Humanized scFv Molecule Specific to an Extracellular Epitope of P2X4R as Therapy for Chronic Pain Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Sequence
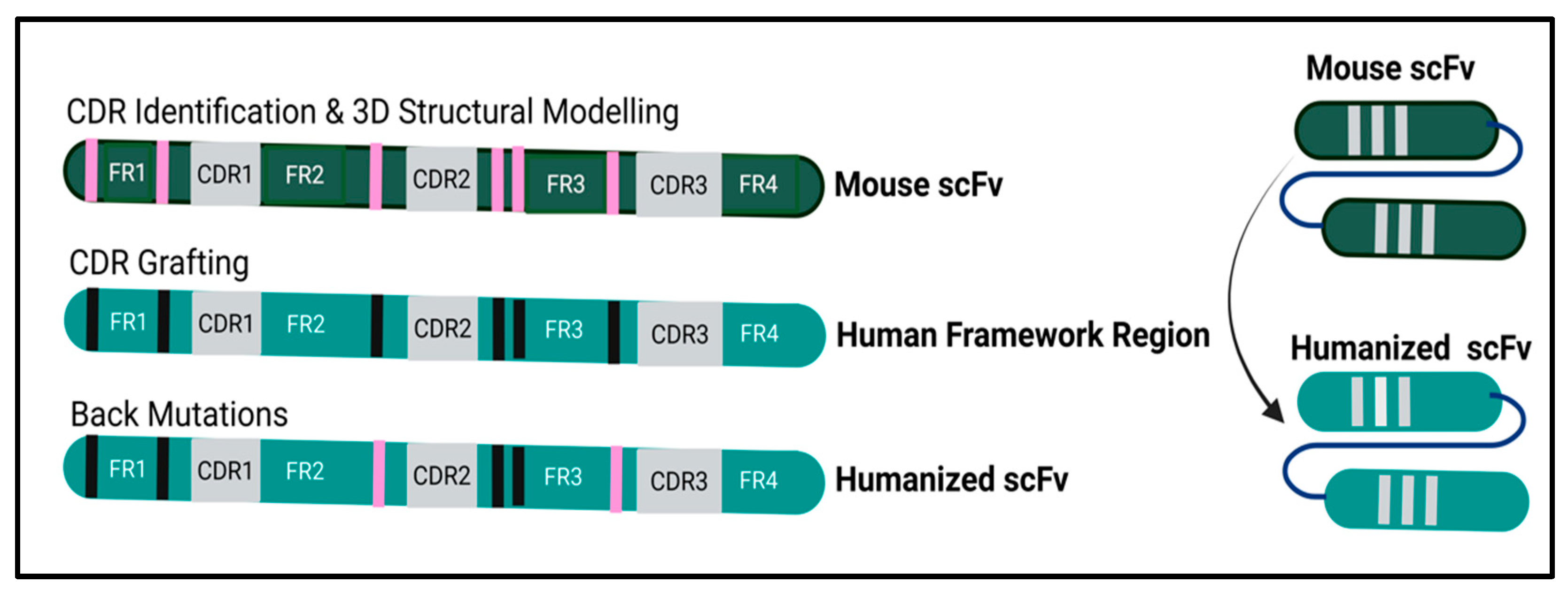
2.2. P2X4R scFv Humanization and Upscaling Overview
2.3. Sequencing the Humanized hscFv
2.4. hscFv Purification
2.5. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
2.6. Western Blot of hscFv Fragments
2.7. Protein Aggregation Analysis by Size-Exclusion Chromatography (SEC-UPLC)
2.8. Purity Analysis by CE-SDS
2.9. Octet RED384 Kinetic Measurements
2.10. Endotoxin Assay
2.11. In Vivo Validation of hscFv Efficacy in FRICT-ION Chronic Trigeminal Neuropathic Pain Model
2.12. Experimental Pain-Related Behavioral Read-Outs
2.12.1. Reflexive Mechanical Threshold Using Von Frey Filaments
2.12.2. Cold Hypersensitivity Threshold
2.12.3. Cognitive-Dependent Anxiety- and Depression-like Behaviors Tested
2.12.4. Power Calculation
3. Results
3.1. Humanization of P2X4R hscFv
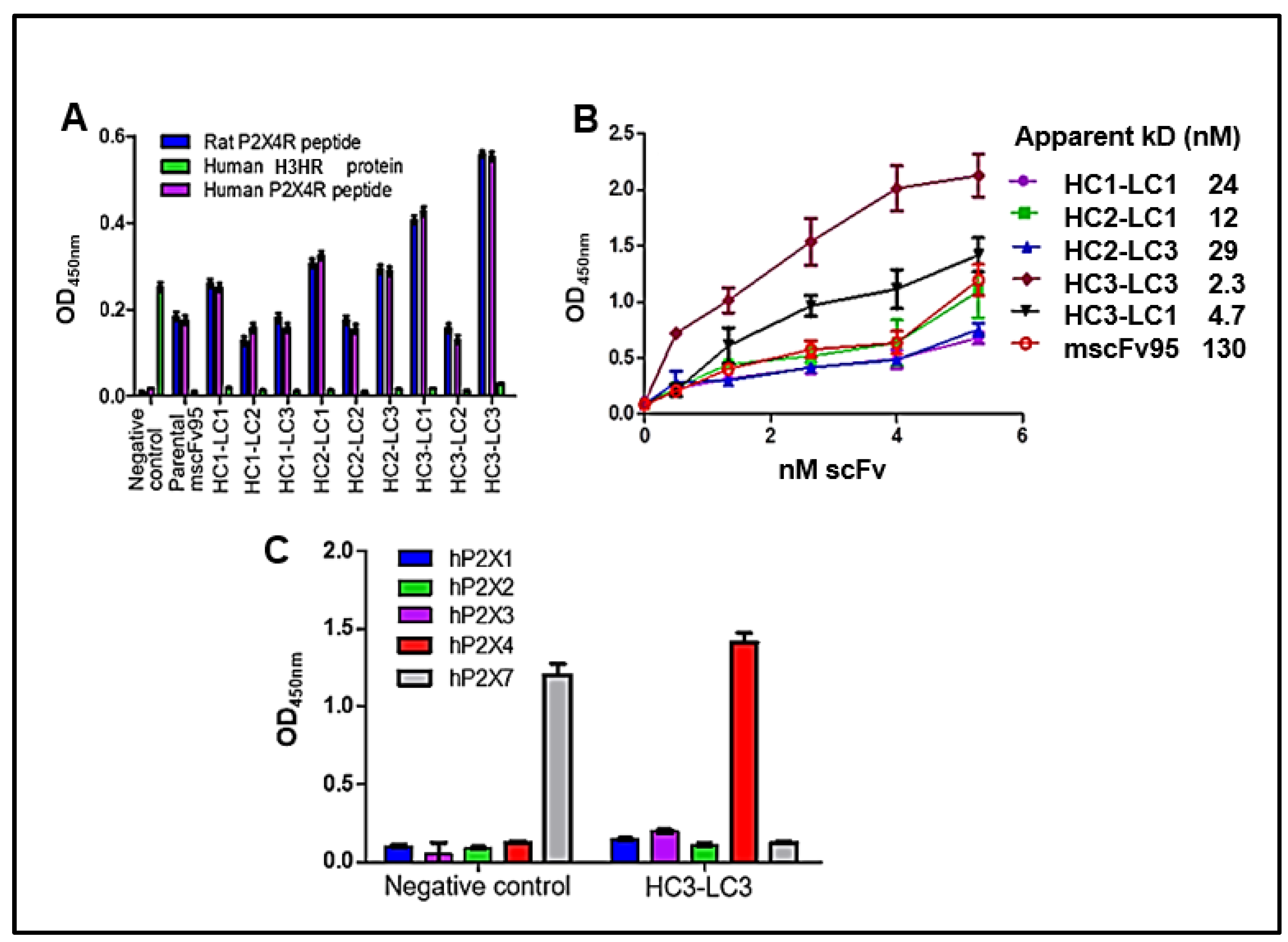
3.2. Characteristics of the Lead HC3-LC3
3.2.1. Specificity Binding by ELISA
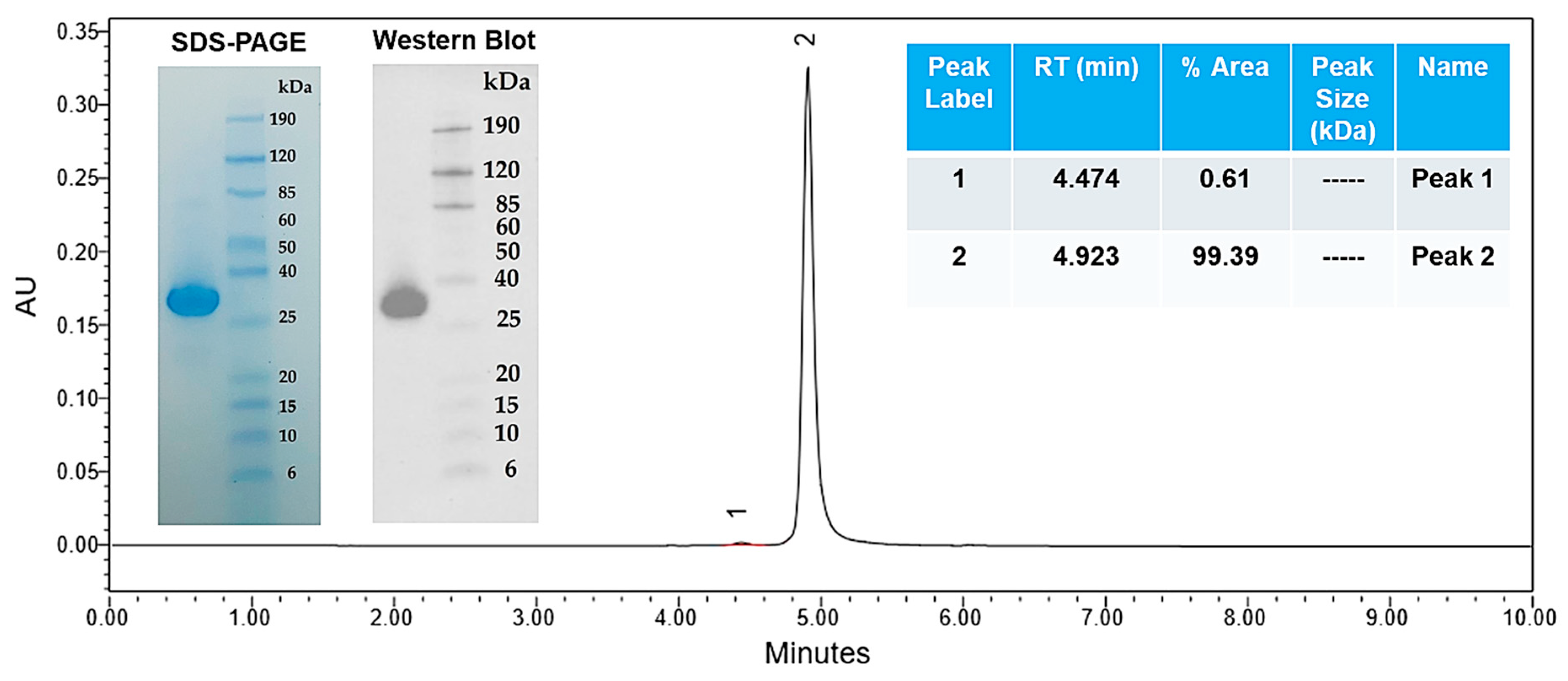
3.2.2. Gel Electrophoresis and Western Blot
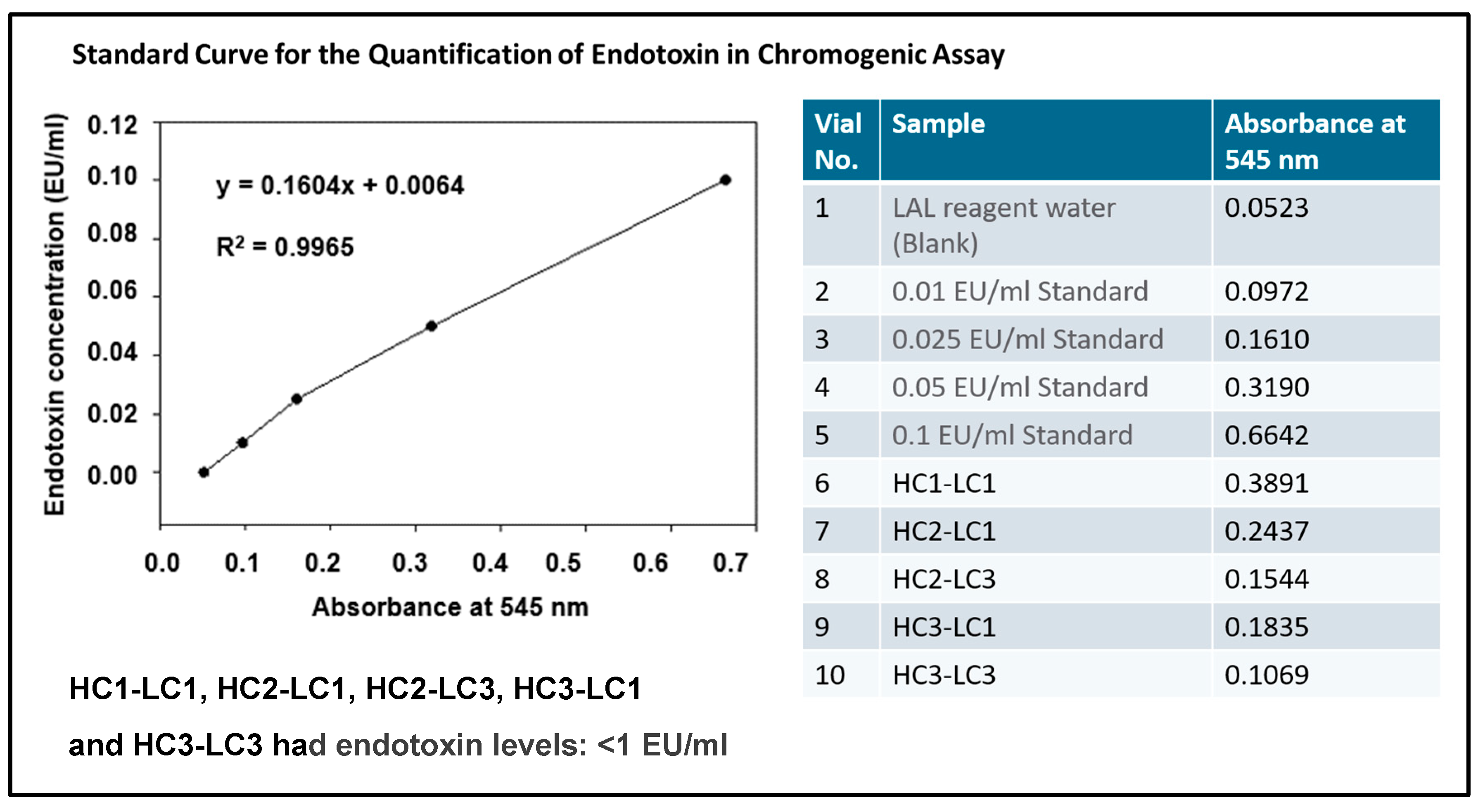
3.2.3. Endotoxin Content
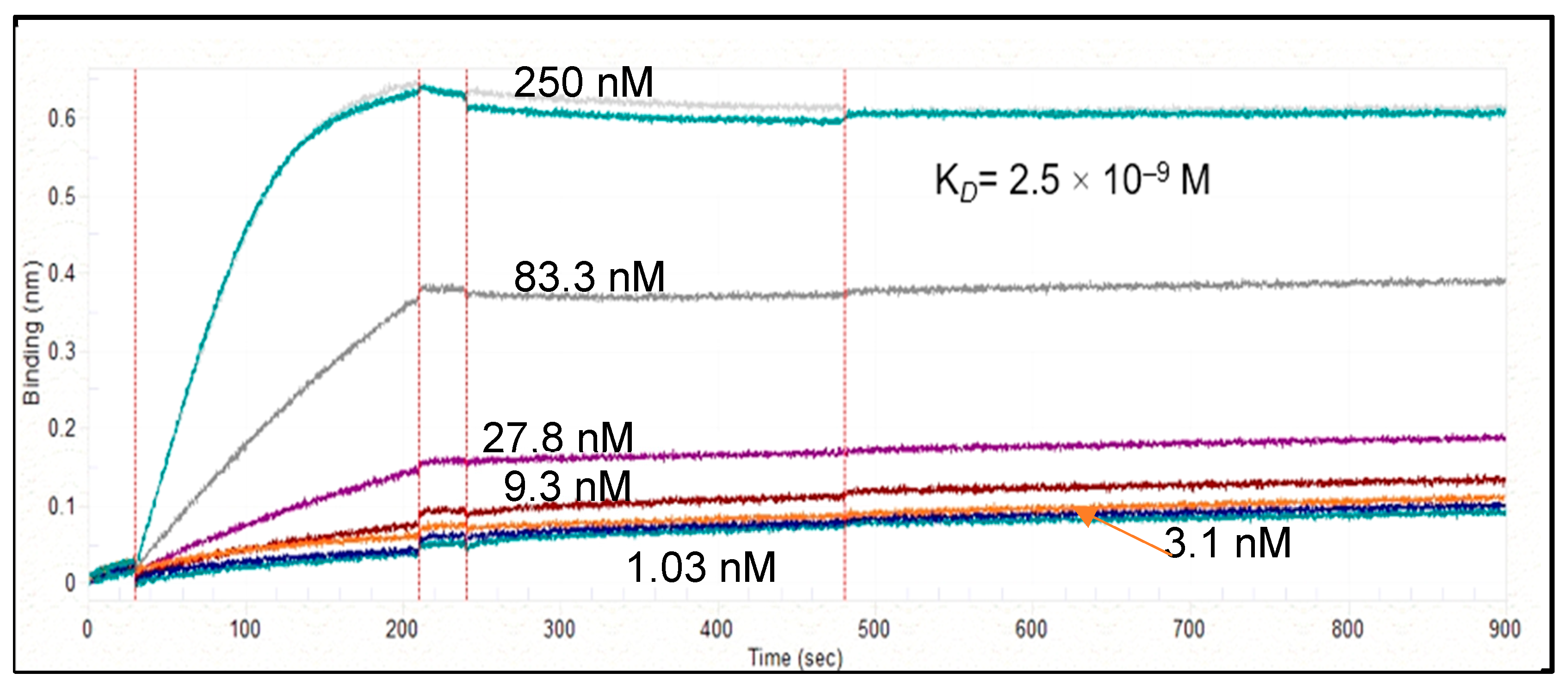
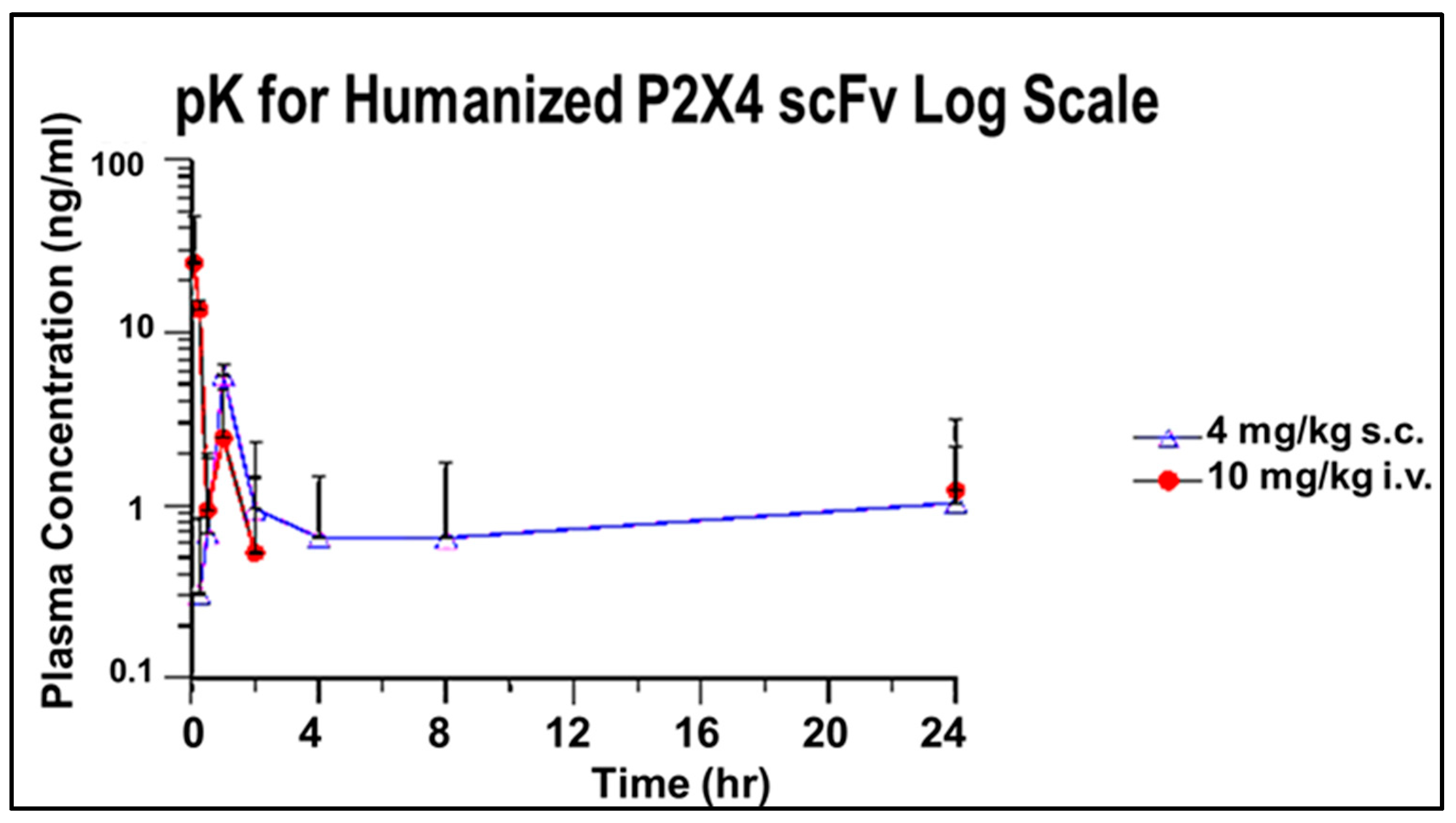
3.2.4. pK for Humanized P2X4 hscFv Biologic
3.3. Validation with Reversal of Nerve Injury Chronic Neuropathic Pain by HC3-LC3 hscFv
3.3.1. Von Frey Mechanical Hypersensitivity
3.3.2. Reversal of Cold Hypersensitivity
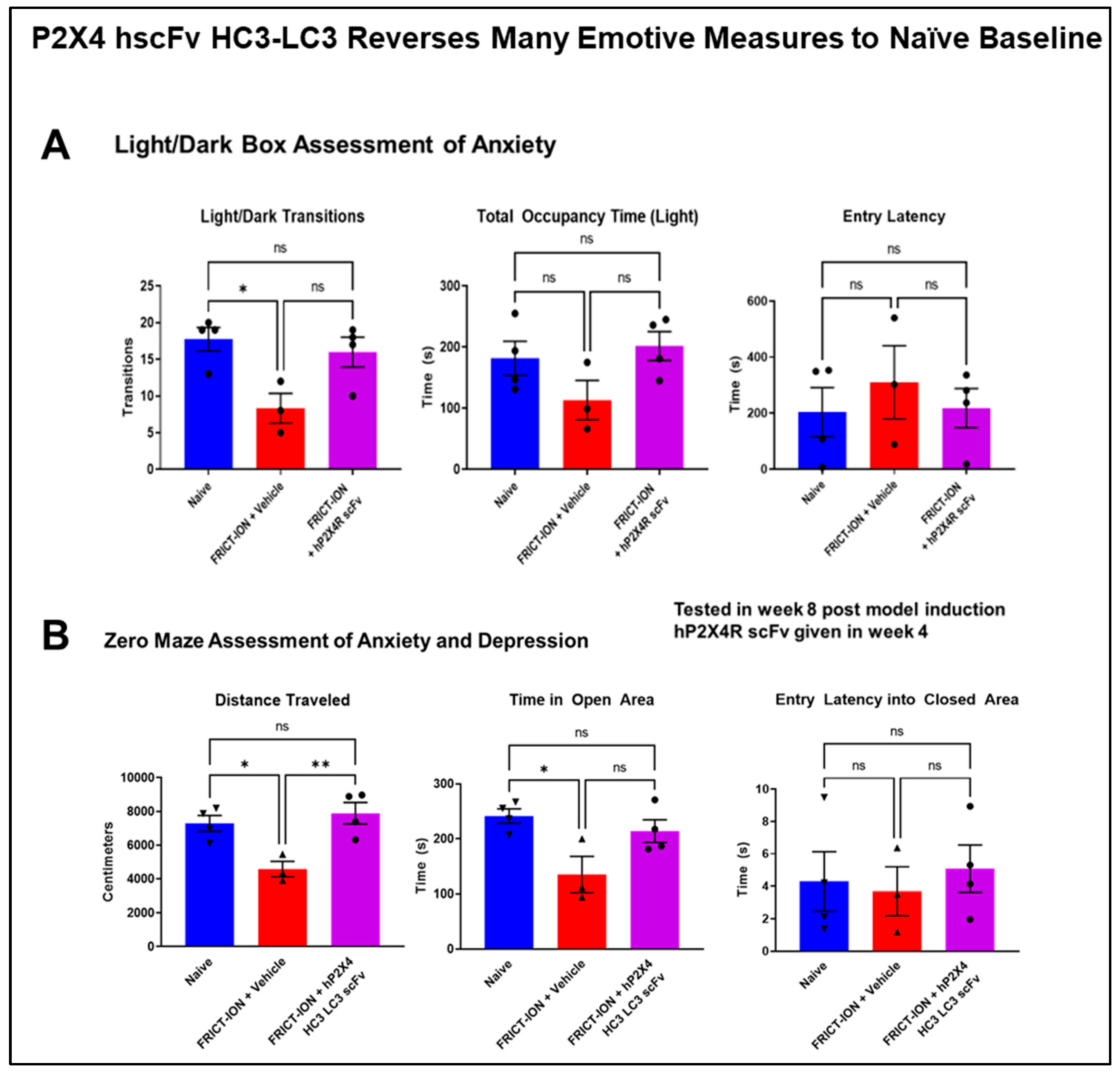
3.3.3. Relief of Anxiety- and Depression-like Behaviors
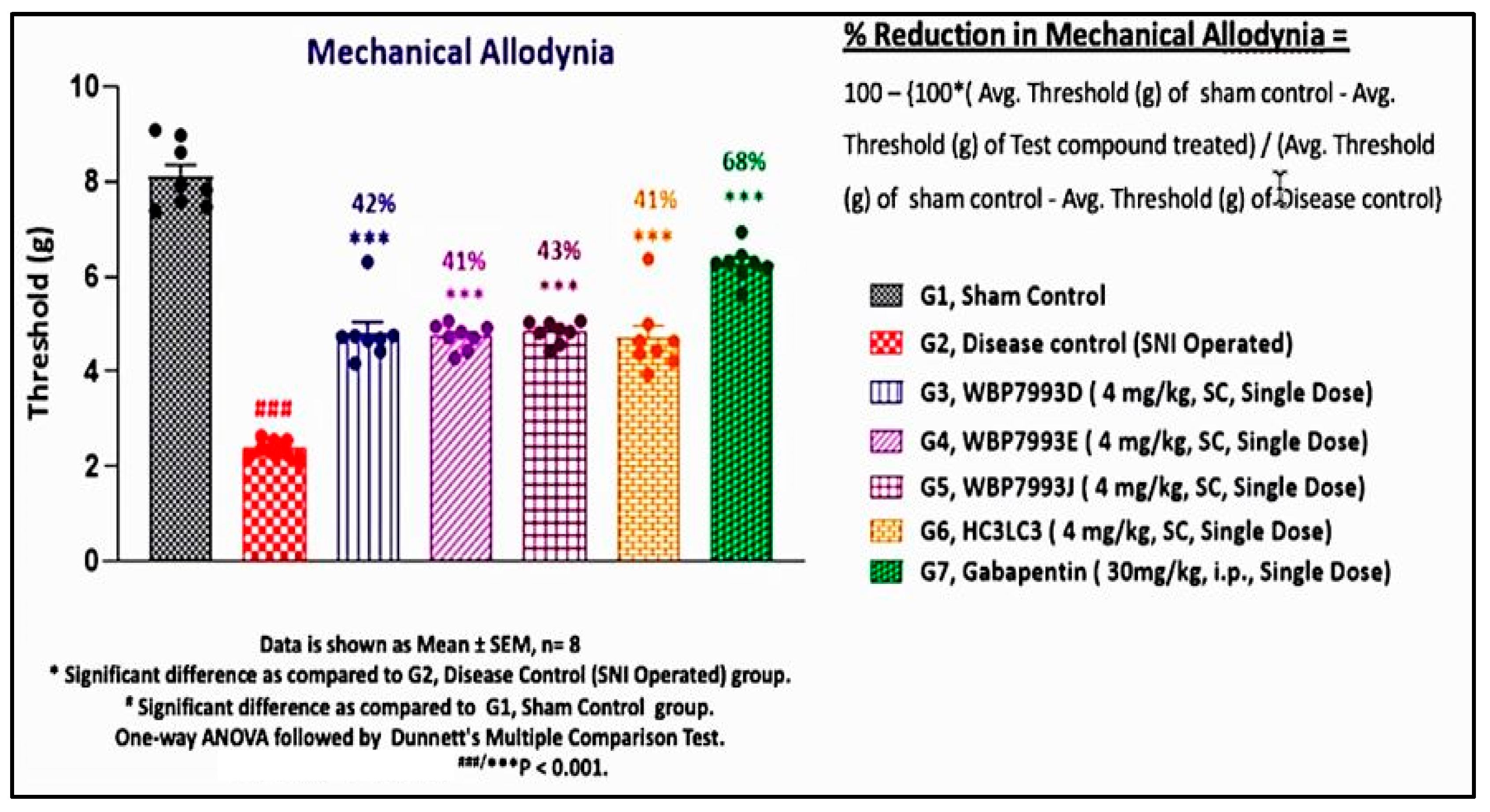
3.4. Validation with Reversal of Spared Nerve Injury (SNI) Chronic Neuropathic Pain by P2X4 hscFv
4. Discussion
