Acute Postural Effects of Spinal Cord Injury: Dual Neural Opioid and Endocrine Non-Opioid Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Lateral Hemisection of the Spinal Cord
2.3. Spinal Cord Transection
2.4. Histological Analysis of SCI
2.5. Analysis of Hindlimb Postural Asymmetry (HL-PA) by the Hands-On and Hands-Off Methods
2.6. Statistical Analysis
2.7. Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
3. Results
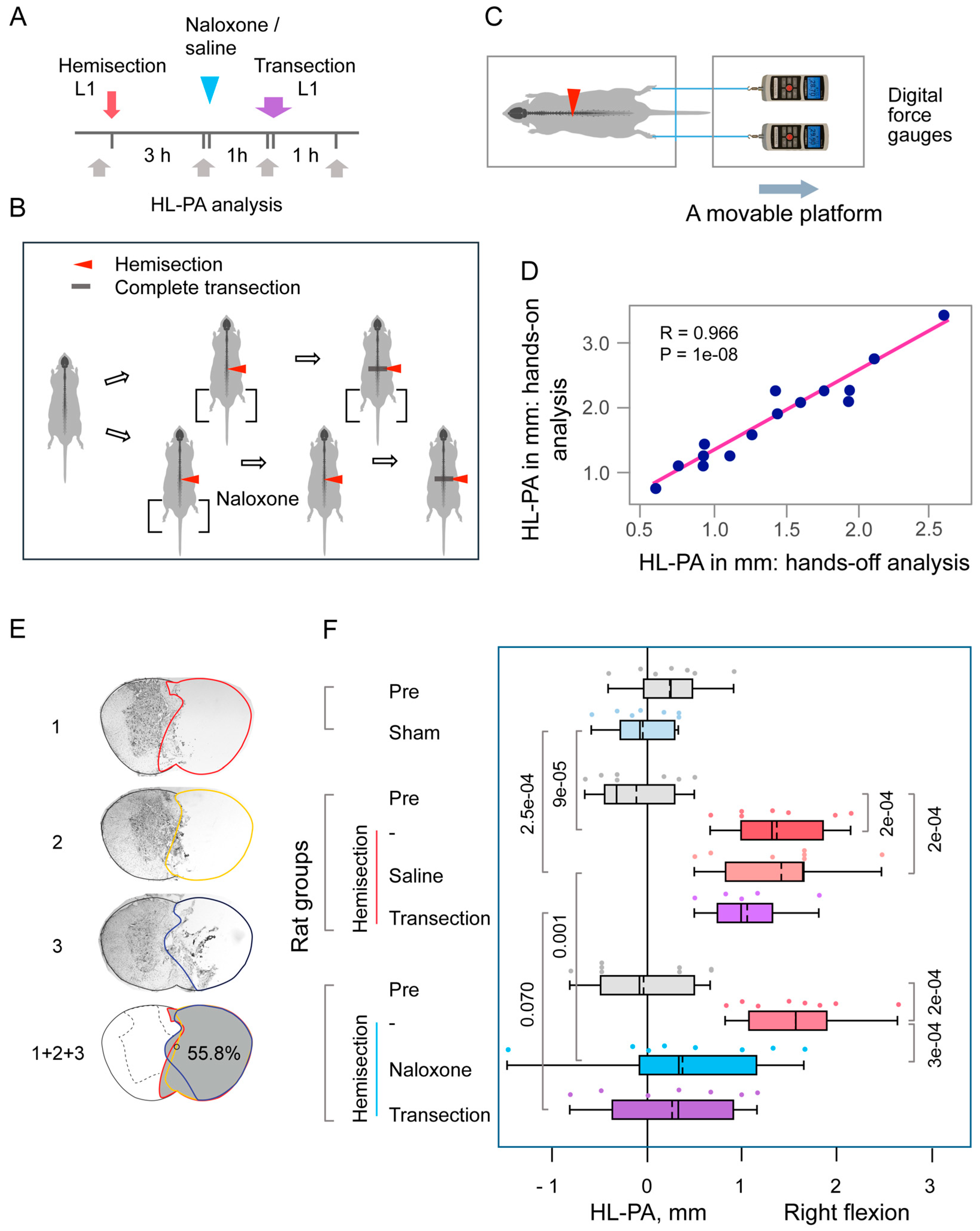
3.1. The Lumbar LHS-Induced HL-PA
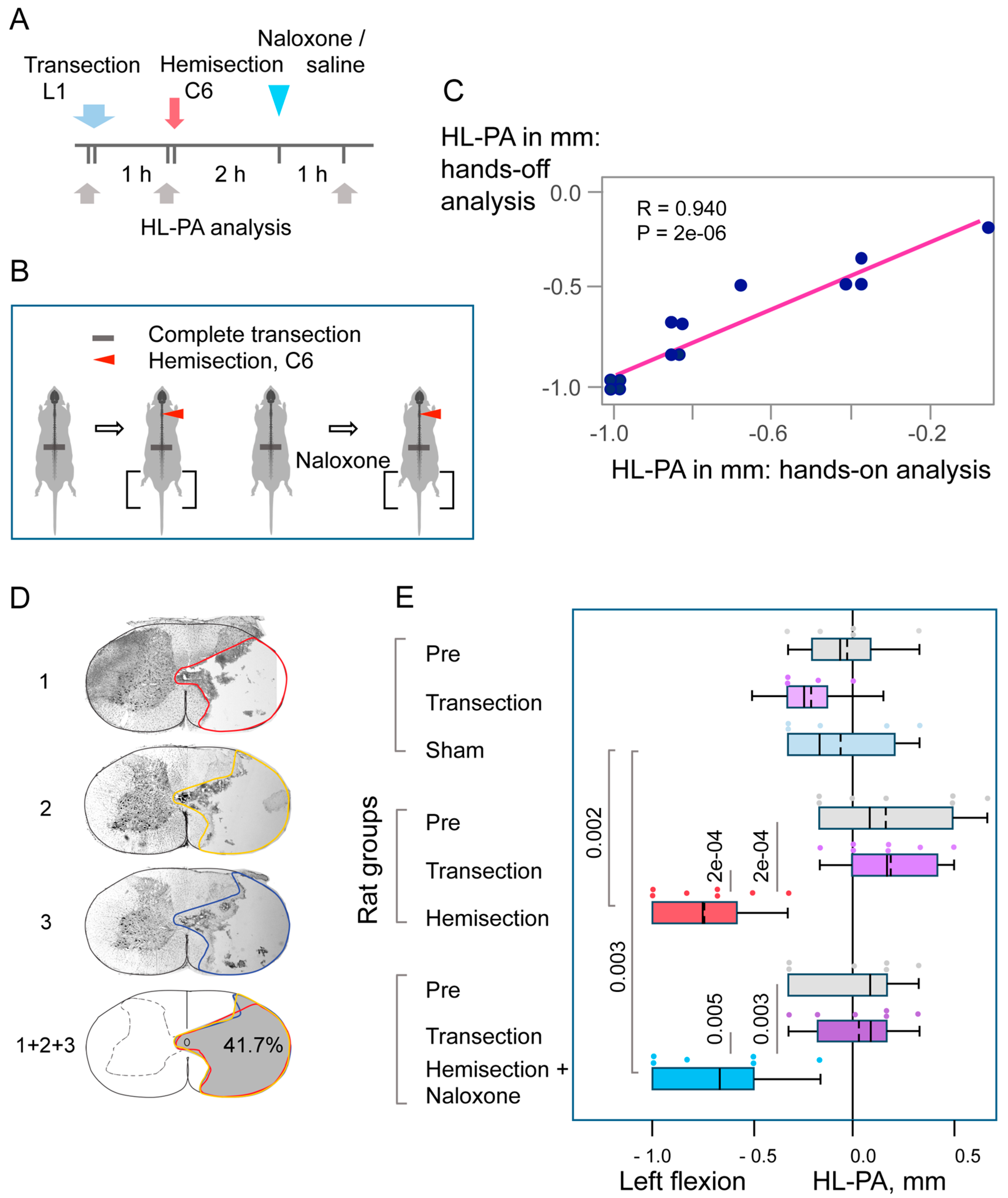
3.2. The Cervical LHS-Induced HL-PA in Rats with Complete Transection of Lumbar Spinal Cords
4. Discussion
4.1. LHS-Induced HL-PA
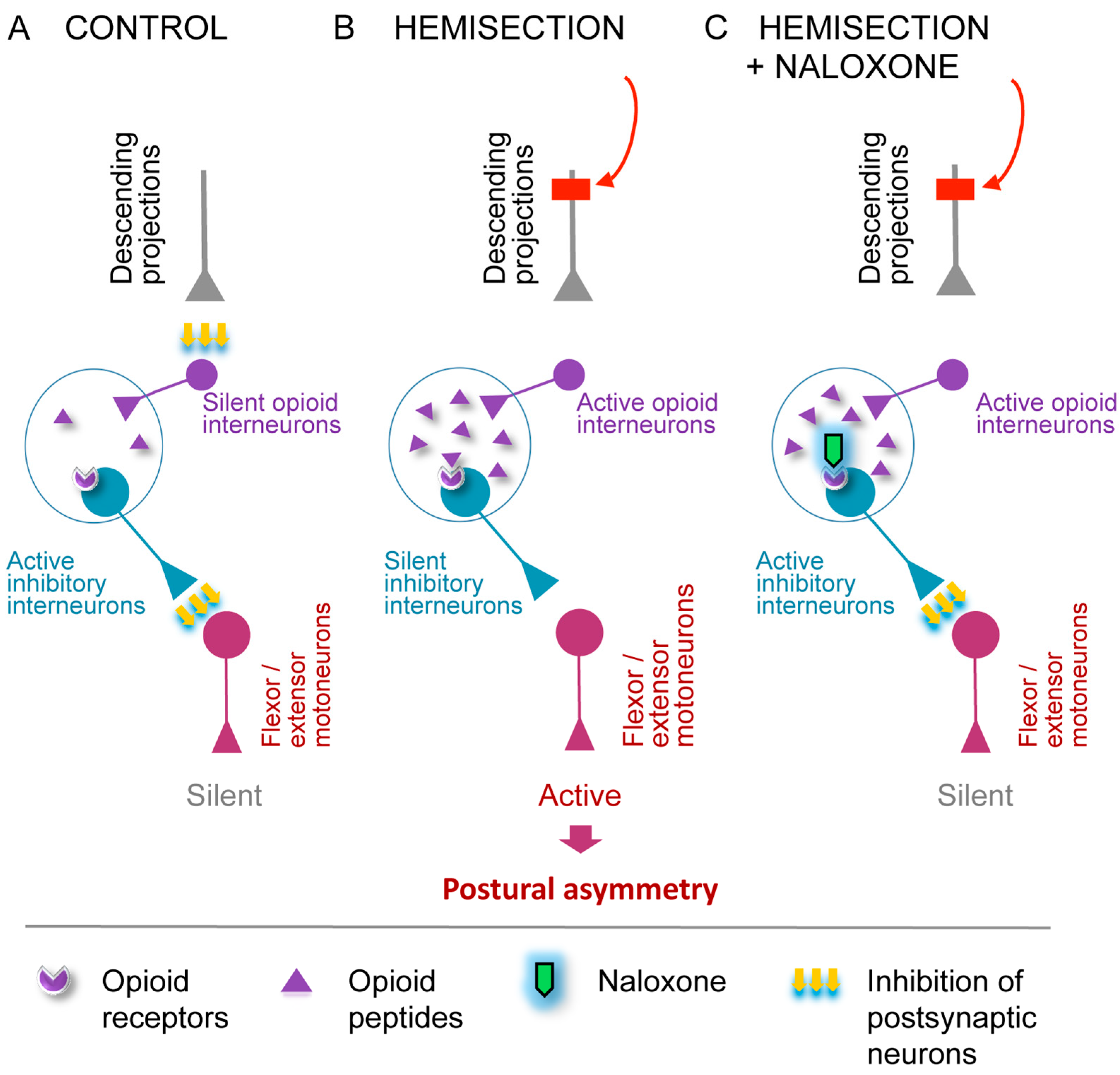
4.2. The Opioid HL-PA Mechanism
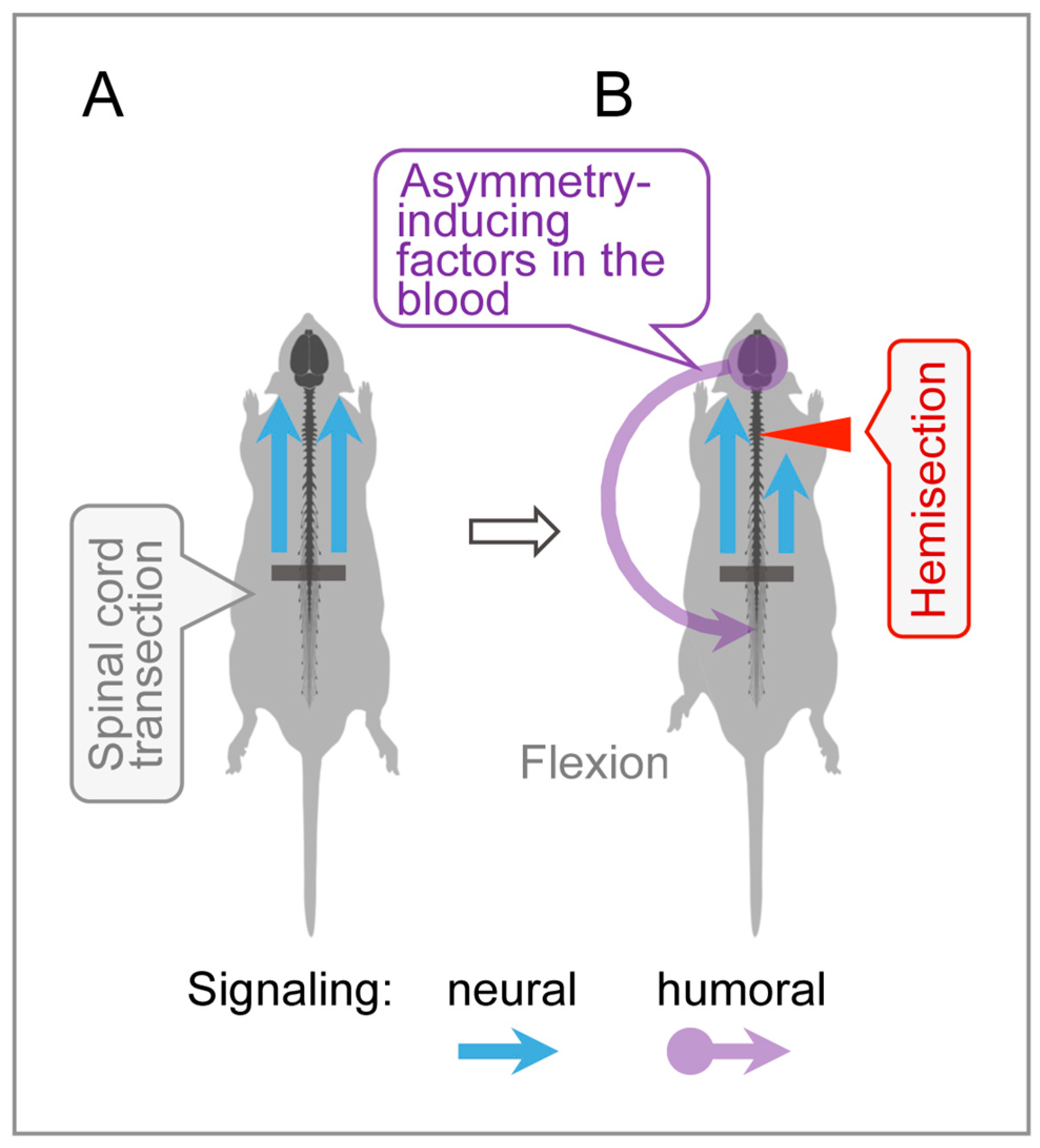
4.3. Humoral Signaling in HL-PA Formation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HL-PA | Hindlimb postural asymmetry |
| LHS | Lateral hemisection of the spinal cord |
| SCI | Spinal cord injury |
References
- Wirz, M.; Zörner, B.; Rupp, R.; Dietz, V. Outcome after Incomplete Spinal Cord Injury: Central Cord versus Brown-Sequard Syndrome. Spinal Cord 2010, 48, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, A.; Arber, S. Functional Local Proprioceptive Feedback Circuits Initiate and Maintain Locomotor Recovery after Spinal Cord Injury. Cell Rep. 2019, 27, 71–85.e3. [Google Scholar] [CrossRef]
- Zörner, B.; Bachmann, L.C.; Filli, L.; Kapitza, S.; Gullo, M.; Bolliger, M.; Starkey, M.L.; Röthlisberger, M.; Gonzenbach, R.R.; Schwab, M.E. Chasing Central Nervous System Plasticity: The Brainstem’s Contribution to Locomotor Recovery in Rats with Spinal Cord Injury. Brain 2014, 137, 1716–1732. [Google Scholar] [CrossRef] [PubMed]
- Aminoff, M.J. The Life and Legacy of Brown-Séquard. Brain 2017, 140, 1525–1532. [Google Scholar] [CrossRef]
