Temperature and WNK-SPAK/OSR1 Kinases Dynamically Regulate Antiviral Human GFP-MxA Biomolecular Condensates in Oral Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Cell Culture
2.2. Plasmids and Transient Transfection
2.3. Live-Cell Fluorescence Imaging
2.4. Phase Transition Experiments and Fluorescence Imaging
2.5. Quantitation of Relative Amounts of GFP-MxA in Condensates vs. Dispersed State in a Cell
2.6. VSV Stock and Virus Infection
2.7. Antibody Reagents and Chemicals
2.8. Statistical Testing
3. Results
3.1. Quantitation of GFP-MxA in Condensed vs. Dispersed Phases at the Single-Cell Level
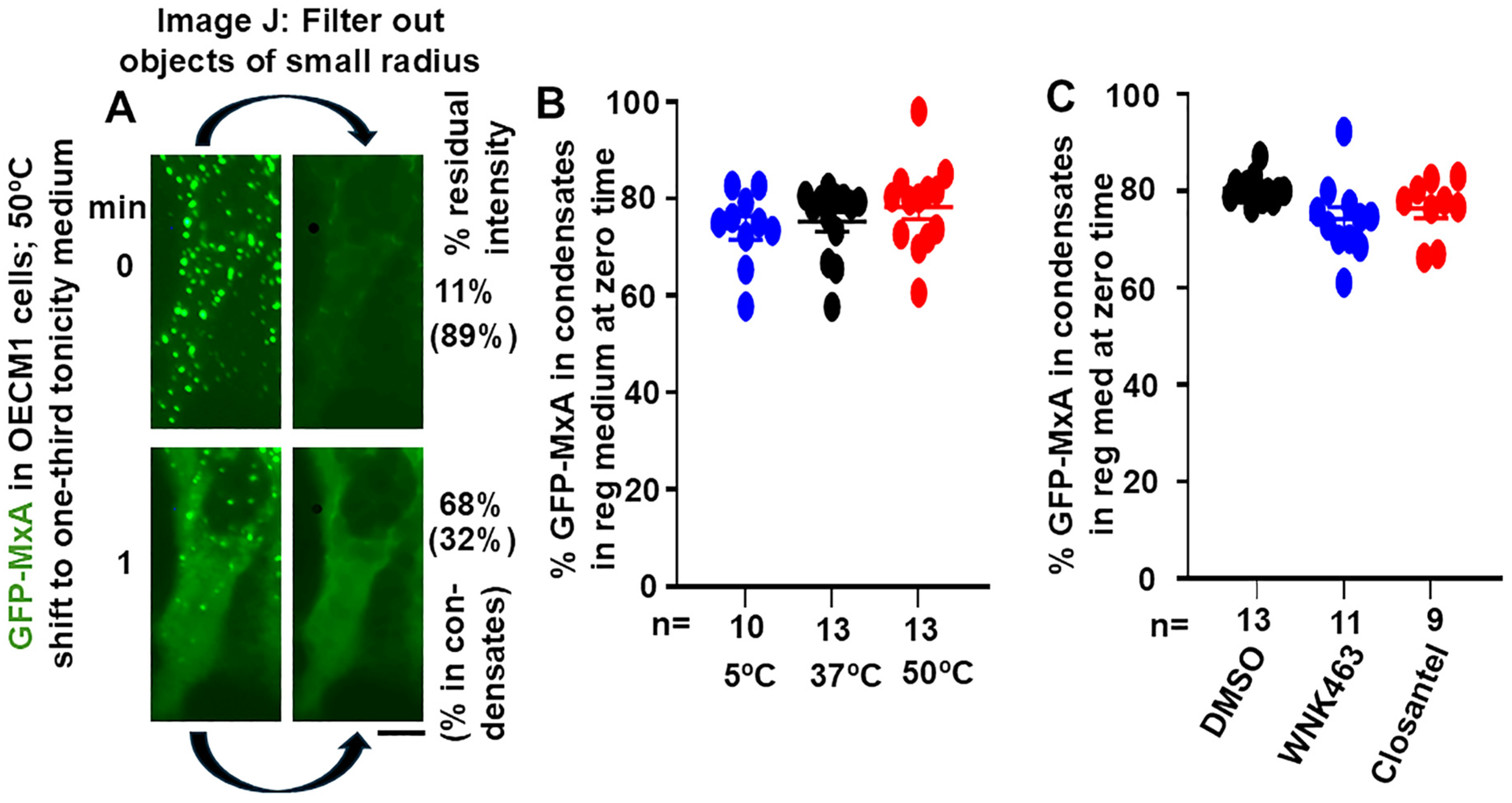
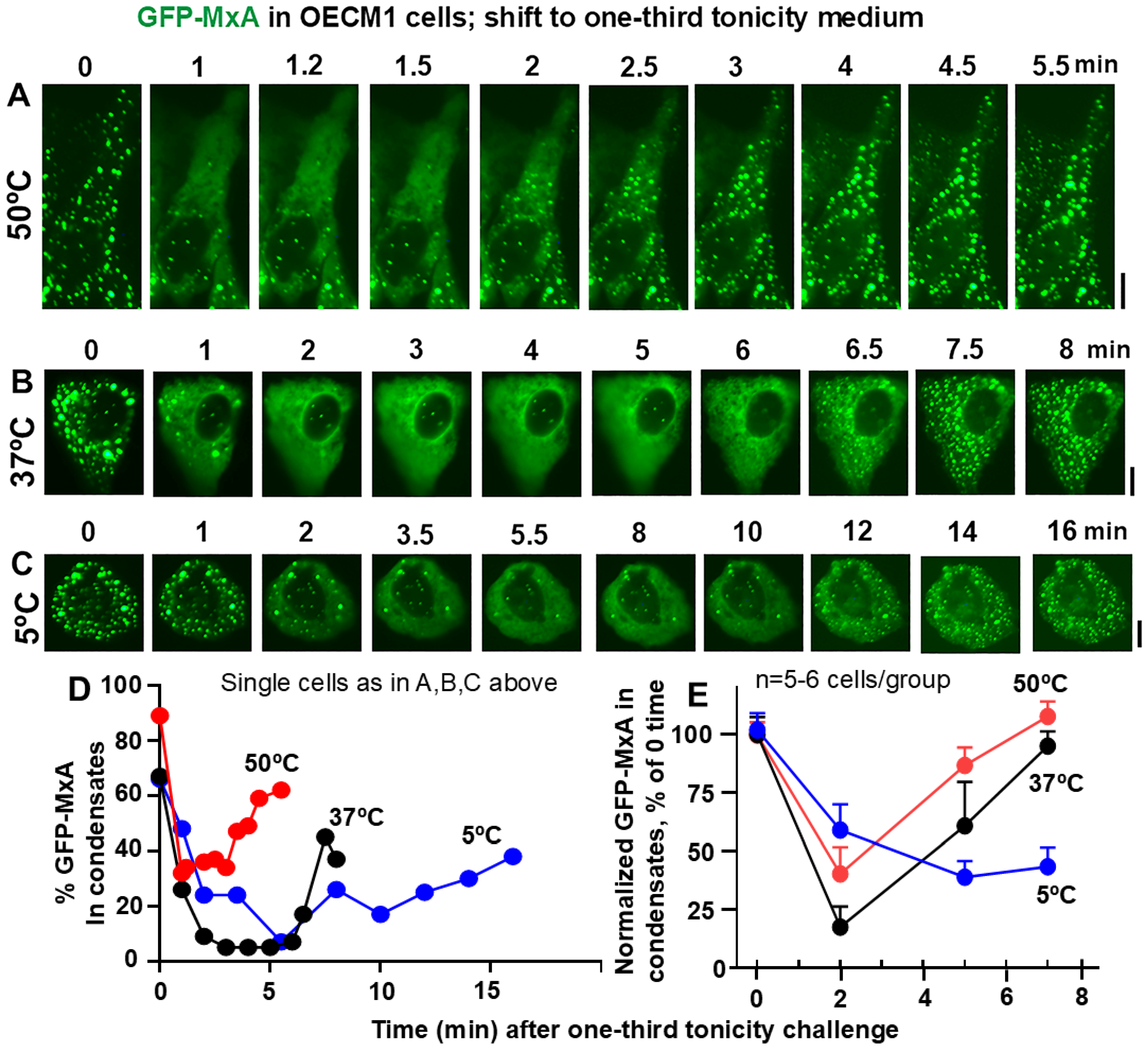
3.2. Temperature Sensitivity of the Spontaneous Reassembly of GFP-MxA Condensates Dispersed by a Hypotonic Challenge
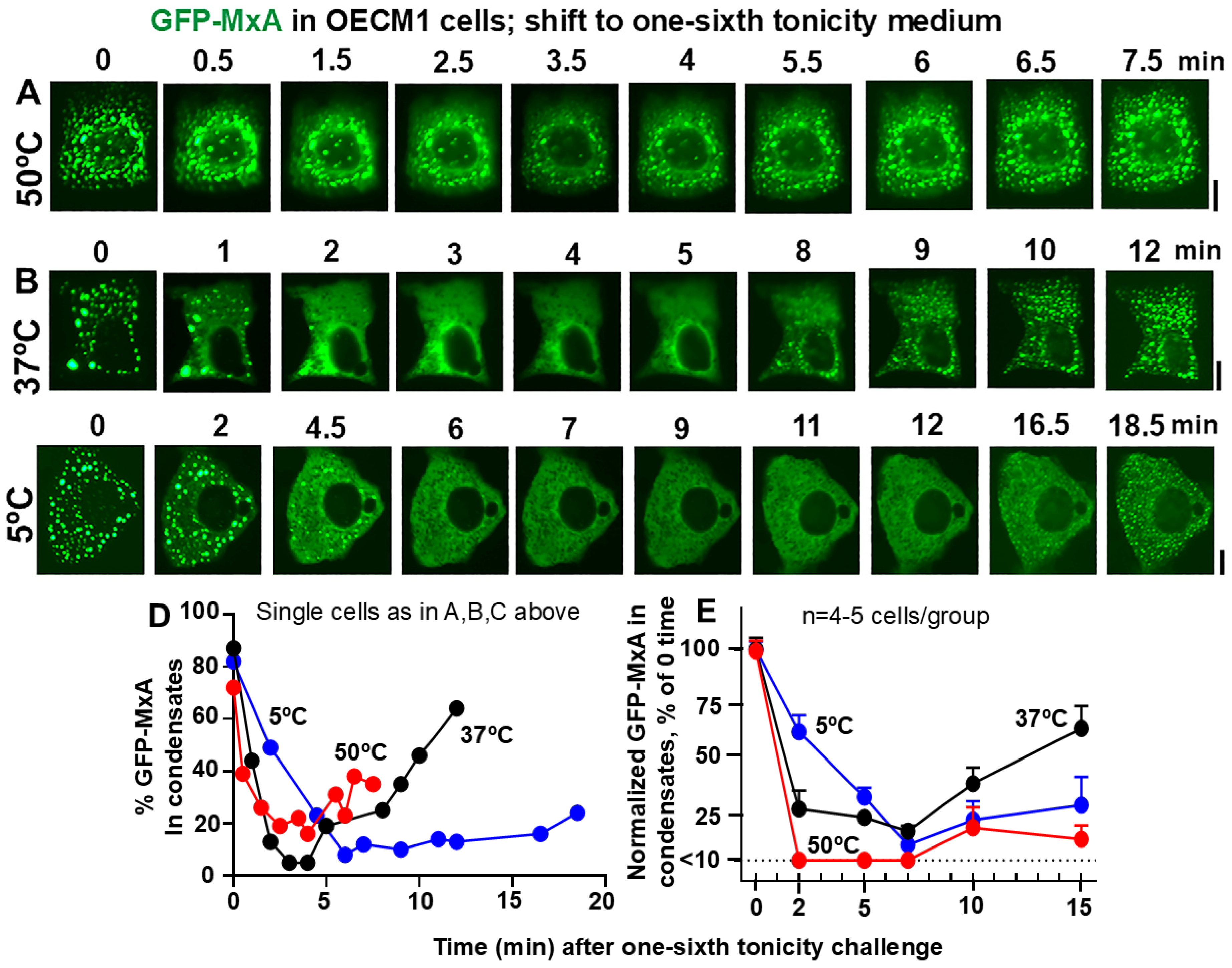
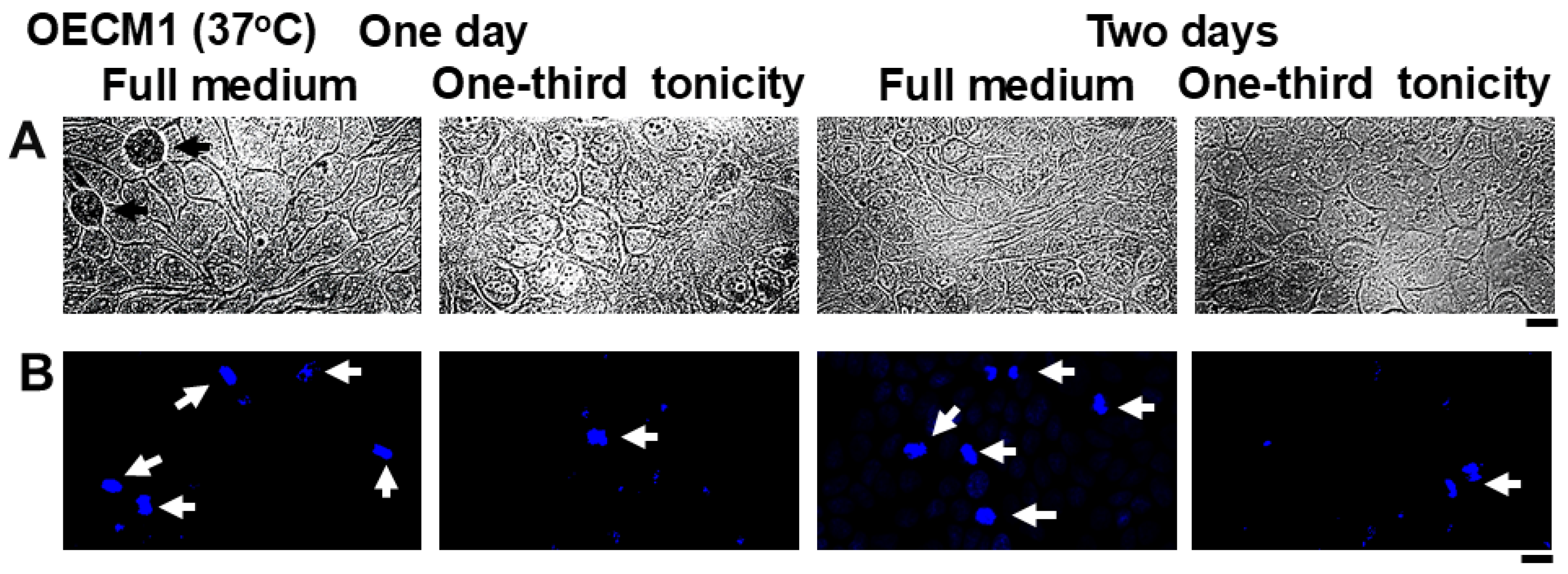
3.3. Resistance of Oral Carcinoma Cells to Saliva-like Hypotonicity
3.4. Involvement of the WNK-SPAK/OSR1 Kinase Pathway in the Dynamic Response of GFP-MxA Condensates to Hypotonicity
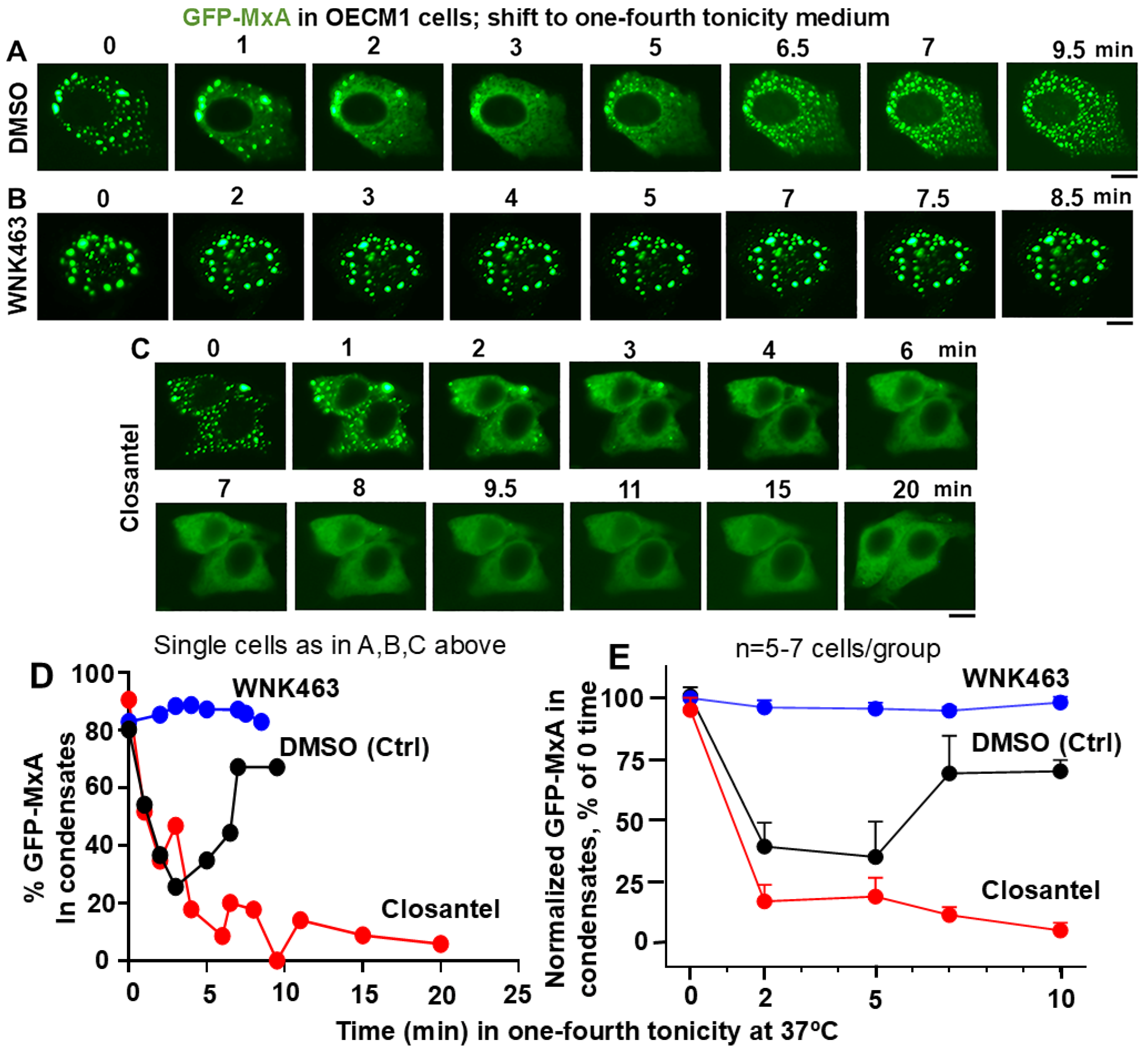
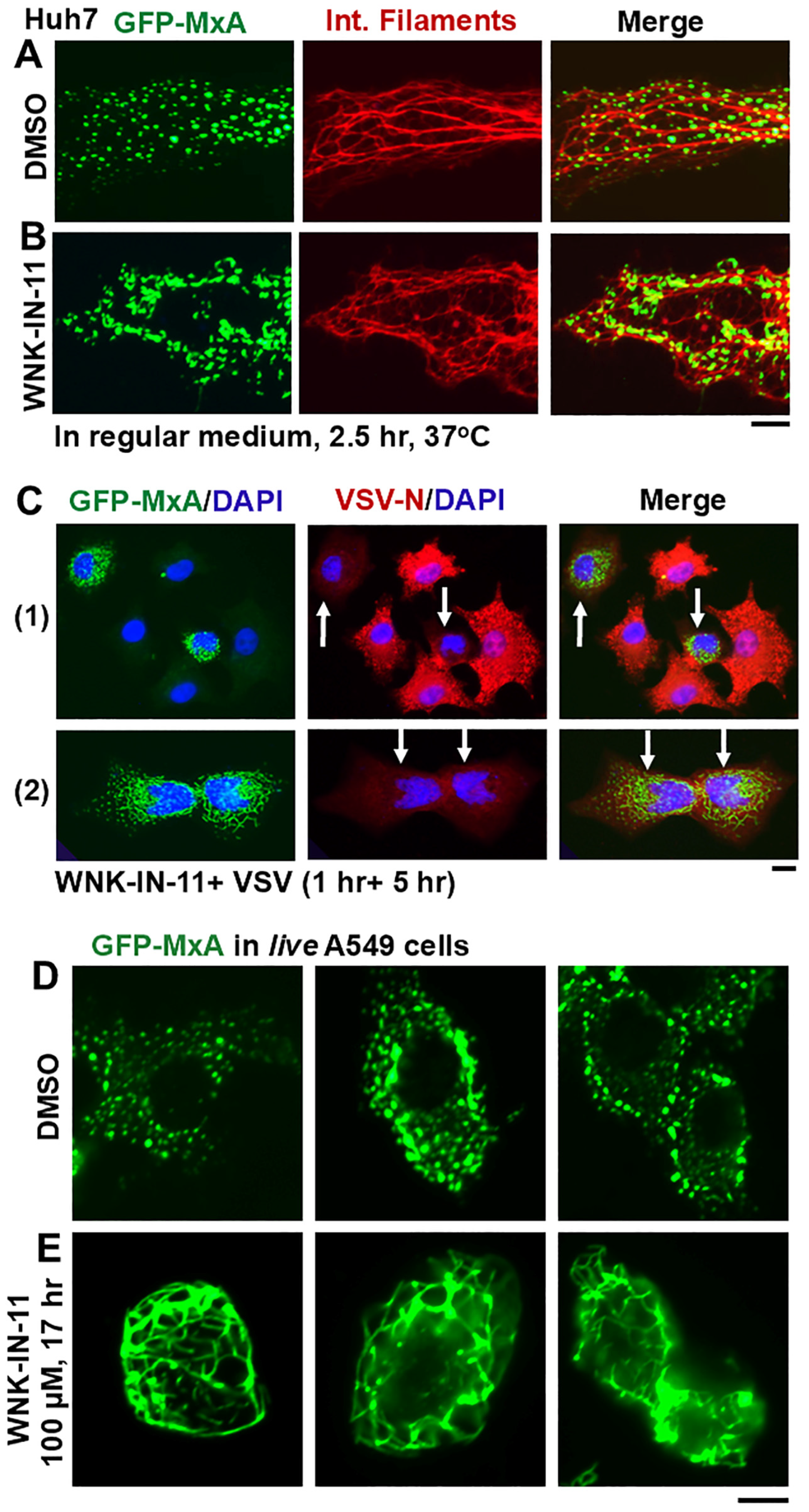
3.5. Dramatic and Rapid Spheroid to Fibril Transition of GFP-MxA Condensates in Live Cells Triggered by the WNK1-Kinase-Selective Inhibitor WNK-IN-11

3.6. Relationship(s) Between GFP-MxA Condensates and the Antiviral Phenotype Against VSV at the Single-Cell Level
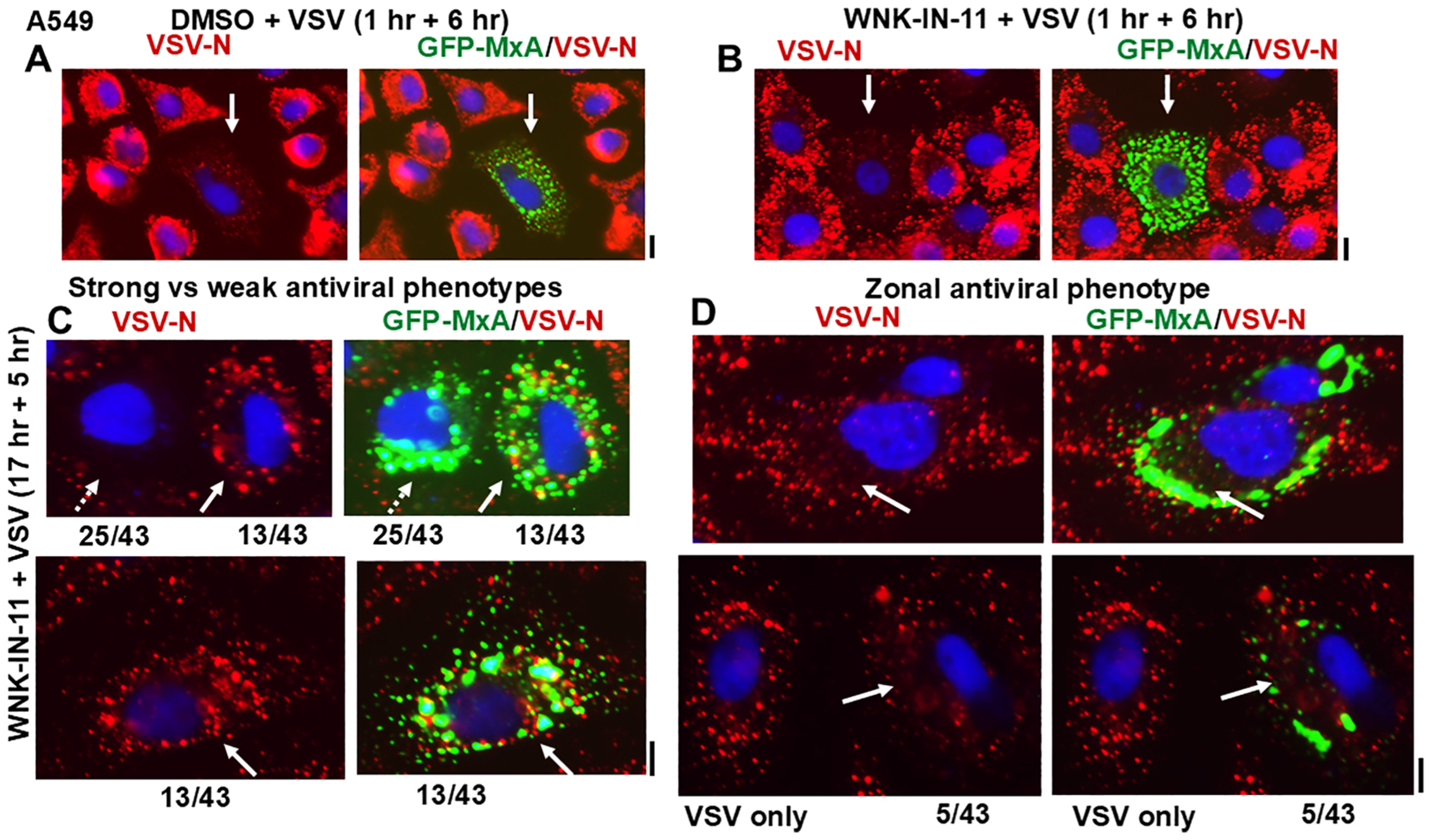
4. Discussion

5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paymaster, J.C. Some observations on oral and pharyngeal carcinomas in the State of Bombay. Cancer 1962, 15, 578–583. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.-K.; Lee, B.-U.; Lee, H.-J.; Cho, N.-P.; Yoon, J.-H.; Choi, H.-R.; Lee, S.-K.; Kim, E.-C. Upregulation of heme oxygenase-1 in oral epithelial dysplasias. Oral. Maxillofac. Surg. 2008, 37, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Mashberg, A.; Meyers, H. Anatomical site and size of 222 early asymptomatic oral squamous cell carcinomas. Cancer 1976, 37, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.A. The permeability of keratinized and non-keratinized oral epithelium to horse radish peroxidase. J. Ultrastruct. Res. 1973, 43, 160–177. [Google Scholar] [CrossRef]
