Alterations of JNK Signaling Pathway Activity in the Rat Retina: Effects of Age, Age-Related Macular Degeneration-like Pathology, and a JNK Inhibitor (IQ-1S)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Treatment of Animals
2.3. Western Blotting
2.4. Electron-Microscopic Examination
2.5. Histological Examination
2.6. Immunohistochemical Examination
2.7. Statistical Analysis
3. Results
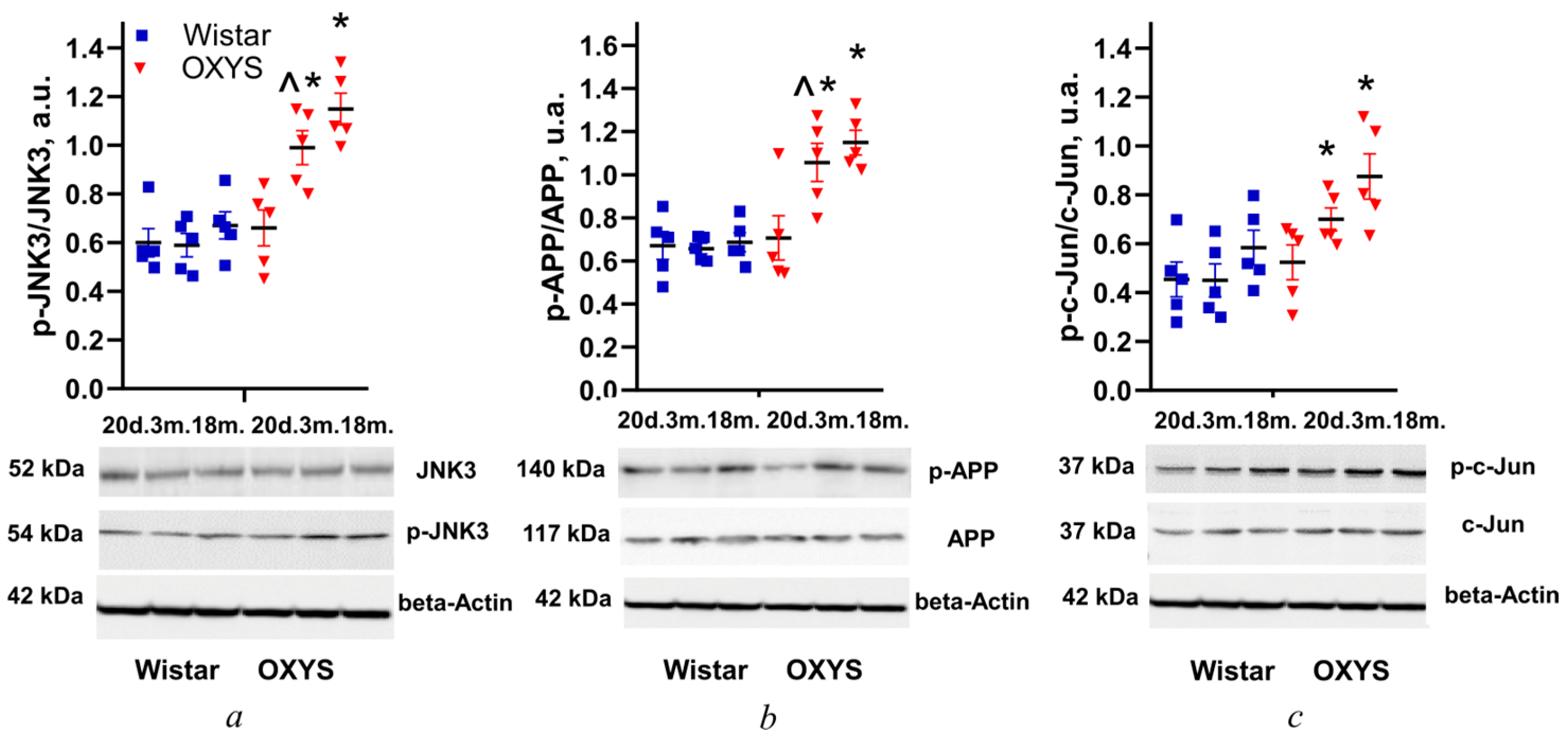
3.1. Changes in Levels of JNK3, APP, and c-Jun Phosphorylation in the Retina of Wistar and OXYS Rats with Age
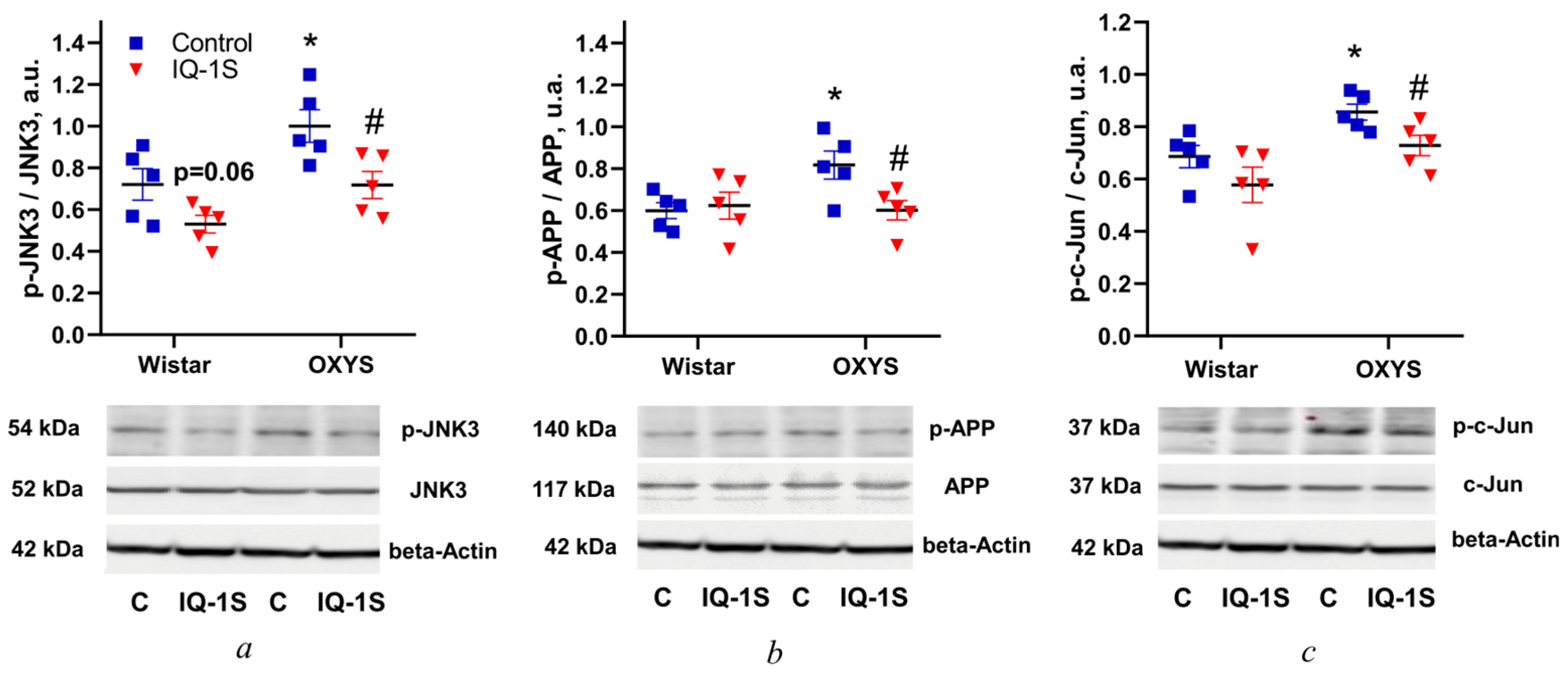
3.2. IQ-1S Prevents the Increase in Levels of JNK3, APP, and c-Jun Phosphorylation in the Rat Retina
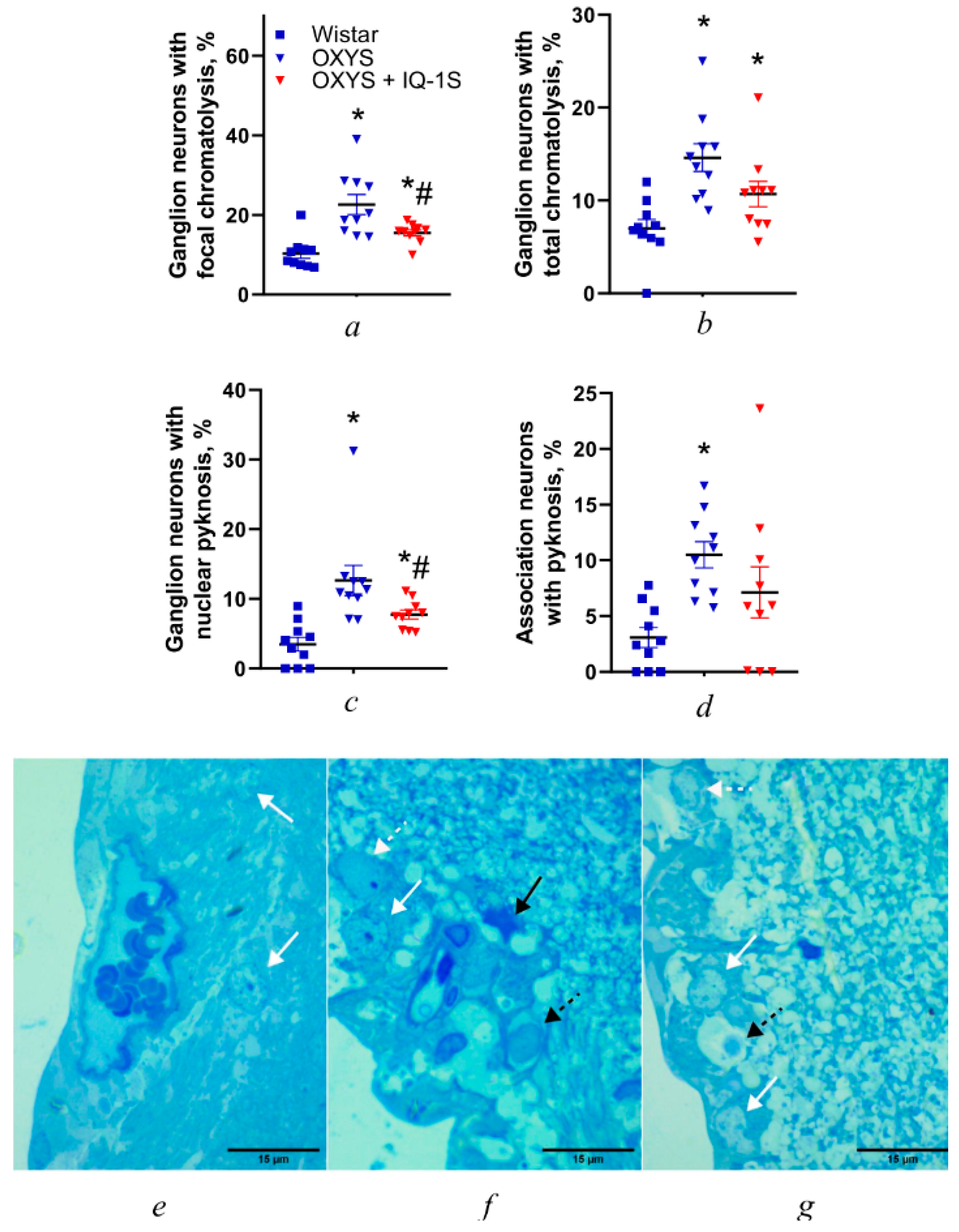
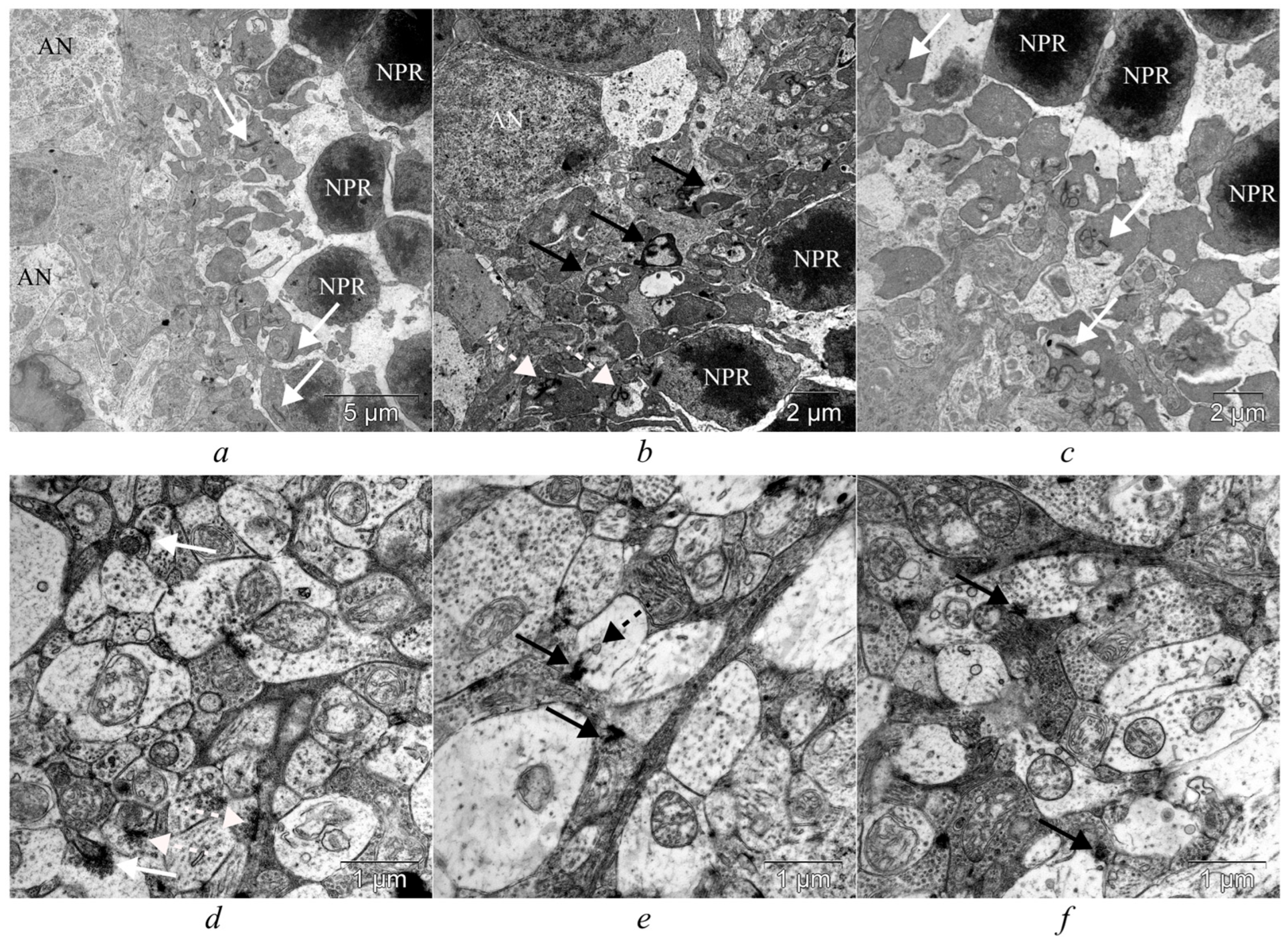
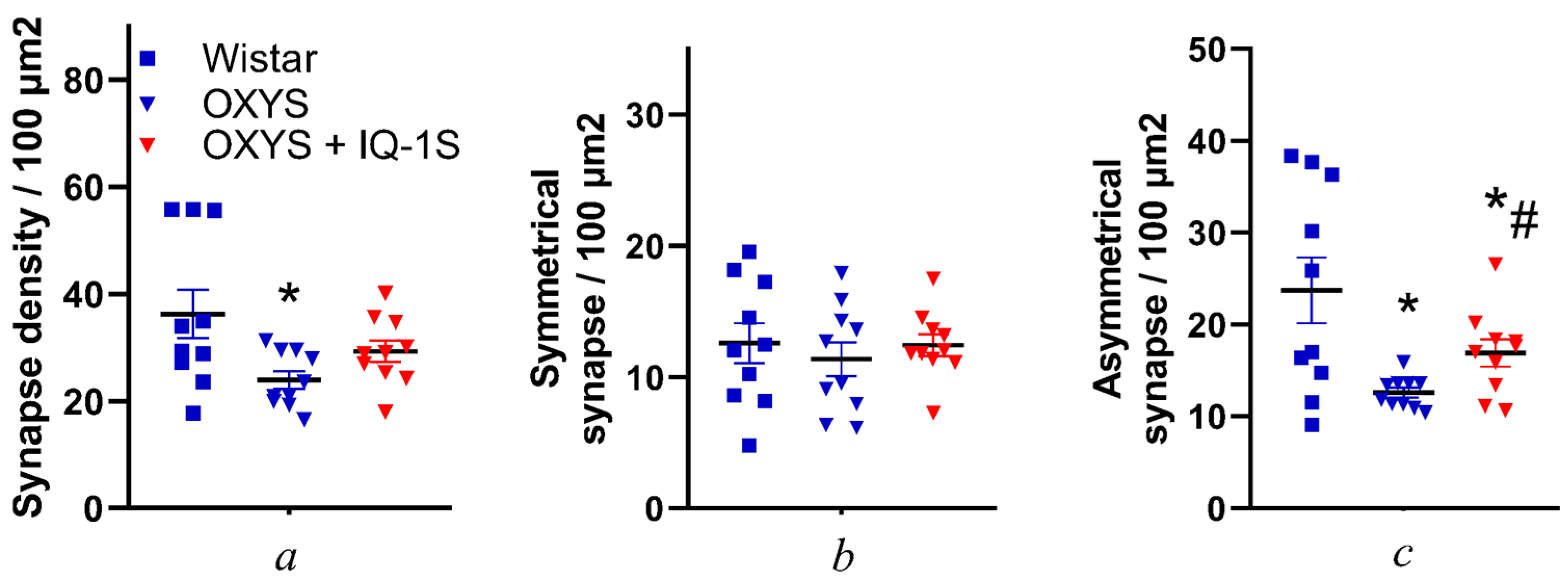
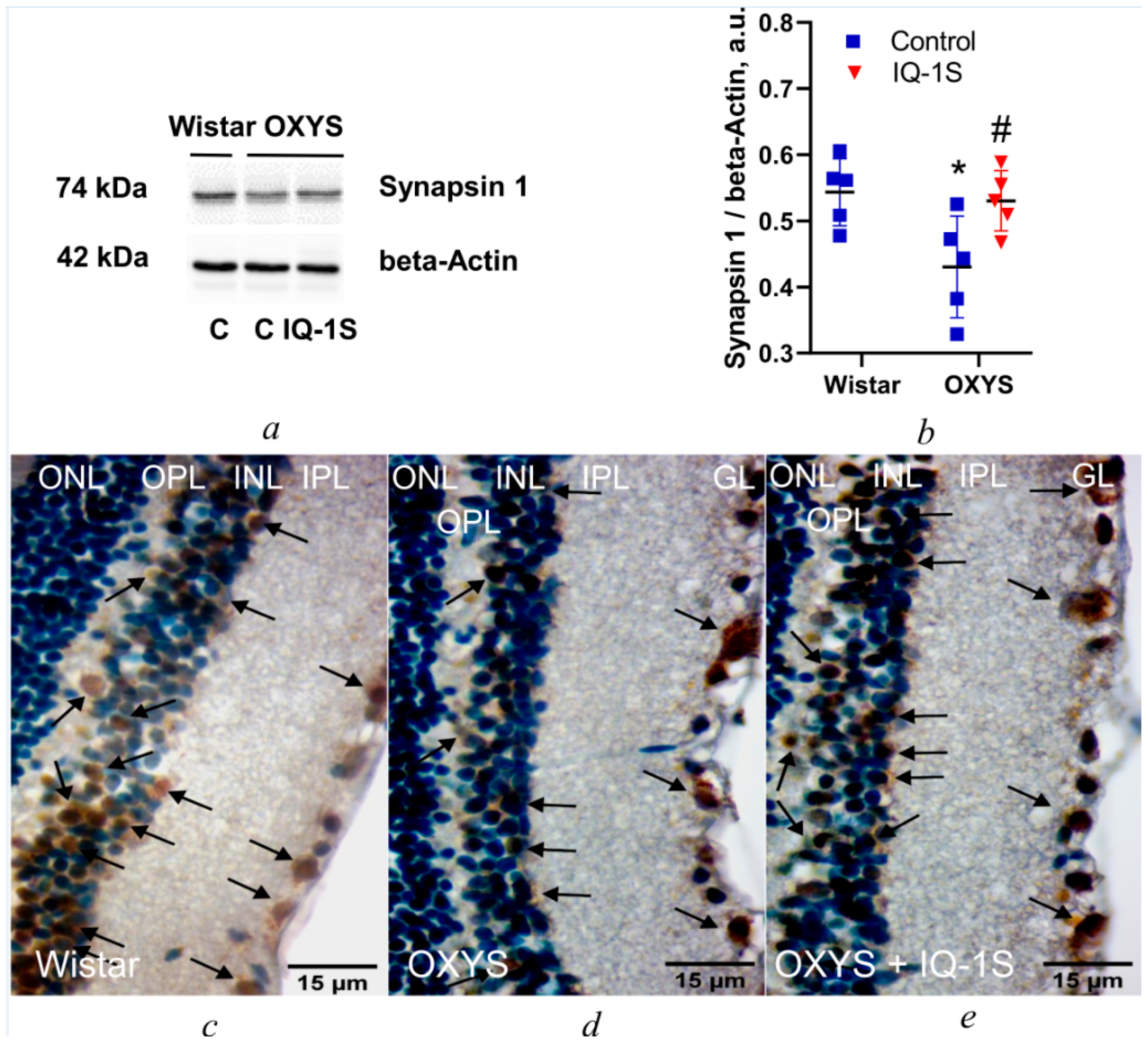
3.3. IQ-1S Attenuates Neurodegeneration in the Ganglion Layer and Associated Synaptic Disorders in the Inner Plexiform Layer of OXYS Rats
3.4. IQ-1S Enhances Synapsin 1 Expression in the OXYS Rat Retina
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACZ | Active contact zone |
| AD | Alzheimer’s disease |
| AMD | Age-related macular degeneration |
| APP | Amyloid beta precursor protein |
| IQ-1S | Sodium salt of 11H-indeno[1,2-b]quinoxalin-11-one oxime |
| JNK | C-Jun N-terminal kinase |
| OPL | Outer plexiform layer |
| RPE | Retinal pigment epithelium |
References
