The Cholesterol Biosynthesis Pathway Plays an Important Role in Chemotherapeutic Drug Response and Metastasis in High-Grade Osteosarcoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Patients
2.3. Cell Cultures
2.4. Chemosensitivity Testing by MTT Colorimetric Assay
2.5. Total RNA Isolation and Expression Analysis
2.6. RNA Sequencing Data Analysis
2.7. Gene Ontology and Pathway Enrichment Analysis
2.8. Protein–Protein Interaction and Identification of Hub Genes and Subnetwork Analysis
2.9. Determination of Gene Expressions by RT-qPCR Analysis
2.10. The Correlation of HMGCR Expression with Overall Survival and Disease-Specific Survival in Sarcoma Samples
2.11. Apoptosis Assay
2.12. Mitochondrial Membrane Potential
2.13. Cell Migration and Invasion Assay
2.14. Gelatin Zymography Assay
2.15. Western Blot Analysis
2.16. Statistical Analysis
3. Results
3.1. Differential Gene Expression Analysis of Osteosarcoma Patients Sensitive and Resistance to Chemotherapy
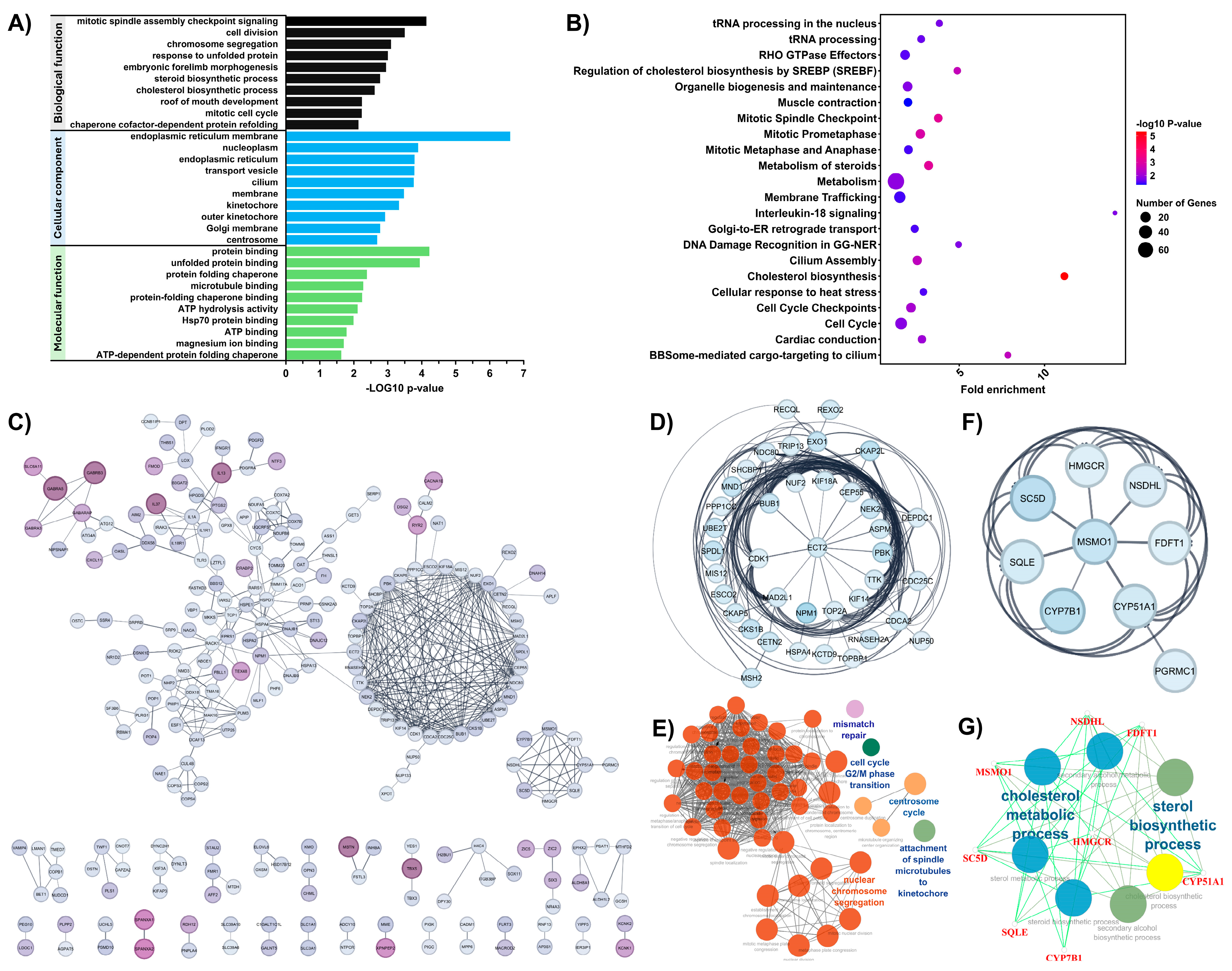
3.2. Functional Enrichment and Protein–Protein Interaction (PPI) Network Analysis of DEGs Associated with Chemotherapeutic Response in OS
3.3. The Role of the Cholesterol Biosynthesis Pathway in the Chemotherapeutic Drug Response of Osteosarcoma Cells
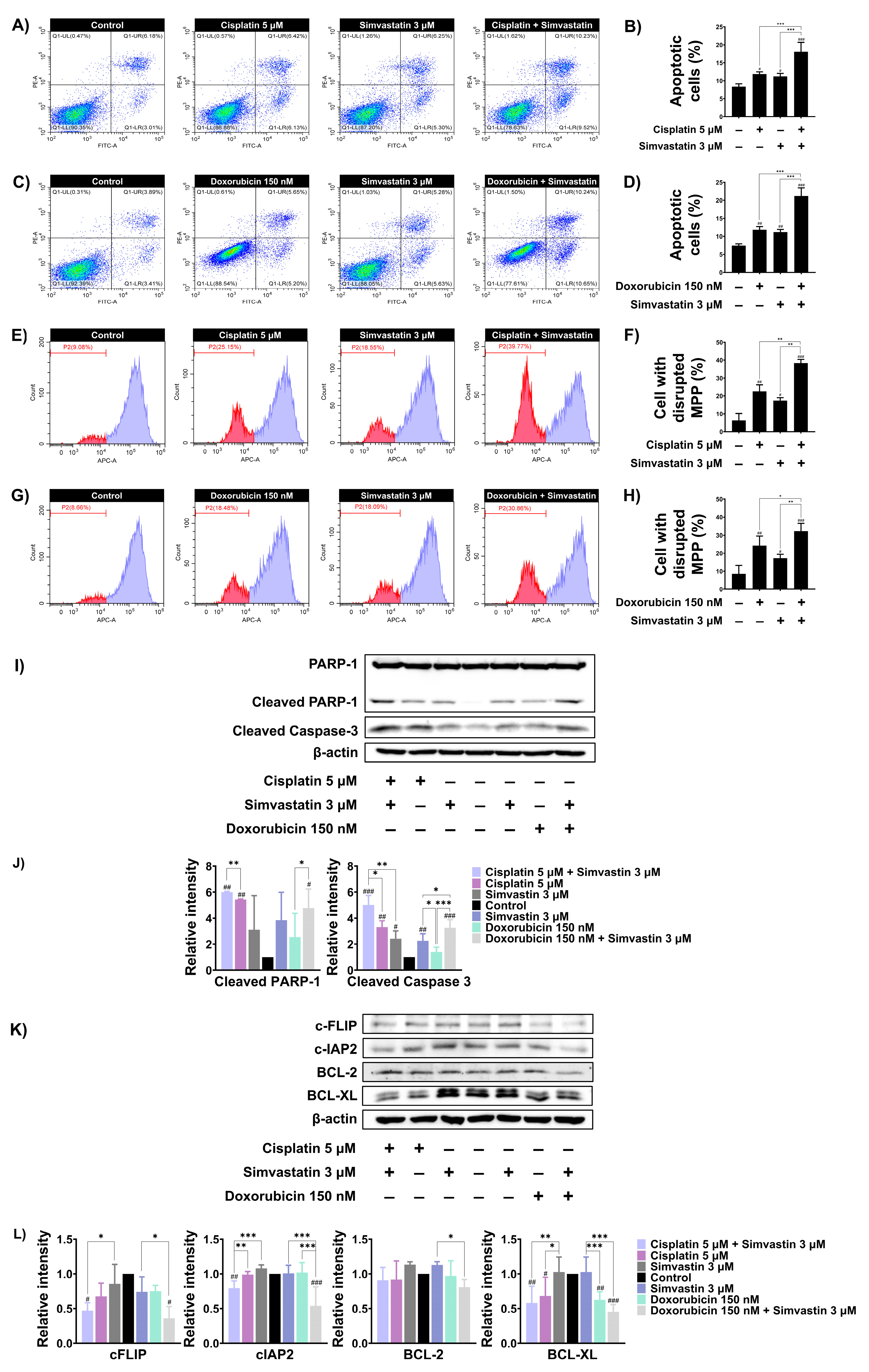
3.4. Simvastatin Enhance Cisplatin and Doxorubicin-Induced SaOS-2 Cells Apoptosis
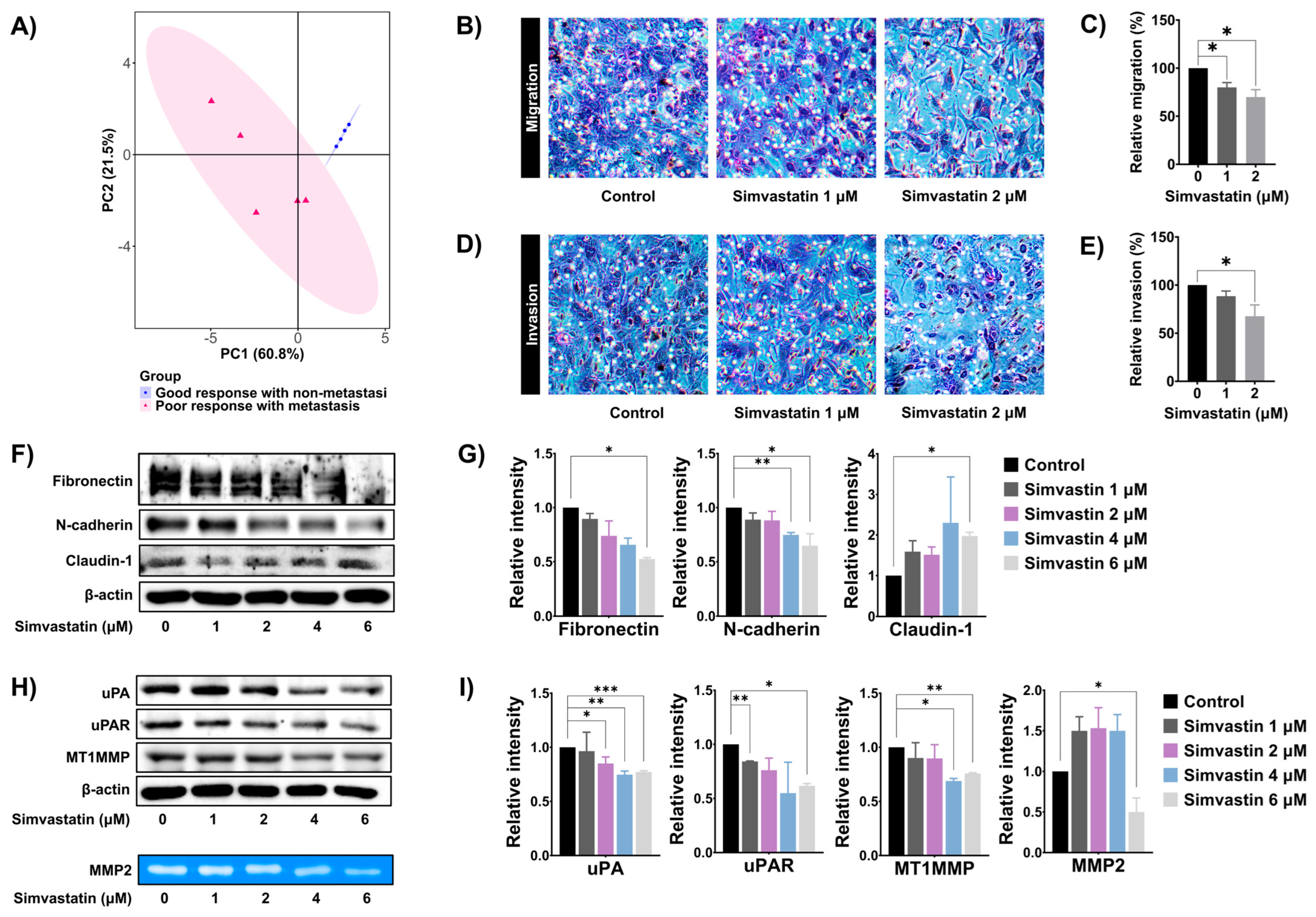
3.5. Simvastatin Reduced SaOS-2 Invasion and Migration
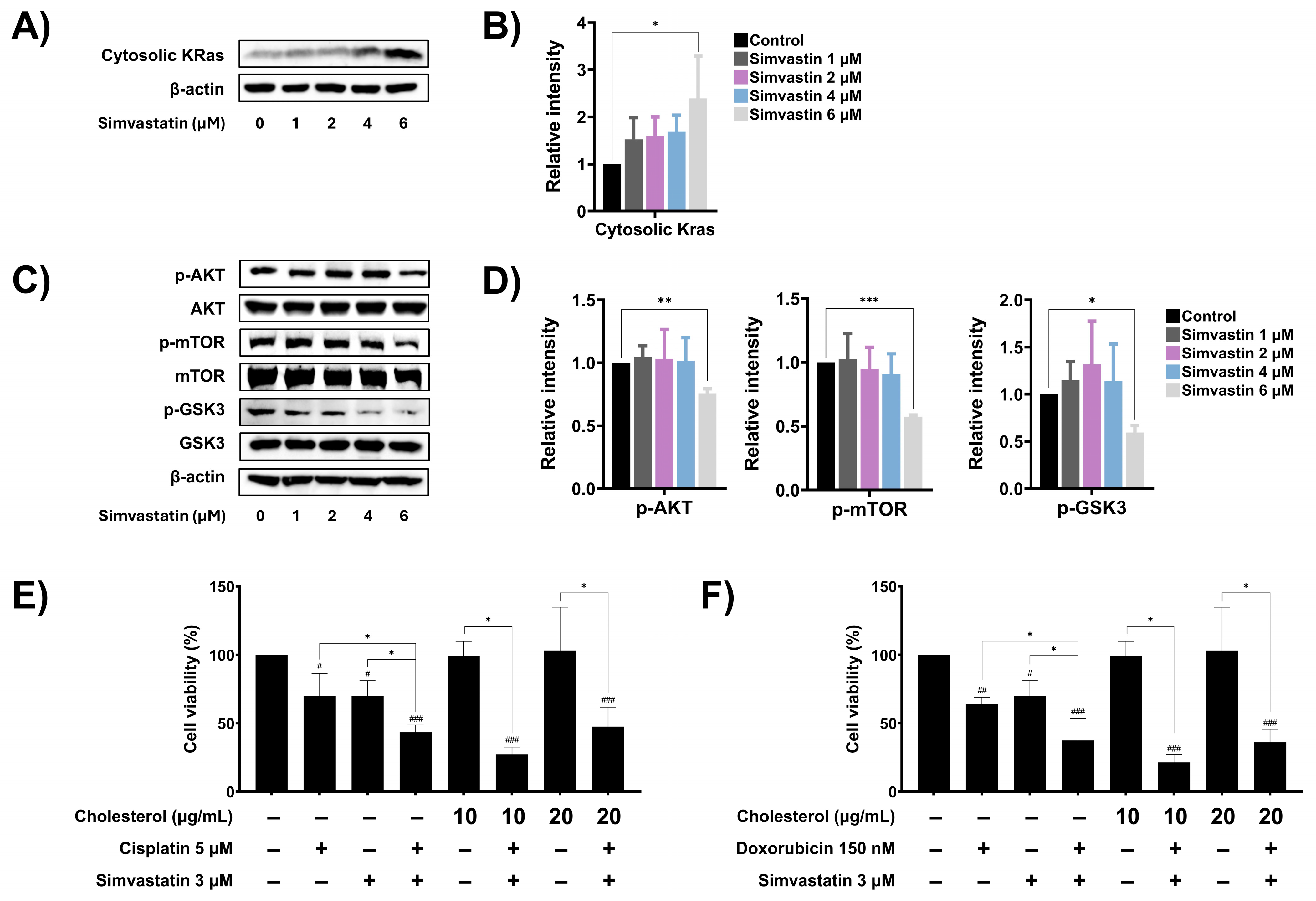
3.6. Simvastatin Modulates Ras Prenylation and Downstream Signaling in SaOS-2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| HGOS | High-grade osteosarcoma |
| OS | Osteosarcoma |
| HMGCR | HMG-CoA reductase |
| DEGs | Differentially expressed genes |
| GO | Gene Ontology |
| MMP | Mitochondrial membrane potential |
| EMT | Epithelial–mesenchymal transition |
| mTOR | Mechanistic target of rapamycin |
