Kisspeptin Administration and mRNA Expression in Adult Syrian Hamsters
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Cycle Tracking
2.3. Histology
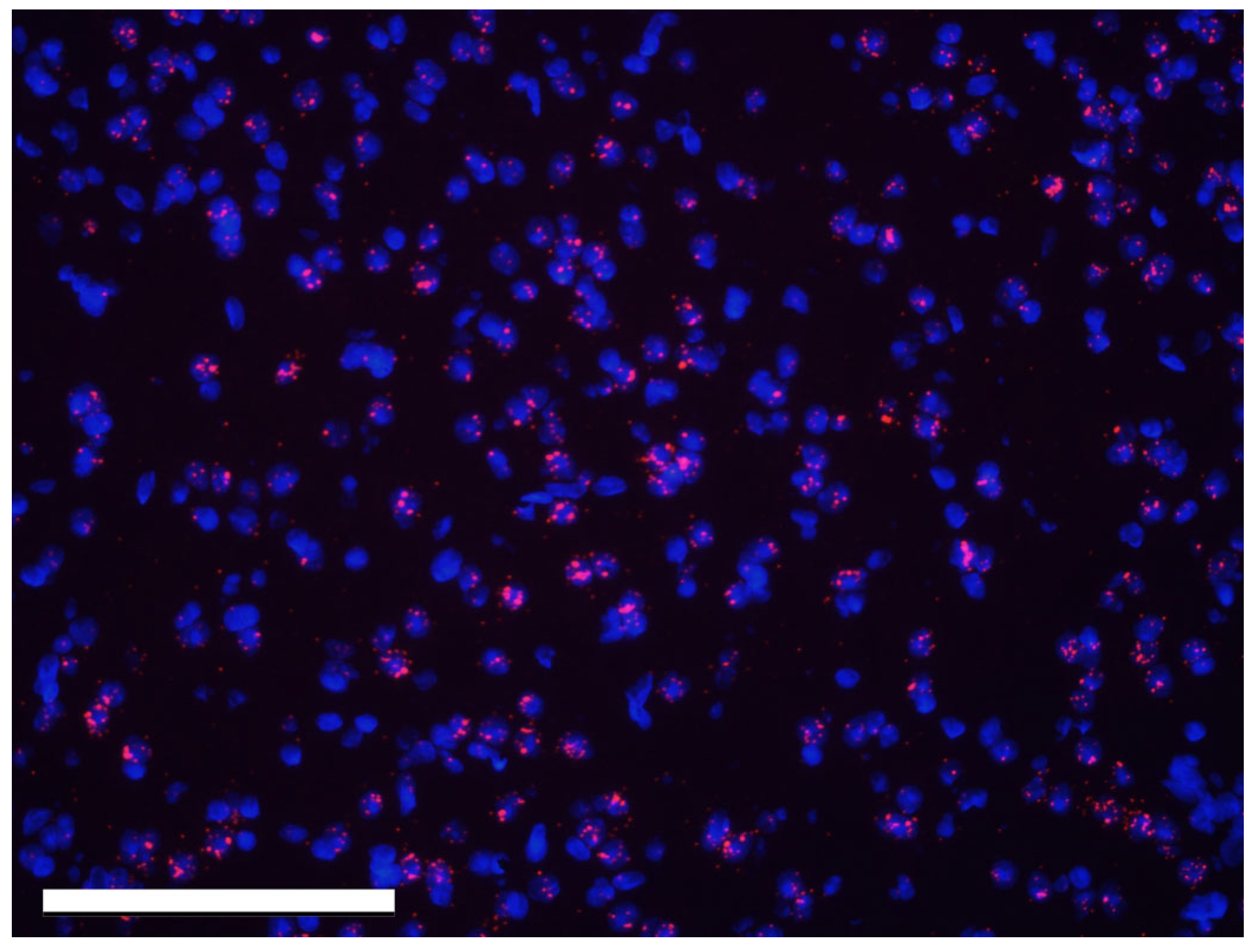
2.4. RNAscope
2.5. Confocal Imaging
2.6. Image Data Collection
2.7. Hormone Treatment to Induce Sexual Receptivity
2.8. Conditioned Place Preference
2.9. Behavior Scoring
2.10. Statistics and Analysis
3. Results
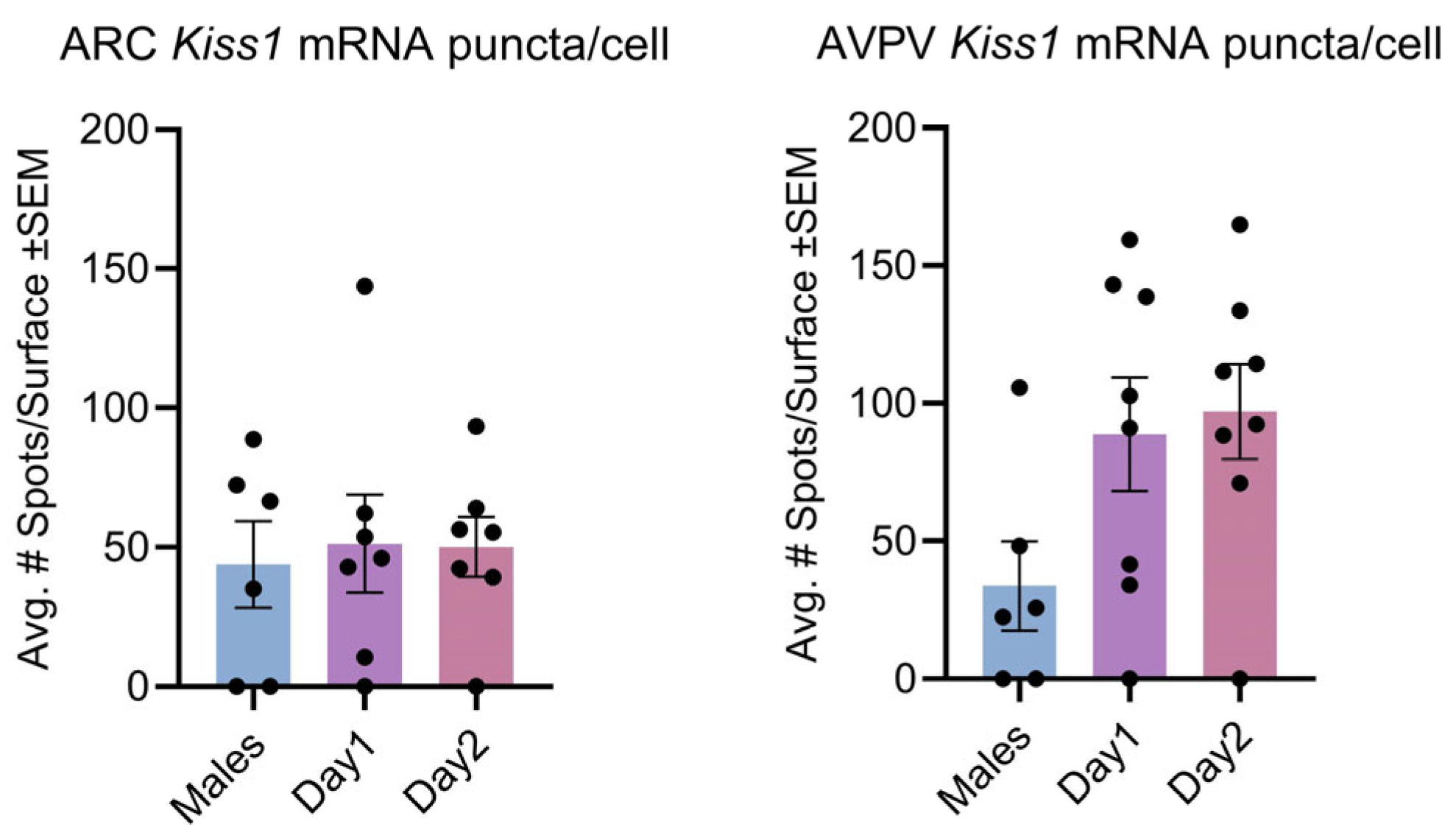
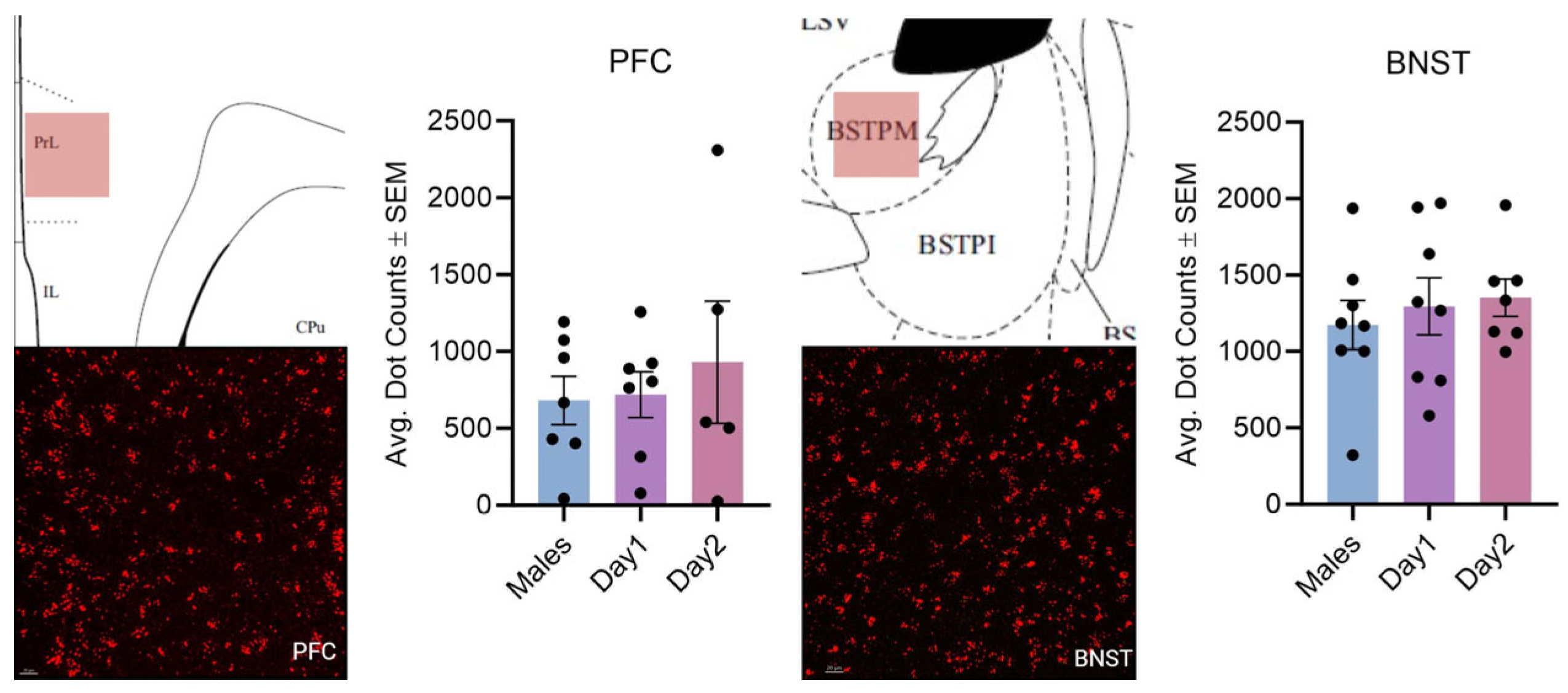
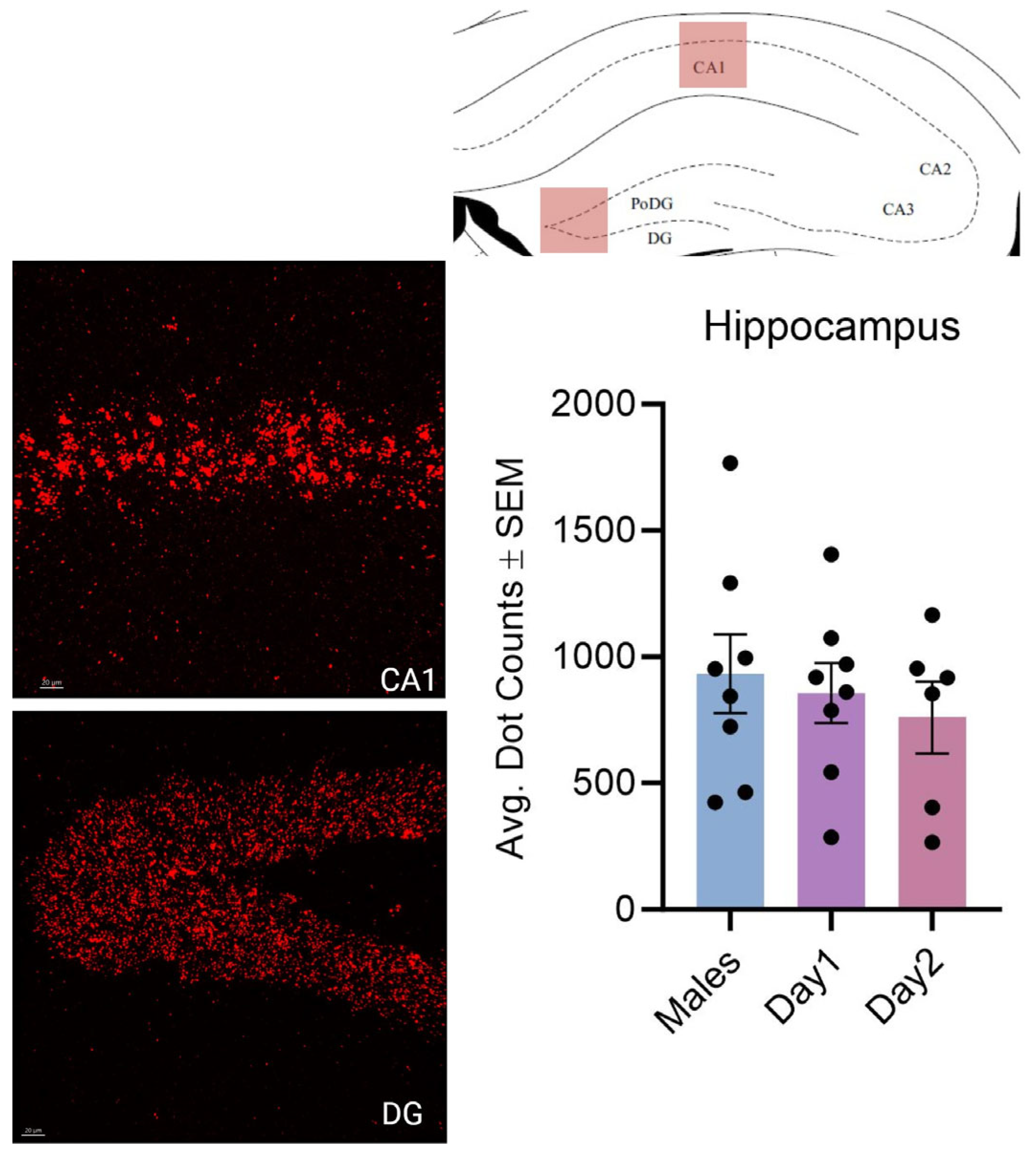
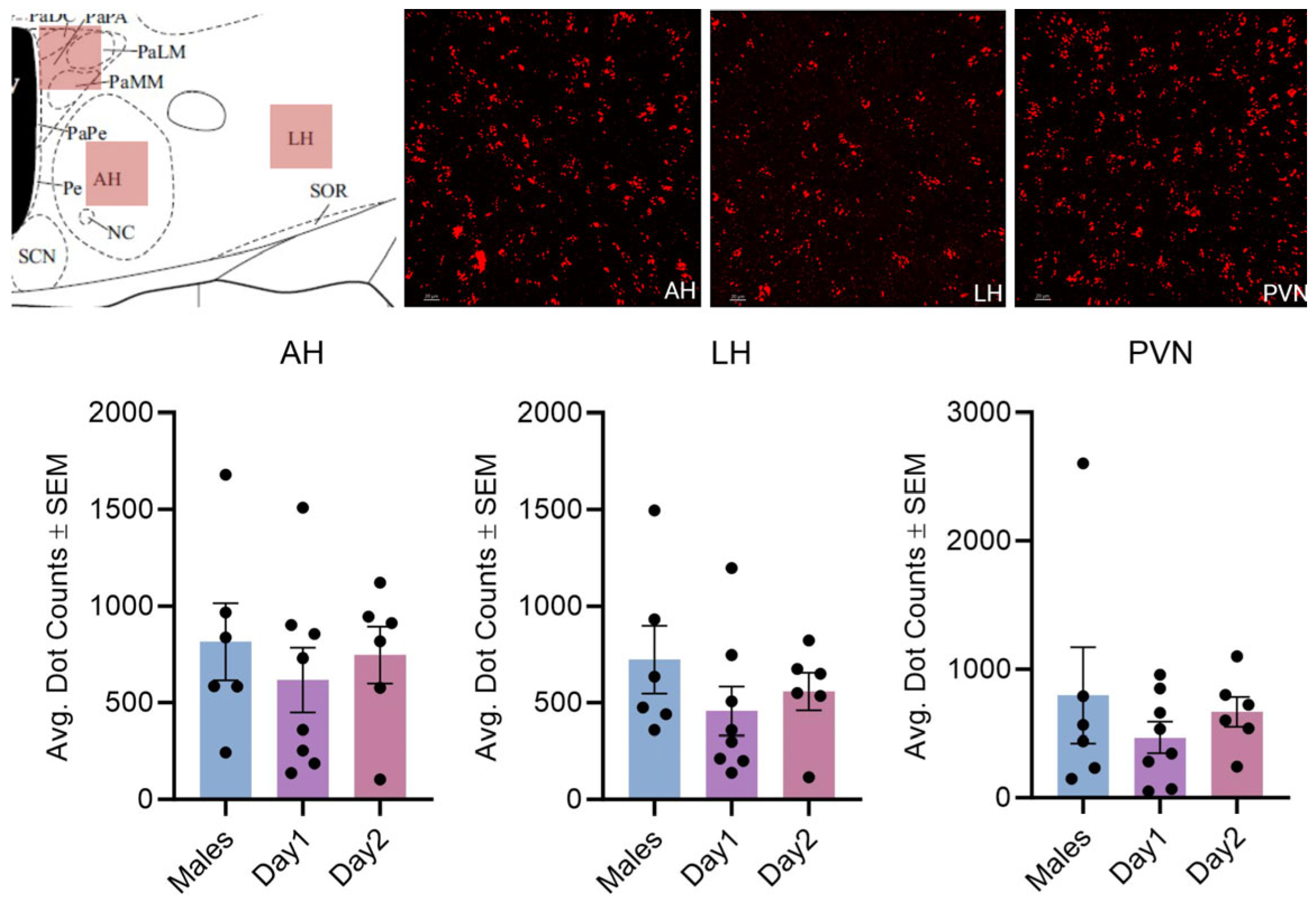
3.1. Effect of Sex and the Estrous Cycle on Kisspeptin mRNA Expression
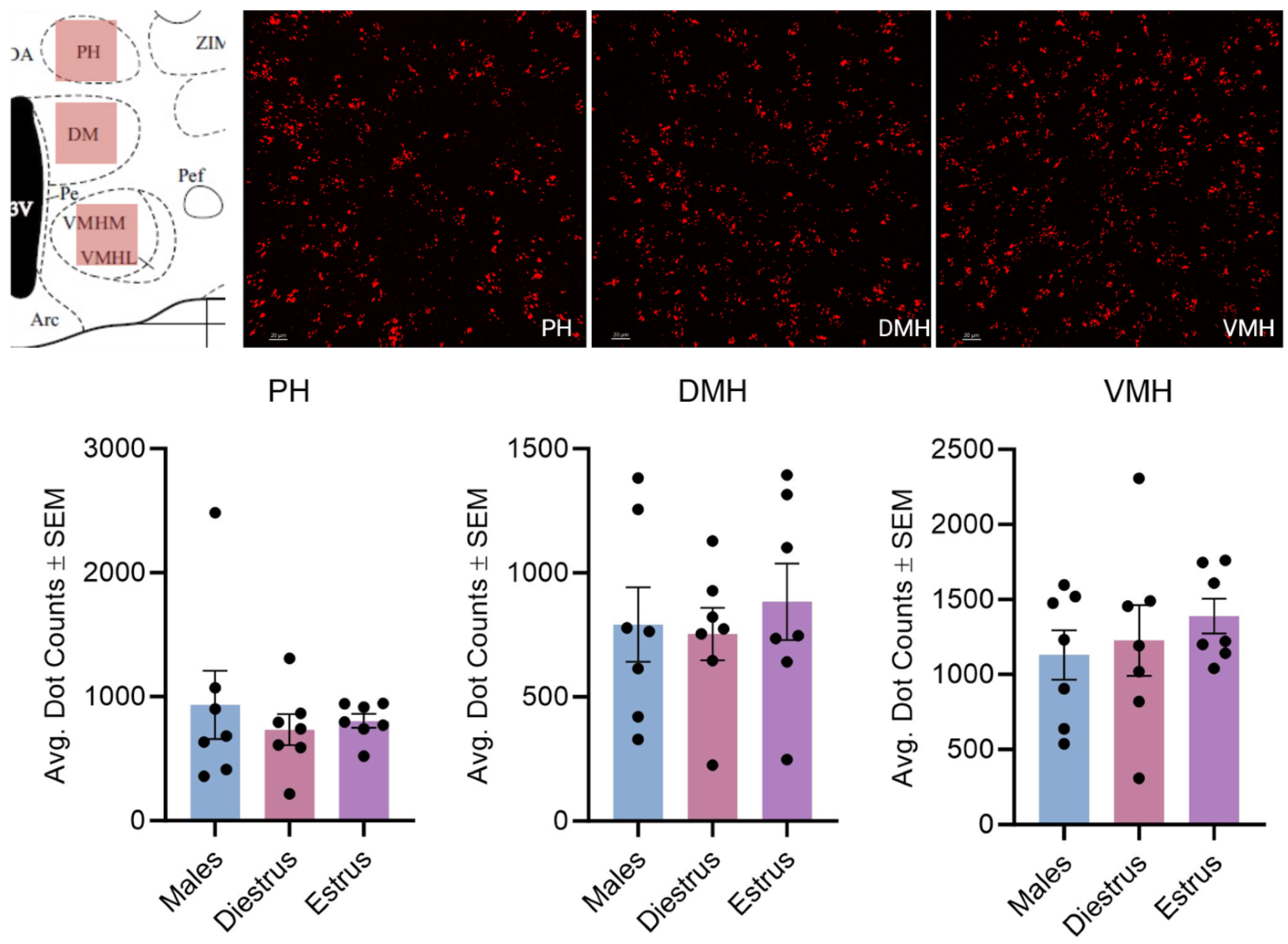
3.2. Effect of Estrous Cycle on Kisspeptin Receptor mRNA Expression
3.3. Effect of Kisspeptin on Lordosis
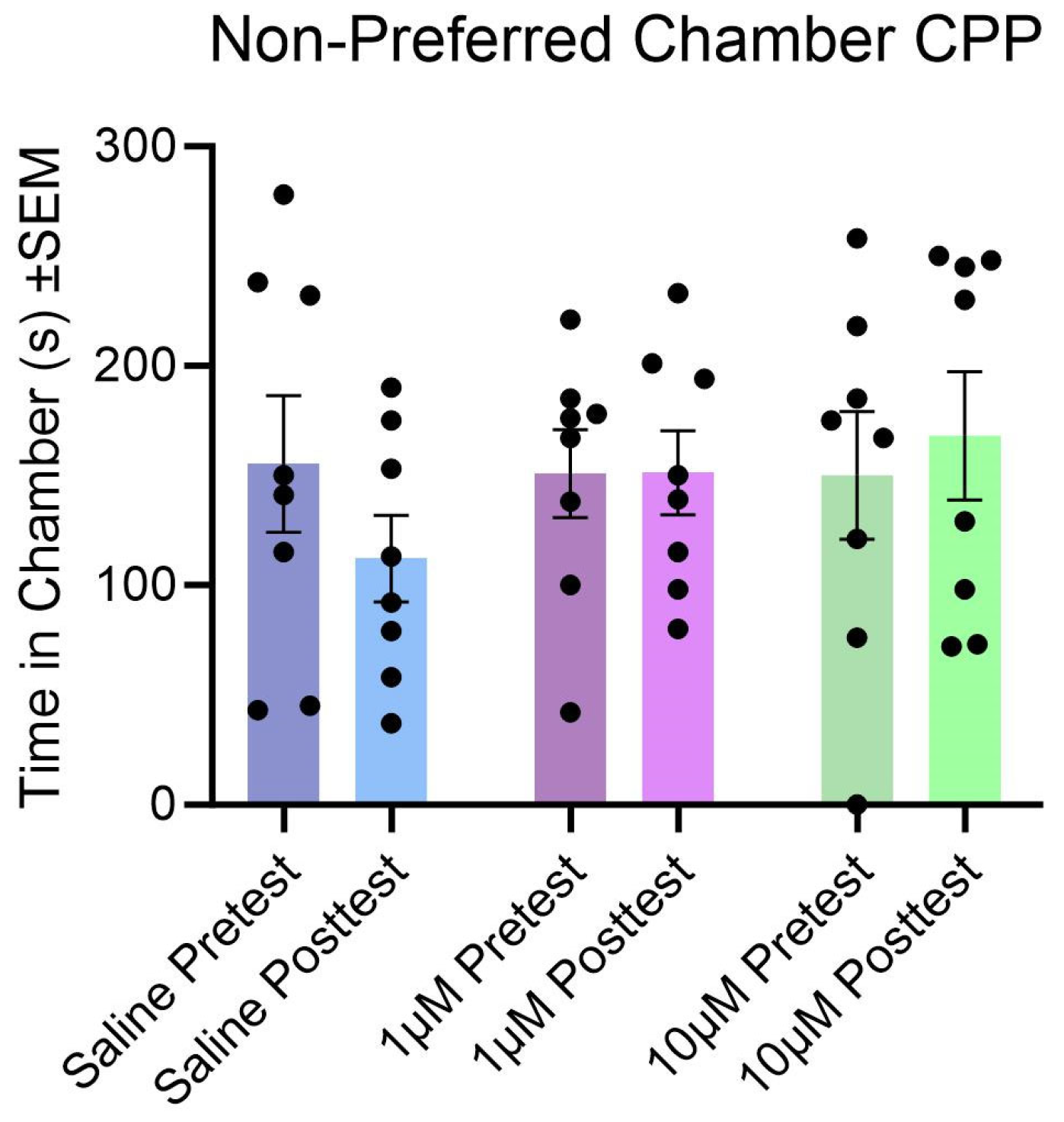
3.4. Effect of Kisspeptin Treatment and Tests on Conditioned Place Preference
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AH | Anterior hypothalamus |
| ARC | Arcuate nucleus of the hypothalamus |
| AVPV | Anteroventral periventricular nucleus |
| BNST | Bed nucleus of the stria terminalis |
| DMH | Dorsomedial hypothalamus |
| Hb | Lateral and medial habenula |
| HPC | Hippocampus (dorsal CA1 and dentate gyrus) |
| LH | Lateral hypothalamus |
| LS | Lateral septum |
| MeAMG | Medial amygdaloid nucleus |
| MS | Medial septum |
| MPOA | Medial preoptic area |
| NAc | Nucleus accumbens |
| PFC | Prefrontal cortex |
| PH | Posterior hypothalamus |
| PVN | Periventricular hypothalamus |
| VMH | Ventromedial hypothalamus |
References
- Navarro, V.M. Metabolic regulation of kisspeptin—The link between energy balance and reproduction. Nature reviews. Endocrinology 2020, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.; d’Anglemont de Tassigny, X.; Colledge, W.H.; Caraty, A.; Herbison, A.E. Distribution of kisspeptin neurones in the adult female mouse brain. J. Neuroendocrinol. 2009, 21, 673–682. [Google Scholar] [CrossRef]
