Modeling Early Stages of Trophectoderm–Endometrium Interactions Using Trophoblastic and Endometrial Organoids and the Generation of Lacunoids/Cystoids
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Embryo Production via Intracytoplasmic Sperm Injection (ICSI)
2.3. Isolation of Trophectoderm Cells
2.4. Derivation of Trophoblastic Organoids and Lacunoids/Cystoids from Trophectoderm Cells
2.5. Derivation of Trophoblastic Organoids and Lacunoids/Cystoids from ICSI Blastocysts
2.6. Generation of Endometrial Organoids (EOs)
2.7. Immunofluorescence Characterization of Trophectoderm, Trophoblastic Organoids, Endometrial Organoids, and Lacunoids/Cystoids
2.8. Effects of Co-Culturing Trophoblastic Organoids with Endometrial Organoids
2.9. Effects of Co-Culturing Trophoblastic Organoids with Day 8 Blastocysts with or Without Attachment to Matrigel Domes
2.10. Real-Time Polymerase Chain Reaction (RT-qPCR)
2.11. Statistical Analysis
3. Results
3.1. Successful Derivation of Trophoblastic Organoids
3.2. Morphological and Structural Features of Trophoblastic Organoids and Lacunoids/Cystoids
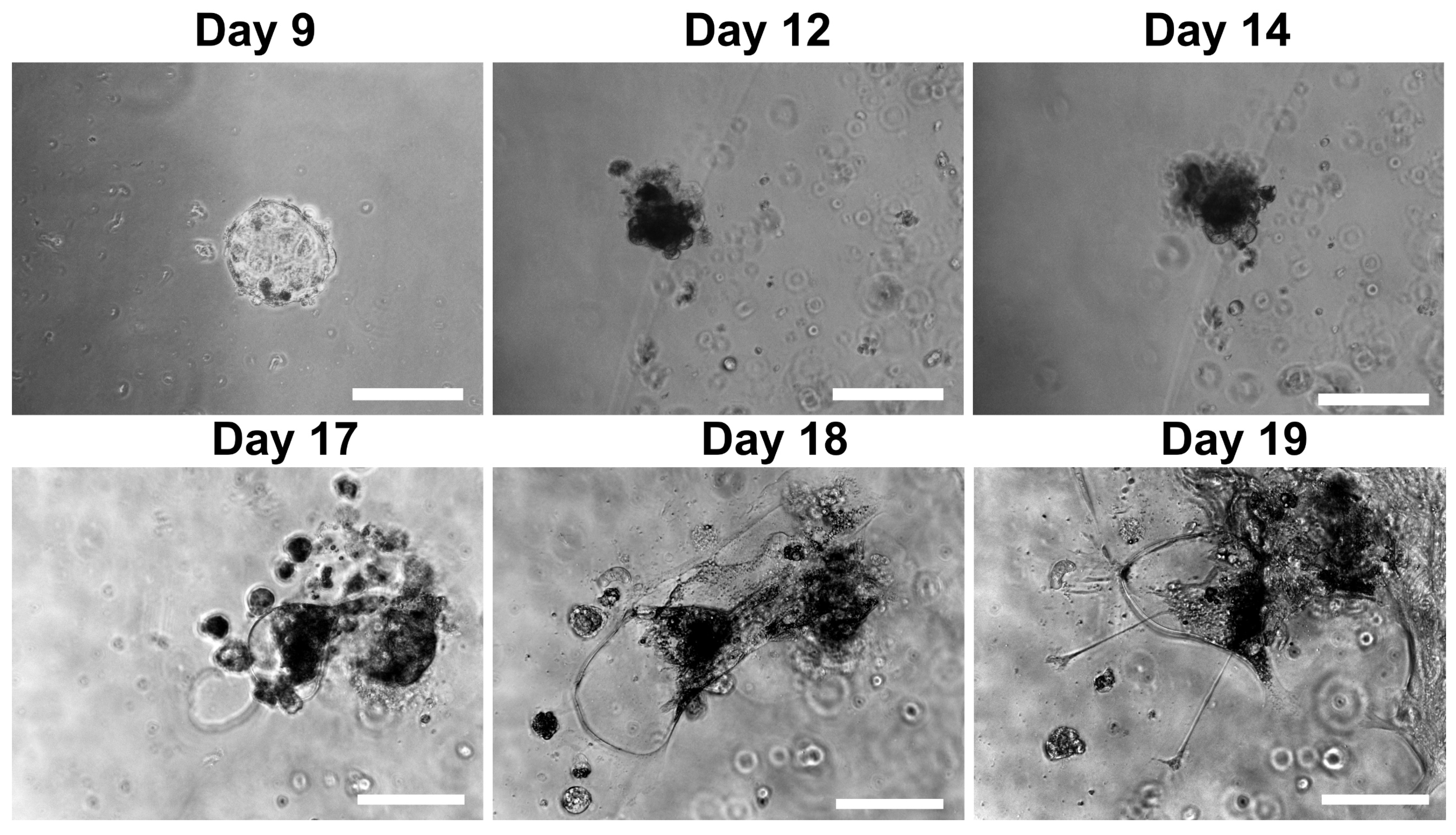
3.3. Effects of Trophoblastic Organoids on Extended Embryo Culture
3.4. Characterization of Sheep Endometrial Organoids
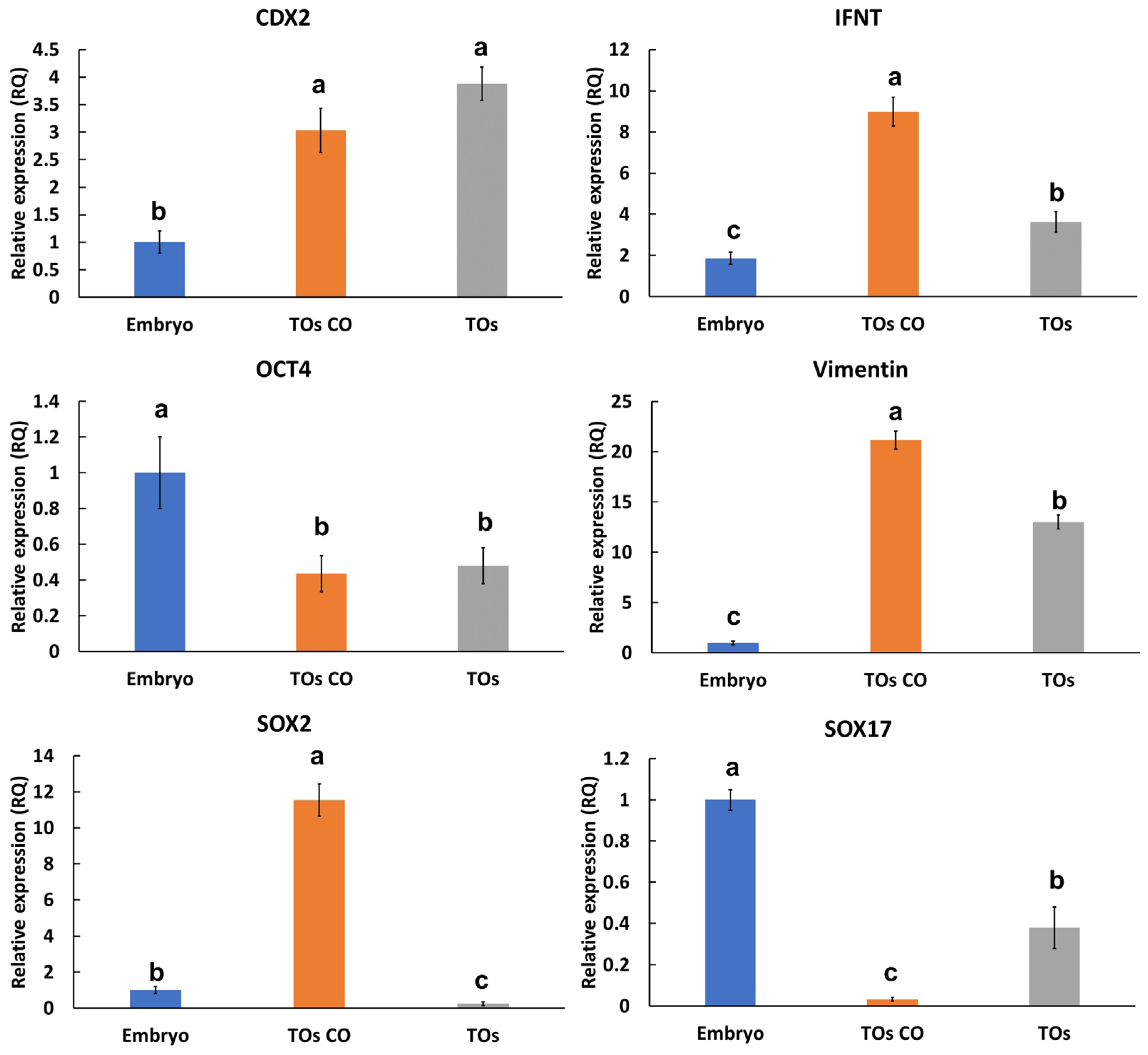
3.5. Interaction of Embryos with Trophoblastic Organoids and Endometrial Organoids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TOs | Trophoblast organoids |
| ICSI | Intracytoplasmic sperm injection |
| EOs | Endometrial organoids |
| 3D | Three-dimensional |
| PBS | Phosphate-buffered saline |
| TLCs | Trophoblastic lacunoids/cystoids |
References
- Burton, G.J.; Jauniaux, E. What is the placenta? Am. J. Obs. Gynecol. 2015, 213, S6.e1–S6.e4. [Google Scholar] [CrossRef]
- Turco, M.Y.; Gardner, L.; Kay, R.G.; Hamilton, R.S.; Prater, M.; Hollinshead, M.S.; McWhinnie, A.; Esposito, L.; Fernando, R.; Skelton, H.; et al. Trophoblast organoids as a model for maternal–fetal interactions during human placentation. Nature 2018, 564, 263–267. [Google Scholar] [PubMed]
- Saadeldin, I.M.; Ehab, S.; Noreldin, A.E.; Swelum, A.A.-A.; Bang, S.; Kim, H.; Yoon, K.Y.; Lee, S.; Cho, J. Current strategies using 3D organoids to establish in vitro maternal-embryonic interaction. J. Vet. Sci. 2024, 25, 24004. [Google Scholar] [CrossRef]
- Haider, S.; Meinhardt, G.; Saleh, L.; Kunihs, V.; Gamperl, M.; Kaindl, U.; Ellinger, A.; Burkard, T.R.; Fiala, C.; Pollheimer, J. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Rep. 2018, 11, 537–551. [Google Scholar]
