An Old New Friend: Folliculo-Stellate Cells in Pituitary Neuroendocrine Tumors
Abstract
1. Introduction
2. Materials and Methods
3. Results
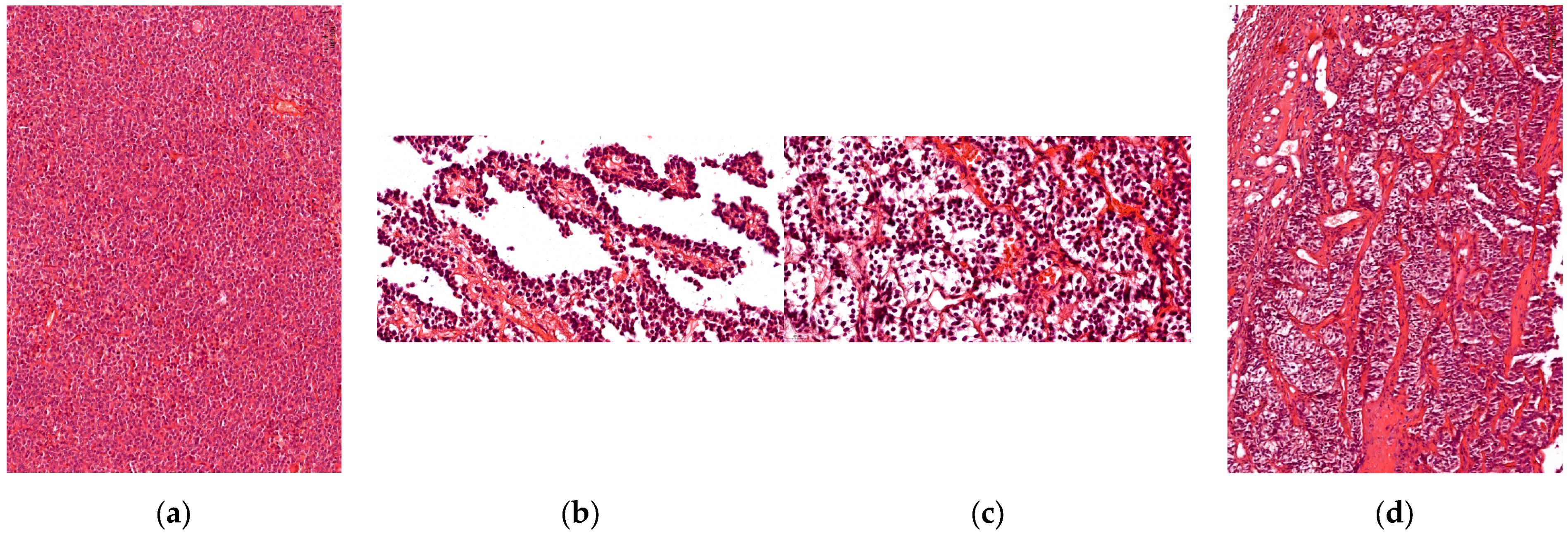
3.1. Histophatological Evaluation
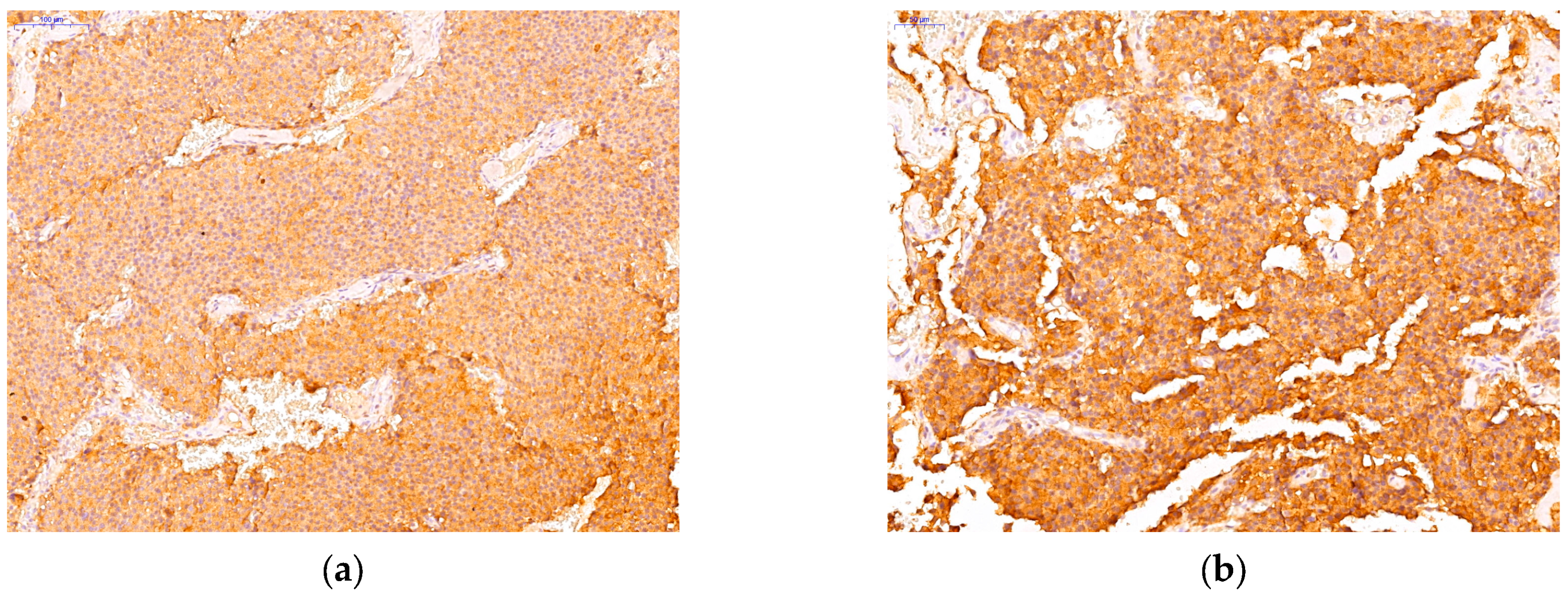
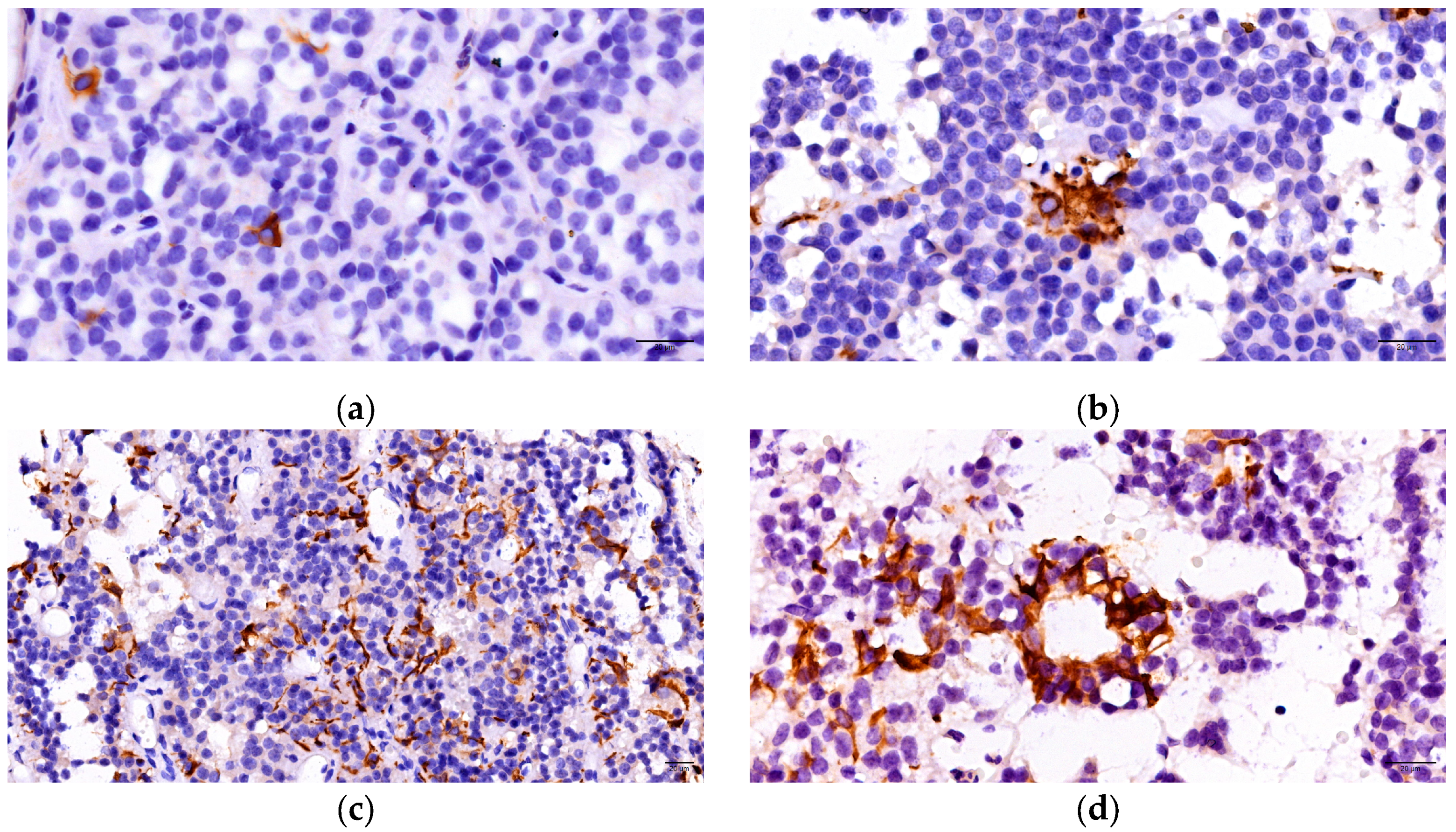
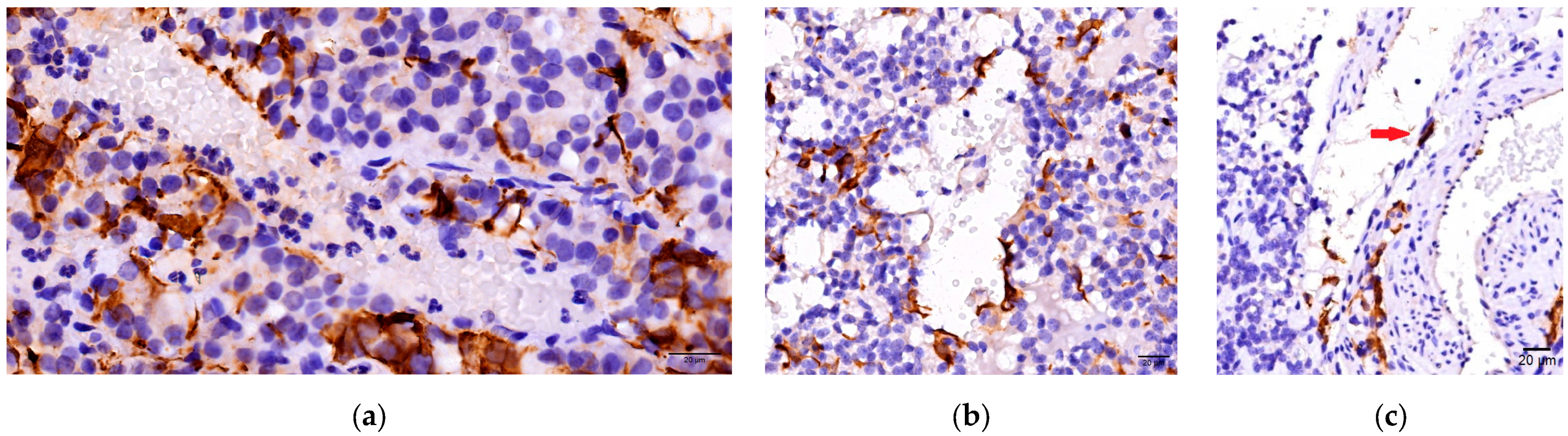
3.2. Immunohistochemichal Analysis
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilie, M.D.; Vasiljevic, A.; Raverot, G.; Bertolino, P. The Microenvironment of Pituitary Tumors-Biological and Therapeutic Implications. Cancers 2019, 11, 1605. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. (Eds.) WHO Classification of Tumours of Endocrine Organs, 4th ed.; International Agency for Research on Cancer IARC: Lyon, France, 2017; print. [Google Scholar]
- Inoshita, N.; Nishioka, H. The 2017 who classification of pituitary adenoma: Overview and comments. Brain Tumor Pathol. 2018, 35, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125 Pt 23, 5591–5596. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Couch, E.F.; Takano, K.; Ogawa, S. The structure and function of folliculo-stellate cells in the anterior pituitary gland. Arch. Histol. Cytol. 1999, 62, 205–218. [Google Scholar] [CrossRef]
- Inoue, K.; Mogi, C.; Ogawa, S.; Tomida, M.; Miyai, S. Are folliculo-stellate cells in the anterior pituitary gland supportive cells or organ-specific stem cells? Arch. Physiol. Biochem. 2002, 110, 50–53. [Google Scholar] [CrossRef]
- Rinehart, J.F.; Farquhar, M.G. Electron microscopic studies of the anterior pituitary gland. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1953, 1, 93–113. [Google Scholar] [CrossRef]
- Vila-Porcile, E. Le réseau des cellules folliculo-stellaires et les follicules de l’adénohypophyse du rat (Pars distalis). Z. Für Zellforsch Und Mikrosk. Anat 1972, 129, 328–369. [Google Scholar] [CrossRef]