- Lesch, C.A.; Squier, C.A.; Cruchley, A.; Williams, D.M.; Speight, P. The permeability of human oral mucosa and skin to water. J. Dent. Res. 1989, 68, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, P.; Eslami, H.; Yousefi, M.; Asgharzadeh, M.; Aghazadeh, M.; Kafil, H.S. Role of oral microbiome on oral cancers: A review. Biomed. Pharmacother. 2016, 84, 552–558. [Google Scholar] [CrossRef]
- Galvin, S.; Moran, G.P.; Healy, C.M. Influence of site and smoking on malignant transformation in the oral cavity: Is microbiome the missing link? Front. Oral. Health 2023, 4, 1166037. [Google Scholar] [CrossRef]
- Lim, Y.X.; D’Silva, N.J. HPV-associated oropharyngeal cancer: In search of surrogate biomarkers for early lesions. Oncogene 2024, 43, 543–554. [Google Scholar] [CrossRef]
- Santhanam, U.; Ray, A.; Sehgal, P.B. Repression of the interleukin 6 gene promoter by p53 and the retinoblastoma susceptibility gene product. Proc. Natl. Acad. Sci. USA 1991, 88, 7605–7609. [Google Scholar] [CrossRef]
- Margulies, L.; Sehgal, P.B. Modulation of the human interleukin-6 promoter (IL-6) and transcription factor C/EBP β (NF-IL6) activity by p53 species. J. Biol. Chem. 1993, 268, 15096–15100. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, P.B. Biomolecular condensates in cancer cell biology: Interleukin-6-induced cytoplasmic and nuclear STAT3/PY-STAT3 condensates in hepatoma cells. Contemp. Oncol. 2019, 23, 16–22. [Google Scholar] [CrossRef]
- Sehgal, P.B. Interleukin-6 at the host-tumor interface: STAT3 in biomolecular condensates in cancer cells. Cells 2022, 11, 1164. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Wang, L.; Long, Y.; Tian, H.; Xu, X.; Ren, Z.; Han, Y.; Jiang, X.; Wu, Z.; Tan, S.; et al. SRSF9 mediates oncogenic RNA splicing of SLC37A4 via liquid-liquid phase separation to promote oral cancer progression. J. Adv. Res. 2025, in press. [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, P.B.; Westley, J.; Lerea, K.M.; DiSenso-Browne, S.; Etlinger, J.D. Biomolecular condensates in cell biology and virology: Phase-separated membraneless organelles (MLOs). Anal. Biochem. 2020, 597, 113691. [Google Scholar] [CrossRef]