4.1. Comparison of Ribosome Display-Generated hscFv Biologic to Other Immunotherapies
4.2. Advantages of Utilizing the Specific Ribosome Display and Humanization Technology Used Here to Generate the P2X4 hscFv
4.3. Significance in the Context of Prior Research
4.4. Strengths and Innovation of the scFv Biologic for Chronic Pain
4.5. Limitations of the Study
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| scFv | Single-chain fragment variable |
| VH | Variable heavy chain |
| VL | Variable light chain |
| CDR | Complementary determining region |
| hscFv | Humanized single-chain fragment variable |
| P2X4R | P2X purinoceptor 4 |
| FRICT-ION | The Foramen Rotundum Inflammatory Constriction of the Trigeminal InfraOrbital Nerve |
| SNI | Spared nerve injury |
| CGRP | Calcitonin gene-related peptide |
| KD | Dissociation constant |
| PCR | Polymerase chain reaction |
| ELISA | Enzyme-linked immunosorbent assay |
| SDS-PAGE | Sodium dodecyl sulfate/polyacrylamide gel electrophoresis |
| SEC-UPLC | Size-exclusion ultraperformance liquid chromatography |
| LAL | Limulus amebocyte lysate |
| IL-6 | Interleukin-6 |
| CD-54 | Cluster of differentiation 54 |
| ANOVA | Analysis of variance |
References
- Nahin, R.L. Pain Prevalence, Chronicity and Impact Within Subpopulations Based on Both Hispanic Ancestry and Race: United States, 2010–2017. J. Pain 2021, 22, 826–851. [Google Scholar] [CrossRef] [PubMed]
- Westlund, K.N.; Montera, M.A.; Goins, A.E.; Alles, S.R.A.; Suri, N.; McIlwrath, S.L.; Bartel, R.; Durvasula, R.V.; Kunamneni, A. Single-Dose P2X4R Single-Chain Fragment Variable Antibody Permanently Reverses Chronic Pain in Male Mice. Int. J. Mol. Sci. 2021, 22, 13612. [Google Scholar] [CrossRef] [PubMed]
- Kunamneni, A.; Ye, C.; Bradfute, S.B.; Durvasula, R. Ribosome display for the rapid generation of high-affinity Zika-neutralizing single-chain antibodies. PLoS ONE 2018, 13, e0205743. [Google Scholar] [CrossRef]