- Han, Q.; Ordaz, J.D.; Liu, N.-K.; Richardson, Z.; Wu, W.; Xia, Y.; Qu, W.; Wang, Y.; Dai, H.; Zhang, Y.P.; et al. Descending Motor Circuitry Required for NT-3 Mediated Locomotor Recovery after Spinal Cord Injury in Mice. Nat. Commun. 2019, 10, 5815. [Google Scholar] [CrossRef]
- Hutson, T.H.; Di Giovanni, S. The Translational Landscape in Spinal Cord Injury: Focus on Neuroplasticity and Regeneration. Nat. Rev. Neurol. 2019, 15, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Asboth, L.; Friedli, L.; Beauparlant, J.; Martinez-Gonzalez, C.; Anil, S.; Rey, E.; Baud, L.; Pidpruzhnykova, G.; Anderson, M.A.; Shkorbatova, P.; et al. Cortico–Reticulo–Spinal Circuit Reorganization Enables Functional Recovery after Severe Spinal Cord Contusion. Nat. Neurosci. 2018, 21, 576–588. [Google Scholar] [CrossRef]
- Han, Q.; Xie, Y.; Ordaz, J.D.; Huh, A.J.; Huang, N.; Wu, W.; Liu, N.; Chamberlain, K.A.; Sheng, Z.-H.; Xu, X.-M. Restoring Cellular Energetics Promotes Axonal Regeneration and Functional Recovery after Spinal Cord Injury. Cell Metab. 2020, 31, 623–641.e8. [Google Scholar] [CrossRef]
- Wang, D.; Tawfik, V.L.; Corder, G.; Low, S.A.; François, A.; Basbaum, A.I.; Scherrer, G. Functional Divergence of Delta and Mu Opioid Receptor Organization in CNS Pain Circuits. Neuron 2018, 98, 90–108.e5. [Google Scholar] [CrossRef]
- Clarke, R.W.; Galloway, F.J.; Harris, J.; Taylor, J.S.; Ford, T.W. Opioidergic Inhibition of Flexor and Extensor Reflexes in the Rabbit. J. Physiol. 1992, 449, 493–501. [Google Scholar] [CrossRef]
- Steffens, H.; Schomburg, E.D. Spinal Motor Actions of the μ-Opioid Receptor Agonist DAMGO in the Cat. Neurosci. Res. 2011, 70, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Faber, E.S.L.; Chambers, J.P.; Brugger, F.; Evans, R.H. Depression of A and C Fibre-evoked Segmental Reflexes by Morphine and Clonidine in the in Vitro Spinal Cord of the Neonatal Rat. Br. J. Pharmacol. 1997, 120, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.; Schomburg, E.D. A Leu-enkephalin Depresses Transmission from Muscle and Skin Non-nociceptors to First-order Feline Spinal Neurones. J. Physiol. 1998, 510, 513–525. [Google Scholar] [CrossRef]
