Insulin Predicts Methotrexate Response by Affecting the Transcription of Methotrexate Target Genes in the Treatment-Naive Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Eligibility Criteria
2.3. Data Collection
2.4. Clinical Outcomes
2.5. Joint Assessment
2.6. RA Classification
2.7. Analysis of RA-Specific Autoantibodies
2.8. Inflammation Index
2.9. Serological Measures
2.10. Isolation and Stimulation of CD4+ Cells
2.11. Transcriptional Sequencing (RNA-Seq)
2.12. Transcriptome Analysis
2.13. Statistical Evaluation
2.14. Data Availability
2.15. Ethical Considerations and Approval
2.16. Use of Generative Artificial Intelligence (GenAI)
3. Results
3.1. MTX Was the Drug of Choice in the Treatment-Naïve First Visit Patients with Severe Inflammatory Arthritis
3.2. Severe Joint Disease Is Linked to a Lack of MTX Response
3.3. Low Insulin Levels Are Associated with a MTX Non-Response
3.4. Development of Predictive Model for MTX Response
3.5. Insulin Levels Secure Robustness of the MTX Response Prediction
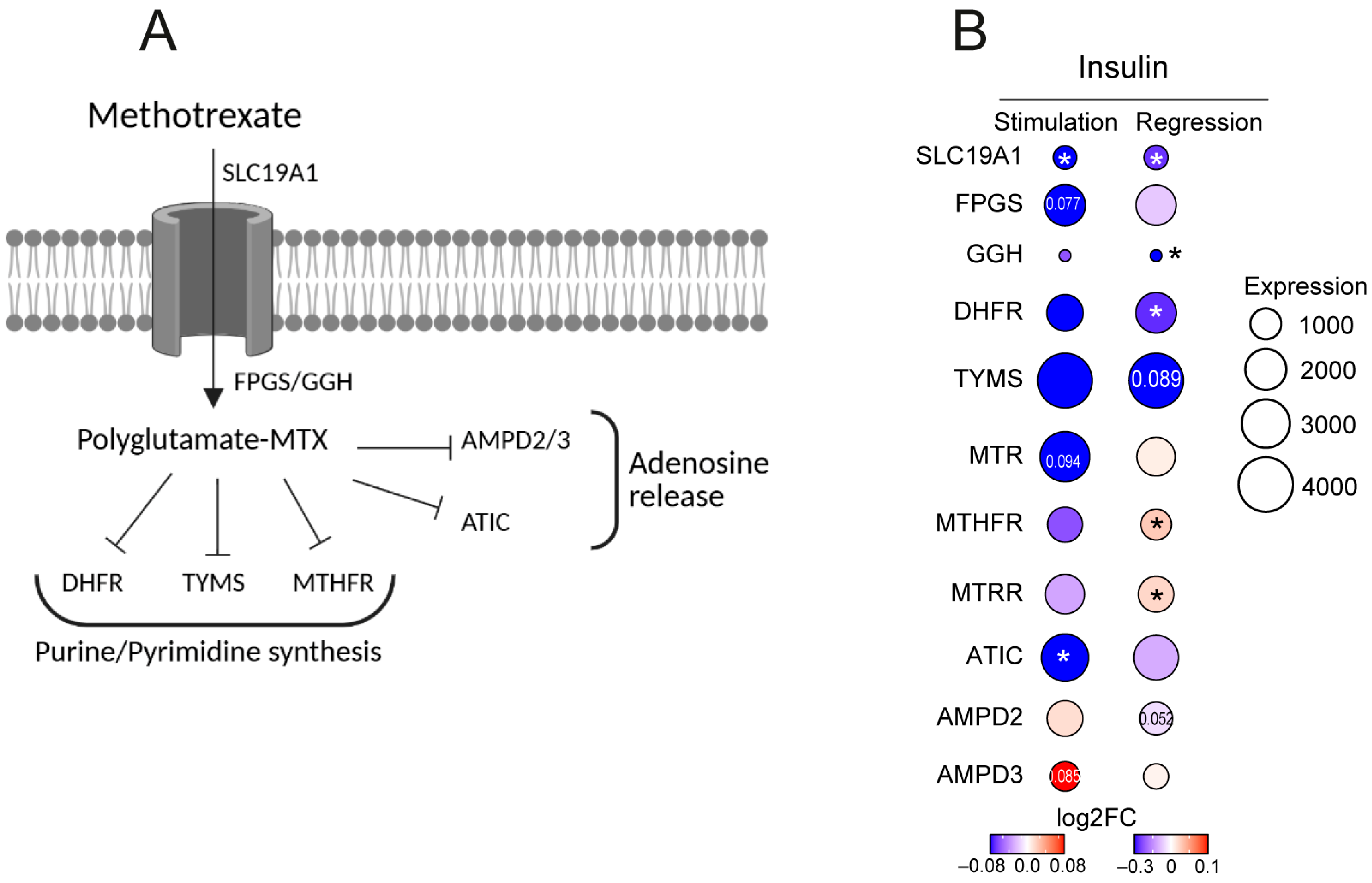
3.6. Insulin Levels Affect Transcription of MTX Metabolizing Enzymes in CD4+ Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RA | Rheumatoid Arthritis |
| EULAR | European League against Rheumatism |
| MTX | Methotrexate |
| MR | Methotrexate responder |
| AUC | Area Under Curve |
| ROC | Receiver operative Characteristics |
| ACPA | Anti-Citrullinated Protein Antibodies |
| RF | Rheumatoid Factor |
| ACR | American College of Rheumatology |
References
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.; Montecucco, C.; Bugatti, S.; Caporali, R. Rheumatoid arthritis treatment: The earlier the better to prevent joint damage. RMD Open 2015, 1 (Suppl. S1), e000057. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewe, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; E Pope, J.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef]
- Bello, A.E.; Perkins, E.L.; Jay, R.; Efthimiou, P. Recommendations for optimizing methotrexate treatment for patients with rheumatoid arthritis. Open Access Rheumatol. 2017, 9, 67–79. [Google Scholar] [CrossRef]
- Roodenrijs, N.M.T.; van der Goes, M.C.; Welsing, P.M.J.; Tekstra, J.; van Laar, J.M.; Lafeber, F.; Bijlsma, J.W.; Jacobs, J.W. Is prediction of clinical response to methotrexate in individual rheumatoid arthritis patients possible? A systematic literature review. Jt. Bone Spine 2020, 87, 13–23. [Google Scholar] [CrossRef]
