The Pathophysiological Role of Vascular Smooth Muscle Cells in Abdominal Aortic Aneurysm
Abstract
1. Introduction
2. Analysis of the Mechanism of Susceptibility to Infrarenal Aneurysms Based on VSMCs
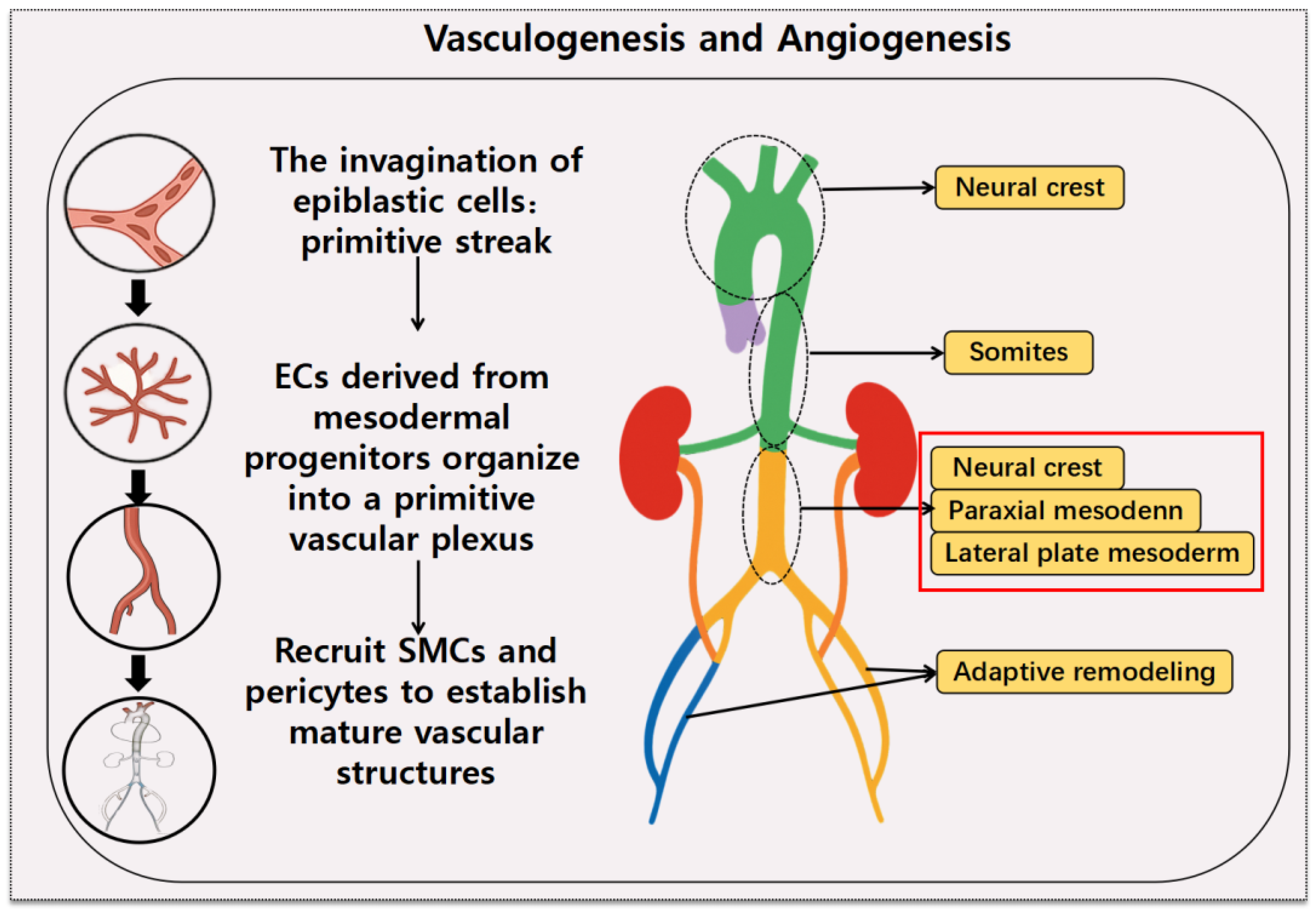
2.1. Origin of VSMCs
2.2. Anatomy, Histological Structure, and Blood Flow Characteristics of the Infrarenal Aorta
3. Cellular Architecture and Functional Regulation of Aortic VSMCs
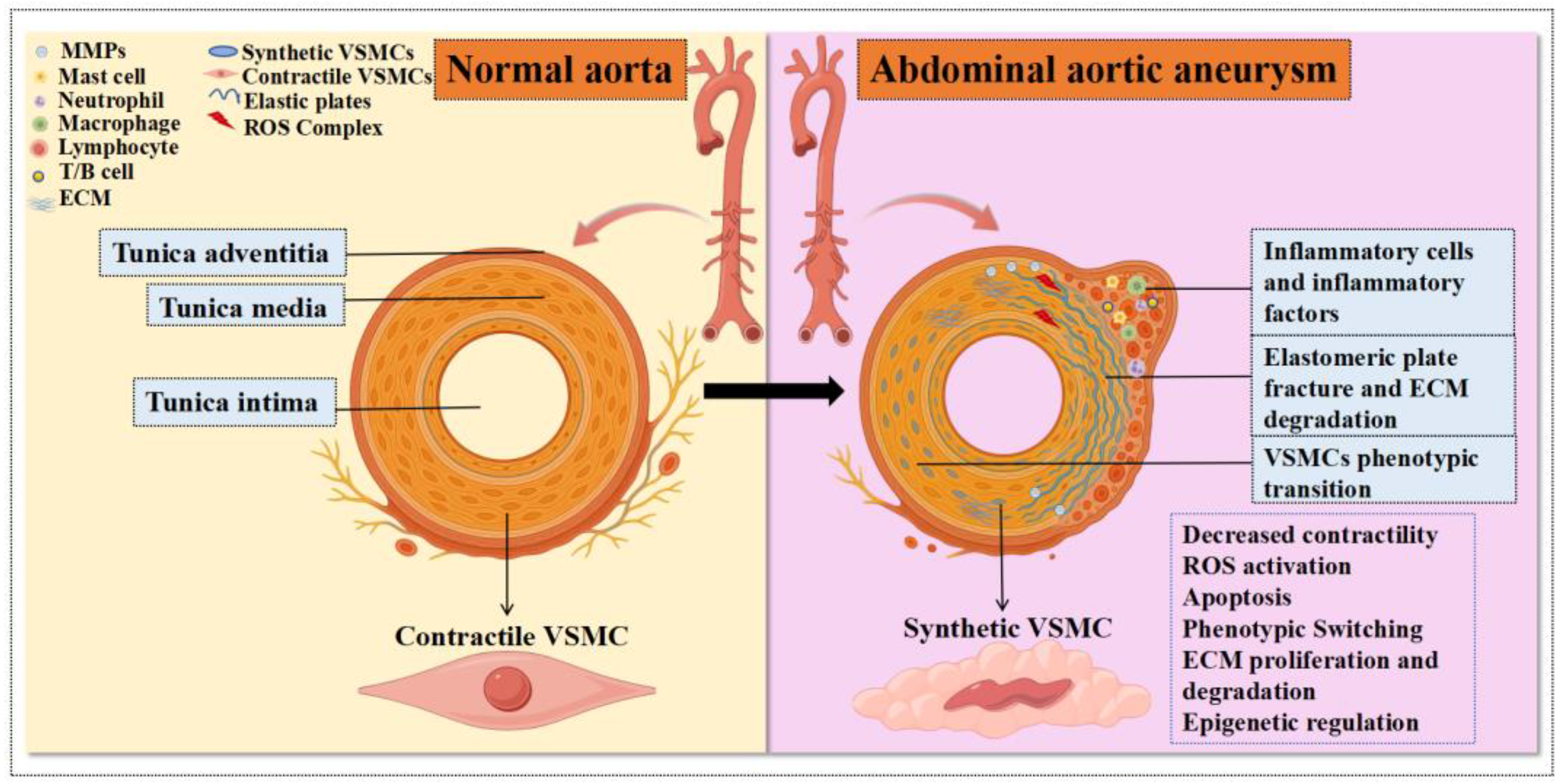
3.1. Structural and Cellular Complexity of the Aortic Wall: Focus on VSMCs
3.2. Regulatory Factors Influencing Aortic VSMC Contractile Function
3.2.1. Intracellular Calcium Ion (Ca2+) Concentration
3.2.2. Mechanical Stimulation, Nitric Oxide (NO), and Prostacyclin (PGI2)
3.2.3. Mitochondrial Energy Supply
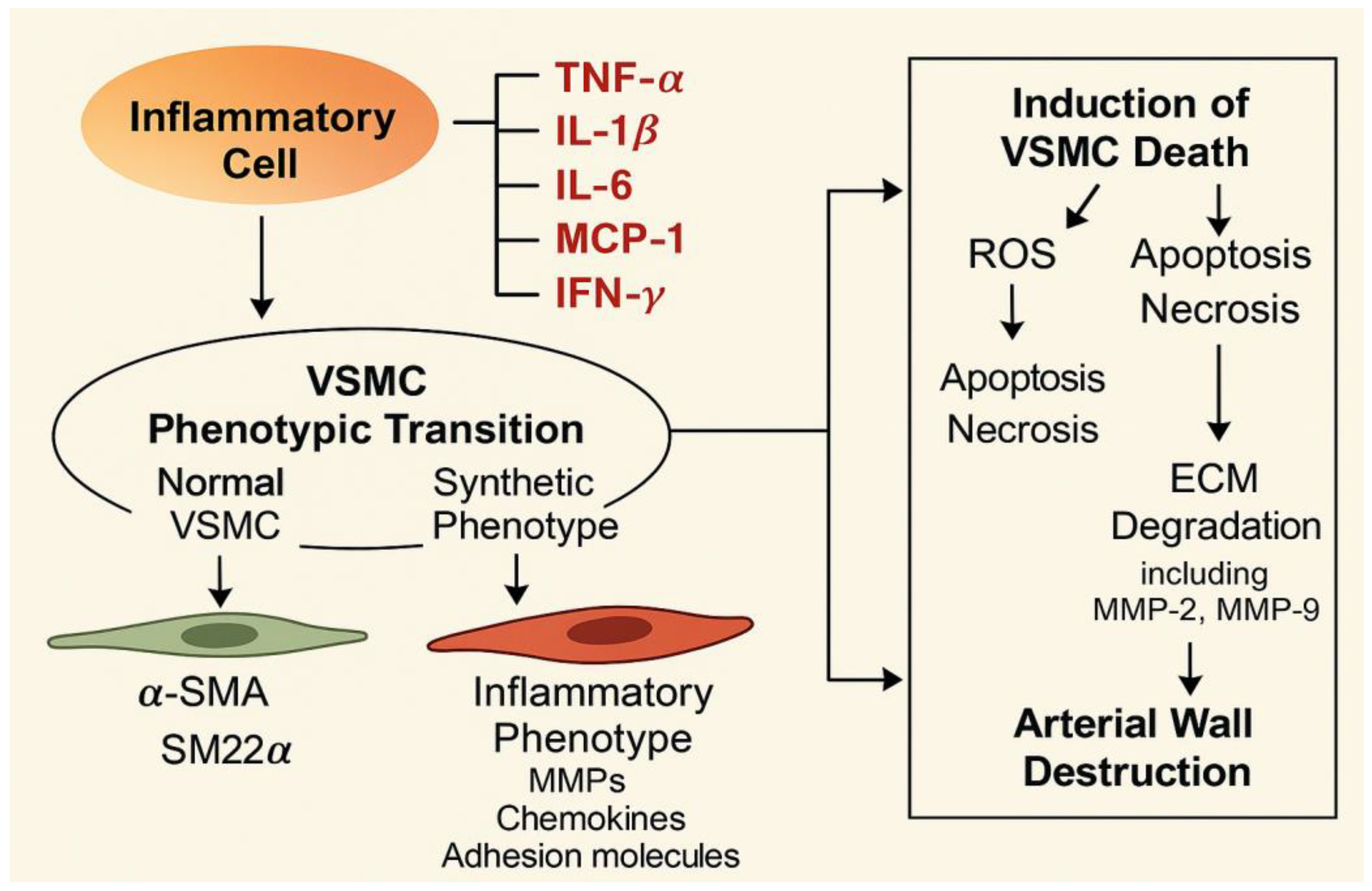
4. Inflammation, Oxidative Stress, and Mitochondrial Dysfunction of the Aortic Wall and VSMCs
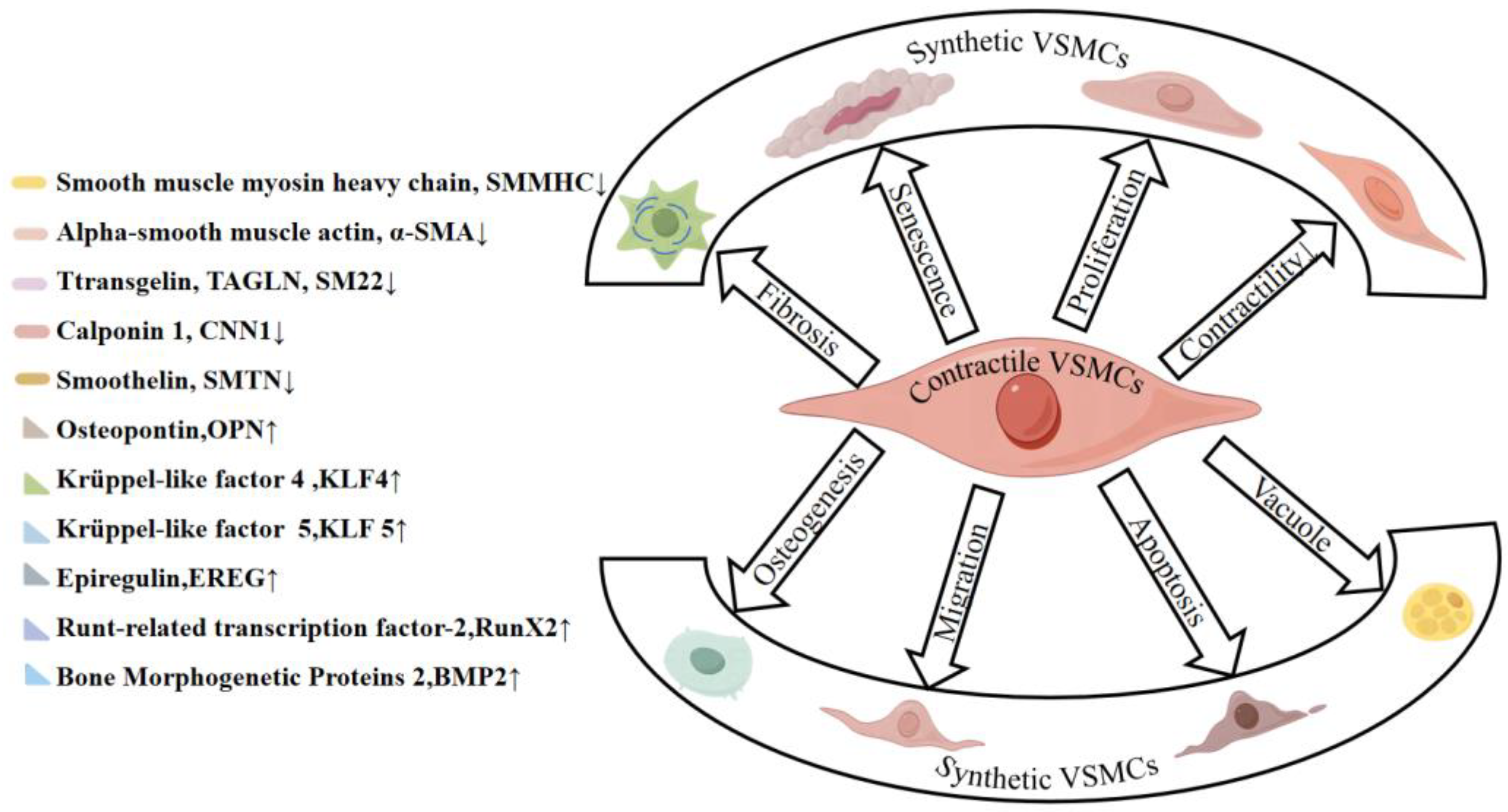
5. Phenotypic Switching of VSMCs
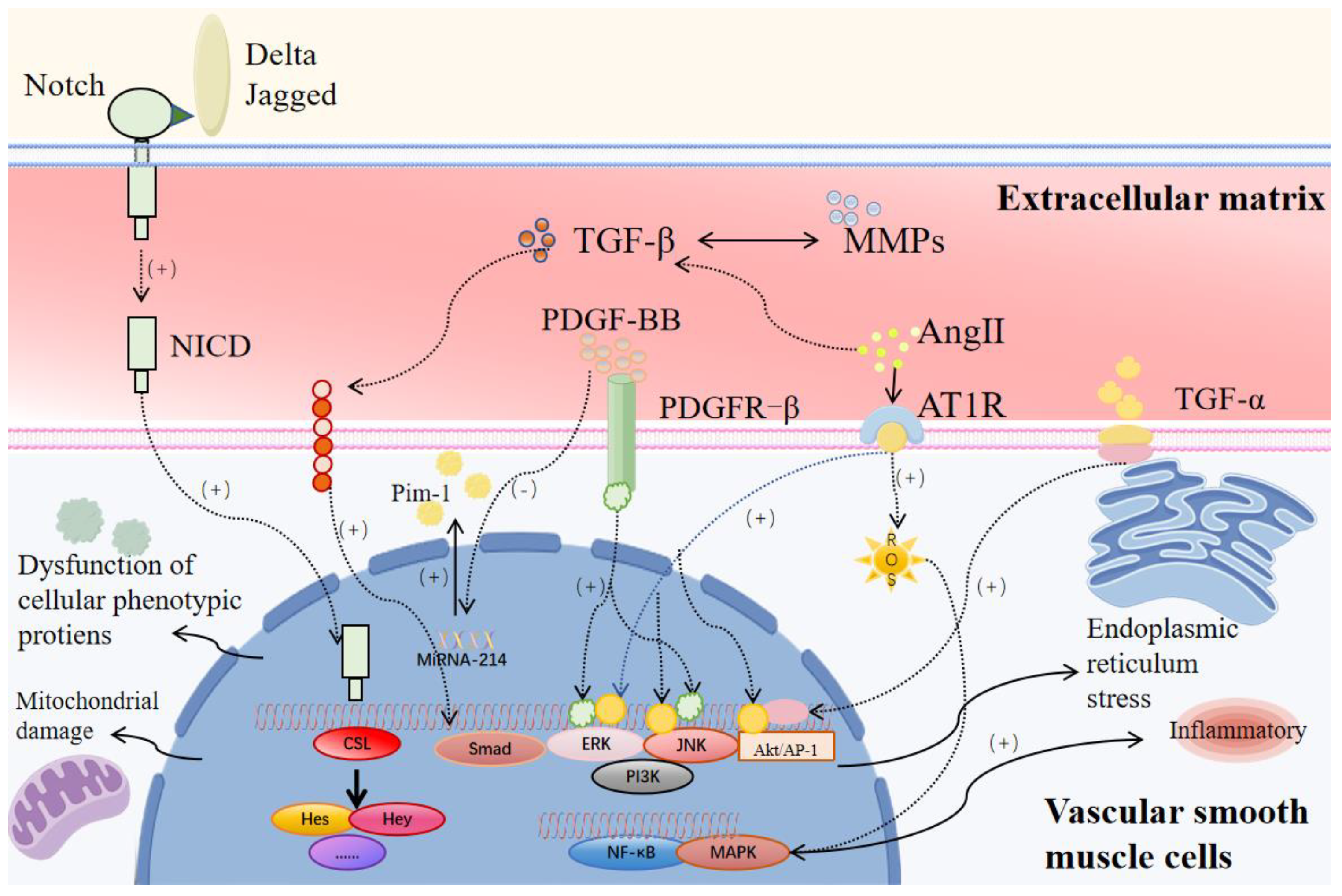
5.1. Cytokines and Signaling Pathways
5.1.1. PDGF Pathway
5.1.2. TGF-β/Smad Pathway
5.1.3. Notch Signaling Pathway
5.1.4. Ang II/AT1R Signaling Pathway
5.1.5. Inflammation-Related Cytokines
5.2. Epigenetic Modifications
5.2.1. DNA Methylation
5.2.2. Histone Modifications
5.2.3. Chromatin Remodeling Complexes
5.2.4. Non-Coding RNA in Abdominal Aortic Aneurysms
6. VSMC Degradation
7. Degradation of ECM by VSMCs
8. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAA | Abdominal Aortic Aneurysm; |
| VSMCs | Vascular Smooth Muscle Cells; |
| TAA | Thoracic Aortic Aneurysm; |
| MMPs | Matrix Metalloproteinases; |
| ECM | Extracellular Matrix; |
| SMCs | Smooth Muscle Cells; |
| TGF-β | Transforming Growth Factor Beta; |
| ROS | Reactive Oxygen Species; |
| α-SMA | Alpha-Smooth Muscle Actin; |
| TAGLN | Transgelin; |
| SM22 | Smooth Muscle Protein 22; |
| CNN1 | Calponin 1; |
| SMTN | Smoothelin; |
| ECIS | Endothelial Cell-Isolation System; |
| LRP1 | Low-Density Lipoprotein Receptor-Related Protein 1; |
| NO | Nitric Oxide; |
| PGI2 | Prostacyclin; |
| cGMP | Cyclic Guanosine Monophosphate; |
| PHB | Prohibitin; |
| OPN | Osteopontin; |
| EREG | Epiregulin; |
| AD | Aortic Dissection; |
| PDGF-BB | Platelet-Derived Growth Factor-BB; |
| TNF-α | Tumor Necrosis Factor Alpha; |
| AS | Atherosclerosis; |
| SMMHC | Smooth Muscle Myosin Heavy Chain; |
| EMT | Epithelial–Mesenchymal Transition; |
| ERK1/2 | Extracellular Signal-Regulated Kinase 1/2; |
| IA | Inflammatory Aneurysm; |
| UC-MSCs | Umbilical Cord Mesenchymal Stem Cells; |
| NF-κB | Nuclear Factor Kappa B; |
| AngII | Angiotensin II; |
| AT1R | Angiotensin II Type 1 Receptor; |
| NICD | Notch Intracellular Domain; |
| PI3K | Phosphoinositide 3-Kinase; |
| Akt | Protein Kinase B; |
| Wnt | Wnt Signaling Pathway; |
| ERK | Extracellular Signal-Regulated Kinase; |
| MAPK | Mitogen-Activated Protein Kinase; |
| JAK | Janus Kinase Janus; |
| STAT | Signal Transducer and Activator of Transcription; |
| ARBs | Angiotensin Receptor Blockers; |
| SUMO | Small Ubiquitin-like Modifier; |
| SENP1 | SUMO-specific Protease 1; |
| SRF | Serum Response Factor; |
| ELK1 | ETS-Like Gene 1; |
| ER | Estrogen Receptor; |
| GSDMD | Gasdermin D; |
| ODC1 | Ornithine Decarboxylase 1; |
| CHOP | C/EBP Homologous Protein; |
| NRP1 | Neuropilin-1; |
| ITGB3 | Integrin Beta-3; |
| GLUT1 | Glucose Transporter 1; |
| A7r5 | Rat Aortic Smooth Muscle Cell Line; |
| TCA | Tricarboxylic Acid Cycle; |
| FAO | Fatty Acid Oxidation; |
| CPT1 | Carnitine Palmitoyltransferase 1; |
| CPT2 | Carnitine Palmitoyltransferase 2; |
| IL-1β | Interleukin-1 Beta; |
| IFN-γ | Interferon Gamma; |
| MCP-1 | Monocyte Chemoattractant Protein-1; |
| FAS | Fatty Acid Synthase; |
| CD95 | Cluster of Differentiation 95; |
| ZBP1 | ZDNA Binding Protein 1; |
| AIM2 | Absent in Melanoma 2; |
| TFEB | Transcription Factor EB; |
| 2HPBCD | 2-Hydroxypropyl-β-Cyclodextrin; |
| BCL-2 | B-Cell Lymphoma 2; |
| SMOC2 | SMAD Family Member 4 Interacting Protein 2; |
| JNK | c-Jun N-Terminal Kinase c-Jun; |
| SAPK | Stress-Activated Protein Kinase; |
| MAPK | Mitogen-Activated Protein Kinase; |
| iNOS | Inducible Nitric Oxide Synthase; |
| CD | Cluster of Differentiation; |
| Nox4 | NADPH Oxidase 4; |
| BAF60α | Bromodomain Adjacent to Zinc Finger 60α; |
| DAMPs | Damage-Associated Molecular Patterns. |
References
- Davis, F.M.; Daugherty, A.; Lu, H.S. Updates of recent aortic aneurysm research. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e83–e90. [Google Scholar] [CrossRef]
- Altobelli, E.; Rapacchietta, L.; Profeta, V.F.; Fagnano, R. Risk factors for abdominal aortic aneurysm in population-based studies: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2018, 15, 2805. [Google Scholar] [CrossRef]
- Johnston, K.W.; Rutherford, R.B.; Tilson, M.D.; Shah, D.M.; Hollier, L.; Stanley, J.C. Suggested standards for reporting on arterial aneurysms. J. Vasc. Surg. 1991, 13, 452–458. [Google Scholar] [CrossRef]
- Howard, D.; Banerjee, A.; Fairhead, J.; Handa, A.; Silver, L.; Rothwell, P. Age-specific incidence, risk factors and outcome of acute abdominal aortic aneurysms in a defined population. J. Br. Surg. 2015, 102, 907–915. [Google Scholar] [CrossRef]
- Golledge, J.; Krishna, S.M.; Wang, Y. Mouse models for abdominal aortic aneurysm. Br. J. Pharmacol. 2022, 179, 792–810. [Google Scholar] [CrossRef]
- Nordon, I.M.; Hinchliffe, R.J.; Loftus, I.M.; Thompson, M.M. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 2011, 8, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Seven, M. Abdominal Aortic Aneurysm. Master’s Thesis, Faculty of Medicine, University of Rijeka, Rijeka, Croatia, 2024. [Google Scholar]
- Sampson, U.K.; Norman, P.E.; Fowkes, F.G.R.; Aboyans, V.; Song, Y.; Harrell, F.E., Jr.; Forouzanfar, M.H.; Naghavi, M.; Denenberg, J.O.; McDermott, M.M. Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010. Glob. Heart 2014, 9, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Doubeni, C.A.; Epling, J.W.; Kubik, M.; Landefeld, C.S. Screening for abdominal aortic aneurysm: US preventive services task force recommendation statement. JAMA 2019, 322, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, K.; Li, T.; Zhai, S. Primary results of abdominal aortic aneurysm screening in the at-risk residents in middle China. BMC Cardiovasc. Disord. 2018, 18, 60. [Google Scholar] [CrossRef]
- Song, P.; He, Y.; Adeloye, D.; Zhu, Y.; Ye, X.; Yi, Q.; Rahimi, K.; Rudan, I.; Group, G.H.E.R. The global and regional prevalence of abdominal aortic aneurysms: A systematic review and modeling analysis. Ann. Surg. 2023, 277, 912–919. [Google Scholar] [CrossRef]
- Lederle, F.A.; Kyriakides, T.C.; Stroupe, K.T.; Freischlag, J.A.; Padberg, F.T., Jr.; Matsumura, J.S.; Huo, Z.; Johnson, G.R. Open versus endovascular repair of abdominal aortic aneurysm. N. Engl. J. Med. 2019, 380, 2126–2135. [Google Scholar] [CrossRef] [PubMed]
- Paravastu, S.C.V.; Jayarajasingam, R.; Cottam, R.; Palfreyman, S.J.; Michaels, J.A.; Thomas, S.M. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst. Rev. 2014, CD004178. [Google Scholar] [CrossRef] [PubMed]
- Quintana, R.A.; Taylor, W.R. Cellular mechanisms of aortic aneurysm formation. Circ. Res. 2019, 124, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Adeyanju, O.; Olajuyin, A.; Guo, X. Abdominal aortic aneurysm formation with a focus on vascular smooth muscle cells. Life 2022, 12, 191. [Google Scholar] [CrossRef]
- Wagenhäuser, M.U.; Mulorz, J.; Krott, K.J.; Bosbach, A.; Feige, T.; Rhee, Y.H.; Chatterjee, M.; Petzold, N.; Böddeker, C.; Ibing, W. Crosstalk of platelets with macrophages and fibroblasts aggravates inflammation, aortic wall stiffening, and osteopontin release in abdominal aortic aneurysm. Cardiovasc. Res. 2024, 120, 417–432. [Google Scholar] [CrossRef]
- Lu, H.; Du, W.; Ren, L.; Hamblin, M.H.; Becker, R.C.; Chen, Y.E.; Fan, Y. Vascular smooth muscle cells in aortic aneurysm: From genetics to mechanisms. J. Am. Heart Assoc. 2021, 10, e023601. [Google Scholar] [CrossRef]
- Sinha, S.; Iyer, D.; Granata, A. Embryonic origins of human vascular smooth muscle cells: Implications for in vitro modeling and clinical application. Cell. Mol. Life Sci. 2014, 71, 2271–2288. [Google Scholar] [CrossRef]
- Amali, A.A.; Sie, L.; Winkler, C.; Featherstone, M. Zebrafish hoxd4a acts upstream of meis1. 1 to direct vasculogenesis, angiogenesis and hematopoiesis. PLoS ONE 2013, 8, e58857. [Google Scholar] [CrossRef]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef]
- Mironov, A.A.; Beznoussenko, G.V. Opinion: On the way towards the new paradigm of atherosclerosis. Int. J. Mol. Sci. 2022, 23, 2152. [Google Scholar] [CrossRef]
- Pouget, C.; Pottin, K.; Jaffredo, T. Sclerotomal origin of vascular smooth muscle cells and pericytes in the embryo. Dev. Biol. 2008, 315, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Gadson, P.F., Jr.; Dalton, M.L.; Patterson, E.; Svoboda, D.D.; Hutchinson, L.; Schram, D.; Rosenquist, T.H. Differential response of mesoderm-and neural crest-derived smooth muscle to TGF-β1: Regulation of c-myb and α1 (I) procollagen genes. Exp. Cell Res. 1997, 230, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Pfaltzgraff, E.R.; Bader, D.M. Heterogeneity in vascular smooth muscle cell embryonic origin in relation to adult structure, physiology, and disease. Dev. Dyn. 2015, 244, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Bernardo, A.S.; Trotter, M.W.; Pedersen, R.A.; Sinha, S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin–dependent disease susceptibility. Nat. Biotechnol. 2012, 30, 165–173. [Google Scholar] [CrossRef]