| GSK3 | Glycogen synthase kinase-3 |
| uPA | Urokinase plasminogen activator |
References
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Pediatr. Adolesc. Osteosarcoma 2009, 152, 3–13. [Google Scholar]
- Sever, N.; Şimşek, F.; Onur, İ.D.; Arvas, H.; Guliyev, T.; Şakalar, T.; Çiçek, C.M.; Orman, S.; Çetin, E.B.; Kayaş, K.; et al. Prognostic Factors in High Grade Osteosarcoma Patients Who Received Neoadjuvant Therapy and Subsequently Underwent Surgery: Data from the Turkish Oncology Group. J. Clin. Med. 2025, 14, 2024. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.D.; Grimm, S.L.; Kanchi, R.S.; Gandhi, T.; Koirala, A.; Yustein, J.T.; Coarfa, C. Identification of an early survival prognostic gene signature for localized osteosarcoma patients. Sci. Rep. 2024, 14, 7327. [Google Scholar] [CrossRef] [PubMed]
- Odri, G.A.; Tchicaya-Bouanga, J.; Yoon, D.J.Y.; Modrowski, D. Metastatic progression of osteosarcomas: A review of current knowledge of environmental versus oncogenic drivers. Cancers 2022, 14, 360. [Google Scholar] [CrossRef]
- Garcia-Ortega, D.Y.; Cabrera-Nieto, S.A.; Caro-Sánchez, H.S.; Cruz-Ramos, M. An overview of resistance to chemotherapy in osteosarcoma and future perspectives. Cancer Drug Resist. 2022, 5, 762. [Google Scholar] [CrossRef]
- Yang, X.; Yang, P.; Shen, J.; Osaka, E.; Choy, E.; Cote, G.; Harmon, D.; Zhang, Z.; Mankin, H.; Hornicek, F. Prevention of multidrug resistance (MDR) in osteosarcoma by NSC23925. Br. J. Cancer 2014, 110, 2896–2904. [Google Scholar] [CrossRef]
- Tippett, V.L.; Tattersall, L.; Ab Latif, N.B.; Shah, K.M.; Lawson, M.A.; Gartland, A. The strategy and clinical relevance of in vitro models of MAP resistance in osteosarcoma: A systematic review. Oncogene 2023, 42, 259–277. [Google Scholar] [CrossRef]
- Menéndez, S.T.; Gallego, B.; Murillo, D.; Rodríguez, A.; Rodríguez, R. Cancer stem cells as a source of drug resistance in bone sarcomas. J. Clin. Med. 2021, 10, 2621. [Google Scholar] [CrossRef]