- Jiang, L.; Kang, Y. Biomolecular condensates: A new lens on cancer biology. BBA Rev. Cancer 2025, 1880, 189245. [Google Scholar] [CrossRef]
- Boija, A.; Klein, I.A.; Young, R.A. Biomolecular condensates and cancer. Cancer Cell 2021, 39, 174–192. [Google Scholar] [CrossRef]
- Han, T.W.; Portz, B.; Young, R.A.; Boija, A.; Klein, I.A. RNA and condensates: Disease implications and therapeutic opportunities. Cell Chem. Biol. 2020, 31, 1593–1609. [Google Scholar] [CrossRef]
- Tripathi, S.; Shimekhi, H.K.; Gorman, S.D.; Chandra, B.; Baggett, D.W.; Park, C.-G.; Somjee, R.; Lang, B.; Hosseini, S.M.H.; Pioso, B.J.; et al. Defining the condensate landscape of fusion oncoproteins. Nat. Commun. 2023, 14, 6008. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Stainier, W.; Dufourt, J.; Lagha, M.; Lehmann, R. Direct observation of translational activation by a ribionucleoprotein granule. Nat. Cell Biol. 2024, 26, 1322–1335. [Google Scholar] [CrossRef]
- Davis, D.; Yuan, H.; Liang, F.X.; Yang, Y.M.; Westley, J.; Petzold, C.; Dancel-Manning, K.; Deng, Y.; Sall, J.; Sehgal, P.B. Human antiviral protein MxA forms novel metastable membraneless cytoplasmic condensates exhibiting rapid reversible tonicity- driven phase transitions. J. Virol. 2019, 93, e01014-19. [Google Scholar] [CrossRef]
- Heinrich, B.S.; Maliga, Z.; Stein, D.A.; Hyman, A.A.; Whelan, S.P.J. Phase transitions drive the formation of vesicular stomatitis virus replication compartments. MBio 2018, 9, e02290-17. [Google Scholar] [CrossRef] [PubMed]
- Dinh, P.X.; Beurs, L.K.; Das, P.B.; Panda, D.; Das, A.; Pattnaik, A.K. Induction of stress granule-like structures in vesicular stomatitis virus-infected cells. J. Virol. 2013, 87, 372–383. [Google Scholar] [CrossRef]
- Nikolic, J.; Bars, R.L.; Lama, Z.; Scrima, N.; Lagaudriere-Gesbet, C.; Gaudin, Y.; Blondel, D. Negri bodies are viral factories with properties of liquid organelles. Nat. Commun. 2017, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Hoenen, T.; Shabman, R.S.; Groseth, A.; Herwig, A.; Weber, M.; Schudt, G.; Dolnik, O.; Basler, C.F.; Becker, S.; Feldmann, H. Inclusion bodies are a site of ebolavirus replication. J. Virol. 2012, 86, 11779–11788. [Google Scholar] [CrossRef]