- Majesky, M.W. Developmental basis of vascular smooth muscle diversity. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1248–1258. [Google Scholar] [CrossRef]
- Cho, M.J.; Lee, M.-R.; Park, J.-G. Aortic aneurysms: Current pathogenesis and therapeutic targets. Exp. Mol. Med. 2023, 55, 2519–2530. [Google Scholar] [CrossRef]
- Golledge, J.; Norman, P.E. Atherosclerosis and Abdominal Aortic Aneurysm: Cause, Response, or Common Risk Factors? Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; Volume 30, pp. 1075–1077. [Google Scholar]
- Cao, G.; Xuan, X.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun. Signal. 2022, 20, 180. [Google Scholar] [CrossRef]
- Diehm, N.; Dick, F.; Schaffner, T.; Schmidli, J.; Kalka, C.; Di Santo, S.; Voelzmann, J.; Baumgartner, I. Novel insight into the pathobiology of abdominal aortic aneurysm and potential future treatment concepts. Prog. Cardiovasc. Dis. 2007, 50, 209–217. [Google Scholar] [CrossRef]
- Halloran, B.G.; Davis, V.A.; McManus, B.M.; Lynch, T.G.; Baxter, B.T. Localization of aortic disease is associated with intrinsic differences in aortic structure. J. Surg. Res. 1995, 59, 17–22. [Google Scholar] [CrossRef]
- Tanweer, O.; Wilson, T.A.; Metaxa, E.; Riina, H.A.; Meng, H. A comparative review of the hemodynamics and pathogenesis of cerebral and abdominal aortic aneurysms: Lessons to learn from each other. J. Cerebrovasc. Endovasc. Neurosurg. 2014, 16, 335–349. [Google Scholar] [CrossRef]
- Dua, M.M.; Dalman, R.L. Hemodynamic influences on abdominal aortic aneurysm disease: Application of biomechanics to aneurysm pathophysiology. Vasc. Pharmacol. 2010, 53, 11–21. [Google Scholar] [CrossRef]
- Taylor, C.A.; Hughes, T.J.; Zarins, C.K. Effect of exercise on hemodynamic conditions in the abdominal aorta. J. Vasc. Surg. 1999, 29, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, H.; Glagov, S. A lamellar unit of aortic medial structure and function in mammals. Circ. Res. 1967, 20, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Zengin, E.; Chalajour, F.; Gehling, U.M.; Ito, W.D.; Treede, H.; Lauke, H.; Weil, J.; Reichenspurner, H.; Kilic, N.; Ergün, S.l. Vascular wall resident progenitor cells: A source for postnatal vasculogenesis. Development 2006, 133, 1543–1551. [Google Scholar] [CrossRef]
- Torsney, E.; Xu, Q. Resident vascular progenitor cells. J. Mol. Cell. Cardiol. 2011, 50, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Majesky, M.W.; Horita, H.; Ostriker, A.; Lu, S.; Regan, J.N.; Bagchi, A.; Dong, X.R.; Poczobutt, J.; Nemenoff, R.A.; Weiser-Evans, M.C. Differentiated smooth muscle cells generate a subpopulation of resident vascular progenitor cells in the adventitia regulated by Klf4. Circ. Res. 2017, 120, 296–311. [Google Scholar] [CrossRef]
- Psaltis, P.J.; Simari, R.D. Vascular wall progenitor cells in health and disease. Circ. Res. 2015, 116, 1392–1412. [Google Scholar] [CrossRef]
- Crosas-Molist, E.; Meirelles, T.; López-Luque, J.; Serra-Peinado, C.; Selva, J.; Caja, L.; Gorbenko del Blanco, D.; Uriarte, J.J.; Bertran, E.; Mendizábal, Y. Vascular smooth muscle cell phenotypic changes in patients with Marfan syndrome. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 960–972. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, H.; Liu, Y.; Adams, S.; Eilken, H.; Stehling, M.; Corada, M.; Dejana, E.; Zhou, B.; Adams, R.H. Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells. Nat. Commun. 2016, 7, 12422. [Google Scholar] [CrossRef]
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis. Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Moos, M.P.; John, N.; Gräbner, R.; Noßmann, S.; Günther, B.; Vollandt, R.d.; Funk, C.D.; Kaiser, B.; Habenicht, A.J. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E–deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.; Bennett, M.R.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef]
- Caplice, N.M.; Doyle, B. Vascular progenitor cells: Origin and mechanisms of mobilization, differentiation, integration, and vasculogenesis. Stem Cells Dev. 2005, 14, 122–139. [Google Scholar] [CrossRef]
- Pohl, U. Connexins: Key players in the control of vascular plasticity and function. Physiol. Rev. 2020, 100, 525–572. [Google Scholar] [CrossRef]
- Cowan, D.B.; Lye, S.J.; Langille, B.L. Regulation of vascular connexin43 gene expression by mechanical loads. Circ. Res. 1998, 82, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Berrier, A.L.; Yamada, K.M. Cell–matrix adhesion. J. Cell. Physiol. 2007, 213, 565–573. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Fu, X.; Yang, S.; Li, Y.; Liu, J.; DiSanto, M.E.; Chen, P.; Zhang, X. The emerging role of cell adhesion molecules on benign prostatic hyperplasia. Int. J. Mol. Sci. 2023, 24, 2870. [Google Scholar] [CrossRef]
- Mui, K.L.; Bae, Y.H.; Gao, L.; Liu, S.-L.; Xu, T.; Radice, G.L.; Chen, C.S.; Assoian, R.K. N-cadherin induction by ECM stiffness and FAK overrides the spreading requirement for proliferation of vascular smooth muscle cells. Cell Rep. 2015, 10, 1477–1486. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.; Yang, C.; Ren, Q.; Zhang, W.; Li, N.; Zhang, M.; Zhang, B.; Zhang, L.; Zhou, X. Maternal High-Sucrose Diet Accelerates Vascular Stiffness in Aged Offspring via Suppressing Cav1. 2 and Contractile Phenotype of Vascular Smooth Muscle Cells. Mol. Nutr. Food Res. 2019, 63, 1900022. [Google Scholar] [CrossRef]
- Huang, H.; Sun, Z.; Hill, M.A.; Meininger, G.A. A calcium mediated mechanism coordinating vascular smooth muscle cell adhesion during kcl activation. Front. Physiol. 2018, 9, 1810. [Google Scholar] [CrossRef]
- Somlyo, A.P.; Somlyo, A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003, 83, 1325–1358. [Google Scholar] [CrossRef] [PubMed]
- Bogunovic, N.; Meekel, J.P.; Micha, D.; Blankensteijn, J.D.; Hordijk, P.L.; Yeung, K.K. Impaired smooth muscle cell contractility as a novel concept of abdominal aortic aneurysm pathophysiology. Sci. Rep. 2019, 9, 6837. [Google Scholar] [CrossRef]
- Au, D.T.; Ying, Z.; Hernández-Ochoa, E.O.; Fondrie, W.E.; Hampton, B.; Migliorini, M.; Galisteo, R.; Schneider, M.F.; Daugherty, A.; Rateri, D.L. LRP1 (low-density lipoprotein receptor–related protein 1) regulates smooth muscle contractility by modulating Ca2+ signaling and expression of cytoskeleton-related proteins. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2651–2664. [Google Scholar] [CrossRef]
- Tomida, S.; Ishima, T.; Nagai, R.; Aizawa, K. T-Type Voltage-Gated Calcium Channels: Potential Regulators of Smooth Muscle Contractility. Int. J. Mol. Sci. 2024, 25, 12420. [Google Scholar] [CrossRef] [PubMed]
- House, S.J.; Potier, M.; Bisaillon, J.; Singer, H.A.; Trebak, M. The non-excitable smooth muscle: Calcium signaling and phenotypic switching during vascular disease. Pflügers Arch.-Eur. J. Physiol. 2008, 456, 769–785. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Yang, F.; Zhang, L. Molecular Mechanism by Which TRPC6 Regulates Calcium Signaling and Neuroinflammation in the Onset and Development of Ischemic Stroke: A Review. Altern. Ther. Health Med. 2024, 30, 170–175. [Google Scholar]
- Yin, Q.; Zang, G.; Li, N.; Sun, C.; Du, R. Agonist-induced Piezo1 activation promote mitochondrial-dependent apoptosis in vascular smooth muscle cells. BMC Cardiovasc. Disord. 2022, 22, 287. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Chen, L.; Zhou, J.; Tang, Z.; Hsu, T.-F.; Wang, Y.; Shih, Y.-T.; Peng, H.-H.; Wang, N.; Guan, Y. Shear stress induces synthetic-to-contractile phenotypic modulation in smooth muscle cells via peroxisome proliferator-activated receptor α/δ activations by prostacyclin released by sheared endothelial cells. Circ. Res. 2009, 105, 471–480. [Google Scholar] [CrossRef]
- Jia, L.; Wang, L.; Wei, F.; Li, C.; Wang, Z.; Yu, H.; Chen, H.; Wang, B.; Jiang, A. Effects of Caveolin-1-ERK1/2 pathway on endothelial cells and smooth muscle cells under shear stress. Exp. Biol. Med. 2020, 245, 21–33. [Google Scholar] [CrossRef]
- Qiao, M.; Li, Y.; Yan, S.; Zhang, R.J.; Dong, H. Modulation of arterial wall remodeling by mechanical stress: Focus on abdominal aortic aneurysm. Vasc. Med. 2025, 30, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Jeremy, J.Y.; Rowe, D.; Emsley, A.M.; Newby, A.C. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovasc. Res. 1999, 43, 580–594. [Google Scholar] [CrossRef]
- Fu, H.; Shen, Q.-R.; Zhao, Y.; Ni, M.; Zhou, C.-C.; Chen, J.-K.; Chi, C.; Li, D.-J.; Liang, G.; Shen, F.-M. Activating α7nAChR ameliorates abdominal aortic aneurysm through inhibiting pyroptosis mediated by NLRP3 inflammasome. Acta Pharmacol. Sin. 2022, 43, 2585–2595. [Google Scholar] [CrossRef]
- Chatzizisis, Y.S.; Coskun, A.U.; Jonas, M.; Edelman, E.R.; Stone, P.H.; Feldman, C.L. Risk stratification of individual coronary lesions using local endothelial shear stress: A new paradigm for managing coronary artery disease. Curr. Opin. Cardiol. 2007, 22, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Tsoi, L.C.; Ma, F.; Wasikowski, R.; Moore, B.B.; Kunkel, S.L.; Gudjonsson, J.E.; Gallagher, K.A. Single-cell transcriptomics reveals dynamic role of smooth muscle cells and enrichment of immune cell subsets in human abdominal aortic aneurysms. Ann. Surg. 2022, 276, 511–521. [Google Scholar] [CrossRef]
