Cancer 2025, 24, 85. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhong, B.; Cao, J.-Y.; Wang, Q.; Liang, J.; Lu, K.; Lu, S.-M. Tumor hypoxia: Classification, detection, and its critical role in cancer progression. Biomol. Biomed. 2025. [Google Scholar] [CrossRef]
- Daskalaki, I.; Gkikas, I.; Tavernarakis, N. Hypoxia and selective autophagy in cancer development and therapy. Front. Cell Dev. Biol. 2018, 6, 104. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Ponneri Babuharisankar, A.; Lin, Y.-C.; Lien, H.-W.; Lo, Y.K.; Chou, H.-Y.; Tangeda, V.; Cheng, L.-C.; Cheng, A.N.; Lee, A.Y.-L. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: Foe or friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.J. Therapeutic strategies of drug repositioning targeting autophagy to induce cancer cell death: From pathophysiology to treatment. J. Hematol. Oncol. 2017, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Jiang, J.; Tian, Z.; Wei, L. Autophagy and tumour chemotherapy. In Autophagy: Biology and Diseases: Clinical Science; Springer: Singapore, 2020; pp. 351–374. [Google Scholar]
- Wei, J.; Wang, B.; Wang, H.; Meng, L.; Zhao, Q.; Li, X.; Xin, Y.; Jiang, X. Radiation-induced normal tissue damage: Oxidative stress and epigenetic mechanisms. Oxidative Med. Cell. Longev. 2019, 2019, 3010342. [Google Scholar] [CrossRef]
- Roy, A.; Bera, S.; Saso, L.; Dwarakanath, B.S. Role of autophagy in tumor response to radiation: Implications for improving radiotherapy. Front. Oncol. 2022, 12, 957373. [Google Scholar] [CrossRef]
- Elshazly, A.M.; Xu, J.; Melhem, N.; Abdulnaby, A.; Elzahed, A.A.; Saleh, T.; Gewirtz, D.A. Is Autophagy Targeting a Valid Adjuvant Strategy in Conjunction with Tyrosine Kinase Inhibitors? Cancers 2024, 16, 2989. [Google Scholar] [CrossRef] [PubMed]
- Akkoc, Y.; Peker, N.; Akcay, A.; Gozuacik, D. Autophagy and cancer dormancy. Front. Oncol. 2021, 11, 627023. [Google Scholar] [CrossRef]
- Ferreira, P.M.P.; de Sousa, R.W.R.; de Oliveira Ferreira, J.R.; Militão, G.C.G.; Bezerra, D.P. Chloroquine and hydroxychloroquine in antitumor therapies based on autophagy-related mechanisms. Pharmacol. Res. 2021, 168, 105582. [Google Scholar] [CrossRef]
- Chen, J.-L.; Wu, X.; Yin, D.; Jia, X.-H.; Chen, X.; Gu, Z.-Y.; Zhu, X.-M. Autophagy inhibitors for cancer therapy: Small molecules and nanomedicines. Pharmacol. Ther. 2023, 249, 108485. [Google Scholar] [CrossRef]
- Shi, T.-T.; Yu, X.-X.; Yan, L.-J.; Xiao, H.-T. Research progress of hydroxychloroquine and autophagy inhibitors on cancer. Cancer Chemother. Pharmacol. 2017, 79, 287–294. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.K.; Saadeldin, M.K.; Salem, A.H.; Ibrahim, S.A.; Shouman, S.; Abdel-Naim, A.B.; Orecchia, R. A critical review of chloroquine and hydroxychloroquine as potential adjuvant agents for treating people with cancer. Future Pharmacol. 2022, 2, 431–443. [Google Scholar] [CrossRef]
- Hassan, A.M.I.A.; Zhao, Y.; Chen, X.; He, C. Blockage of autophagy for cancer therapy: A comprehensive review. Int. J. Mol. Sci. 2024, 25, 7459. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Rahman, M.A.; Kabir, M.T.; Behl, T.; Mathew, B.; Perveen, A.; Barreto, G.E.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Ashraf, G.M. Multifarious roles of mTOR signaling in cognitive aging and cerebrovascular dysfunction of Alzheimer’s disease. Iubmb Life 2020, 72, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.; El-Houjeiri, L.; Pause, A. mTOR pathways in cancer and autophagy. Cancers 2018, 10, 18. [Google Scholar] [CrossRef]
