COP1 Deficiency in BRAFV600E Melanomas Confers Resistance to Inhibitors of the MAPK Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Western Blots
2.3. Immunoprecipitations
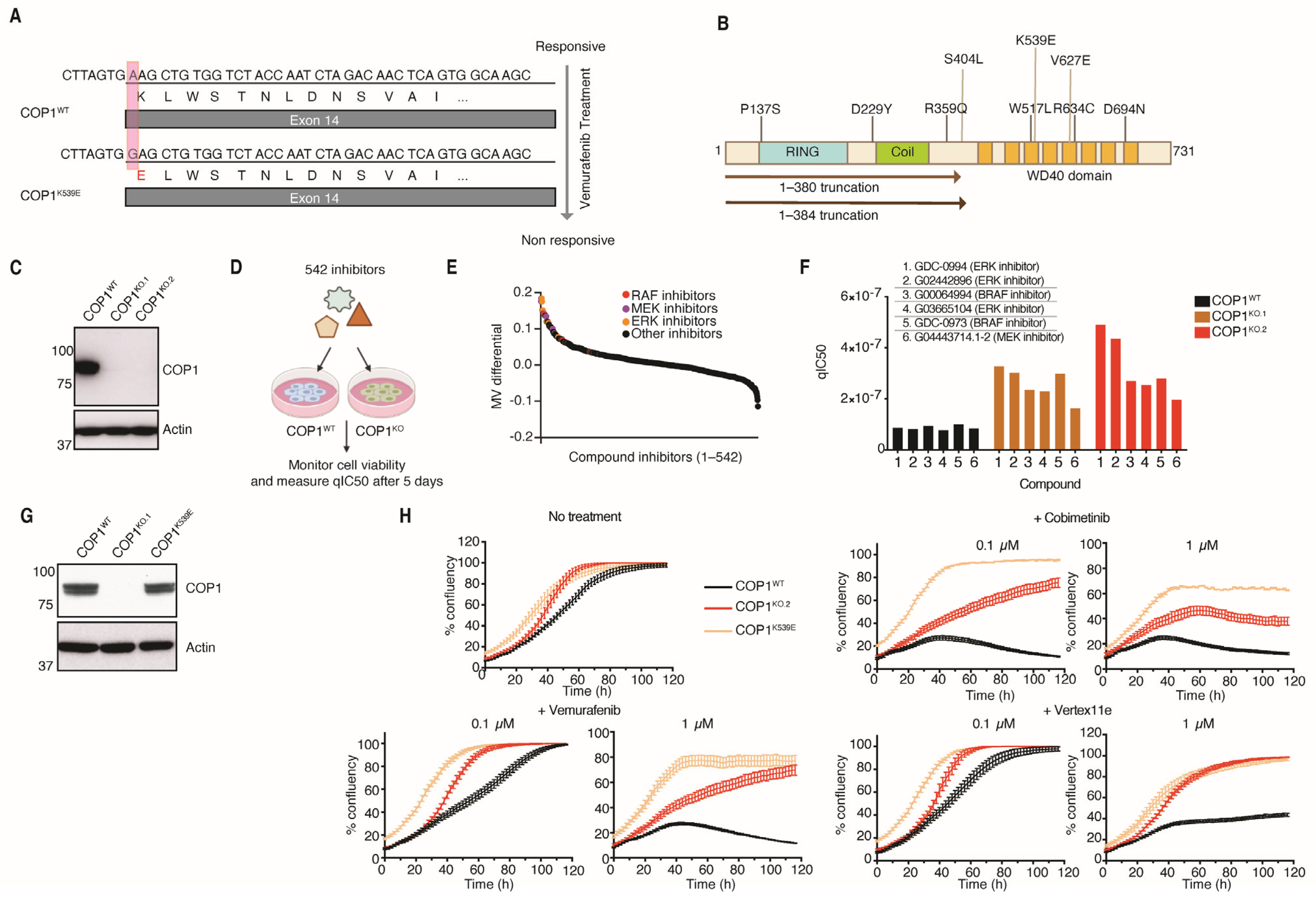
2.4. Chemical Genomic Screen
2.5. Cell Confluency Assays
2.6. Mass Spectrometry-Based Proteomics
2.7. Data Processing
2.8. RNA-Sequencing
2.9. RNA-Seq Differential Gene Expression Analysis
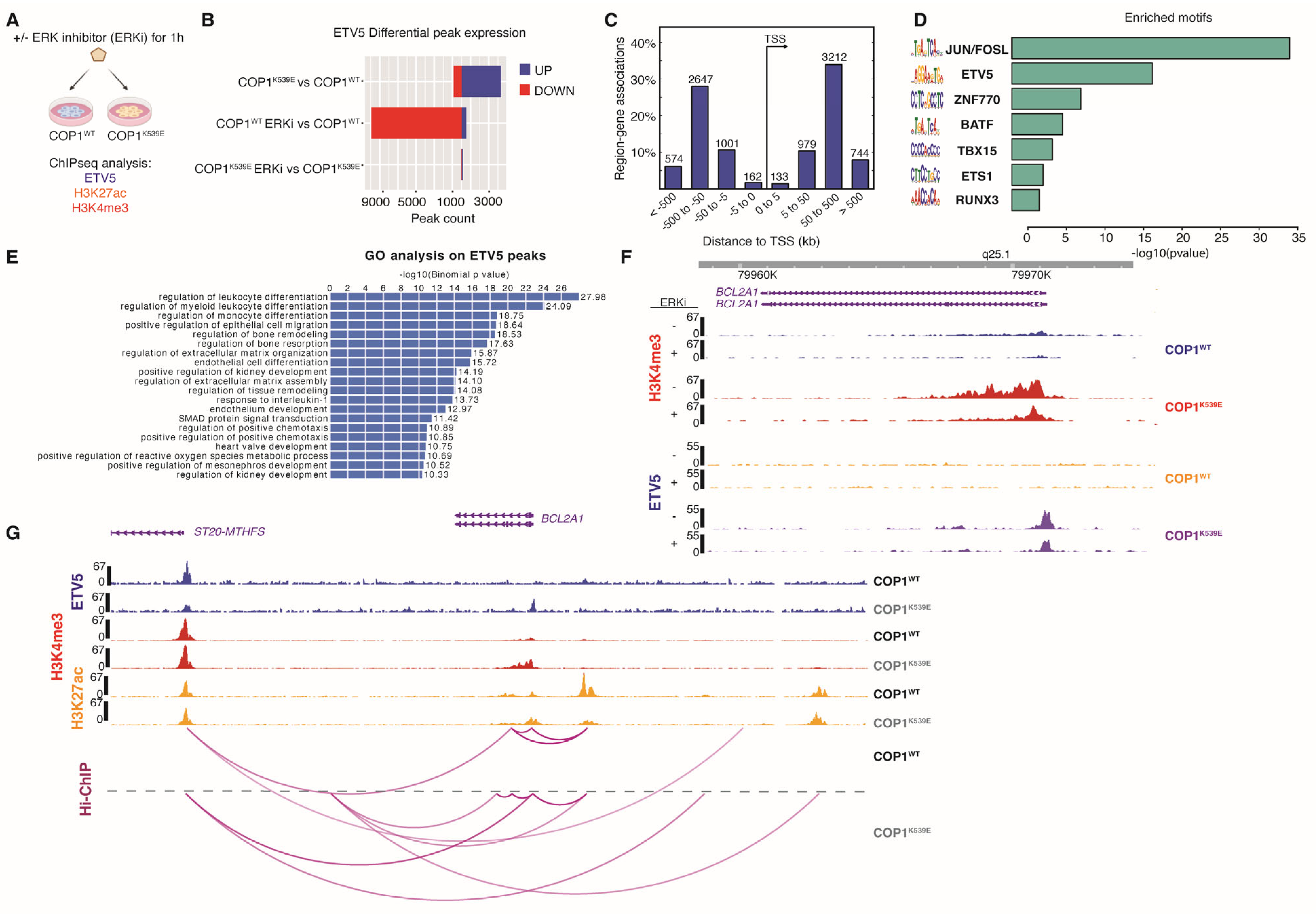
2.10. ChIP-Sequencing
2.11. Hi-ChIP
2.12. ChIP-Seq Differential Binding Analysis and Ontology Enrichment
3. Results and Discussion
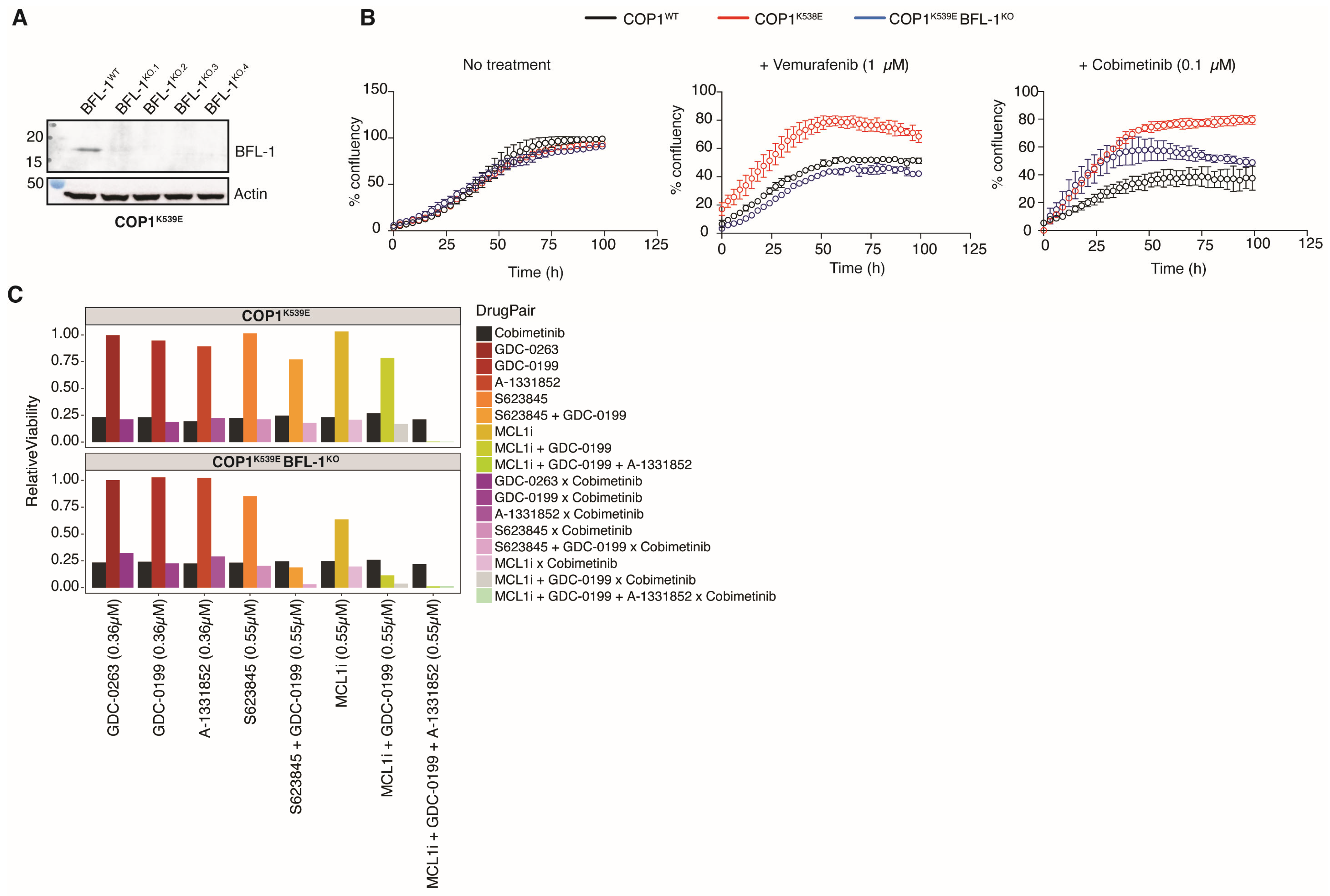
3.1. Deletion or Inactivation of COP1 Renders Melanoma Cells Less Sensitive to Inhibitors of the MAPK Pathway
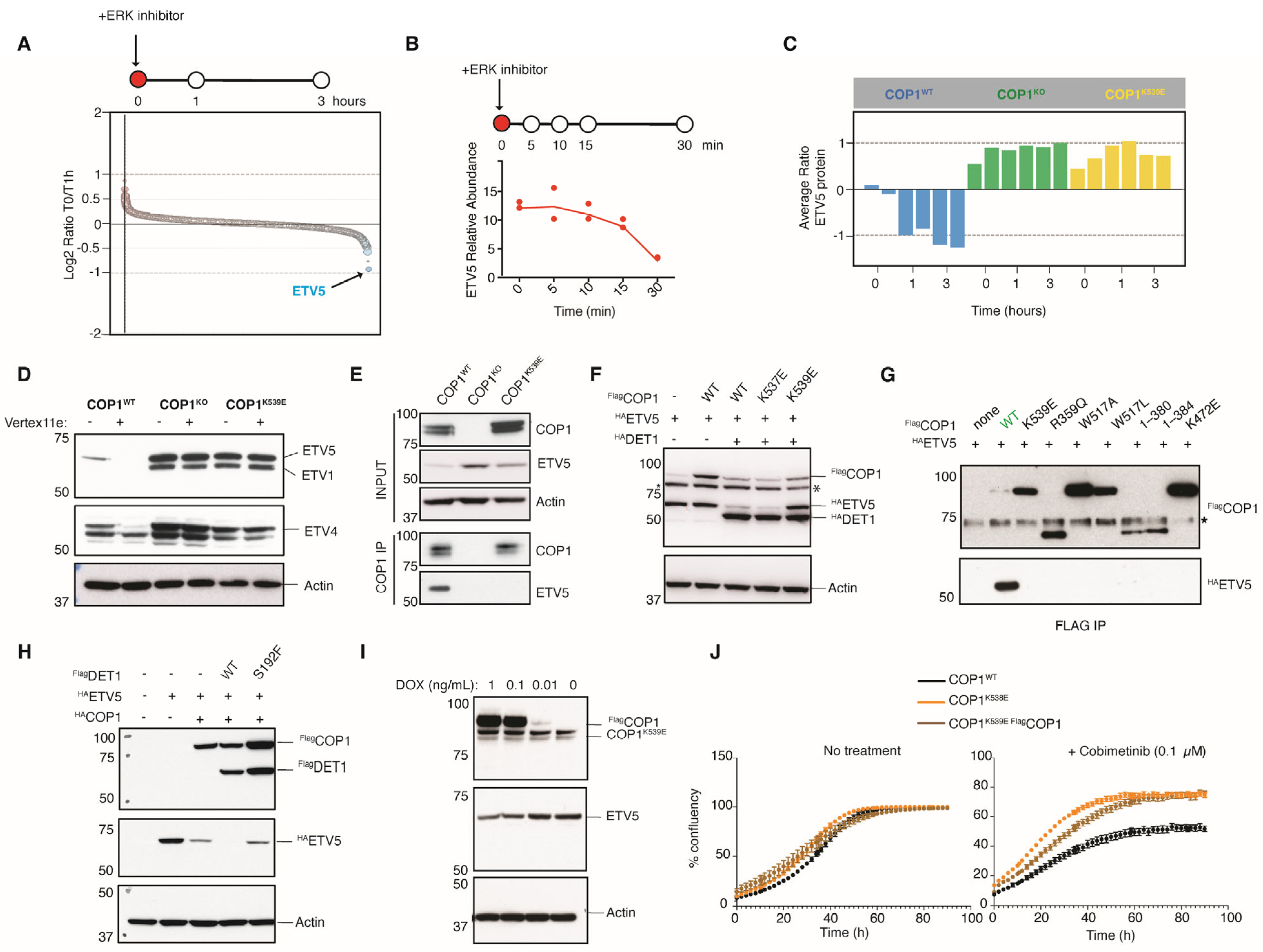
3.2. COP1 Substrates ETV1, ETV4, and ETV5 Are Rapidly Eliminated upon ERK Inhibition
3.3. COP1K539E Fails to Interact with the Transcription Factor ETV5
3.4. Mutation of DET1 in a Vemurafenib-Resistant Melanoma
3.5. ETV5 Protein Abundance Correlates with Resistance to MAPK Pathway Inhibition
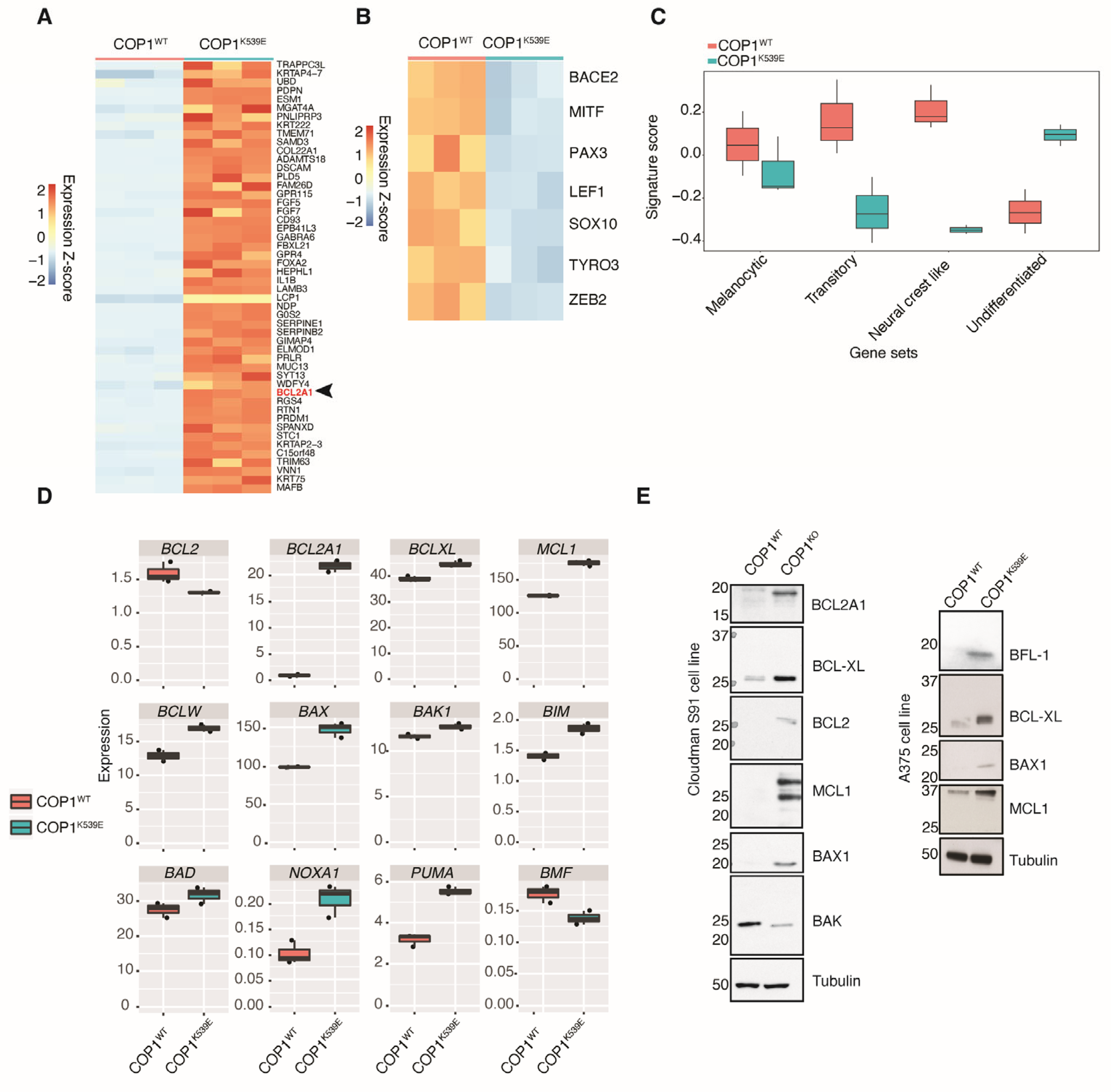
3.6. COP1K539E Elicits a Gene Signature in Melanoma Cells That Is Associated with Resistance to MAPK Pathway Inhibitors
3.7. COP1K539E Increases Expression of BCL2A1
3.8. ETV5-Driven Expression of BCL2A1 Confers Resistance to MAPK Pathway Inhibitors