- Qin, H.-L.; Bao, J.-H.; Tang, J.-J.; Xu, D.-Y.; Shen, L. Arterial remodeling: The role of mitochondrial metabolism in vascular smooth muscle cells. Am. J. Physiol.-Cell Physiol. 2023, 324, C183–C192. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Jin, J.; Li, S.-L.; Yan, J.; Zhen, C.-L.; Gao, J.-L.; Zhang, Y.-H.; Zhang, Y.-Q.; Shen, X.; Zhang, L.-S. Mitochondrial fission of smooth muscle cells is involved in artery constriction. Hypertension 2016, 68, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Mizrak, D.; Feng, H.; Yang, B. Dissecting the heterogeneity of human thoracic aortic aneurysms using single-cell transcriptomics. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 919–930. [Google Scholar] [CrossRef]
- Lahiri, V.; Klionsky, D.J. PHB2/prohibitin 2: An inner membrane mitophagy receptor. Cell Res. 2017, 27, 311–312. [Google Scholar] [CrossRef]
- Tavris, B.S.; Peters, A.S.; Böckler, D.; Dihlmann, S. Mitochondrial Dysfunction and Increased DNA Damage in Vascular Smooth Muscle Cells of Abdominal Aortic Aneurysm (AAA-SMC). Oxidative Med. Cell. Longev. 2023, 2023, 6237960. [Google Scholar] [CrossRef]
- Jia, Y.; Mao, C.; Ma, Z.; Huang, J.; Li, W.; Ma, X.; Zhang, S.; Li, M.; Yu, F.; Sun, Y. PHB2 maintains the contractile phenotype of VSMCs by counteracting PKM2 splicing. Circ. Res. 2022, 131, 807–824. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jia, L.; Zhao, C.; Wang, H.; Dai, Z.; Jing, Y.; Jiang, B.; Xin, S. Mitochondrial quality control in abdominal aortic aneurysm: From molecular mechanisms to therapeutic strategies. FASEB J. 2023, 37, e22969. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Lu, Y.; Wei, J.; Wu, J.; Yang, J.; Cai, Z. Abdominal aortic aneurysm: Roles of inflammatory cells. Front. Immunol. 2021, 11, 609161. [Google Scholar] [CrossRef]
- Wortmann, M.; Skorubskaya, E.; Peters, A.S.; Hakimi, M.; Böckler, D.; Dihlmann, S. Necrotic cell debris induces a NF-κB-driven inflammasome response in vascular smooth muscle cells derived from abdominal aortic aneurysms (AAA-SMC). Biochem. Biophys. Res. Commun. 2019, 511, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; Qu, W. Metformin inhibits LPS-induced inflammatory response in VSMCs by regulating TLR4 and PPAR-γ. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4988–4995. [Google Scholar]
- Wen, H.; Wang, M.; Gong, S.; Li, X.; Meng, J.; Wen, J.; Wang, Y.; Zhang, S.; Xin, S. Human umbilical cord mesenchymal stem cells attenuate abdominal aortic aneurysm progression in sprague-dawley rats: Implication of vascular smooth muscle cell phenotypic modulation. Stem Cells Dev. 2020, 29, 981–993. [Google Scholar] [CrossRef]
- Mi, T.; Nie, B.; Zhang, C.; Zhou, H. The elevated expression of osteopontin and NF-κB in human aortic aneurysms and its implication. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 602–607. [Google Scholar] [CrossRef]
- Han, M.S.; Jung, D.Y.; Morel, C.; Lakhani, S.A.; Kim, J.K.; Flavell, R.A.; Davis, R.J. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 2013, 339, 218–222. [Google Scholar] [CrossRef]
- Magnani, F.; Mattevi, A. Structure and mechanisms of ROS generation by NADPH oxidases. Curr. Opin. Struct. Biol. 2019, 59, 91–97. [Google Scholar] [CrossRef]
- Xiong, W.; Mactaggart, J.; Knispel, R.; Worth, J.; Zhu, Z.; Li, Y.; Sun, Y.; Baxter, B.T.; Johanning, J. Inhibition of reactive oxygen species attenuates aneurysm formation in a murine model. Atherosclerosis 2009, 202, 128–134. [Google Scholar] [CrossRef]
- He, F.; Zuo, L. Redox roles of reactive oxygen species in cardiovascular diseases. Int. J. Mol. Sci. 2015, 16, 27770–27780. [Google Scholar] [CrossRef] [PubMed]
- Krylatov, A.V.; Maslov, L.N.; Voronkov, N.S.; Boshchenko, A.A.; Popov, S.V.; Gomez, L.; Wang, H.; Jaggi, A.S.; Downey, J.M. Reactive oxygen species as intracellular signaling molecules in the cardiovascular system. Curr. Cardiol. Rev. 2018, 14, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Han, Y.; Ren, T.; Fang, H.; Xu, X.; Lun, Y.; Jiang, H.; Xin, S.; Zhang, J. AEBP1 promotes the occurrence and development of abdominal aortic aneurysm by modulating inflammation via the NF-κB pathway. J. Atheroscler. Thromb. 2020, 27, 255–270. [Google Scholar] [CrossRef]
- Zhong, L.; He, X.; Si, X.; Wang, H.; Li, B.; Hu, Y.; Li, M.; Chen, X.; Liao, W.; Liao, Y. SM22α (smooth muscle 22α) prevents aortic aneurysm formation by inhibiting smooth muscle cell phenotypic switching through suppressing reactive oxygen species/NF-κB (nuclear factor-κB). Arterioscler. Thromb. Vasc. Biol. 2019, 39, e10–e25. [Google Scholar] [CrossRef]
- Sanchez-Infantes, D.; Nus, M.; Navas-Madronal, M.; Fite, J.; Perez, B.; Barros-Membrilla, A.J.; Soto, B.; Martinez-Gonzalez, J.; Camacho, M.; Rodriguez, C.; et al. Oxidative Stress and Inflammatory Markers in Abdominal Aortic Aneurysm. Antioxidants 2021, 10, 602. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.H.; Hsu, L.A.; Tsai, H.Y.; Yeh, Y.H.; Lu, C.Y.; Chen, P.C.; Wang, J.C.; Chiu, Y.L.; Lin, C.Y.; Hsu, Y.J. Aldehyde dehydrogenase 2 protects against abdominal aortic aneurysm formation by reducing reactive oxygen species, vascular inflammation, and apoptosis of vascular smooth muscle cells. FASEB J. 2020, 34, 9498–9511. [Google Scholar] [CrossRef]
- Shi, J.; Yang, Y.; Cheng, A.; Xu, G.; He, F. Metabolism of vascular smooth muscle cells in vascular diseases. Am. J. Physiol.-Heart Circ. Physiol. 2020, 319, H613–H631. [Google Scholar] [CrossRef]
- Chung, J.; Lachapelle, K.; Wener, E.; Cartier, R.; De Varennes, B.; Fraser, R.; Leask, R.L. Energy loss, a novel biomechanical parameter, correlates with aortic aneurysm size and histopathologic findings. J. Thorac. Cardiovasc. Surg. 2014, 148, 1082–1089. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Hermann, M.; Saks, V.; Hengster, P.; Margreiter, R. The cell-type specificity of mitochondrial dynamics. Int. J. Biochem. Cell Biol. 2009, 41, 1928–1939. [Google Scholar] [CrossRef]
- Marchi, S.; Guilbaud, E.; Tait, S.W.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2023, 23, 159–173. [Google Scholar] [CrossRef]
- Xia, D.; Chen, Y.; Luo, G.; Wei, D. Atherosclerosis: From the Disruption of Mitochondrial Membrane Potential to the Potential Interventional Strategies. Curr. Med. Chem. 2023, 30, 4355–4373. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.; Hruz, M.M.M. Structural analysis of the GLUT1 facilitative glucose transporter. Mol. Membr. Biol. 2001, 18, 183–193. [Google Scholar] [CrossRef]
- Hall, J.L.; Chatham, J.C.; Eldar-Finkelman, H.; Gibbons, G.H. Upregulation of glucose metabolism during intimal lesion formation is coupled to the inhibition of vascular smooth muscle cell apoptosis: Role of GSK3β. Diabetes 2001, 50, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Hsu, S.-C.; Lee, H.-S.; Lin, S.-H.; Tsai, C.-S.; Huang, S.-M.; Shih, C.-C.; Hsu, Y.-J. Enhanced expression of glucose transporter-1 in vascular smooth muscle cells via the Akt/tuberous sclerosis complex subunit 2 (TSC2)/mammalian target of rapamycin (mTOR)/ribosomal S6 protein kinase (S6K) pathway in experimental renal failure. J. Vasc. Surg. 2013, 57, 475–485. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, J.; Liu, M.; He, L.; Zhang, K.; Yang, Y.; Yang, X.; Zhou, H.; Tang, M.; Lu, L. Warburg effect is involved in apelin-13-induced human aortic vascular smooth muscle cells proliferation. J. Cell. Physiol. 2019, 234, 14413–14421. [Google Scholar] [CrossRef] [PubMed]
- Pfleger, J.; He, M.; Abdellatif, M. Mitochondrial complex II is a source of the reserve respiratory capacity that is regulated by metabolic sensors and promotes cell survival. Cell Death Dis. 2015, 6, e1835. [Google Scholar] [CrossRef]
- Perez, J.; Hill, B.G.; Benavides, G.A.; Dranka, B.P.; Darley-Usmar, V.M. Role of cellular bioenergetics in smooth muscle cell proliferation induced by platelet-derived growth factor. Biochem. J. 2010, 428, 255–267. [Google Scholar] [CrossRef]
- Salabei, J.K.; Hill, B.G. Mitochondrial fission induced by platelet-derived growth factor regulates vascular smooth muscle cell bioenergetics and cell proliferation. Redox Biol. 2013, 1, 542–551. [Google Scholar] [CrossRef]
- Chen, X.; Austin, E.D.; Talati, M.; Fessel, J.P.; Farber-Eger, E.H.; Brittain, E.L.; Hemnes, A.R.; Loyd, J.E.; West, J. Oestrogen inhibition reverses pulmonary arterial hypertension and associated metabolic defects. Eur. Respir. J. 2017, 50, 1602337. [Google Scholar] [CrossRef]
- Barron, J.T.; Kopp, S.J.; Tow, J.; Parrillo, J.E. Fatty acid, tricarboxylic acid cycle metabolites, and energy metabolism in vascular smooth muscle. Am. J. Physiol.-Heart Circ. Physiol. 1994, 267, H764–H769. [Google Scholar] [CrossRef]
- Scheede-Bergdahl, C.; Bergdahl, A. Adaptation of mitochondrial expression and ATP production in dedifferentiating vascular smooth muscle cells. Can. J. Physiol. Pharmacol. 2017, 95, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Y.; Zhou, Y.; Chen, Q.; Luo, Z.; Ren, Y.; Chen, X.; Chen, G. Iron and copper: Critical executioners of ferroptosis, cuproptosis and other forms of cell death. Cell Commun. Signal. 2023, 21, 327. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Chen, Y.; Li, K.; Zhan, R.; Zhao, M.; Xu, Y.; Lin, Z.; Fu, Y.; He, Q.; Tang, P.C. Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection. Eur. Heart J. 2021, 42, 4373–4385. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, Z.; Yang, L.; He, L.; Liu, H.; Yang, S.; Xu, Q.; Li, Y.; Li, W.; Li, Y. ANK Deficiency-Mediated Cytosolic Citrate Accumulation Promotes Aortic Aneurysm. Circ. Res. 2024, 135, 1175–1192. [Google Scholar] [CrossRef]