- Velasco, M.E.; Roessmann, U.; Gambetti, P. The presence of glial fibrillary acidic protein in the human pituitary gland. J. Neuropathol. Exp. Neurol. 1982, 41, 150–163. [Google Scholar] [CrossRef]
- Devnath, S.; Inoue, K. An insight to pituitary folliculo-stellate cells. J. Neuroendocrinol. 2008, 20, 687–691. [Google Scholar] [CrossRef]
- Shirasawa, N.; Kihara, H.; Yamaguchi, S.; Yoshimura, F. Pituitary folliculo-stellate cells immunostained with S-100 protein antiserum in postnatal, castrated and thyroidectomized rats. Cell Tissue Res. 1983, 231, 235–249. [Google Scholar] [CrossRef]
- Roberts, W.G.; Palade, G.E. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J. Cell Sci. 1995, 108 Pt 6, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Drewett, N.; Jacobi, J.M.; Willgoss, D.A.; Lloyd, H.M. Apoptosis in the anterior pituitary gland of the rat: Studies with estrogen and bromocriptine. Neuroendocrinology 1993, 57, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, F.; Soji, T.; Kiguchi, Y. Relationship between the follicular cells and marginal layer cells of the anterior pituitary. Endocrinol. Jpn. 1977, 24, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, H.; Llena, J.F.; Hirano, A. Immunohistochemical study of folliculostellate cells in pituitary lesions. Endocr. Pathol. 1991, 2, 155–160. [Google Scholar] [CrossRef]
- Delfin, L.; Mete, O.; Asa, S.L. Follicular cells in pituitary neuroendocrine tumors. Hum. Pathol. 2021, 114, 1–8. [Google Scholar] [CrossRef]
- Trouillas, J.; Jaffrain-Rea, M.L.; Vasiljevic, A.; Raverot, G.; Roncaroli, F.; Villa, C.C. How to classify the pituitary neuroendocrine tumors (PitNET)s in 2020. Cancers 2020, 12, 514. [Google Scholar] [CrossRef]
- Asa, S.L.; Casar-Borota, O.; Chanson, P.; Delgrange, E.; Earls, P.; Ezzat, S.; Grossman, A.; Ikeda, H.; Inoshita, N.; Karavitaki, N.; et al. From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): An International Pituitary Pathology Club proposal. Endocr. Relat. Cancer 2017, 24, C5–C8. Available online: https://erc.bioscientifica.com/view/journals/erc/24/4/C5.xml (accessed on 3 March 2025). [CrossRef]