- Alenquer, M.; Vale-Costa, S.; Sousa, A.L.; Etibor, T.A.; Ferreira, F.; Amorim, M.J. Influenza A virus ribonucleoproteins form liquid organelles at endoplasmic reticulum exit sites. Nat. Commun. 2019, 10, 1629. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, L.; Qin, Z.; Wang, J.; Zheng, X.; Wei, L.; Zhang, X.; Zhang, X.; Liu, C.; Li, Z.; et al. Phase separation of Epstein-Barr virus EBNA2 and its coactivator EBNALP controls gene expression. J. Virol. 2020, 94, e01771-19. [Google Scholar] [CrossRef]
- Cubuk, J.; Alston, J.J.; Incicco, J.J.; Singh, S.; Stuchell-Brereton, M.D.; Ward, M.D.; Zimmerman, M.I.; Vithani, N.; Griffith, D.; Wagoner, J.A.; et al. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. Nat. Commun. 2021, 12, 1936. [Google Scholar] [CrossRef]
- Perdikari, T.M.; Murthy, A.C.; Ryan, V.H.; Watters, S.; Naik, M.T.; Fawzi, N.L. SARS-CoV-2 nucleocapsid protein undergoes liquid-liquid phase separation stimulated by RNA and partitions into phases of human ribonucleoproteins. EMBO J. 2020, 39, e106478. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.; Yuan, H.; Yang, Y.M.; Liang, F.X.; Sehgal, P.B. Interferon-α-induced cytoplasmic MxA structures in hepatoma Huh7 and primary endothelial cells. Contemp. Oncol. 2018, 22, 86–94. [Google Scholar] [CrossRef]
- Haller, O.; Kochs, G. Interferon-induced mx proteins: Dynamin-like GTPases with antiviral activity. Traffic 2002, 3, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Staeheli, P.; Kochs, G. Interferon-induced Mx proteins in antiviral host defense. Biochimie 2007, 89, 812–818. [Google Scholar] [CrossRef]
- Haller, O.; Staeheli, P.; Schwemmle, M.; Kochs, G. Mx GTPases: Dynamin-like antiviral machines of innate immunity. Trends Microbiol. 2015, 23, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Verhelst, J.; Hulpiau, P.; Saelens, X. Mx proteins: Antiviral gatekeepers that restrain the uninvited. Microbiol. Mol. Biol. Rev. 2013, 77, 551–566. [Google Scholar] [CrossRef]
- Staeheli, P.; Pavlovic, J. Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J. Virol. 1991, 65, 4498–4501. [Google Scholar] [CrossRef]