- Pascual, F. Trophoblast Organoids: A New Tool for Studying Placental Development. Environ. Health Perspect. 2022, 130, 54003. [Google Scholar] [CrossRef]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.-E.; Stephenson, E.; Polański, K.; Goncalves, A. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar]
- Arutyunyan, A.; Roberts, K.; Troule, K.; Wong, F.C.K.; Sheridan, M.A.; Kats, I.; Garcia-Alonso, L.; Velten, B.; Hoo, R.; Ruiz-Morales, E.R.; et al. Spatial multiomics map of trophoblast development in early pregnancy. Nature 2023, 616, 143–151. [Google Scholar] [CrossRef]
- Sheridan, M.A.; Fernando, R.C.; Gardner, L.; Hollinshead, M.S.; Burton, G.J.; Moffett, A.; Turco, M.Y. Establishment and differentiation of long-term trophoblast organoid cultures from the human placenta. Nat. Protoc. 2020, 15, 3441–3463. [Google Scholar] [CrossRef]
- Yang, L.; Liang, P.; Yang, H.; Coyne, C.B. Trophoblast organoids with physiological polarity model placental structure and function. J. Cell Sci. 2024, 137, jcs261528. [Google Scholar] [CrossRef]
- Wu, H.; Huang, X.Y.; Sun, M.X.; Wang, Y.; Zhou, H.Y.; Tian, Y.; He, B.; Li, K.; Li, D.Y.; Wu, A.P.; et al. Zika virus targets human trophoblast stem cells and prevents syncytialization in placental trophoblast organoids. Nat. Commun. 2023, 14, 5541. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.H.; Wang, Y.X.; Liu, T.H. A comprehensive review of human trophoblast fusion models: Recent developments and challenges. Cell Death Discov. 2023, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Kobayashi, E.H.; Kobayashi, N.; Oike, A.; Okae, H.; Arima, T. Unique features and emerging in vitro models of human placental development. Reprod. Med. Biol. 2020, 19, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Horii, M.; Touma, O.; Bui, T.; Parast, M.M. Modeling human trophoblast, the placental epithelium at the maternal fetal interface. Reproduction 2020, 160, R1–R11. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Han, A.; Bang, S.; Kang, H.; Kim, H.; Abady, M.M.; Jeong, J.-S.; Kwon, H.-J.; Lee, S.; Cho, J. Generation of porcine endometrial organoids and their use as a model for enhancing embryonic attachment and elongation. Reproduction 2023, 167, e230429. [Google Scholar] [CrossRef]
- Moffett, A. Organoid cultures as model systems to study disorders of the human endometrium and placenta. Obstet. Gynaecol. Reprod. Med. 2023, 33, 268–270. [Google Scholar] [CrossRef]
- Wen, L.; Tang, F. Organoid research on human early development and beyond. Med. Rev. 2022, 2, 512–523. [Google Scholar] [CrossRef]
- Enders, A.C. Transition from Lacunar to Villous Stage of Implantation in the Macaque, Including Establishment of the Trophoblastic Shell. Acta Anat. 1995, 152, 151–169. [Google Scholar] [CrossRef]
