Enhancing Antiviral Immunity in the Gastrointestinal Epithelium: The Role of Fibroblast–Endothelium Interaction and Melatonin
Abstract
1. Introduction
2. Materials and Methods
2.1. The Cell Culture
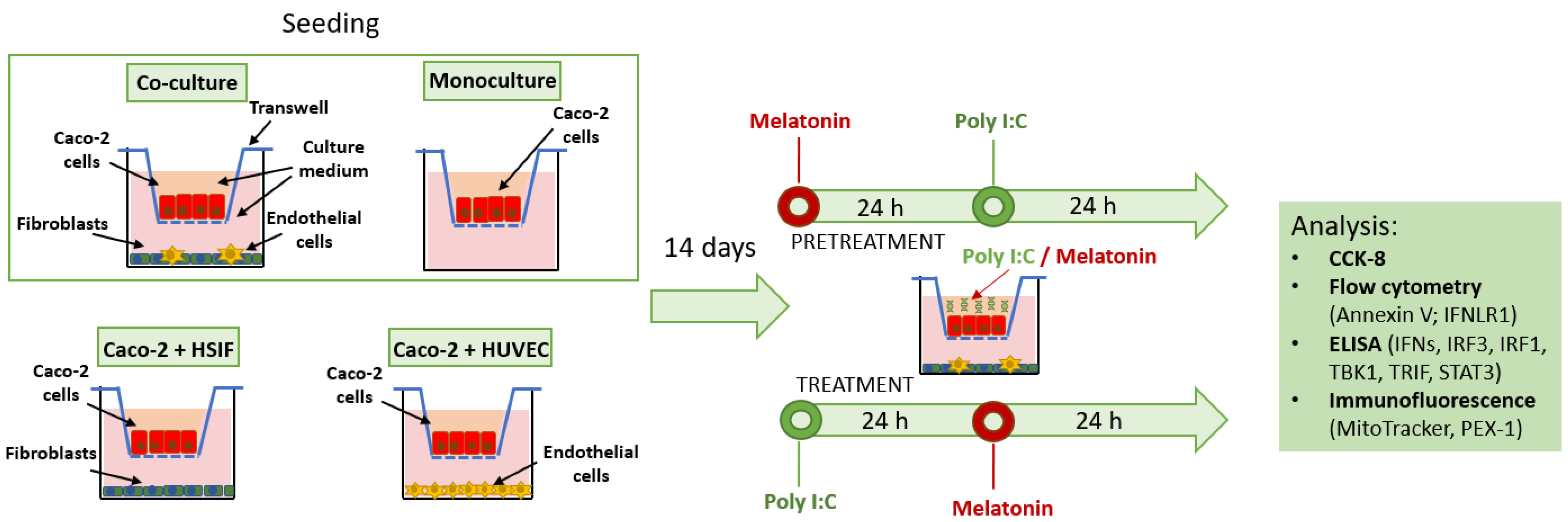
2.2. The Development of the Co-Culture GI Model
2.3. Poly I:C Stimulation and Melatonin Treatment
2.4. Immunofluorescence Microscopy and Image Analysis
2.5. The Cell Viability Assays
2.6. The Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Flow Cytometry
2.8. The Statistical Analysis
3. Results
3.1. The Development of the Co-Culture GI Model
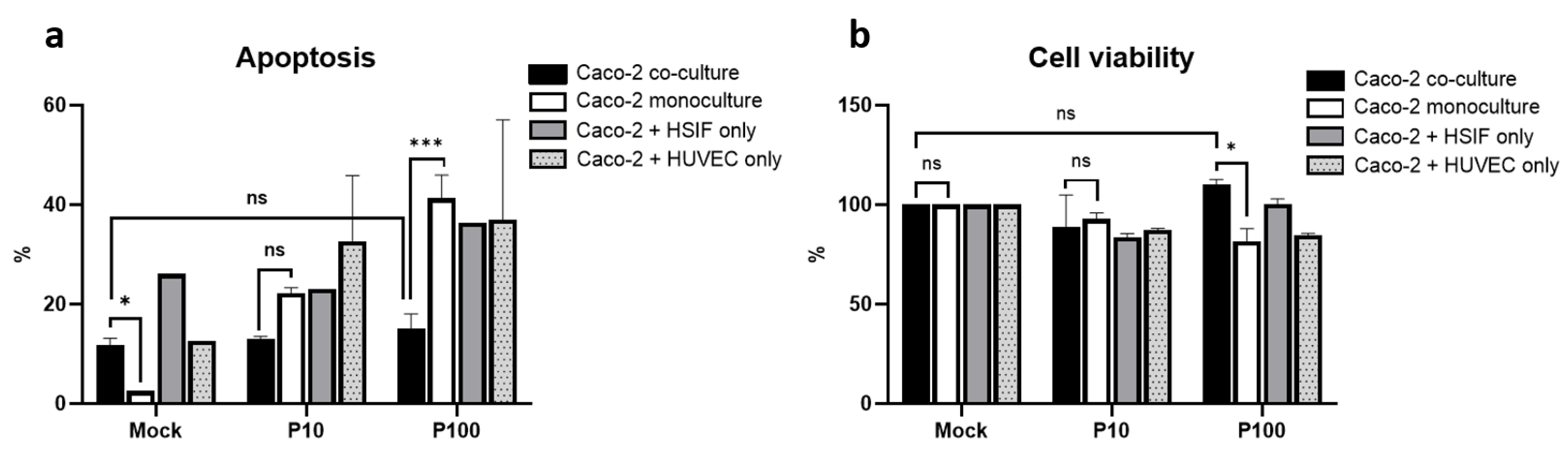
3.2. Apoptosis and Viability Following Poly I:C Stimulation
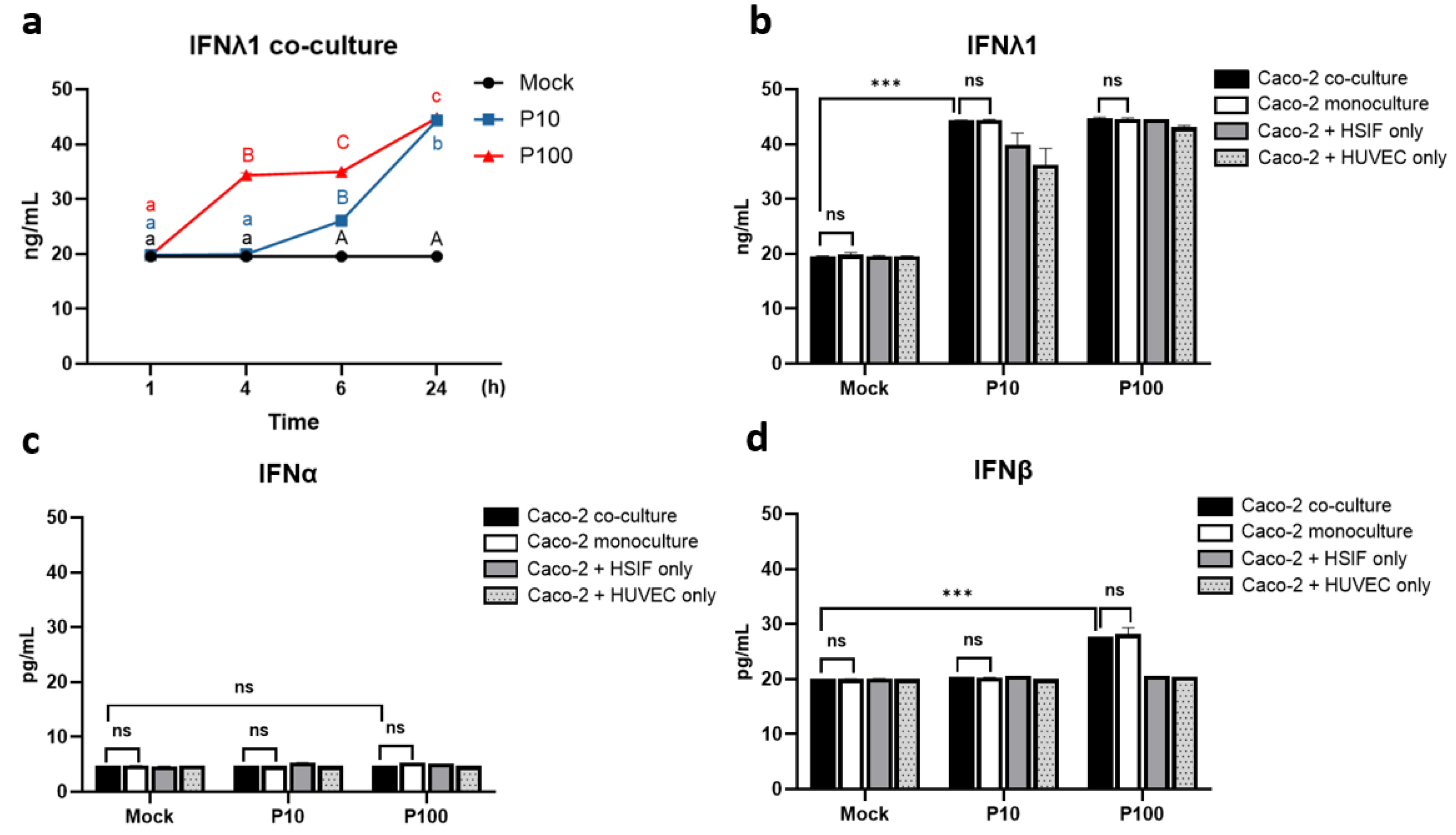
3.3. IFN Kinetics and Expression in the Co-Culture
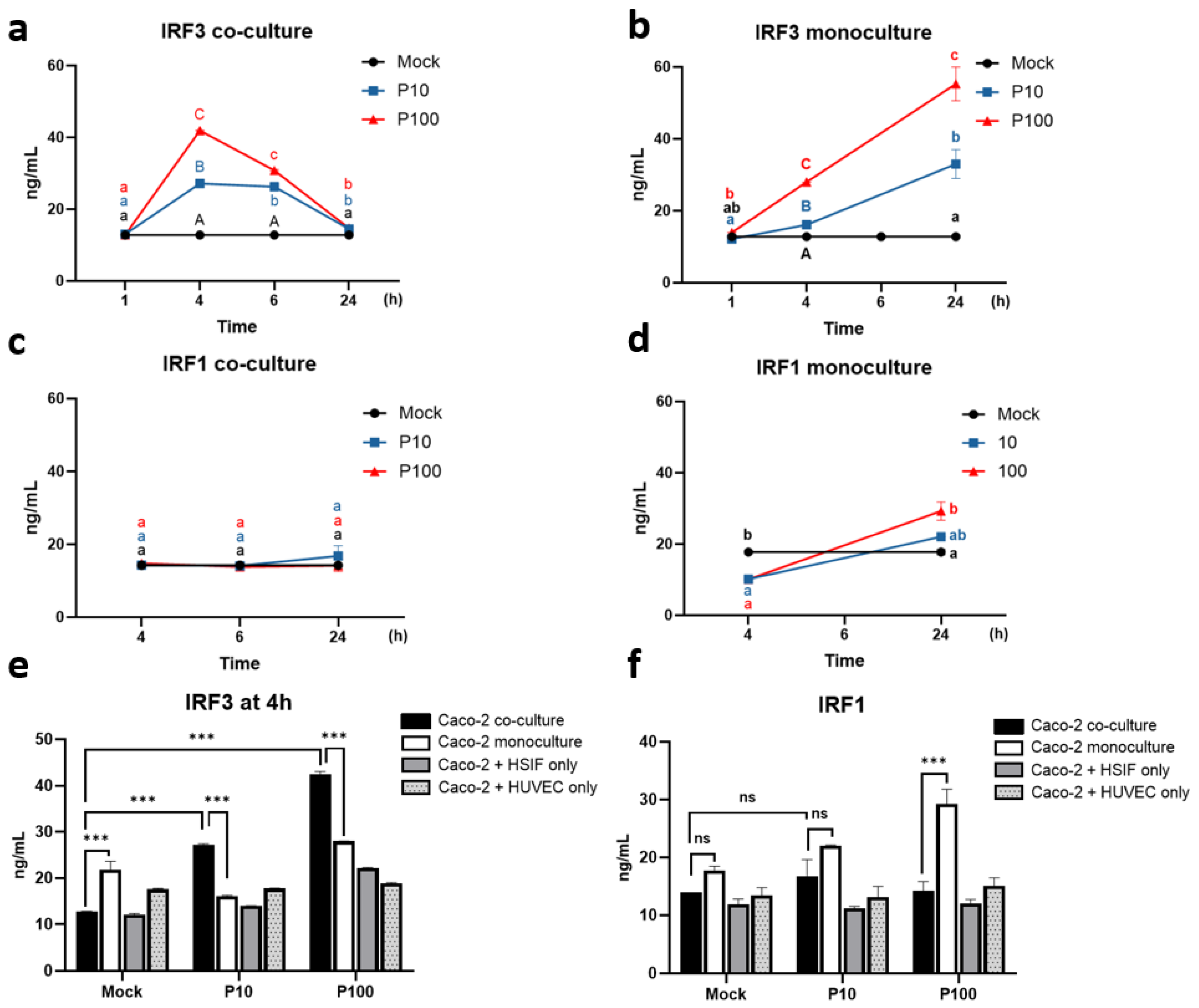
3.4. Distinct Regulation of IRF3 and IRF1 in the Co-Cultured Caco-2 Cells During the Antiviral Response
3.5. The TRIF-TBK1 Signaling Axis Is Pre-Activated and Sustained in GI Co-Cultures Following Poly I:C Stimulation
3.6. Sustained IFNLR1 Expression Supports Robust IFNλ1 Signaling in Co-Cultures
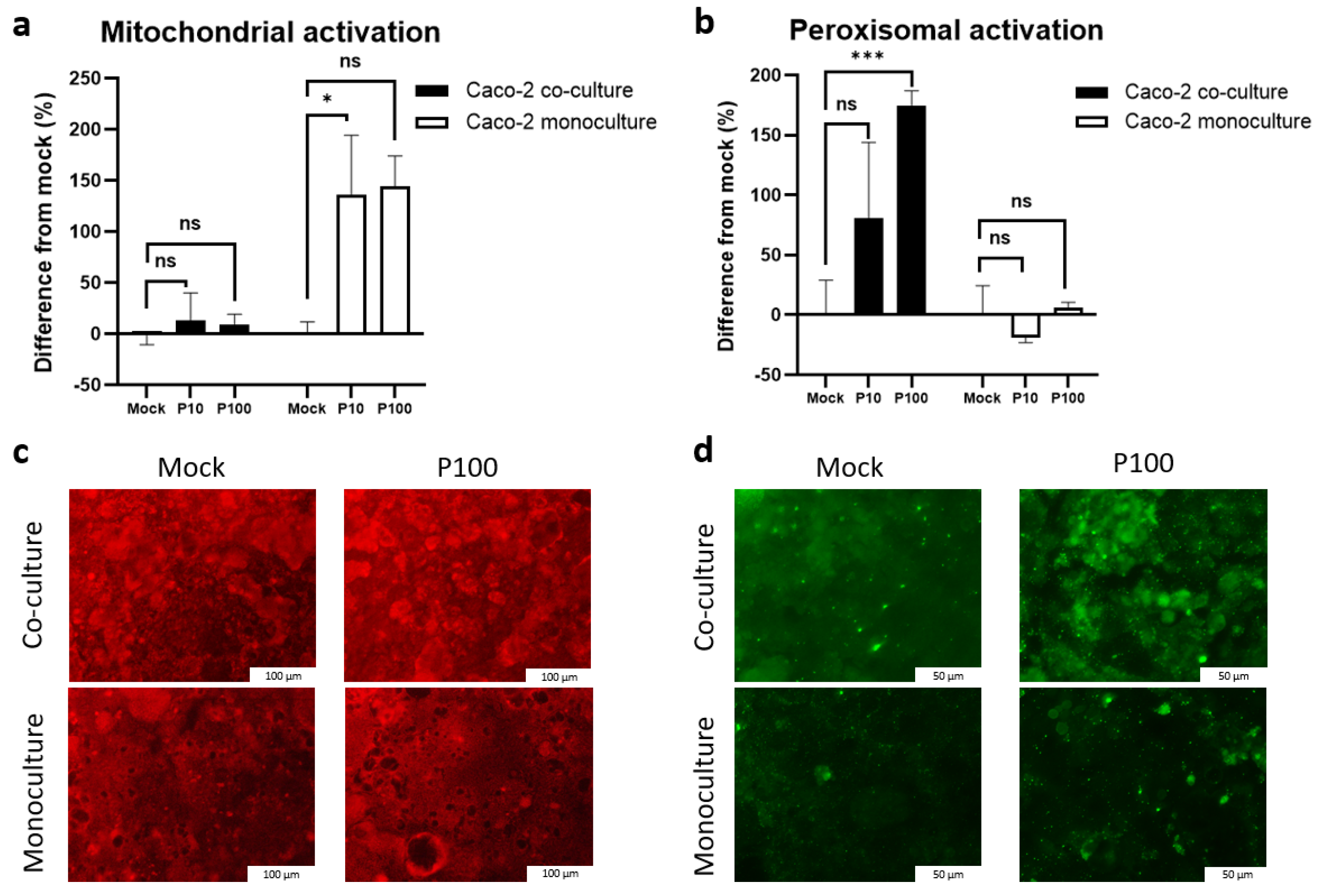
3.7. Mitochondrial and Peroxisomal Activation Diverges Between Monoculture and Co-Culture Models
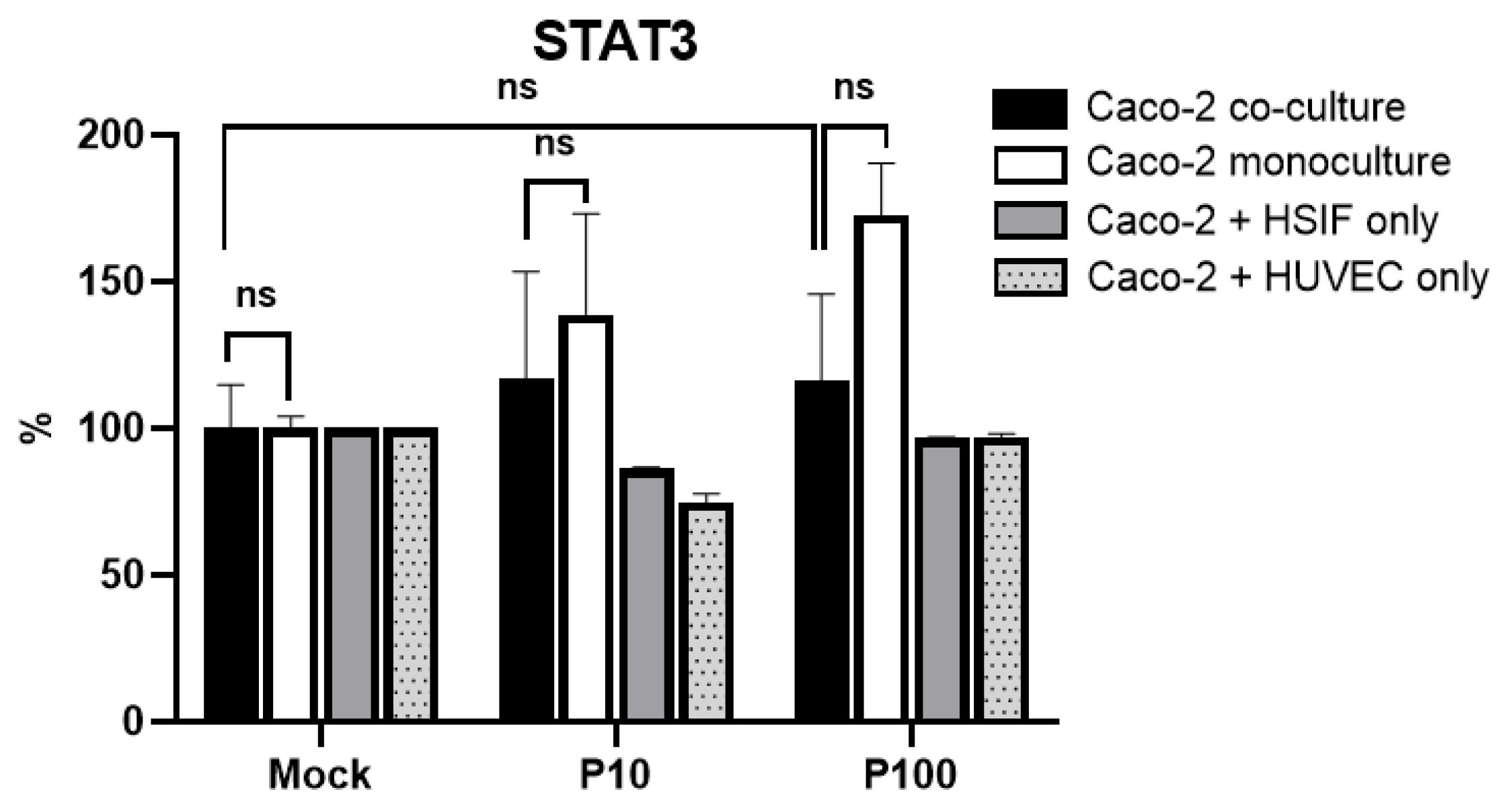
3.8. STAT3 Signaling Across Models Does Not Mirror TBK1/IFNLR1 Patterns
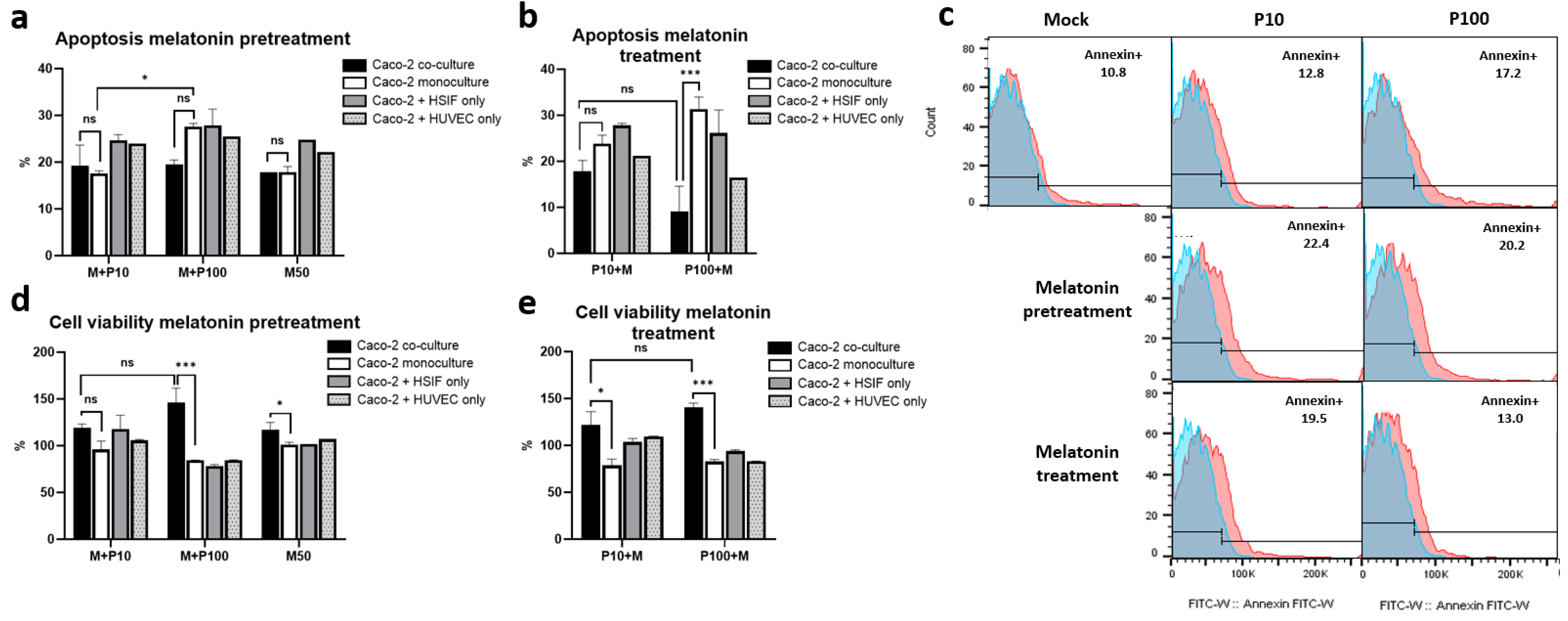
3.9. Melatonin Mitigates Poly I:C-Induced Apoptosis and Enhances Cell Viability in GI Epithelial Models
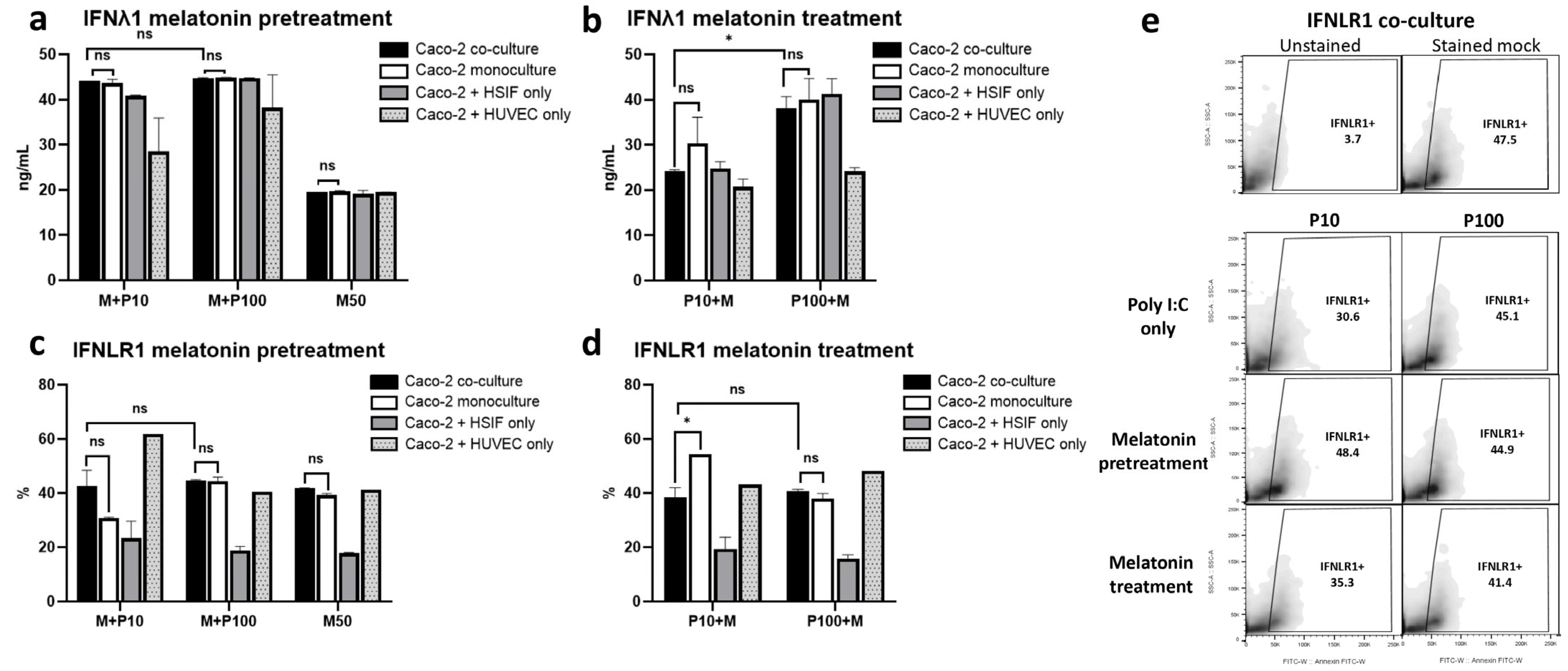
3.10. Melatonin Maintains IFNλ1 and IFNLR1 Signaling, Especially with Pretreatment