- Kunamneni, A.; Clarke, E.C.; Ye, C.; Bradfute, S.B.; Durvasula, R. Generation and Selection of a Panel of Pan-Filovirus Single-Chain Antibodies using Cell-Free Ribosome Display. Am. J. Trop. Med. Hyg. 2019, 101, 198–206. [Google Scholar] [CrossRef]
- Kunamneni, A.; Montera, M.A.; Durvasula, R.; Alles, S.R.A.; Goyal, S.; Westlund, K.N. Rapid Generation and Molecular Docking Analysis of Single-Chain Fragment Variable (scFv) Antibody Selected by Ribosome Display Targeting Cholecystokinin B Receptor (CCK-BR) for Reduction of Chronic Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 11035. [Google Scholar] [CrossRef]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef] [PubMed]
- Torkashvand, F.; Vaziri, B. Main Quality Attributes of Monoclonal Antibodies and Effect of Cell Culture Components. Iran. Biomed. J. 2017, 21, 131–141. [Google Scholar] [CrossRef]
- Montera, M.A.; Westlund, K.N. Minimally Invasive Oral Surgery Induction of the FRICT-ION Chronic Neuropathic Pain Model. Bio Protoc. 2020, 10, e3591. [Google Scholar] [CrossRef]
- Tsuda, M.; Beggs, S.; Salter, M.W.; Inoue, K. Microglia and intractable chronic pain. Glia 2013, 61, 55–61. [Google Scholar] [CrossRef]
- Mapplebeck, J.C.S.; Beggs, S.; Salter, M.W. Sex differences in pain: A tale of two immune cells. Pain 2016, 157 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef]
- Mapplebeck, J.C.S.; Dalgarno, R.; Tu, Y.; Moriarty, O.; Beggs, S.; Kwok, C.H.T.; Halievski, K.; Assi, S.; Mogil, J.S.; Trang, T.; et al. Microglial P2X4R-Evoked Pain Hypersensitivity Is Sexually Dimorphic in Rats. Pain 2018, 159, 1752–1763. [Google Scholar] [CrossRef]