- Xu, Z.; Kaga, S.; Mochiduki, A.; Tsubomizu, J.; Adachi, S.; Sakai, T.; Inoue, K.; Adachi, A.A. Immunocytochemical localization of kisspeptin neurons in the rat forebrain with special reference to sexual dimorphism and interaction with GnRH neurons. Endocr. J. 2012, 59, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Adekunbi, D.A.; Li, X.F.; Lass, G.; Shetty, K.; Adegoke, O.A.; Yeo, S.H.; Colledge, W.H.; Lightman, S.L.; O’Byrne, K.T. Kisspeptin neurones in the posterodorsal medial amygdala modulate sexual partner preference and anxiety in male mice. J. Neuroendocrinol. 2018, 30, e12572. [Google Scholar] [CrossRef] [PubMed]
- Cravo, R.M.; Margatho, L.O.; Osborne-Lawrence, S.; Donato, J., Jr.; Atkin, S.; Bookout, A.L.; Rovinsky, S.; Frazão, R.; Lee, C.E.; Gautron, L.; et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 2011, 173, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, K.M.; Costanza, L.; Kauffman, A.S.; Stephens, S.B.Z. The molecular phenotype of kisspeptin neurons in the medial amygdala of female mice. Front. Endocrinol. 2023, 14, 1093592. [Google Scholar] [CrossRef]
- Kim, J.; Semaan, S.J.; Clifton, D.K.; Steiner, R.A.; Dhamija, S.; Kauffman, A.S. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology 2011, 152, 2020–2030. [Google Scholar] [CrossRef]
- Pineda, R.; Plaisier, F.; Millar, R.P.; Ludwig, M. Amygdala kisspeptin neurons: Putative mediators of olfactory control of the gonadotropic axis. Neuroendocrinology 2017, 104, 223–238. [Google Scholar] [CrossRef]
- Gottsch, M.L.; Navarro, V.M.; Zhao, Z.; Glidewell-Kenney, C.; Weiss, J.; Jameson, J.L.; Clifton, D.K.; Levine, J.E.; Steiner, R.A. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J. Neurosci. 2009, 29, 9390–9395. [Google Scholar] [CrossRef]
- Navarro, V.M.; Gottsch, M.L.; Chavkin, C.; Okamura, H.; Clifton, D.K.; Steiner, R.A. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 2009, 29, 11859–11866. [Google Scholar] [CrossRef]