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.A.; Whalen, D.M.; Özen, A.; Wongchenko, M.J.; Yin, J.; Yen, I.; Schaefer, G.; Mayfield, J.D.; Chmielecki, J.; Stephens, P.J.; et al. Activation Mechanism of Oncogenic Deletion Mutations in BRAF, EGFR, and HER2. Cancer Cell 2016, 29, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Ravnan, M.C.; Matalka, M.S. Vemurafenib in Patients with BRAF V600E Mutation-Positive Advanced Melanoma. Clin. Ther. 2012, 34, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Atkins, M.B. Treatment of BRAF-Mutant Melanoma: The Role of Vemurafenib and Other Therapies. Clin. Pharmacol. Ther. 2013, 95, 24–31. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Infante, J.R.; Daud, A.; Gonzalez, R.; Kefford, R.F.; Sosman, J.; Hamid, O.; Schuchter, L.; Cebon, J.; Ibrahim, N.; et al. Combined BRAF and MEK Inhibition in Melanoma with BRAF V600 Mutations. N. Engl. J. Med. 2012, 367, 1694–1703. [Google Scholar] [CrossRef]
- Marais, R.; Wynne, J.; Treisman, R. The SRF Accessory Protein Elk-1 Contains a Growth Factor-Regulated Transcriptional Activation Domain. Cell 1993, 73, 381–393. [Google Scholar] [CrossRef]
- Pratilas, C.A.; Taylor, B.S.; Ye, Q.; Viale, A.; Sander, C.; Solit, D.B.; Rosen, N. V600E BRAF Is Associated with Disabled Feedback Inhibition of RAF–MEK Signaling and Elevated Transcriptional Output of the Pathway. Proc. Natl. Acad. Sci. USA 2009, 106, 4519–4524. [Google Scholar] [CrossRef]
- Dry, J.R.; Pavey, S.; Pratilas, C.A.; Harbron, C.; Runswick, S.; Hodgson, D.; Chresta, C.; McCormack, R.; Byrne, N.; Cockerill, M.; et al. Transcriptional Pathway Signatures Predict MEK Addiction and Response to Selumetinib (AZD6244). Cancer Res. 2010, 70, 2264–2273. [Google Scholar] [CrossRef]