- Charles, A.K.; Faye-Petersen, O.M. Human Placental Development from Conception to Term. In Pathobiology of Human Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 2322–2341. [Google Scholar]
- Leiser, R.; Beier, H.M. Morphological Studies of Lacunar Formation in the Early Rabbit Placenta. In Placental Vascularization and Blood Flow; Springer: Berlin/Heidelberg, Germany, 1988; pp. 97–110. [Google Scholar]
- Saadeldin, I.M.; Swelum, A.A.-A.; Elsafadi, M.; Mahmood, A.; Yaqoob, S.H.; Alfayez, M.; Alowaimer, A.N. Effects of all-trans retinoic acid on the in vitro maturation of camel (Camelus dromedarius) cumulus-oocyte complexes. J. Reprod. Dev. 2019, 65, 215–221. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Mara, L.; Chessa, B.; Chessa, F.; Parham, A.; Dattena, M. Optimizing injection time of GFP plasmid into sheep zygote. Reprod. Domest. Anim. 2021, 56, 467–475. [Google Scholar] [CrossRef]
- Swelum, A.A.; Saadeldin, I.M.; Bahadi, M.; Afifi, M.; Al-Mutary, M.; Alowaimer, A.N. The effect of heterologous seminal plasma from ram, buck or camel on the freezability of ram semen. Vet. Med. 2018, 63, 500–512. [Google Scholar] [CrossRef]
- Ressaissi, Y.; Anzalone, D.A.; Palazzese, L.; Czernik, M.; Loi, P. The impaired development of sheep ICSI derived embryos is not related to centriole dysfunction. Theriogenology 2021, 159, 7–12. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Kim, B.; Lee, B.; Jang, G. Effect of different culture media on the temporal gene expression in the bovine developing embryos. Theriogenology 2011, 75, 995–1004. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Kim, S.J.; Lee, B.C. Blastomeres aggregation as an efficient alternative for trophoblast culture from porcine parthenogenetic embryos. Dev. Growth Differ. 2015, 57, 362–368. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Swelum, A.A.-A.; Elsafadi, M.; Moumen, A.F.; Alzahrani, F.A.; Mahmood, A.; Alfayez, M.; Alowaimer, A.N. Isolation and characterization of the trophectoderm from the Arabian camel (Camelus dromedarius). Placenta 2017, 57, 113–122. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Kim, S.J.; Choi, Y.B.; Lee, B.C. Post-maturation zona perforation improves porcine parthenogenetic trophoblast culture. Placenta 2014, 35, 286–288. [Google Scholar] [CrossRef]
- Ralston, A.; Cox, B.J.; Nishioka, N.; Sasaki, H.; Chea, E.; Rugg-Gunn, P.; Guo, G.; Robson, P.; Draper, J.S.; Rossant, J. Gata3 regulates trophoblast development downstream of Tead4 and in parallel to Cdx2. Development 2010, 137, 395–403. [Google Scholar] [CrossRef]