- Sun, L.-Y.; Lyu, Y.-Y.; Zhang, H.-Y.; Shen, Z.; Lin, G.-Q.; Geng, N.; Wang, Y.-L.; Huang, L.; Feng, Z.-H.; Guo, X. Nuclear receptor NR1D1 regulates abdominal aortic aneurysm development by targeting the mitochondrial tricarboxylic acid cycle enzyme aconitase-2. Circulation 2022, 146, 1591–1609. [Google Scholar] [CrossRef]
- Gao, J.; Chen, Y.; Wang, H.; Li, X.; Li, K.; Xu, Y.; Xie, X.; Guo, Y.; Yang, N.; Zhang, X. Gasdermin D deficiency in vascular smooth muscle cells ameliorates abdominal aortic aneurysm through reducing putrescine synthesis. Adv. Sci. 2023, 10, 2204038. [Google Scholar] [CrossRef]
- Lai, C.-H.; Chang, C.-W.; Lee, F.-T.; Kuo, C.-H.; Hsu, J.-H.; Liu, C.-P.; Wu, H.-L.; Yeh, J.-L. Targeting vascular smooth muscle cell dysfunction with xanthine derivative KMUP-3 inhibits abdominal aortic aneurysm in mice. Atherosclerosis 2020, 297, 16–24. [Google Scholar] [CrossRef]
- Song, T.; Zhao, S.; Luo, S.; Chen, C.; Liu, X.; Wu, X.; Sun, Z.; Cao, J.; Wang, Z.; Wang, Y. SLC44A2 regulates vascular smooth muscle cell phenotypic switching and aortic aneurysm. J. Clin. Investig. 2024, 134, e173690. [Google Scholar] [CrossRef]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Vascular smooth muscle cell in atherosclerosis. Acta Physiol. 2015, 214, 33–50. [Google Scholar] [CrossRef]
- Raines, E.W. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004, 15, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.; Lizneva, D.; Yuen, T. The role of PDGF-BB in the bone-vascular relationship during aging. J. Clin. Investig. 2021, 131, e153644. [Google Scholar] [CrossRef]
- Zhou, J.; Shao, L.; Yu, J.; Huang, J.; Feng, Q. PDGF-BB promotes vascular smooth muscle cell migration by enhancing Pim-1 expression via inhibiting miR-214. Ann. Transl. Med. 2021, 9, 1728. [Google Scholar] [CrossRef]
- Tian, J.; Fu, Y.; Li, Q.; Xu, Y.; Xi, X.; Zheng, Y.; Yu, L.; Wang, Z.; Yu, B.; Tian, J. Differential expression and bioinformatics analysis of CircRNA in PDGF-BB-induced vascular smooth muscle cells. Front. Genet. 2020, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-H.; Park, H.-S.; Lee, D.-H.; Jo, J.-H.; Heo, K.-S.; Myung, C.-S. Regulation of autophagy by controlling Erk1/2 and mTOR for platelet-derived growth factor-BB-mediated vascular smooth muscle cell phenotype shift. Life Sci. 2021, 267, 118978. [Google Scholar] [CrossRef]
- Swaminathan, G.; Stoilov, I.; Broekelmann, T.; Mecham, R.; Ramamurthi, A. Phenotype-based selection of bone marrow mesenchymal stem cell-derived smooth muscle cells for elastic matrix regenerative repair in abdominal aortic aneurysms. J. Tissue Eng. Regen. Med. 2018, 12, e60–e70. [Google Scholar] [CrossRef]
- Chen, J.; Cui, X.; Qian, Z.; Li, Y.; Kang, K.; Qu, J.; Li, L.; Gou, D. Multi-omics analysis reveals regulators of the response to PDGF-BB treatment in pulmonary artery smooth muscle cells. BMC Genom. 2016, 17, 781. [Google Scholar] [CrossRef]
- Song, X.; Shi, J.; Liu, J.; Liu, Y.; Yu, Y.; Qiu, Y.; Cao, Z.; Pan, Y.; Yuan, X.; Chu, Y. Recombinant truncated latency-associated peptide alleviates liver fibrosis in vitro and in vivo via inhibition of TGF-β/Smad pathway. Mol. Med. 2022, 28, 80. [Google Scholar] [CrossRef]
- Ghosh, J.; Murphy, M.O.; Turner, N.; Khwaja, N.; Halka, A.; Kielty, C.M.; Walker, M.G. The role of transforming growth factor β1 in the vascular system. Cardiovasc. Pathol. 2005, 14, 28–36. [Google Scholar] [CrossRef]
- Bobik, A. Transforming growth factor-βs and vascular disorders. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1712–1720. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Gillis, E.; Van Laer, L.; Loeys, B.L. Genetics of thoracic aortic aneurysm: At the crossroad of transforming growth factor-β signaling and vascular smooth muscle cell contractility. Circ. Res. 2013, 113, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Milewicz, D.M.; Guo, D.-C.; Tran-Fadulu, V.; Lafont, A.L.; Papke, C.L.; Inamoto, S.; Kwartler, C.S.; Pannu, H. Genetic basis of thoracic aortic aneurysms and dissections: Focus on smooth muscle cell contractile dysfunction. Annu. Rev. Genom. Hum. Genet. 2008, 9, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Liang, X.; Dai, F.; Guan, H.; Sun, J.; Yao, W. RhoA/ROCK pathway activation is regulated by AT1 receptor and participates in smooth muscle migration and dedifferentiation via promoting actin cytoskeleton polymerization. Int. J. Mol. Sci. 2020, 21, 5398. [Google Scholar] [CrossRef]
- Belmadani, S.; Zerfaoui, M.; Boulares, H.A.; Palen, D.I.; Matrougui, K. Microvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, αvβ3-integrin, and TGF-β1 in response to ANG II and high glucose. Am. J. Physiol.-Heart Circ. Physiol. 2008, 295, H69–H76. [Google Scholar] [CrossRef]
- Da, X.; Li, Z.; Huang, X.; He, Z.; Yu, Y.; Tian, T.; Xu, C.; Yao, Y.; Wang, Q.K. AGGF1 therapy inhibits thoracic aortic aneurysms by enhancing integrin α7-mediated inhibition of TGF-β1 maturation and ERK1/2 signaling. Nat. Commun. 2023, 14, 2265. [Google Scholar] [CrossRef]
- Dai, X.; Shen, J.; Priyanka Annam, N.; Jiang, H.; Levi, E.; Schworer, C.M.; Tromp, G.; Arora, A.; Higgins, M.; Wang, X.-F. SMAD3 deficiency promotes vessel wall remodeling, collagen fiber reorganization and leukocyte infiltration in an inflammatory abdominal aortic aneurysm mouse model. Sci. Rep. 2015, 5, 10180. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massagué, J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Rombouts, K.B.; van Merrienboer, T.A.; Ket, J.C.; Bogunovic, N.; van der Velden, J.; Yeung, K.K. The role of vascular smooth muscle cells in the development of aortic aneurysms and dissections. Eur. J. Clin. Investig. 2022, 52, e13697. [Google Scholar] [CrossRef]
- Elmarasi, M.; Elmakaty, I.; Elsayed, B.; Elsayed, A.; Zein, J.A.; Boudaka, A.; Eid, A.H. Phenotypic switching of vascular smooth muscle cells in atherosclerosis, hypertension, and aortic dissection. J. Cell. Physiol. 2024, 239, e31200. [Google Scholar] [CrossRef]
- He, X.; Li, X.; Han, Y.; Chen, G.; Xu, T.; Cai, D.; Sun, Y.; Wang, S.; Lai, Y.; Teng, Z. CircRNA Chordc1 protects mice from abdominal aortic aneurysm by contributing to the phenotype and growth of vascular smooth muscle cells. Mol. Ther.-Nucleic Acids 2022, 27, 81–98. [Google Scholar] [CrossRef]
- Lin, F.; Yang, X. TGF-β signaling in aortic aneurysm: Another round of controversy. J. Genet. Genom. 2010, 37, 583–591. [Google Scholar] [CrossRef]
- Meester, J.A.; Vandeweyer, G.; Pintelon, I.; Lammens, M.; Van Hoorick, L.; De Belder, S.; Waitzman, K.; Young, L.; Markham, L.W.; Vogt, J. Loss-of-function mutations in the X-linked biglycan gene cause a severe syndromic form of thoracic aortic aneurysms and dissections. Genet. Med. 2017, 19, 386–395. [Google Scholar] [CrossRef]
- Yorn, C.; Kim, H.; Jeong, K. Influence of DNA methylation on vascular smooth muscle cell phenotypic switching. Int. J. Mol. Sci. 2024, 25, 3136. [Google Scholar] [CrossRef]
- Tingting, T.; Wenjing, F.; Qian, Z.; Hengquan, W.; Simin, Z.; Zhisheng, J.; Shunlin, Q. The TGF-β pathway plays a key role in aortic aneurysms. Clin. Chim. Acta 2020, 501, 222–228. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, L.; Gong, Y.; Zhang, C.; Yang, Y.; Wang, W.; Qin, L. Isovaleroylbinankadsurin A ameliorates atherosclerosis and restenosis by promoting LXRα signaling pathway and inhibiting TGF-β1 and FHL1 signaling pathway. Phytomedicine 2025, 139, 156451. [Google Scholar] [CrossRef]
- High, F.A.; Zhang, M.; Proweller, A.; Tu, L.; Parmacek, M.S.; Pear, W.S.; Epstein, J.A. An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. J. Clin. Investig. 2007, 117, 353–363. [Google Scholar] [CrossRef]
- Boucher, J.; Gridley, T.; Liaw, L. Molecular pathways of notch signaling in vascular smooth muscle cells. Front. Physiol. 2012, 3, 81. [Google Scholar] [CrossRef]
- Sharma, N.; Dev, R.; Ruiz-Rosado, J.d.D.; Partida-Sanchez, S.; Guerau-de-Arellano, M.; Dhakal, P.; Kuivaniemi, H.; Hans, C.P. Pharmacological inhibition of Notch signaling regresses pre-established abdominal aortic aneurysm. Sci. Rep. 2019, 9, 13458. [Google Scholar] [CrossRef]
- Hans, C.P.; Sharma, N.; Dev, R.; Blain, J.M.; Tonniges, J.; Agarwal, G. DAPT, a potent Notch inhibitor regresses actively growing abdominal aortic aneurysm via divergent pathways. Clin. Sci. 2020, 134, 1555–1572. [Google Scholar] [CrossRef]
- Zheng, Y.-H.; Li, F.-D.; Tian, C.; Ren, H.-L.; Du, J.; Li, H.-H. Notch γ-secretase inhibitor dibenzazepine attenuates angiotensin II-induced abdominal aortic aneurysm in ApoE knockout mice by multiple mechanisms. PLoS ONE 2013, 8, e83310. [Google Scholar] [CrossRef]
- Breikaa, R. Investigating the Role of Endothelial Cell-Expressed Jag1 on Smooth Muscle Function and Vascular Homeostasis. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2022. [Google Scholar]
- Xie, M.; Li, X.; Chen, L.; Zhang, Y.; Chen, L.; Hua, H.; Qi, J. The crosstalks between vascular endothelial cells, vascular smooth muscle cells, and adventitial fibroblasts in vascular remodeling. Life Sci. 2024, 361, 123319. [Google Scholar] [CrossRef]