- Schwemmle, M.; Weining, K.C.; Richter, M.F.; Shumacher, B.; Staeheli, P. Vesicular stomatitis virus transcription inhibited by purified MxA protein. Virology 1996, 206, 545–554. [Google Scholar] [CrossRef]
- Steiner, F.; Pavlovic, J. Subcellular localization of MxB determines its antiviral potential against influenza virus. J. Virol. 2020, 94, e00125-20. [Google Scholar] [CrossRef]
- Kochs, G.; Haener, M.; Aebi, U.; Haller, O. Self-assembly of human MxA GTPase into highly ordered dynamin-like oligomers. J. Biol. Chem. 2002, 277, 14172–14176. [Google Scholar] [CrossRef]
- Kochs, G.; Janzen, C.; Hohenberg, H.; Haller, O. Antivirally active MxA protein sequesters La Crosse virus nucleocapsid protein into perinuclear complexes. Proc. Natl. Acad. Sci. USA 2002, 99, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Gao, S.; von der Malsburg, A.; Daumke, O.; Kochs, G. Dynamin-like MxA GTPase: Structural insights into oligomerization and implications for antiviral activity. J. Biol. Chem. 2010, 285, 28419–28424. [Google Scholar] [CrossRef]
- Nigg, P.E.; Pavlovic, J. Oligomerization and GTP-binding requirements of MxA for viral target recognition and antiviral activity against influenza A virus. J. Biol. Chem. 2015, 290, 29893–29906. [Google Scholar] [CrossRef]
- Dick, A.; Graf, L.; Olal, D.; von der Malsburg, A.; Gao, S.; Kochs, G.; Daumke, O. Role of nucleotide binding and GTPase domain dimerization in dynamin-like myxovirus resistance protein A for GTPase activation and antiviral activity. J. Biol. Chem. 2015, 290, 12779–12792. [Google Scholar] [CrossRef]
- Graf, L.; Dick, A.; Sendker, F.; Barth, E.; Marz, M.; Daumke, O.; Kochs, G. Effects of allelic variations in the human myxovirus resistance protein A on its antiviral activity. J. Biol. Chem. 2018, 293, 3056–3072. [Google Scholar] [CrossRef]
- Janzen, C.; Kochs, G.; Haller, O. A monomeric GTPase-negative MxA mutant with antiviral activity. J. Virol. 2000, 74, 8202–8206. [Google Scholar] [CrossRef] [PubMed]
- DiPaolo, C.; Hefti, H.P.; Meli, M.; Landis, H.; Pavlovic, J. Intramolecular backfolding of the carboxyl-terminal end of MxA protein is a prerequisite for its oligomerization. J. Biol. Chem. 1999, 274, 32071–32078. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, P.B.; Yuan, H.; Centone, A.; DiSenso-Browne, S.V. Oral antiviral defesnse: Saliva- and beverage-like hypotonicity dynamically regulate formation of membraneless biomolecular condensates of antiviral human MxA in oral epithelial cells. Cells 2024, 13, 590. [Google Scholar] [CrossRef]