- Slack, C.; Alic, N.; Foley, A.; Cabecinha, M.; Hoddinott, M.P.; Partridge, L. The Ras-Erk-ETS-Signaling Pathway Is a Drug Target for Longevity. Cell 2015, 162, 72–83. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK Signalling Pathway and Tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Baek, K.; Scott, D.C.; Schulman, B.A. NEDD8 and Ubiquitin Ligation by Cullin-RING E3 Ligases. Curr. Opin. Struct. Biol. 2021, 67, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Petroski, M.D.; Deshaies, R.J. Function and Regulation of Cullin–RING Ubiquitin Ligases. Nat. Rev. Mol. Cell Biol. 2005, 6, 9–20. [Google Scholar] [CrossRef]
- Karayel, O.; Soung, A.; Gurung, H.; Schubert, A.F.; Klaeger, S.; Kschonsak, M.; Al-Maraghi, A.; Bhat, A.A.; Alshabeeb Akil, A.S.; Dugger, D.L.; et al. Impairment of DET1 Causes Neurological Defects and Lethality in Mice and Humans. Proc. Natl. Acad. Sci. USA 2025, 122, 9–20. [Google Scholar] [CrossRef]
- Burgess, A.E.; Loughran, T.A.; Turk, L.S.; Nyvall, H.G.; Dunlop, J.L.; Jamieson, S.A.; Curry, J.R.; Burke, J.E.; Filipcik, P.; Brown, S.H.J.; et al. DET1 Dynamics Underlie Cooperative Ubiquitination by CRL4 DET1-COP1 Complexes. Sci. Adv. 2025, 11, eadq4187. [Google Scholar] [CrossRef]
- Pick, E.; Lau, O.-S.; Tsuge, T.; Menon, S.; Tong, Y.; Dohmae, N.; Plafker, S.M.; Deng, X.W.; Wei, N. Mammalian DET1 Regulates Cul4A Activity and Forms Stable Complexes with E2 Ubiquitin-Conjugating Enzymes. Mol. Cell. Biol. 2007, 27, 4708–4719. [Google Scholar] [CrossRef] [PubMed]