- Home, P.; Ray, S.; Dutta, D.; Bronshteyn, I.; Larson, M.; Paul, S. GATA3 Is Selectively Expressed in the Trophectoderm of Peri-implantation Embryo and Directly Regulates Cdx2 Gene Expression. J. Biol. Chem. 2009, 284, 28729–28737. [Google Scholar] [CrossRef]
- Pötgens, A.J.G.; Drewlo, S.; Kokozidou, M.; Kaufmann, P. Syncytin: The major regulator of trophoblast fusion? Recent developments and hypotheses on its action. Hum. Reprod. Update 2004, 10, 487–496. [Google Scholar] [CrossRef]
- Liang, X.; Qiu, X.; Ma, Y.; Xu, W.; Chen, S.; Zhang, P.; Liu, M.; Lin, X. KRT18 regulates trophoblast cell migration and invasion which are essential for embryo implantation. Reprod. Biol. Endocrinol. 2023, 21, 78. [Google Scholar] [CrossRef]
- Siriwardena, D.; Munger, C.; Penfold, C.; Kohler, T.N.; Weberling, A.; Linneberg-Agerholm, M.; Slatery, E.; Ellermann, A.L.; Bergmann, S.; Clark, S.J.; et al. Marmoset and human trophoblast stem cells differ in signaling requirements and recapitulate divergent modes of trophoblast invasion. Cell Stem Cell 2024, 31, 1427–1446.e1428. [Google Scholar] [CrossRef]
- Han, Q.; Zheng, L.; Liu, Z.; Luo, J.; Chen, R.; Yan, J. Expression of β-catenin in human trophoblast and its role in placenta accreta and placenta previa. J. Int. Med. Res. 2018, 47, 206–214. [Google Scholar] [CrossRef]
- Pera, M.; Keramari, M.; Razavi, J.; Ingman, K.A.; Patsch, C.; Edenhofer, F.; Ward, C.M.; Kimber, S.J. Sox2 Is Essential for Formation of Trophectoderm in the Preimplantation Embryo. PLoS ONE 2010, 5, 0013952. [Google Scholar] [CrossRef]
- Niakan, K.K.; Eggan, K. Analysis of human embryos from zygote to blastocyst reveals distinct gene expression patterns relative to the mouse. Dev. Biol. 2013, 375, 54–64. [Google Scholar] [CrossRef]
- Mathew, D.J.; Peterson, K.D.; Senn, L.K.; Oliver, M.A.; Ealy, A.D. Ruminant conceptus-maternal interactions: Interferon-tau and beyond. J. Anim. Sci. 2022, 100, skac123. [Google Scholar] [CrossRef]
- Bott, R.C.; Ashley, R.L.; Henkes, L.E.; Antoniazzi, A.Q.; Bruemmer, J.E.; Niswender, G.D.; Bazer, F.W.; Spencer, T.E.; Smirnova, N.P.; Anthony, R.V.; et al. Uterine Vein Infusion of Interferon Tau (IFNT) Extends Luteal Life Span in Ewes1. Biol. Reprod. 2010, 82, 725–735. [Google Scholar] [CrossRef]
- Bazer, F.W.; Ott, T.L.; Spencer, T.E. Pregnancy recognition in ruminants, pigs and horses: Signals from the trophoblast. Theriogenology 1994, 41, 79–94. [Google Scholar] [CrossRef]
- Farin, C.E.; Imakawa, K.; Hansen, T.R.; McDonnell, J.J.; Murphy, C.N.; Farin, P.W.; Roberts, R.M. Expression of Trophoblastic Interferon Genes in Sheep and Cattle1. Biol. Reprod. 1990, 43, 210–218. [Google Scholar] [CrossRef]