- Xu, Z.; Han, X.; Ou, D.; Liu, T.; Li, Z.; Jiang, G.; Liu, J.; Zhang, J. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl. Microbiol. Biotechnol. 2020, 104, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Ihlamur, M.; Akgül, B.; Zengin, Y.; Korkut, Ş.V.; Kelleci, K.; Abamor, E.Ş. The mTOR Signaling pathway and mTOR Inhibitors in cancer: Next-generation inhibitors and approaches. Curr. Mol. Med. 2024, 24, 478–494. [Google Scholar] [CrossRef]
- Masaki, N.; Aoki, Y.; Obara, K.; Kubota, Y.; Bouvet, M.; Miyazaki, J.; Hoffman, R.M. Targeting autophagy with the synergistic combination of chloroquine and rapamycin as a novel effective treatment for well-differentiated liposarcoma. Cancer Genom. Proteom. 2023, 20, 317–322. [Google Scholar] [CrossRef]
- Tian, Y.; Song, W.; Li, D.; Cai, L.; Zhao, Y. Resveratrol as a natural regulator of autophagy for prevention and treatment of cancer. OncoTargets Ther. 2019, 12, 8601–8609. [Google Scholar] [CrossRef]
- Utpal, B.K.; Mokhfi, F.Z.; Zehravi, M.; Sweilam, S.H.; Gupta, J.K.; Kareemulla, S.; Rao, A.; Kumar, V.V.; Krosuri, P.; Prasad, D. Resveratrol: A Natural Compound Targeting the PI3K/Akt/mTOR Pathway in Neurological Diseases. Mol. Neurobiol. 2024, 62, 5579–5608. [Google Scholar] [CrossRef]
- Yang, R.; Dong, H.; Jia, S.; Yang, Z. Resveratrol as a modulatory of apoptosis and autophagy in cancer therapy. Clin. Transl. Oncol. 2022, 24, 1219–1230. [Google Scholar] [CrossRef]
- Zhu, Y.; Bu, S. Curcumin induces autophagy, apoptosis, and cell cycle arrest in human pancreatic cancer cells. Evid.-Based Complement. Altern. Med. 2017, 2017, 5787218. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.U.; Woo, S.M.; Lee, H.-S.; Kim, S.H.; Min, K.-J.; Kwon, T.K. mTORC1/2 inhibitor and curcumin induce apoptosis through lysosomal membrane permeabilization-mediated autophagy. Oncogene 2018, 37, 5205–5220. [Google Scholar] [CrossRef]
- Arena, A.; Romeo, M.A.; Benedetti, R.; Masuelli, L.; Bei, R.; Gilardini Montani, M.S.; Cirone, M. New insights into curcumin-and resveratrol-mediated anti-cancer effects. Pharmaceuticals 2021, 14, 1068. [Google Scholar] [CrossRef]
- Yuan, W.; Fang, W.; Zhang, R.; Lyu, H.; Xiao, S.; Guo, D.; Ali, D.W.; Michalak, M.; Chen, X.-Z.; Zhou, C. Therapeutic strategies targeting AMPK-dependent autophagy in cancer cells. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2023, 1870, 119537. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Li, C.-F.; Cai, Z.; Zhang, X.; Jin, G.; Zhang, W.-N.; Xu, C.; Wang, C.-Y.; Morrow, J.; Zhang, S. The critical role of AMPK in driving Akt activation under stress, tumorigenesis and drug resistance. Nat. Commun. 2018, 9, 4728. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, M.Y.; Golmohammadi, M.; Yumashev, A.; Hjazi, A.; Toama, M.A.; AbdRabou, M.A.; Gehlot, A.; Alwaily, E.R.; Shirsalimi, N.; Yadav, P.K. Effects of metformin on cancers in experimental and clinical studies: Focusing on autophagy and AMPK/mTOR signaling pathways. Cell Biochem. Funct. 2024, 42, e4071. [Google Scholar] [CrossRef]
- Xia, H.; Tai, X.-J.; Cheng, W.; Wu, Y.; He, D.; Wang, L.-F.; Liu, H.; Zhang, S.-Y.; Sun, Y.-T.; Liu, H.-Z. Metformin inhibits the growth of SCLC cells by inducing autophagy and apoptosis via the suppression of EGFR and AKT signalling. Sci. Rep. 2025, 15, 6081. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-B. Metformin promotes apoptosis but suppresses autophagy in glucose-deprived H4IIE hepatocellular carcinoma cells. Diabetes Metab. J. 2015, 39, 518. [Google Scholar] [CrossRef]