- Gil-Martínez, M.; Santos-Ramos, P.; Fernández-Rodríguez, M.; Abraldes, M.J.; Rodríguez-Cid, M.J.; Santiago-Varela, M.; Fernández-Ferreiro, A.; Gómez-Ulla, F. Pharmacological Advances in the Treatment of Age-Related Macular Degeneration. Curr. Med. Chem. 2020, 27, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-Related Macular Degeneration. Med. Clin. N. Am. 2021, 105, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.D.; Tarachand, S.P.; Abdulwahab, Q.; Samuel, M.A. Structure, Function, and Molecular Landscapes of the Aging Retina. Annu. Rev. Vis. Sci. 2023, 9, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Gierke, K.; Lux, U.T.; Regus-Leidig, H.; Brandstätter, J.H. The First Synapse in Vision in the Aging Mouse Retina. Front. Cell. Neurosci. 2023, 17, 1291054. [Google Scholar] [CrossRef]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Musi, C.; Bonadonna, C.; Borsello, T. Synaptic Alterations as a Common Phase in Neurological and Neurodevelopmental Diseases: JNK Is a Key Mediator in Synaptic Changes. Neural Regen. Res. 2023, 18, 531. [Google Scholar] [CrossRef]
- Li, Y.; You, L.; Nepovimova, E.; Adam, V.; Heger, Z.; Jomova, K.; Valko, M.; Wu, Q.; Kuca, K. C-Jun N-Terminal Kinase Signaling in Aging. Front. Aging Neurosci. 2024, 16, 1453710. [Google Scholar] [CrossRef]
- Kim, B.J.; Zack, D.J. The Role of C-Jun N-Terminal Kinase (JNK) in Retinal Degeneration and Vision Loss. Adv. Exp. Med. Biol. 2018, 1074, 351–357. [Google Scholar]