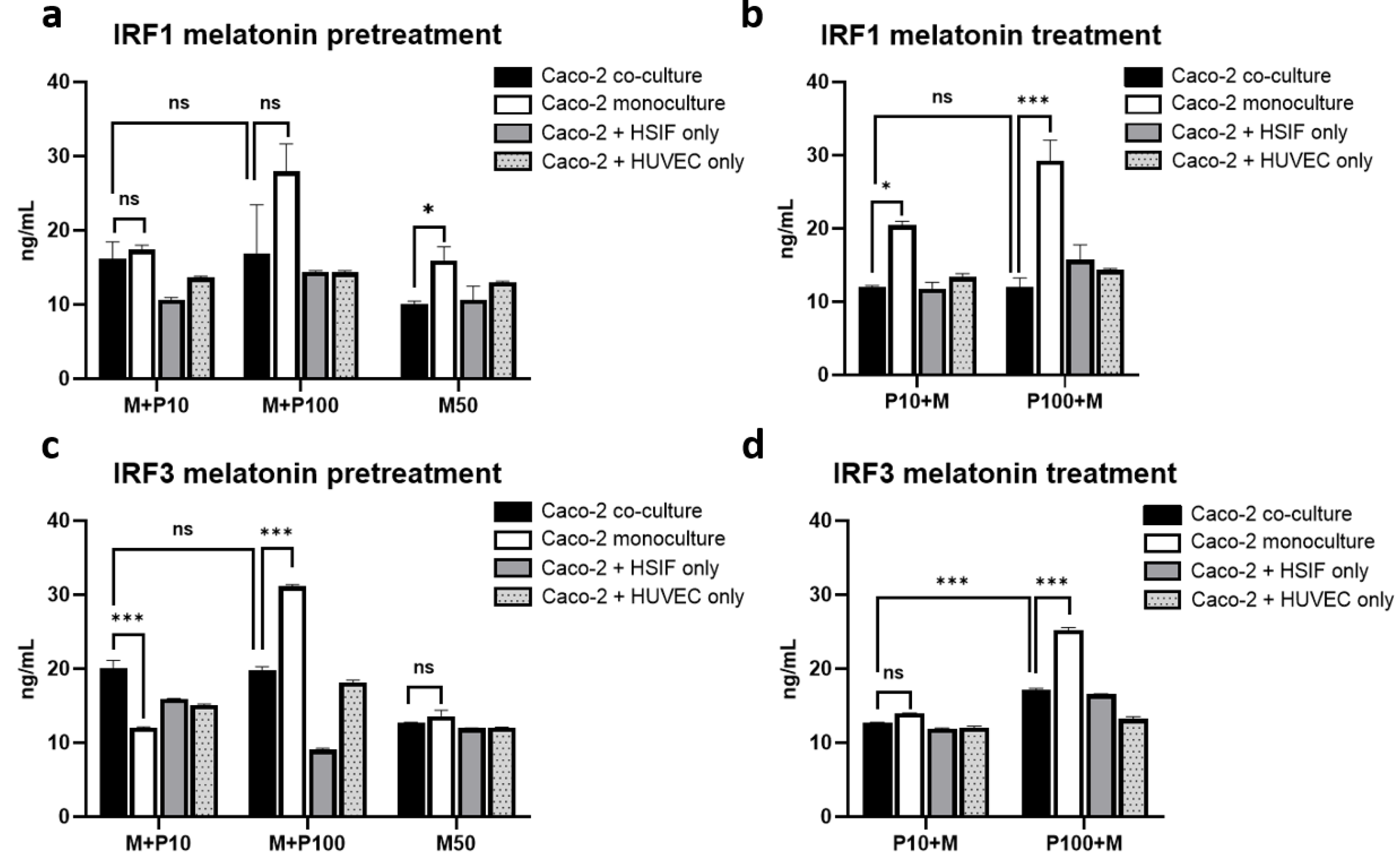
3.11. Melatonin Represses IRF1 and IRF3 Signaling Preferentially in Co-Cultures
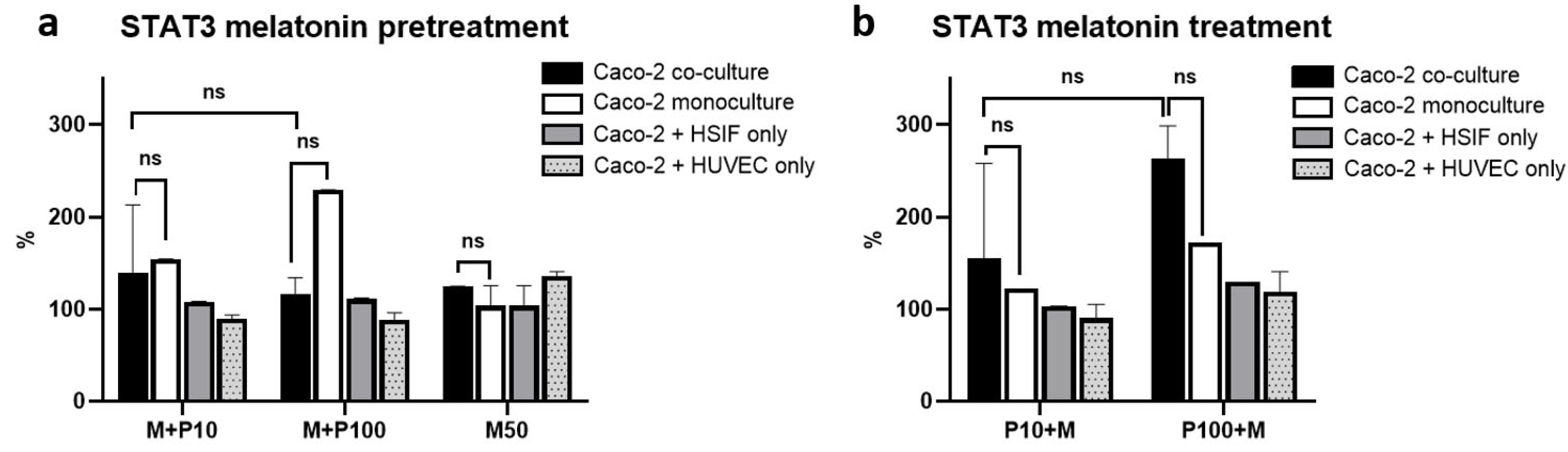
3.12. STAT3 Activation Is Attenuated by Melatonin in Co-Cultures but Elevated in Monocultures
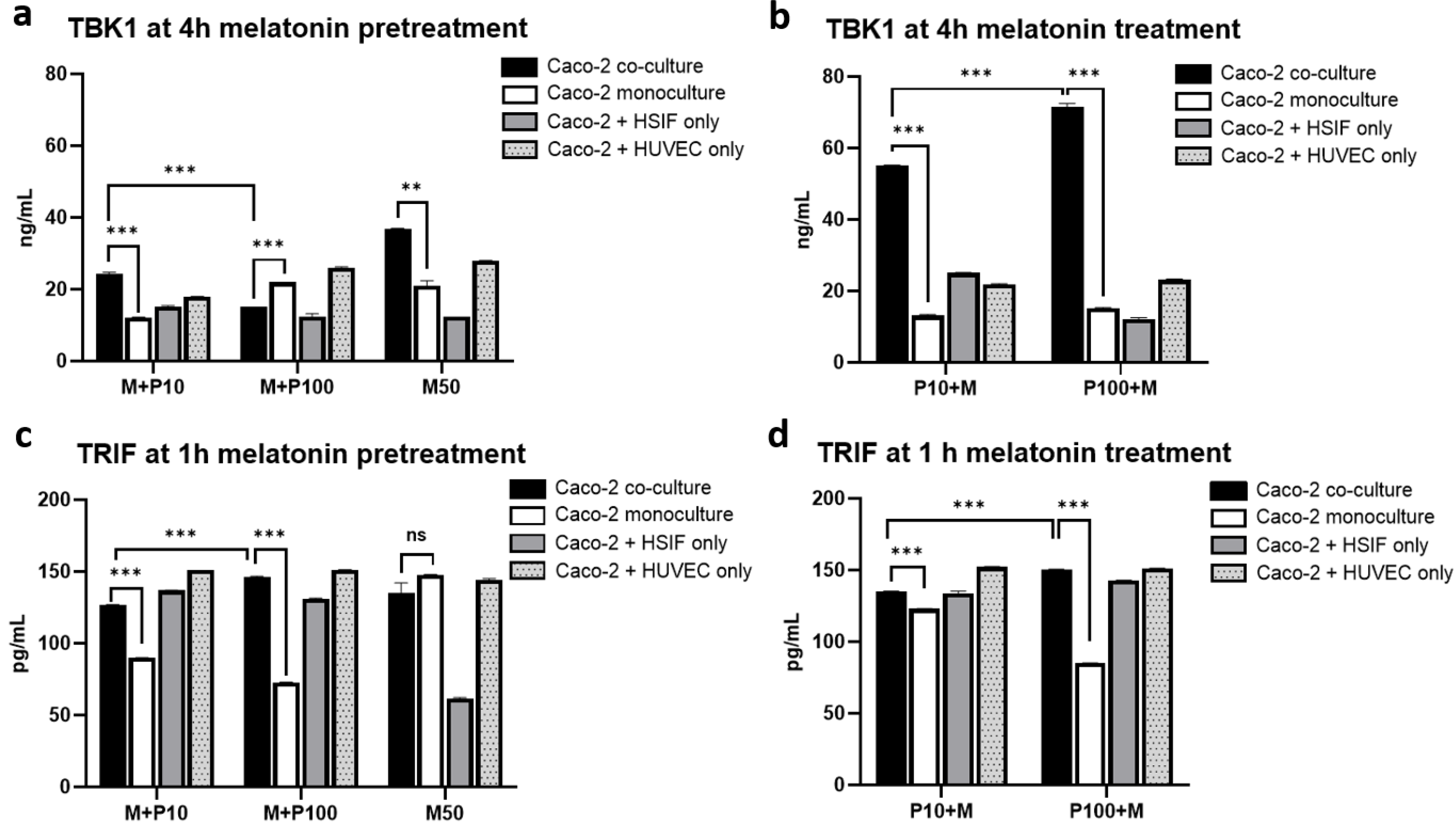
3.13. Melatonin Enhances the TBK1 Expression Specifically in Co-Cultures Without Altering TRIF Levels
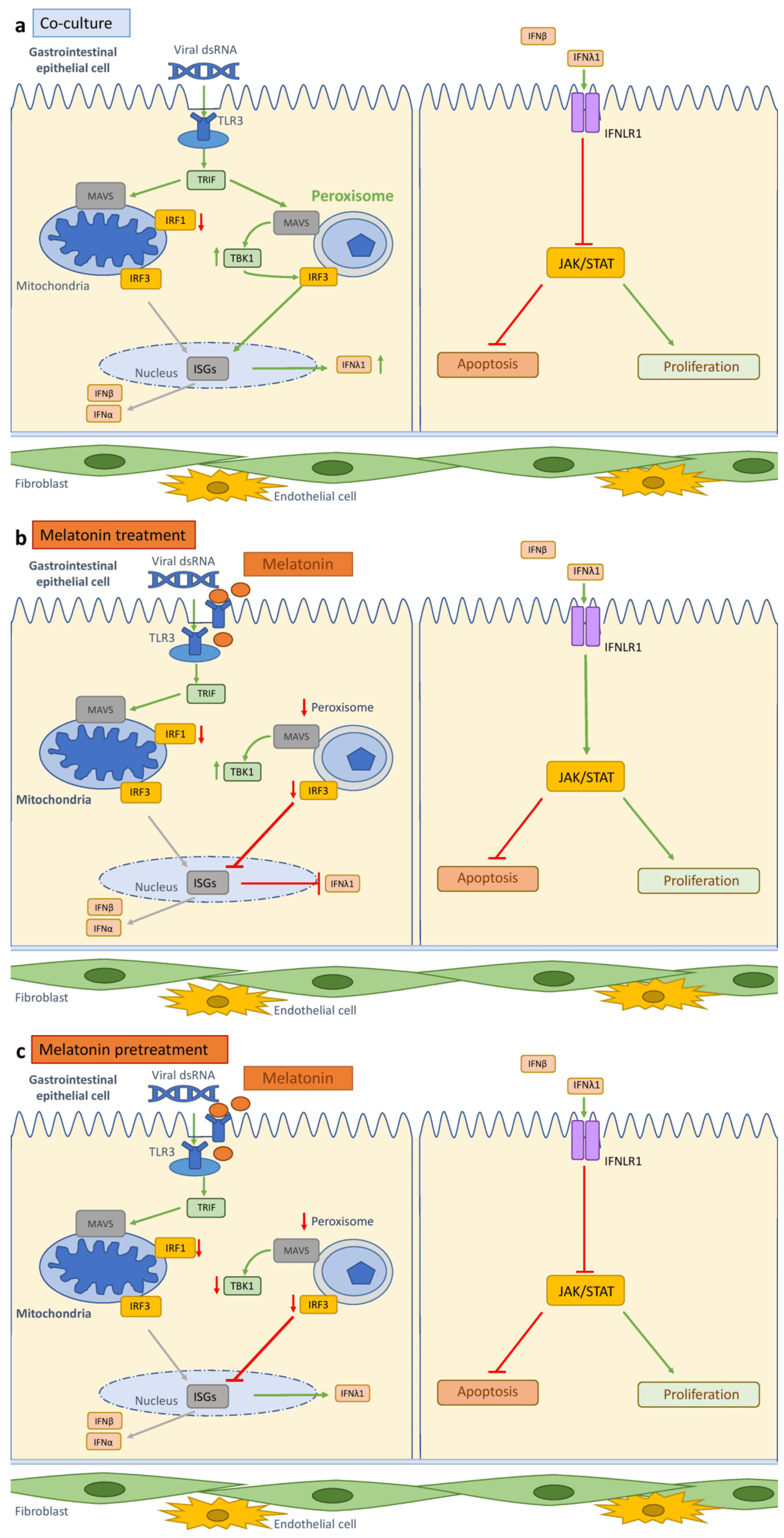
3.14. Melatonin Modulates Organelle Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Caco-2 | Human Epithelial Colorectal Adenocarcinoma Cell Line |
| CCK-8 | Cell Counting Kit-8 |
| CLD | Compact Letter Display |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FBS | Fetal Bovine Serum |
| GI | Gastrointestinal Tract |
| HSIF | Human Small Intestinal Fibroblast |
| HUVEC | Human Umbilical Vein Endothelial Cell |
| IFN | Interferon |
| IFNα | Interferon Alpha |
| IFNβ | Interferon Beta |
| IFNλ1 | Interferon Lambda 1 |
| IFNLR1 | Interferon Lambda Receptor 1 |
| IRF1 | Interferon Regulatory Factor 1 |
| IRF3 | Interferon Regulatory Factor 3 |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription |
| LSGS | Low Serum Growth Supplement |
| MEM | Minimum Essential Medium |
| MFI | Median Fluorescence Intensity |
| NEAA | Non-Essential Amino Acid |
| PBS | Phosphate-Buffered Saline |
| PFA | Paraformaldehyde |
| PI | Propidium Iodide |
| Poly I:C | Polyinosinic–Polycytidylic Acid |
| P/S | Penicillin/Streptomycin |
| ROI | Region of Interest |
| SMA | Smooth Muscle Actin |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TBK1 | TANK-Binding Kinase 1 |
| TLR3 | Toll-Like Receptor 3 |