- Molbay, M.; Kipmen-Korgun, D.; Korkmaz, G.; Ozekinci, M.; Turkay Korgun, E. Human Trophoblast Progenitor Cells Express and Release Angiogenic Factors. Int. J. Mol. Cell. Med. 2018, 7, 203–211. [Google Scholar] [CrossRef]
- Chung, I.B.; Yelian, F.D.; Zaher, F.M.; Gonik, B.; Evans, M.I.; Diamond, M.P.; Svinarich, D.M. Expression and regulation of vascular endothelial growth factor in a first trimester trophoblast cell line. Placenta 2000, 21, 320–324. [Google Scholar] [CrossRef]
- Enders, A.C.; King, B.F. Early stages of trophoblastic invasion of the maternal vascular system during implantation in the macaque and baboon. Am. J. Anat. 1991, 192, 329–346. [Google Scholar] [CrossRef]
- Enders, A.C.; Lantz, K.C.; Peterson, P.E.; Hendrickx, A.G. From blastocyst to placenta: The morphology of implantation in the baboon. Hum. Reprod. Update 1997, 3, 561–573. [Google Scholar] [CrossRef]
- Bills, V.L.; Hamdollah-Zadeh, M.; Soothill, P.W.; Harper, S.J.; Bates, D.O. The role of VEGF-A165b in trophoblast survival. BMC Pregnancy Childbirth 2014, 14, 278. [Google Scholar] [CrossRef]
- Fan, X.; Muruganandan, S.; Shallie, P.D.; Dhal, S.; Petitt, M.; Nayak, N.R. VEGF Maintains Maternal Vascular Space Homeostasis in the Mouse Placenta through Modulation of Trophoblast Giant Cell Functions. Biomolecules 2021, 11, 1062. [Google Scholar] [CrossRef]
- Ghosh, D.; Sharkey, A.M.; Charnock-Jones, D.S.; Dhawan, L.; Dhara, S.; Smith, S.K.; Sengupta, J. Expression of vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) in conceptus and endometrium during implantation in the rhesus monkey. Mol. Hum. Reprod. 2000, 6, 935–941. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhang, L.; Shi, Y.; Wang, J.; Yan, H. Wnt/β-catenin signaling pathway in trophoblasts and abnormal activation in preeclampsia. Mol. Med. Rep. 2017, 16, 1007–1013. [Google Scholar] [CrossRef]
- Kiewisz, J.; Wasniewski, T.; Kmiec, Z. Participation of WNT andβ-Catenin in Physiological and Pathological Endometrial Changes: Association with Angiogenesis. BioMed Res. Int. 2015, 2015, 854056. [Google Scholar] [CrossRef]
- Yu, N.; Wu, J.-L.; Xiao, J.; Fan, L.; Chen, S.-H.; Li, W. HIF-1α regulates angiogenesis via Notch1/STAT3/ETBR pathway in trophoblastic cells. Cell Cycle 2019, 18, 3502–3512. [Google Scholar] [CrossRef]
- Taghizadeh, E.; Tazik, K.; Taheri, F.; Shayankia, G.; Gheibihayat, S.M.; Saberi, A. Abnormal angiogenesis associated with HIF-1α/VEGF signaling pathway in recurrent miscarriage along with therapeutic goals. Gene Rep. 2022, 26, 101483. [Google Scholar] [CrossRef]