- Sehgal, P.B.; Yuan, H.; Jin, Y. Rapid reversible osmoregulation of cytoplasmic biomolecular condensates of human interferon-α-induced antiviral MxA GTPase. Int. J. Mol. Sci. 2022, 23, 12739. [Google Scholar] [CrossRef]
- Mahanonda, R.; Sa-Ard-Iam, N.; Rerkyen, P.; Thitithanyanont, A.; Subbalekha, K.; Pichyangkul, S. MxA expression induced by α-defensin in healthy human periodontal tissue. Eur. J. Immunol. 2012, 42, 946–956. [Google Scholar] [CrossRef]
- Imangulli, M.M.; Swaim, W.D.; League, S.C.; Gress, R.E.; Pavletic, S.Z.; Hakim, F.T. Increased T-bet+ cytotoxic effectors and type I interferon-mediated processes in chronic graft-versus-host disease of the oral mucosa. Blood 2009, 113, 3620–3630. [Google Scholar] [CrossRef] [PubMed]
- Rodriquez-Hernandez, C.J.; Sokolski, K.J.; Stocke, K.S.; Dukka, H.; Jin, S.; Metzler, M.A.; Zaitsev, K.; Shpak, B.; Shen, D.; Miller, D.P.; et al. Microbiome-mediated incapacitation of interferon lambda production in the oral mucosa. Proc. Natl. Acad. Sci. USA 2021, 118, e2105170118. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.T.B.R.; Ferreira, M.C.D.; Guare, R.O.; Diniz, M.B.; Rösing, C.K.; Rodrigues, J.A.; Duarte, D.A. Gingivitis and salivary osmolality in children with cerebral palsy. Int. J. Paediatr. Dent. 2016, 26, 463–470. [Google Scholar] [CrossRef]
- Ruiz, L.A.; Diniz, M.B.; Loyola-Rodriguez, J.P.; Habibe, C.; Garrubbo, C.; Santos, M. A controlled study comparing salivary osmolality, caries experience and caries risk in patients with cerebral palsy. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e211–e215. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Barnett, C. Relationships between the acidity and osmolality of popular beverages and reported postprandial heartburn. Gasteroenterology 1995, 108, 125–131. [Google Scholar] [CrossRef]
- Gresz, V.; Kwon, T.H.; Hurley, P.T.; Varga, G.; Zelles, T.; Nielsen, S.; Case, R.M.; Steward, M.C. Identification and localization of aquaporin water channels in human salivary glands. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G247–G254. [Google Scholar] [CrossRef]