- Terminel, M.N.; Bassil, C.; Rau, J.; Trevino, A.; Ruiz, C.; Alaniz, R.; Hook, M.A. Morphine-Induced Changes in the Function of Microglia and Macrophages after Acute Spinal Cord Injury. BMC Neurosci. 2022, 23, 58. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.; Hemphill, A.; Araguz, K.; Cunningham, R.; Stefanov, A.; Weise, L.; Hook, M.A. Adverse Effects of Repeated, Intravenous Morphine on Recovery after Spinal Cord Injury in Young, Male Rats Are Blocked by a Kappa Opioid Receptor Antagonist. J. Neurotrauma 2022, 39, 1741–1755. [Google Scholar] [CrossRef]
- Yue, W.W.S.; Touhara, K.K.; Toma, K.; Duan, X.; Julius, D. Endogenous Opioid Signalling Regulates Spinal Ependymal Cell Proliferation. Nature 2024, 634, 407–414. [Google Scholar] [CrossRef]
- Lukoyanov, N.; Watanabe, H.; Carvalho, L.S.; Kononenko, O.; Sarkisyan, D.; Zhang, M.; Andersen, M.S.; Lukoyanova, E.A.; Galatenko, V.; Tonevitsky, A.; et al. Left-Right Side-Specific Endocrine Signaling Complements Neural Pathways to Mediate Acute Asymmetric Effects of Brain Injury. eLife 2021, 10, e65247. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Nosova, O.; Sarkisyan, D.; Andersen, M.S.; Zhang, M.; Rorick-Kehn, L.; Clausen, F.; Gawel, K.; Kehr, J.; Hallberg, M.; et al. Ipsilesional versus Contralesional Postural Deficits Induced by Unilateral Brain Trauma: A Side Reversal by Opioid Mechanism. Brain Commun. 2020, 2, fcaa208. [Google Scholar] [CrossRef]
- Watanabe, H.; Nosova, O.; Sarkisyan, D.; Andersen, M.S.; Carvalho, L.; Galatenko, V.; Bazov, I.; Lukoyanov, N.; Maia, G.H.; Hallberg, M.; et al. Left-Right Side-Specific Neuropeptide Mechanism Mediates Contralateral Responses to a Unilateral Brain Injury. eNeuro 2021, 8, ENEURO.0548-20.2021. [Google Scholar] [CrossRef]