- Lv, L.; Jiang, Y.; Yin, S.; Hu, Y.; Chen, C.; Ma, W.; Jiang, S.; Zhou, P. Mammosomatotroph and mixed somatotroph-lactotroph adenoma in acromegaly: A retrospective study with long-term follow-up. Endocrine 2019, 66, 310–318. [Google Scholar] [CrossRef]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef]
- Voit, D.; Saeger, W.; Lüdecke, D.K. Folliculo-stellate cells in pituitary adenomas of patients with acromegaly. Pathol. Res. Pract. 1999, 195, 143–147. [Google Scholar] [CrossRef]
- Coates, P.J.; Doniach, I. Development of folliculo-stellate cells in the human pituitary. Acta Endocrinol. 1988, 119, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.; Kovacs, K.; Stefaneanu, L.; Horvath, E.; Cheng, Z. S-100 protein immunopositivity in human nontumorous hypophyses and pituitary adenomas. Endocr. Pathol. 1992, 3, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Mailloux, J. Analysis of S-100 protein positive folliculo-stellate cells in rat pituitary tissues. Am. J. Pathol. 1988, 133, 338–346. [Google Scholar] [PubMed]
- Ekici, A.I.D.; Eren, B.; Türkmen, N.; Comunoğlu, N.; Fedakar, R. Clusterin expression in non-neoplastic adenohypophyses and pituitary adenomas: Cytoplasmic clusterin localization in adenohypophysis is related to aging. Endocr. Pathol. 2008, 19, 47–53. [Google Scholar] [CrossRef]
- Sultana, P.; Novotny, J. Clusterin: A double-edged sword in cancer and neurological disorders. EXCLI J. 2024, 23, 912–936. [Google Scholar]
- Chesnokova, V.; Zonis, S.; Zhou, C.; Ben-Shlomo, A.; Wawrowsky, K.; Toledano, Y.; Tong, Y.; Kovacs, K.; Scheithauer, B.; Melmed, S.; et al. Lineage-specific restraint of pituitary gonadotroph cell adenoma growth. PLoS ONE 2011, 6, e17924. [Google Scholar] [CrossRef]