- Palomer, X.; Wang, J.-R.; Escalona, C.; Wu, S.; Wahli, W.; Vázquez-Carrera, M. Targeting AMPK as a potential treatment for hepatic fibrosis in MASLD. Trends Pharmacol. Sci. 2025, 46, 551–566. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Warrier, S.; Rangarajan, A. Rethinking AMPK: A Reversible Switch Fortifying Cancer Cell Stress-Resilience. Yale J. Biol. Med. 2025, 98, 33. [Google Scholar] [CrossRef]
- Psara, E.; Poulios, E.; Papadopoulou, S.K.; Tolia, M.; Vasios, G.K.; Giaginis, C. Intermittent fasting against cancer development and progression: Highlighting potential anticancer molecular mechanisms. Anti-Cancer Agents Med. Chem.-Anti-Cancer Agents 2023, 23, 1889–1909. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, J.; Li, K.; Deng, L.; Wang, H. Combination of an Autophagy Inducer and an Autophagy Inhibitor: A Smarter Strategy Emerging in Cancer Therapy. Front. Pharmacol. 2020, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, C.; Zhu, W.; Li, X.; Chen, T.; Liu, Q.; Zhou, S.; Zhang, T.C.; Ma, W. Chemotherapeutic drugs induce oxidative stress associated with DNA repair and metabolism modulation. Life Sci. 2022, 289, 120242. [Google Scholar] [CrossRef]
- Agalakova, N.I. Chloroquine and Chemotherapeutic Compounds in Experimental Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 945. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Cheng, X.; Kuang, J.; Feng, H.; Chen, L.; Liang, J.; Shen, X.; Yuen, S.; Peng, C.; Shen, B. CQ sensitizes human pancreatic cancer cells to gemcitabine through the lysosomal apoptotic pathway via reactive oxygen species. Mol. Oncol. 2018, 12, 529–544. [Google Scholar] [CrossRef]
- Rahman, M.A.; Jalouli, M.; Bhajan, S.K.; Al-Zharani, M.; Harrath, A.H. A Comprehensive Review of Nanoparticle-Based Drug Delivery for Modulating PI3K/AKT/mTOR-Mediated Autophagy in Cancer. Int. J. Mol. Sci. 2025, 26, 1868. [Google Scholar] [CrossRef]
- Sareen, G.; Mohan, M.; Mannan, A.; Dua, K.; Singh, T.G. A new era of cancer immunotherapy: Vaccines and miRNAs. Cancer Immunol. Immunother. 2025, 74, 163. [Google Scholar] [CrossRef]
- Chen, D.; Ling, X.; Wang, Y.; Zhang, Q.; He, X.; Dong, Z.; Li, M.; He, Q. Autophagy-activating aluminum hydroxide nanovaccine for enhanced antigen presentation and anti-tumor immunity. J. Control. Release 2025, 377, 223–235. [Google Scholar] [CrossRef]
- Jiang, G.M.; Tan, Y.; Wang, H.; Peng, L.; Chen, H.T.; Meng, X.J.; Li, L.L.; Liu, Y.; Li, W.F.; Shan, H. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol. Cancer 2019, 18, 17. [Google Scholar] [CrossRef]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.U. Regulation of the innate immune system by autophagy: Monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ. 2019, 26, 715–727. [Google Scholar] [CrossRef]
- Delmas, D.; Hermetet, F.; Aires, V. PD-1/PD-L1 Checkpoints and Resveratrol: A Controversial New Way for a Therapeutic Strategy. Cancers 2021, 13, 4509. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zheng, L.; Qi, C. Myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment and their targeting in cancer therapy. Mol. Cancer 2025, 24, 5. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Jalouli, M.; Yadab, M.K.; Al-Zharani, M. Progress in drug delivery systems based on nanoparticles for improved glioblastoma therapy: Addressing challenges and investigating opportunities. Cancers 2025, 17, 701. [Google Scholar] [CrossRef]