- Watanabe, H.; Kobikov, Y.; Nosova, O.; Sarkisyan, D.; Galatenko, V.; Carvalho, L.; Maia, G.H.; Lukoyanov, N.; Lavrov, I.; Ossipov, M.H.; et al. The Left-Right Side-Specific Neuroendocrine Signaling from Injured Brain: An Organizational Principle. Function 2024, 5, zqae013. [Google Scholar] [CrossRef]
- Lin, X.-J.; Wen, S.; Deng, L.-X.; Dai, H.; Du, X.; Chen, C.; Walker, M.J.; Zhao, T.-B.; Xu, X.-M. Spinal Cord Lateral Hemisection and Asymmetric Behavioral Assessments in Adult Rats. JoVE 2020, 157, 57126. [Google Scholar] [CrossRef]
- Webb, A.A.; Muir, G.D. Compensatory Locomotor Adjustments of Rats with Cervical or Thoracic Spinal Cord Hemisections. J. Neurotrauma 2002, 19, 239–256. [Google Scholar] [CrossRef]
- Hultborn, H.; Malmsten, J. Changes in Segmental Reflexes Following Chronic Spinal Cord Hemisection in the Cat: I.Increased Monosynaptic and Polysynaptic Ventral Root Discharges. Acta Physiol. Scand. 1983, 119, 405–422. [Google Scholar] [CrossRef]
- Gossard, J.-P.; Delivet-Mongrain, H.; Martinez, M.; Kundu, A.; Escalona, M.; Rossignol, S. Plastic Changes in Lumbar Locomotor Networks after a Partial Spinal Cord Injury in Cats. J. Neurosci. 2015, 35, 9446–9455. [Google Scholar] [CrossRef]
- Miller, J.F.; Paul, K.D.; Lee, R.H.; Rymer, W.Z.; Heckman, C.J. Restoration of Extensor Excitability in the Acute Spinal Cat by the 5-HT2 Agonist DOI. J. Neurophysiol. 1996, 75, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Musienko, P.E.; Zelenin, P.V.; Orlovsky, G.N.; Deliagina, T.G. Facilitation of Postural Limb Reflexes with Epidural Stimulation in Spinal Rabbits. J. Neurophysiol. 2010, 103, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Frigon, A.; Johnson, M.D.; Heckman, C.J. Altered Activation Patterns by Triceps Surae Stretch Reflex Pathways in Acute and Chronic Spinal Cord Injury. J. Neurophysiol. 2011, 106, 1669–1678. [Google Scholar] [CrossRef]