- Suurväli, J.; Boudinot, P.; Kanellopoulos, J.; Rüütel Boudinot, S. P2X4: A fast and sensitive purinergic receptor. Biomed. J. 2017, 40, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.M.; Malek, N.; Edye, M.; Jager, S.B.; McMurray, S.; McMahon, S.B.; Denk, F. Sex Differences in Peripheral Not Central Immune Responses to Pain-Inducing Injury. Sci. Rep. 2017, 7, 16460. [Google Scholar] [CrossRef]
- Paige, C.; Maruthy, G.B.; Mejia, G.; Dussor, G.; Price, T. Spinal Inhibition of P2XR or P38 Signaling Disrupts Hyperalgesic Priming in Male, but Not Female, Mice. Neuroscience 2018, 385, 133–142. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci. Methods. 1994, 53, 55–63. [Google Scholar] [CrossRef]
- File, S.E.; Lippa, A.S.; Beer, B.; Lippa, M.T. Animal tests of anxiety. Curr. Protoc. Pharmacol. 2004, 27, 5.38.1–5.38.21. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, I.; Bohren, Y.; Waltisperger, E.; Sage-Ciocca, D.; Yin, J.C.; Freund-Mercier, M.J.; Barrot, M. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol. Psychiatry 2011, 70, 946–953. [Google Scholar] [CrossRef]
- Yalcin, I.; Megat, S.; Barthas, F.; Waltisperger, E.; Kremer, M.; Salvat, E.; Barrot, M. The sciatic nerve cuffing model of neuropathic pain in mice. J. Vis. Exp. 2014, 16, 51608. [Google Scholar]
- David, D.J.; Samuels, B.A.; Rainer, Q.; Wang, J.W.; Marsteller, D.; Mendez, I.; Drew, M.; Craig, D.A.; Guiard, B.P.; Guilloux, J.P.; et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 2009, 62, 479–493. [Google Scholar] [CrossRef]
- Gao, S.H.; Huang, K.; Tu, H.; Adler, A.S. Monoclonal antibody humanness score and its applications. BMC Biotechnol. 2013, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Xu, Q.; Shi, Y.; Zhao, R.; Li, H.; Zheng, J.; Liu, F.; Wan, Y.; Wei, B. Pathology of pain and its implications for therapeutic interventions. Signal Transduct. Target. Ther. 2024, 9, 155. [Google Scholar]