- Suwińska, A.; Czołowska, R.; Ożdżeński, W.; Tarkowski, A.K. Blastomeres of the mouse embryo lose totipotency after the fifth cleavage division: Expression of Cdx2 and Oct4 and developmental potential of inner and outer blastomeres of 16- and 32-cell embryos. Dev. Biol. 2008, 322, 133–144. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Koo, O.J.; Kang, J.T.; Kwon, D.K.; Park, S.J.; Kim, S.J.; Moon, J.H.; Oh, H.J.; Jang, G.; Lee, B.C. Paradoxical effects of kisspeptin: It enhances oocyte in vitro maturation but has an adverse impact on hatched blastocysts during in vitro culture. Reprod. Fertil. Dev. 2012, 24, 656–668. [Google Scholar] [CrossRef]
- Cecchele, A.; Cermisoni, G.C.; Giacomini, E.; Pinna, M.; Vigano, P. Cellular and Molecular Nature of Fragmentation of Human Embryos. Int. J. Mol. Sci. 2022, 23, 1349. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Lin, Y.-P.; Cheng, E.-H.; Chen, C.-H.; Huang, C.-C.; Lin, P.-Y.; Lee, T.-H.; Lee, M.-S. The presence of vacuoles in blastocysts is negatively associated with euploidy and live birth rates. Fertil. Steril. 2023, 120, 298–304. [Google Scholar] [CrossRef]
- Saraste, A. Morphologic criteria and detection of apoptosis. Herz 1999, 24, 189–195. [Google Scholar] [CrossRef]
- Otun, H.A.; Lash, G.E.; Innes, B.A.; Bulmer, J.N.; Naruse, K.; Hannon, T.; Searle, R.F.; Robson, S.C. Effect of tumour necrosis factor-α in combination with interferon-γ on first trimester extravillous trophoblast invasion. J. Reprod. Immunol. 2011, 88, 1–11. [Google Scholar] [CrossRef]
- Moindjie, H.; Santos, E.D.; Gouesse, R.-J.; Swierkowski-Blanchard, N.; Serazin, V.; Barnea, E.R.; Vialard, F.; Dieudonné, M.-N. Preimplantation factor is an anti-apoptotic effector in human trophoblasts involving p53 signaling pathway. Cell Death Dis. 2016, 7, e2504. [Google Scholar] [CrossRef]
- Xing, F.; Kong, L.; Chen, S. Apoptosis of cultured human trophoblasts induced by tumor necrosis factor-alpha and interferon-gamma. Zhonghua Fu Chan Ke Za Zhi 1999, 34, 476–478. [Google Scholar]
- Yamauchi, H.; Katayama, K.-I.; Ueno, M.; He, X.J.; Mikami, T.; Uetsuka, K.; Doi, K.; Nakayama, H. Essential role of p53 in trophoblastic apoptosis induced in the developing rodent placenta by treatment with a DNA-damaging agent. Apoptosis 2007, 12, 1743–1754. [Google Scholar] [CrossRef]
- Oldak, B.; Wildschutz, E.; Bondarenko, V.; Comar, M.-Y.; Zhao, C.; Aguilera-Castrejon, A.; Tarazi, S.; Viukov, S.; Pham, T.X.A.; Ashouokhi, S.; et al. Complete human day 14 post-implantation embryo models from naive ES cells. Nature 2023, 622, 562–573. [Google Scholar] [CrossRef]