- Roa, J.; Vigo, E.; Castellano, J.M.; Navarro, V.M.; Fernández-Fernández, R.; Casanueva, F.F.; Dieguez, C.; Aguilar, E.; Pinilla, L.; Tena-Sempere, M. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female rat. Endocrinology 2006, 147, 2864–2878. [Google Scholar] [CrossRef]
- Smith, J.T.; Clifton, D.K.; Steiner, R.A. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction 2006, 131, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, H.; Bartram, S.; Charalambides, M.M.; Murthy, S.; Petitt, T.; Pradeep, A.; Vineall, O.; Abaraonye, I.; Lancaster, A.; Koysombat, K.; et al. Kisspeptin-neuron control of LH pulsatility and ovulation. Front. Endocrinol. 2022, 13, 951938. [Google Scholar] [CrossRef] [PubMed]
- Weems, P.W.; Goodman, R.L.; Lehman, M.N. Neural mechanisms controlling seasonal reproduction: Principles derived from the sheep model and its comparison with hamsters. Front. Neuroendocrinol. 2015, 37, 43–51. [Google Scholar] [CrossRef]
- Desroziers, E.; Mikkelsen, J.; Simonneaux, V.; Keller, M.; Tillet, Y.; Caraty, A.; Franceschini, I. Mapping of kisspeptin fibres in the brain of the pro-oestrous rat. J. Neuroendocrinol. 2010, 22, 1101–1112. [Google Scholar] [CrossRef]
- Patel, B.; Koysombat, K.; Mills, E.G.; Tsoutsouki, J.; Comninos, A.N.; Abbara, A.; Dhillo, W.S. The emerging therapeutic potential of kisspeptin and neurokinin B. Endocr. Rev. 2024, 45, 30–68. [Google Scholar] [CrossRef] [PubMed]
- Velasco, I.; Daza-Dueñas, S.; Torres, E.; Ruiz-Pino, F.; Vázquez, M.J.; Tena-Sempere, M. Kisspeptins centrally modulate food intake and locomotor activity in mice independently of gonadal steroids in a sexually dimorphic manner. J. Neuroendocrinol. 2024, 36, e13433. [Google Scholar] [CrossRef]
- Hudson, A.D.; Kauffman, A.S. Metabolic actions of kisspeptin signaling: Effects on body weight, energy expenditure, and feeding. Pharmacol. Ther. 2022, 231, 107974. [Google Scholar] [CrossRef]
- Saito, R.; Tanaka, K.; Nishimura, H.; Nishimura, K.; Sonoda, S.; Ueno, H.; Motojima, Y.; Yoshimura, M.; Maruyama, T.; Yamamoto, Y.; et al. Centrally administered kisspeptin suppresses feeding via nesfatin-1 and oxytocin in male rats. Peptides 2019, 112, 114–124. [Google Scholar] [CrossRef]