- Gwinnutt, J.M.; Leggett, S.; Lunt, M.; Barton, A.; Hyrich, K.L.; Walker-Bone, K.; Verstappen, S.M.M.; RAMS and BRAGGSS co-investigators. Predictors of presenteeism, absenteeism and job loss in patients commencing methotrexate or biologic therapy for rheumatoid arthritis. Rheumatology 2020, 59, 2908–2919. [Google Scholar] [CrossRef]
- Carini, C.; Hunter, E.; Scottish Early Rheumatoid Arthritis Inception cohort Investigators; Ramadass, A.S.; Green, J.; Akoulitchev, A.; McInnes, I.B.; Goodyear, C.S. Chromosome conformation signatures define predictive markers of inadequate response to methotrexate in early rheumatoid arthritis. J. Transl. Med. 2018, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.A.; van der Kooij, S.M.; le Cessie, S.; Kievit, W.; Barerra, P.; Allaart, C.F.; Huizinga, T.W.J.; Guchelaar, H.; Pharmacogenetics Collaborative Research Group. A clinical pharmacogenetic model to predict the efficacy of methotrexate monotherapy in recent-onset rheumatoid arthritis. Arthritis Rheum. 2007, 56, 1765–1775. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.A.; de Vries-Bouwstra, J.K.; Heijmans, B.T.; Slagboom, P.E.; Goekoop-Ruiterman, Y.P.; Allaart, C.F.; Kerstens, P.J.S.M.; van Zeben, D.; Breedveld, F.C.; Dijkmans, B.A.C.; et al. Efficacy and toxicity of methotrexate in early rheumatoid arthritis are associated with single-nucleotide polymorphisms in genes coding for folate pathway enzymes. Arthritis Rheum. 2006, 54, 1087–1095. [Google Scholar] [CrossRef]
- Jenko, B.; Tomsic, M.; Jekic, B.; Milic, V.; Dolzan, V.; Praprotnik, S. Clinical Pharmacogenetic Models of Treatment Response to Methotrexate Monotherapy in Slovenian and Serbian Rheumatoid Arthritis Patients: Differences in Patient’s Management May Preclude Generalization of the Models. Front. Pharmacol. 2018, 9, 20. [Google Scholar] [CrossRef]
- Stuhlmuller, B.; Mans, K.; Tandon, N.; Bonin, M.O.; Smiljanovic, B.; Sorensen, T.A.; Schendel, P.; Martus, P.; Listing, J.; Detert, J.; et al. Genomic stratification by expression of HLA-DRB4 alleles identifies differential innate and adaptive immune transcriptional patterns—A strategy to detect predictors of methotrexate response in early rheumatoid arthritis. Clin. Immunol. 