| TRIF | TIR-Domain-Containing Adapter-Inducing Interferon-β |
References
- Neurath, M.F.; Artis, D.; Becker, C. The intestinal barrier: A pivotal role in health, inflammation, and cancer. Lancet Gastroenterol. Hepatol. 2025, 10, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Julio-Pieper, M.; López-Aguilera, A.; Eyzaguirre-Velásquez, J.; Olavarría-Ramírez, L.; Ibacache-Quiroga, C.; Bravo, J.A.; Cruz, G. Gut Susceptibility to Viral Invasion: Contributing Roles of Diet, Microbiota and Enteric Nervous System to Mucosal Barrier Preservation. Int. J. Mol. Sci. 2021, 22, 4734. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; Xue, B.; Deng, R.; Huang, X.; Xu, Y.; Chen, S.; Tian, R.; Wang, X.; Xun, Z.; et al. IRF1 Promotes the Innate Immune Response to Viral Infection by Enhancing the Activation of IRF3. J. Virol. 2020, 94, e01231-20. [Google Scholar] [CrossRef]
- Pervolaraki, K.; Talemi, S.R.; Albrecht, D.; Bormann, F.; Bamford, C.; Mendoza, J.L.; Garcia, K.C.; McLauchlan, J.; Höfer, T.; Stanifer, M.L.; et al. Differential induction of interferon stimulated genes between type I and type III interferons is independent of interferon receptor abundance. PLoS Pathog. 2018, 14, e1007420. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.K.; Chow, E.K.; Goodnough, J.B.; Yeh, W.C.; Cheng, G. Differential requirement for TANK-binding kinase-1 in type I interferon responses to toll-like receptor activation and viral infection. J. Exp. Med. 2004, 199, 1651–1658. [Google Scholar] [CrossRef]