- Ozasa, Y.; Akazawa, H.; Qin, Y.; Tateno, K.; Ito, K.; Kudo-Sakamoto, Y.; Yano, M.; Yabumoto, C.; Naito, A.T.; Oka, T. Notch activation mediates angiotensin II-induced vascular remodeling by promoting the proliferation and migration of vascular smooth muscle cells. Hypertens. Res. 2013, 36, 859–865. [Google Scholar] [CrossRef]
- Pan, L.; Gross, K.W. Transcriptional regulation of renin: An update. Hypertension 2005, 45, 3–8. [Google Scholar] [CrossRef]
- Tang, Y.; Urs, S.; Liaw, L. Hairy-related transcription factors inhibit notch-induced smooth muscle α-actin expression by interfering with notch intracellular Domain/CBF-1 complex interaction with the CBF-1–binding site. Circ. Res. 2008, 102, 661–668. [Google Scholar] [CrossRef]
- Boulos, N.; Helle, F.; Dussaule, J.-C.; Placier, S.; Milliez, P.; Djudjaj, S.; Guerrot, D.; Joutel, A.; Ronco, P.; Boffa, J.-J. Notch3 is essential for regulation of the renal vascular tone. Hypertension 2011, 57, 1176–1182. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Penninger, J.M. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ. J. 2013, 77, 301–308. [Google Scholar] [CrossRef]
- Tan, W.S.D.; Liao, W.; Zhou, S.; Mei, D.; Wong, W.-S.F. Targeting the renin–angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr. Opin. Pharmacol. 2018, 40, 9–17. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Ohto-Nakanishi, T.; Penninger, J.M. Trilogy of ACE2: A peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010, 128, 119–128. [Google Scholar] [CrossRef]
- Perlot, T.; Penninger, J.M. ACE2–From the renin–angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013, 15, 866–873. [Google Scholar] [CrossRef]
- Osumi, H.; Matsusaka, S.; Wakatsuki, T.; Suenaga, M.; Shinozaki, E.; Mizunuma, N. Angiotensin II type-1 receptor blockers enhance the effects of bevacizumab-based chemotherapy in metastatic colorectal cancer patients. Mol. Clin. Oncol. 2015, 3, 1295–1300. [Google Scholar] [CrossRef]
- Bosnyak, S.; Jones, E.S.; Christopoulos, A.; Aguilar, M.-I.; Thomas, W.G.; Widdop, R.E. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin. Sci. 2011, 121, 297–303. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005, 436, 112–116. [Google Scholar] [CrossRef]
- Matysiak-Burzyńska, Z.E.; Nowakowska, M.; Domińska, K.; Kowalska, K.; Płuciennik, E.; Piastowska-Ciesielska, A.W. Silencing of angiotensin receptor 1 interferes with angiotensin II oncogenic activity in endometrial cancer. J. Cell. Biochem. 2018, 119, 9110–9121. [Google Scholar] [CrossRef]
- Speck, D.; Kleinau, G.; Szczepek, M.; Kwiatkowski, D.; Catar, R.; Philippe, A.; Scheerer, P. Angiotensin and endothelin receptor structures with implications for signaling regulation and pharmacological targeting. Front. Endocrinol. 2022, 13, 880002. [Google Scholar] [CrossRef]
- Daugherty, A.; Cassis, L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor−/− mice. Ann. N. Y. Acad. Sci. 1999, 892, 108–118. [Google Scholar] [CrossRef]
- Dong, C.X.; Malecki, C.; Robertson, E.; Hambly, B.; Jeremy, R. Molecular mechanisms in genetic aortopathy–signaling pathways and potential interventions. Int. J. Mol. Sci. 2023, 24, 1795. [Google Scholar] [CrossRef]
- Norambuena-Soto, I.; Ocaranza, M.P.; Cancino-Arenas, N.; Sanhueza–Olivares, F.; Villar-Fincheira, P.; Leiva–Navarrete, S.; Mancilla-Medina, C.; Moya, J.; Novoa, U.; Jalil, J.E. Angiotensin-(1–9) prevents vascular remodeling by decreasing vascular smooth muscle cell dedifferentiation through a FoxO1-dependent mechanism. Biochem. Pharmacol. 2020, 180, 114190. [Google Scholar] [CrossRef]
- Savoia, C.; Burger, D.; Nishigaki, N.; Montezano, A.; Touyz, R.M. Angiotensin II and the vascular phenotype in hypertension. Expert Rev. Mol. Med. 2011, 13, e11. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Yang, X.; Liu, K.; Zhang, X.; Zuo, X.; Ye, R.; Wang, Z.; Shi, R.; Meng, Q. Signaling pathways in vascular function and hypertension: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 168. [Google Scholar] [CrossRef]
- Karasaki, K.; Kokubo, H.; Bumdelger, B.; Kaji, N.; Sakai, C.; Ishida, M.; Yoshizumi, M. Angiotensin II Type 1 Receptor Blocker Prevents Abdominal Aortic Aneurysm Progression in Osteoprotegerin-Deficient Mice via Upregulation of Angiotensin (1–7). J. Am. Heart Assoc. 2023, 12, e027589. [Google Scholar] [CrossRef]
- Hackam, D.G.; Thiruchelvam, D.; Redelmeier, D.A. Angiotensin-converting enzyme inhibitors and aortic rupture: A population-based case-control study. Lancet 2006, 368, 659–665. [Google Scholar] [CrossRef]
- Bicknell, C.D.; Kiru, G.; Falaschetti, E.; Powell, J.T.; Poulter, N.R.; Collaborators, A.; Collaborators, A. An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: A randomized placebo-controlled trial (AARDVARK). Eur. Heart J. 2016, 37, 3213–3221. [Google Scholar] [CrossRef]
- Kristensen, K.E.; Torp-Pedersen, C.; Gislason, G.H.; Egfjord, M.; Rasmussen, H.B.; Hansen, P.R. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in patients with abdominal aortic aneurysms: Nation-wide cohort study. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 733–740. [Google Scholar] [CrossRef]
- Sweeting, M.J.; Thompson, S.G.; Brown, L.C.; Greenhalgh, R.M.; Powell, J.T. Use of angiotensin converting enzyme inhibitors is associated with increased growth rate of abdominal aortic aneurysms. J. Vasc. Surg. 2010, 52, 1–4. [Google Scholar] [CrossRef]
- Tian, K.; Thanigaimani, S.; Gibson, K.; Golledge, J. Systematic Review Examining the Association Between Angiotensin Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Prescription and Abdominal Aortic Aneurysm Growth and Events. Eur. J. Vasc. Endovasc. Surg. 2024, 68, 180–187. [Google Scholar] [CrossRef]
- Iida, Y.; Xu, B.; Schultz, G.M.; Chow, V.; White, J.J.; Sulaimon, S.; Hezi-Yamit, A.; Peterson, S.R.; Dalman, R.L. Efficacy and mechanism of angiotensin II receptor blocker treatment in experimental abdominal aortic aneurysms. PLoS ONE 2012, 7, e49642. [Google Scholar] [CrossRef]
- Kaschina, E.; Schrader, F.; Sommerfeld, M.; Kemnitz, U.R.; Grzesiak, A.; Krikov, M.; Unger, T. Telmisartan prevents aneurysm progression in the rat by inhibiting proteolysis, apoptosis and inflammation. J. Hypertens. 2008, 26, 2361–2373. [Google Scholar] [CrossRef]
- Krueger, F.; Kappert, K.; Foryst-Ludwig, A.; Kramer, F.; Clemenz, M.; Grzesiak, A.; Sommerfeld, M.; Paul Frese, J.; Greiner, A.; Kintscher, U. AT1-receptor blockade attenuates outward aortic remodeling associated with diet-induced obesity in mice. Clin. Sci. 2017, 131, 1989–2005. [Google Scholar] [CrossRef]
- Sakaue, T.; Suzuki, J.; Hamaguchi, M.; Suehiro, C.; Tanino, A.; Nagao, T.; Uetani, T.; Aono, J.; Nakaoka, H.; Kurata, M. Perivascular adipose tissue angiotensin II type 1 receptor promotes vascular inflammation and aneurysm formation. Hypertension 2017, 70, 780–789. [Google Scholar] [CrossRef]
- Xuan, H.; Xu, B.; Wang, W.; Tanaka, H.; Fujimura, N.; Miyata, M.; Michie, S.A.; Dalman, R.L. Inhibition or deletion of angiotensin II type 1 receptor suppresses elastase-induced experimental abdominal aortic aneurysms. J. Vasc. Surg. 2018, 67, 573–584.e572. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Sukhova, G.; Schwartz, D.; Libby, P. Proliferating arterial smooth muscle cells after balloon injury express TNF-α but not interleukin-1 or basic fibroblast growth factor. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-C.; Wang, C.-P.; Chen, J.-H.; Lin, H.-H. Anti-atherosclerotic effect of Hibiscus leaf polyphenols against tumor necrosis factor-alpha-induced abnormal vascular smooth muscle cell migration and proliferation. Antioxidants 2019, 8, 620. [Google Scholar] [CrossRef]
- Fan, W.; Liu, Y.; Li, C.; Qu, X.; Zheng, G.; Zhang, Q.; Pan, Z.; Wang, Y.; Rong, J. microRNA-331-3p maintains the contractile type of vascular smooth muscle cells by regulating TNF-α and CD14 in intracranial aneurysm. Neuropharmacology 2020, 164, 107858. [Google Scholar] [CrossRef]
- Zhuang, J.; Luan, P.; Li, H.; Wang, K.; Zhang, P.; Xu, Y.; Peng, W. The Yin–Yang dynamics of DNA methylation is the key regulator for smooth muscle cell phenotype switch and vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 84–97. [Google Scholar] [CrossRef]
- Satta, R.; Maloku, E.; Zhubi, A.; Pibiri, F.; Hajos, M.; Costa, E.; Guidotti, A. Nicotine decreases DNA methyltransferase 1 expression and glutamic acid decarboxylase 67 promoter methylation in GABAergic interneurons. Proc. Natl. Acad. Sci. USA 2008, 105, 16356–16361. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, D.; Xu, T. DNA methylation aberrant in atherosclerosis. Front. Pharmacol. 2022, 13, 815977. [Google Scholar] [CrossRef] [PubMed]
- Warsi, A.A.; Davies, B.; Morris-Stiff, G.; Hullin, D.; Lewis, M.H. Abdominal aortic aneurysm and its correlation to plasma homocysteine, and vitamins. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 75–79. [Google Scholar] [CrossRef]
- Krishna, S.M.; Dear, A.; Craig, J.M.; Norman, P.E.; Golledge, J. The potential role of homocysteine mediated DNA methylation and associated epigenetic changes in abdominal aortic aneurysm formation. Atherosclerosis 2013, 228, 295–305. [Google Scholar] [CrossRef]
- Lipp, C.; Lohoefer, F.; Reeps, C.; Rudelius, M.; Baummann, M.; Heemann, U.; Eckstein, H.-H.; Pelisek, J. Expression of a disintegrin and metalloprotease in human abdominal aortic aneurysms. J. Vasc. Res. 2012, 49, 198–206. [Google Scholar] [CrossRef]