- Stengel, A.; Wang, L.; Goebel-Stengel, M.; Taché, Y. Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. Neuroreport 2011, 22, 253–257. [Google Scholar] [CrossRef]
- Velasco, I.; León, S.; Barroso, A.; Ruiz-Pino, F.; Heras, V.; Torres, E.; León, M.; Ruohonen, S.T.; García-Galiano, D.; Romero-Ruiz, A.; et al. Gonadal hormone-dependent vs. -independent effects of kisspeptin signaling in the control of body weight and metabolic homeostasis. Metab. Clin. Ex. 2019, 98, 84–94. [Google Scholar] [CrossRef]
- Mills, E.G.A.; O’Byrne, K.T.; Comninos, A.N. The Roles of the Amygdala Kisspeptin System. Semin. Reprod. Med. 2019, 37, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.G.; Yang, L.; Abbara, A.; Dhillo, W.S.; Comninos, A.N. Current Perspectives on kisspeptin’s role in behaviour. Front. Endocrinol. 2022, 13, 928143. [Google Scholar] [CrossRef]
- Piet, R. Circadian and kisspeptin regulation of the preovulatory surge. Peptides 2023, 163, 170981. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J. Can kisspeptin be a new treatment for sexual dysfunction? Trends Endocrinol. Metab. 2025. [Google Scholar] [CrossRef]
- Lehman, M.N.; Merkley, C.M.; Coolen, L.M.; Goodman, R.L. Anatomy of the kisspeptin neural network in mammals. Brain Res. 2010, 1364, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, M.; Parhar, I.S. Hypothalamic kisspeptin and kisspeptin receptors: Species variation in reproduction and reproductive behaviours. Front. Neuroendocrinol. 2022, 64, 100951. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, L.K.; Lisk, R.D. Organization and expression of agonistic and socio-sexual behavior in golden hamsters over the estrous cycle and after ovariectomy. Physiol. Behav. 1983, 31, 477–482. [Google Scholar] [CrossRef]