- Richardson, S.M.; Wurtz, L.D.; Collier, C.D. Ninety percent or greater tumor necrosis is associated with survival and social determinants of health in patients with osteosarcoma in the National Cancer Database. Clin. Orthop. Relat. Res. 2023, 481, 512–522. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Zhang, X.; Chen, W.; Dai, Z. Exosomes derived from FN14-overexpressing BMSCs activate the NF-κB signaling pathway to induce PANoptosis in osteosarcoma. Apoptosis 2025, 30, 880–893. [Google Scholar] [CrossRef]
- Han, J.; Ding, R.; Xiong, X. Expression of NUF2 and CD52 and Their Correlation with Chemotherapy Resistance and Prognosis in Patients with Non-Small Cell Lung Cancer. J. Biochem. Mol. Toxicol. 2025, 39, e70308. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wu, Z. FOXM1-mediated NUF2 expression confers temozolomide resistance to human glioma cells by regulating autophagy via the PI3K/AKT/mTOR signaling pathway. Neuropathology 2022, 42, 430–446. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Sha, T.; Li, H. Knockdown of NUF2-derived exosomes can inhibit the migration and autophagy of BC cells and improve resistance to doxorubicin. Tissue Cell 2024, 89, 102455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, Y.; Zhu, L.; Cheng, J.; Li, Y. MAD2L1-mediated NANOG nuclear translocation: A critical factor in lung cancer chemoresistance. Cell. Signal. 2025, 132, 111811. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Yan, J.; Peng, K.; Zhou, H. Single-cell sequencing reveals MYC targeting gene MAD2L1 is associated with prostate cancer bone metastasis tumor dormancy. BMC Urol. 2022, 22, 37. [Google Scholar] [CrossRef]
- Thoidingjam, S.; Sriramulu, S.; Hassan, O.; Brown, S.L.; Siddiqui, F.; Movsas, B.; Gadgeel, S.; Nyati, S. BUB1 Inhibition Overcomes Radio-and Chemoradiation Resistance in Lung Cancer. Cancers 2024, 16, 3291. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Kong, L.; Pan, Z.; Chen, G. BUB1 Promotes Gemcitabine Resistance in Pancreatic Cancer Cells by Inhibiting Ferroptosis. Cancers 2024, 16, 1540. [Google Scholar] [CrossRef]