- Vats, S.; Sundquist, K.; Wang, X.; Zarrouk, M.; Ågren-Witteschus, S.; Sundquist, J.; Gottsäter, A.; Memon, A.A. Associations of global DNA methylation and homocysteine levels with abdominal aortic aneurysm: A cohort study from a population-based screening program in Sweden. Int. J. Cardiol. 2020, 321, 137–142. [Google Scholar] [CrossRef]
- Toghill, B.J.; Saratzis, A.; Freeman, P.J.; Sylvius, N.; Bown, M.J. SMYD2 promoter DNA methylation is associated with abdominal aortic aneurysm (AAA) and SMYD2 expression in vascular smooth muscle cells. Clin. Epigenet. 2018, 10, 29. [Google Scholar] [CrossRef]
- Alexander, M.R.; Owens, G.K. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu. Rev. Physiol. 2012, 74, 13–40. [Google Scholar] [CrossRef]
- Hoshikawa, Y.; Matsuda, Y.; Suzuki, S.; Okada, Y.; Tabata, T.; Matsumura, Y.; Kondo, T. Osteopontin may be responsible for pulmonary vascular remodeling. Chest 2005, 128, 621S. [Google Scholar] [CrossRef]
- Lesauskaite, V.; Epistolato, M.C.; Castagnini, M.; Urbonavicius, S.; Tanganelli, P. Expression of matrix metalloproteinases, their tissue inhibitors, and osteopontin in the wall of thoracic and abdominal aortas with dilatative pathology. Hum. Pathol. 2006, 37, 1076–1084. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Z.; Jia, J.; Du, T.; Zhang, N.; Tang, Y.; Fang, Y.; Fang, D. Overview of histone modification. Histone Mutat. Cancer 2021, 1283, 1–16. [Google Scholar]
- Rodríguez-Castañeda, F.; Lemma, R.B.; Cuervo, I.; Bengtsen, M.; Moen, L.M.; Ledsaak, M.; Eskeland, R.; Gabrielsen, O.S. The SUMO protease SENP1 and the chromatin remodeler CHD3 interact and jointly affect chromatin accessibility and gene expression. J. Biol. Chem. 2018, 293, 15439–15454. [Google Scholar] [CrossRef]
- Han, Y.; Tanios, F.; Reeps, C.; Zhang, J.; Schwamborn, K.; Eckstein, H.-H.; Zernecke, A.; Pelisek, J. Histone acetylation and histone acetyltransferases show significant alterations in human abdominal aortic aneurysm. Clin. Epigenet. 2016, 8, 3. [Google Scholar] [CrossRef]
- Galán, M.; Varona, S.; Orriols, M.; Rodríguez, J.A.; Aguiló, S.; Dilmé, J.; Camacho, M.; Martínez-González, J.; Rodriguez, C. Induction of histone deacetylases (HDACs) in human abdominal aortic aneurysm: Therapeutic potential of HDAC inhibitors. Dis. Models Mech. 2016, 9, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Vinh, A.; Gaspari, T.A.; Liu, H.B.; Dousha, L.F.; Widdop, R.E.; Dear, A.E. A novel histone deacetylase inhibitor reduces abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E-deficient mice. J. Vasc. Res. 2008, 45, 143–152. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.; Chen, Y.; Pober, J.S.; Zhou, M.; Zhou, J.H.; Min, W. SRF SUMOylation modulates smooth muscle phenotypic switch and vascular remodeling. Nat. Commun. 2024, 15, 6919. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, H.; Huang, S.; Yin, L.; Wang, F.; Luo, P.; Huang, H. Epigenetic regulation in cardiovascular disease: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2022, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, Y.; Zhao, G.; Deng, Y.; Chen, Y.E.; Zhang, J. SWI/SNF complex in vascular smooth muscle cells and its implications in cardiovascular pathologies. Cells 2024, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Zhao, G.; Zhao, Y.; Lu, H.; Xiong, W.; Liang, W.; Sun, J.; Wang, H.; Zhu, T.; Rom, O. BAF60a deficiency in vascular smooth muscle cells prevents abdominal aortic aneurysm by reducing inflammation and extracellular matrix degradation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2494–2507. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, Y.; Lu, H.; Chang, Z.; Liu, H.; Wang, H.; Liang, W.; Liu, Y.; Zhu, T.; Rom, O. BAF60c prevents abdominal aortic aneurysm formation through epigenetic control of vascular smooth muscle cell homeostasis. J. Clin. Investig. 2022, 132, e158309. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, C.-Q.; Peng, G.-Y.; Huang, H.-y.; Yu, Y.-s.; Ji, Z.-C.; Shen, Z.-Y. MicroRNA-134-5p regulates media degeneration through inhibiting VSMC phenotypic switch and migration in thoracic aortic dissection. Mol. Ther. Nucleic Acids 2019, 16, 284–294. [Google Scholar] [CrossRef]
- Bi, S.; Peng, Q.; Liu, W. MicroRNA-342-5p activates the Akt signaling pathway by downregulating PIK3R1 to modify the proliferation and differentiation of vascular smooth muscle cells. Exp. Ther. Med. 2020, 20, 239. [Google Scholar] [CrossRef]
- Si, X.; Chen, Q.; Zhang, J.; Zhou, W.; Chen, L.; Chen, J.; Deng, N.; Li, W.; Liu, D.; Wang, L. MicroRNA-23b prevents aortic aneurysm formation by inhibiting smooth muscle cell phenotypic switching via FoxO4 suppression. Life Sci. 2022, 288, 119092. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, M.; Zhang, J.; Lv, M.; Li, Y.; Yang, X.; Liu, A.; Wu, Z. MiR-29b downregulation induces phenotypic modulation of vascular smooth muscle cells: Implication for intracranial aneurysm formation and progression to rupture. Cell. Physiol. Biochem. 2017, 41, 510–518. [Google Scholar] [CrossRef]
- Farina, F.M.; Hall, I.F.; Serio, S.; Zani, S.; Climent, M.; Salvarani, N.; Carullo, P.; Civilini, E.; Condorelli, G.; Elia, L. miR-128-3p is a novel regulator of vascular smooth muscle cell phenotypic switch and vascular diseases. Circ. Res. 2020, 126, e120–e135. [Google Scholar] [CrossRef]
- Cluzel, G.L.; Ryan, P.M.; Herisson, F.M.; Caplice, N.M. High-fidelity porcine models of metabolic syndrome: A contemporary synthesis. Am. J. Physiol.-Endocrinol. Metab. 2022, 322, E366–E381. [Google Scholar] [CrossRef]
- Tao, W.; Hong, Y.; He, H.; Han, Q.; Mao, M.; Hu, B.; Zhang, H.; Huang, X.; You, W.; Liang, X. MicroRNA-199a-5p aggravates angiotensin II–induced vascular smooth muscle cell senescence by targeting Sirtuin-1 in abdominal aortic aneurysm. J. Cell. Mol. Med. 2021, 25, 6056–6069. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.-S.; Nguyen, P.; Wang, K.-C.; Weiss, A.; Kuo, Y.-C.; Chiu, J.-J.; Shyy, J.Y.; Chien, S. Regulation of vascular smooth muscle cell turnover by endothelial cell–secreted microRNA-126: Role of shear stress. Circ. Res. 2013, 113, 40–51. [Google Scholar] [CrossRef]
- Maegdefessel, L.; Spin, J.M.; Raaz, U.; Eken, S.M.; Toh, R.; Azuma, J.; Adam, M.; Nagakami, F.; Heymann, H.M.; Chernugobova, E. miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat. Commun. 2014, 5, 5214. [Google Scholar] [CrossRef]
- Yu, X.; Xu, J.-f.; Song, M.; Zhang, L.; Li, Y.-h.; Han, L.; Tang, M.-x.; Zhang, W.; Zhong, M.; Wang, Z.-h. Associations of circulating microRNA-221 and 222 with the severity of coronary artery lesions in acute coronary syndrome patients. Angiology 2022, 73, 579–587. [Google Scholar] [CrossRef]
- Luo, Y.; Xiong, W.; Dong, S.; Liu, F.; Liu, H.; Li, J. MicroRNA-146a promotes the proliferation of rat vascular smooth muscle cells by downregulating p53 signaling. Mol. Med. Rep. 2017, 16, 6940–6945. [Google Scholar] [CrossRef]
- Zhao, L.; Ouyang, Y.; Bai, Y.; Gong, J.; Liao, H. miR-155-5p inhibits the viability of vascular smooth muscle cell via targeting FOS and ZIC3 to promote aneurysm formation. Eur. J. Pharmacol. 2019, 853, 145–152. [Google Scholar] [CrossRef]
- Lin, W.; Hou, L.; Tang, J.; Huang, A.; Jia, Z. Mir-195-5p targets Smad7 regulation of the Wnt/β-catenin pathway to promote osteogenic differentiation of vascular smooth muscle cells. BMC Cardiovasc. Disord. 2024, 24, 221. [Google Scholar] [CrossRef]
- Yang, X.; Dong, M.; Wen, H.; Liu, X.; Zhang, M.; Ma, L.; Zhang, C.; Luan, X.; Lu, H.; Zhang, Y. MiR-26a contributes to the PDGF-BB-induced phenotypic switch of vascular smooth muscle cells by suppressing Smad1. Oncotarget 2017, 8, 75844. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, B.; Zhang, X.; Zhang, R.; Lu, Q.; Shi, F.; Xu, J.; Deng, L. miRNA-92a inhibits vascular smooth muscle cell phenotypic modulation and may help prevent in-stent restenosis. Mol. Med. Rep. 2023, 27, 40. [Google Scholar] [CrossRef]
- Liu, Y. Atherosclerotic Conditions Promote the Packaging of Functional microRNA-92 into Endothelial Microvesicles. Ph.D. Thesis, Rheinische Friedrich-Wilhelms-Universität Bonn, Bonn, Germany, 2019. [Google Scholar]
- Hall, I.F.; Climent, M.; Quintavalle, M.; Farina, F.M.; Schorn, T.; Zani, S.; Carullo, P.; Kunderfranco, P.; Civilini, E.; Condorelli, G. Circ_Lrp6, a circular RNA enriched in vascular smooth muscle cells, acts as a sponge regulating miRNA-145 function. Circ. Res. 2019, 124, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.-H.; Chang, N.-B.; Yao, Q.-P.; Li, T.; Zhu, X.-L.; Cao, Y.; Jiang, M.-J.; Cheng, Y.-S.; Jiang, R.; Jiang, J. Suppression of circDcbld1 alleviates intimal hyperplasia in rat carotid artery by targeting miR-145-3p/Neuropilin-1. Mol. Ther. Nucleic Acids 2019, 18, 999–1008. [Google Scholar] [CrossRef]
- Zeng, Z.; Xia, L.; Fan, S.; Zheng, J.; Qin, J.; Fan, X.; Liu, Y.; Tao, J.; Liu, Y.; Li, K. Circular RNA CircMAP3K5 acts as a MicroRNA-22-3p sponge to promote resolution of intimal hyperplasia via TET2-mediated smooth muscle cell differentiation. Circulation 2021, 143, 354–371. [Google Scholar] [CrossRef]
- Thompson, R.W.; Liao, S.; Curci, J.A. Vascular smooth muscle cell apoptosis in abdominal aortic aneurysms. Coron. Artery Dis. 1997, 8, 623–632. [Google Scholar] [CrossRef]
- Lopez-Candales, A.; Holmes, D.R.; Liao, S.; Scott, M.J.; Wickline, S.A.; Thompson, R.W. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am. J. Pathol. 1997, 150, 993. [Google Scholar]