- Zhou, H.H.; Jin, T.-T.; Qin, B.; Turndorf, H. Suppression of Spinal Cord Motoneuron Excitability Correlates with Surgical Immobility during Isoflurane Anesthesia. Anesthesiology 1998, 88, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Fuchigami, T.; Kakinohana, O.; Hefferan, M.P.; Lukacova, N.; Marsala, S.; Platoshyn, O.; Sugahara, K.; Yaksh, T.L.; Marsala, M. Potent Suppression of Stretch Reflex Activity after Systemic or Spinal Delivery of Tizanidine in Rats with Spinal Ischemia-Induced Chronic Spastic Paraplegia. Neuroscience 2011, 194, 160–169. [Google Scholar] [CrossRef]
- Jankowska, E. Interneuronal Relay in Spinal Pathways from Proprioceptors. Prog. Neurobiol. 1992, 38, 335–378. [Google Scholar] [CrossRef]
- Valero-Cabré, A.; Forés, J.; Navarro, X. Reorganization of Reflex Responses Mediated by Different Afferent Sensory Fibers After Spinal Cord Transection. J. Neurophysiol. 2004, 91, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Lavrov, I.; Gerasimenko, Y.; Burdick, J.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Integrating Multiple Sensory Systems to Modulate Neural Networks Controlling Posture. J. Neurophysiol. 2015, 114, 3306–3314. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J. Pathophysiology of Spastic Paresis. I: Paresis and Soft Tissue Changes. Muscle Nerve 2005, 31, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, J.; Pradines, M.; Gracies, J.-M.; Bo Nielsen, J. On Denny-Brown’s ‘Spastic Dystonia’—What Is It and What Causes It? Clin. Neurophysiol. 2018, 129, 89–94. [Google Scholar] [CrossRef]
- Marinelli, L.; Currà, A.; Trompetto, C.; Capello, E.; Serrati, C.; Fattapposta, F.; Pelosin, E.; Phadke, C.; Aymard, C.; Puce, L.; et al. Spasticity and Spastic Dystonia: The Two Faces of Velocity-Dependent Hypertonia. J. Electromyogr. Kinesiol. 2017, 37, 84–89. [Google Scholar] [CrossRef]
- Sheean, G.; McGuire, J.R. Spastic Hypertonia and Movement Disorders: Pathophysiology, Clinical Presentation, and Quantification. PMR 2009, 1, 827–833. [Google Scholar] [CrossRef]