- Ding, Y.; Gao, J.; Chen, J.; Ren, J.; Jiang, J.; Zhang, Z.; Tong, X.; Zhao, J. BUB1b impairs chemotherapy sensitivity via resistance to ferroptosis in lung adenocarcinoma. Cell Death Dis. 2024, 15, 525. [Google Scholar] [CrossRef]
- Cicirò, Y.; Ragusa, D.; Sala, A. Expression of the checkpoint kinase BUB1 is a predictor of response to cancer therapies. Sci. Rep. 2024, 14, 4461. [Google Scholar] [CrossRef]
- Martinez, M.J.; Lyles, R.D.; Peinetti, N.; Grunfeld, A.M.; Burnstein, K.L. Inhibition of the serine/threonine kinase BUB1 reverses taxane resistance in prostate cancer. Iscience 2023, 26, 107681. [Google Scholar] [CrossRef]
- González-Ortiz, A.; Galindo-Hernández, O.; Hernández-Acevedo, G.N.; Hurtado-Ureta, G.; García-González, V. Impact of cholesterol-pathways on breast cancer development, a metabolic landscape. J. Cancer 2021, 12, 4307. [Google Scholar] [CrossRef] [PubMed]
- Qusairy, Z.; Gangloff, A.; Leung, S.O.A. Dysregulation of cholesterol homeostasis in ovarian cancer. Curr. Oncol. 2023, 30, 8386–8400. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Sugihara, K.; Miura, T.; Hoshi, D.; Kohno, S.; Takahashi, C.; Hirata, E.; Kiyokawa, E. Cholesterol synthesis is essential for the growth of liver metastasis-prone colorectal cancer cells. Cancer Sci. 2024, 115, 3817–3828. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Li, J.; Xu, H.; Zhao, Y.; Yu, X.; Shi, S. Functional significance of cholesterol metabolism in cancer: From threat to treatment. Exp. Mol. Med. 2023, 55, 1982–1995. [Google Scholar] [CrossRef]
- Buranrat, B.; Senggunprai, L.; Prawan, A.; Kukongviriyapan, V. Effects of Simvastatin in Combination with Anticancer Drugs on Proliferation and Migration in Cholangiocarcinoma Cells. Indian J. Pharm. Sci. 2022, 84, 72–79. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, W.; Zhang, Y.; Yao, M.; Ying, L.; Zhu, L. Inhibitory effect of simvastatin in nasopharyngeal carcinoma cells. Exp. Ther. Med. 2019, 17, 4477–4484. [Google Scholar] [CrossRef]