- Chesnokova, V.; Zonis, S.; Wawrowsky, K.; Tani, Y.; Ben-Shlomo, A.; Ljubimov, V.; Mamelak, A.; Bannykh, S.; Melmed, S. Clusterin and FOXL2 act concordantly to regulate pituitary gonadotroph adenoma growth. Mol. Endocrinol. 2012, 26, 2092–2103. [Google Scholar] [CrossRef]
- Asa, S.L.; Faiman, G.H.; Mohamed, A.; Mete, O. Multilineage Pituitary Neuroendocrine Tumors Expressing TPIT and SF1: A Clinicopathological Series of Six Tumors. Endocr. Pathol. 2024, 35, 349–353. [Google Scholar] [CrossRef]
- Doğukan, F.M.; Karatay, H.; Yüzkan, S.; Burhan, Ş.; Erkan, B.; Yılmaz-Özgüven, B. Clinicopathologic Correlates of PIT1 and SF1-Multilineage Pituitary Neuroendocrine Tumors and the Diagnostic Utility of NKX2.2 Immunohistochemistry in Pituitary Pathology. Arch. Pathol. Lab. Med. 2025, 149, 83–89. [Google Scholar] [CrossRef]
- Neou, M.; Villa, C.; Armignacco, R.; Jouinot, A.; Raffin-Sanson, M.-L.; Septier, A.; Letourneur, F.; Diry, S.; Diedisheim, M.; Izac, B.; et al. Pangenomic Classification of Pituitary Neuroendocrine Tumors. Cancer Cell. 2020, 37, 123–134.e5. [Google Scholar] [CrossRef]
- Tang, H.; Wang, X.; Bie, Z.; Yang, Z.; Wang, B.; Li, S.; Liu, P. Pituitary adenomas with multiple cell lineage combinations: Clinicopathological features and short-term prognosis. J. Neurosurg. 2023, 139, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Roca, E.; Mattogno, P.P.; Porcelli, T.; Poliani, L.; Belotti, F.; Schreiber, A.; Maffezzoni, F.; Fontanella, M.M.; Doglietto, F. Plurihormonal ACTH-GH Pituitary Adenoma: Case Report and Systematic Literature Review. World Neurosurg. 2018, 114, e158–64. [Google Scholar] [CrossRef] [PubMed]
- Soukup, J.; Česák, T.; Hornychová, H.; Michalová, K.; Michnová, Ľ.; Netuka, D.; Čáp, J.; Gabalec, F. Stem Cell Transcription Factor Sox2 Is Expressed in a Subset of Folliculo-stellate Cells of Growth Hormone-Producing Pituitary Neuroendocrine Tumours and Its Expression Shows No Association with Tumour Size or IGF1 Levels: A Clinicopathological Study of. Endocr. Pathol. 