- Wertz, I.E. Human De-Etiolated-1 Regulates C-Jun by Assembling a CUL4A Ubiquitin Ligase. Science 2004, 303, 1371–1374. [Google Scholar] [CrossRef]
- Deng, X.W.; Caspar, T.; Quail, P.H. Cop1: A Regulatory Locus Involved in Light-Controlled Development and Gene Expression in Arabidopsis. Genes Dev. 1991, 5, 1172–1182. [Google Scholar] [CrossRef]
- Marine, J.-C. Spotlight on the Role of COP1 in Tumorigenesis. Nat. Rev. Cancer 2012, 12, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Vitari, A.C.; Leong, K.G.; Newton, K.; Yee, C.; O’Rourke, K.; Liu, J.; Phu, L.; Vij, R.; Ferrando, R.; Couto, S.S.; et al. COP1 Is a Tumour Suppressor That Causes Degradation of ETS Transcription Factors. Nature 2011, 474, 403–406. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, Q.; Huang, Y.; Song, J.; Tomaino, R.; Ehrenberger, T.; Lim, E.; Liu, W.; Bronson, R.T.; Bowden, M.; et al. Phosphorylation of ETS1 by Src Family Kinases Prevents Its Recognition by the COP1 Tumor Suppressor. Cancer Cell 2014, 26, 222–234. [Google Scholar] [CrossRef]
- Keeshan, K.; Bailis, W.; Dedhia, P.H.; Vega, M.E.; Shestova, O.; Xu, L.; Toscano, K.; Uljon, S.N.; Blacklow, S.C.; Pear, W.S. Transformation by Tribbles Homolog 2 (Trib2) Requires Both the Trib2 Kinase Domain and COP1 Binding. Blood 2010, 116, 4948–4957. [Google Scholar] [CrossRef] [PubMed]