- Huang, C.-l.; Huang, Y.-n.; Yao, L.; Li, J.-p.; Zhang, Z.-h.; Huang, Z.-q.; Chen, S.-x.; Zhang, Y.-l.; Wang, J.-f.; Chen, Y.-x. Thoracic perivascular adipose tissue inhibits VSMC apoptosis and aortic aneurysm formation in mice via the secretome of browning adipocytes. Acta Pharmacol. Sin. 2023, 44, 345–355. [Google Scholar] [CrossRef]
- Wang, J.; Da, X.; Chen, Y.; Yuan, A.; Pu, J. Glutamine Protects against Mouse Abdominal Aortic Aneurysm through Modulating VSMC Apoptosis and M1 Macrophage Activation. Int. J. Med. Sci. 2024, 21, 1414. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, N.; Zhao, Y. Resveratrol Inhibits Abdominal Aortic Aneurysm Progression by Reducing Extracellular Matrix Degradation, Apoptosis, Autophagy, and Inflammation of Vascular Smooth Muscle Cells via Upregulation of HMOX1. J. Endovasc. Ther. 2023; ahead-of-print. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, Y.; Li, Q.; Tan, J.; Fu, X.; Liang, Y.; Tuo, Y.; Liu, L.; Zhou, X.; LiuFu, D. Spatiotemporal ATF3 Expression Determines VSMC Fate in Abdominal Aortic Aneurysm. Circ. Res. 2024, 134, 1495–1511. [Google Scholar] [CrossRef]
- Liang, K.; Cui, M.; Fu, X.; Ma, J.; Zhang, K.; Zhang, D.; Zhai, S. LncRNA Xist induces arterial smooth muscle cell apoptosis in thoracic aortic aneurysm through miR-29b-3p/Eln pathway. Biomed. Pharmacother. 2021, 137, 111163. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, D.; Xu, R.; Zhai, S.; Zhang, K. Inhibition of XIST attenuates abdominal aortic aneurysm in mice by regulating apoptosis of vascular smooth muscle cells through miR-762/MAP2K4 axis. Microvasc. Res. 2022, 140, 104299. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Hong, Y.; Mai, C.; Yang, H.; Wu, Z.; Gao, X.; Zeng, W.; Deng, X.; Liu, B.; Zhang, Y. Transcriptome analysis reveals therapeutic potential of NAMPT in protecting against abdominal aortic aneurysm in human and mouse. Bioact. Mater. 2024, 34, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Sun, J.; Liang, W.; Chang, Z.; Rom, O.; Zhao, Y.; Zhao, G.; Xiong, W.; Wang, H.; Zhu, T. Cyclodextrin prevents abdominal aortic aneurysm via activation of vascular smooth muscle cell transcription factor EB. Circulation 2020, 142, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wei, M.; Zhang, D.; Zhai, S.; Liu, H. PANoptosis in vascular smooth muscle cells regulated by TNF-α/IL-1β can be a new target for alleviating the progression of abdominal aortic aneurysm. Physiol. Genom. 2024, 56, 158–166. [Google Scholar] [CrossRef]
- Martinez-Pinna, R.; Lindholt, J.S.; Madrigal-Matute, J.; Blanco-Colio, L.M.; Esteban-Salan, M.; Torres-Fonseca, M.M.; Lefebvre, T.; Delbosc, S.; Laustsen, J.; Driss, F. From tissue iron retention to low systemic haemoglobin levels, new pathophysiological biomarkers of human abdominal aortic aneurysm. Thromb. Haemost. 2014, 112, 87–95. [Google Scholar] [CrossRef]
- Liao, F.; Wang, L.; Wu, Z.; Luo, G.; Qian, Y.; He, X.; Ding, S.; Pu, J. Disulfiram protects against abdominal aortic aneurysm by ameliorating vascular smooth muscle cells pyroptosis. Cardiovasc. Drugs Ther. 2023, 37, 1–14. [Google Scholar] [CrossRef]
- Cai, H.; Li, H.; Xiao, X.; Wang, S.; Liu, R.; Qin, Y.; Zhou, Y.; Yao, C. TRAF6 promotes abdominal aortic aneurysm development by activating macrophage pyroptosis via the NLRP3/Caspase1/GSDMD pathway. FASEB J. 2025, 39, e70318. [Google Scholar] [CrossRef]
- Ye, B.; Fan, X.; Fang, Z.; Mao, C.; Lin, L.; Wu, J.; Zheng, W.; Cai, X.; Huang, W.; Lv, Y. Macrophage-derived GSDMD promotes abdominal aortic aneurysm and aortic smooth muscle cells pyroptosis. Int. Immunopharmacol. 2024, 128, 111554. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.; Zong, J.; Wang, Z.; Jiang, S.; Fu, X.; He, X.; Li, X.; Xue, Q.; Wang, J.-X. miR-564: A potential regulator of vascular smooth muscle cells and therapeutic target for aortic dissection. J. Mol. Cell. Cardiol. 2022, 170, 100–114. [Google Scholar] [CrossRef]
- Sun, L.; Li, X.; Luo, Z.; Li, M.; Liu, H.; Zhu, Z.; Wang, J.; Lu, P.; Wang, L.; Yang, C. Purinergic receptor P2X7 contributes to abdominal aortic aneurysm development via modulating macrophage pyroptosis and inflammation. Transl. Res. 2023, 258, 72–85. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, J.; Zhao, M.; Liu, J.; Xu, Y.; Peng, S.; Pan, W.; Wei, C.; Zheng, Z.; Liu, S. EDIL3/Del-1 prevents aortic dissection through enhancing internalization and degradation of apoptotic vascular smooth muscle cells. Autophagy 2024, 20, 2405–2425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, K.; Zhang, W.; Zhao, Z.; Chang, F.; Du, J.; Zhang, X.; Bao, K.; Zhang, C.; Shi, L. Ganglioside GM3 protects against abdominal aortic aneurysm by suppressing ferroptosis. Circulation 2024, 149, 843–859. [Google Scholar] [CrossRef]
- Zou, H.-X.; Qiu, B.-Q.; Lai, S.-Q.; Huang, H.; Zhou, X.-L.; Gong, C.-W.; Wang, L.-J.; Yuan, M.-M.; He, A.-D.; Liu, J.-C. Role of ferroptosis-related genes in Stanford type a aortic dissection and identification of key genes: New insights from bioinformatic analysis. Bioengineered 2021, 12, 9976–9990. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xiong, Y.; Liu, Y.; Li, Y.; Zhou, H.; Wu, K. Ferrostatin-1 inhibits ferroptosis of vascular smooth muscle cells and alleviates abdominal aortic aneurysm formation through activating the SLC7A11/GPX4 axis. FASEB J. 2024, 38, e23401. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.R.; Bellotti, P.; Valisno, J.A.C.; Su, G.; Sharma, S.; Kollareth, D.J.M.; Hartman, J.B.; Adithan, A.; Spinosa, M.; Kamat, M. Pharmacologic Inhibition of Ferroptosis Attenuates Experimental Abdominal Aortic Aneurysm Formation. bioRxiv 2024. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; Cui, Y.; Gan, N.; Xiang, Q.; Li, M.; Zeng, W.; Zheng, X.-L.; Dai, X.; Peng, J. Inhibition of VSMC Ferroptosis Mitigates Pathological Vascular Remodeling: A Novel Therapeutic Strategy for Abdominal Aortic Aneurysm. J. Cardiovasc. Transl. Res. 2025, 1–15. [Google Scholar] [CrossRef]
- Hu, X.; Hu, L.; Si, X.; Feng, Q.; Ma, Y.; Liu, Z.; He, X.; Shi, B. Comprehensive Bioinformatics Analysis Reveals the Role of Shared Cuproptosis-and Ferroptosis-Related DEG DLD in Abdominal Aortic Aneurysm. J. Cell. Mol. Med. 2025, 29, e70399. [Google Scholar] [CrossRef]
- Mutailipu, M.; Zhang, M.; Ding, W.; Fan, Y.; Ye, Y.; Lu, Z. Identification of cuproptosis-related biomarkers in aortic dissection: New insights from bioinformatic analysis. Res. Sq. 2023; preprint. [Google Scholar] [CrossRef]
- Xiao, X.; Deng, Z.; Huang, Z.; Liang, C.; Chen, Z.; Xiao, X.; Liu, D. Comprehensive analysis the role of cuproptosis related genes in abdominal aortic aneurysm. Ann. Med. Surg. 2025, 87, 1282–1294. [Google Scholar] [CrossRef]
- Van den Bergh, G.; Opdebeeck, B.; D’Haese, P.C.; Verhulst, A. The vicious cycle of arterial stiffness and arterial media calcification. Trends Mol. Med. 2019, 25, 1133–1146. [Google Scholar] [CrossRef]
- Vine, N.; Powell, J.T. Metalloproteinases in degenerative aortic disease. Clin. Sci. 1991, 81, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Newman, K.M.; Ogata, Y.; Malon, A.M.; Irizarry, E.; Gandhi, R.H.; Nagase, H.; Tilson, M.D. Identification of matrix metalloproteinases 3 (stromelysin-1) and 9 (gelatinase B) in abdominal aortic aneurysm. Arterioscler. Thromb. J. Vasc. Biol. 1994, 14, 1315–1320. [Google Scholar] [CrossRef]
- Nishimura, K.; Ohgi, S.; Nanba, E. Expression of MMP-2, MMP-9 and TIMP-1 in the Wall of Abdominal Aortic Aneurysms. Yonago Acta Medica 2001, 44, 25–35. [Google Scholar]
- Stepien, K.L.; Bajdak-Rusinek, K.; Fus-Kujawa, A.; Kuczmik, W.; Gawron, K. Role of extracellular matrix and inflammation in abdominal aortic aneurysm. Int. J. Mol. Sci. 2022, 23, 11078. [Google Scholar] [CrossRef] [PubMed]
- Davis, V.; Persidskaia, R.; Baca-Regen, L.; Itoh, Y.; Nagase, H.; Persidsky, Y.; Ghorpade, A.; Baxter, B.T. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1625–1633. [Google Scholar] [CrossRef]
- Miyagawa, K.; Ogata, T.; Ueyama, T.; Kasahara, T.; Nakanishi, N.; Naito, D.; Taniguchi, T.; Hamaoka, T.; Maruyama, N.; Nishi, M. Loss of MURC/Cavin-4 induces JNK and MMP-9 activity enhancement in vascular smooth muscle cells and exacerbates abdominal aortic aneurysm. Biochem. Biophys. Res. Commun. 2017, 487, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Ramella, M.; Boccafoschi, F.; Bellofatto, K.; Follenzi, A.; Fusaro, L.; Boldorini, R.; Casella, F.; Porta, C.; Settembrini, P.; Cannas, M. Endothelial MMP-9 drives the inflammatory response in abdominal aortic aneurysm (AAA). Am. J. Transl. Res. 2017, 9, 5485. [Google Scholar]
- Wang, X.; Wang, M.; Zhou, Z.; Zou, X.; Song, G.; Zhang, Q.; Zhou, H. SMOC2 promoted vascular smooth muscle cell proliferation, migration, and extracellular matrix degradation by activating BMP/TGF-β1 signaling pathway. J. Clin. Biochem. Nutr. 2023, 73, 116. [Google Scholar] [CrossRef]
- Longo, G.M.; Buda, S.J.; Fiotta, N.; Xiong, W.; Griener, T.; Shapiro, S.; Baxter, B.T. MMP-12 has a role in abdominal aortic aneurysms in mice. Surgery 2005, 137, 457–462. [Google Scholar] [CrossRef]
- Koch, A.E.; Kunkel, S.L.; Pearce, W.H.; Shah, M.R.; Parikh, D.; Evanoff, H.L.; Haines, G.K.; Burdick, M.D.; Strieter, R.M. Enhanced production of the chemotactic cytokines interleukin-8 and monocyte chemoattractant protein-1 in human abdominal aortic aneurysms. Am. J. Pathol. 1993, 142, 1423. [Google Scholar]