- Baude, M.; Nielsen, J.B.; Gracies, J.-M. The Neurophysiology of Deforming Spastic Paresis: A Revised Taxonomy. Ann. Phys. Rehabil. Med. 2019, 62, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Denny-Brown, D. The Cerebral Control of Movement; Liverpool University Press: Liverpool, UK, 1966; pp. 210–217. [Google Scholar]
- Denny-Brown, D. Preface: Historical Aspects of the Relation of Spasticity to Movement. In Spasticity: Disordered Motor Control; Feldman, R.G., Young, R.R., Koella, W.P., Eds.; Yearbook Medical: Chicago, IL, USA, 1980; pp. 1–16. [Google Scholar]
- Pingel, J.; Bartels, E.M.; Nielsen, J.B. New Perspectives on the Development of Muscle Contractures Following Central Motor Lesions. J. Physiol. 2017, 595, 1027–1038. [Google Scholar] [CrossRef]
- Kononenko, O.; Galatenko, V.; Andersson, M.; Bazov, I.; Watanabe, H.; Zhou, X.W.; Iatsyshyna, A.; Mityakina, I.; Yakovleva, T.; Sarkisyan, D.; et al. Intra- and Interregional Coregulation of Opioid Genes: Broken Symmetry in Spinal Circuits. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 1953–1963. [Google Scholar] [CrossRef]
- Serafin, E.K.; Paranjpe, A.; Brewer, C.L.; Baccei, M.L. Single-Nucleus Characterization of Adult Mouse Spinal Dynorphin-Lineage Cells and Identification of Persistent Transcriptional Effects of Neonatal Hindpaw Incision. Pain 2021, 162, 203–218. [Google Scholar] [CrossRef]
- Schomburg, E.D. Spinal Sensorimotor Systems and Their Supraspinal Control. Neurosci. Res. 1990, 7, 265–340. [Google Scholar] [CrossRef]
- François, A.; Low, S.A.; Sypek, E.I.; Christensen, A.J.; Sotoudeh, C.; Beier, K.T.; Ramakrishnan, C.; Ritola, K.D.; Sharif-Naeini, R.; Deisseroth, K.; et al. A Brainstem-Spinal Cord Inhibitory Circuit for Mechanical Pain Modulation by GABA and Enkephalins. Neuron 2017, 93, 822–839.e6. [Google Scholar] [CrossRef] [PubMed]
- Baskin, D.S.; Hosobuchi, Y. Naloxone Reversal of Ischaemic Neurological Deficits in Man. Lancet 1981, 318, 272–275. [Google Scholar] [CrossRef]