2016, 171, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Sysojev, A.O.; Saevarsdottir, S.; Diaz-Gallo, L.M.; Silberberg, G.N.; Alfredsson, L.; Klareskog, L.; Baecklund, E.; Björkman, L.; Kastbom, A.; Rantapää-Dahlqvist, S.; et al. Genome-wide investigation of persistence with methotrexate treatment in early rheumatoid arthritis. Rheumatology 2024, 63, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Yang, S.; Wang, Y.; Yu, L.; Zhang, B.; Rao, Z.; Gao, J.; Tu, S. (1)H NMR-based metabolomic analysis for identifying serum biomarkers to evaluate methotrexate treatment in patients with early rheumatoid arthritis. Exp. Ther. Med. 2012, 4, 165–171. [Google Scholar] [CrossRef]
- Maciejewski, M.; Sands, C.; Nair, N.; Ling, S.; Verstappen, S.; Hyrich, K.; Barton, A.; Ziemek, D.; Lewis, M.R.; Plant, D. Prediction of response of methotrexate in patients with rheumatoid arthritis using serum lipidomics. Sci. Rep. 2021, 11, 7266. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, J.; Lequerre, T.; Bacquet, H.; Vittecoq, O. Rheumatoid arthritis, insulin resistance, and diabetes. Jt. Bone Spine 2017, 84, 411–416. [Google Scholar] [CrossRef]
- Giles, J.T.; Danielides, S.; Szklo, M.; Post, W.S.; Blumenthal, R.S.; Petri, M.; Schreiner, P.J.; Budoff, M.; Detrano, R.; Bathon, J.M. Insulin resistance in rheumatoid arthritis: Disease-related indicators and associations with the presence and progression of subclinical atherosclerosis. Arthritis Rheumatol. 2015, 67, 626–636. [Google Scholar] [CrossRef]
- Yu, M.B.; Firek, A.; Langridge, W.H.R. Predicting methotrexate resistance in rheumatoid arthritis patients. Inflammopharmacology 2018, 26, 699–708. [Google Scholar] [CrossRef]
- Cooles, F.A.H.; Tarn, J.; Lendrem, D.W.; Naamane, N.; Lin, C.M.; Millar, B.; Maney, N.J.; E Anderson, A.; Thalayasingam, N.; Diboll, J.; et al. Interferon-alpha-mediated therapeutic resistance in early rheumatoid arthritis implicates epigenetic reprogramming. Ann. Rheum. Dis. 2022, 81, 1214–1223. [Google Scholar] [CrossRef]
- Cooles, F.A.H.; Anderson, A.E.; Lendrem, D.W.; Norris, J.; Pratt, A.G.; Hilkens, C.M.U.; Isaacs, J.D. The interferon gene signature is increased in patients with early treatment-naive rheumatoid arthritis and predicts a poorer response to initial therapy. J. Allergy Clin. Immunol. 2018, 141, 445–448.e4. [Google Scholar] [CrossRef]
- Sestan, M.; Marinovic, S.; Kavazovic, I.; Cekinovic, D.; Wueest, S.; Turk Wensveen, T.; Brizić, I.; Jonjić, S.; Konrad, D.; Wensveen, F.M.; et al. Virus-Induced Interferon-gamma Causes Insulin Resistance in Skeletal Muscle and Derails Glycemic Control in Obesity. Immunity 2018, 49, 164–177.e6. [Google Scholar] [CrossRef]