- Abdoul-Azize, S.; Buquet, C.; Li, H.; Picquenot, J.-M.; Vannier, J.-P. Integration of Ca2+ signaling regulates the breast tumor cell response to simvastatin and doxorubicin. Oncogene 2018, 37, 4979–4993. [Google Scholar] [CrossRef]
- Buranrat, B.; Suwannaloet, W.; Naowaboot, J. Simvastatin potentiates doxorubicin activity against MCF-7 breast cancer cells. Oncol. Lett. 2017, 14, 6243–6250. [Google Scholar] [CrossRef]
- Dewidar, S.A.; Hamdy, O.; Soliman, M.M.; El Gayar, A.M.; El-Mesery, M. Enhanced therapeutic efficacy of doxorubicin/cyclophosphamide in combination with pitavastatin or simvastatin against breast cancer cells. Med. Oncol. 2023, 41, 7. [Google Scholar] [CrossRef]
- Chu, Z.; Fang, L.; Xiang, Y.; Ding, Y. Research progress on cholesterol metabolism and tumor therapy. Discov. Oncol. 2025, 16, 647. [Google Scholar] [CrossRef]
- Simigdala, N.; Gao, Q.; Pancholi, S.; Roberg-Larsen, H.; Zvelebil, M.; Ribas, R.; Folkerd, E.; Thompson, A.; Bhamra, A.; Dowsett, M.; et al. Cholesterol biosynthesis pathway as a novel mechanism of resistance to estrogen deprivation in estrogen receptor-positive breast cancer. Breast Cancer Res. 2016, 18, 58. [Google Scholar] [CrossRef] [PubMed]
- Cousins, A.; Olivares, O.; Markert, E.; Manoharan, A.; Bubnova, X.; Bresolin, S.; Degn, M.; Li, Z.; Silvestri, D.; McGregor, G.; et al. Central nervous system involvement in childhood acute lymphoblastic leukemia is linked to upregulation of cholesterol biosynthetic pathways. Leukemia 2022, 36, 2903–2907. [Google Scholar] [CrossRef] [PubMed]