- Zhao, Y.; Ding, M.; Yan, F.; Yin, J.; Shi, W.; Yang, N.; Zhao, H.; Fang, Y.; Huang, Y.; Zheng, Y.; et al. Inhibition of the JAK2/STAT3 Pathway and Cell Cycle Re-Entry Contribute to the Protective Effect of Remote Ischemic Pre-Conditioning of Rat Hindlimbs on Cerebral Ischemia/Reperfusion Injury. CNS Neurosci. Ther. 2022, 29, 866–877. [Google Scholar] [CrossRef]
- Nakano, R.; Nakayama, T.; Sugiya, H. Biological Properties of JNK3 and Its Function in Neurons, Astrocytes, Pancreatic β-Cells and Cardiovascular Cells. Cells 2020, 9, 19. [Google Scholar] [CrossRef]
- Yan, H.; He, L.; Lv, D.; Yang, J.; Yuan, Z. The Role of the Dysregulated JNK Signaling Pathway in the Pathogenesis of Human Diseases and Its Potential Therapeutic Strategies: A Comprehensive Review. Biomolecules 2024, 14, 243. [Google Scholar] [CrossRef] [PubMed]
- Devi, B.; Jangid, K.; Kumar, N.; Kumar, V.; Kumar, V. Identification of Potential JNK3 Inhibitors through Virtual Screening, Molecular Docking and Molecular Dynamics Simulation as Therapeutics for Alzheimer’s Disease. Mol. Divers. 2024, 28, 4361–4380. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, Y.; Guan, Z.; Esmaeili, S.; Xiao, Z.; Kuriakose, D. JNK3 inhibitors as promising pharmaceuticals with neuroprotective properties. Cell Adhes. Migr. 2024, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kuca, K.; Wu, W.; Wang, X.; Nepovimova, E.; Musilek, K.; Wu, Q. Hypothesis: JNK Signaling Is a Therapeutic Target of Neurodegenerative Diseases. Alzheimer’s Dement. 2022, 18, 152–158. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Khlebnikov, A.I.; Potapov, A.S.; Kovrizhina, A.R.; Matveevskaya, V.V.; Belyanin, M.L.; Atochin, D.N.; Zanoza, S.O.; Gaidarzhy, N.M.; Lyakhov, S.A.; et al. Synthesis, Biological Evaluation, and Molecular Modeling of 11H-Indeno[1,2-b]Quinoxalin-11-One Derivatives and Tryptanthrin-6-Oxime as c-Jun N-Terminal Kinase Inhibitors. Eur. J. Med. Chem. 2019, 161, 179–191. [Google Scholar] [CrossRef]
- Atochin, D.N.; Schepetkin, I.A.; Khlebnikov, A.I.; Seledtsov, V.I.; Swanson, H.; Quinn, M.T.; Huang, P.L. A Novel Dual NO-Donating Oxime and c-Jun N-Terminal Kinase Inhibitor Protects against Cerebral Ischemia-Reperfusion Injury in Mice. Neurosci. Lett. 2016, 618, 45–49. [Google Scholar] [CrossRef]
- Nie, Z.; Xia, X.; Zhao, Y.; Zhang, S.; Zhang, Y.; Wang, J. JNK Selective Inhibitor, IQ-1S, Protects the Mice against Lipopolysaccharides-Induced Sepsis. Bioorganic Med. Chem. 2021, 30, 115945. [Google Scholar] [CrossRef]
- Zhdankina, A.A.; Osipenko, A.N.; Tikhonov, D.I.; Logvinov, S.V.; Plotnikov, M.B.; Khlebnikov, A.I.; Kolosova, N.G. The IQ-1S JNK (c-Jun N-Terminal Kinase) Inhibitor Suppresses Premature Aging of OXYS Rat Brain. Neurochem. J. 2023, 17, 369–379. [Google Scholar] [CrossRef]
- Muraleva, N.A.; Zhdankina, A.A.; Khlebnikov, A.I.; Kolosova, N.G. JNK signaling pathway activity alterations in the rat hippocampus: Effect of age, Alzheimer’s Disease-like pathology development and the JNK Inhibitor IQ-1S. Biochemistry 2025, 90, 265–275. [Google Scholar] [CrossRef]