- Darling, N.J.; Mobbs, C.L.; González-Hau, A.L.; Freer, M.; Przyborski, S. Bioengineering Novel in vitro Co-culture Models That Represent the Human Intestinal Mucosa With Improved Caco-2 Structure and Barrier Function. Front. Bioeng. Biotechnol. 2020, 8, 992. [Google Scholar] [CrossRef]
- Su, R.; Shereen, M.A.; Zeng, X.; Liang, Y.; Li, W.; Ruan, Z.; Li, Y.; Liu, W.; Liu, Y.; Wu, K.; et al. The TLR3/IRF1/Type III IFN Axis Facilitates Antiviral Responses against Enterovirus Infections in the Intestine. mBio 2020, 11, e02540-20. [Google Scholar] [CrossRef]
- Riemann, D.; Espie, C.A.; Altena, E.; Arnardottir, E.S.; Baglioni, C.; Bassetti, C.L.A.; Bastien, C.; Berzina, N.; Bjorvatn, B.; Dikeos, D.; et al. The European Insomnia Guideline: An update on the diagnosis and treatment of insomnia 2023. J. Sleep Res. 2023, 32, e14035. [Google Scholar] [CrossRef]
- Kitidee, K.; Samutpong, A.; Pakpian, N.; Wisitponchai, T.; Govitrapong, P.; Reiter, R.J.; Wongchitrat, P. Antiviral effect of melatonin on Japanese encephalitis virus infection involves inhibition of neuronal apoptosis and neuroinflammation in SH-SY5Y cells. Sci. Rep. 2023, 13, 1–17. [Google Scholar] [CrossRef]
- Lempesis, I.G.; Georgakopoulou, V.E.; Reiter, R.J.; Spandidos, D.A. A mid pandemic night’s dream: Melatonin, from harbinger of anti-inflammation to mitochondrial savior in acute and long COVID-19 (Review). Int. J. Mol. Med. 2024, 53, 1–13. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and inflammation—Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef]