- Ndoja, A.; Reja, R.; Lee, S.; Webster, J.D.; Ngu, H.; Rose, C.J.; Kirkpatrick, D.S.; Modrusan, Z.; Chen, Y.-J.J.; Dugger, D.L.; et al. Ubiquitin Ligase COP1 Suppresses Neuroinflammation by Degrading C/EBPβ in Microglia. Cell 2020, 182, 1156–1169.e12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Newton, K.; Kummerfeld, S.K.; Webster, J.; Kirkpatrick, D.S.; Phu, L.; Eastham-Anderson, J.; Liu, J.; Lee, W.P.; Wu, J.; et al. Transcription Factor Etv5 Is Essential for the Maintenance of Alveolar Type II Cells. Proc. Natl. Acad. Sci. USA 2017, 114, 3903–3908. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.J.; Brennan, M.S.; O’Reilly, L.A.; Ottina, E.; Czabotar, P.E.; Whitlock, E.; Fairlie, W.D.; Tai, L.; Strasser, A.; Herold, M.J. Characterisation of a Novel A1-Specific Monoclonal Antibody. Cell Death Dis. 2014, 5, e1553. [Google Scholar] [CrossRef]
- Gangoda, L.; Teh, C.E.; Dengler, M.A.; Best, S.A.; Weeden, C.E.; Tai, L.; Lee, E.F.; Fairlie, W.D.; Sutherland, K.D.; Harrison, L.C.; et al. Characterization of a Novel Human BFL-1-Specific Monoclonal Antibody. Cell Death Differ. 2020, 27, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.; Rad, R.; Gygi, S.P.; Haas, W. MS3 Eliminates Ratio Distortion in Isobaric Multiplexed Quantitative Proteomics. Nat. Methods 2011, 8, 937–940. [Google Scholar] [CrossRef]
- McAlister, G.C.; Nusinow, D.P.; Jedrychowski, M.P.; Wühr, M.; Huttlin, E.L.; Erickson, B.K.; Rad, R.; Haas, W.; Gygi, S.P. MultiNotch MS3 Enables Accurate, Sensitive, and Multiplexed Detection of Differential Expression across Cancer Cell Line Proteomes. Anal. Chem. 2014, 86, 7150–7158. [Google Scholar] [CrossRef]