- Chushi, L.; Wei, W.; Kangkang, X.; Yongzeng, F.; Ning, X.; Xiaolei, C. HMGCR is up-regulated in gastric cancer and promotes the growth and migration of the cancer cells. Gene 2016, 587, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Yu, P.; Chen, X.-S.; Wei, H.; Cao, S.-J.; Zhang, M.; Zhang, Y.; Tao, Y.-G.; Cao, D.-S.; Qiu, F.; et al. Identification of HMGCR as the anticancer target of physapubenolide against melanoma cells by in silico target prediction. Acta Pharmacol. Sin. 2022, 43, 1594–1604. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Yang, Y.; Lu, Y.; Li, X. Antidiabetic drug metformin suppresses tumorigenesis through inhibition of mevalonate pathway enzyme HMGCS1. J. Biol. Chem. 2022, 298, 102678. [Google Scholar] [CrossRef]
- Tilija Pun, N.; Jeong, C.H. Statin as a Potential Chemotherapeutic Agent: Current Updates as a Monotherapy, Combination Therapy, and Treatment for Anti-Cancer Drug Resistance. Pharmaceuticals 2021, 14, 470. [Google Scholar] [CrossRef]
- Calvillo-Argüelles, O.; Abdel-Qadir, H.; Michalowska, M.; Billia, F.; Suntheralingam, S.; Amir, E.; Thavendiranathan, P. Cardioprotective Effect of Statins in Patients With HER2-Positive Breast Cancer Receiving Trastuzumab Therapy. Can. J. Cardiol. 2019, 35, 153–159. [Google Scholar] [CrossRef]
- Moon, D.C.; Lee, H.S.; Lee, Y.I.; Chung, M.J.; Park, J.Y.; Park, S.W.; Song, S.Y.; Chung, J.B.; Bang, S. Concomitant Statin Use Has a Favorable Effect on Gemcitabine-Erlotinib Combination Chemotherapy for Advanced Pancreatic Cancer. Yonsei Med. J. 2016, 57, 1124–1130. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Duan, J.; Wang, H.; Zhang, Y.; Qiao, K.; Wang, J. Cholesterol depletion sensitizes gallbladder cancer to cisplatin by impairing DNA damage response. Cell Cycle 2019, 18, 3337–3350. [Google Scholar] [CrossRef]
- Koi, C.; Izumi, H.; Kurita, T.; Nguyen, T.T.; Murakami, M.; Yoshiura, Y.; Hachisuga, T.; Morimoto, Y. Lovastatin induced Kruppel like factor 2 (KLF2), Kruppel like factor 6 (KLF6) and Ras homolog family member B (RHOB) genes and preferentially led to viability reduction of Cisplatin-resistant cells. Oncotarget 2017, 8, 106429–106442. [Google Scholar] [CrossRef]