- Šeškutė, M.; Žukaitė, D.; Laucaitytė, G.; Inčiūraitė, R.; Malinauskas, M.; Jankauskaitė, L. Antiviral Effect of Melatonin on Caco-2 Cell Organoid Culture: Trick or Treat? Int. J. Mol. Sci. 2024, 25, 11872. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A. Gastrointestinal melatonin: Localization, function, and clinical relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, S.; Son, M.; Cheon, J.H.; Park, Y.S. Melatonin controls microbiota in colitis by goblet cell differentiation and antimicrobial peptide production through Toll-like receptor 4 signalling. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bhushal, S.; Wolfsmüller, M.; Selvakumar, T.A.; Kemper, L.; Wirth, D.; Hornef, M.W.; Hauser, H.; Köster, M. Cell Polarization and Epigenetic Status Shape the Heterogeneous Response to Type III Interferons in Intestinal Epithelial Cells. Front. Immunol. 2017, 8, 671. [Google Scholar] [CrossRef]
- Kayama, H.; Takeda, K. Regulation of intestinal epithelial homeostasis by mesenchymal cells. Inflamm. Regen. 2024, 44, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Eenjes, E.; Grommisch, D.; Genander, M. Functional Characterization and Visualization of Esophageal Fibroblasts Using Organoid Co-Cultures. J. Vis. Exp. 2023, 191, e64905. [Google Scholar] [CrossRef] [PubMed]
- Hemann, E.A.; Gale, M., Jr.; Savan, R. Interferon Lambda Genetics and Biology in Regulation of Viral Control. Front. Immunol. 2017, 8, 1707. [Google Scholar] [CrossRef]
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef]
- Wright, A.P.; Nice, T.J. Role of type-I and type-III interferons in gastrointestinal homeostasis and pathogenesis. Curr. Opin. Immunol. 2024, 86, 102412. [Google Scholar] [CrossRef]