- Baboota, R.K.; Spinelli, R.; Erlandsson, M.C.; Brandao, B.B.; Lino, M.; Yang, H.; Mardinoglu, A.; Bokarewa, M.I.; Boucher, J.; Kahn, C.R.; et al. Chronic hyperinsulinemia promotes human hepatocyte senescence. Mol. Metab. 2022, 64, 101558. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, M.C.; Malmhall-Bah, E.; Chandrasekaran, V.; Andersson, K.M.E.; Nilsson, L.M.; Toyra Silfversward, S.; Pullerits, R.; Bokarewa, M.I. Insulin Sensitivity Controls Activity of Pathogenic CD4+ T Cells in Rheumatoid Arthritis. Cells 2024, 13, 2124. [Google Scholar] [CrossRef] [PubMed]
- Deane, K.D.; O’Donnell, C.I.; Hueber, W.; Majka, D.S.; Lazar, A.A.; Derber, L.A.; Gilliland, W.R.; Edison, J.D.; Norris, J.M.; Robinson, W.H.; et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 2010, 62, 3161–3172. [Google Scholar] [CrossRef] [PubMed]
- Isgren, A.; Forslind, K.; Erlandsson, M.; Axelsson, C.; Andersson, S.; Lund, A.; Bokarewa, M. High survivin levels predict poor clinical response to infliximab treatment in patients with rheumatoid arthritis. Semin. Arthritis Rheum. 2012, 41, 652–657. [Google Scholar] [CrossRef]
- Levitsky, A.; Erlandsson, M.C.; van Vollenhoven, R.F.; Bokarewa, M.I. Serum survivin predicts responses to treatment in active rheumatoid arthritis: A post hoc analysis from the SWEFOT trial. BMC Med. 2015, 13, 247. [Google Scholar] [CrossRef]
- Wessels, J.A.; Kooloos, W.M.; De Jonge, R.; De Vries-Bouwstra, J.K.; Allaart, C.F.; Linssen, A.; Collee, G.; De Sonnaville, P.; Lindemans, J.; Huizinga, T.W.J.; et al. Relationship between genetic variants in the adenosine pathway and outcome of methotrexate treatment in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2006, 54, 2830–2839. [Google Scholar] [CrossRef]
- Mirjafari, H.; Farragher, T.M.; Verstappen, S.M.; Yates, A.; Bunn, D.; Marshall, T.; Lunt, M.; Symmons, D.P.; Bruce, I.N. Seropositivity is associated with insulin resistance in patients with early inflammatory polyarthritis: Results from the Norfolk Arthritis Register (NOAR): An observational study. Arthritis Res. Ther. 2011, 13, R159. [Google Scholar] [CrossRef]


| MTX Responders (n = 92) | Non-Responders (n = 80) | No MTX (n = 85) | |
|---|---|---|---|
| Female, n (%) | 61 (66.3) | 55 (68.75) | 59 (69.4) |
| Age, y | 58 [22–89] | 51 [22–88] p = 0.0045 | 52.5 [17–90] |
| Smokers, n (%) | 39 (42.4) | 29 (36.3) | 29 (34.1) |
| Diabetes mellitus, n | 10 (10.9) | 7 (8.7) | 6 (7.06) |
| RA antibodies, pos | 50 (54.3) | 56 (70) p = 0.037 | 23 (27) p < 0.0001 |