- Kirkpatrick, D.S.; Bustos, D.; Dogan, T.; Chan, J.; Phu, L.; Young, A.; Friedman, L.S.; Belvin, M.; Song, Q.; Bakalarski, C.E.; et al. Phosphoproteomic Characterization of DNA Damage Response in Melanoma Cells Following MEK/PI3K Dual Inhibition. Proc. Natl. Acad. Sci. USA 2013, 110, 19426–19431. [Google Scholar] [CrossRef]
- Zhuang, G.; Yu, K.; Jiang, Z.; Chung, A.; Yao, J.; Ha, C.; Toy, K.; Soriano, R.; Haley, B.; Blackwood, E.; et al. Phosphoproteomic Analysis Implicates the MTORC2-FoxO1 Axis in VEGF Signaling and Feedback Activation of Receptor Tyrosine Kinases. Sci. Signal. 2013, 6, ra25. [Google Scholar] [CrossRef]
- Wu, T.D.; Watanabe, C.K. GMAP: A Genomic Mapping and Alignment Program for MRNA and EST Sequences. Bioinformatics 2005, 21, 1859–1875. [Google Scholar] [CrossRef]
- Xie, Y.; Cao, Z.; Wong, E.W.; Guan, Y.; Ma, W.; Zhang, J.Q.; Walczak, E.G.; Murphy, D.; Ran, L.; Sirota, I.; et al. COP1/DET1/ETS Axis Regulates ERK Transcriptome and Sensitivity to MAPK Inhibitors. J. Clin. Investig. 2018, 128, 1442–1457. [Google Scholar] [CrossRef] [PubMed]
- Wongchenko, M.J.; McArthur, G.A.; Dréno, B.; Larkin, J.; Ascierto, P.A.; Sosman, J.; Andries, L.; Kockx, M.; Hurst, S.D.; Caro, I.; et al. Gene Expression Profiling in BRAF-Mutated Melanoma Reveals Patient Subgroups with Poor Outcomes to Vemurafenib That May Be Overcome by Cobimetinib plus Vemurafenib. Clin. Cancer Res. 2017, 23, 5238–5245. [Google Scholar] [CrossRef]
- Uljon, S.; Xu, X.; Durzynska, I.; Stein, S.; Adelmant, G.; Marto, J.A.; Pear, W.S.; Blacklow, S.C. Structural Basis for Substrate Selectivity of the E3 Ligase COP1. Structure 2016, 24, 687–696. [Google Scholar] [CrossRef]