- Pott, J.; Stockinger, S. Type I and III Interferon in the Gut: Tight Balance between Host Protection and Immunopathology. Front. Immunol. 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Odendall, C.; Dixit, E.; Stavru, F.; Bierne, H.; Franz, K.M.; Durbin, A.F.; Boulant, S.; Gehrke, L.; Cossart, P.; Kagan, J.C. Diverse intracellular pathogens activate type III interferon expression from peroxisomes. Nat. Immunol. 2014, 15, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Forero, A.; Ozarkar, S.; Li, H.; Lee, C.H.; Hemann, E.A.; Nadjsombati, M.S.; Hendricks, M.R.; So, L.; Green, R.; Roy, C.N.; et al. Differential Activation of the Transcription Factor IRF1 Underlies the Distinct Immune Responses Elicited by Type I and Type III Interferons. Immunity 2019, 51, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Dixit, E.; Boulant, S.; Zhang, Y.; Lee, A.S.; Odendall, C.; Shum, B.; Hacohen, N.; Chen, Z.J.; Whelan, S.P.; Fransen, M.; et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell 2010, 141, 668–681. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Marques, M.; Ramos, B.; Kagan, J.C.; Ribeiro, D. Emerging roles of peroxisomes in viral infections. Trends Cell Biol. 2022, 32, 124–139. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Maestroni, G.J. The immunotherapeutic potential of melatonin. Expert Opin. Investig. Drugs 2001, 10, 467–476. [Google Scholar] [CrossRef]
- Reiter, R.J.; Ma, Q.; Sharma, R. Melatonin in Mitochondria: Mitigating Clear and Present Dangers. Physiology (Bethesda) 2020, 35, 86–95. [Google Scholar] [CrossRef]