- Kaplon, H.; Reichert, J.M. Antibodies to watch in 2019. mAbs 2018, 11, 219–238. [Google Scholar] [CrossRef]
- Alpaugh, M.; Cicchetti, F. A brief history of antibody-based therapy. Neurobiol. Dis. 2019, 130, 104504. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.G.; McMahon, S.B. ATP as a peripheral mediator of pain. J. Auton. Nerv. Syst. 2000, 81, 187–194. [Google Scholar] [CrossRef]
- Bortolato, M.; Yardley, M.M.; Khoja, S.; Godar, S.C.; Asatryan, L.; Finn, D.A.; Alkana, R.L.; Louie, S.G.; Davies, D.L. Pharmacological insights into the role of P2X4 receptors in behavioural regulation: Lessons from ivermectin. Int. J. Neuropsychopharmacol. 2013, 16, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Kuboyama, K.; Inoue, T.; Nagata, K.; Tozaki-Saitoh, H.; Inoue, K. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol. Pain 2009, 5, 28. [Google Scholar] [CrossRef]
- Tsuda, M.; Shigemoto-Mogami, Y.; Koizumi, S.; Mizokoshi, A.; Kohsaka, S.; Salter, M.W.; Inoue, K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003, 424, 778–783. [Google Scholar] [CrossRef]
- He, M.; Taussig, M.J. Ribosome display: Cell-free protein display technology. Brief. Funct. Genom. Proteomic. 2002, 1, 204–212. [Google Scholar] [CrossRef]
- Toleikis, L.; Broders, O.; Dübel, S. Cloning single-chain antibody fragments (scFv) from hybridoma cells. Methods Mol. Med. 2004, 94, 447–458. [Google Scholar]
- He, M.; Edwards, B.M.; Kastelic, D.; Taussig, M.J. Eukaryotic ribosome display with in situ DNA recovery. Methods Mol. Biol. 2012, 805, 75–85. [Google Scholar] [PubMed]
- Kunamneni, A.; Montera, M.; Gott, K.; Durvasula, R.; Westlund, K. Generating highly potent and efficacious antibodies to the cholecystokinin B (CCK-B) receptor by ribosome display for the treatment of neuropathic pain. FASEB J. 2019, 3, lb31. [Google Scholar] [CrossRef]