- Papalambros, E.; Sigala, F.; Georgopoulos, S.; Menekakos, C.; Giatromanolaki, A.; Bastounis, E.; Sivridis, E. Immunohistochemical expression of metalloproteinases MMP-2 and MMP-9 in abdominal aortic aneurysms: Correlation with symptoms and aortic diameter. Int. J. Mol. Med. 2003, 12, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Fang, T. The expression and significance of NF-κB, MMP1, and MMP2 in rats with abdominal aortic aneurysm. Cell. Mol. Biol. 2020, 66, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Curci, J.A.; Liao, S.; Huffman, M.D.; Shapiro, S.D.; Thompson, R.W. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J. Clin. Investig. 1998, 102, 1900–1910. [Google Scholar] [CrossRef]
- Mao, D.; Lee, J.K.; VanVickle, S.J.; Thompson, R.W. Expression of collagenase-3 (MMP-13) in human abdominal aortic aneurysms and vascular smooth muscle cells in culture. Biochem. Biophys. Res. Commun. 1999, 261, 904–910. [Google Scholar] [CrossRef]
- Wilson, W.R.W.; Anderton, M.; Schwalbe, E.C.; Jones, J.L.; Furness, P.N.; Bell, P.R.; Thompson, M.M. Matrix metalloproteinase-8 and-9 are increased at the site of abdominal aortic aneurysm rupture. Circulation 2006, 113, 438–445. [Google Scholar] [CrossRef]
- Brophy, C.M.; Marks, W.H.; Reilly, J.M.; Tilson, M.D. Decreased tissue inhibitor of metalloproteinases (TIMP) in abdominal aortic aneurysm tissue: A preliminary report. J. Surg. Res. 1991, 50, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Elmore, J.R.; Keister, B.F.; Franklin, D.P.; Youkey, J.R.; Carey, D.J. Expression of matrix metalloproteinases and TIMPs in human abdominal aortic aneurysms. Ann. Vasc. Surg. 1998, 12, 221–228. [Google Scholar] [CrossRef]
- Eskandari, M.K.; Vijungco, J.D.; Flores, A.; Borensztajn, J.; Shively, V.; Pearce, W.H. Enhanced abdominal aortic aneurysm in TIMP-1-deficient mice1. J. Surg. Res. 2005, 123, 289–293. [Google Scholar] [CrossRef]
- Hu, M.; Meganathan, I.; Zhu, J.; MacArthur, R.; Kassiri, Z. Loss of TIMP3, but not TIMP4, exacerbates thoracic and abdominal aortic aneurysm. J. Mol. Cell. Cardiol. 2023, 184, 61–74. [Google Scholar] [CrossRef]
- Zhong, S.; Khalil, R.A. A Disintegrin and Metalloproteinase (ADAM) and ADAM with thrombospondin motifs (ADAMTS) family in vascular biology and disease. Biochem. Pharmacol. 2019, 164, 188–204. [Google Scholar] [CrossRef]
- Zhang, P.; Shen, M.; Fernandez-Patron, C.; Kassiri, Z. ADAMs family and relatives in cardiovascular physiology and pathology. J. Mol. Cell. Cardiol. 2016, 93, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Giebeler, N.; Zigrino, P. A disintegrin and metalloprotease (ADAM): Historical overview of their functions. Toxins 2016, 8, 122. [Google Scholar] [CrossRef]
- Mizoguchi, T.; MacDonald, B.T.; Bhandary, B.; Popp, N.R.; Laprise, D.; Arduini, A.; Lai, D.; Zhu, Q.M.; Xing, Y.; Kaushik, V.K. Coronary disease association with ADAMTS7 is due to protease activity. Circ. Res. 2021, 129, 458–470. [Google Scholar] [CrossRef]
- Geng, L.; Wang, W.; Chen, Y.; Cao, J.; Lu, L.; Chen, Q.; He, R.; Shen, W. Elevation of ADAM10, ADAM17, MMP-2 and MMP-9 expression with media degeneration features CaCl2-induced thoracic aortic aneurysm in a rat model. Exp. Mol. Pathol. 2010, 89, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Chute, M.; Hu, M.; Winkelaar, G.; Owen, C.A.; Oudit, G.Y.; Kassiri, Z. ADAM (a disintegrin and metalloproteinase) 15 deficiency exacerbates Ang II (angiotensin II)–Induced aortic remodeling leading to abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1918–1934. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Barallobre-Barreiro, J.; Mayr, U.; Lu, R.; Didangelos, A.; Baig, F.; Lynch, M.; Catibog, N.; Joshi, A.; Barwari, T. Role of ADAMTS-5 in aortic dilatation and extracellular matrix remodeling. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1537–1548. [Google Scholar] [CrossRef]
- Qiu, R.; Chen, S.; Gao, P.; Luo, K.; Feng, X.; Yuan, H.; Wu, X.; Li, G. ADAM10 attenuates the development of abdominal aortic aneurysms in a mouse model. Mol. Med. Rep. 2021, 24, 774. [Google Scholar]
- Jiao, T.; Yao, Y.; Zhang, B.; Hao, D.-C.; Sun, Q.-F.; Li, J.-B.; Yuan, C.; Jing, B.; Wang, Y.-P.; Wang, H.-Y. Role of MicroRNA-103a targeting ADAM10 in abdominal aortic aneurysm. BioMed Res. Int. 2017, 2017, 9645874. [Google Scholar] [CrossRef]
- Shen, G.; Sun, Q.; Yao, Y.; Li, S.; Liu, G.; Yuan, C.; Li, H.; Xu, Y.; Wang, H. Role of ADAM9 and miR-126 in the development of abdominal aortic aneurysm. Atherosclerosis 2020, 297, 47–54. [Google Scholar] [CrossRef]
- Oller, J.; Méndez-Barbero, N.; Ruiz, E.J.; Villahoz, S.; Renard, M.; Canelas, L.I.; Briones, A.M.; Alberca, R.; Lozano-Vidal, N.; Hurlé, M.A. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat. Med. 2017, 23, 200–212. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhao, G.; He, L.; Fu, Y.; Yu, C.; Wang, Z.; Zhao, T.; Cao, F.; Gao, Y. Postnatal deficiency of ADAMTS1 ameliorates thoracic aortic aneurysm and dissection in mice. Exp. Physiol. 2018, 103, 1717–1731. [Google Scholar] [CrossRef] [PubMed]
- Fashandi, A.Z.; Spinosa, M.; Salmon, M.; Su, G.; Montgomery, W.; Mast, A.; Lu, G.; Hawkins, R.B.; Cullen, J.M.; Sharma, A.K. Female mice exhibit abdominal aortic aneurysm protection in an established rupture model. J. Surg. Res. 2020, 247, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Lu, G.; Su, G.; McEvoy, B.; Sadiq, O.; DiMusto, P.D.; Laser, A.; Futchko, J.S.; Henke, P.K.; Eliason, J.L. Phosphorylation of AKT and abdominal aortic aneurysm formation. Am. J. Pathol. 2014, 184, 148–158. [Google Scholar] [CrossRef] [PubMed]
| RNA | Animal Model/Sample/Cell | Modeling Method | Downstream Molecule/ Signaling Pathway | Promotes (+)/Inhibits (−) VSMC Phenotypic Switching | VSMC Differentiation Markers | VSMC Dedifferentiation Markers | Other Cytokine/Gene | Disease | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| MicroRNA-134-5p | Mice | High-fat Diet + Chronic AngII Infusion | STAT 5 B/ITGB 1 Pathway | Inhibition (−) | α-SMA SM22α CNN1 SMMHC | MMP2 MMP9 MMP12 | ADAMTS1 ADAMTS4 ADAMTS7 Col2A1 VEGFA SMAD6 MKNK1 | Thoracic aortic dissection | [199] |
| MicroRNA-342-5p | MOVAS cells | MOVAS cells were transfected with miR-342-5p mimics | Akt Pathway | Activation (+) | α-SMA | Vimentin | —— | —— | [200] |
| MicroRNA-23b | Mice | Chronic infusion of AngII on the backgroun of APOE knockout mice. | FoxO4 Pathway | Inhibition (−) | α-SMA SM22α CNN1 | —— | —— | Abdominal aortic aneurysm | [201] |
| MicroRNA-126-5p | Mice | Chronic infusion of AngII on the background of APOE knockout mice. | VEPH1 Pathway | Activation (+) | α-SMA SMMHC | PCNA Vimentin | MMP2 MMP9 | Abdominal aortic aneurysm | [78] |
| MicroRNA-29b | —— | —— | ATG14 Pathway | Inhibition (−) | α-SMA SM22α SM-MHC | —— | MMP-2 MMP-3 TNF-α Beclin-1 ATG5 ATG14 p62 Beclin-1 ATG5 ATG14 | Intracranial aneurysm | [202] |
| MicroRNA-128-3p | Mice | ApoE−/− animals of either gender with a hypercholesterolemic diet for 16 weeks | KLF4 Pathway | Inhibition (−) | α-SMA CNN1 SMMHC SM22α | —— | —— | Atherosclerosis and carotid stenosis | [203] |
| MicroRNA-564 | Mice | AngII infects cells | SKI/NRGN Pathway | Inhibition (−) | α-SMA MHC | —— | —— | Abdominal aortic dissection | [204] |
| MicroRNA-199a-5p- | AAA-VSMCs from patients | SIRT1/ROS Pathway | Activation (+) | α-SMA | P53 p21 | Abdominal aortic aneurysm | [205] | ||
| MicroRNA-126 | Mice | Carotid artery ligation | Argonaute2 Pathway | Inhibition (−) | α-SMA | —— | —— | Atherosclerosis | [206] |
| MicroRNA-24 | Murine | Porcine-pancreatic-elastase (PPE) and AngII infusion | MAPK/NF-κB Pathway | Inhibition (−) | —— | —— | IL-6 IL-8 IL-1β TLR4 | Abdominal aortic aneurysm | [207] |
| MicroRNA-221/222 | Human samples | —— | —— | —— | —— | —— | —— | Acute coronary syndrome | [208] |
| MicroRNA-146a | Rat VSMCs | Artificially synthesized miR-146a mimics was transfected into cultured primary rat VSMCs in vitro | p53 Pathway | Activation (+) | —— | CCK-8 Cyclin D1 | Caspase-3 PTEN | —— | [209] |
| MicroRNA-155-5p | HASMC | H2O2 or NaAsO2 suppressed viability and induced apoptosis of VSMCs | FOS/ZIC 3 Pathway | Activation (+) | —— | Cyclin A Cyclin B Cyclin D | Caspase-3 Bcl-2 | Abdominal aortic aneurysm | [210] |
| MicroRNA-195-5p | Rat VSMCs | Osteogenic induction of VSMCs by β-glycerophosphate (β-GP) | Wnt/β-catenin Pathway | Activation (+) | —— | Runt Runx2 BMP2 ALP OCN Smad7 | IL-6 TNF-α | —— | [211] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, D.; Zhang, M.; Zhang, Y.; Shi, Y.; Liu, X.; Wu, X.; Yang, Z. The Pathophysiological Role of Vascular Smooth Muscle Cells in Abdominal Aortic Aneurysm. Cells 2025, 14, 1009. https://doi.org/10.3390/cells14131009
Shi D, Zhang M, Zhang Y, Shi Y, Liu X, Wu X, Yang Z. The Pathophysiological Role of Vascular Smooth Muscle Cells in Abdominal Aortic Aneurysm. Cells. 2025; 14(13):1009. https://doi.org/10.3390/cells14131009
Chicago/Turabian StyleShi, Dou, Mo Zhang, Yuhan Zhang, Yang Shi, Xing Liu, Xianxian Wu, and Zhiwei Yang. 2025. "The Pathophysiological Role of Vascular Smooth Muscle Cells in Abdominal Aortic Aneurysm" Cells 14, no. 13: 1009. https://doi.org/10.3390/cells14131009
APA StyleShi, D., Zhang, M., Zhang, Y., Shi, Y., Liu, X., Wu, X., & Yang, Z. (2025). The Pathophysiological Role of Vascular Smooth Muscle Cells in Abdominal Aortic Aneurysm. Cells, 14(13), 1009. https://doi.org/10.3390/cells14131009