- Shen, M.R.; Chou, C.Y.; Ellory, J.C. Volume-sensitive KCl cotransport associated with human cervical carcinogenesis. Pflugers Archiv. 2000, 440, 751–760. [Google Scholar] [CrossRef]
- Bize, I.; Guvenc, B.; Robb, A.; Buchbinder, G.; Brugnara, C. Serine/threonine protein phosphatases a2d regulation of K-Cl cotransport in human erythrocytes. Am. J. Physiol. 1999, 277, C926–C936. [Google Scholar] [CrossRef]
- Akella, R.; Humphreys, J.M.; Sekulski, K.; He, H.; Durbacz, M.; Chakravarthy, S.; Liwocha, J.; Mohammed, Z.J.; Brautigam, C.A.; Goldsmith, E.J.; et al. Osmosensing by WNK kinases. Mol. Biol. Cell 2021, 32, 1614–1623. [Google Scholar] [CrossRef]
- Boyd-Shiwarski, C.R.; Shiwarski, D.J.; Griffths, S.E.; Beacham, R.T.; Norrell, L.; Morrison, D.E.; Wang, J.; Mann, J.; Tennant, W.; Anderson, E.N.; et al. WNK kinases sense molecular crowding and rescue cell volume via phase separation. Cell 2022, 185, 4488–4506. [Google Scholar] [CrossRef]
- Xiu, M.; Li, Y.; Gao, Y. An update regarding the role of WNK kinases in cancer. Cell Death Dis. 2022, 13, 795. [Google Scholar] [CrossRef]
- Teixeira, L.R.; Akella, R.; Humphreys, J.M.; He, H.; Goldsmith, E.J. Water and chloride as allosteric inhibitors in WNK kinase osmosensing. eLife 2024, 12, RP88224. [Google Scholar] [CrossRef]
- Carey, B.L.; Ahmed, M.; Puckett, S.; Lyles, D.S. Early steps of the virus replication cycle are inhibited in prostate cancer cells resistant to oncolytic vesicular stomatitis virus. J. Virol. 2008, 82, 12104–12115. [Google Scholar] [CrossRef]
- Sehgal, P.B.; Yuan, H.; Scott, M.F.; Deng, Y.; Liang, F.-X.; Mackiewicz, A. Murine GFP-Mx1 forms phase-separated nuclear condensates and associates with cytoplasmic intermediate filaments: Novel antiviral activity against vesicular stomatitis virus. J. Biol. Chem. 2020, 294, 15218–15234. [Google Scholar]
- Thomson, M.N.; Cuevas, C.A.; Bewarder, T.M.; Dittmayer, C.; Miller, L.N.; Si, J.; Cornelius, R.J.; Su, X.-T.; Yang, C.-L.; McCormick, J.A.; et al. WNK bodies cluster WNK4 and SPAK/OSR1 to promote NCC activation in hypokalemia. Am. J. Physiol. Renal Physiol. 2020, 318, F216–F228. [Google Scholar] [CrossRef]