- Zhdankina, A.A.; Tikhonov, D.I.; Logvinov, S.V.; Plotnikov, M.B.; Khlebnikov, A.I.; Kolosova, N.G. Suppression of Age-Related Macular Degeneration-like Pathology by c-Jun N-Terminal Kinase Inhibitor IQ-1S. Biomedicines 2023, 11, 395. [Google Scholar] [CrossRef]
- Markovets, A.M.; Saprunova, V.B.; Zhdankina, A.A.; Fursova, A.Z.H.; Bakeeva, L.E.; Kolosova, N.G. Alterations of Retinal Pigment Epithelium Cause AMD-like Retinopathy in Senescence-Accelerated OXYS Rats. Aging 2011, 3, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, N.G.; Stefanova, N.A.; Korbolina, E.E.; Fursova, A.Z.H.; Kozhevnikova, O.S. The Senescence-Accelerated Oxys Rats–a Genetic Model of Premature Aging and Age-Dependent Degenerative Diseases. Adv. Gerontol. 2014, 27, 336–340. [Google Scholar] [PubMed]
- Sukhorukov, V.; Magnaeva, A.; Baranich, T.; Gofman, A.; Voronkov, D.; Gulevskaya, T.; Glinkina, V.; Illarioshkin, S. Brain Neurons during Physiological Aging: Morphological Features, Autophagic and Mitochondrial Contribution. Int. J. Mol. Sci. 2022, 23, 10695. [Google Scholar] [CrossRef] [PubMed]
- Moon, L.D.F. Chromatolysis: Do Injured Axons Regenerate Poorly When Ribonucleases Attack Rough Endoplasmic Reticulum, Ribosomes and RNA? Dev. Neurobiol. 2018, 78, 1011–1024. [Google Scholar] [CrossRef]
- Dowling, J.E. Synaptic Organization of the Frog Retina: An Electron Microscopic Analysis Comparing the Retinas of Frogs and Primates. Proc. R. Soc. Lond. 1968, 170, 205–228. [Google Scholar] [CrossRef]