- Tashima, T. Smart Strategies for Therapeutic Agent Delivery into Brain across the Blood-Brain Barrier Using Receptor-Mediated Transcytosis. Chem. Pharm. Bull. 2020, 68, 316–325. [Google Scholar] [CrossRef]
- Ayoub, M.A.; Crépieux, P.; Koglin, M.; Parmentier, M.; Pin, J.P.; Poupon, A.; Reiter, E.; Smit, M.; Steyaert, J.; Watier, H.; et al. Antibodies targeting G protein-coupled receptors: Recent advances and therapeutic challenges. mAbs 2017, 9, 735–741. [Google Scholar] [CrossRef] [PubMed]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef]
- Deng, B.; Lv, W.; Duan, W.; Liu, Y.; Li, Z.; Song, X.; Cui, C.; Qi, X.; Wang, X.; Li, C. FGF9 modulates Schwann cell myelination in developing nerves and induces a pro-inflammatory environment during injury. J. Cell Biochem. 2018, 119, 8643–8658. [Google Scholar] [CrossRef]
- Burnstock, G. P2X receptors in sensory neurones. Br. J. Anaesth. 2000, 84, 476–488. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 10811083. [Google Scholar] [CrossRef]
- Bernier, L.P.; Ase, A.R.; Séguéla, P. P2X receptor channels in chronic pain pathways. Br. J. Pharmacol. 2018, 175, 2219–2230. [Google Scholar] [CrossRef]
- Cohen, S.P.; Mao, J. Neuropathic pain: Mechanisms and their clinical implications. BMJ 2014, 348, f7656. [Google Scholar] [CrossRef]
- Mika, J.; Zychowska, M.; Popiolek-Barczyk, K.; Rojewska, E.; Przewlocka, B. Importance of glial activation in neuropathic pain. Eur. J. Pharmacol. 2013, 716, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Ulmann, L.; Hirbec, H.; Rassendren, F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010, 29, 2290–2300. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yamashita, T.; Sasaki, A.; Nakata, E.; Kohno, K.; Masuda, T.; Tozaki-Saitoh, H.; Imai, T.; Kuraishi, Y.; Tsuda, M.; et al. A novel P2X4 receptor-selective antagonist produces anti-allodynic effect in a mouse model of herpetic pain. Sci. Rep. 2016, 6, 32461. [Google Scholar] [CrossRef]