- Borland, J.M.; Kohut-Jackson, A.L.; Peyla, A.C.; Hall, M.A.; Mermelstein, P.G.; Meisel, R.L. Female Syrian hamster analyses of bremelanotide, a US FDA approved drug for the treatment of female hypoactive sexual desire disorder. Neuropharmacology 2025, 267, 110299. [Google Scholar] [CrossRef]
- Hedges, V.L.; Chakravarty, S.; Nestler, E.J.; Meisel, R.L. Delta FosB overexpression in the nucleus accumbens enhances sexual reward in female Syrian hamsters. Genes. Brain Behav. 2009, 8, 442–449. [Google Scholar] [CrossRef]
- Klingerman, C.M.; Patel, A.; Hedges, V.L.; Meisel, R.L.; Schneider, J.E. Food restriction dissociates sexual motivation, sexual performance, and the rewarding consequences of copulation in female Syrian hamsters. Behav. Brain Res. 2011, 223, 356–370. [Google Scholar] [CrossRef]
- Meisel, R.L.; Joppa, M.A. Conditioned place preference in female hamsters following aggressive or sexual encounters. Physiol. Behav. 1994, 56, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Orsini, M.W. The external vaginal phenomena characterizing the stages of the estrous cycle, pregnancy, pseudo-pregnancy, lactation and the anestrous hamster. Mesocricetus Auratus Waterhouse Proc. Anim. Care Panel 1961, 11, 193–206. [Google Scholar]
- Abdulhay, A.; Benton, N.A.; Klingerman, C.M.; Krishnamoorthy, K.; Brozek, J.M.; Schneider, J.E. Estrous cycle fluctuations in sex and ingestive behavior are accentuated by exercise or cold ambient temperatures. Horm. Behav. 2014, 66, 135–147. [Google Scholar] [CrossRef]
- Kirby, H.R.; Maguire, J.J.; Colledge, W.H.; Davenport, A.P. International Union of Basic and Clinical Pharmacology. LXXVII. Kisspeptin receptor nomenclature, distribution, and function. Pharmacol. Rev. 2010, 62, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.A.L.; Kohut-Jackson, A.L.; Peyla, A.C.; Friedman, G.D.; Simco, N.J.; Borland, J.M.; Meisel, R.L. Melanocortin receptor 3 and 4 mRNA expression in the adult female Syrian hamster brain. Front. Mol. Neuroscie 2023, 16, 1038341. [Google Scholar] [CrossRef]