- Sterling, P.; Matthews, G. Structure and Function of Ribbon Synapses. Trends Neurosci. 2005, 28, 20–29. [Google Scholar] [CrossRef]
- Wässle, H. Parallel Processing in the Mammalian Retina. Nat. Rev. Neurosci. 2004, 5, 747–757. [Google Scholar] [CrossRef]
- Demb, J.B. Cellular Mechanisms for Direction Selectivity in the Retina. Neuron 2007, 55, 179–186. [Google Scholar] [CrossRef]
- Eltokhi, A.; Santuy, A.; Merchan-Perez, A.; Sprengel, R. Glutamatergic Dysfunction and Synaptic Ultrastructural Alterations in Schizophrenia and Autism Spectrum Disorder: Evidence from Human and Rodent Studies. Int. J. Mol. Sci. 2020, 22, 26. [Google Scholar] [CrossRef]
- Cheng, J.H.T. Stimulation Induces Gradual Increases in the Thickness and Curvature of Postsynaptic Density of Hippocampal CA1 Neurons in Slice Cultures. Mol. Brain 2019, 12, 44. [Google Scholar] [CrossRef]
- Cesca, F.; Baldelli, P.; Valtorta, F.; Benfenati, F. The Synapsins: Key Actors of Synapse Function and Plasticity. Prog. Neurobiol. 2010, 91, 313–348. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Zulfiqar, A.; Arguelles, S.; Rasekhian, M.; Nabavi, S.F.; Silva, A.S.; Nabavi, S.M. Map Kinase Signaling as Therapeutic Target for Neurodegeneration. Pharmacol. Res. 2020, 160, 105090. [Google Scholar] [CrossRef] [PubMed]
- Tiziana, B.; Gianluigi, F. JNK Signalling: A Possible Target to Prevent Neurodegeneration. Curr. Pharm. Des. 2007, 13, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, M.R.; Chaudhary, S.; Sharma, Y.; Singh, T.A.; Mishra, A.K.; Sharma, S.; Mehdi, M.M. Aging, oxidative stress and degenerative diseases: Mechanisms, complications and emerging therapeutic strategies. Biogerontology 2023, 24, 609–662. [Google Scholar] [CrossRef]