- Ma, H.; Meng, J.; Wang, J.; Hearty, S.; Dolly, J.O.; O’Kennedy, R. Targeted delivery of a SNARE protease to sensory neurons using a single chain antibody (scFv) against the extracellular domain of P2X(3) inhibits the release of a pain mediator. Biochem. J. 2014, 462, 247–256. [Google Scholar] [CrossRef]
- Schaffitzel, C.; Hanes, J.; Jermutus, L.; Plückthun, A. Ribosome display: An in vitro method for selection and evolution of antibodies from libraries. J. Immunol. Methods 1999, 231, 119–135. [Google Scholar] [CrossRef]
- Liu, X.G.; Lu, S.; Liu, D.Q.; Zhang, L.; Zhang, L.X.; Yu, X.L.; Liu, R.T. ScFv-conjugated superparamagnetic iron oxide nanoparticles for MRI-based diagnosis in transgenic mouse models of Parkinson's and Huntington’s diseases. Brain Res. 2019, 1707, 141–153. [Google Scholar] [CrossRef]
- Ahmad, Z.A.; Yeap, S.K.; Ali, A.M.; Ho, W.Y.; Alitheen, N.B.; Hamid, M. scFv antibody: Principles and clinical application. Clin. Dev. Immunol. 2012, 12, 980250. [Google Scholar] [CrossRef] [PubMed]
- Škrlj, N.; Dolinar, M. New engineered antibodies against prions. Bioengineered 2014, 5, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.C.; McLear, J.A.; Messer, A. Engineered antibody therapies to counteract mutant huntingtin and related toxic intracellular proteins. Prog. Neurobiol. 2012, 97, 190–204. [Google Scholar] [CrossRef]
- Angelini, A.; Miyabe, Y.; Newsted, D.; Kwan, B.H.; Miyabe, C.; Kelly, R.L.; Jamy, M.N.; Luster, A.D.; Wittrup, K.D. Directed evolution of broadly crossreactive chemokine-blocking antibodies efficacious in arthritis. Nat. Commun. 2018, 9, 1461. [Google Scholar] [CrossRef]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef] [PubMed]