- Kung, J.E.; Jura, N. The Pseudokinase TRIB 1 Toggles an Intramolecular Switch to Regulate COP 1 Nuclear Export. EMBO J. 2019, 38, e99708. [Google Scholar] [CrossRef] [PubMed]
- Rizos, H.; Menzies, A.M.; Pupo, G.M.; Carlino, M.S.; Fung, C.; Hyman, J.; Haydu, L.E.; Mijatov, B.; Becker, T.M.; Boyd, S.C.; et al. BRAF Inhibitor Resistance Mechanisms in Metastatic Melanoma: Spectrum and Clinical Impact. Clin. Cancer Res. 2014, 20, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, J.; Robert, L.; Paraiso, K.; Galvan, C.; Sheu, K.M.; Lay, J.; Wong, D.J.L.; Atefi, M.; Shirazi, R.; Wang, X.; et al. Multi-Stage Differentiation Defines Melanoma Subtypes with Differential Vulnerability to Drug-Induced Iron-Dependent Oxidative Stress. Cancer Cell 2018, 33, 890–904.e5. [Google Scholar] [CrossRef]
- Long, J.E.; Wongchenko, M.J.; Nickles, D.; Chung, W.-J.; Wang, B.; Riegler, J.; Li, J.; Li, Q.; Sandoval, W.; Eastham-Anderson, J.; et al. Therapeutic Resistance and Susceptibility Is Shaped by Cooperative Multi-Compartment Tumor Adaptation. Cell Death Differ. 2019, 26, 2416–2429. [Google Scholar] [CrossRef]
- Haq, R.; Yokoyama, S.; Hawryluk, E.B.; Jönsson, G.B.; Frederick, D.T.; McHenry, K.; Porter, D.; Tran, T.-N.; Love, K.T.; Langer, R.; et al. BCL2A1 Is a Lineage-Specific Antiapoptotic Melanoma Oncogene That Confers Resistance to BRAF Inhibition. Proc. Natl. Acad. Sci. USA 2013, 110, 4321–4326. [Google Scholar] [CrossRef]