- Muraleva, N.A.; Kolosova, N.G. Alteration of the MEK1/2–ERK1/2 Signaling Pathway in the Retina Associated with Age and Development of AMD-Like Retinopathy. Biochemistry 2023, 88, 179–188. [Google Scholar] [CrossRef]
- Muraleva, N.A.; Kolosova, N.G. P38 MAPK Signaling in the Retina: Effects of Aging and Age-Related Macular Degeneration. Int. J. Mol. Sci. 2023, 24, 11586. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Salminen, A.; Haapasalo, A.; Soininen, H.; Hiltunen, M. Age-related macular degeneration (AMD): Alzheimer’s disease in the eye? J. Alzheimer’s Dis. 2011, 24, 615–631. [Google Scholar] [CrossRef]
- Blasiak, J.; Sobczuk, P.; Pawlowska, E.; Kaarniranta, K. Interplay between aging and other factors of the pathogenesis of age-related macular degeneration. Ageing Res. Rev. 2022, 81, 101735. [Google Scholar] [CrossRef]
- De Wilde, M.C.; Overk, C.R.; Sijben, J.W.; Masliah, E. Meta-analysis of Synaptic Pathology in Alzheimer’s Disease Reveals Selective Molecular Vesicular Machinery Vulnerability. Alzheimer’s Dement. 2016, 12, 633–644. [Google Scholar] [CrossRef]
- Burke, S.N.; Barnes, C.A. Neural Plasticity in the Ageing Brain. Nat. Rev. Neurosci. 2006, 7, 30–40. [Google Scholar] [CrossRef]