- Shahbazi, M.N.; Jedrusik, A.; Vuoristo, S.; Recher, G.; Hupalowska, A.; Bolton, V.; Fogarty, N.M.E.; Campbell, A.; Devito, L.G.; Ilic, D.; et al. Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 2016, 18, 700–708. [Google Scholar] [CrossRef]
- Alexandrova, M.; Manchorova, D.; You, Y.; Mor, G.; Dimitrova, V.; Dimova, T. Functional HLA-C expressing trophoblast spheroids as a model to study placental–maternal immune interactions during human implantation. Sci. Rep. 2022, 12, 10224. [Google Scholar] [CrossRef]
- Tachi, S.; Tachi, C.; Lindner, H.R. Ultrastructural Features of Blastocyst Attachment and Trophoblastic Invasion in the Rat. Reproduction 1970, 21, 37–56. [Google Scholar] [CrossRef]
- Potts, M.; Psychoyos, A. Evolution of the ultrastructure of the ovoendometrial connections under the influence of estrogen in the rat during experimental retardation of nidation. C R Acad. Hebd. Seances Acad. Sci. D 1967, 264, 370–373. [Google Scholar] [PubMed]
- Potts, D.M. The ultrastructure of implantation in the mouse. J. Anat. 1968, 103, 77–90. [Google Scholar] [PubMed]
- Zhai, J.; Xiao, Z.; Wang, Y.; Wang, H. Human embryonic development: From peri-implantation to gastrulation. Trends Cell Biol. 2022, 32, 18–29. [Google Scholar] [CrossRef]
- Enders, A.C. Implantation in the Macaque: Expansion of the Implantation Site During the First Week of Implantation. Placenta 2007, 28, 794–802. [Google Scholar] [CrossRef]
- Enders, A.C.; Lopata, A. Implantation in the marmoset monkey: Expansion of the early implantation site. Anat. Rec. 1999, 256, 279–299. [Google Scholar] [CrossRef]
- Seo, H.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A. Immunohistochemical Examination of Trophoblast Syncytialization during Early Placentation in Sheep. Int. J. Mol. Sci. 2019, 20, 4530. [Google Scholar] [CrossRef]










| Component | Source | Catalog Number | Working Conc. |
|---|---|---|---|
| Advanced DMEM/F12 | Life Technologies, Carlsbad, CA, USA | 12634010 | 1X |
| ALK-4, -5, -7 inhibitor (A83-01) | System Biosciences, Palo Alto, CA, USA | ZRD-A8-02 | 500 nM |
| B27 supplement minus vitamin A | Life Technologies | 12587010 | 1X |
| L-glutamine | Life Technologies | 25030-024 | 2 mM |
| N2 supplement | Life Technologies | 17502048 | 1X |
| N-Acetyl-L-cysteine | Sigma-Aldrich | A9165-5G | 1.25 mM |
| Nicotinamide | Sigma-Aldrich | N0636 | 10 nM |
| Primocin | Invivogen, San Diego, CA, USA | ant-pm-1 | 100 μg/mL |
| Recombinant human EGF | Peprotech, Cranbury, NJ, USA | AF-100-15 | 50 ng/mL |
| Recombinant human FGF-10 | Peprotech | 100-26 | 100 ng/mL |
| Recombinant human HGF | Peprotech | 100-39 | 50 ng/mL |