- Lei, X.; Xu, Z.; Huang, L.; Huang, Y.; Tu, S.; Xu, L.; Liu, D. The potential influence of melatonin on mitochondrial quality control: A review. Front. Pharmacol. 2024, 14, 1332567. [Google Scholar] [CrossRef]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Pal, P.K.; Sarkar, S.; Chattopadhyay, A.; Tan, D.X.; Bandyopadhyay, D. Enterochromaffin cells as the source of melatonin: Key findings and functional relevance in mammals. Melatonin Res. 2019, 2, 61–82. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Bahammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin antioxidative defense: Therapeutical implications for aging and neurodegenerative processes. Neurotox. Res. 2013, 23, 267–300. [Google Scholar] [CrossRef] [PubMed]
- Juybari, K.B.; Pourhanifeh, M.H.; Hosseinzadeh, A.; Hemati, K.; Mehrzadi, S. Melatonin potentials against viral infections including COVID-19: Current evidence and new findings. Virus Res. 2020, 287, 198108. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, G.J.M. Role of Melatonin in Viral, Bacterial and Parasitic Infections. Biomolecules 2024, 14, 356. [Google Scholar] [CrossRef]
- Andersen, L.P.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef]



| Cells | Medium Composition |
|---|---|
| Caco-2 | MEM+ 10% FBS (Gibco, Life Technologies NZ Ltd., Auckland, New Zealand) + 1% NEAAs (Gibco, Life Technologies Limited, Paisley, UK) + 1% P/S (Gibco, Life Technologies Limited, Paisley, UK) |
| HSIF | DMEM (Gibco, Life Technologies Limited, Paisley, UK) + 10% FBS + 1% P/S |
| HUVEC | HLVEC Basal Medium (Gibco, Life Technologies Corp., Grand Island, NY, USA) + 10% FBS + 1% P/S + LSGS (Gibco; Cascade Biologics; Life Technologies Corp., Grand Island, NY, USA) |
| Analyte and Manufacturer | Detection Limit |
|---|---|
| IFNλ1 (Abcam, Cambridge, UK) | 13.72 ng/mL |