- Hosobuchi, Y.; Baskin, D.S.; Woo, S.K. Reversal of Induced Ischemic Neurologic Deficit in Gerbils by the Opiate Antagonist Naloxone. Science 1982, 215, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Baskin, D.S.; Kieck, C.F.; Hosobuchi, Y. Naloxone Reversal and Morphine Exacerbation of Neurologic Deficits Secondary to Focal Cerebral Ischemia in Baboons. Brain Res. 1984, 290, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Jabaily, J.; Davis, J.N. Naloxone Administration to Patients with Acute Stroke. Stroke 1984, 15, 36–39. [Google Scholar] [CrossRef]
- Namba, S.; Nishigaki, S.; Fujiwara, N.; Wani, T.; Namba, Y.; Masaoka, T. Opiate-Antagonist Reversal of Neurological Deficits –Experimental and Clinical Studies–. Psychiatry Clin. Neurosci. 1986, 40, 61–79. [Google Scholar] [CrossRef]
- Skarphedinsson, J.O.; Delle, M.; Hoffman, P.; Thorén, P. The Effects of Naloxone on Cerebral Blood Flow and Cerebral Function during Relative Cerebral Ischemia. J. Cereb. Blood Flow Metab. 1989, 9, 515–522. [Google Scholar] [CrossRef]
- Hans, P.; Brichant, J.F.; Longerstay, E.; Damas, F.; Remacle, J.M. Reversal of Neurological Deficit with Naloxone: An Additional Report. Intensive Care Med. 1992, 18, 362–363. [Google Scholar] [CrossRef]
- Baskin, D.S.; Widmayer, M.A.; Browning, J.L.; Heizer, M.L.; Schmidt, W.K. Evaluation of Delayed Treatment of Focal Cerebral Ischemia with Three Selective Kappa-Opioid Agonists in Cats. Stroke 1994, 25, 2047–2053. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Z.; Wu, J.; Quan, W.; Xiao, W.; Chew, H.; Jiang, C.; Li, D. Naloxone Attenuates Ischemic Brain Injury in Rats through Suppressing the NIK/IKKα/NF-κB and Neuronal Apoptotic Pathways. Acta Pharmacol. Sin. 2019, 40, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Gironi, M.; Martinelli-Boneschi, F.; Sacerdote, P.; Solaro, C.; Zaffaroni, M.; Cavarretta, R.; Moiola, L.; Bucello, S.; Radaelli, M.; Pilato, V.; et al. A Pilot Trial of Low-Dose Naltrexone in Primary Progressive Multiple Sclerosis. Mult. Scler. 