- Gu, X.; Xiang, D.; Zhu, H.; He, X.; Yang, C.; Chen, R. Dual role of autophagy in bone metastasis: Mechanistic insights and therapeutic targeting. Am. J. Clin. Exp. Urol. 2025, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Chen, M.; Cao, F.; Huang, H.; Zhan, R.; Zheng, X. Chloroquine, an autophagy inhibitor, potentiates the radiosensitivity of glioma initiating cells by inhibiting autophagy and activating apoptosis. BMC Neurol. 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Cufí, S.; Vazquez-Martin, A.; Oliveras-Ferraros, C.; Corominas-Faja, B.; Cuyàs, E.; López-Bonet, E.; Martin-Castillo, B.; Joven, J.; Menendez, J.A. The anti-malarial chloroquine overcomes primary resistance and restores sensitivity to trastuzumab in HER2-positive breast cancer. Sci. Rep. 2013, 3, 2469. [Google Scholar] [CrossRef]
- Mauvezin, C.; Neufeld, T.P. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy 2015, 11, 1437–1438. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Yu, M.; Ma, K.; Ning, L. Inhibition of autophagy by 3-methyladenine promotes migration and invasion of colon cancer cells through epithelial mesenchymal transformation. Transl. Cancer Res. 2022, 11, 2834. [Google Scholar] [CrossRef]
- Alayev, A.; Berger, S.M.; Kramer, M.Y.; Schwartz, N.S.; Holz, M.K. The combination of rapamycin and resveratrol blocks autophagy and induces apoptosis in breast cancer cells. J. Cell. Biochem. 2015, 116, 450–457. [Google Scholar] [CrossRef]
- Demirkesen, Ş.; İriağaç, Y.; Şeber, E.S.; Aral, C. Melatonin enhances everolimus efficacy in breast cancer by suppressing mTOR pathway activation and promoting apoptosis and mitochondrial function. BMC Pharmacol. Toxicol. 2025, 26, 1–12. [Google Scholar] [CrossRef]
- Rangwala, R.; Chang, Y.C.; Hu, J.; Algazy, K.M.; Evans, T.L.; Fecher, L.A.; Schuchter, L.M.; Torigian, D.A.; Panosian, J.T.; Troxel, A.B. Combined MTOR and autophagy inhibition: Phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy 2014, 10, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Park, W.Y.; Song, G.; Boo, M.; Kim, H.I.; Park, J.Y.; Jung, S.J.; Choi, M.; Kim, B.; Kim, Y.D.; Kim, M.-H. Anmyungambi decoction ameliorates obesity through activation of non-shivering thermogenesis in Brown and white adipose tissues. Antioxidants 2022, 12, 49. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, S.; Cheng, X.; Wu, J.; Wu, L.; Wang, Y.; Wang, X.; Bao, J.; Yu, H. Curcumin induces autophagic cell death in human thyroid cancer cells. Toxicol. Vitr. 2022, 78, 105254. [Google Scholar] [CrossRef]
- Chen, C.; Wang, H.; Geng, X.; Zhang, D.; Zhu, Z.; Zhang, G.; Hou, J. Metformin exerts anti-AR-negative prostate cancer activity via AMPK/autophagy signaling pathway. Cancer Cell Int. 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Cao, P.; Jiang, X.; Xi, Z. Sunitinib induces autophagy via suppressing Akt/mTOR pathway in renal cell carcinoma. Beijing Da Xue Xue Bao Yi Xue Ban J. Peking Univ. Health Sci. 2016, 48, 584–589. [Google Scholar]
- Jawad, M.H.; Jabir, M.S.; Ozturk, K.; Sulaiman, G.M.; Abomughaid, M.M.; Albukhaty, S.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-Azzawi, W.K.; Najm, M.A. Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin. Nanotechnol. Rev. 2023, 12, 20230148. [Google Scholar] [CrossRef]
- Bahar, E.; Kim, J.-Y.; Kim, D.-C.; Kim, H.-S.; Yoon, H. Combination of niraparib, cisplatin and twist knockdown in cisplatin-resistant ovarian cancer cells potentially enhances synthetic lethality through ER-stress mediated mitochondrial apoptosis pathway. Int. J. Mol. Sci. 2021, 22, 3916. [Google Scholar] [CrossRef]