- Gangoda, L.; Schenk, R.L.; Tai, L.; Szeto, P.; Cheung, J.G.; Strasser, A.; Lessene, G.; Shackleton, M.; Herold, M.J. Removal of BFL-1 Sensitises Some Melanoma Cells to Killing by BH3 Mimetic Drugs. Cell Death Dis. 2022, 13, 301. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndoja, A.; Rose, C.M.; Lin, E.; Reja, R.; Petrovic, J.; Kummerfeld, S.; Blair, A.; Rizos, H.; Modrusan, Z.; Martin, S.; et al. COP1 Deficiency in BRAFV600E Melanomas Confers Resistance to Inhibitors of the MAPK Pathway. Cells 2025, 14, 975. https://doi.org/10.3390/cells14130975
Ndoja A, Rose CM, Lin E, Reja R, Petrovic J, Kummerfeld S, Blair A, Rizos H, Modrusan Z, Martin S, et al. COP1 Deficiency in BRAFV600E Melanomas Confers Resistance to Inhibitors of the MAPK Pathway. Cells. 2025; 14(13):975. https://doi.org/10.3390/cells14130975
Chicago/Turabian StyleNdoja, Ada, Christopher M. Rose, Eva Lin, Rohit Reja, Jelena Petrovic, Sarah Kummerfeld, Andrew Blair, Helen Rizos, Zora Modrusan, Scott Martin, and et al. 2025. "COP1 Deficiency in BRAFV600E Melanomas Confers Resistance to Inhibitors of the MAPK Pathway" Cells 14, no. 13: 975. https://doi.org/10.3390/cells14130975
APA StyleNdoja, A., Rose, C. M., Lin, E., Reja, R., Petrovic, J., Kummerfeld, S., Blair, A., Rizos, H., Modrusan, Z., Martin, S., Kirkpatrick, D. S., Heidersbach, A., Sun, T., Haley, B., Karayel, O., Newton, K., & Dixit, V. M. (2025). COP1 Deficiency in BRAFV600E Melanomas Confers Resistance to Inhibitors of the MAPK Pathway. Cells, 14(13), 975. https://doi.org/10.3390/cells14130975