2020, 31, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Mogi, C.; Miyai, S.; Nishimura, Y.; Fukuro, H.; Yokoyama, K.; Takaki, A.; Inoue, K. Differentiation of skeletal muscle from pituitary folliculo-stellate cells and endocrine progenitor cells. Exp. Cell Res. 2004, 292, 288–294. [Google Scholar] [CrossRef]
- Le Tissier, P.R.; Mollard, P. Renewing an old interest: Pituitary folliculostellate cells. J. Neuroendocrinol. 2021, 33, e13053. [Google Scholar] [CrossRef]
- Carreno, G.; Gonzalez-Meljem, J.M.; Haston, S.; Martinez-Barbera, J.P. Stem cells and their role in pituitary tumorigenesis. Mol. Cell Endocrinol. 2017, 445, 27–34. [Google Scholar] [CrossRef]
- Würth, R.; Thellung, S.; Corsaro, A.; Barbieri, F.; Florio, T. Experimental Evidence and Clinical Implications of Pituitary Adenoma Stem Cells. Front. Endocrinol. 2020, 11, 54. [Google Scholar] [CrossRef]
- Lenders, N.F.; Thompson, T.J.; Chui, J.; Low, J.; Inder, W.J.; Earls, P.E.; McCormack, A.I. Pituitary tumours without distinct lineage differentiation express stem cell marker SOX2. Pituitary 2024, 27, 248–258. [Google Scholar] [CrossRef]
- Koyama, C.; Matsumoto, H.; Sakai, T.; Wakabayashi, K.; Ito, A.; Couch, E.F.; Inoue, K. Pituitary Folliculo-Stellate-Like Cells Stimulate Somatotroic Pituitary Tumor Growth in Nude Mice. Endocr. Pathol. 1995, 6, 67–75. [Google Scholar] [CrossRef]
- Horvath, E.; Kovacs, K. Folliculo-stellate cells of the human pituitary: A type of adult stem cell? Ultrastruct. Pathol. 2002, 26, 219–228. [Google Scholar] [CrossRef]
- Kurotani, R.; Tahara, S.; Sanno, N.; Teramoto, A.; Mellon, P.L.; Inoue, K.; Yoshimura, S.; Osamura, R.Y. Expression of Ptx1 in the adult rat pituitary glands and pituitary cell lines: Hormone-secreting cells and folliculo-stellate cells. Cell Tissue Res. 1999, 298, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, K.; Fujiwara, K.; Yoshida, S.; Nakakura, T.; Arae, K.; Tsukada, T.; Hasegawa, R.; Takigami, S.; Ohsako, S.; Yashiro, T.; et al. Isolation and characterisation of CD9-positive pituitary adult stem/progenitor cells in rats. Sci. Rep. 2018, 8, 5533. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, K.; Fujiwara, K.; Yoshida, S.; Tsukada, T.; Hasegawa, R.; Takigami, S.; Ohsako, S.; Yashiro, T.; Kato, T.; Kato, Y. CX3CL1/CX3CR1-signalling in the CD9/S100β/SOX2-positive adult pituitary stem/progenitor cells modulates differentiation into endothelial cells. Histochem. Cell Biol. 2020, 153, 385–396. [Google Scholar] [CrossRef] [PubMed]
| Expression Pattern | Company | Clone | Dilution Factor | |
|---|---|---|---|---|
| GH | Cytoplasmic | Dako Agilent 1 | Polyclonal rabbit anti-human | 1:400 |
| PRL | Cytoplasmic | Dako Agilent | Polyclonal rabbit anti-human | 1:300 |
| TSH | Cytoplasmic | Thermo Fisher Scientific 2 | TSH01 + TSH02 | 1:400 |
| ACTH | Cytoplasmic | Dako Agilent | C93 | 1:50 |
| FSH | Cytoplasmic | Thermo Fisher Scientific | FSH03 | 1:500 |
| LH | Cytoplasmic | Thermo Fisher Scientific | LH01 | 1:500 |
| PIT1 | Nuclear | Thermo Fisher Scientific | Rabbit polyclonal antibody | 1:500 |
| Anti-Tpit | Nuclear | Abcam 3 | CL6251 | 1:1000 |
| SF1 | Nuclear | Invitrogen (Thermo Fisher Scientific) | SF1 antibody PA5-79984 | 1:500 |
| GFAP | Cytoplasmic | Leica Bond 4 | GA5 | ready to use |
| GH | PRL | TSH | ACTH | FSH | LH | PIT1 | TPIT | SF1 | |
|---|---|---|---|---|---|---|---|---|---|
| Somatotroph (n = 19) | + (n = 19) | - | - | - | - | - | + (n = 19) | - | - |
| Mammosomatotroph (n = 16) | + (n = 16) | + (n = 16) | - | - | - | - | + (n = 16) | - | - |
| Plurihormonal PIT-1 positive (n = 5) | + (n = 5) | + (n = 4) | + (n = 5) | - | - | - | + (n = 5) | - | - |
| Corticotroph (n = 7) | - | - | - | + (n = 7) | - | - | - | + (n = 7) | - |
| Gonadotroph (n = 14) | - | - | - | - | + (n = 8) | + (n = 14) | - | - | + (n = 14) |