- Zhou, Y.; Rucker, E.B., III; Zhou, B.P. Autophagy regulation in the development and treatment of breast cancer. Acta Biochim. Biophys. Sin. 2016, 48, 60–74. [Google Scholar] [CrossRef]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Levine, B.; Green, D.R.; Kroemer, G. Pharmacological modulation of autophagy: Therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov. 2017, 16, 487–511. [Google Scholar] [CrossRef]




| Drug Name | Cancer Type | Mechanism of Action | ADCD | Ref. |
|---|---|---|---|---|
| Chloroquine (CQ) | Glioblastoma, Breast Cancer | Inhibits autophagosome-lysosome fusion, preventing degradation of cellular components | Inhibition | [134] |
| Hydroxychloroquine (HCQ) | Pancreatic Cancer, Lung Cancer | Blocks lysosomal acidification, disrupting autophagic flux | Inhibition | [135] |
| Bafilomycin A1 | Liver Cancer, Leukemia | Inhibits lysosomal acidification, blocking autophagosome clearance | Inhibition | [136] |
| 3-Methyladenine (3-MA) | Colorectal Cancer, Lung Cancer | Blocks PI3K-mediated autophagy initiation | Inhibition | [137] |
| Rapamycin | Renal Cell Carcinoma, Breast Cancer | mTORC1 inhibitor, induces autophagy leading to ADCD | Activation | [138] |
| Everolimus | Neuroendocrine Tumors, Breast Cancer | mTORC1 inhibitor, enhances autophagic cell death in tumors | Activation | [139] |
| Temsirolimus | Renal Cell Carcinoma, Lymphoma | mTORC1 inhibition promotes sustained autophagy and tumor regression | Activation | [140] |
| Resveratrol | Colon Cancer, Melanoma | Induces oxidative stress and autophagic cell death via AMPK activation | Activation | [141] |
| Curcumin | Lung Cancer, Pancreatic Cancer | Triggers autophagy through Beclin-1 upregulation, enhancing ADCD | Activation | [142] |
| Metformin | Prostate Cancer, Ovarian Cancer | Activates AMPK, inhibits mTOR signaling, promoting autophagic cell death | Activation | [143] |
| Sunitinib | Renal Cell Carcinoma, Gastrointestinal Stromal Tumors | Induces autophagy-dependent cell death through mTORC1 inhibition | Activation | [144] |
| Doxorubicin | Breast Cancer, Osteosarcoma | Enhances autophagy-dependent apoptosis via ROS generation | Activation | [145] |
| Cisplatin | Ovarian Cancer, Bladder Cancer | Induces ER stress-mediated autophagic cell death | Activation | [146] |
| Vinblastine | Lymphoma, Breast Cancer | Disrupt microtubules, triggering autophagic stress and ADCD | Activation | [147] |
| Carbamazepine | Glioblastoma, Pancreatic Cancer | Enhances autophagic flux and degradation of damaged proteins | Activation | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.A.; Jalouli, M.; Al-Zharani, M.; Hoque Apu, E.; Harrath, A.H. Mechanistic Insights into Autophagy-Dependent Cell Death (ADCD): A Novel Avenue for Cancer Therapy. Cells 2025, 14, 1072. https://doi.org/10.3390/cells14141072
Rahman MA, Jalouli M, Al-Zharani M, Hoque Apu E, Harrath AH. Mechanistic Insights into Autophagy-Dependent Cell Death (ADCD): A Novel Avenue for Cancer Therapy. Cells. 2025; 14(14):1072. https://doi.org/10.3390/cells14141072
Chicago/Turabian StyleRahman, Md Ataur, Maroua Jalouli, Mohammed Al-Zharani, Ehsanul Hoque Apu, and Abdel Halim Harrath. 2025. "Mechanistic Insights into Autophagy-Dependent Cell Death (ADCD): A Novel Avenue for Cancer Therapy" Cells 14, no. 14: 1072. https://doi.org/10.3390/cells14141072
APA StyleRahman, M. A., Jalouli, M., Al-Zharani, M., Hoque Apu, E., & Harrath, A. H. (2025). Mechanistic Insights into Autophagy-Dependent Cell Death (ADCD): A Novel Avenue for Cancer Therapy. Cells, 14(14), 1072. https://doi.org/10.3390/cells14141072