- Morin, L.P.; Wood, R.I. A Stereotaxic Atlas of the Golden Hamster Brain; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Goodman, R.L.; Lehman, M.N. Kisspeptin neurons from mice to men: Similarities and differences. Endocrinology 2012, 153, 5105–5118. [Google Scholar] [CrossRef]
- Smith, J.T. Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: Comparative aspects. Peptides 2009, 30, 94–102. [Google Scholar] [CrossRef]
- Vargas Trujillo, M.; Kalil, B.; Ramaswamy, S.; Plant, T.M. Estradiol upregulates kisspeptin expression in the preoptic area of both the male and female rhesus monkey (Macaca mulatta): Implications for the hypothalamic control of ovulation in highly evolved primates. Neuroendocrinology 2017, 105, 77–89. [Google Scholar] [CrossRef]
- Wang, L.; Moenter, S.M. Differential roles of hypothalamic AVPV and arcuate kisspeptin neurons in estradiol feedback regulation of female reproduction. Neuroendocrinology 2020, 110, 172–184. [Google Scholar] [CrossRef]
- Ansel, L.; Bolborea, M.; Bentsen, A.H.; Klosen, P.; Mikkelsen, J.D.; Simonneaux, V. Differential regulation of kiss1 expression by melatonin and gonadal hormones in male and female Syrian hamsters. J. Biol. Rhythms 2010, 25, 81–91. [Google Scholar] [CrossRef]
- Greives, T.J.; Mason, A.O.; Scotti, M.A.; Levine, J.; Ketterson, E.D.; Kriegsfeld, L.J.; Demas, G.E. Environmental control of kisspeptin: Implications for seasonal reproduction. Endocrinology 2007, 148, 1158–1166. [Google Scholar] [CrossRef]
- Kauffman, A.S.; Park, J.H.; McPhie-Lalmansingh, A.A.; Gottsch, M.L.; Bodo, C.; Hohmann, J.G.; Pavlova, M.N.; Rohde, A.D.; Clifton, D.K.; Steiner, R.A.; et al. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J. Neurosci. 2007, 27, 8826–8835. [Google Scholar] [CrossRef]