- Zhu, W.; Begum, G.; Pointer, K.; Clark, P.A.; Yang, S.S.; Lin, S.H.; Kahle, K.T.; Kuo, J.S.; Sun, D. WMK1-OSR1 kinase-mediated phospho-activation of Na+-K+-2Cl- cotransporter facilitates glioma migration. Mol. Cancer 2014, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.L., III. Water and sodium balance. In Merck Manual Professional Version; Merck & Co., Inc.: Rahway, NJ, USA, 2024. [Google Scholar]
- Haas, B.R.; Cuddapah, V.A.; Watkins, S.; Rohn, K.J.; Dy, T.E.; Sontheimer, H. With-No-Lysine kinase 3 (WNK3 stimulates glioma invasion by regulating cell volume. Am. J. Physiol. Cell Physiol. 2011, 301, C1150–C1160. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, D.; Yu, S.; Ma, M.; Fan, K.; Chen, J.; Xiu, M.; Xie, K.; Li, Y.; Gao, Y. WNK1 interaction with KEAP1 promotes NRF2 stabilization to enhance the oxidative stress response in hepatocellular carcinoma. Cancer Res. 2024, 84, 2776–2791. [Google Scholar] [CrossRef]
- Kim, S.; Kehri, J.H. Inhibition of WNK kinases in NK cells disrupts cellular osmoregulation and control of tumor metastasis. J. Innate Immunol. 2024, 16, 451–469. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sehgal, P.B.; Yuan, H.; DiSenso-Browne, S.V. Temperature and WNK-SPAK/OSR1 Kinases Dynamically Regulate Antiviral Human GFP-MxA Biomolecular Condensates in Oral Cancer Cells. Cells 2025, 14, 947. https://doi.org/10.3390/cells14130947
Sehgal PB, Yuan H, DiSenso-Browne SV. Temperature and WNK-SPAK/OSR1 Kinases Dynamically Regulate Antiviral Human GFP-MxA Biomolecular Condensates in Oral Cancer Cells. Cells. 2025; 14(13):947. https://doi.org/10.3390/cells14130947
Chicago/Turabian StyleSehgal, Pravin B., Huijuan Yuan, and Susan V. DiSenso-Browne. 2025. "Temperature and WNK-SPAK/OSR1 Kinases Dynamically Regulate Antiviral Human GFP-MxA Biomolecular Condensates in Oral Cancer Cells" Cells 14, no. 13: 947. https://doi.org/10.3390/cells14130947
APA StyleSehgal, P. B., Yuan, H., & DiSenso-Browne, S. V. (2025). Temperature and WNK-SPAK/OSR1 Kinases Dynamically Regulate Antiviral Human GFP-MxA Biomolecular Condensates in Oral Cancer Cells. Cells, 14(13), 947. https://doi.org/10.3390/cells14130947