| Recombinant human Noggin | Peprotech | 120-10c | 100 ng/mL |
| Recombinant human Rspondin-1 | Peprotech | 120-38 | 500 ng/mL |
| Rock inhibitor (Y-27632) | Sigma-Aldrich | Y0503 | 10 µM |
| Species | Model | Major Finding | Ref. | Notes |
|---|---|---|---|---|
| Human | Synthetic embryo model (blastoids) | Multiple cavities between the outer sides of a syncytiotrophoblast-like layer | [60] | On day 6 of blastoid formation |
| Human | IVF-derived embryo | Cavities in the outer layer of attached trophoblasts | [61] | Day 11 |
| Human | Human first-trimester trophoblast cell spheroid outgrowth | Cavities central to spheroid attachment and at the periphery | [62] | Cultured 3D spheroids forming 2D outgrowths |
| Human | Trophoblast-enriched cell (villi from first-trimester placental tissue) | Lacunae present within the syncytial areas | [2] | Similarly to Carnegie stage 5b embryos (approximately 9 days after fertilization) |
| Marmoset | Trophoblast stem cell differentiation in vitro | Peri-implantation trophoblast-like stem cells form floating vesicles at the periphery | [32] | Day 5 of floating after passaging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadeldin, I.M.; Alshehri, B.; AlThubyani, M.; Almohanna, F.H.; Matic, G.; Swelum, A.A.; Coskun, S.; Awartani, K.A.; Assiri, A.M. Modeling Early Stages of Trophectoderm–Endometrium Interactions Using Trophoblastic and Endometrial Organoids and the Generation of Lacunoids/Cystoids. Cells 2025, 14, 1051. https://doi.org/10.3390/cells14141051
Saadeldin IM, Alshehri B, AlThubyani M, Almohanna FH, Matic G, Swelum AA, Coskun S, Awartani KA, Assiri AM. Modeling Early Stages of Trophectoderm–Endometrium Interactions Using Trophoblastic and Endometrial Organoids and the Generation of Lacunoids/Cystoids. Cells. 2025; 14(14):1051. https://doi.org/10.3390/cells14141051
Chicago/Turabian StyleSaadeldin, Islam M., Budur Alshehri, Maha AlThubyani, Falah H. Almohanna, Goran Matic, Ayman A. Swelum, Serdar Coskun, Khalid A. Awartani, and Abdullah M. Assiri. 2025. "Modeling Early Stages of Trophectoderm–Endometrium Interactions Using Trophoblastic and Endometrial Organoids and the Generation of Lacunoids/Cystoids" Cells 14, no. 14: 1051. https://doi.org/10.3390/cells14141051
APA StyleSaadeldin, I. M., Alshehri, B., AlThubyani, M., Almohanna, F. H., Matic, G., Swelum, A. A., Coskun, S., Awartani, K. A., & Assiri, A. M. (2025). Modeling Early Stages of Trophectoderm–Endometrium Interactions Using Trophoblastic and Endometrial Organoids and the Generation of Lacunoids/Cystoids. Cells, 14(14), 1051. https://doi.org/10.3390/cells14141051