| IFNβ (Abcam, Cambridge, UK) | 9.38 pg/mL |
| IFNα (Invitrogen, Bender MedSystems GmbH, Vienna, Austria) | 7.8 pg/mL |
| phospho-STAT3 (Tyr705; Invitrogen, Thermo Fisher Scientific, Life Technologies Corp., Carlsbad, CA, USA) | Semi-quantitative |
| IRF1 (ELK Biotechnology, Denver, USA) | 0.32 ng/mL |
| IRF3 (ELK Biotechnology, Denver, USA) | 0.16 ng/mL |
| TRIF (ELK Biotechnology, Denver, USA) | 78.13 pg/mL |
| TBK1 (ELK Biotechnology, Denver, USA) | 0.32 ng/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šeškutė, M.; Laucaitytė, G.; Inčiūraitė, R.; Malinauskas, M.; Jankauskaitė, L. Enhancing Antiviral Immunity in the Gastrointestinal Epithelium: The Role of Fibroblast–Endothelium Interaction and Melatonin. Cells 2025, 14, 990. https://doi.org/10.3390/cells14130990
Šeškutė M, Laucaitytė G, Inčiūraitė R, Malinauskas M, Jankauskaitė L. Enhancing Antiviral Immunity in the Gastrointestinal Epithelium: The Role of Fibroblast–Endothelium Interaction and Melatonin. Cells. 2025; 14(13):990. https://doi.org/10.3390/cells14130990
Chicago/Turabian StyleŠeškutė, Milda, Goda Laucaitytė, Rūta Inčiūraitė, Mantas Malinauskas, and Lina Jankauskaitė. 2025. "Enhancing Antiviral Immunity in the Gastrointestinal Epithelium: The Role of Fibroblast–Endothelium Interaction and Melatonin" Cells 14, no. 13: 990. https://doi.org/10.3390/cells14130990
APA StyleŠeškutė, M., Laucaitytė, G., Inčiūraitė, R., Malinauskas, M., & Jankauskaitė, L. (2025). Enhancing Antiviral Immunity in the Gastrointestinal Epithelium: The Role of Fibroblast–Endothelium Interaction and Melatonin. Cells, 14(13), 990. https://doi.org/10.3390/cells14130990