- Ozaki, S.; Higo, S.; Iwata, K.; Saeki, H.; Ozawa, H. Region-specific changes in brain kisspeptin receptor expression during estrogen depletion and the estrous cycle. Histochem. Cell Biol. 2019, 152, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Herbison, A.E.; de Tassigny, X.d.; Doran, J.; Colledge, W.H. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 2010, 151, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hernández, V.S.; Zetter, M.A.; Hernández-Pérez, O.R.; Hernández-González, R.; Camacho-Arroyo, I.; Eiden, L.E.; Millar, R.P. Kisspeptin fiber and receptor distribution analysis suggests its potential role in central sensorial processing and behavioral state control. J. Neuroendocrinol. 2025, 37, e70007. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, V.S.; Zetter, M.A.; Hernandez-Perez, O.R.; Hernandez-Gonzalez, R.; Camacho-Arroyo, I.S.; Millar, R.P.; Eiden, L.E.; Zhang, L. Comprehensive chemoanatomical mapping, and the gonadal regulation, of seven kisspeptin neuronal populations in the mouse brain. J. Neuroendocrinol. 2025, 37, e70019. [Google Scholar] [CrossRef]
- Bedos, M.; Ponce, E.; Corona, R.; Paredes, R.G. Kisspeptin participates in the positive reward state induced by paced mating and modulates sexual receptivity and paced mating behavior in female rats. Horm. Behav. 2025, 167, 105671. [Google Scholar] [CrossRef]
- Greives, T.J.; Long, K.L.; Burns, C.M.; Demas, G.E. Response to exogenous kisspeptin varies according to sex and reproductive condition in Siberian hamsters (Phodopus sungorus). Gen. Comp. Endocrinol. 2011, 170, 172–179. [Google Scholar] [CrossRef]
- Borland, J.M.; Aiani, L.M.; Norvelle, A.; Grantham, K.N.; O’Laughlin, K.; Terranova, J.I.; Frantz, K.J.; Albers, H.E. Sex-dependent regulation of social reward by oxytocin receptors in the ventral tegmental area. Neuropsychopharmacol. 2019, 44, 785–792. [Google Scholar] [CrossRef]
- Kastin, A.J.; Galina, Z.H.; Horvath, A.; Olson, R.D. Some principles in the peptide field. J. Allergy Clin. Immunol. 1987, 79, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Thurston, L.; Hunjan, T.; Ertl, N.; Wall, M.B.; Mills, E.G.; Suladze, S.; Patel, B.; Alexander, E.C.; Muzi, B.; Bassett, P.A.; et al. Effects of kisspeptin administration in women with Hypoactive Sexual Desire Disorder: A randomized clinical trial. JAMA Netw. Open 2022, 5, e2236131. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.G.; Ertl, N.; Wall, M.B.; Thurston, L.; Yang, L.; Suladze, S.; Hunjan, T.; Phylactou, M.; Patel, B.; Muzi, B.; et al. Effects of kisspeptin on sexual brain processing and penile tumescence in men with Hypoactive Sexual Desire Disorder: A randomized clinical trial. JAMA Netw. Open 2023, 6, e2254313. [Google Scholar] [CrossRef] [PubMed]
- Comninos, A.N.; Wall, M.B.; Demetriou, L.; Shah, A.J.; Clarke, S.A.; Narayanaswamy, S.; Nesbitt, A.; Izzi-Engbeaya, C.; Prague, J.K.; Abbara, A.; et al. Kisspeptin modulates sexual and emotional brain processing in humans. J. Clin. Investig. 2017, 127, 709–719. [Google Scholar] [CrossRef]
- Meisel, R.L.; Mullins, A.J. Sexual experience in female rodents: Cellular mechanisms and functional consequences. Brain Res. 2006, 1126, 56–65. [Google Scholar] [CrossRef]
- Kohut-Jackson, A.L.; Borland, J.M.; Meisel, R.L. Modeling female sexual desire: An overview and commentary. In Women’s Health Problems—A Global Perspective; Kabir, R., Parsa, A.D., Lakhno, I.V., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, M.A.L.; Reeder, P.L.; Borland, J.M.; Meisel, R.L. Kisspeptin Administration and mRNA Expression in Adult Syrian Hamsters. Cells 2025, 14, 992. https://doi.org/10.3390/cells14130992
Hall MAL, Reeder PL, Borland JM, Meisel RL. Kisspeptin Administration and mRNA Expression in Adult Syrian Hamsters. Cells. 2025; 14(13):992. https://doi.org/10.3390/cells14130992
Chicago/Turabian StyleHall, Megan A. L., Peyton L. Reeder, Johnathan M. Borland, and Robert L. Meisel. 2025. "Kisspeptin Administration and mRNA Expression in Adult Syrian Hamsters" Cells 14, no. 13: 992. https://doi.org/10.3390/cells14130992
APA StyleHall, M. A. L., Reeder, P. L., Borland, J. M., & Meisel, R. L. (2025). Kisspeptin Administration and mRNA Expression in Adult Syrian Hamsters. Cells, 14(13), 992. https://doi.org/10.3390/cells14130992