| RF, n (%) | 40 (43.5) | 49 (61.25) p = 0.021 | 17 (20.0) p = 0.00073 |
| ACPA, n (%) | 40 (43.5) | 52 (65) p = 0.0055 | 12 (14.1) p < 0.0001 |
| RF + ACPA, n (%) | 30 (32.6) | 45 (56.25) p = 0.0020 | 6 (7.06) p < 0.0001 |
| RA classification score | 5.85 [1–10] | 6.89 [1–10] p = 0.0005 | 4.07 [1–9] p < 0.0001 |
| ≥6 points, n (%) | 55 (59.8) | 62 (77.5) p = 0.0059 | 20 (23.5) p < 0.0001 |
| Swollen joints, n | 5.11 [1–20] | 7.06 [0–22] | 2.74 [0–10] |
| Inflammation Index | 1.50 (0–4) | 1.85(0–4) p = 0.064 | 1.13 (0–4) p = 0.0003 |
| MTX at 1y, n (%) | 80 (87) | 68 (85) | 0 |
| MTX dose at 1y, mg/w | 16.4 (0–25) | 14.9 (0–25) | 0 |
| Other DMARDs, n | 2 (1.47) | 20 (25) p < 0.0001 | 14 (16.5) |
| Biologics, n | 0 (0) | 46 (57.5) | 5 (5.9) p = 0.0059 |
| Tested DMARDs, n | 1.03 (1–4) | 1.84 (1–3) p < 0.0001 | 0.29 (0–2) p < 0.0001 |
| OC at 1st visit, n (%) | 51 (76) | 58 (58) p = 0.022 | 23 (27.1) p = 0.0014 |
| OC at 1 year, n (%) | 23 (25) | 31 (38.75) p = 0.056 | 8 (9.4) p = 0.0066 |
| Remission at 1 y, n (%) | 51 (55.4) | 21 (26.3) p = 0.0001 | 68 (80) p = 0.00052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundgren, V.M.E.; Erlandsson, M.C.; Chandrasekaran, V.; Töyrä Silfverswärd, S.; Pullerits, R.; Bokarewa, M.I. Insulin Predicts Methotrexate Response by Affecting the Transcription of Methotrexate Target Genes in the Treatment-Naive Rheumatoid Arthritis. Cells 2025, 14, 964. https://doi.org/10.3390/cells14130964
Lundgren VME, Erlandsson MC, Chandrasekaran V, Töyrä Silfverswärd S, Pullerits R, Bokarewa MI. Insulin Predicts Methotrexate Response by Affecting the Transcription of Methotrexate Target Genes in the Treatment-Naive Rheumatoid Arthritis. Cells. 2025; 14(13):964. https://doi.org/10.3390/cells14130964
Chicago/Turabian StyleLundgren, Victoria M. E., Malin C. Erlandsson, Venkataragavan Chandrasekaran, Sofia Töyrä Silfverswärd, Rille Pullerits, and Maria I. Bokarewa. 2025. "Insulin Predicts Methotrexate Response by Affecting the Transcription of Methotrexate Target Genes in the Treatment-Naive Rheumatoid Arthritis" Cells 14, no. 13: 964. https://doi.org/10.3390/cells14130964
APA StyleLundgren, V. M. E., Erlandsson, M. C., Chandrasekaran, V., Töyrä Silfverswärd, S., Pullerits, R., & Bokarewa, M. I. (2025). Insulin Predicts Methotrexate Response by Affecting the Transcription of Methotrexate Target Genes in the Treatment-Naive Rheumatoid Arthritis. Cells, 14(13), 964. https://doi.org/10.3390/cells14130964