- Chou, C.-W.; Lin, C.-H.; Hsiao, T.-H.; Lo, C.-C.; Hsieh, C.-Y.; Huang, C.-C.; Sher, Y.-P. Therapeutic effects of statins against lung adenocarcinoma via p53 mutant-mediated apoptosis. Sci. Rep. 2019, 9, 20403. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Matuszewska, K.; Glogova, A.; Petrik, J. Mutant p53, the Mevalonate Pathway and the Tumor Microenvironment Regulate Tumor Response to Statin Therapy. Cancers 2022, 14, 3500. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.F.; Zheng, L.; Lee, K.J.; Kim, D.H.; Kim, C.S.; Cai, D.Q.; Wu, Z.; Qin, J.W.; Yu, Y.H.; Kim, S.K. HMG-CoA reductase inhibitors induce apoptosis of lymphoma cells by promoting ROS generation and regulating Akt, Erk and p38 signals via suppression of mevalonate pathway. Cell Death Dis. 2013, 4, e518. [Google Scholar] [CrossRef] [PubMed]
- Buranrat, B.; Senggunprai, L.; Prawan, A.; Kukongviriyapan, V. Simvastatin and atorvastatin as inhibitors of proliferation and inducers of apoptosis in human cholangiocarcinoma cells. Life Sci. 2016, 153, 41–49. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Garcia-Saez, A.J. Mechanisms of BCL-2 family proteins in mitochondrial apoptosis. Nat. Rev. Mol. Cell Biol. 2023, 24, 732–748. [Google Scholar] [CrossRef]
- Boice, A.; Bouchier-Hayes, L. Targeting apoptotic caspases in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118688. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Tang, Q.; Liang, B.; Zhang, L.; Li, X.; Li, H.; Jing, W.; Jiang, Y.; Zhou, F.; Zhang, J.; Meng, Y.; et al. Enhanced CHOLESTEROL biosynthesis promotes breast cancer metastasis via modulating CCDC25 expression and neutrophil extracellular traps formation. Sci. Rep. 2022, 12, 17350. [Google Scholar] [CrossRef]