- Samuel, M.A.; Zhang, Y.; Meister, M.; Sanes, J.R. Age-Related Alterations in Neurons of the Mouse Retina. J. Neurosci. 2011, 31, 16033–16044. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muraleva, N.A.; Tikhonov, D.I.; Zhdankina, A.A.; Plotnikov, M.B.; Khlebnikov, A.I.; Logvinov, S.V.; Kolosova, N.G. Alterations of JNK Signaling Pathway Activity in the Rat Retina: Effects of Age, Age-Related Macular Degeneration-like Pathology, and a JNK Inhibitor (IQ-1S). Cells 2025, 14, 896. https://doi.org/10.3390/cells14120896
Muraleva NA, Tikhonov DI, Zhdankina AA, Plotnikov MB, Khlebnikov AI, Logvinov SV, Kolosova NG. Alterations of JNK Signaling Pathway Activity in the Rat Retina: Effects of Age, Age-Related Macular Degeneration-like Pathology, and a JNK Inhibitor (IQ-1S). Cells. 2025; 14(12):896. https://doi.org/10.3390/cells14120896
Chicago/Turabian StyleMuraleva, Natalia A., Dmitry I. Tikhonov, Anna A. Zhdankina, Mark B. Plotnikov, Andrei I. Khlebnikov, Sergey V. Logvinov, and Nataliya G. Kolosova. 2025. "Alterations of JNK Signaling Pathway Activity in the Rat Retina: Effects of Age, Age-Related Macular Degeneration-like Pathology, and a JNK Inhibitor (IQ-1S)" Cells 14, no. 12: 896. https://doi.org/10.3390/cells14120896
APA StyleMuraleva, N. A., Tikhonov, D. I., Zhdankina, A. A., Plotnikov, M. B., Khlebnikov, A. I., Logvinov, S. V., & Kolosova, N. G. (2025). Alterations of JNK Signaling Pathway Activity in the Rat Retina: Effects of Age, Age-Related Macular Degeneration-like Pathology, and a JNK Inhibitor (IQ-1S). Cells, 14(12), 896. https://doi.org/10.3390/cells14120896