- Röhn, T.A.; Ralvenius, W.T.; Paul, J.; Borter, P.; Hernandez, M.; Witschi, R.; Grest, P.; Zeilhofer, H.U.; Bachmann, M.F.; Jennings, G.T. A virus-like particle-based anti-nerve growth factor vaccine reduces inflammatory hyperalgesia: Potential long-term therapy for chronic pain. J. Immunol. 2011, 186, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Introduction to Purinergic Signaling. Methods Mol. Biol. 2020, 2041, 1–15. [Google Scholar] [PubMed]



| Variable Region Chain | Species | T20 Analyzer Score (Full Length) | T20 Analyzer Score (Framework Only) |
|---|---|---|---|
| mscFv95-HC_parental | Mus musculus | 74.5 | 78.3 |
| hscFv95.HC1 | Homo sapiens | 74.51 | 78.33 |
| hscFv95.HC2 | Homo sapiens | 68.93 | 84.88 |
| hscFv95.HC3 | Homo sapiens | 82.09 | 90.92 |
| mscFv95-LC_parental | Mus musculus | 76.9 | 82.3 |
| hscFv95.LC1 | Homo sapiens | 76 | 81.06 |
| hscFv95.LC2 | Homo sapiens | 88.7 | 96.9 |
| hscFv95.LC3 | Homo sapiens | 89.39 | 96.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunamneni, A.; Westlund, K.N. Humanized scFv Molecule Specific to an Extracellular Epitope of P2X4R as Therapy for Chronic Pain Management. Cells 2025, 14, 953. https://doi.org/10.3390/cells14130953
Kunamneni A, Westlund KN. Humanized scFv Molecule Specific to an Extracellular Epitope of P2X4R as Therapy for Chronic Pain Management. Cells. 2025; 14(13):953. https://doi.org/10.3390/cells14130953
Chicago/Turabian StyleKunamneni, Adinarayana, and Karin N. Westlund. 2025. "Humanized scFv Molecule Specific to an Extracellular Epitope of P2X4R as Therapy for Chronic Pain Management" Cells 14, no. 13: 953. https://doi.org/10.3390/cells14130953
APA StyleKunamneni, A., & Westlund, K. N. (2025). Humanized scFv Molecule Specific to an Extracellular Epitope of P2X4R as Therapy for Chronic Pain Management. Cells, 14(13), 953. https://doi.org/10.3390/cells14130953