| Unusual Plurihormonal (n = 11) | + (n = 11) | - | + (n = 1) | + (n = 7) | - | + (n = 4) | + (n = 11) | + (n = 7) | + (n = 4) |
| Null cell (n = 5) | - | - | - | - | - | - | - | - | - |
| PitNET Subtype | Absent | Isolated | Small Groups | Diffuse Networks |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Somatotroph | 10 (12.99) | 1 (1.3) | 4 (5.19) | 4 (5.19) |
| Mammosomatotroph | 0 (0) | 5 (6.49) | 6 (7.79) | 5 (6.49) |
| Plurihormonal PIT-1 positive | 0 (0) | 3 (3.9) | 1 (1.3) | 1 (1.3) |
| Corticotroph | 2 (2.6) | 2 (2.6) | 2 (2.6) | 1 (1.3) |
| Gonadotroph | 4 (5.19) | 3 (3.9) | 2 (2.6) | 5 (6.49) |
| Unusual plurihormonal | 2 (2.6) | 2 (2.6) | 3 (3.9) | 4 (5.19) |
| Null cell | 4 (5.19) | 0 (0) | 1 (1.3) | 0 (0) |
| Total | 22 (29.9) | 16 (20.78) | 19 (24.68) | 20 (25.97) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nastase, V.-N.; Burcea, I.F.; Dumitriu-Stan, R.I.; Ceausu, A.R.; Zara, F.; Poiana, C.; Raica, M. An Old New Friend: Folliculo-Stellate Cells in Pituitary Neuroendocrine Tumors. Cells 2025, 14, 1019. https://doi.org/10.3390/cells14131019
Nastase V-N, Burcea IF, Dumitriu-Stan RI, Ceausu AR, Zara F, Poiana C, Raica M. An Old New Friend: Folliculo-Stellate Cells in Pituitary Neuroendocrine Tumors. Cells. 2025; 14(13):1019. https://doi.org/10.3390/cells14131019
Chicago/Turabian StyleNastase, Valeria-Nicoleta, Iulia Florentina Burcea, Roxana Ioana Dumitriu-Stan, Amalia Raluca Ceausu, Flavia Zara, Catalina Poiana, and Marius Raica. 2025. "An Old New Friend: Folliculo-Stellate Cells in Pituitary Neuroendocrine Tumors" Cells 14, no. 13: 1019. https://doi.org/10.3390/cells14131019
APA StyleNastase, V.-N., Burcea, I. F., Dumitriu-Stan, R. I., Ceausu, A. R., Zara, F., Poiana, C., & Raica, M. (2025). An Old New Friend: Folliculo-Stellate Cells in Pituitary Neuroendocrine Tumors. Cells, 14(13), 1019. https://doi.org/10.3390/cells14131019