- Atale, N.; Wells, A. Statins as Secondary Preventive Agent to Limit Breast Cancer Metastatic Outgrowth. Int. J. Mol. Sci. 2025, 26, 1300. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Hu, J.W.; He, X.R.; Jin, W.L.; He, X.Y. Statins: A repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 2021, 40, 241. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Bachmann, H.S. Regulation of protein prenylation. Biomed. Pharmacother. 2023, 164, 114915. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, C.; Arévalo-Alameda, C.; Castellano, E. The Importance of Being PI3K in the RAS Signaling Network. Genes 2021, 12, 1094. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Juarez, D.; Fruman, D.A. Targeting the Mevalonate Pathway in Cancer. Trends Cancer 2021, 7, 525–540. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhamwang, A.; Pruksakorn, D.; Dejkriengkraikul, P.; Sangphukieo, A.; Dissook, S.; Yodkeeree, S. The Cholesterol Biosynthesis Pathway Plays an Important Role in Chemotherapeutic Drug Response and Metastasis in High-Grade Osteosarcoma. Cells 2025, 14, 993. https://doi.org/10.3390/cells14130993
Sukhamwang A, Pruksakorn D, Dejkriengkraikul P, Sangphukieo A, Dissook S, Yodkeeree S. The Cholesterol Biosynthesis Pathway Plays an Important Role in Chemotherapeutic Drug Response and Metastasis in High-Grade Osteosarcoma. Cells. 2025; 14(13):993. https://doi.org/10.3390/cells14130993
Chicago/Turabian StyleSukhamwang, Amonnat, Dumnoensun Pruksakorn, Pornngarm Dejkriengkraikul, Apiwat Sangphukieo, Sivamoke Dissook, and Supachai Yodkeeree. 2025. "The Cholesterol Biosynthesis Pathway Plays an Important Role in Chemotherapeutic Drug Response and Metastasis in High-Grade Osteosarcoma" Cells 14, no. 13: 993. https://doi.org/10.3390/cells14130993
APA StyleSukhamwang, A., Pruksakorn, D., Dejkriengkraikul, P., Sangphukieo, A., Dissook, S., & Yodkeeree, S. (2025). The Cholesterol Biosynthesis Pathway Plays an Important Role in Chemotherapeutic Drug Response and Metastasis in High-Grade Osteosarcoma. Cells, 14(13), 993. https://doi.org/10.3390/cells14130993