2008, 14, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Bakalkin, G.Y.; Kobylyansky, A.G.; Nagornaya, L.V.; Yarygin, K.N.; Titov, M.I. Met-Enkephalin-Induced Release into the Blood of a Factor Causing Postural Asymmetry. Peptides 1986, 7, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Brodal, A. Neurological Anatomy in Relation to Clinical Medicine, 3rd ed.; Oxford University Press: New York, NY, USA, 1981; Volume 10, p. 584. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; Lee, C.L. Memory Traces in Primate Spinal Cord Produced by Operant Conditioning of H-Reflex. J. Neurophysiol. 1989, 61, 563–572. [Google Scholar] [CrossRef]
- Lee, T.-K.; Lois, J.H.; Troupe, J.H.; Wilson, T.D.; Yates, B.J. Transneuronal Tracing of Neural Pathways That Regulate Hindlimb Muscle Blood Flow. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 292, R1532–R1541. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, H.; Lavrov, I.; Hallberg, M.; Schouenborg, J.; Zhang, M.; Bakalkin, G. Acute Postural Effects of Spinal Cord Injury: Dual Neural Opioid and Endocrine Non-Opioid Mechanism. Cells 2025, 14, 980. https://doi.org/10.3390/cells14130980
Watanabe H, Lavrov I, Hallberg M, Schouenborg J, Zhang M, Bakalkin G. Acute Postural Effects of Spinal Cord Injury: Dual Neural Opioid and Endocrine Non-Opioid Mechanism. Cells. 2025; 14(13):980. https://doi.org/10.3390/cells14130980
Chicago/Turabian StyleWatanabe, Hiroyuki, Igor Lavrov, Mathias Hallberg, Jens Schouenborg, Mengliang Zhang, and Georgy Bakalkin. 2025. "Acute Postural Effects of Spinal Cord Injury: Dual Neural Opioid and Endocrine Non-Opioid Mechanism" Cells 14, no. 13: 980. https://doi.org/10.3390/cells14130980
APA StyleWatanabe, H., Lavrov, I., Hallberg, M., Schouenborg, J., Zhang, M., & Bakalkin, G. (2025). Acute Postural Effects of Spinal Cord Injury: Dual Neural Opioid and Endocrine Non-Opioid Mechanism. Cells, 14(13), 980. https://doi.org/10.3390/cells14130980







